Subsampling of Regional-Scale Database for improving Multivariate Analysis Interpretation of Groundwater Chemical Evolution and Ion Sources
Abstract
:1. Introduction
2. Hydrogeological Context
3. The Statistical Treatment of the Regional Database
4. Methodology
4.1. Sample Grouping as Hydrogeochemical Poles (RHPs)
4.2. Detection Ratio of the Key Chemical Elements
4.3. Multivariate Analysis
5. Results
5.1. Identification of the Regional Hydrogeochemical Poles (RHPs)
5.2. Chemical Characteristics of the Subset versus the Regional-Scale Database
5.3. Results Comparison between Subset and Regional-Scale Database
5.3.1. Hierarchical Cluster Analysis Results
5.3.2. Principal Component Analysis Results
5.3.3. New Graphical Fields and Interpretations Improvement
5.4. Factor Analysis
6. Discussion
6.1. Evolution of Recharge Groundwater (Clusters 1 and 2; Factor 4)
6.2. Fingerprint of the Laflamme Seawater (Cluster 4; Factor 1)
(microcline) (illite)
6.3. Fingerprinting of the Precambrian Shield Brines (Cluster 3; Factor 3)
6.4. Sources of Dissolved Fluoride in Groundwater (Factor 2)
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvin, C.R. Methods of Multivariate Analysis; Wiley Interscience: Hoboken, NJ, USA, 2002. [Google Scholar]
- Farnham, I.M.; Johannesson, K.H.; Singh, A.K.; Hodge, V.F.; Stetzenbach, K.J. Factor analytical approaches for evaluating groundwater trace element chemistry data. Anal. Chim. Acta 2003, 490, 123–138. [Google Scholar] [CrossRef]
- Brown, C.E. Applied Multivariate Statistics in Geohydrology and Related Sciences; Springer: Berlin, Germany, 1998. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality: Recommendations, (Vol. 1); World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Walter, J.; Chesnaux, R.; Cloutier, V.; Gaboury, D. The influence of water/rock—Water/clay interactions and mixing in the salinization processes of groundwater. J. Hydrol. Reg. Stud. 2017, 13, 168–188. [Google Scholar] [CrossRef]
- Laurin, A.F.; Sharma, K.N.M. Mistassini, Peribonka, Saguenay Rivers Area: Grenville 1965–1967; Ministère des Richesses Naturelles, Direction générale des mines, Geological Exploration Service: Québec, QC, Canada, 1975. [Google Scholar]
- Desbiens, S.; Lespérance, P.J. Stratigraphy of the Ordovician of the Lac Saint-Jean and Chicoutimi outliers, Quebec. Can. J. Earth Sci. 1989, 26, 1185–1202. [Google Scholar] [CrossRef]
- CERM-PACES. Résultats du Programme D’acquisition de Connaissances sur les Eaux Souterraines du Saguenay-Lac-Saint-Jean, Centre D’études sur les Ressources Minérales; Université du Québec à Chicoutimi: Saguenay, QC, Canada, 2013. [Google Scholar]
- Walter, J.; Rouleau, A.; Chesnaux, R.; Lambert, M.; Daigneault, R. Characterization of general and singular features of major aquifer systems in the Saguenay-Lac-Saint-Jean region. Can. Water Resour. J./Revue Can. Ressour. Hydr. 2018, 43, 75–91. [Google Scholar] [CrossRef]
- Dionne, J.; Laverdière, C. Sites fossilifères du golfe de Laflamme. Rev. Géogr. Montr. 1969, 23, 259–270. [Google Scholar]
- LaSalle, P.; Tremblay, G. Dépôts meubles: Saguenay Lac Saint-Jean; Ministère des Richesses Naturelles, Direction générales de la Recherche Géologique et Minière: Québec, QC, Canada, 1978. [Google Scholar]
- Daigneault, R.; Cousineau, P.; Leduc, E.; Beaudoin, G.; Milette, S.; Horth, N.; Roy, D.; Lamothe, M.; Allard, G. Rapport Final sur les Travaux de Cartographie des Formations Superficielles Réalisés dans le Territoire Municipalisé du Saguenay-Lac-Saint-Jean (Québec) Entre 2009 et 2011; Gouvernemental Report for the Ministere des Ressources Naturelles et de la Faune (Québec): Québec, QC, Canada, 2011. [Google Scholar]
- Bouchard, R.; Dion, D.J.; Tavenas, F. Origine de la préconsolidation des argiles du Saguenay, Québec. Can. Geotech. J. 1983, 20, 315–328. [Google Scholar] [CrossRef]
- Health Canada. Guidelines for Canadian Drinking Water Quality—Summary Table; Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada: Ottawa, ON, Canada, 2014. [Google Scholar]
- Edmunds, W.M.; Smedley, P.L. Fluoride in Natural Waters. In Essentials of Medical Geology; Springer: Dordrecht, The Netherlands, 2013; pp. 311–336. [Google Scholar]
- Toth, J. Groundwater as a geologic agent: an overview of the causes, processes, and manifestations. Hydrogeol. J. 1999, 7, 1–14. [Google Scholar] [CrossRef]
- Bucher, K.; Stober, I. Fluids in the upper continental crust. Geofluids 2010, 10, 241–253. [Google Scholar]
- Chebotarev, I.I. Metamorphism of natural waters in the crust of weathering 1-2-3. Geochim. Cosmochim. Acta 1955, 8, 22–48. [Google Scholar] [CrossRef]
- Tóth, J. A conceptual model of the groundwater regime and the hydrogeologic environment. J. Hydrol. 1970, 10, 164–176. [Google Scholar] [CrossRef]
- Ingebritsen, S.E.; Sanford, W.E. Groundwater in Geologic Processes; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- StatSoft Inc. STATISTICA Data Analysis Software System, Version 12. 2013. Available online: http://www.statsoft.com (accessed on 21 January 2019).
- Box, G.E.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. Ser. B Methodol. 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Davis, J. Statistics and Data Analysis in Geology; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Gascoyne, M.; Kamineni, D.C. The hydrogeochemistry of fractured plutonic rocks in the Canadian. Appl. Hydrogeol. 1994, 2, 43–49. [Google Scholar] [CrossRef]
- Cloutier, V.; Lefebvre, R.; Savard, M.M.; Therrien, R. Desalination of a sedimentary rock aquifer system invaded by Pleistocene Champlain Sea water and processes controlling groundwater geochemistry. Environ. Earth Sci. 2010, 59, 977–994. [Google Scholar] [CrossRef]
- Cloutier, V.; Lefebvre, R.; Therrien, R.; Savard, M.M. Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J. Hydro. 2008, 353, 294–313. [Google Scholar] [CrossRef]
- Gascoyne, M. Evolution of redox conditions and groundwater composition in recharge-discharge environments on the Canadian Shield. Hydrogeol. J. 1997, 5, 4–18. [Google Scholar] [CrossRef]
- Edmunds, W.; Guendouz, A.H.; Mamou, A.; Moulla, A.; Shand, P.; Zouari, K. “Groundwater evolution in the Continental Intercalaire aquifer of southern Algeria and Tunisia: Trace element and isotopic indicators”. Appl. Geochem. 2003, 18, 805–822. [Google Scholar] [CrossRef]
- Hatva, T. Iron and Manganese in Groundwater in Finland: Occurrence in Glacifluvial Aquifers and Removal by Biofiltration; National Board of Waters and the Environment: Helsinki, Finland, 1989; Volume 4. [Google Scholar]
- Wang, Y.; Jiao, J.J.; Cherry, J.A.; Lee, C.M. Contribution of the aquitard to the regional groundwater hydrochemistry of the underlying confined aquifer in the Pearl River Delta, China. Sci. Total Environ. 2013, 461, 663–671. [Google Scholar] [CrossRef]
- Miao, Z.; Brusseau, M.; Carroll, K.C.; Carreón-Diazconti, C.; Johnson, B. Sulfate reduction in groundwater: Characterization and applications for remediation. Environ. Geochem. Health 2012, 34, 539–550. [Google Scholar] [CrossRef]
- Gravel, J.-Y. Minéralogie de l’argile Champlain de St-Jean-Vianney; Université Laval: Québec, QC, Canada, 1974. [Google Scholar]
- Frape, S.; Fritz, P. The chemistry and isotopic composition of saline groundwaters from the Sudbury Basin, Ontario. Can. J. Earth Sci. 1982, 19, 645–661. [Google Scholar] [CrossRef]
- Bottomley, D.J.; Katz, A.; Chan, L.H.; Starinsky, A.; Douglas, M.; Clark, I.D.; Raven, K.G. The origin and evolution of Canadian Shield brines: Evaporation or freezing of seawater? New lithium isotope and geochemical evidence from the Slave craton. Chem. Geol. 1999, 155, 295–320. [Google Scholar] [CrossRef]
- Frape, S.K.; Fritz, P. Geochemical trends for groundwaters from the Canadian Shield. Saline water and gases in crystalline rocks. Geol. Assoc. Can. Spec. Pap. 1987, 33, 19–38. [Google Scholar]
- Frape, S.K.; Fritz, P.; McNutt, R.H. Water-rock interaction and chemistry of groundwaters from the Canadian Shield. Geochim. Cosmochim. Acta 1984, 48, 1617–1627. [Google Scholar] [CrossRef]
- Beaucaire, C.; Michard, G. Origin of dissolved minor elements (Li, Rb, Sr, Ba) in superificial waters in a granitic area. Geochem. J. 1982, 16, 247–258. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; United States Geological Survey Water Supply Paper 2254; U.S. Geological Survey: Alexandria, VA, USA, 1985. [Google Scholar]
- Edmunds, W.; Smedley, P. Residence time indicators in groundwater: The East Midlands Triassic sandstone aquifer. Appl. Geochem. 2000, 15, 737–752. [Google Scholar] [CrossRef]
- Gascoyne, M.; Ross, J.D.; Watson, R.L.; Kamineni, D.C. Soluble salts in a Canadian Shield granite as contributors to groundwater salinity. In Proceedings of the 6th International Symposium on Water-Rock Interactions, Malvern, UK, 3–12 August 1989; pp. 247–249. [Google Scholar]
- Kamineni, D.C. Halogen-bearing minerals in plutonic rocks: A possible source of chlorine in saline groundwater in the Canadian Shield. In Saline Water and Gases in Crystalline Rocks; Geological Association of Canada Special Paper: St John’s, NL, Canada, 1987; Volume 33, pp. 69–79. [Google Scholar]
- Goldberg, E.; Broecker, W.; Gross, M.; Turekian, K. Radioactivity in the Marine Environment; National Academy of Science: Washington, DC, USA, 1971; p. 137. [Google Scholar]
- Wen, D.; Zhang, F.; Zhang, E.; Wang, C.; Han, S.; Zheng, Y. Arsenic, fluoride and iodine in groundwater of China. J. Geochem. Explor. 2013, 135, 1–21. [Google Scholar] [CrossRef]
- Battaleb-Looie, S.; Moore, F.; Jafari, H.; Jacks, G.; Ozsvath, D. Hydrogeochemical evolution of groundwaters with excess fluoride concentrations from Dashtestan, South of Iran. Environ. Earth Sci. 2012, 67, 1173–1182. [Google Scholar] [CrossRef]
- Walter, J. Les eaux Souterraines à Salinité Élevée Autour du lac Saint-Jean, Québec: Origines et Incidences; Université du Québec à Chicoutimi: Chicoutimi, QC, Canada, 2010. [Google Scholar]
- Chae, G.T.; Yun, S.T.; Kwon, M.J.; Kim, Y.S.; Mayer, B. Batch dissolution of granite and biotite in water: Implication for fluorine geochemistry in groundwater. Geochem. J. 2006, 40, 95–102. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater, Pollution; AA Balkema Publishers: Amsterdam, The Netherlands, 2005. [Google Scholar]
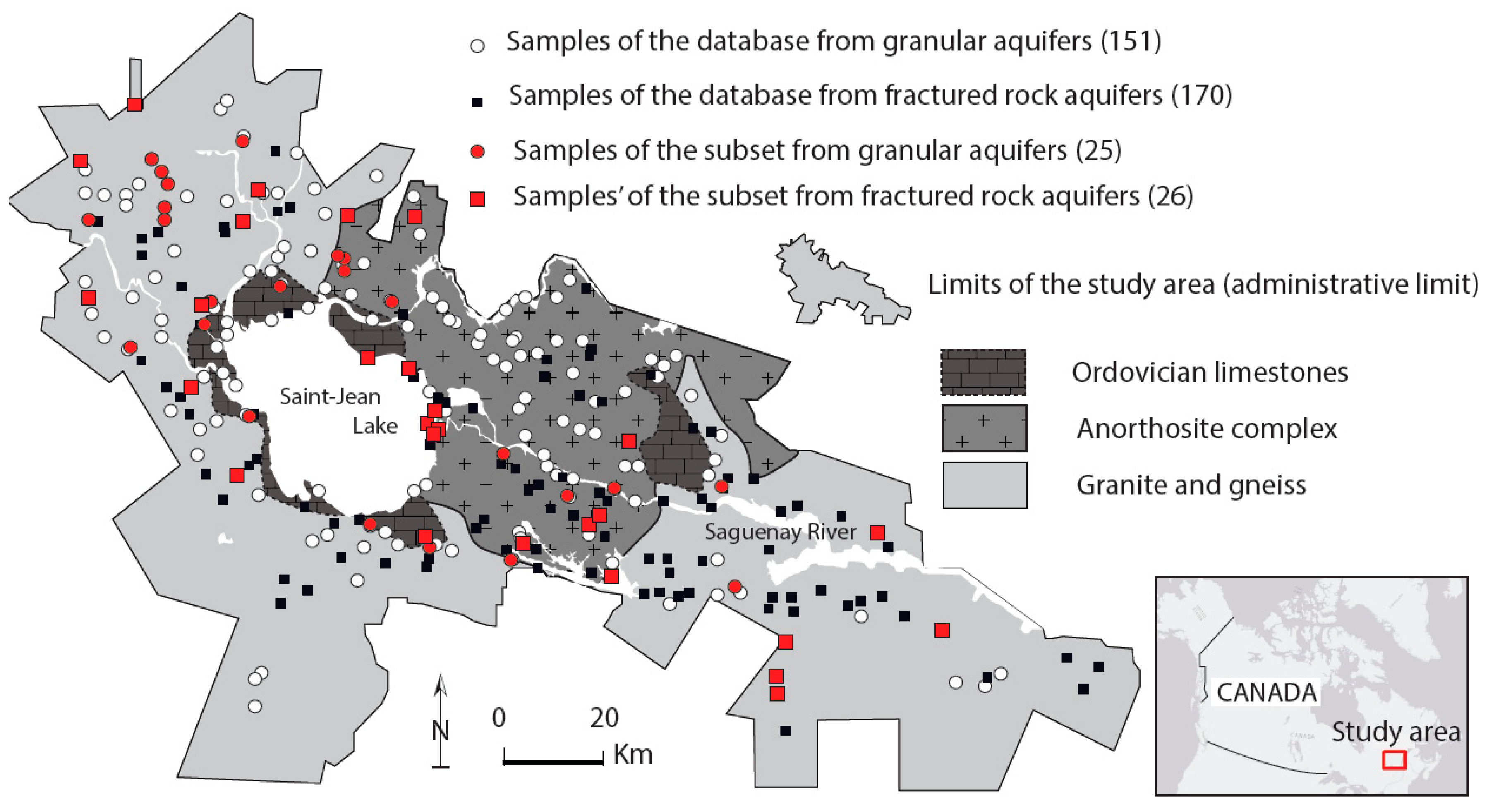


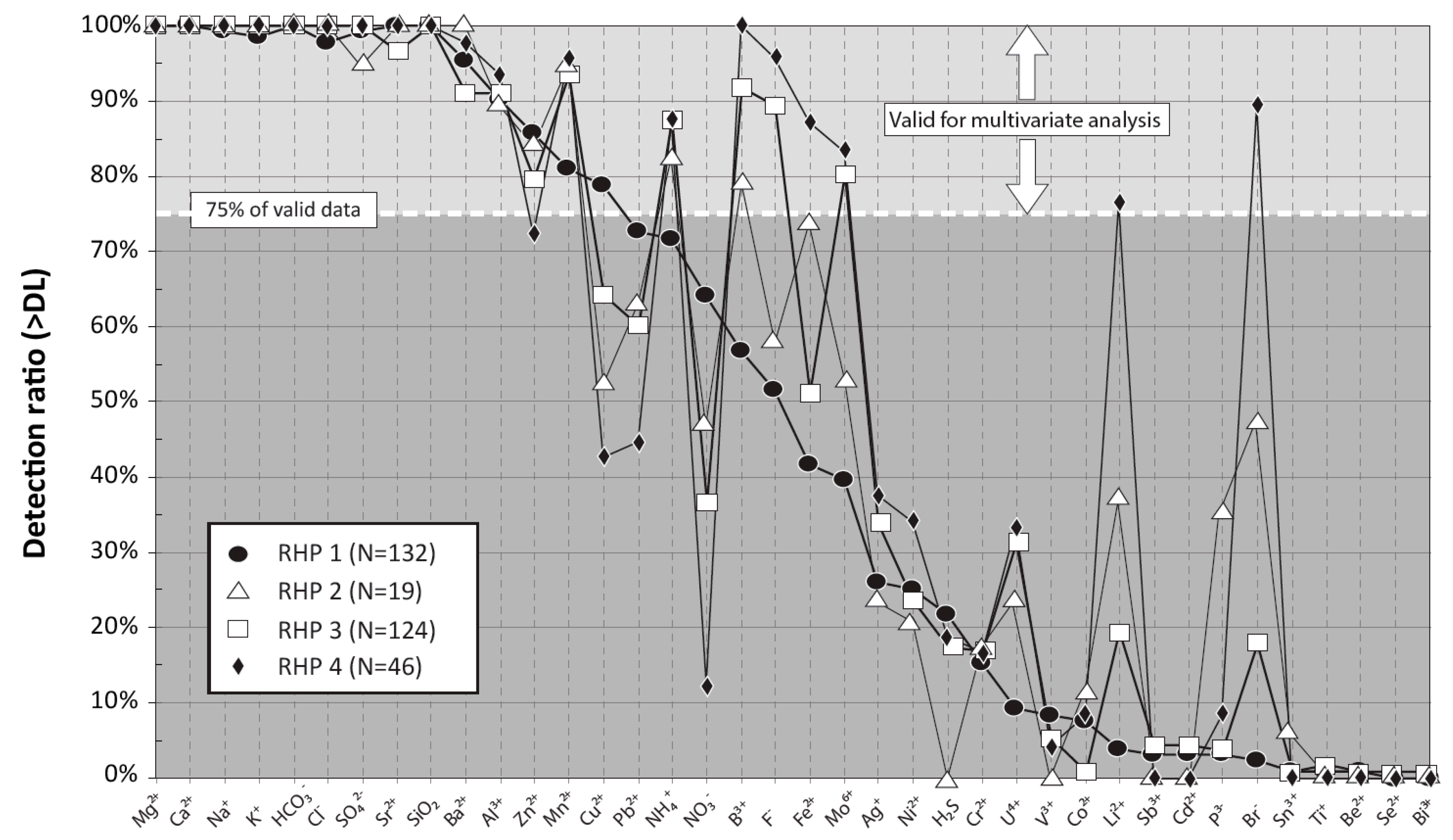





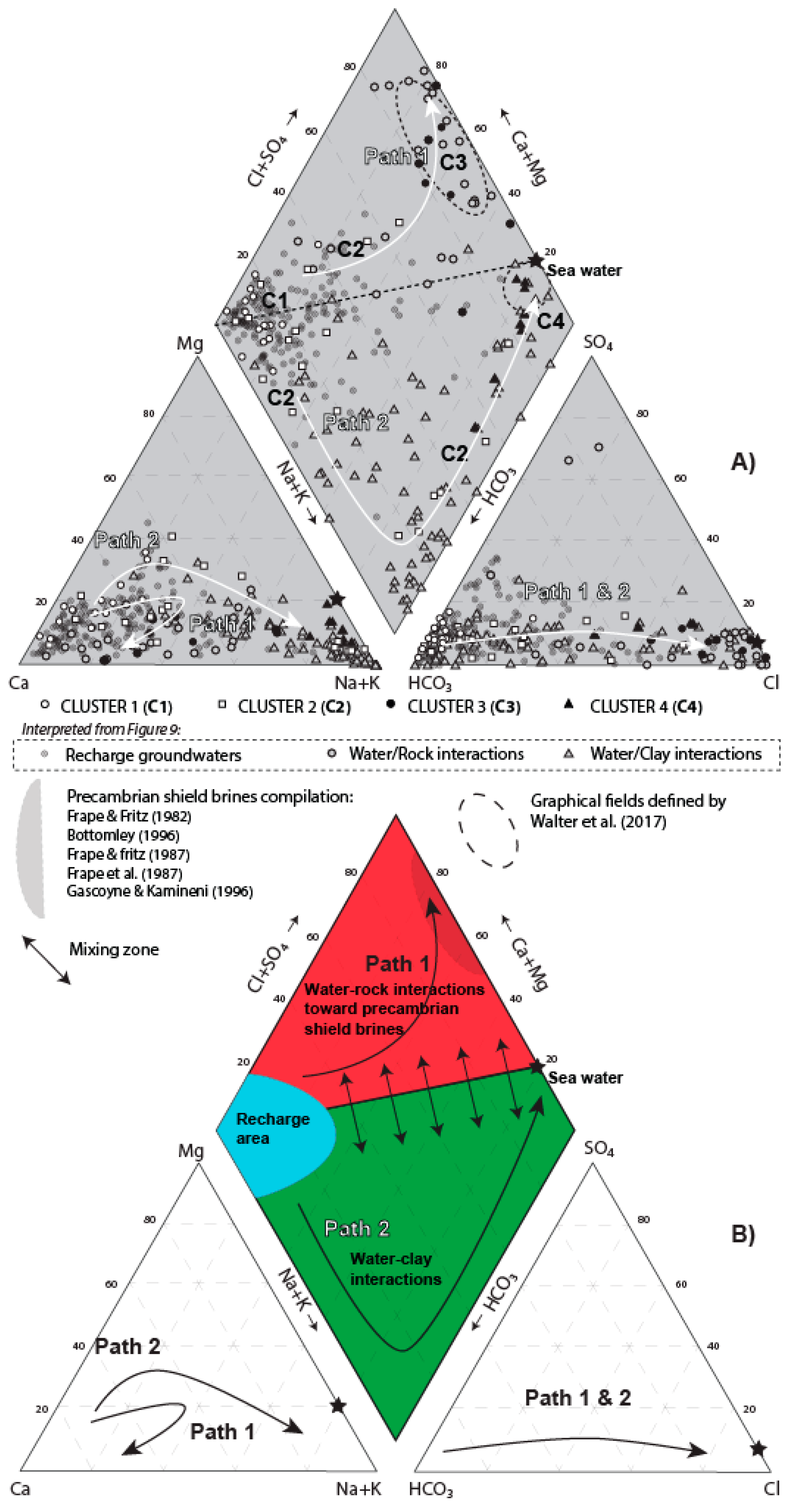

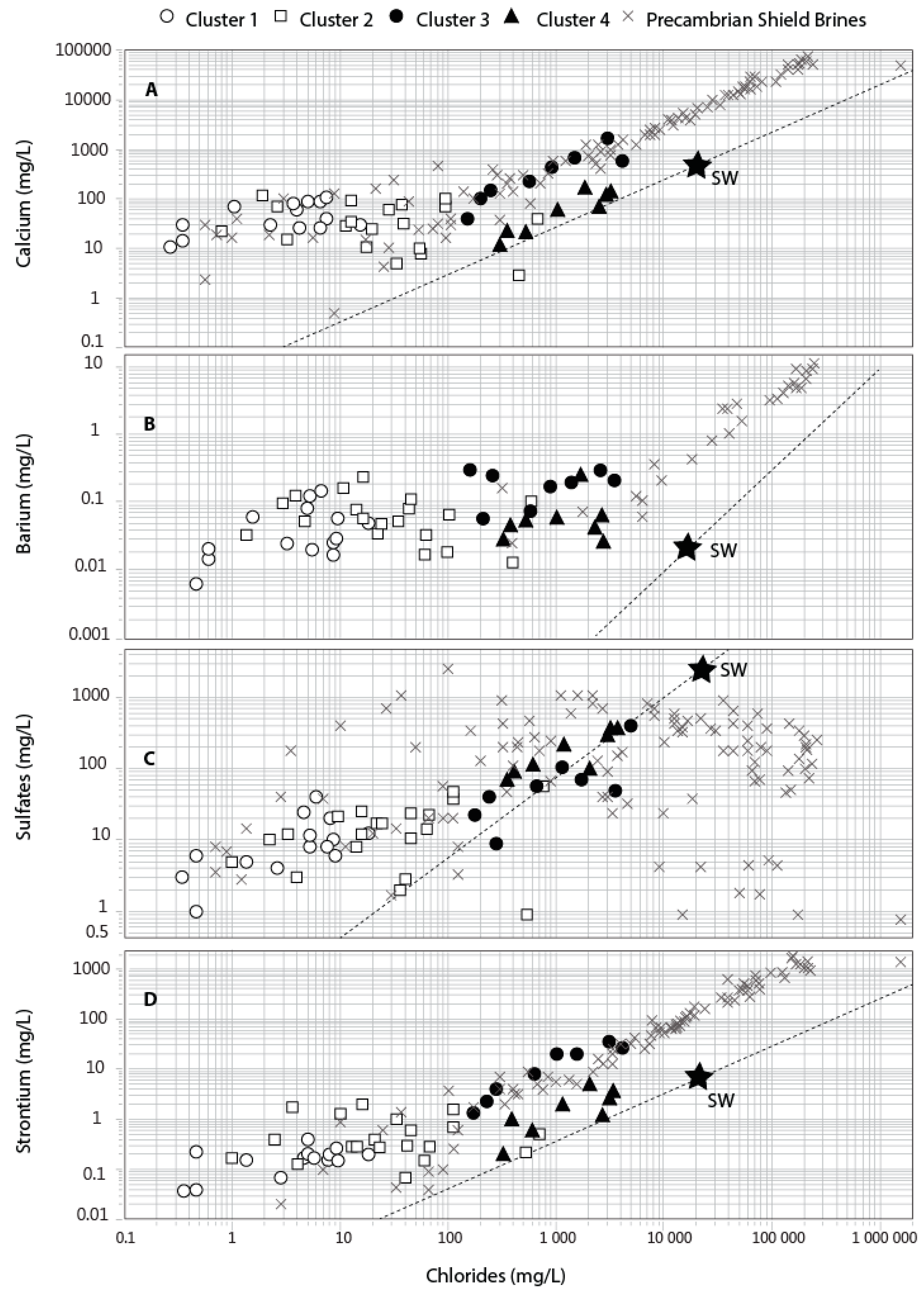
| PARAMETER | RHP 1: HCO3_Granular | RHP 2: Cl_Granular | RHP 3: HCO3_Rock | RHP 4: Cl_Rock | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | 25 | 75 | Min | Max | N | Median | 25 | 75 | Min | Max | N | Median | 25 | 75 | Min | Max | N | Median | 25 | 75 | Min | Max | |
| TDS (mg/L) | 132 | 170.2 | 62.9 | 282.2 | 12.1 | 836.7 | 19 | 1167.9 | 174.4 | 1679.6 | 37.3 | 6107.6 | 124 | 291.7 | 207.7 | 388.2 | 62.4 | 2266.7 | 46 | 1862.0 | 725.4 | 2857.2 | 391.3 | 8266.6 |
| Temperature (Celcius) * | 129 | 8.02 | 7.02 | 9.75 | 4.01 | 15.60 | 19 | 7.83 | 7.55 | 9.40 | 6.47 | 12.18 | 124 | 7.41 | 6.90 | 8.13 | 5.97 | 13.30 | 46 | 7.38 | 6.92 | 8.00 | 4.90 | 11.30 |
| Redox potential (mV) * | 120 | 97.2 | 79.7 | 130.4 | −255.7 | 743.9 | 17 | 68.9 | −21.2 | 107.5 | −147.0 | 161.1 | 104 | 22.7 | −92.0 | 100.7 | −375.6 | 680.0 | 43 | −12.8 | −65.4 | 78.4 | −326.0 | 720.1 |
| pH * | 130 | 6.98 | 6.03 | 7.81 | 4.38 | 10.08 | 19 | 7.20 | 5.50 | 7.61 | 4.93 | 8.71 | 124 | 7.85 | 7.06 | 8.42 | 4.49 | 9.74 | 46 | 7.64 | 7.26 | 7.99 | 5.18 | 10.06 |
| Dissolved oxygen (mg/L) * | 117 | 3.62 | 0.72 | 7.30 | 0.00 | 90.00 | 17 | 0.73 | 0.00 | 2.76 | 0.00 | 6.22 | 101 | 0.00 | 0.00 | 0.98 | 0.00 | 25.10 | 41 | 0.05 | 0.00 | 0.33 | 0.00 | 9.90 |
| Sodium ** | 131 | 3.80 | 1.90 | 10.00 | 0.87 | 240.00 | 19 | 220.00 | 13.00 | 490.00 | 3.80 | 1900.00 | 124 | 25.00 | 7.70 | 60.00 | 1.60 | 840.00 | 46 | 315.00 | 130.00 | 570.00 | 20.00 | 2500.00 |
| Magnesium ** | 132 | 2.60 | 0.95 | 6.75 | 0.15 | 26.00 | 19 | 13.40 | 1.60 | 25.00 | 0.31 | 160.00 | 124 | 4.30 | 2.10 | 7.20 | 0.01 | 26.00 | 46 | 15.90 | 7.80 | 45.00 | 3.00 | 220.00 |
| Potassium ** | 130 | 1.30 | 0.72 | 2.70 | 0.14 | 8.50 | 19 | 13.00 | 1.40 | 21.00 | 0.40 | 64.00 | 124 | 1.90 | 1.30 | 3.70 | 0.12 | 41.00 | 46 | 4.50 | 3.10 | 18.10 | 0.54 | 82.00 |
| Calcium ** | 132 | 19.50 | 7.30 | 46.00 | 1.20 | 130.00 | 19 | 21.00 | 11.00 | 60.00 | 2.20 | 200.00 | 124 | 25.00 | 8.60 | 48.00 | 0.04 | 170.00 | 46 | 110.00 | 44.00 | 380.00 | 2.10 | 1500.00 |
| Bicarbonates ***** | 132 | 108.58 | 32.33 | 183.00 | 7.32 | 427.00 | 19 | 207.40 | 26.84 | 380.64 | 6.10 | 695.40 | 124 | 170.80 | 112.85 | 219.60 | 21.52 | 553.88 | 46 | 158.60 | 117.12 | 244.00 | 6.10 | 683.20 |
| Chloride *** | 129 | 3.40 | 0.90 | 10.00 | 0.16 | 170.00 | 19 | 310.00 | 55.00 | 690.00 | 9.50 | 3000.00 | 124 | 11.00 | 4.00 | 35.00 | 0.07 | 1100.00 | 46 | 815.00 | 260.00 | 1600.00 | 64.00 | 4200.00 |
| Sulfates *** | 131 | 5.20 | 3.30 | 10.00 | 0.20 | 41.00 | 18 | 44.50 | 8.60 | 120.00 | 2.40 | 420.00 | 124 | 14.00 | 7.60 | 18.00 | 0.10 | 250.00 | 46 | 42.50 | 15.00 | 130.00 | 0.50 | 530.00 |
| Barium ** | 126 | 0.0250 | 0.0100 | 0.0500 | 0.0030 | 0.3000 | 19 | 0.0540 | 0.0370 | 0.0800 | 0.0130 | 0.6500 | 112 | 0.0635 | 0.0235 | 0.1250 | 0.0060 | 1.2000 | 46 | 0.0820 | 0.0400 | 0.2200 | 0.0120 | 2.8000 |
| Boron ** | 75 | 0.0120 | 0.0073 | 0.0210 | 0.0050 | 0.3500 | 15 | 0.2900 | 0.0160 | 0.3800 | 0.0130 | 0.6600 | 115 | 0.0500 | 0.0240 | 0.1200 | 0.0040 | 3.4000 | 46 | 0.2650 | 0.1400 | 0.4500 | 0.0060 | 1.9000 |
| Strontium ** | 132 | 0.0910 | 0.0390 | 0.2100 | 0.0140 | 2.4000 | 19 | 0.3700 | 0.1500 | 1.4000 | 0.0160 | 7.8000 | 121 | 0.2800 | 0.1100 | 0.7400 | 0.0030 | 4.3000 | 46 | 3.750 | 0.89 | 13 | 0.1900 | 37.0000 |
| Silicium ** | 132 | 5.9500 | 4.7500 | 7.6500 | 2.5000 | 14.0000 | 19 | 7.1000 | 6.0000 | 9.1000 | 1.3000 | 13.0000 | 124 | 5.6000 | 4.9000 | 6.7000 | 2.4000 | 16.0000 | 46 | 5.4500 | 4.6000 | 7.7000 | 0.2200 | 15.0000 |
| Manganese ** | 107 | 0.0071 | 0.0014 | 0.0530 | 0.0004 | 2.4000 | 18 | 0.0380 | 0.0110 | 0.0710 | 0.0070 | 0.2800 | 117 | 0.0140 | 0.0040 | 0.0420 | 0.0004 | 1.0000 | 44 | 0.0620 | 0.0245 | 0.1020 | 0.0010 | 1.1000 |
| Fluoride **** | 68 | 0.2000 | 0.1000 | 0.6000 | 0.1000 | 2.3000 | 11 | 1.3000 | 0.9000 | 1.7000 | 0.1000 | 2.9000 | 112 | 0.8500 | 0.4000 | 1.6000 | 0.1000 | 4.9000 | 44 | 1.3000 | 0.7100 | 1.6500 | 0.0600 | 3.0000 |
| Aluminium ** | 119 | 0.0075 | 0.0041 | 0.0180 | 0.0020 | 0.1600 | 17 | 0.0120 | 0.0063 | 0.0290 | 0.0020 | 0.1700 | 114 | 0.0070 | 0.0039 | 0.0097 | 0.0010 | 0.2300 | 43 | 0.0070 | 0.0057 | 0.0120 | 0.0020 | 0.0260 |
| Bromide *** | 3 | 0.4000 | 0.4000 | 0.5220 | 0.4000 | 0.5220 | 9 | 4.1050 | 1.6000 | 8.0000 | 1.1000 | 11.0000 | 24 | 0.5000 | 0.2000 | 2.0345 | 0.1000 | 15.8340 | 41 | 7.1000 | 2.3780 | 18.0000 | 0.2000 | 45.0000 |
| Iron ** | 55 | 0.1100 | 0.0490 | 0.7800 | 0.0250 | 13.0000 | 14 | 0.1900 | 0.1300 | 1.3000 | 0.0410 | 5.4000 | 65 | 0.0980 | 0.0460 | 0.2400 | 0.0020 | 13.0000 | 40 | 0.1200 | 0.0465 | 0.4100 | 0.0050 | 35.0000 |
| Lithium ** | 5 | 0.0120 | 0.0110 | 0.0130 | 0.0030 | 0.0180 | 7 | 0.0180 | 0.0120 | 0.0300 | 0.0090 | 0.0310 | 26 | 0.0110 | 0.0030 | 0.0150 | 0.0010 | 0.6000 | 35 | 0.0300 | 0.0130 | 0.0700 | 0.0030 | 0.5700 |
| Zinc ** | 113 | 0.0180 | 0.0097 | 0.0540 | 0.0020 | 0.7100 | 16 | 0.0190 | 0.0125 | 0.0500 | 0.0070 | 0.1900 | 98 | 0.0130 | 0.0072 | 0.0250 | 0.0020 | 0.3500 | 34 | 0.0100 | 0.0067 | 0.0120 | 0.0020 | 0.1000 |
| Lead ** | 96 | 0.0003 | 0.0002 | 0.0007 | 0.0001 | 0.0073 | 12 | 0.0003 | 0.0002 | 0.0010 | 0.0001 | 0.0021 | 74 | 0.0003 | 0.0002 | 0.0006 | 0.0001 | 0.0020 | 21 | 0.0006 | 0.0003 | 0.0080 | 0.0001 | 0.0100 |
| Ammonium **** | 94 | 0.0600 | 0.0400 | 0.1000 | 0.0200 | 0.8500 | 14 | 0.4650 | 0.0600 | 1.1000 | 0.0400 | 2.4000 | 98 | 0.0950 | 0.0500 | 0.2100 | 0.0200 | 0.7900 | 21 | 0.5100 | 0.1200 | 1.6000 | 0.0300 | 5.7000 |
| Copper ** | 104 | 0.0056 | 0.0020 | 0.0130 | 0.0010 | 0.3500 | 10 | 0.0060 | 0.0020 | 0.0390 | 0.0010 | 0.0800 | 79 | 0.0038 | 0.0013 | 0.0120 | 0.0010 | 0.2600 | 20 | 0.0030 | 0.0014 | 0.0100 | 0.0010 | 0.0540 |
| Molybdene ** | 52 | 0.0015 | 0.0007 | 0.0024 | 0.0010 | 0.0086 | 9 | 0.0020 | 0.0015 | 0.0030 | 0.0010 | 0.0060 | 90 | 0.0019 | 0.0011 | 0.0032 | 0.0010 | 0.0240 | 20 | 0.0020 | 0.0013 | 0.0050 | 0.0010 | 0.0150 |
| Nickel ** | 33 | 0.0018 | 0.0014 | 0.0039 | 0.0010 | 0.0110 | 4 | 0.0020 | 0.0013 | 0.0020 | 0.0010 | 0.0020 | 29 | 0.0019 | 0.0011 | 0.0030 | 0.0010 | 0.0200 | 16 | 0.0020 | 0.0010 | 0.0020 | 0.0010 | 0.0150 |
| Silver ** | 34 | 0.0002 | 0.0001 | 0.0003 | 0.0001 | 0.0007 | 4 | 0.0003 | 0.0001 | 0.0003 | 0.0001 | 0.0003 | 38 | 0.0002 | 0.0002 | 0.0003 | 0.0001 | 0.0020 | 9 | 0.0002 | 0.0001 | 0.0010 | 0.0001 | 0.0090 |
| Uranium ** | 12 | 0.0016 | 0.0011 | 0.0022 | 0.0010 | 0.0034 | 4 | 0.0020 | 0.0012 | 0.0020 | 0.0010 | 0.0020 | 35 | 0.0029 | 0.0014 | 0.0055 | 0.0010 | 0.0200 | 8 | 0.0020 | 0.0018 | 0.0030 | 0.0020 | 0.0100 |
| Chromium ** | 20 | 0.0010 | 0.0006 | 0.0014 | 0.0010 | 0.0021 | 3 | 0.0010 | 0.0005 | 0.0010 | 0.0010 | 0.0010 | 19 | 0.0012 | 0.0006 | 0.0016 | 0.0010 | 0.0110 | 4 | 0.0010 | 0.0009 | 0.0020 | 0.0010 | 0.0020 |
| Nitrate *** | 84 | 0.3150 | 0.1000 | 0.9700 | 0.0200 | 8.6000 | 8 | 0.8300 | 0.2500 | 1.7000 | 0.0900 | 3.7000 | 41 | 0.2000 | 0.1000 | 0.7000 | 0.0200 | 4.4000 | 3 | 0.11 | 0.1000 | 1.2000 | 0.1000 | 1.2000 |
| Sulfide *** | 5 | 0.3200 | 0.3000 | 0.5900 | 0.0600 | 0.7800 | 0 | - | - | - | - | - | 8 | 0.1350 | 0.0550 | 0.7050 | 0.0300 | 16.0000 | 3 | 0.1000 | 0.0400 | 1.4000 | 0.0400 | 1.4000 |
| Cobalt ** | 10 | 0.0023 | 0.0010 | 0.0026 | 0.0010 | 0.0066 | 2 | 0.0010 | - | - | - | - | 1 | 0.0013 | - | - | - | - | 2 | 0.0010 | - | - | - | - |
| Inorganic phosphorus **** | 4 | 0.1050 | 0.0550 | 0.3200 | 0.0400 | 0.5000 | 6 | 0.0650 | 0.0500 | 0.0900 | 0.0400 | 0.1100 | 4 | 0.3250 | 0.1750 | 0.4250 | 0.0500 | 0.5000 | 2 | 0.0500 | - | - | - | - |
| Vanadium ** | 11 | 0.0027 | 0.0022 | 0.0042 | 0.0020 | 0.0069 | 0 | - | - | - | - | - | 6 | 0.0026 | 0.0026 | 0.0031 | 0.0020 | 0.0030 | 1 | 0.0020 | - | - | - | - |
| Antimony ** | 4 | 0.0023 | 0.0013 | 0.0042 | 0.0010 | 0.0052 | 0 | - | - | - | - | - | 5 | 0.0016 | 0.0012 | 0.0020 | 0.0010 | 0.0020 | 0 | - | - | - | - | - |
| Cadmium ** | 4 | 0.0003 | 0.0003 | 0.0005 | 0.0002 | 0.0007 | 0 | - | - | - | - | - | 5 | 0.0006 | 0.0003 | 0.0007 | 0.0003 | 0.0010 | 0 | - | - | - | - | - |
| Selenium ** | 0 | - | - | - | - | - | 0 | - | - | - | - | - | 1 | 0.0420 | - | - | - | - | 0 | - | - | - | - | - |
| Tin ** | 1 | 0.0021 | - | - | - | - | 1 | 0.0030 | - | - | - | - | 1 | 0.0011 | - | - | - | - | 0 | - | - | - | - | - |
| Titanium ** | 1 | 0.0044 | - | - | - | - | 0 | - | - | - | - | - | 2 | 0.0012 | - | - | - | - | 0 | - | - | - | - | - |
| Beryllium ** | 1 | 0.0044 | - | - | - | - | 0 | - | - | - | - | - | 1 | 0.0008 | - | - | - | - | 0 | - | - | - | - | - |
| Bismuth ** | 0 | - | - | - | - | - | 0 | - | - | - | - | - | 1 | 0.0007 | - | - | - | - | 0 | - | - | - | - | - |
| Regional Hydrogeochemical Pole (RHP) | Iron | Molybdenum | Fluoride | Nitrates | Copper | Lead | Boron | Bromides | Lithium | Ammonium |
|---|---|---|---|---|---|---|---|---|---|---|
| RHP 1: Bicarbonate groundwater from granular sediments (HCO3-Gran) | X | X | X | X | X | X | ||||
| RHP 2: Brackish groundwater from granular sediments (Cl-Gran) | X | X | X | X | X | X | ||||
| RHP 3: Bicarbonate groundwater from fractured rock (HCO3-Rock) | X | X | X | X | X | |||||
| RHP 4: Brackish groundwater from fractured rock (Cl-Rock) | X | X | X | X | X | X | X |
| N | %Detect | Median | 25 | 75 | Min | Max | Multivariate | |
|---|---|---|---|---|---|---|---|---|
| TDS (mg/L) | 51 | 100% | 427.2 | 238.3 | 1359.6 | 72.0 | 7291.4 | |
| Temperature (Celcius) * | 50 | 98% | 7.66 | 7.13 | 8.56 | 5.97 | 15.60 | |
| Redox potential (mV) * | 47 | 92% | 79.4 | −27.1 | 101 | −245.7 | 524.9 | |
| pH * | 50 | 98% | 7.63 | 7.23 | 8.09 | 4.49 | 10.06 | |
| Dissolved oxygen (mg/L) * | 38 | 75% | 0.17 | 0.00 | 1.82 | 0.00 | 8.63 | |
| Sodium ** | 51 | 100% | 40.00 | 7.50 | 350.00 | 1.50 | 1900.00 | x |
| Magnesium ** | 51 | 100% | 8.70 | 3.90 | 15.00 | 1.50 | 160.00 | x |
| Potassium ** | 51 | 100% | 3.50 | 2.40 | 7.50 | 0.54 | 64.00 | x |
| Calcium ** | 51 | 100% | 55.00 | 21.00 | 81.00 | 2.10 | 1500.00 | x |
| Bicarbonates ***** | 51 | 100% | 207.40 | 118.34 | 280.60 | 6.10 | 695.40 | x |
| Chloride *** | 50 | 98% | 37.00 | 6.40 | 510.00 | 0.40 | 4200.00 | |
| Sulfates *** | 51 | 100% | 18.00 | 9.20 | 63.00 | 1.00 | 430.00 | x |
| Barium ** | 50 | 98% | 0.055 | 0.027 | 0.110 | 0.006 | 0.320 | x |
| Boron ** | 47 | 92% | 0.120 | 0.016 | 0.320 | 0.006 | 0.750 | x |
| Strontium ** | 51 | 100% | 0.400 | 0.180 | 1.900 | 0.033 | 37.000 | x |
| Silicium ** | 51 | 100% | 6.500 | 5.400 | 8.100 | 0.220 | 13.000 | x |
| Manganese ** | 50 | 98% | 0.031 | 0.011 | 0.067 | 0.001 | 0.900 | x |
| Fluoride **** | 48 | 94% | 0.800 | 0.300 | 1.300 | 0.100 | 2.400 | x |
| Aluminium ** | 44 | 86% | 0.007 | 0.005 | 0.013 | 0.002 | 0.087 | |
| Bromide *** | 21 | 41% | 3.5000 | 1.6000 | 11.0000 | 0.1000 | 45.0000 | |
| Iron ** | 41 | 80% | 0.1500 | 0.0900 | 0.3600 | 0.0320 | 3.4000 | x |
| Lithium ** | 22 | 43% | 0.0180 | 0.0120 | 0.0370 | 0.0050 | 0.5700 | |
| Zinc ** | 44 | 86% | 0.0145 | 0.0077 | 0.0375 | 0.0016 | 0.1500 | |
| Lead ** | 33 | 65% | 0.0004 | 0.0002 | 0.0006 | 0.0001 | 0.0024 | |
| Ammonium **** | 46 | 90% | 0.2300 | 0.0600 | 0.5100 | 0.0200 | 3.0000 | x |
| Copper ** | 33 | 65% | 0.0027 | 0.0014 | 0.0110 | 0.0006 | 0.0350 | |
| Molybdene ** | 45 | 88% | 0.0018 | 0.0014 | 0.0029 | 0.0006 | 0.0150 | x |
| Nickel ** | 19 | 37% | 0.0019 | 0.0011 | 0.0039 | 0.0010 | 0.0110 | |
| Silver ** | 21 | 41% | 0.0002 | 0.0002 | 0.0003 | 0.0001 | 0.0090 | |
| Uranium ** | 17 | 33% | 0.0018 | 0.0011 | 0.0054 | 0.0010 | 0.0096 | |
| Chromium ** | 6 | 12% | 0.0013 | 0.0007 | 0.0018 | 0.0005 | 0.0024 | |
| Nitrate *** | 28 | 55% | 0.3000 | 0.1000 | 0.7500 | 0.0200 | 6.3000 | |
| Cobalt ** | 2 | 4% | 0.0023 | 0.0019 | 0.0026 | 0.0019 | 0.0026 | |
| Inorganic phosphorus **** | 9 | 18% | 0.0500 | 0.0443 | 0.0600 | 0.0400 | 0.0900 | |
| Vanadium ** | 3 | 6% | 0.0023 | 0.0023 | 0.0069 | 0.0023 | 0.0069 | |
| Antimony ** | 2 | 4% | 0.0017 | 0.0014 | 0.0020 | 0.0014 | 0.0020 | |
| Cadmium ** | 2 | 4% | 0.0007 | 0.0006 | 0.0007 | 0.0006 | 0.0007 | |
| Selenium ** | 0 | 0% | - | - | - | - | - | |
| Tin ** | 1 | 2% | 0.0034 | - | - | - | - | |
| Titanium ** | 0 | 0% | - | - | - | - | - | |
| Beryllium ** | 0 | 0% | - | - | - | - | - | |
| Bismuth ** | 0 | 0% | - | - | - | - | - |
| Sample | Regional Hydrogeochemical Pole (RHP) | Aquifer Type | Water Type | Detection Limit (DL) | 0.03 | 0.01 | 0.1 | 0.05 | 2 | 0.5 | 2 | 0.001 | 0.001 | 0.0001 | 0.001 | 0.0002 | 0.001 | |||||||
| TDS | T | Eh | pH | O.D | Sodium | Magnesium | Potassium | Calcium | Bicarbonate | Chloride | Sulfate | Aluminium | Antimony | Silver | Baryum | Cadmium | Chromium | |||||||
| (mg/L) | (mV) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | 0.001 | 0.001 | 0.0001 | ||||||
| 1 | 1 | Granular | Ca-HCO3 | 326.51 | 6.3 | 55.7 | 6.29 | UnK | 4.2 | 3.5 | 2.7 | 70 | 207.4 | 8.6 | 11 | 0.0075 | - | 0.00015 | 0.026 | - | - | Cobalt | Copper | Manganese |
| 2 | 1 | Granular | Ca-HCO3 | 229.64 | 11 | 5.63 | 4.2 | UnK | 9.3 | 11 | 3.3 | 24 | 146.4 | 5.4 | 9.4 | - | - | 0.00018 | 0.02 | - | - | (mg/L) | (mg/L) | (mg/L) |
| 3 | 1 | Granular | Na-HCO3 | 403.8 | 13 | 43.1 | 5.64 | UnK | 48 | 15 | 5.9 | 21 | 256.2 | 24 | 18 | 0.0097 | - | 0.00016 | 0.043 | - | - | - | 0.0007 | 0.062 |
| 4 | 1 | Granular | Ca-HCO3 | 84.707 | 7 | 64.5 | 5.56 | UnK | 4.4 | 2.1 | 1.3 | 9.6 | 51.24 | 0.4 | 3.3 | 0.0095 | - | 0.00018 | 0.0055 | - | 0.0018 | - | 0.012 | 0.014 |
| 5 | 1 | Granular | Ca-HCO3 | 459.44 | 7.6 | 79.4 | 8.04 | 0 | 40 | 11 | 3.1 | 56 | 292.8 | 35 | 2.1 | 0.036 | 0.0014 | 0.00069 | 0.055 | - | - | - | 0.011 | 0.00081 |
| 6 | 1 | Granular | Na-HCO3 | 217.36 | 9.8 | 88.7 | 8.22 | 0 | 16 | 8.7 | 7.1 | 15 | 146.4 | 4.3 | 3.5 | 0.0022 | - | - | 0.05 | - | - | - | 0.0092 | 0.00089 |
| 7 | 1 | Granular | Ca-HCO3 | 427.22 | 6.9 | 90.6 | 7.73 | 1.7 | 3.6 | 11 | 3.9 | 81 | 268.4 | 6.4 | 41 | 0.003 | - | - | 0.14 | - | - | - | 0.0009 | 0.042 |
| 8 | 1 | Granular | Ca-HCO3 | 335.39 | 6.6 | 90.2 | 8.01 | 8.63 | 4 | 6.1 | 2.5 | 64 | 219.6 | 4.8 | 25 | - | - | - | 0.08 | - | - | - | - | 0.021 |
| 9 | 1 | Granular | Ca-HCO3 | 247.93 | 6.8 | 98.9 | 7.57 | 8.48 | 13 | 14 | 4.8 | 21 | 170.8 | 1.1 | 5 | 0.0039 | - | 0.0001 | 0.031 | - | - | - | 0.0027 | 0.039 |
| 10 | 1 | Ca-HCO3 | 228.6 | 6 | 91.6 | 8.33 | 0 | 13 | 11 | 5.6 | 24 | 134.2 | 14 | 9.2 | 0.0044 | - | 0.00012 | 0.078 | - | - | - | - | 0.015 | |
| 11 | 1 | Granular | Ca-HCO3 | 396.69 | 7.1 | 120.4 | 7.39 | 3.33 | 7.6 | 10 | 2.4 | 76 | 256.2 | 9.1 | 23 | 0.022 | - | 0.0001 | 0.027 | - | - | - | 0.016 | 0.011 |
| 12 | 1 | Granular | Ca-HCO3 | 200.13 | 7.7 | 116.4 | 7.63 | 2.85 | 9.5 | 9.7 | 3.6 | 23 | 118.34 | 8.3 | 10 | 0.0088 | - | - | 0.017 | - | - | - | 0.0009 | 0.033 |
| 13 | 1 | Granular | Ca-HCO3 | 311.59 | 8.1 | 97.8 | 7.49 | 0.77 | 13 | 8.2 | 3.5 | 46 | 207.4 | 9.6 | 7 | 0.0053 | - | - | 0.055 | - | - | - | 0.0059 | 0.03 |
| 14 | 1 | Granular | Ca-HCO3 | 238.27 | 9.1 | UnK | 7.67 | 2.45 | 1.5 | 3 | 0.77 | 55 | 170.8 | 1.4 | 5.8 | 0.002 | - | - | 0.058 | - | 0.00071 | - | 0.0019 | 0.0024 |
| 15 | 1 | Granular | Ca-HCO3 | 454.73 | 9.1 | −75 | 6.93 | 0.72 | 7.5 | 15 | 5.6 | 84 | 280.6 | 16 | 30 | 0.0063 | - | 0.00014 | 0.06 | - | - | - | 0.015 | 0.032 |
| 16 | 1 | Ca-HCO3 | 463.85 | 16 | 6.4 | 6.84 | 1.57 | 5.6 | 4 | 7.5 | 110 | 305 | 2.6 | 12 | 0.022 | - | - | 0.094 | - | - | - | 0.0012 | 0.094 | |
| 17 | 2 | Granular | Na-Cl | 5573.4 | 7.5 | 27.9 | 6.47 | UnK | 1600 | 100 | 55 | 60 | 695.4 | 2700 | 330 | 0.0046 | - | 0.00016 | 0.039 | - | - | 0.0026 | 0.0006 | 0.9 |
| 18 | 2 | Granular | Na-Cl | 1359.6 | 9.5 | −21.2 | 7.31 | UnK | 350 | 13 | 13 | 9.8 | 536.8 | 340 | 79 | 0.012 | - | 0.00028 | 0.029 | - | 0.00098 | 0.0019 | 0.0035 | 0.66 |
| 19 | 2 | Granular | Na-Cl | 3576 | 7.8 | −132 | 7.61 | 0.73 | 980 | 52 | 21 | 150 | 317.2 | 1900 | 120 | 0.0021 | - | 0.00033 | 0.25 | - | - | - | - | 0.071 |
| 20 | 2 | Granular | Na-Cl | 1352.8 | 7.8 | 104.9 | 8.1 | 0 | 220 | 15 | 11 | 200 | 183 | 630 | 63 | 0.02 | - | - | 0.07 | - | - | - | 0.0011 | 0.031 |
| 21 | 2 | Granular | Na-Cl | 1387.7 | 7.5 | 107.5 | 8.71 | 0 | 410 | 18 | 14 | 19 | 207.4 | 570 | 130 | 0.013 | - | - | 0.053 | - | - | - | - | 0.055 |
| 22 | 2 | Granular | Na-Cl | 1679.6 | 7.6 | −147 | 7.41 | 0 | 490 | 20 | 34 | 36 | 341.6 | 690 | 68 | 0.087 | - | - | 0.094 | - | - | - | - | 0.061 |
| 23 | 2 | Granular | Na-Cl | 6107.6 | 7.6 | −97.9 | 7.2 | 0 | 1900 | 160 | 64 | 100 | 463.6 | 3000 | 420 | - | - | - | 0.064 | - | 0.00052 | - | - | 0.011 |
| 24 | 2 | Granular | Na-Cl | 2709.1 | 9.4 | −8.3 | 8.09 | 0 | 830 | 33 | 31 | 58 | 380.64 | 1100 | 250 | 0.007 | 0.0005 | - | 0.06 | - | - | - | - | 0.15 |
| 25 | 2 | Granular | Na-Cl | 1283.2 | 9.4 | −75.3 | 8.47 | 0.11 | 370 | 13.4 | 15 | 21 | 353.8 | 390 | 100 | 0.007 | 0.0005 | - | 0.043 | - | - | - | - | 0.098 |
| 26 | 3 | Rock | Ca-HCO3 | 125.52 | 11 | 133.5 | 4.7 | UnK | 4.3 | 2.1 | 2.5 | 23 | 70.76 | 3 | 4.8 | 0.0076 | - | - | 0.022 | - | - | - | 0.002 | 0.067 |
| 27 | 3 | Rock | Ca-HCO3 | 372.21 | 7.9 | 95.2 | 5.58 | UnK | 25 | 7.2 | 2.8 | 60 | 231.8 | 10 | 26 | 0.0096 | - | 0.00029 | 0.16 | - | - | - | - | 0.018 |
| 28 | 3 | Rock | Ca-HCO3 | 72.019 | 7.9 | 100.5 | 4.49 | UnK | 2.1 | 1.5 | 0.92 | 11 | 40.26 | - | 4 | 0.0095 | - | 0.00041 | 0.018 | - | - | - | 0.026 | 0.0025 |
| 29 | 3 | Rock | Ca-HCO3 | 229.08 | 6.6 | 42.5 | 6.02 | UnK | 21 | 6.3 | 2.4 | 25 | 102.48 | 45 | 12 | 0.023 | - | 0.00021 | 0.087 | - | 0.0017 | - | 0.029 | 0.0087 |
| 30 | 3 | Rock | Ca-HCO3 | 393.04 | 6.3 | −182 | 7.55 | 0 | 20 | 6.7 | 2.8 | 71 | 207.4 | 45 | 25 | 0.0072 | - | 0.00018 | 0.11 | 0.00061 | - | - | 0.035 | 0.0094 |
| 31 | 3 | Rock | Ca-HCO3 | 153.57 | 6 | 524.9 | 8.06 | 0.22 | 5.4 | 4.3 | 1 | 24 | 104.92 | 0.5 | 1.1 | 0.007 | - | - | 0.015 | - | - | - | 0.0049 | 0.038 |
| 32 | 3 | Rock | Ca-HCO3 | 322.78 | 8.8 | UnK | 7.44 | 5.59 | 5.5 | 7.5 | 2.9 | 58 | 219.6 | 5.1 | 13 | 0.0048 | 0.002 | - | 0.12 | - | - | - | 0.028 | 0.015 |
| 33 | 3 | Rock | Na-HCO3 | 474.48 | 7.7 | 93 | 8.64 | 0 | 110 | 3 | 3 | 10 | 292.8 | 22 | 18 | 0.017 | - | - | 0.037 | - | - | - | 0.0034 | 0.048 |
| 34 | 3 | Rock | Na-HCO3 | 512.36 | 7.3 | 111.5 | 8.01 | 0 | 140 | 2.6 | 3.3 | 7.1 | 256.2 | 65 | 24 | 0.0078 | - | - | 0.03 | - | - | - | 0.0098 | 0.0019 |
| 35 | 3 | Rock | Ca-HCO3 | 162.56 | 7.8 | 101.5 | 6.19 | 6.46 | 7.5 | 3.9 | 1.5 | 28 | 69.54 | 19 | 13 | 0.0092 | - | - | 0.047 | 0.00073 | - | - | - | 0.013 |
| 36 | 3 | Rock | Ca-HCO3 | 92.998 | 7.1 | 101 | 7.83 | 0.9 | 2.5 | 2.8 | 2.1 | 11 | 50.02 | 0.5 | 6.3 | 0.0067 | - | - | 0.018 | - | - | - | - | 0.0035 |
| 37 | 3 | Rock | Ca-HCO3 | 304.9 | 7.2 | 307.2 | 7.53 | 4.73 | 8.4 | 6.8 | 1.8 | 63 | 207.4 | 3.5 | 14 | - | - | 0.00016 | 0.13 | - | - | - | 0.01 | 0.0043 |
| 38 | 3 | Rock | Ca-HCO3 | 508.64 | UnK | UnK | UnK | UnK | 47 | 12 | 3.7 | 77 | 195.2 | 110 | 45 | 0.058 | - | - | 0.065 | - | - | - | 0.002 | 0.0041 |
| 39 | 3 | Rock | Ca-HCO3 | 207.73 | 7.3 | −98.9 | 8.21 | 1.18 | 20 | 5.7 | 1.4 | 26 | 111.02 | 16 | 15 | 0.0054 | - | - | 0.24 | - | - | - | 0.0025 | 0.013 |
| 40 | 3 | Rock | Na-HCO3 | 497.36 | 8.6 | UnK | 8.06 | 0 | 130 | 3 | 5.3 | 7.3 | 256.2 | 64 | 16 | 0.02 | - | - | 0.015 | - | - | - | 0.002 | 0.065 |
| 41 | 3 | Rock | Na-HCO3 | 401.03 | 7.7 | 58.1 | 8.54 | 0.87 | 100 | 1.9 | 4.4 | 4.7 | 231.8 | 39 | 3.3 | - | - | - | - | - | - | - | 0.014 | 0.009 |
| 42 | 4 | Rock | Na-Cl | 498.99 | 7.3 | −29.9 | 7.62 | UnK | 110 | 7.8 | 3.2 | 32 | 134.2 | 170 | 25 | 0.0029 | - | 0.0012 | 0.32 | - | - | - | 0.0014 | 0.013 |
| 43 | 4 | Rock | Ca-Cl | 630.02 | 6.6 | −27.1 | 7.72 | UnK | 75 | 3.3 | 0.54 | 130 | 104.92 | 280 | 9.5 | 0.0028 | - | - | 0.22 | - | - | - | - | 0.015 |
| 44 | 4 | Rock | Ca-Cl | 5227.9 | 8.5 | −246 | 8.6 | 0 | 570 | 3 | 1.6 | 1500 | 6.1 | 3000 | 53 | 0.0035 | - | - | 0.26 | - | - | - | - | 0.025 |
| 45 | 4 | Rock | Ca-Cl | 2113.4 | 9.2 | 82.9 | 7.78 | 0 | 260 | 60 | 4.5 | 400 | 231.8 | 1000 | 110 | 0.026 | - | 0.0002 | 0.16 | - | - | - | - | 0.19 |
| 46 | 4 | Rock | Ca-Cl | 590.73 | 7.4 | 91.1 | 8.53 | 0 | 84 | 17 | 1.6 | 94 | 104.92 | 230 | 45 | 0.0037 | - | - | 0.054 | - | - | - | 0.0015 | 0.11 |
| 47 | 4 | Rock | Ca-Cl | 391.26 | 6.7 | 129.6 | 7.54 | 7.55 | 45 | 3.9 | 0.65 | 62 | 117.12 | 110 | 40 | - | - | - | 0.018 | - | - | - | 0.0006 | 0.046 |
| 48 | 4 | Rock | Na-Cl | 1794.8 | 7.3 | 102.5 | 10.1 | 0 | 560 | 12 | 20 | 2.1 | 683.2 | 510 | 1 | 0.0048 | - | 0.009 | 0.012 | - | - | - | - | 0.081 |
| 49 | 4 | Rock | Ca-Cl | 2857.2 | 8.4 | −102 | 7.82 | 0 | 380 | 54 | 3.6 | 580 | 158.6 | 1600 | 81 | 0.0072 | - | - | 0.18 | - | - | - | - | - |
| 50 | 4 | Rock | Na-Cl | 7291.4 | 7.8 | −21.8 | 7.59 | 1.82 | 1800 | 140 | 16 | 520 | 134.2 | 4200 | 420 | 0.0091 | - | 0.00015 | 0.2 | - | 0.0024 | - | 0.0012 | 0.0092 |
| 51 | 4 | Rock | Na-Cl | 6301 | 7.2 | −100 | 7.23 | 0 | 1900 | 160 | 64 | 120 | 427 | 3200 | 430 | - | - | - | 0.024 | - | - | - | 0.0026 | 0.077 |
| Sample | Regional Hydrogeochemical Pole (RHP) | Aquifer Type | Water Type | 0.001 | 0.001 | 0.002 | 0.004 | 0.001 | 0.01 | 0.01 | 0.002 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.1 | 0.0001 | 0.001 | 0.02 | 0.1 | 0.1 | 0.1 | 0.04 |
| Molybdenum | Nickel | Zinc | Boron | Iron | Lithium | Selenium | Strontium | Tin | Titan | Vanadium | Berillium | Bismuth | Silicium | Lead | Uranium | Amonium | Bromide | Fluoride | Nitrate | Phosphorus | ||||
| (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | ||||
| 1 | 1 | Granular | Ca-HCO3 | 0.0006 | - | 0.0073 | - | 0.062 | - | - | 0.19 | - | - | - | - | - | 6.2 | 0.00031 | - | 0.05 | - | - | 6.3 | - |
| 2 | 1 | Granular | Ca-HCO3 | 0.0006 | - | 0.058 | 0.01 | 0.14 | 0.013 | - | 0.21 | - | - | - | - | - | 9.6 | 0.00022 | - | 0.06 | - | 0.2 | 0.9 | - |
| 3 | 1 | Granular | Na-HCO3 | 0.0031 | - | 0.012 | 0.068 | - | - | - | 0.27 | - | - | - | - | - | 7.1 | 0.00041 | 0.001 | 0.08 | - | 0.7 | 0.3 | - |
| 4 | 1 | Granular | Ca-HCO3 | 0.00087 | - | 0.055 | 0.011 | - | - | - | 0.033 | - | - | 0.0023 | - | - | 5.7 | 0.0014 | - | 0.04 | - | 0.6 | 0.2 | - |
| 5 | 1 | Granular | Ca-HCO3 | - | - | 0.004 | 0.13 | 0.78 | 0.018 | - | 0.96 | - | - | - | - | - | 8.1 | 0.00016 | - | 0.51 | - | 0.4 | - | - |
| 6 | 1 | Granular | Na-HCO3 | 0.003 | - | 0.0021 | 0.011 | 0.13 | - | - | 0.14 | - | - | - | - | - | 7.7 | 0.00014 | - | 0.28 | - | 0.3 | 0.02 | - |
| 7 | 1 | Granular | Ca-HCO3 | 0.0018 | 0.0029 | 0.0052 | 0.0055 | 1.1 | - | - | 0.2 | - | - | - | - | - | 5.1 | 0.00049 | - | 0.02 | - | 0.2 | - | - |
| 8 | 1 | Granular | Ca-HCO3 | 0.0013 | 0.0021 | - | 0.011 | 0.11 | - | - | 0.17 | - | - | - | - | - | 4.4 | - | - | - | - | 0.1 | 0.1 | - |
| 9 | 1 | Granular | Ca-HCO3 | 0.0017 | 0.011 | 0.015 | 0.015 | - | - | - | 0.16 | - | - | - | - | - | 8.6 | 0.00051 | - | 0.07 | - | 0.2 | 0.5 | - |
| 10 | 1 | Ca-HCO3 | 0.0029 | - | 0.0023 | 0.016 | 0.081 | - | - | 0.35 | - | - | - | - | - | 8 | 0.00022 | - | 0.23 | - | 0.8 | - | - | |
| 11 | 1 | Granular | Ca-HCO3 | 0.0015 | 0.0019 | 0.017 | 0.014 | - | - | - | 0.21 | - | - | - | - | - | 5.7 | 0.00023 | - | 0.05 | - | 0.1 | 0.51 | - |
| 12 | 1 | Granular | Ca-HCO3 | 0.00075 | - | 0.024 | 0.0072 | 0.3 | - | - | 0.16 | - | - | - | - | - | 8.1 | - | - | 0.07 | - | 0.1 | 0.8 | - |
| 13 | 1 | Granular | Ca-HCO3 | 0.0021 | 0.0016 | 0.026 | 0.015 | 3.4 | - | - | 0.14 | - | - | - | - | - | 6.3 | - | 0.0011 | - | - | 0.3 | 0.3 | - |
| 14 | 1 | Granular | Ca-HCO3 | 0.00083 | - | - | 0.02 | 2.4 | - | - | 0.14 | - | - | - | - | - | 5.7 | - | - | 0.05 | - | 0.2 | 0.02 | - |
| 15 | 1 | Granular | Ca-HCO3 | 0.0022 | 0.0031 | 0.09 | 0.017 | 2 | - | - | 0.31 | - | - | - | - | - | 6.1 | 0.0002 | - | 0.34 | - | 0.1 | - | |
| 16 | 1 | Ca-HCO3 | - | 0.0042 | 0.072 | 0.025 | 0.21 | - | - | 0.46 | - | - | 0.0069 | - | - | 6.5 | 0.00015 | - | 0.4 | - | 0.1 | 2.1 | - | |
| 17 | 2 | Granular | Na-Cl | 0.0015 | 0.0016 | 0.044 | 0.57 | 0.19 | 0.018 | - | 1.4 | - | - | - | - | - | 8.8 | - | 0.0018 | 2.2 | 10 | 0.9 | - | - |
| 18 | 2 | Granular | Na-Cl | 0.0044 | - | 0.026 | 0.36 | 0.13 | - | - | 0.19 | - | - | - | - | - | 6.9 | 0.00021 | 0.0021 | 0.47 | 1.1 | 1.8 | - | 0.07 |
| 19 | 2 | Granular | Na-Cl | 0.0016 | 0.0011 | 0.015 | 0.38 | 1 | 0.031 | - | 5.8 | - | - | - | - | - | 9.1 | 0.00019 | - | 1.4 | 7 | 1.7 | - | 0.05 |
| 20 | 2 | Granular | Na-Cl | 0.0028 | - | 0.0066 | 0.17 | 0.29 | 0.012 | - | 7.8 | - | - | - | - | - | 6.5 | - | 0.0011 | 0.41 | 8 | 1 | - | - |
| 21 | 2 | Granular | Na-Cl | 0.0055 | - | 0.15 | 0.19 | 0.19 | - | - | 0.71 | - | - | - | - | - | 6.7 | 0.0001 | - | 0.81 | 1.6 | 1.7 | 0.46 | - |
| 22 | 2 | Granular | Na-Cl | 0.00056 | - | 0.0092 | 0.29 | 3.1 | 0.017 | - | 0.49 | - | - | - | - | - | 9.2 | - | - | 0.46 | 2.8 | 0.7 | - | 0.09 |
| 23 | 2 | Granular | Na-Cl | 0.0015 | - | - | 0.66 | 1.3 | 0.03 | - | 2.9 | - | - | - | - | - | 13 | - | 0.0012 | 2.4 | 11 | 1.1 | - | 0.04 |
| 24 | 2 | Granular | Na-Cl | 0.003 | - | 0.014 | 0.6 | 0.19 | 0.02 | - | 1.9 | - | - | - | - | - | 9.1 | - | 0.0011 | 1.1 | 4.1 | 1.3 | 0.074 | - |
| 25 | 2 | Granular | Na-Cl | 0.003 | - | 0.011 | 0.36 | 0.047 | 0.009 | - | 0.98 | - | - | - | - | - | 7.7 | - | 0.0011 | 1.1 | 1.4 | 1.6 | 0.074 | - |
| 26 | 3 | Rock | Ca-HCO3 | - | - | 0.052 | - | 0.046 | - | - | 0.068 | - | - | - | - | - | 5.6 | 0.001 | - | 0.03 | - | 0.1 | 3.5 | - |
| 27 | 3 | Rock | Ca-HCO3 | 0.00099 | 0.0012 | 0.0086 | 0.12 | - | - | - | 1.2 | - | - | - | - | - | 3.4 | 0.00067 | - | 0.06 | - | 0.8 | 0.21 | - |
| 28 | 3 | Rock | Ca-HCO3 | - | - | 0.038 | 0.0086 | 0.12 | - | - | 0.18 | - | - | - | - | - | 5.6 | 0.00042 | - | 0.32 | - | 0.2 | 0.1 | - |
| 29 | 3 | Rock | Ca-HCO3 | 0.002 | 0.0011 | 0.014 | 0.024 | 0.09 | - | - | 0.33 | - | - | - | - | - | 6.8 | 0.00045 | - | 0.09 | - | 0.6 | - | - |
| 30 | 3 | Rock | Ca-HCO3 | 0.0011 | 0.0039 | 0.028 | 0.03 | 0.32 | - | - | 0.72 | - | - | - | - | - | 6.7 | 0.00032 | - | 0.08 | - | 0.4 | - | - |
| 31 | 3 | Rock | Ca-HCO3 | 0.008 | 0.0012 | 0.036 | 0.021 | 0.032 | - | - | 0.23 | - | - | - | - | - | 5.5 | 0.00041 | 0.0013 | 0.05 | - | 0.9 | - | - |
| 32 | 3 | Rock | Ca-HCO3 | - | - | 0.0081 | - | 0.071 | - | - | 0.43 | - | - | - | - | - | 4.9 | 0.00047 | - | 0.03 | - | - | 0.7 | - |
| 33 | 3 | Rock | Na-HCO3 | 0.0015 | - | - | 0.18 | 0.12 | - | - | 0.4 | - | - | - | - | - | 6.1 | - | 0.0054 | 0.29 | 0.1 | 2.3 | 0.02 | 0.05 |
| 34 | 3 | Rock | Na-HCO3 | 0.0027 | - | - | 0.14 | 0.032 | - | - | 0.28 | - | - | - | - | - | 5.3 | 0.0024 | 0.0089 | 0.06 | 0.2 | 2.3 | 0.5 | - |
| 35 | 3 | Rock | Ca-HCO3 | - | 0.0011 | 0.037 | - | 0.033 | - | - | 0.18 | - | - | - | - | - | 8.3 | 0.00055 | - | - | - | - | 3.2 | - |
| 36 | 3 | Rock | Ca-HCO3 | 0.002 | - | 0.012 | 0.011 | 0.043 | - | - | 0.041 | - | - | 0.0023 | - | - | 8.5 | 0.00029 | - | 0.04 | - | 0.4 | 0.2 | - |
| 37 | 3 | Rock | Ca-HCO3 | 0.0029 | - | 0.011 | 0.022 | - | - | - | 2 | - | - | - | - | - | 6.1 | 0.00088 | 0.0054 | 0.05 | - | 1.7 | 1.7 | - |
| 38 | 3 | Rock | Ca-HCO3 | 0.0018 | 0.0021 | 0.02 | 0.057 | 0.36 | - | - | 1.6 | - | - | - | - | - | 7.1 | 0.00043 | - | 0.11 | - | 1 | - | - |
| 39 | 3 | Rock | Ca-HCO3 | 0.0012 | 0.0058 | 0.032 | 0.042 | - | - | - | 2.2 | - | - | - | - | - | 4.5 | 0.002 | - | 0.16 | - | 0.8 | 0.1 | - |
| 40 | 3 | Rock | Na-HCO3 | 0.0029 | 0.0039 | 0.011 | 0.3 | - | - | - | 0.16 | - | - | - | - | - | 6.7 | 0.00058 | 0.0055 | 0.23 | - | 1.3 | 0.1 | - |
| 41 | 3 | Rock | Na-HCO3 | 0.00065 | - | 0.13 | 0.46 | 0.19 | 0.011 | - | 0.077 | - | - | - | - | - | 6.5 | - | - | 0.45 | - | 1.2 | 0.4 | - |
| 42 | 4 | Rock | Na-Cl | 0.0016 | - | - | 0.25 | 0.066 | 0.013 | - | 1.4 | - | - | - | - | - | 5.6 | 0.00019 | - | 0.51 | 2 | 1 | - | - |
| 43 | 4 | Rock | Ca-Cl | 0.0078 | - | 0.0041 | 0.22 | 0.15 | 0.07 | - | 4.1 | - | - | - | - | - | 8.2 | - | 0.0019 | - | 3.5 | 1.9 | - | - |
| 44 | 4 | Rock | Ca-Cl | 0.0065 | - | 0.012 | 0.14 | 0.7 | 0.57 | - | 37 | - | - | - | - | - | 4.5 | - | - | 0.12 | 45 | 1.3 | - | - |
| 45 | 4 | Rock | Ca-Cl | 0.0017 | 0.001 | 0.0029 | 0.32 | 0.1 | 0.084 | - | 18 | - | - | - | - | - | 5.2 | 0.0015 | - | 1.7 | 15 | 1.3 | - | 0.06 |
| 46 | 4 | Rock | Ca-Cl | 0.0036 | - | 0.0016 | 0.15 | 0.13 | 0.014 | - | 2.3 | - | - | - | - | - | 3.4 | 0.00011 | 0.0036 | 0.07 | 3 | 1.6 | - | - |
| 47 | 4 | Rock | Ca-Cl | 0.0015 | - | 0.0041 | 0.037 | - | 0.021 | - | 0.69 | - | - | - | - | - | 5.4 | - | 0.0096 | - | 0.2 | 0.7 | 0.11 | - |
| 48 | 4 | Rock | Na-Cl | 0.006 | 0.001 | 0.0067 | 0.35 | 0.15 | 0.01 | - | 0.22 | - | - | - | - | - | 0.22 | - | - | 1.8 | 1.9 | 1.6 | - | - |
| 49 | 4 | Rock | Ca-Cl | 0.0059 | - | 0.064 | 0.75 | - | 0.082 | - | 22 | - | - | - | - | - | 5 | 0.00062 | - | 1.3 | 24 | 2.4 | - | - |
| 50 | 4 | Rock | Na-Cl | 0.015 | - | 0.1 | 0.46 | 0.27 | 0.12 | - | 27 | - | - | - | - | - | 4.8 | - | - | 3 | 19 | 0.8 | - | 0.04 |
| 51 | 4 | Rock | Na-Cl | 0.0014 | - | - | 0.68 | 1.3 | 0.037 | - | 3.7 | - | - | - | - | - | 11 | - | 0.0016 | 2.8 | 12 | 1.1 | - | - |
| Cluster 1 (N = 15) | Cluster 2 (N=20) | Cluster 3 (N = 8) | Cluster 4 (N = 8) | |
|---|---|---|---|---|
| Water Type | Na-Cl (0)/Ca-Cl (0) | Na-Cl (2)/Ca-Cl (1) | Na-Cl(3)/Ca-Cl(5) | Na-Cl (8)/Ca-Cl (0) |
| Na-HCO3 (0)/Ca-HCO3 (15) | Na-HCO3 (6)/Ca-HCO3 (11) | Na-HCO3 (0)/Ca-HCO3 (0) | Na-HCO3 (0)/Ca-HCO3 (0) | |
| Aquifer Type | Rock (6)/Granular (9) | Rock (12)/Granular (8) | Rock (7)/Granular (1) | Rock (1)/Granular (7) |
| Hydrogeological Context * | UnC (10)/C (0)/UnK (6) | UnC (6)/C (7)/UnK (7) | UnC (5)/C (0)/UnK (3) | UnC (1)/C (6)/UnK (1) |
| Median | Median | Median | Median | |
| TDS (mg/L) | 229.6 | 402.4 | 1733.1 | 3142.6 |
| Temperature (Celcius) ** | 7.68 | 7.58 | 7.77 | 7.69 |
| Redox potential (mV) ** | 100.5 | 84.05 | −24.45 | −48.25 |
| pH ** | 7.49 | 7.57 | 7.80 | 7.46 |
| Dissolved oxygen (mg/l) ** | 2.65 | 0.00 | 0.00 | 0.00 |
| Sodium | 4.40 | 32.50 | 240.00 | 905.00 |
| Magnesium | 4.30 | 7.00 | 16.00 | 42.50 |
| Potassium | 2.50 | 4.05 | 3.40 | 26.00 |
| Calcium | 28.00 | 25.50 | 300.00 | 59.00 |
| Bicarbonates | 146.40 | 231.80 | 134.20 | 403.82 |
| Chloride | 5.10 | 29.50 | 815.00 | 1500.00 |
| Sulfates | 9.40 | 15.50 | 58.00 | 190.00 |
| Barium | 0.026 | 0.058 | 0.190 | 0.048 |
| Boron | 0.010 | 0.050 | 0.235 | 0.475 |
| Strontium | 0.180 | 0.375 | 12.900 | 1.650 |
| Silicium | 5.700 | 6.600 | 5.100 | 8.950 |
| Manganese | 0.014 | 0.014 | 0.079 | 0.061 |
| Fluoride | 0.200 | 0.750 | 1.300 | 1.450 |
| Aluminium | 0.007 | 0.008 | 0.005 | 0.006 |
| Bromide | <DL | <DL | 11.500 | 5.553 |
| Iron | 0.071 | 0.105 | 0.140 | 0.190 |
| Lithium | <DL | <DL | 0.076 | 0.019 |
| Zinc | 0.0240 | 0.0110 | 0.005 | 0.015 |
| Lead | <DL | <DL | <DL | <DL |
| Ammonium | 0.040 | 0.195 | 0.460 | 1.282 |
| Copper | 0.006 | 0.001 | <<DL | <DL |
| Molybdenium | 0.001 | 0.002 | 0.0048 | 0.002 |
| Nickel | <DL | 0.001 | <<DL | <DL |
| Silver | <DL | 0.0001 | <DL | <DL |
| Uranium | <DL | <DL | <DL | 0.001 |
| Chromium | <DL | <DL | <DL | <DL |
| Nitrate | 0.300 | 0.060 | <DL | <DL |
| Sulfide | NA | NA | <DL | <DL |
| Cobalt | <DL | <DL | <DL | <DL |
| Inorganic phosphorus | <DL | <DL | <DL | 0.042 |
| Vanadium | <DL | <DL | <DL | <DL |
| Antimony | <DL | <DL | <DL | <DL |
| Cadmium | <DL | <DL | <DL | <DL |
| Selenium | <DL | <DL | <DL | <DL |
| Tin | <DL | <DL | <DL | <DL |
| Titanium | <DL | <DL | <DL | <DL |
| Beryllium | <DL | <DL | <DL | <DL |
| Bismuth | <DL | <DL | <DL | <DL |
| Parameter | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| Potassium | 0.87 | 0.21 | −0.03 | 0.24 |
| Bicarbonates | 0.71 | 0.24 | −0.22 | 0.22 |
| Magnesium | 0.70 | 0.22 | 0.45 | 0.15 |
| Silicium | 0.67 | −0.34 | 0.08 | 0.00 |
| Ammonium | 0.64 | 0.49 | 0.05 | 0.28 |
| Fluoride | 0.16 | 0.90 | 0.07 | −0.12 |
| Boron | 0.50 | 0.78 | 0.15 | 0.10 |
| Molybdenum | −0.12 | 0.77 | 0.20 | 0.04 |
| Sodium | 0.60 | 0.70 | 0.23 | 0.03 |
| Calcium | 0.02 | −0.05 | 0.93 | 0.20 |
| Barium | −0.11 | 0.15 | 0.83 | 0.15 |
| Strontium | 0.21 | 0.47 | 0.79 | 0.09 |
| Sulfates | 0.61 | 0.22 | 0.62 | −0.03 |
| Iron | 0.26 | −0.14 | 0.08 | 0.82 |
| Manganese | 0.14 | 0.16 | 0.40 | 0.80 |
| Explained variance | 3.77 | 3.33 | 3.10 | 1.63 |
| Explained variance (%) | 25.1 | 22.2 | 20.6 | 10.8 |
| Cumulative % of variance | 25.1 | 47.3 | 68.0 | 78.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walter, J.; Chesnaux, R.; Gaboury, D.; Cloutier, V. Subsampling of Regional-Scale Database for improving Multivariate Analysis Interpretation of Groundwater Chemical Evolution and Ion Sources. Geosciences 2019, 9, 139. https://doi.org/10.3390/geosciences9030139
Walter J, Chesnaux R, Gaboury D, Cloutier V. Subsampling of Regional-Scale Database for improving Multivariate Analysis Interpretation of Groundwater Chemical Evolution and Ion Sources. Geosciences. 2019; 9(3):139. https://doi.org/10.3390/geosciences9030139
Chicago/Turabian StyleWalter, Julien, Romain Chesnaux, Damien Gaboury, and Vincent Cloutier. 2019. "Subsampling of Regional-Scale Database for improving Multivariate Analysis Interpretation of Groundwater Chemical Evolution and Ion Sources" Geosciences 9, no. 3: 139. https://doi.org/10.3390/geosciences9030139
APA StyleWalter, J., Chesnaux, R., Gaboury, D., & Cloutier, V. (2019). Subsampling of Regional-Scale Database for improving Multivariate Analysis Interpretation of Groundwater Chemical Evolution and Ion Sources. Geosciences, 9(3), 139. https://doi.org/10.3390/geosciences9030139





