Follow the High Subcritical Water
Abstract
:1. Introduction
2. Methods
3. Results
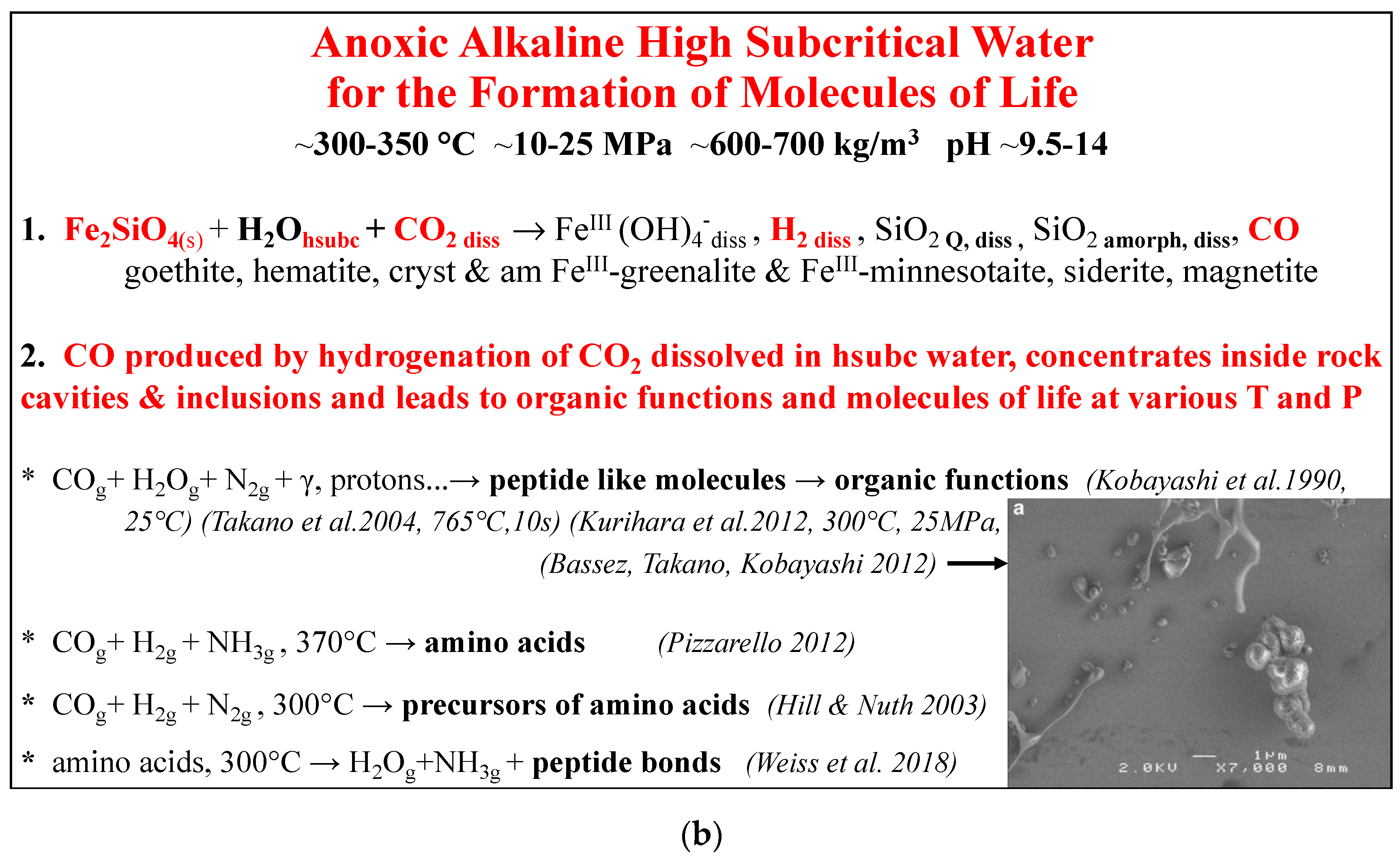
3.1. Five Processes in High Subcritical Water for the Anoxic Formation of Ferric Minerals and Molecules of Life
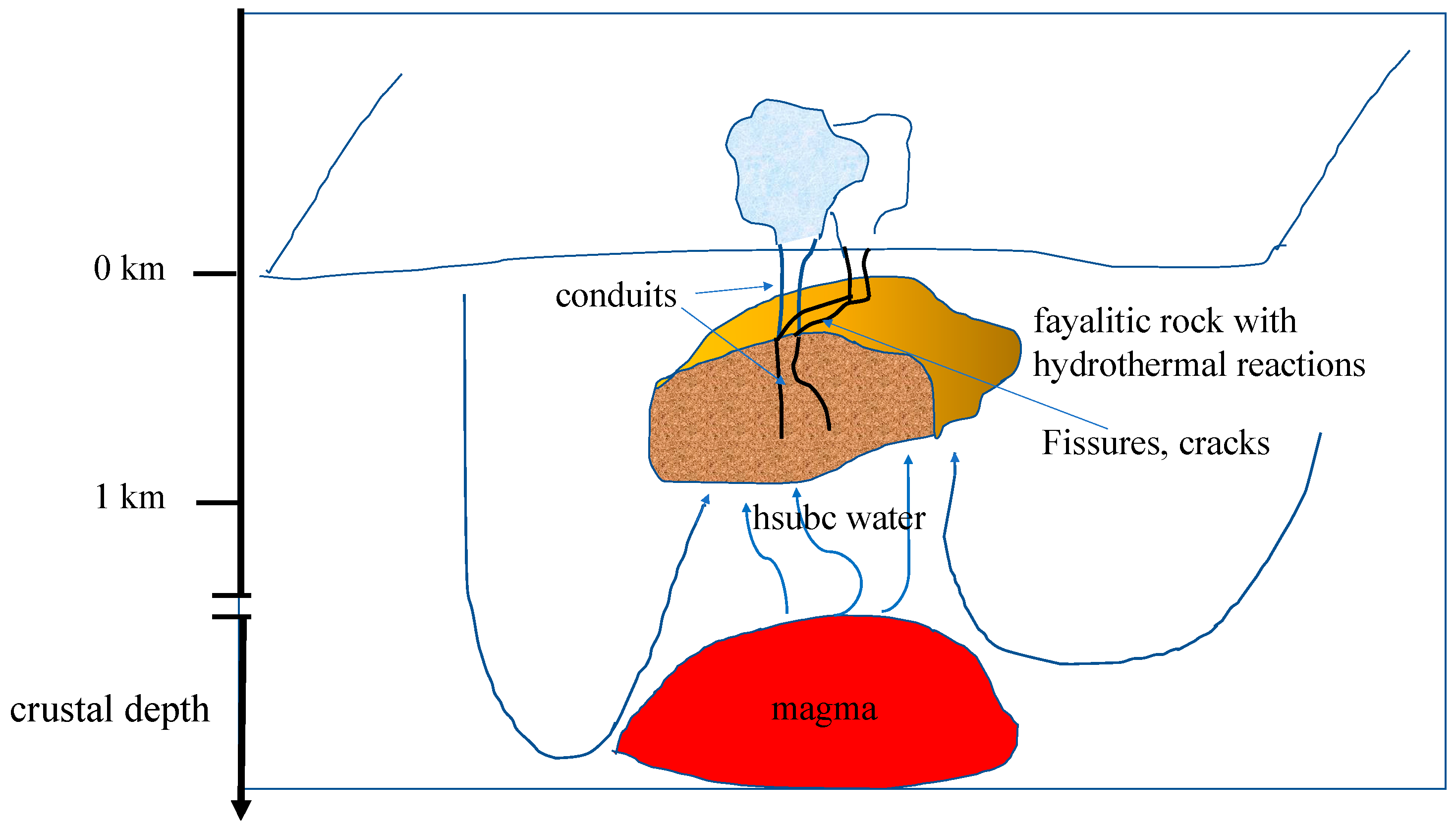
3.2. Formation of Ferric Minerals in Geological Terrains and on Early Earth: A Proposed Alkaline High Subcritical Water–Ferrous Silicate Rock Interaction for the Banded Iron Formations
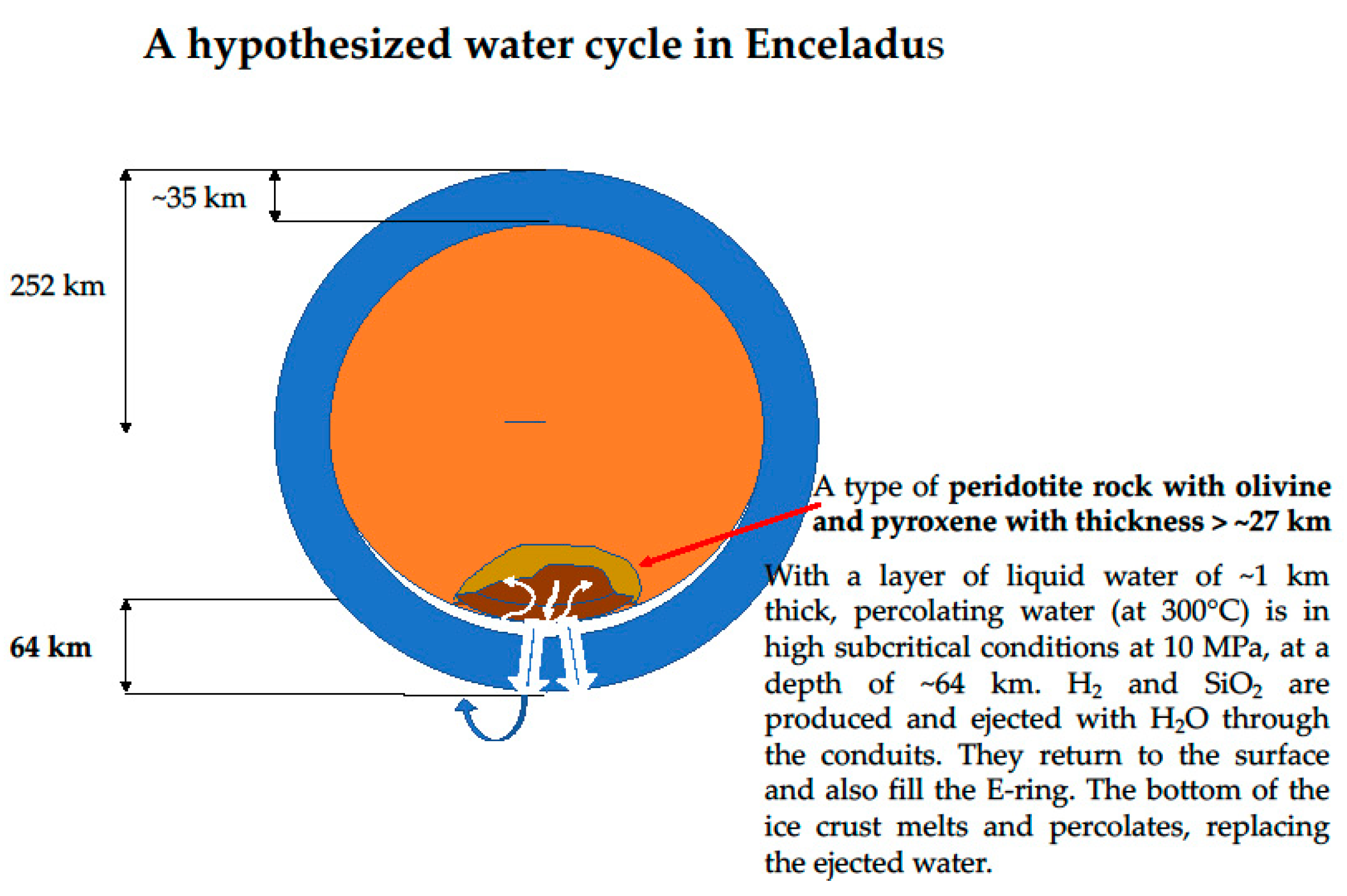
3.3. The Case of Enceladus
3.3.1. Composition of the Plume and Structure of Enceladus
3.3.2. A Plausible High Subcritical Water-Ferromagnesian Silicate Rock Interaction on Enceladus
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hubbard, S.G.; Naderi, F.M.; Garvin, J.B. Following the water, the new program for Mars exploration. Acta Astronaut. 2002, 51, 337–350. [Google Scholar] [CrossRef]
- Bassez, M.-P. Geochemical origin of biological molecules. EGU’2013, PS8.1, Oral 9 April 2013, Vienna, Austria. Geophys. Res. Abstr. 2013, 15, EGU2013-22. [Google Scholar]
- Bassez, M.-P. Water, Air, Earth and Cosmic Radiation. Orig. Life Evol. Biosph. 2015, 45, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Bassez, M.-P. Geobiotropy. In Proceedings of the 47th Lunar Planetary Science Conference 2016, Abstract. 1853. LPSC’2016, Session 804, The Woodlands, TX, USA, 21–25 March 2016. [Google Scholar]
- Bassez, M.-P. Anoxic and oxic oxidation of rocks containing Fe(II)Mg-silicates and Fe(II)-monosulfidesas source of Fe(III)-minerals and hydrogen. Geobiotropy. Orig. Life Evol. Biosph. 2017, 47, 453–480. [Google Scholar] [CrossRef] [PubMed]
- Bassez, M.-P. Ferromagnesian silicate and ferrosulfide rocks as a source of magnetite and hydrogen. Procedia Earth Planet. Sci. 2017, 17, 492–495. [Google Scholar] [CrossRef]
- Bassez, M.-P. Water near its supercritical point and at alkaline pH for the production of ferric oxides and silicates in anoxic conditions. A new hypothesis for the synthesis of minerals observed in Banded Iron Formations and for the related geobiotropic chemistry inside fluid inclusions. Orig. Life Evol. Biosph. 2018, 48, 289–320. [Google Scholar] [CrossRef] [PubMed]
- Bassez, M.-P. High subcritical water–rock interaction for the formation of ferric minerals, in the absence of oxygen, UV light and microorganisms. In Proceedings of the EDP-Science, E3S Web of Conference, WRI16 2019, Tomsk, Russia, 21–26 July 2019. In press. [Google Scholar]
- Konhauser, K.O.; Planavsky, N.J.; Hardisty, D.S.; Robbins, L.J.; Warchola, T.J.; Haugaard, R.; Lalonde, S.V.; Partin, C.A.; Oonk, P.B.H.; Tsikos, H.; et al. Iron formations: A global record of Neoarchaean to Palaeoproterozoic environmental history. Earth Sci. Rev. 2017, 172, 140–177. [Google Scholar] [CrossRef] [Green Version]
- Czaja, D.A.; van Kranendonk, J.M.; Beard, L.B.; Johnson, M.C. A multistage origin for Neoarchean layered hematite-magnetite iron formation from the Weld Range, Yilgarn Craton, Western Australia. Chem. Geol. 2018, 488, 125–137. [Google Scholar] [CrossRef]
- Hegler, F.; Posth, R.N.; Jiang, J.; Kappler, A. Physiology of phototrophic iron(II)-oxidizing bacteria: Implications for modern and ancient environments. FEMS Microbiol. Ecol. 2008, 66, 250–260. [Google Scholar] [CrossRef]
- Madigan, T.M. Anoxygenic phototrophic bacteria from extreme environments. Photosynth. Res. 2003, 76, 157–171. [Google Scholar] [CrossRef]
- Scholes, D.G.; Fleming, R.G.; Olaya-Castro, A.; van Grondelle, R. Lessons from nature about solar light harvesting. Nat. Chem. 2011, 3, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Witzel, T.; Bassez, M.-P. Etude bibliographique de l’eau supercritique. In Report-1rst Year, IUT-Chemistry; Université Robert Schuman: Strasbourg, France, 1997. [Google Scholar]
- Bassez, M.-P. La structure de l’eau supercritique et l’origine de la vie. In Sciences et Technologies: Regards croisés; sciences pour l’ingénieur, informatique, mathématiques, biologie, biochimie, chimie; L’Harmattan: Paris, France, 1999; pp. 583–591. ISBN 2-7384-7367-9. [Google Scholar]
- Bassez, M.-P. Is high-pressure water the cradle of life? J. Phys. Condens Matter 2003, 15, L353–L361. [Google Scholar] [CrossRef]
- Bassez, M.-P. Prebiotic synthesis under hydrothermal conditions. C. R. Chimi. Acad. Des. Sci. Paris 2009, 12, 801–807. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J.; Martin, W. Serpentinization as a source at the origin of life. Geobiology 2010, 8, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Chivot, J. Thermodynamique Des Produits De Corrosion; Andra: Châtenay-Malabry, France, 2004. [Google Scholar]
- Timm, F.; Möller, P. The relation between electric and redox potential: Evidence from laboratory and field measurements. J. Geochem. Explor. 2001, 72, 115–128. [Google Scholar] [CrossRef]
- Cook, G.W.; Olive, P.R. Pourbaix diagrams for the iron-water system extended to high & low supercritical conditions. Corros. Sci. 2012, 55, 326–331. [Google Scholar] [CrossRef]
- Smith, R.L., Jr.; Fang, Z. Properties and phase equilibria of fluid mixtures as the basis for developing green chemical processes. Fluid Phase Equilib. 2011, 302, 65–73. [Google Scholar] [CrossRef]
- Karasek, P.; Stavikova, L.; Planeta, J.; Hohnova, B.; Roth, M. Solubility of fused silica in sub- and supercritical water: Estimation from a thermodynamic model. J. Supercrit. Fluids 2013, 83, 72–77. [Google Scholar] [CrossRef]
- Shock, E.L.; Helgeson, H.C.; Sverjensky, D.A. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Standard partial molal properties of inorganic neutral species. Geochim. Cosmochim. Acta 1989, 53, 2157–2183. [Google Scholar] [CrossRef]
- Qafoku, O.; Kovarik, L.; Kukkadapu, K.R.; Ilton, S.E.; Arey, W.B.; Tucek, J.; Felmy, R.A. Fayalite dissolution and siderite formation in water-saturated supercritical CO2. Chem. Geol. 2012, 332–333, 124–135. [Google Scholar] [CrossRef]
- Tosca, N.J.; Guggenheim, S.; Pufahl, P.K. An authigenic origin for Precambrian greenalite: Implications for iron formation and the chemistry of ancient seawater. GSA Bull. 2016, 128, 511–530. [Google Scholar] [CrossRef]
- Milesi, V.; Guyot, F.; Brunet, F.; Richard, L.; Recham, N.; Benedetti, M.; Dairou, J.; Prinzhofer, A. Formation of CO2, H2 and condensed carbon from siderite dissolution in the 200–300 °C range and at 50 MPa. Geochim. Cosmochim. Acta 2015, 154, 201–211. [Google Scholar] [CrossRef]
- Sabatier, P.; Senderens, J.B. Nouvelles synthèses du méthane. Comptes Rendus des Séances de l’académie des Sciences 1902, 134, 514–516. [Google Scholar]
- Sabatier, P.; Senderens, J.B. Hydrogénation directe des oxydes du carbone en présence de divers métaux divisés. Comptes Rendus des Séances de l’académie des Sciences 1902, 134, 689–691. [Google Scholar]
- Anderson, R.B. The Fischer–Tropsch Synthesis; Academic Press, Inc.: Orlando, FL, USA, 1984; p. 301. [Google Scholar]
- de Smit, E.; Weckhuyse, B.M. The renaissance of iron-based Fischer–Tropsch synthesis: On the multifaceted catalyst deactivation behaviour. Chem. Soc. Rev. 2008, 37, 2758–2781. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Seyfried, W.E., Jr. Experimental study of abiotic synthesis processes in a hydrothermal flow system. In Proceedings of the 40th Lunar Planetary Science Conference 2009, Abstract 2504, LPSC’2009, Session 104, The Woodlands, TX, USA, 23 March 2009. [Google Scholar]
- Schlesinger, G.; Miller, S.L. Prebiotic synthesis in atmospheres containing CH4, CO, and CO2. J. Mol. Evol. 1983, 19, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Tsuchiya, M.; Oshima, T.; Yanagawa, H. Abiotic synthesis of amino acids and imidazole by proton irradiation of simulated primitive earth atmospheres. Orig. Life Evol. Biosph. 1990, 20, 99–109. [Google Scholar] [CrossRef]
- Takano, Y.; Marumo, K.; Yabashi, S.; Kaneko, T.; Kobayashi, K. Pyrolysis of complex organics following high-energy proton irradiation of a simple inorganic gas mixture. Appl. Phys. Lett. 2004, 85, 1633–1635. [Google Scholar] [CrossRef]
- Kurihara, H.; Yabuta, H.; Kaneko, T.; Obayashi, Y.; Takano, Y.; Kobayashi, K. Characterization of organic aggregates formed by heating products of simulated primitive Earth atmosphere experiments. Chem. Lett. 2012, 41, 441–443. [Google Scholar] [CrossRef]
- Bassez, M.-P.; Takano, Y.; Kobayashi, K. Prebiotic organic microstructures. Orig. Life Evol. Biosph. 2012, 42, 307–316. [Google Scholar] [CrossRef]
- Pizzarello, S. Catalytic syntheses of amino acids and their significance for nebular and planetary chemistry. Meteorit. Planet. Sci. 2012, 47, 1291–1296. [Google Scholar] [CrossRef]
- Hill, H.G.M.; Nuth, J. The catalytic potential of cosmic dust: Implications for prebiotic chemistry in the solar nebula and other protoplanetary systems. Astrobiology 2003, 3, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.I.; Muth, C.; Drumm, R.; Kirchner, K.O. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophys. 2018, 11, 2–15. [Google Scholar] [CrossRef]
- Xing, T.; Zhu, W.; Fusseis, F.; Lisabeth, H. Generating porosity during olivine carbonation via dissolutionchannels and expansion cracks. Solid Earth 2018, 9, 879–896. [Google Scholar] [CrossRef]
- Zhu, W.; Fusseis, F.; Lisabeth, H.P.; Xing, T.; Xiao, X.; De Andrade, V.; Karato, S.I. Experimental evidence of reaction-induced fracturing during olivine carbonation. Geophys. Res. Lett. 2016, 43, 9535–9543. [Google Scholar] [CrossRef] [Green Version]
- Beukes, J.N.; Gutzmer, J. Origin and Paleoenvironmental significance of major iron formations at the Archean-Paleoproterozoic boundary. Soc. Econ. Geol. Rev. 2008, 15, 5–47. [Google Scholar]
- Dekkers, M.J. Magnetic properties of natural goethite: III. Magnetic behaviour and properties of minerals originating from goethite dehydration during thermal demagnetization. Geoph. J. Int. 1990, 103, 233–250. [Google Scholar] [CrossRef]
- Barley, E.M.; Pickard, L.A.; Sylvester, J.P. Emplacement of a large igneous province as a possible cause of banded iron formation 2.45 billion years ago. Nature 1997, 385, 55–58. [Google Scholar] [CrossRef]
- Haugaard, R.; Pecoits, E.; Lalonde, S.; Rouxel, O.; Konhauser, K. The Joffre banded iron formation, Hamersley group, Western Australia: Assessing the paleoenvironment through detailed petrology and chemostratigraphy. Precambrian Res. 2016, 273, 12–37. [Google Scholar] [CrossRef]
- Hofmann, A.; Karykowski, B.; Mason, P.; Chunnet, G.; Arndt, N. Barberton drilling project-Buck Reef Chert core BARB3. In Proceedings of the EGU’2013, Vienna, Austria, 7–12 April 2013; Volume 15. EGU2013-EG12227. [Google Scholar]
- Hendrix, A.R.; Hurford, T.A.; Barge, L.M.; Bland, M.T.; Bowman, J.S.; Brinkckerhoff, W.; Buratti, B.J.; Cable, M.L.; Castillo-Rogez, J.; Collims, G.C.; et al. The NASA Roadmap to Ocean Worlds. Astrobiology 2019, 19, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekine, Y.; Shibuya, T.; Kamata, S. E Enceladus: Evidence and unsolved questions for an Ice-Covered Habitable world. In Astrobiology. from the Origins of Life to the Search of Extraterrestrial Intelligence; Yamagishi, A., Kakegawa, T., Usui, T., Eds.; Springer Nature: Singapore, 2019; pp. 1189–1220. [Google Scholar]
- Bassez, M.-P. La recherche de vie dans l’univers. In Regard vers l’Univers Conference; University of Strasbourg: Strasbourg, France, Available online: https://pod.unistra.fr/video/8916-la-recherche-de-vie-dans-lunivers and http://chemphys.u-strasbg.fr/mpb/teach/originevie.html; (accessed on 31 January 2013).
- Waite, J.H., Jr.; Lewis, W.S.; Magee, B.A.; Lunine, J.I.; McKinnon, W.B.; Glein, C.R.; Mousis, O.; Young, D.T.; Brockwell, T.; Westlake, J.; et al. Liquid Water on Enceladus from observations of ammonia and 40Ar in the plume. Nat. Lett. 2009, 460, 487–491. [Google Scholar] [CrossRef]
- Waite, J.H.; Magee, B.; Brockwell, T.; Zolotov, M.Y.; Teolis, B.; Lewis, W.S.; The INMS Team. Enceladus’ Plume Composition. EPSC Abstr. 2011, 6, EPSC-DPS2011-61-4. [Google Scholar]
- Waite, J.H.; Glein, C.R.; Perryman, R.S.; Teolis, B.D.; Magee, B.A.; Miller, G.; Grimes, J.; Perry, M.E.; Miller, K.E.; Bouquet, A.; et al. Cassini finds molecular hydrogen in the Enceladus plume: Evidence for hydrothermal processes. Science 2017, 356, 155–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postberg, F.; Kempf, S.; Schmidt, J.; Brilliantov, N.; Beinsen, A.; Abel, B.; Buck, U.; Srama, R. Sodium salts in E-ring ice grains from an ocean below the surface of Enceladus. Nature 2009, 459, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Zolotov, M.Y. An oceanic composition on early and today’s Enceladus. Geophys. Res. Lett. 2007, 34, L23203. [Google Scholar] [CrossRef]
- Glein, C.R.; Baross, J.A.; Waite, J.H., Jr. The pH of Enceladus’ ocean. Geochim. Cosmochim. Acta 2015, 162, 202–219. [Google Scholar] [CrossRef]
- Hsu, H.-W.; Postberg, F.; Sekine, Y.; Shibuya, T.; Kempf, S.; Horanyi, M.; Juhasz, A.; Altobelli, N.; Suzuki, K.; Masaki, Y.; et al. Ongoing hydrothermal activities within Enceladus. Nature 2015, 519, 207–210. [Google Scholar] [CrossRef]
- Sekine, Y.; Shibuya, T.; Postberg, F.; Hsu, H.W.; Suzuki, K.; Masaki, Y.; Kuwatani, T.; Mori, M.; Hong, P.K.; Yoshieaki, M.; et al. High-temperature water–rock interactions and hydrothermal environments in the chondrite-like core of Enceladus. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Postberg, F.; Khawaja, N.; Abel, B.; Choblet, G.; Glein, C.R.; Gudipati, M.S.; Henderson, B.L.; Hsu, H.-W.; Kempf, S.; Klenner, F.; et al. Macromolecular organic compounds from the depths of Enceladus. Nature 2018, 528, 564–568. [Google Scholar] [CrossRef]
- Combe, J.-P.; McCord, T.B.; Matson, D.L.; Johnson, T.V.; Davies, A.G.; Scipioni, F.; Tosi, F. Nature, distribution and origin of CO2 on Enceladus. Icarus 2019, 317, 491–508. [Google Scholar] [CrossRef]
- Guzman, M.; Lorenz, R.; Hurley, D.; Farrell, W.; Spencer, J.; Hansen, C.; Hurford, T.; Ibea, J.; Carlson, P.; McKay, C.P. Collecting amino acids in the Enceladus plume. Int. J. Astrobiol. 2019, 18, 47–59. [Google Scholar] [CrossRef]
- Spencer, J.R.; Pearl, J.C.; Segura, M.; Flasar, F.M.; Mamoutkine, A.; Romani, P.; Buratti, B.; Hendrix, A.; Spilker, L.J.; Lopes, R.M.C. Cassini encounters Enceladus: Background and the discovery of a south polar hot spot. Science 2006, 311, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Howett, C.J.A.; Spencer, J.R.; Pearl, J.; Segura, M. High heat flow from Enceladus’ south polar regionmeasured using 10–600 cm−1 Cassini/CIRS data. J. Geophys. Res. 2011, 116, 1–15. [Google Scholar] [CrossRef]
- Spencer, J.R.; Howett, C.J.A.; Verbiscer, A.; Hurford, T.A.; Segura, M.; Spencer, D.C. Enceladus heat flow from high spatial resolution thermal emission observations. EPSC Abstr. 2013, 8, EPSC2013-840-1. [Google Scholar]
- Hedman, M.M.; Gosmeyer, C.M.; Nicholson, P.D.; Sotin, C.; Brown, R.H.; Clark, R.N.; Baines, K.H.; Buratti, B.J.; Showalter, M.R. An observed correlation between plume activity and tidal stresses on Enceladus. Nature 2013, 500, 182–184. [Google Scholar] [CrossRef]
- Iess, L.; Stevenson, D.J.; Parisi, M.; Hemingway, D.; Jacobson, J.I.; Lunine, J.I.; Nimmo, F.; Armstrong, J.W.; Asmar, S.W.; Ducci, M.; et al. The gravity field and interior structure of Enceladus. Science 2014, 344, 78–80. [Google Scholar] [CrossRef]
- McKinnon, W.B. Effects of Enceladus’s rapid synchronous spin on interpretation of Cassini gravity. Geophys. Res. Lett. 2015, 42, 2137–2143. [Google Scholar] [CrossRef]
- Thomas, P.C.; Tajeddine, R.; Tiscareno, M.S.; Burns, J.A.; Joseph, J.; Loredo, T.J.; Helfenstein, P.; Porco, C. Enceladus’s measured physical libration requires a global surface ocean. Icarus 2016, 264, 37–47. [Google Scholar] [CrossRef]
- Cadek, O.; Tobie, G.; Van Hoolst, T.; Massé, M.; Choblet, G.; Lefèvre, A.; Mitri, G.; Baland, R.-M.; Behounkova, M.; Bourgeois, O.; et al. Enceladus’s internal ocean and ice shell constrained from Cassinigravity, shape, and libration data. Geophys. Res. Lett. 2016, 43, 5653–5660. [Google Scholar] [CrossRef]
- Lunine, J.I. Ocean worlds exploration. Acta Astronaut. 2017, 131, 123–130. [Google Scholar] [CrossRef]
- Jean-Baptiste, P.; Fourré, E.; Charlou, J.-L.; German, C.R.; Radford-Knoery, J. Helium isotopes at the Rainbow hydrothermal site (Mid-Atlantic Ridge, 36°14’N). Earth Planet. Sci. Lett. 2004, 221, 325–335. [Google Scholar] [CrossRef]
- Thurnherr, A.M.; Richards, K.J. Hydrography and high-temperature heat flux of the Rainbow hydrothermal site (36°14N, Mid-Atlantic Ridge). J. Geophys. Res. 2001, 106, 9411–9426. [Google Scholar] [CrossRef]
- Mügler, C.; Jean-Baptiste, P.; Florian, P.; Charlou, J.-L. Modeling of hydrogen production by serpentinization in ultramafic-hosted hydrothermal systems: Application to the Rainbow field. Geofluids 2016, 16, 476–489. [Google Scholar] [CrossRef]
- Lodders, K.; Amari, S. Presolar grains from meteorites: Remnants from the early times of the solar system. Chem. Erde 2005, 65, 93–166. [Google Scholar] [CrossRef] [Green Version]
- Gounelle, M.; Meibom, A. The origin of short-lived radionuclides and the astrophysical environment of solar system formation. Astrophys. J. 2008, 680, 781–792. [Google Scholar] [CrossRef]
- Malamud, U.; Prialnik, D. Modeling serpentinization: Applied to the early evolution of Enceladus and Mimas. Icarus 2013, 225, 763–774. [Google Scholar] [CrossRef]
- Travis, B.J.; Schubert, G. Keeping Enceladus warm. Icarus 2015, 250, 32–42. [Google Scholar] [CrossRef]
- Porco, C.C.; Helfenstein, P.; Thomas, P.C.; Ingersoll, A.P.; Wisdom, J.; West, R.; Neukum, G.; Denk, T.; Wagner, R.; Roatsch, T.; et al. Cassini observes the active south pole of Enceladus. Science 2006, 311, 1393–1401. [Google Scholar] [CrossRef]
- Dougherty, M.K.; Cao, H.; Khurana, K.K.; Hunt, G.J.; Provan, G.; Kellock, S.; Burton, M.E.; Burk, T.A.; Bunce, E.J.; Cowley, S.W.H.; et al. Saturn’s magnetic field revealed by the Cassini Grande Finale. Science 2018, 362, 1–8. [Google Scholar] [CrossRef]
- Le Duc, S. Théorie Physico-Chimique de La vie et Générations Spontanées; A. Poinat: Paris, France, 1910; p. 202. [Google Scholar]
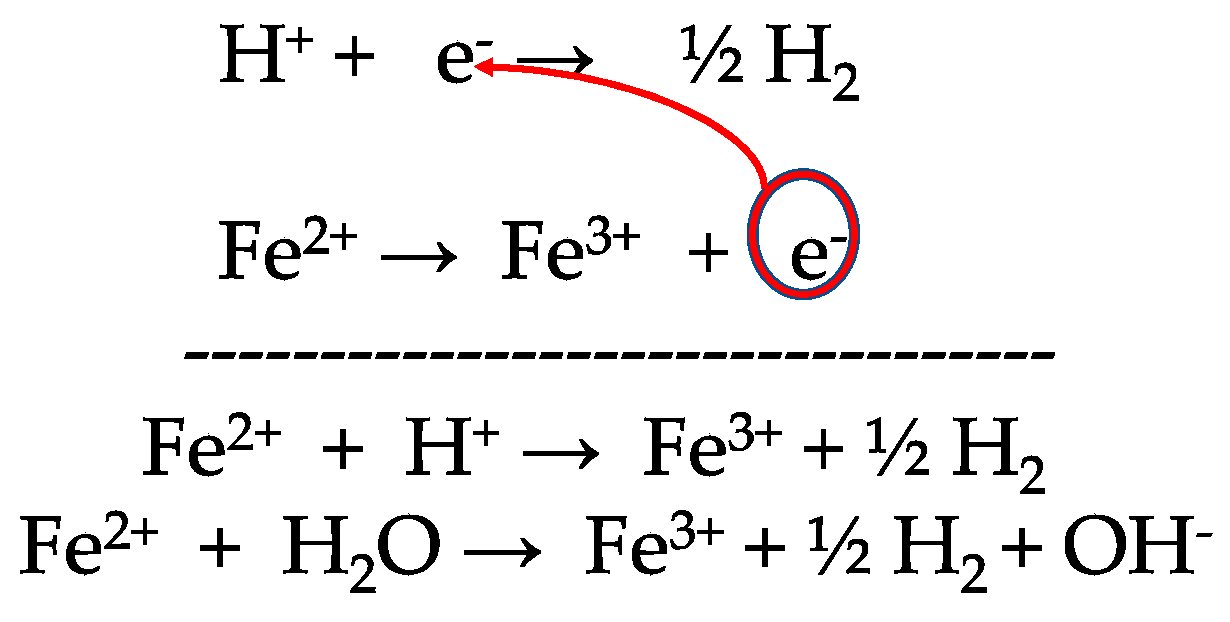




| Reaction No. | Equation for the Reaction | ΔrH° in kJ/mol | ΔrG° in kJ/mol |
|---|---|---|---|
| 1 | 3H2 + 2CO → C2H2 + 2H2O | −41.8 | +70.22 |
| 2 | H2 + 4CO → C2H2 + 2CO2 | −121.22 | + 30.10 |
| 3 | 5H2 + 2CO2 → C2H2 + 4H2O | +37.62 | +110.35 |
| 4 | H2O +5CO → C2H2 + 3CO2 | −160.59 | +10.03 |
| 5 | H2 +2CO → C6H12 + CO2 | −185.17 | −1.75 |
| 6 | 2H2 +CO → C6H12 + H2O | −146.34 | +10.99 |
| Mineral | Density (g/cm3) |
|---|---|
| hematite | 5–5.3 |
| magnetite | 5.18 |
| fayalite | 4.4 |
| ferrosilite | 3.96 |
| goethite | 4.28 |
| siderite | 3.96 |
| riebeckite | 3.30 |
| minnesotaite | 3.1 |
| ankerite | 3.1 |
| dolomite | 2.86 |
| greenalite | 2.85–3.15 |
| calcite | 2.71 |
| stilpnomelane | 2.59–2.96 |
| quartz | 2.59–2.65 |
| hsubc water | 0.7–0.6 |
| Parameter | Value | Reference |
|---|---|---|
| Radius | 252.1 ± 0.2 km | [Int. Space Station] [78] |
| Mean density | 1608.3 ± 4.5 kg/m3 | Porco 2006 [78] |
| Ice crust density | 900 kg/m3 | Chosen for the calculation |
| hsubc water density at 10MPa (and 300 °C) | 700 kg/m3 | Educed from Figure 2 in [21] |
| Acceleration of gravity | 0.113 m/s2 | Travis 2015 [77] |
| Thickness of the ice crust | 30–40 km | Iess 2014 [66] |
| Thickness of the water layer at the south pole | 1 km | Hypothesized in this work |
| Core density (with 35 km ice crust & 1 km water) | 2000 kg/m3 | Calculated in this work |
| Depth inside the core where P = 10 MPa | 28 km | Calculated in this work |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassez, M.-P. Follow the High Subcritical Water. Geosciences 2019, 9, 249. https://doi.org/10.3390/geosciences9060249
Bassez M-P. Follow the High Subcritical Water. Geosciences. 2019; 9(6):249. https://doi.org/10.3390/geosciences9060249
Chicago/Turabian StyleBassez, Marie-Paule. 2019. "Follow the High Subcritical Water" Geosciences 9, no. 6: 249. https://doi.org/10.3390/geosciences9060249
APA StyleBassez, M. -P. (2019). Follow the High Subcritical Water. Geosciences, 9(6), 249. https://doi.org/10.3390/geosciences9060249





