Arboreal Epiphytes in the Soil-Atmosphere Interface: How Often Are the Biggest “Buckets” in the Canopy Empty?
Abstract
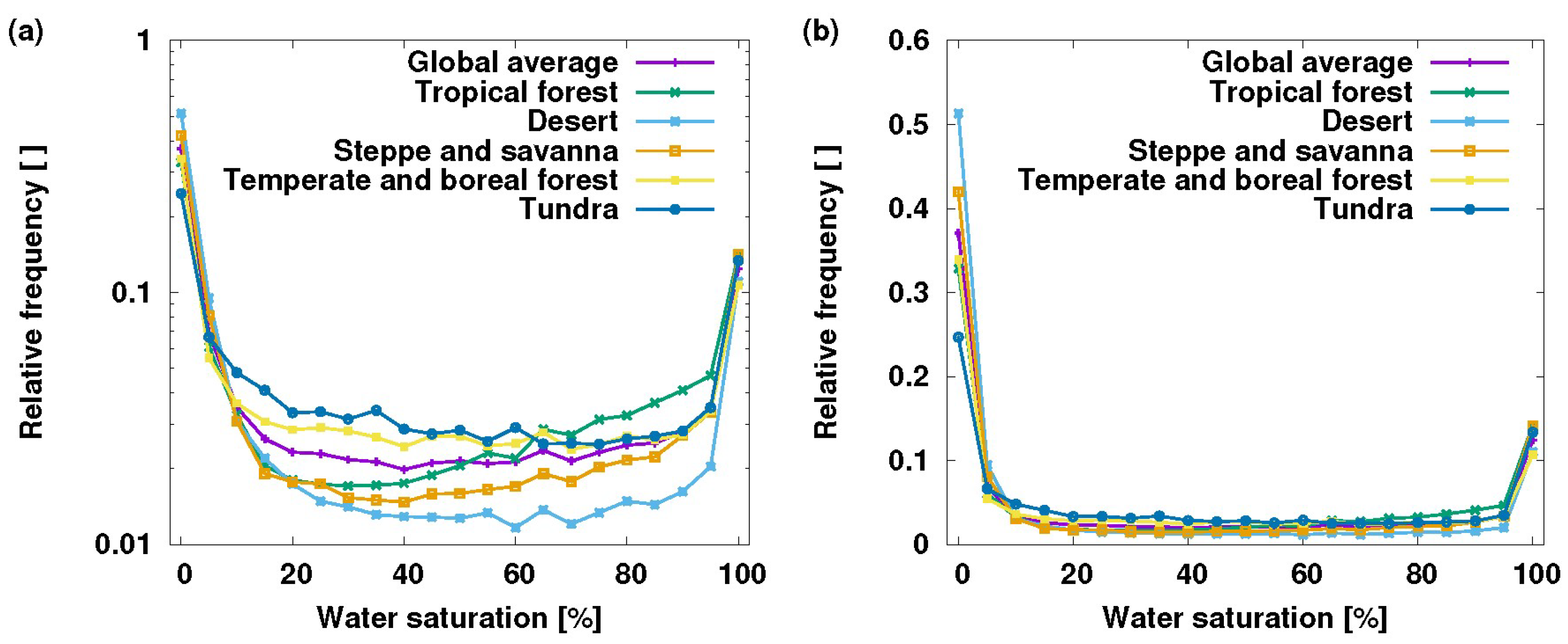
:1. Introduction: How Big Is the Epiphyte Bucket?
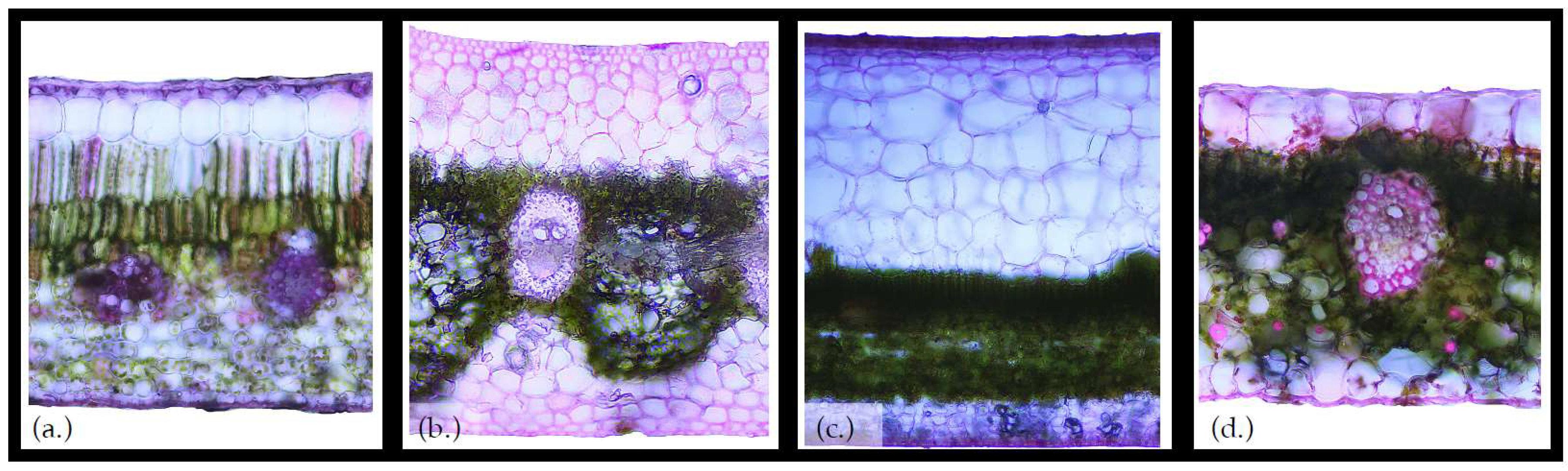
Anatomy of the Epiphyte Bucket
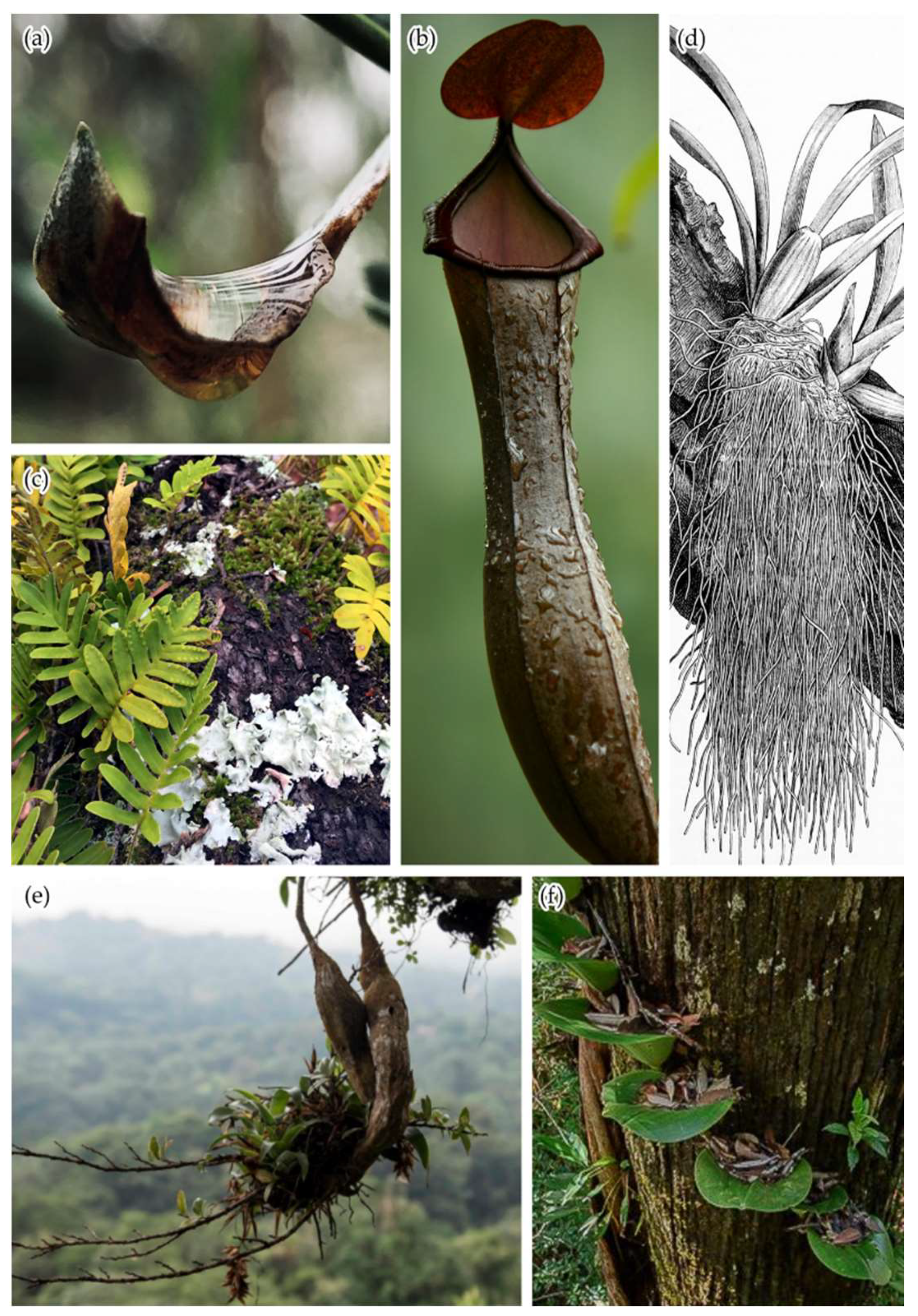
2. Open Buckets: Non-Vascular Epiphytes, Phytotelmata, and Canopy Soils
2.1. Non-Vascular Epiphytes
2.2. Phytotelmata
2.3. Canopy Soils
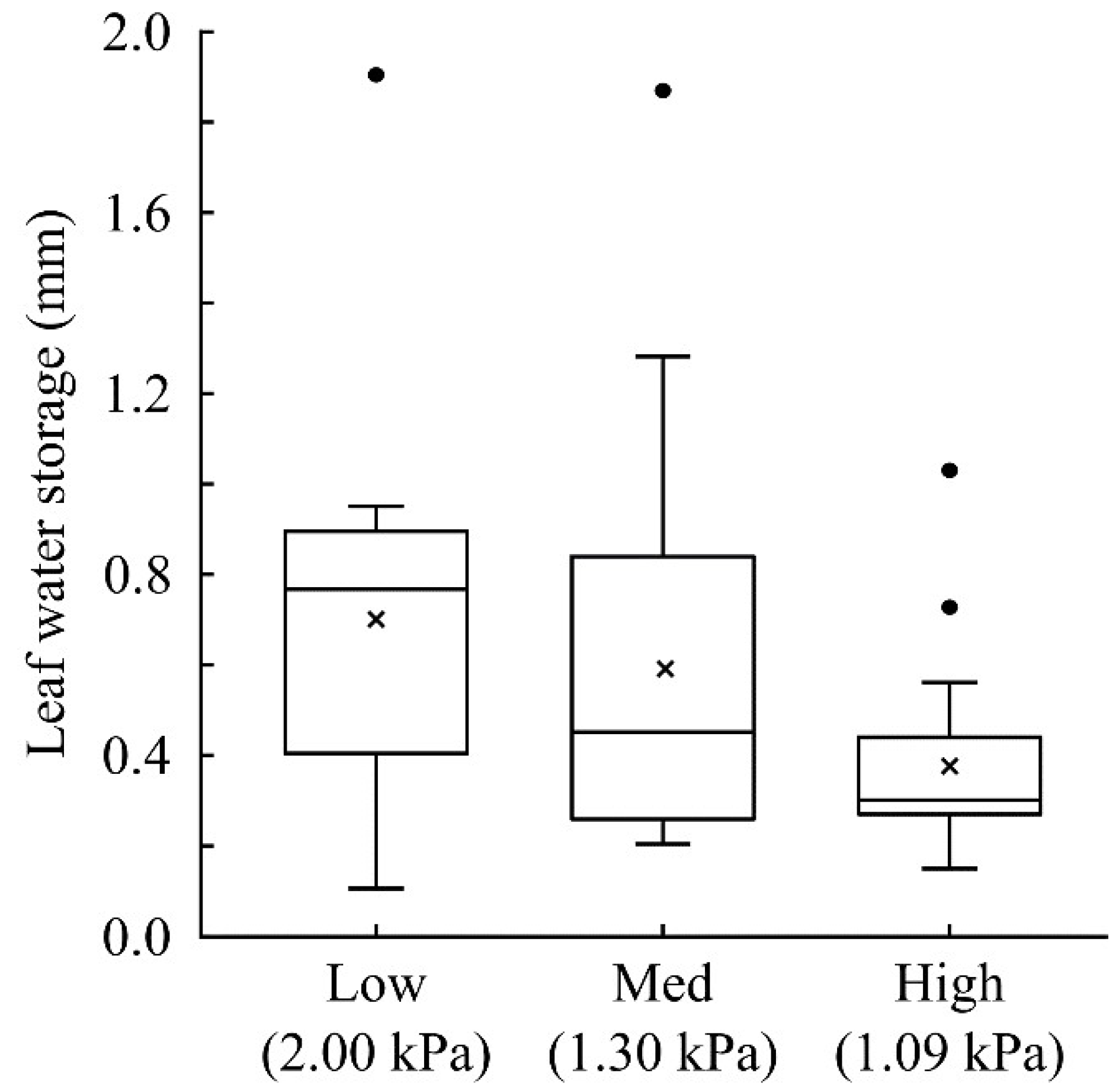
3. Vascular Epiphytes: Do Their Ecophysiological Mechanisms Keep the Bucket Full?
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Manabe, S.; Smagorinsky, J.; Strickler, R.F. Simulated climatology of a general circulation model with a hydrologic cycle. Mon. Weather Rev. 1965, 93, 769–798. [Google Scholar] [CrossRef]
- Manabe, S. Climate and the ocean circulation. Mon. Weather Rev. 1969, 97, 739–774. [Google Scholar] [CrossRef]
- Gerrits, A.; Savenije, H. Forest floor interception. In Forest Hydrology and Biogeochemistry; Springer: Heidelberg, Germany, 2011; pp. 445–454. [Google Scholar]
- Robock, A.; Vinnikov, K.Y.; Schlosser, C.A.; Speranskaya, N.A.; Xue, Y. Use of midlatitude soil moisture and meteorological observations to validate soil moisture simulations with biosphere and bucket models. J. Clim. 1995, 8, 15–35. [Google Scholar] [CrossRef]
- Savenije, H.H. The importance of interception and why we should delete the term evapotranspiration from our vocabulary. Hydrol. Process. 2004, 18, 1507–1511. [Google Scholar] [CrossRef]
- Carlyle-Moses, D.E.; Gash, J.H. Rainfall interception loss by forest canopies. In Forest Hydrology and Biogeochemistry; Springer: Heidelberg, Germany, 2011; pp. 407–423. [Google Scholar]
- Good, S.P.; Moore, G.W.; Miralles, D.G. A mesic maximum in biological water use demarcates biome sensitivity to aridity shifts. Nat. Ecol. Evol. 2017, 1, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Van der Ent, R.; Wang-Erlandsson, L.; Keys, P.; Savenije, H. Contrasting roles of interception and transpiration in the hydrological cycle—Part 2: Moisture recycling. Earth Syst. Dyn. 2014, 5, 471–489. [Google Scholar] [CrossRef]
- Davies-Barnard, T.; Valdes, P.; Jones, C.; Singarayer, J. Sensitivity of a coupled climate model to canopy interception capacity. Clim. Dyn. 2014, 42, 1715–1732. [Google Scholar] [CrossRef]
- Rutter, A.; Kershaw, K.; Robins, P.; Morton, A. A predictive model of rainfall interception in forests, 1. Derivation of the model from observations in a plantation of corsican pine. Agric. Meteorol. 1971, 9, 367–384. [Google Scholar] [CrossRef]
- Klaassen, W.; Bosveld, F.; de Water, E. Water storage and evaporation as constituents of rainfall interception. J. Hydrol. 1998, 212, 36–50. [Google Scholar] [CrossRef]
- Van Stan, J.T., II; Pypker, T.G. A review and evaluation of forest canopy epiphyte roles in the partitioning and chemical alteration of precipitation. Sci. Total Environ. 2015, 536, 813–824. [Google Scholar] [CrossRef]
- Callaway, R.M.; Reinhart, K.O.; Moore, G.W.; Moore, D.J.; Pennings, S.C. Epiphyte host preferences and host traits: Mechanisms for species-specific interactions. Oecologia 2002, 132, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Zotz, G. The role of vascular epiphytes in the ecosystem. In Plants on Plants–The Biology of Vascular Epiphytes; Springer: Cham, Switzerland, 2016; pp. 229–243. [Google Scholar]
- Porada, P.; Van Stan, J.T., II; Kleidon, A. Significant contribution of non-vascular vegetation to global rainfall interception. Nat. Geosci. 2018, 11, 563–567. [Google Scholar] [CrossRef]
- Liu, S. Estimation of rainfall storage capacity in the canopies of cypress wetlands and slash pine uplands in north-central florida. J. Hydrol. 1998, 207, 32–41. [Google Scholar] [CrossRef]
- Xiao, Q.; McPherson, E.G. Surface water storage capacity of twenty tree species in Davis, California. J. Environ. Qual. 2016, 45, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Pypker, T.G.; Levia, D.F.; Staelens, J.; Van Stan, J.T., II. Canopy structure in relation to hydrological and biogeochemical fluxes. In Forest Hydrology and Biogeochemistry; Springer: Cham, Switzerland, 2011; pp. 371–388. [Google Scholar]
- Gotsch, S.G.; Nadkarni, N.; Amici, A. The functional roles of epiphytes and arboreal soils in tropical montane cloud forests. J. Trop. Ecol. 2016, 32, 455–468. [Google Scholar] [CrossRef]
- Jarvis, A. Measuring & modelling the impact of land-use change in tropical hillsides: The role of cloud interception to epiphytes. Adv. Eviron. Modell. Monit. 2000, 1, 118–148. [Google Scholar]
- Nieder, J.; Prosperí, J.; Michaloud, G. Epiphytes and their contribution to canopy diversity. In Tropical Forest Canopies: Ecology and Management; Springer: Cham, Switzerland, 2001; pp. 51–63. [Google Scholar]
- Gotsch, S.G.; Nadkarni, N.; Darby, A.; Glunk, A.; Dix, M.; Davidson, K.; Dawson, T.E. Life in the treetops: Ecophysiological strategies of canopy epiphytes in a tropical montane cloud forest. Ecol. Monogr. 2015, 85, 393–412. [Google Scholar] [CrossRef]
- Kranner, I.; Beckett, R.; Hochman, A.; Nash, T.H., III. Desiccation-tolerance in lichens: A review. Bryologist 2008, 111, 576–593. [Google Scholar] [CrossRef]
- Proctor, M.C.; Oliver, M.J.; Wood, A.J.; Alpert, P.; Stark, L.R.; Cleavitt, N.L.; Mishler, B.D. Desiccation-tolerance in bryophytes: A review. Bryologist 2007, 110, 595–621. [Google Scholar] [CrossRef]
- Hietz, P. Ecology and ecophysiology of epiphytes in tropical montane cloud forests. In Tropical Montane Cloud Forests: Science for Conservation and Management; Cambridge University Press: Cambridge, UK, 2010; pp. 67–76. [Google Scholar]
- Hölscher, D.; Köhler, L.; van Dijk, A.I.; Bruijnzeel, L.S. The importance of epiphytes to total rainfall interception by a tropical montane rain forest in Costa Rica. J. Hydrol. 2004, 292, 308–322. [Google Scholar] [CrossRef]
- Holwerda, F.; Bruijnzeel, L.; Muñoz-Villers, L.; Equihua, M.; Asbjornsen, H. Rainfall and cloud water interception in mature and secondary lower montane cloud forests of central Veracruz, Mexico. J. Hydrol. 2010, 384, 84–96. [Google Scholar] [CrossRef]
- Pypker, T.G.; Unsworth, M.H.; Bond, B.J. The role of epiphytes in rainfall interception by forests in the Pacific Northwest. II. Field measurements at the branch and canopy scale. Can. J. For. Res. 2006, 36, 819–832. [Google Scholar] [CrossRef] [Green Version]
- Tobón, C.; Köhler, L.; Frumau, K.; Bruijnzeel, L.; Burkard, R.; Schmid, S. Water dynamics of epiphytic vegetation in a lower montane cloud forest: Fog interception, storage and evaporation. In Tropical Montane Cloud Forests: Science for Conservation and Management; Cambridge University Press: Cambridge, UK, 2010; pp. 261–267. [Google Scholar]
- Van Stan, J.T., II; Stubbins, A.; Bittar, T.; Reichard, J.S.; Wright, K.A.; Jenkins, R.B. Tillandsia usneoides (L.) L. (Spanish moss) water storage and leachate characteristics from two maritime oak forest settings. Ecohydrology 2015, 8, 988–1004. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Van Ek, R. Rainfall interception in two tropical montane rain forests, colombia. Hydrol. Process. 1990, 4, 311–326. [Google Scholar] [CrossRef]
- Clark, K.L.; Nadkarni, N.M.; Gholz, H.L. Retention of inorganic nitrogen by epiphytic bryophytes in a tropical montane forest. Biotropica 2005, 37, 328–336. [Google Scholar] [CrossRef]
- Nilsson, M.-C.; Wardle, D.A. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 2005, 3, 421–428. [Google Scholar] [CrossRef]
- Stark, L.R. Ecology of desiccation tolerance in bryophytes: A conceptual framework and methodology. Bryologist 2017, 120, 130–165. [Google Scholar] [CrossRef]
- Benzing, D.H. Vascular Epiphytes: General Biology and Related Biota; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Van Stan, J.T., II; (Georgia Southern University, Statesboro, GA, USA). Photograph: Phytotelma at Charles H. Herty Pines Nature Preserve. Unpublished work. 2018. [Google Scholar]
- Marshall, C.; (Bristol, UK). Photograph: Kew Gardens pitcher plant. Unpublished work. 2014. [Google Scholar]
- Van Stan, J.T., II; (Georgia Southern University, Statesboro, GA, USA). Photograph: Saturated Pleopeltis Polypodiodes, foliose lichen, and mosses on a tree branch. Unpublished work. 2018. [Google Scholar]
- Von Marilaun, A.K. The Natural History of Plants, their Forms, Growth, Reproduction, and Distribution; Blackie and Son: London, UK, 1895. [Google Scholar]
- Gotsch, S.G.; (Franklin & Marshall College, Lancaster, PA, USA). Photograph: Lignotuber portrait low. Unpublished work. 2017. [Google Scholar]
- Lee, C.I.C.; (Borneo, Indonesia). Photograph: Leaflitter caught by epiphytic leaves. Unpublished work. 2016. [Google Scholar]
- Nadkarni, N.M. Canopy roots: Convergent evolution in rainforest nutrient cycles. Science 1981, 214, 1023–1024. [Google Scholar] [CrossRef]
- Sanford, R.L. Apogeotropic roots in an amazon rain forest. Science 1987, 235, 1062–1064. [Google Scholar] [CrossRef]
- Orlovich, D.A.; Draffin, S.J.; Daly, R.A.; Stephenson, S.L. Piracy in the high trees: Ectomycorrhizal fungi from an aerial ‘canopy soil’ microhabitat. Mycologia 2013, 105, 52–60. [Google Scholar] [CrossRef]
- Jones, S., Jr.; Coile, N. The Distribution of the Vascular Flora of Georgia; University of Georgia: Athens, GO, USA, 1988; pp. 87–110. [Google Scholar]
- Vance, E.D.; Nadkarni, N.M. Root biomass distribution in a moist tropical montane forest. Plant Soil 1992, 142, 31–39. [Google Scholar] [CrossRef]
- Zotz, G.; Schickenberg, N.; Albach, D. The velamen radicum is common among terrestrial monocotyledons. Ann. Bot. 2017, 120, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzing, D.; Ott, D.; Friedman, W. Roots of Sobralia macrantha (orchidaceae): Structure and function of the velamen-exodermis complex. Am. J. Bot. 1982, 69, 608–614. [Google Scholar] [CrossRef]
- Went, F.W. Soziologie der epiphyten eines tropischen urwaldes. Annales du Jardin Botanique de Buitenzorg 1940, 50, 1–98. [Google Scholar]
- Zotz, G.; Winkler, U. Aerial roots of epiphytic orchids: The velamen radicum and its role in water and nutrient uptake. Oecologia 2013, 171, 733–741. [Google Scholar] [CrossRef]
- Ng, C.K.Y.; Hew, C.S. Orchid pseudobulbs—‘False’ bulbs with a genuine importance in orchid growth and survival! Sci. Hortic. 2000, 83, 165–172. [Google Scholar] [CrossRef]
- Zotz, G. Functional anatomy and morphology. In Plants on Plants–The Biology of Vascular Epiphytes; Springer: Cham, Switzerland, 2016; pp. 67–93. [Google Scholar]
- Treseder, K.K.; Davidson, D.W.; Ehleringer, J.R. Absorption of ant-provided carbon dioxide and nitrogen by a tropical epiphyte. Nature 1995, 375, 137–139. [Google Scholar] [CrossRef]
- Ellwood, M.D.; Foster, W.A. Doubling the estimate of invertebrate biomass in a rainforest canopy. Nature 2004, 429, 549–551. [Google Scholar] [CrossRef]
- Nadkarni, N.M.; Matelson, T.J. Fine litter dynamics within the tree canopy of a tropical cloud forest. Ecology 1991, 72, 2071–2082. [Google Scholar] [CrossRef]
- Coxson, D.; Nadkarni, N. Ecological roles of epiphytes in nutrient cycles of forest ecosystems. In Forest Canopies; Academic Press: San Diego, CA, USA, 1995; pp. 495–543. [Google Scholar]
- Van Stan, J.T., II; Underwood, S.J.; Friesen, J. Urban forestry: An underutilized tool in water management. In Advances in Chemical Polltion Environmental Management and Protection; Elsevier: Amsterdam, The Netherlands, 2018; Volume 3, pp. 35–61. [Google Scholar]
- Ilek, A.; Kucza, J.; Szostek, M. The effect of stand species composition on water storage capacity of the organic layers of forest soils. Eur. J. For. Res. 2015, 134, 187–197. [Google Scholar] [CrossRef]
- Zotz, G.; Thomas, V. How much water is in the tank? Model calculations for two epiphytic bromeliads. Ann. Bot. 1999, 83, 183–192. [Google Scholar] [CrossRef]
- Lange, O.; Büdel, B.; Heber, U.; Meyer, A.; Zellner, H.; Green, T. Temperate rainforest lichens in New Zealand: High thallus water content can severely limit photosynthetic CO2 exchange. Oecologia 1993, 95, 303–313. [Google Scholar] [CrossRef]
- Porada, P.; Weber, B.; Elbert, W.; Pöschl, U.; Kleidon, A. Estimating global carbon uptake by lichens and bryophytes with a process-based model. Biogeosciences 2013, 10, 6989–7033. [Google Scholar] [CrossRef] [Green Version]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C. Terrestrial ecoregions of the world: A new map of life on eartha new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Weedon, G.; Gomes, S.; Viterbo, P.; Shuttleworth, W.J.; Blyth, E.; Österle, H.; Adam, J.; Bellouin, N.; Boucher, O.; Best, M. Creation of the watch forcing data and its use to assess global and regional reference crop evaporation over land during the twentieth century. J. Hydrometeorol. 2011, 12, 823–848. [Google Scholar] [CrossRef]
- Teale, N.G.; Mahan, H.; Bleakney, S.; Berger, A.; Shibley, N.; Frauenfeld, O.W.; Quiring, S.M.; Rapp, A.D.; Roark, E.B.; Washington-Allen, R. Impacts of vegetation and precipitation on throughfall heterogeneity in a tropical pre-montane transitional cloud forest. Biotropica 2014, 46, 667–676. [Google Scholar] [CrossRef]
- Aparecido, L.M.T.; Miller, G.R.; Cahill, A.T.; Moore, G.W. Comparison of tree transpiration under wet and dry canopy conditions in a Costa Rican premontane tropical forest. Hydrol. Process. 2016, 30, 5000–5011. [Google Scholar] [CrossRef]
- Mulligan, M. Modeling the tropics-wide extent and distribution of cloud forest and cloud forest loss, with implications for conservation priority. In Tropical Montane Cloud Forests: Science for Conservation and Management; Cambridge University Press: Cambridge, UK, 2010; pp. 16–38. [Google Scholar]
- Oliveira, R.S.; Eller, C.B.; Bittencourt, P.R.; Mulligan, M. The hydroclimatic and ecophysiological basis of cloud forest distributions under current and projected climates. Ann. Bot. 2014, 113, 909–920. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, G.; Zotz, G. Ecophysiological consequences of differences in plant size: In situ carbon gain and water relations of the epiphytic bromeliad, Vriesea sanguinolenta. Plant Cell Environ. 2001, 24, 101–111. [Google Scholar] [CrossRef]
- Zotz, G.; Laube, S. Tank function in the epiphytic bromeliad, Catopsis sessiliflora. Ecotropica 2005, 11, 63–68. [Google Scholar]
- Troeh, F.; Thompson, L. Soils and Soil Fertility, 6th ed.; Wiley-Blackwell: New York, NY, USA, 2005. [Google Scholar]
- Gotsch, S.G.; Davidson, K.; Murray, J.G.; Duarte, V.J.; Draguljić, D. Vapor pressure deficit predicts epiphyte abundance across an elevational gradient in a tropical montane region. Am. J. Bot. 2017, 104, 1790–1801. [Google Scholar] [CrossRef] [Green Version]
- Coenders-Gerrits, A.M.J.; Schilperoort, B.; Jiménez-Rodríguez, C. Evaporative Processes on Vegetation: An Inside Look. In Precipitation Partitioning by Vegetation: A Global Synthesis; Springer: Cham, Switzerland, in press; Chapter 3.
- Stuart, T.S. Revival of respiration and photosynthesis in dried leaves of polypodium polypodioides. Planta 1968, 83, 185–206. [Google Scholar] [CrossRef]
- Aparecido, L.M.; Miller, G.R.; Cahill, A.T.; Moore, G.W. Leaf surface traits and water storage retention affect photosynthetic responses to leaf surface wetness among wet tropical forest and semiarid savanna plants. Tree Physiol. 2017, 37, 1285–1300. [Google Scholar] [CrossRef]
- McAdam, S.A.; Brodribb, T.J. Ancestral stomatal control results in a canalization of fern and lycophyte adaptation to drought. New Phytol. 2013, 198, 429–441. [Google Scholar] [CrossRef]
- Oppenheimer, H.; Halevy, A. Anabiosis of Ceterach officinarum Lam. et DC. Bull. Res. Council Israel 1962, 11D3, 127–147. [Google Scholar]
- Pina, A.L.; Zandavalli, R.B.; Oliveira, R.S.; Martins, F.R.; Soares, A.A. Dew absorption by the leaf trichomes of Combretum leprosum in the Brazilian semiarid region. Funct. Plant Biol. 2016, 43, 851–861. [Google Scholar] [CrossRef]
- Schwerbrock, R.; Leuschner, C. Foliar water uptake, a widespread phenomenon in temperate woodland ferns? Plant Ecol. 2017, 218, 555–563. [Google Scholar] [CrossRef]
- Dawson, T.E.; Goldsmith, G.R. The value of wet leaves. New Phytol. 2018, 219, 1156–1169. [Google Scholar] [CrossRef] [Green Version]
- Berry, Z.C.; Emery, N.C.; Gotsch, S.G.; Goldsmith, G.R. Foliar water uptake: Processes, pathways, and integration into plant water budgets. Plant Cell Environ. 2019, 42, 410–423. [Google Scholar] [CrossRef]
- Lawton, R.O.; Nair, U.S.; Pielke, R.A.; Welch, R.M. Climatic impact of tropical lowland deforestation on nearby montane cloud forests. Science 2001, 294, 584–587. [Google Scholar]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef]
- Pounds, J.A.; Fogden, M.P.; Campbell, J.H. Biological response to climate change on a tropical mountain. Nature 1999, 398, 611–618. [Google Scholar] [CrossRef]
- Darby, A.; Draguljic, D.; Glunk, A.; Gotsch, S. Habitat moisture is an important driver of patterns of sap flow and water balance in tropical montane cloud forest epiphytes. Oecologia 2016, 182, 357–371. [Google Scholar] [CrossRef]
- Williams, C.; Murray, J.; Glunk, A.; Dawson, T.; Nadkarni, N.; Gotsch, S. Vascular epiphyte responses to experimental drought: Implications for community turnover in a Costa Rican cloud forest. Funct. Ecol. 2019. in review. [Google Scholar]
- Ogburn, R.M.; Edwards, E.J. The ecological water-use strategies of succulent plants. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 55, pp. 179–225. [Google Scholar]
- Rascio, N.; Rocca, N.L. Resurrection plants: The puzzle of surviving extreme vegetative desiccation. Crit. Rev. Plant Sci. 2005, 24, 209–225. [Google Scholar] [CrossRef]
- Scott, P. Resurrection plants and the secrets of eternal leaf. Ann. Bot. 2000, 85, 159–166. [Google Scholar] [CrossRef]
- Schiller, P.; Heilmeier, H.; Hartung, W. Abscisic acid (aba) relations in the aquatic resurrection plant Chamaegigas intrepidus under naturally fluctuating environmental conditions. New Phytol. 1997, 136, 603–611. [Google Scholar] [CrossRef]
- Schiller, P.; Wolf, R.; Hartung, W. A scanning electron microscopical study of hydrated and desiccated submerged leaves of the aquatic resurrection plant Chamaegigas intrepidus. Flora 1999, 194, 97–102. [Google Scholar] [CrossRef]
- Van Stan, J.T., II; Wagner, S.; Guillemette, F.; Whitetree, A.; Lewis, J.; Silva, L.; Stubbins, A. Temporal dynamics in the concentration, flux, and optical properties of tree-derived dissolved organic matter in an epiphyte-laden oak-cedar forest. J. Geophys. Res. Biogeosci. 2017, 122, 2982–2997. [Google Scholar] [CrossRef]
- McDonnell, J.J. Beyond the water balance. Nat. Geosci. 2017, 10, 396. [Google Scholar] [CrossRef]
- Benzing, D.H. Vulnerabilities of tropical forests to climate change: The significance of resident epiphytes. In Potential Impacts of Climate Change on Tropical Forest Ecosystems; Springer: Cham, Switzerland, 1998; pp. 379–400. [Google Scholar]
- Andrade, J.L. Dew deposition on epiphytic bromeliad leaves: An important event in a Mexican tropical dry deciduous forest. J. Trop. Ecol. 2003, 19, 479–488. [Google Scholar] [CrossRef]
- Stanton, D.E.; Huallpa Chávez, J.; Villegas, L.; Villasante, F.; Armesto, J.; Hedin, L.O.; Horn, H. Epiphytes improve host plant water use by microenvironment modification. Funct. Ecol. 2014, 28, 1274–1283. [Google Scholar] [CrossRef]
- Zotz, G. Vascular epiphytes in the temperate zones—A review. Plant Ecol. 2005, 176, 173–183. [Google Scholar] [CrossRef]
- Esseen, P.-A.; Renhorn, K.-E.; Pettersson, R.B. Epiphytic lichen biomass in managed and old-growth boreal forests: Effect of branch quality. Ecol. Appl. 1996, 6, 228–238. [Google Scholar] [CrossRef]



| Element | Range of Surface Water Storage Capacities (mm) |
|---|---|
| Leaves | 0.04–2.2 [16,17] |
| Bark | 0.2–5.9 [16,18] |
| Epiphytes | 0.4–16.6 [15,19,20] |
| Canopy roots | Unknown |
| Canopy soils | Unknown |
| Total canopy | 0.04–19 [15] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hargis, H.; Gotsch, S.G.; Porada, P.; Moore, G.W.; Ferguson, B.; Van Stan, J.T., II. Arboreal Epiphytes in the Soil-Atmosphere Interface: How Often Are the Biggest “Buckets” in the Canopy Empty? Geosciences 2019, 9, 342. https://doi.org/10.3390/geosciences9080342
Hargis H, Gotsch SG, Porada P, Moore GW, Ferguson B, Van Stan JT II. Arboreal Epiphytes in the Soil-Atmosphere Interface: How Often Are the Biggest “Buckets” in the Canopy Empty? Geosciences. 2019; 9(8):342. https://doi.org/10.3390/geosciences9080342
Chicago/Turabian StyleHargis, Hailey, Sybil G. Gotsch, Philipp Porada, Georgianne W. Moore, Briana Ferguson, and John T. Van Stan, II. 2019. "Arboreal Epiphytes in the Soil-Atmosphere Interface: How Often Are the Biggest “Buckets” in the Canopy Empty?" Geosciences 9, no. 8: 342. https://doi.org/10.3390/geosciences9080342
APA StyleHargis, H., Gotsch, S. G., Porada, P., Moore, G. W., Ferguson, B., & Van Stan, J. T., II. (2019). Arboreal Epiphytes in the Soil-Atmosphere Interface: How Often Are the Biggest “Buckets” in the Canopy Empty? Geosciences, 9(8), 342. https://doi.org/10.3390/geosciences9080342






