4. Vetulicolians
These enigmatic creatures, typified by the eponymous genus
Vetulicola, have the general appearance of a segmented tadpole. They were originally identified as arthropods due to the apparently segmented character of the ‘tail’ in some genera, but the complete absence of jointed appendages plus the recognition of a series of five pairs of gill structures [
45] in the anterior part of the animal led to a revised placement with the deuterostomes. They are best known from
Lagerstätten such as Chengjiang [
13] and have recently been reported from the newly discovered Qingjiang biota [
16].
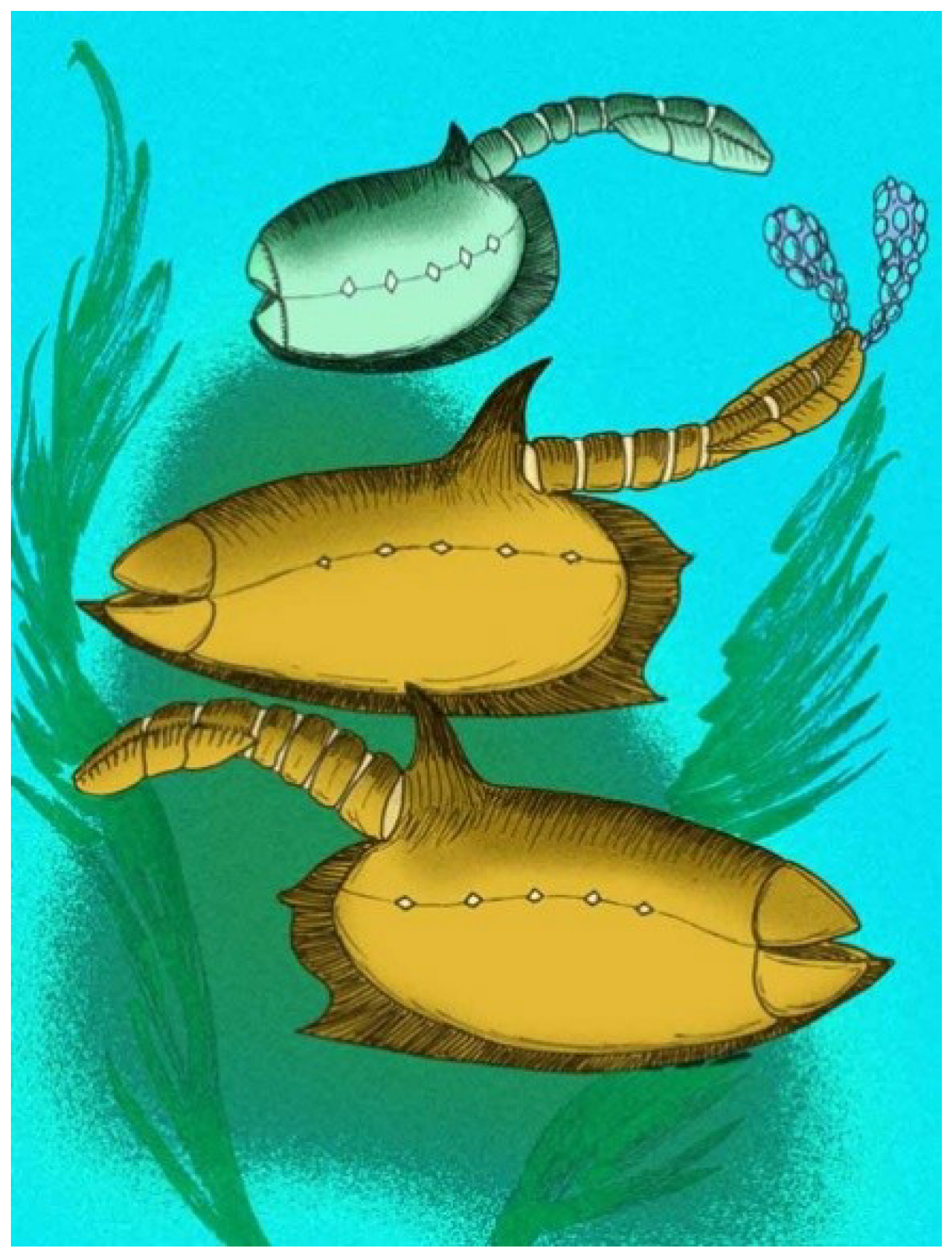
The overall shape of vetulicolians is somewhat reminiscent of bivalved crustaceans, but any resemblance to anterior tagmosis of crustaceans is superficial. The lateral line in vetulicolians represents a potential plane of flexure and this may be a characteristic of their unusual body plan.
Diego García-Bellido and his coauthors [
46] concluded that “the common ancestor of the Vetulicolia + Tunicata include distinct anterior and posterior body regions—the former being non-fusiform and used for filter feeding and the latter originally segmented—plus a terminal mouth, absence of pharyngeal bars, the notochord restricted to the posterior body region, and the gut extending to the end of the tail.”
Two informal groups of vetulicolians are recognized here, the “soft shell vetulicolians” and the “hard shell vetulicolians”. Soft shell vetulicolians lack segmentation in their posterior section or tail; lack a prominent constriction between the anterior and posterior portions of the body; and have a very blunt, rectangular anterior. Hard shell vetulicolians have a segmented posterior section, and may have a tuberculate anterior section, at least as juveniles.
4.1. Banffia
Banffia constricta (
Figure 7), the first described putative vetulicolian, is a common constituent of the Middle Cambrian Burgess Shale biota.
Banffia can attain a length of 10 cm. Originally assigned to the annelids, a lack of appendages has proved to be an insurmountable obstacle for assigning
Banffia to Arthropoda.
As is characteristic for vetulicolians,
Banffia is divided into an anterior part and a posterior part. The posterior has a characteristic twist and numerous (40–50) narrow segments. There is a “crossing pattern” twisted section at the constriction between the anterior and posterior sections. The anus is at the end of the posterior section and occurs in the center of a forked structure called the caudal notch [
47]. The anterior section or ‘carapace’ is elongate pill-shaped and largely smooth. The anterior tip of the animal bears a corona-like structure of concentric rings or ‘circlets’. The structure is presumed to be the mouth of the animal.
Fine anastomosing ridges on
Banffia, identified as “micro-ichnofossils” [
47], are in fact part of the structure of the animal and may represent vascular canals of some type. Comparable features occur on
Vetulicola longbaoshanensis [
15]. No ‘gill slits’ are present in
Banffia, and this has impeded comparisons with other vetulicolians. J.-B. Caron [
47] sees the “presence of mid-gut diverticulae in
Banffia” to represent a possible link to protostomes.
Banffia episoma of Utah has a considerably diminished anterior section [
48].
Banffia confusa from Chengjiang has gill slits on its anterior section and is thus securely placed in the Vetulicolia [
47].
4.2. Beidazoon
Beidazoon venustum is considered a ‘dwarf vetulicolian’ reaching only 8–14 mm in length [
49]. Tubercles occur on the anterior section. Thus, the possibility that the fossil may represent a juvenile cannot be excluded.
Beidazoon is one of the ‘hard shell’ vetulicolians [
49].
4.3. Bullivetula
Bullivetula variola is considered here to possibly be synonymous with (a juvenile of)
Beidazoon venustum. Pronounced tubercles and pits occur on the anterior section [
50].
4.4. Didazoon
Somewhat resembling
Pomatrum,
Didazoon haoae (
Figure 8) has an anterior section divided into six segments, a posterior section divided into seven segments, and a creased junction between the two [
51].
Didazoon is one of the ‘soft shell’ vetulicolians [
49]. Five gill ports have been identified on either side of the anterior section. The tail section is advanced dorsally.
4.5. Heteromorphus
Heteromorphus confusus was originally assigned to
Banffia, and the possibility exists that more genera and species will need to be described with ongoing study of these morphologically variable fossils [
50].
Heteromorphus is one of the ‘soft shell’ vetulicolians [
49].
4.6. Heteromorphus Subtype New Species Form A
This undescribed form is a ‘soft shell’ vetulicolian with an apparently unsegmented posterior region (‘tail’) that is “covered with numerous wrinkles” [
49]. The rather thick tail is thought to be either an extension of both the dorsal and ventral partitions of the anterior section, or, more probably, only an extension of the dorsal part of the anterior section [
49].
4.7. Nesonektris
Nesonektris aldridgei (
Figure 9) is a vetulicolian described in 2014 from the Cambrian Emu Bay Shale Konservat-Lagerstätte of Kangaroo Island, Australia. It has a rather rectangular anterior portion and a laterally flattened, paddle-like posterior section. Narrow, diamond-shaped intersegmental membranes occur between posterior section segments. Gill structures are not evident on the anterior section, but a lateral groove appears on both right and left sides of the anterior section along the line where one would expect to find gill structures [
46].
The animal attained a length of 1.7 cm. An “axial, rod-like structure in the posterior body region,” filling much of the “tail cavity,” has been interpreted as a notochord [
46], thus lending support to the deuterostome interpretation of vetulicolians. An alternate interpretation is that the thick rod structure represents a wide posterior gut region, although an alimentary tract interpretation would be difficult to reconcile with fragmentation of this linear structure into segments intepreted as notochordal discs [
46].
4.8. Ooedigera
Ooedigera’s relatively large size (up to 42 mm) and tuberculate ornamentation on its anterior section sets it apart from other vetulicolians.
Ooedigera (
Figure 10) is known from the lower Cambrian Sirius Passet Lagerstätte of Greenland [
52]. If the tuberculate ornamentation is considered a juvenile trait, as in the possible juveniles
Beidazoon and
Bullivetula, then
Ooedigera might be considered to be a neotenous form.
4.9. Pomatrum
Pomatrum ventralis has a typical overall vetulicolian body form, with a chicken-egg-shaped anterior portion and a flattened, beaver-tail-like posterior section or ‘tail’.
Pomatrum (
Figure 11) is one of the ‘soft shell’ vetulicolians [
49].
Pomatrum is remarkable for its circlet mouth consisting of concentric zones of 30 or more plates. Five gill structures (G
1–G
5) are present on either side of the anterior portion [
49]. The anterior section is smooth apart from some transverse grooves. The posterior region has ten or more segments, with the anterior-most partitions becoming fainter and more closely spaced [
49].
4.10. Skeemella
Skeemella, one of the strangest animals assigned to the vetulicolians, occurs in Middle Cambrian strata of Utah, USA [
53].
Skeemella has a relatively small anterior section and a highly elongated posterior section that is divided into dorsal and ventral plates or half-rings. A telson-like, double-pointed or forked posterior tip occurs at the end of the vermiform posterior section. This structure is presumed here to be homologous to the caudal notch of
Banffia.
Skeemella’s placement in the Vetulicolia is controversial.
Skeemella has a general morphology that is similar to the 7–8 stage Sytox green fluorescence nuclear-stained embryo of the millipede
Strigamia [
54], and the resemblence may not be entirely superficial if both
Skeemella and
Strigamia had dorsal expression of the segment polarity gene
engrailed.
Skeemella is nevertheless considered here to be both deuterostome and vetulicolian, with the dorsal-ventral partition in the posterior region, with ‘lateral line’ partition between them on both sides of the animal, representing an extension of the ‘gill’-bearing lateral line structure seen on the anterior end of vetulicolians. Some type of
Hox-related genetic modification has evidently led to caudal extension of the vetulicolian lateral line in
Skeemella.
4.11. Vetulicola
The type species of the genus is
Vetulicola cuneata [
50], and it remains the best known species in the genus.
Vetulicola (
Figure 12) is one of the ‘hard shell’ vetulicolians [
49].
Vetulicola has a ‘quadrate carapace’ [
55] with a ventral keel, a pointed dorsal fin, and a segmented, dorsally advanced arthropod-like posterior section. Spindle-shaped gill structures are present, showing what are interpreted as gill pouches, gill filaments, and gill slits [
4,
15,
49]. Up to three orders of faint annulations occur on the anterior body section. Seven segments are seen on the posterior section.
Vetulicola rectangulata has a relatively slender posterior region (
Figure 12) but it is otherwise similar to
V. cuneata.
Vetulicola longbaoshanensis from the Gushan Biota has more elaborate gill structures [
15], somewhat resembling those of an undescribed new taxon of vetulicolian from the Qingjiang Biota ([
16],
Supplementary Materials).
4.12. Vetulicolian gen. et sp. Indet. A
This vetulicolian, 28 mm in length, has a posterior section that is similar to
Ooedigera peeli but “with a broader flattened area ventrally than dorsally” [
52]. The single known specimen has a relatively smooth surface.
4.13. Xidazoon
Xidazoon [
56] is a possible junior synonym of
Pomatrum.
4.14. Yuyuanozoon
Known only from the holotype,
Yuyuanozoon magnificissimi (
Figure 13) is another vetulicolian from Chengjiang [
43]. Its anterior section is ovoid and comparatively smooth, with five pairs of gill structures arranged in a chain-like array on either side of the anterior section of the animal.
Yuyuanozoon reaches over 20 cm in length. As typical for vetulicolians, its posterior section is divided into seven segments. The posterior section is cylindrical, rather than being flattened as in some other vetulicolians. The anterior section has six segments.
The large mouth region [
57,
58] suggests that
Yuyuanozoon may have been well suited to filter feeding strategies similar to those of a modern salp or doliodid, although any similarities are likely to be superficial. An unidentified modern doliolid (possibly genus
Doliolum) bears resemblance to the anterior section of
Yuyuanozoon magnificissimi [
Figure 13], which might imply a filter feeding lifestyle for the latter.
5. Shenzianyuloma nov. gen.
A new species of vetulicolian (
Shenzianyuloma yunnanense nov. gen. nov. sp.) from the early Cambrian Chengjiang Biota (Burgess Shale-Type (BST) deposit; Maotianshan Shale, 518 Ma), represents the earliest example of ‘angelfish’ body form in a nectobenthic deuterostome (
Figure 14,
Figure 15,
Figure 16,
Figure 17,
Figure 18,
Figure 19,
Figure 20,
Figure 21,
Figure 22,
Figure 23 and
Figure 24). Although the exact locality of the holotype is unknown, a specimen of the brachiopod
Diadonga pista occurs on the same slab (
Figure 14), in accordance with matrix characteristics (yellow shale), indicating that the specimen is indeed derived from the Maotianshan Shale.
The fossil was acquired in February 2019 from a crystal and fossil vendor located in Lianyungang, Jiangsu, China. This online purchase rescued an important specimen that was at risk of being lost to science by vanishing into a private collection. The specimen (IGM 5008) is now available for study by the paleontological research community.
Shenzianyuloma yunnanense nov. gen. nov. sp. is preserved as a compression fossil in yellow Chengjiang shale. The perimeter/margins of the fossil seem to be intact, suggesting that the animal in life had a slender profile as viewed from the front or back. The proximal part of the tail and midpoint of the flanks experienced minor deformation that led to displacement of some of the gill structures and, significantly, de-nesting of one or more of the myomere cones. The gill structures have the shape of ovoid capsules, and one of these may have split apart, allowing gill filament-like structures to project downward (
Figure 17 and
Figure 18).
In spite of the central disturbance, the fossilized animal is largely intact and gives a good representation of the shape of the creature. Shenzianyuloma yunnanense nov. gen. nov. sp. is not a decayed or mutilated specimen of a known taxon.
The rock sample bearing the fossil has dimensions 12 cm × 7.8 cm, and is 1.4 cm thick. The preserved length of the animal is 5 cm, with an anterior body region 4 cm deep (
Figure 14 and
Figure 15). It has a dorsal fin (
Figure 14 and
Figure 15) with a saw tooth margin. The anterior of the animal is blunt, with a ventral beak.
Although its posterior is not segmented, Shenzianyuloma yunnanense nov. gen. nov. sp. has four clearly defined segments in the ventral part of its anterior portion, whereas the dorsal part of the anterior section where the dorsal fin attaches is smooth, almost domal.
Segment 1 of the ventral anterior forms the ‘mandible’ of the beak. This does appear to be a beak-like structure, as opposed to being some sort of spinose projection, due to its similarity to the ‘wedge beak’ known from
Vetulicola cuneata. Segments 2–4 increase in depth in a posterior direction, with segment 4 extending downward to form the edge of a ventral keel. The posterior section seems to continue as an extension of this segmented ventral part of the anterior section. This would be inverse to the pattern proposed for
Heteromorphus subtype new species Form A [
49], where the posterior section is thought to be likely an extension of the dorsal half of the anterior section.
The dorsal part of the anterior section appears to be unsegmented, rather developing a triangular fin that mirrors the profile of ventral segments 1–4 to give the angelfish body shape. The dorsal fin has blunt teeth on its leading edge that increase in size in an anterior direction. The base of the dorsal fin develops a thin dark line that parallels the fin insertion at the top of the animal’s back (
Figure 16). The line is approximately 0.4 mm from the base of the fin.
Three (of a presumed original five) gill structures are preserved at the interface between the segmented ventral and smooth dorsal parts of the anterior section (
Figure 15). The posterior-most reserved gill structure preserves paired gill filaments that trail downward from the gill structure (
Figure 17 and
Figure 18).
The posterior section of
Shenzianyuloma yunnanense nov. gen. nov. sp. provides key information about the affinities of this creature. The ‘tail’ shows evidence for both a notochord and a distinct posterior gut trace. Both the notochord and the gut trace are surrounded above and below by anteriorly slanting myotomes (
Figure 19,
Figure 20,
Figure 21 and
Figure 22).
The notochord is preserved as a light-colored band that runs along the dorsal half of the tail. Its width is approximately 3–4 mm diameter, and about 1.3 cm of notochord length is preserved (
Figure 19,
Figure 20,
Figure 21 and
Figure 22). Two prominent nodes along its length (to the right of center in
Figure 22) likely represent notochordal discs [
46].
The gut trace is preserved as a linear structure with 7 mm preserved length. It is approximately 0.35 mm in width with a distinct, very narrow central canal about 0.05 mm wide. Fine linear structures project at high angles to the central tube of the alimentary tract (
Figure 22 and
Figure 23); these are interpreted here as gut diverticula [
47]. They are definitely projections from the central gut trace, rather than being cracks or fractures of some type in the vicinity of the gut.
The diverticula connect to the main gut trace at a variety of high angles (
Figure 23). In
Banffia, diverticula usually connect with the intestinal trace at approximate right angles, but not infrequently, are offset at high angles [
47] similar to the arrangement seen in
Figure 23.
A similar morphology is seen in
Pomatrum cf.
ventralis, specimen YKLP 10914a [
50], where an alimentary tract is ventral to a linear structure flanked by “numerous transverse lines” [
50] that may very well represent a notochord surrounded by myotome cones.
Disturbance to the central part of the animal displaced gill structures and caused delamination or de-nesting of the myotome cones in the tail region (
m* in
Figure 21). This was a key occurrence, as it demonstrated the presence of cone-in-cone myomere structure in
Shenzianyuloma yunnanense nov. gen. nov. sp. Connective tissues were apparently not yet as strong as those in a modern fish, allowing the delamination to occur.
Muscle fibers are visible in the myotome immediately posterior to the delaminated myotome, just to the right of the middle
m in
Figure 21.
Vetulicolian Locomotion
The seemingly awkward body shape of virtually all vetulicolians begs the question of how these creatures were able to swim and to maintain their position in the water column. The roughly triangular, angelfish-like body form of angelfish vetulicolian (Shenzianyuloma yunnanense nov. gen. nov. sp.), complete with a protruding lower ‘jaw,’ is well known from the freshwater angelfish Pterophyllum, the marine angelfish Pomacanthus, and a variety of fossil fishes including Eoplatex papilio and the pycnodont Proscinetes. Lacking pectoral fins or their functional equivalents, Shenzianyuloma yunnanense nov. gen. nov. sp. would presumably have been incapable of labriform swimming, and would have relied instead on less-efficient tail propulsion. Jets of water from the gill structures may have also helped to propel angelfish vetulicolian forward. The dorsal fin on the anterior section, as well as its denticulate leading margin, imply at least a certain degree of controlled forward motion and hydrodynamic stability in the water column.
6. Developmental Biology of Chordate/Vetulicolian Origins
Several aspects should be mentioned with regard to developmental biology when considering the origin of Chordata and related Cambrian animals. For example, the distinction between the tiny pharyngeal teeth of
Haikouella and the larger pharyngeal teeth of
Yunnanozoon, homologous features [
7] between the two genera, represents the range of variation seen in the early chordate anterior scleritome.
The body division into two halves in vetulicolians has been invoked as an illustration of Romer’s somato-visceral theory [
51,
59]. It seems reasonable to speculate that a discontinuity in all-
trans-retinoic acid (ATRA)-FGF morphogen signaling molecules has occurred at the vetulicolian midline; thus, in vetulicolians, the anterior part of the animal and the posterior part of the animal may have been dominated by different molecular signaling regimes. For example, a dual retinoic acid gradient is involved in down-regulating FGF8 to permit somitogenesis. Interestingly, the gradient is at a minimum value at the head/tail junction, and reaches a maximum in the anterior section [
60].
The (ATRA)-FGF double-sided retinoic acid gradient signal isolation seems to be particularly pronounced in
Skeemella [
53]. Somite/segmentation formation is dramatically favored in the posterior section and more subdued (although still present) in the anterior section, suggesting that the same posterior-generated signaling factor is influencing segmentation in both the posterior (influence strong) and anterior (influence weak) sections, with the presumed ATRA gradient dropping off sharply across the anterior–posterior sections division [
61]. Here, we see a potential unification of the morphogenetic field and gradient signaling approaches, with a morphogenetic field discontinuity demarcating fields of morphogen signaling factor influence.
The serial gene duplications known to have occurred in vertebrates, gnathostomes and teleost fish evidently cannot be linked to the “evolution of complexity in vertebrates” [
29].
7. Conclusions
Among living chordates, molecular phylogeny shows that tunicates are more closely related to vertebrates (they jointly form the Olfactores) than vertebrates are to lancelets (Cephalochordata [
61,
62]). Curiously, in the latter analysis, “the monophyly of deuterostomes remained poorly supported” [
62]. The reliability of molecular clocks based on these data (“most major lineages of deuterostomes arose prior to the Cambrian Explosion” [
61]) is open to question, considering that few if any deuterostome fossils are known from strata deposited before the Cambrian boundary. In any case, the general consensus is that vertebrates first appeared in the Cambrian [
63].
A putative exception to this general rule of Cambrian chordate appearance is the identification of
Burykhia hunti from Ediacaran strata of the White Sea region, Russia and
Ausia fenestrata from the Nama Group in Namibia, as the oldest known ascidians [
64]. It seems highly unlikely that members of the Ausiidae are urochordates, considering that their morphology consists of tubes or bags with serial perforations. The presence of a zig-zag medial suture in
Burykhia suggests rather that the fossils are more properly allied with frondose ‘vendobiont’ Ediacarans such as
Charnia,
Rangea,
Phyllozoon and
Pteridinium.
It seems quite plausible that the advent of deuterostomes near the Cambrian boundary involved both a reversal of gut polarity and a two-sided retinoic acid gradient, with a gradient discontinuity at the midpoint of the organism that is reflected in the characteristic division of vetulicolians into morphologically distinct anterior and posterior sections.
The posterior section of
Shenzianyuloma yunnanense nov. gen. nov. sp. demonstrates that vetulicolians are indeed deuterostomes allied to chordates [
65], due to
Shenzianyuloma’s notochord (with discs) in a position that is dorsal to an alimentary tract with diverticula, and also due to the presence of myotome cones [
66] with preserved muscle fibers.
It is now possible to review the current state of knowledge regarding Cambrian chordates, vetulicolians and their affinities. All of these animals are deuterostomes (i.e., the blastopore becomes the anus during early ontogeny), although of course, other deuterostomes are also known from the early Cambrian (cambroernids, echinoderms and hemichordates).
Three groups may be recognized among the Cambrian chordates. Urochordates, characterized by the presence of a branchial basket, are represented by Cheungkongella and Shankouclava. Cephalochordates, characterized by a full notochord, are represented by four genera: Cathaymyrus Pikaia (with question), Zhongjianichthys, and Zhongxiniscus. Vertebrates, characterized by a neural crest, are also represented by four genera: Haikouella, Haikouichthys, Metaspriggina and Myllokunmingia.
Vetulicolians are characterized by their bipartite body form and by five pairs of gill structures. Two informal groups of vetulicolians can be recognized, the “soft shell vetulicolians” and the “hard shell vetulicolians”. Soft shell vetulicolians lack segmentation in their posterior section or tail; lack a prominent constriction between the anterior and posterior portions of the body; and have a very blunt, rectangular anterior. Hard shell vetulicolians have a segmented posterior section, and may have a tuberculate anterior section, at least as juveniles.
Soft shell vetulicolians are represented by: Didazoon, Heteromorphus, Pomatrum and Xidazoon. Hard shell vetulicolians are represented by six genera: Beidazoon, Bullivetula, Nesonektris, Ooedigera, Vetulicola and Yuyuanozoon.
Skeemella and Shenzianyuloma are unique, unusual vetulicolians that cannot be placed in either the soft shell or hard shell clade. Yunnanozoan, although surely a bilaterian deuterostome, cannot be confidently placed in either the chordates or the vetulicolians.
Taken together, these chordate and vetulicolian genera document an important part of the explosive radiation of deuterostomes at the base of the Cambrian. Although debate is likely to continue, the preponderance of the evidence does suggest that vetulicolians and yunnanozoans are indeed deuterostomes [
47,
50,
52,
53,
67,
68,
69,
70,
71]. Although deuterostomes, the extremely odd character set in vetulicolians also suggests that they may very well represent a higher taxon of phylum rank. Great caution, however, is needed in order to avoid circular reasoning with regard to the use of gross morphology to make such phylogenetic claims. The five pairs of gill structures in vetulicolians could in fact represent mid-gut glands of some sort, although this seems unlikely. The bizarre carpoids, with (like vetulicolians) a bipartite body division are now considered to be regular echinoderms with no gill slits and a feeding arm with a normal water vascular system [
72]. Still, the mythical chimaera-like nature of many of the early deuterostomes suggests that the group may very well require the erection of multiple extinct phylum-level taxa to avoid systematic shoehorning.
Aldridge et al. [
50] note that although the vetulicolians “appear to form a monophyletic clade, it is premature to accord them phylum rank without resolution of their phylogenetic position.” This remains a cogent observation, for indeed, with
Shenzianyuloma yunnanense nov. gen. nov. sp. displaying evidence for conical myomeres surrounding a notochord with discs, the possibility that
Shenzianyuloma and other vetulicolians represent stem chordates must be seriously considered.
8. Systematic Paleontology
Repository data: IGM, Institute of Geology Museum, Departmento de Paleontología, Cuidad Universitaria, Delegacíon de Coyoacán, 04510, México; YKLP, Key Laboratory for Palaeobiology, Yunnan University, Kunming, China.
Kingdom Animalia
Superphylum Deuterostomia
Phylum Vetulicolia
Genus Shenzianyuloma nov. gen.
Diagnosis: Vetulicolian with a body divided into a broad, laterally flattened anterior section and relatively narrow posterior or tail region. The anterior section is divided into a relatively smooth dorsal part with a denticulate dorsal fin and a segmented or partitioned ventral part. The dorsal fin has a thin ridge that parallels the base of the fin. A wedge beak occurs at the anterior junction of the dorsal and ventral parts. Five gill structures with filaments occur between the dorsal and ventral parts of the anterior section. The dorsal and ventral parts of the anterior section have approximately similar (but inverted relative to one another) profiles. The unsegmented posterior or tail region develops a notochord, a gut trace with diverticula, and at least seven myomeres. The posterior tip of the tail region is unknown.
Description: This vetulicolian has an unsegmented posterior section (‘tail’) and anterior section that is segmented only in its ventral part, where four segments occur. Gill structures occur between the dorsal and ventral parts of the anterior section. At the anterior of the animal, a wedge beak forms from segment 1 at the juncture between the dorsal and ventral anterior section parts. The sagittal crest bears a dorsal fin with a denticulate anterior margin. The dorsal fin has blunt teeth on its leading edge that increase in size toward the front of the fin. The base of the dorsal fin shows a thin dark linear ridge that parallels the fin insertion at the top of the animal’s back, at a distance of 0.4 mm from the fin base (
Figure 16).
A thin keel forms the ventral margin of the anterior section, and the anterior-ventral profile roughly matches that of the main dorsal fin. The dorsal part of the narrow, dorsally advanced posterior section bears three fins, two larger and flap-shaped and a posterior one that is much smaller.
The posterior section has both a notochord (with notochordal discs) and distinct gut trace (with diverticula). Both notochord and gut/intestine are surrounded by conical myotomes. Extremely fine lines project at approximate right angles to the central tube of the alimentary tract (
Figure 22); these represent gut diverticula [
44].
Discussion: The posterior tip of Shenzianyuloma yunnanense nov. gen. nov. sp. is unknown due to breakage in the holotype. The presence of myotomes strongly suggest that Shenzianyuloma yunnanense nov. gen. nov. sp. is both a deuterostome and a chordate.
Shenzianyuloma yunnanense nov. gen. nov. sp. resembles “
Heteromorphus subtype new species Form A” [
46] in being a ‘soft shell’ vetulicolian form that: lacks segmentation in its posterior section or tail; lacks a prominent constriction between the anterior and posterior portions of the body; and has a very blunt, rectangular anterior.
Shenzianyuloma yunnanense nov. gen. nov. sp. differs from “
Heteromorphus subtype new species Form A” by having: a narrower tail that lacks wrinkles; anteriorly slanting myotome-like structures; a wedge beak; and several flap- or scoop-like fins along the dorsal edge of its posterior section. A gut trace extends to the preserved end of the tail in
Shenzianyuloma yunnanense nov. gen. nov. sp. As in
Haikouella and
Myllokunmingia, gill filaments that proceed from the gill structure in
Shenzianyuloma appear to be paired.
Sagittal plane expansion and lateral flattening of the anterior section of
Shenzianyuloma provides a striking morphological contrast to the dorsal-ventral flattening of the anterior section of the Burgess Shale vetulicolid
Banffia constricta (
Figure 7), thereby expanding our knowledge of the range of form in this important group of early deuterostomes.
The dorsal fin in
Shenzianyuloma is comparable to the dorsal fin of
Myllokunmingia, although
Myllokunmingia’s dorsal fin has a comparatively lower profile [
4] and lacks the denticles on the leading edge of the fin.
Shenzianyuloma yunnanense nov. gen. nov. sp. resembles “
Heteromorphus subtype new species Form A” [
46] in being a ‘soft shell’ form that: lacks segmentation in its posterior section or tail; lacks a prominent constriction between the anterior and posterior portions of the body; and has a very blunt, rectangular anterior end.
Shenzianyuloma yunnanense nov. gen. nov. sp. differs from “
Heteromorphus subtype new species Form A” by having: a narrower tail that lacks wrinkles, but has anteriorly slanting myotome-like structures, and a wedge beak. A gut trace extends to the preserved end of the tail in
Shenzianyuloma yunnanense nov. gen. nov. sp. As in
Haikouella and
Myllokunmingia, gill filaments that proceed from the gill structure in
Shenzianyuloma appear to be paired.
A unique ‘dorsal fin’ in
Shenzianyuloma yunnanense nov. gen. nov. sp. mirrors the profile of the ventral cuticular plate. The anterior end of the latter forms a ‘wedge beak’ similar to that described from
Vetulicola cuneata. The wedge beak-bearing cuticular plate consists of three articulating sections, implying a dentary-like mobility in what appears to be the earliest beak-like ‘jaw’ structure reported from a deuterostome. Whether or not
Shenzianyuloma yunnanense nov. gen. nov. sp. also has a vertical mouth (‘M’ in [
46]) as inferred for “
Heteromorphus subtype new species Form A” is unknown. If so,
Shenzianyuloma may have had a dual oral apparatus, a biting beak at the lower part of the mouth (i.e., jaw with horizontal opening) and a blunt, elongate ‘M’ mouth opening that forms a vertical gape for the purposes of filter feeding.
The anteriorly slanting myotome-like structures of Shenzianyuloma yunnanense nov. gen. nov. sp. strongly support the hypothesis that vetulicolians are deuterostomes rather than arthropod-like protostomes.
Etymology: The genus name is a neuter noun formed as a combination and rearrangement of the Chinese word for angelfish (Shénxiān yú, 神仙鱼) and an anagram of the genus name for the ocean sunfish, Mola.
Holotype: Field sample 1 of 6/25/2019; IGM 5008.
Description: Same as for genus, by monotypy.
Etymology: The trivial name, in honor of Yunnan, China, is rendered here “yunnanense” rather than “yunnanensis” to agree in gender with the neuter noun Shenzianyuloma.
Age and Locality Information: Early Cambrian Chengjiang Biota (Burgess Shale-Type (BST) deposit; Maotianshan Shale, 518 Ma).