Preharvest Methyl Jasmonate Treatment Increased the Antioxidant Activity and Glucosinolate Contents of Hydroponically Grown Pak Choi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Quantification of Chls
2.3. GSLs Analysis
2.4. Total Phenolics Content
2.5. Total Flavonoids Content
2.6. Antioxidant Activities
2.6.1. DPPH Radical Scavenging Activity
2.6.2. ABTS Radical Scavenging Activity
2.6.3. Ferric-Reducing Antioxidant Power (FRAP)
2.6.4. Oxygen Radical Absorbance Capacity (ORAC)
2.7. Statistical Analysis
3. Results and Discussion
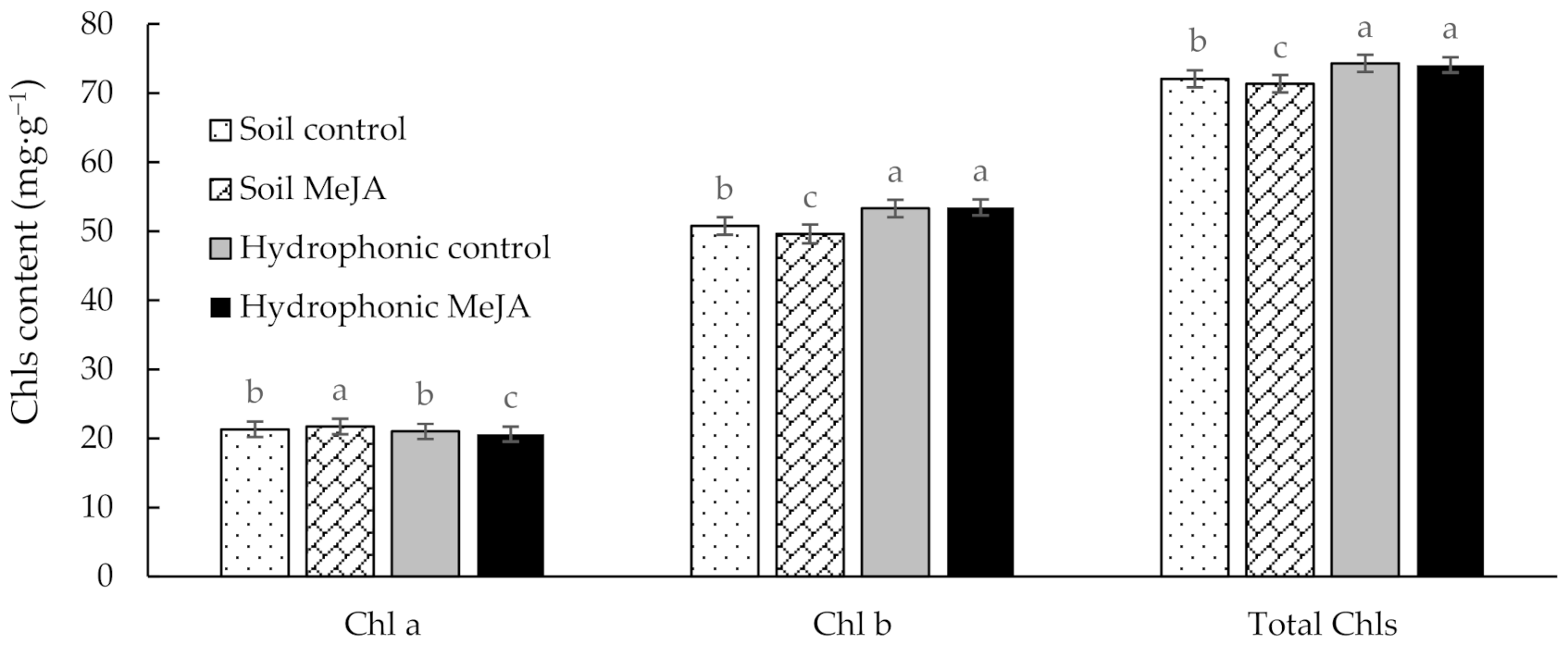
3.1. Effect of MeJA Treatment on Chl Contents of Pak Choi Grown on Soil and in a Hydroponic System
3.2. Effects of the MeJA Treatment on GSL Contents of Pak Choi Grown in Soil and in a Hydroponic System
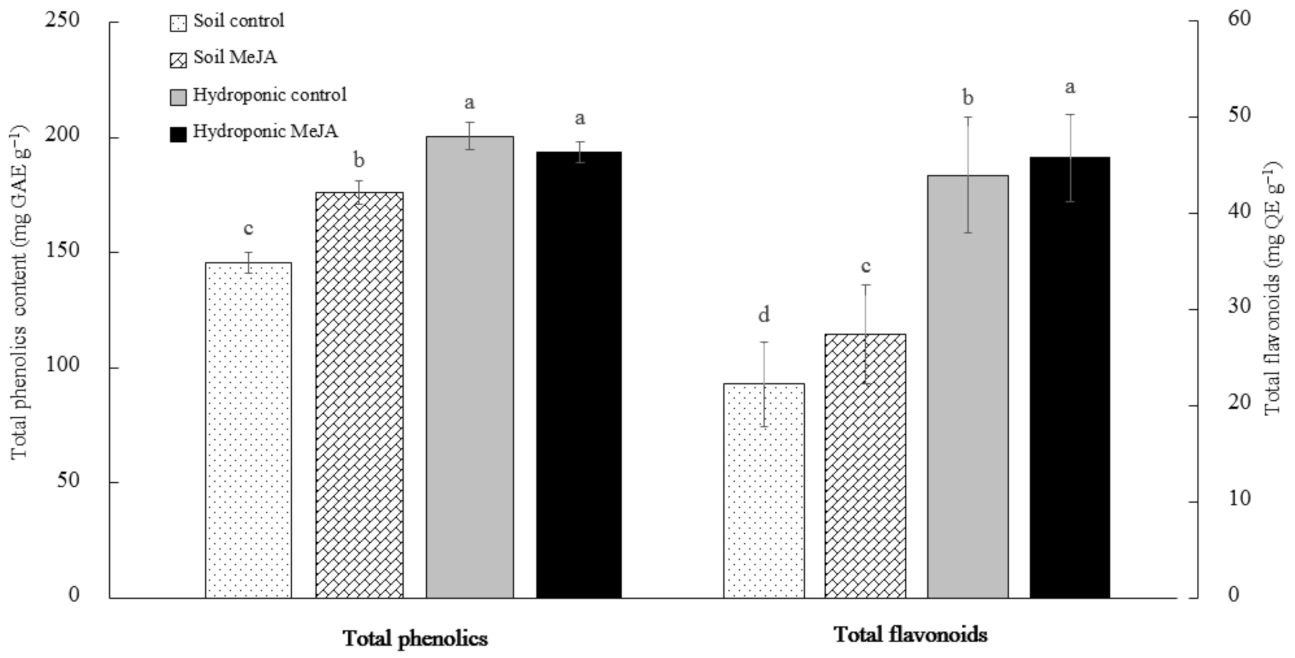
3.3. Effects of MeJA Treatment on the Total Phenolic and Flavonoid Contents of Pak Choi Grown in Soil and in the Hydroponic System
3.4. Effects of the MeJA Treatment on the Antioxidant Activities of Pak Choi Grown in Soil and in the Hydroponic System
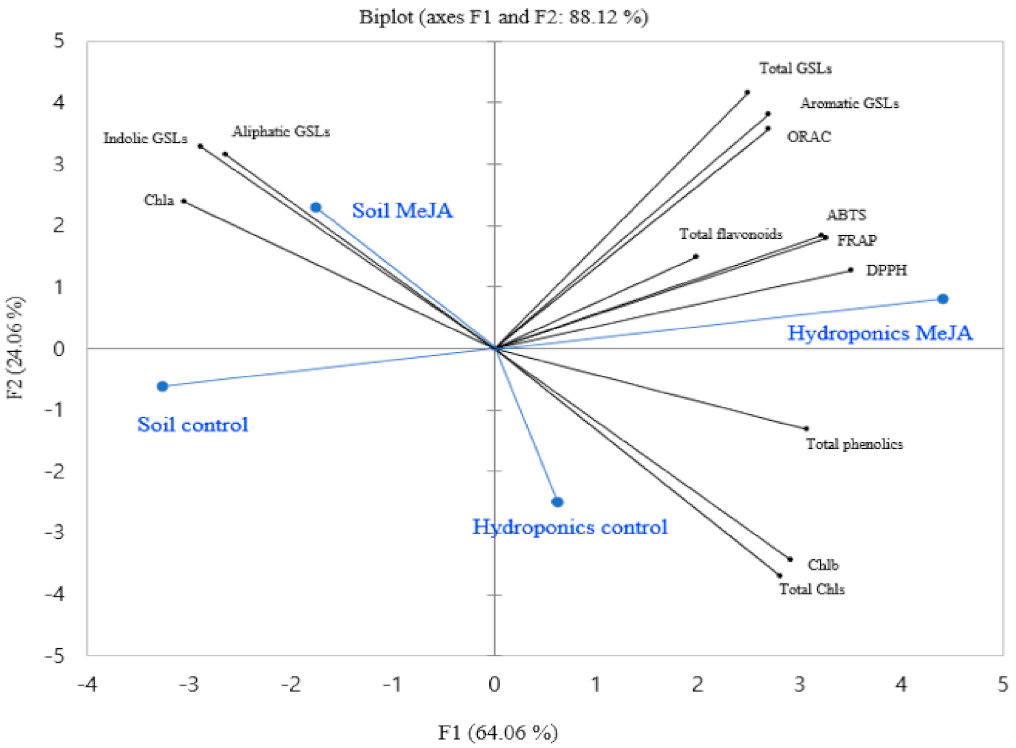
3.5. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO, F.A.O. Fruit and Vegetables for Health: Report of a Joint FAO/WHO Workshop, 1–3 September 2004, Kobe, Japan; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Shimeles, T.; Do, S.P.; Mu, H.S.; Cheon, S.J. Review on factors affecting the quality and antioxidant properties of tomatoes. Afr. J. Biotechnol. 2017, 16, 1678–1687. [Google Scholar] [CrossRef] [Green Version]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Głąbska, D.; Guzek, D.; Groele, B.; Gutkowska, K. Fruit and vegetable intake and mental health in adults: A systematic review. Nutrients 2020, 12, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isic, A. A Study of Flavonols in Bok Choy and their Anti-Cancer Properties. Ph.D. Thesis, Victoria University, Melbourne, Australia, August 2017. [Google Scholar]
- Lule, S.U.; Xia, W. Food phenolics, pros and cons: A review. Food Rev. Int. 2005, 21, 367–388. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479–3485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Yan, X.; Chen, S. Regulation of plant glucosinolate metabolism. Planta 2007, 226, 1343–1352. [Google Scholar] [CrossRef]
- Kim, M.J.; Chiu, Y.C.; Kim, N.K.; Park, H.M.; Lee, C.H.; Juvik, J.A.; Ku, K.M. Cultivar-specific changes in primary and secondary metabolites in pak choi (Brassica rapa, Chinensis group) by methyl jasmonate. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, S.R.; Rhee, J.; Choi, C.S.; Jo, J.S.; Shin, Y.K.; Lee, J.G. Profiling of individual desulfo-glucosinolate content in cabbage head (Brassica oleracea var. Capitata) germplasm. Molecules 2020, 25, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baenas, N.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P.; Moreno, D.A. Influence of cooking methods on glucosinolates and isothiocyanates content in novel cruciferous foods. Foods 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, H.R.; Tallontire, A.M.; Dougill, A.J. Exploring the Contribution of Vertical Farming to Sustainable Intensification from the Point of View of the Innovator and the Farmer; Sustainability Research Institute: Leeds, UK, 2019; pp. 1–44. [Google Scholar]
- Nair, A.; Irish, L. Commercial Production of Pak Choi; 2016; Iowa State University Extension and Outreach: Ames, Iowa; pp. 1–4. [Google Scholar]
- Kalantari, F.; Tahir, O.M.; Joni, R.A.; Fatemi, E. Opportunities and challenges in sustainability of vertical farming: A review. J. Landsc. Ecol. Repub. 2018, 11, 35–60. [Google Scholar] [CrossRef] [Green Version]
- Ku, K.M.; Juvik, J.A. Environmental stress and methyl jasmonate-mediated changes in flavonoid concentrations and antioxidant activity in broccoli florets and kale leaf tissues. HortScience 2013, 48, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, S. Methyl Jasmonate; Exemption from the Requirement of a Tolerance; Federal Register. A Rule by the Environmental Protection Agency (EPA). Fed. Regist. 2013, 78, 22789–22794. [Google Scholar]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24. [Google Scholar] [CrossRef] [Green Version]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A. Exogenous methyl jasmonate treatment increases glucosinolate biosynthesis and quinone reductase activity in kale leaf tissue. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Han, N.; Ku, K.M.; Kim, J. Postharvest variation of major glucosinolate and their hydrolytic products in Brassicoraphanus ‘BB1’. Postharvest Biol. Technol. 2019, 154, 70–78. [Google Scholar] [CrossRef]
- Tezcan, F.; Gültekin-Özgüven, M.; Diken, T.; Özçelik, B.; Erim, F.B. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009, 115, 873–877. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, J.-H.; Park, S.-D.; Choi, S.-Y.; Seong, J.-H.; Moon, K.-D. Preparation and antioxidant activity of health drink with extract powders from safflower (Carthamus tinctorius L.) seed. Korean J. Food Sci. Technol. 2002, 34, 617–624. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Limantara, L.; Dettling, M.; Indrawati, R.; Brotosudarmo, T.H.P. Analysis on the chlorophyll content of commercial green leafy vegetables. Procedia Chem. 2015, 14, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Chen, J.; Stamps, R.H.; Li, Y. Correlation of visual quality grading and SPAD reading of green-leaved foliage plants. J. Plant Nutr. 2005, 28, 1215–1225. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, D. Increased consumption of green leafy vegetables, but not fruit, vegetables or fruit and vegetables combined, is associated with reduced incidence of type 2 diabetes. Evid. Based. Med. 2011, 16, 27–28. [Google Scholar] [CrossRef]
- Endo, Y.; Usuki, R.; Kaneda, T. Antioxidant effects of chlorophyll and pheophytin on the autoxidation of oils in the dark. I. Comparison of the inhibitory effects. J. Am. Oil Chem. Soc. 1985, 62, 1375–1378. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Rezai, S.; Etemadi, N.; Nikbakht, A.; Yousefi, M.; Majidi, M.M. Effect of light intensity on leaf morphology, photosynthetic capacity, and chlorophyll content in sage (Salvia officinalis L.). Hortic. Sci. Technol. 2018, 36, 46–57. [Google Scholar] [CrossRef]
- Van Dam, N.M.; Witjes, L.; Svatoš, A. Interactions between aboveground and belowground induction of glucosinolates in two wild Brassica species. New Phytol. 2004, 161, 801–810. [Google Scholar] [CrossRef]
- Smetanska, I.; Krumbein, A.; Schreiner, M.; Knorr, D. Influence of salicylic acid and methyl jasmonate on glucosinolate levels in turnip. J. Hortic. Sci. Biotechnol. 2007, 82, 690–694. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wang, X.; Choi, J.H. Effect of methyl jasmonate on phenolics, isothiocyanate, and metabolic enzymes in radish sprout (Raphanus sativus L.). J. Agric. Food Chem. 2006, 54, 7263–7269. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011, 129, 35–44. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Manuel Domínguez, J.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Carlos Parajó, J. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Sokół-Łetowska, A.; Oszmiański, J.; Wojdyło, A. Antioxidant activity of the phenolic compounds of hawthorn, pine and skullcap. Food Chem. 2007, 103, 853–859. [Google Scholar] [CrossRef]
- Becker, T.; Juvik, J. The Role of Glucosinolate Hydrolysis Products from Brassica Vegetable Consumption in Inducing Antioxidant Activity and Reducing Cancer Incidence. Diseases 2016, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates—A review. LWT Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Haile, M.; Bae, H.M.; Kang, W.H. Comparison of the antioxidant activities and volatile compounds of coffee beans obtained using digestive bio-processing (elephant dung coffee) and commonly known processing methods. Antioxidants 2020, 9. [Google Scholar] [CrossRef]


| Treatments | GSLs Content (µg/g DW) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aliphatic | Indolic | Aromatic | Total GSLs | |||||||||||||
| PGT | GRA | GIB | SIN | GAS | GNP | GBN | Total | GBS | 4HGB | 4MTGB | NGB | Total | GNT | |||
| Soil | Control | 33.9a | 89.9b | 4.8a | 4.9ab | 20.0bc | 1190.3a | 119.6b | 1463.4a | 70.1b | 7.8b | 74.1a | 17.9a | 169.9b | 299.2c | 1932.5c |
| MeJA | 29.5a | 102.7a | 2.1c | 4.4b | 22.1b | 1121.8b | 174.6a | 1457.2a | 200.2a | 1.7c | 63.2b | 18.3a | 283.3a | 5866.8b | 7607.4b | |
| Hydroponics | Control | 19.6b | 94.5b | 2.7b | 5.3a | 16.5c | 488.2d | 78.1c | 704.9c | 56.0c | 10.1a | 46.7c | 10.5b | 123.3c | 255.4c | 1083.5d |
| MeJA | 17.6b | 72.8c | 1.5d | 3.0c | 34.5a | 576.7c | 127.0b | 833.0b | 30.7d | 0.5c | 35.8d | 3.3c | 70.3d | 12145.1a | 13048.4a | |
| Parameters | Sample Concentration (mg·mL−1) | Treatments | |||
|---|---|---|---|---|---|
| Soil | Hydroponics | ||||
| Control | MeJA | Control | MeJA | ||
| DPPH (%) | 1.25 | 39.06 ± 0.69d | 41.30 ± 0.38c | 42.52 ± 0.06b | 47.75 ± 0.29a |
| 2.5 | 51.26 ± 0.25c | 57.09 ± 0.46b | 56.91 ± 0.08b | 68.83 ± 0.33a | |
| 5 | 73.78 ± 0.60d | 82.40 ± 0.67c | 84.71 ± 0.17b | 92.84 ± 0.14a | |
| ABTS (%) | 1.25 | 12.88 ± 0.24c | 14.39 ± 0.54b | 13.34 ± 0.70c | 18.11 ± 0.15a |
| 2.5 | 20.24 ± 0.47b | 19.92 ± 1.1b | 20.33 ± 0.81b | 27.80 ± 0.12a | |
| 5 | 32.20 ± 0.53d | 33.95 ± 0.21b | 33.07 ± 0.17c | 49.05 ± 0.32a | |
| FRAP (Absorbance) | 1.25 | 0.18 ± 0.002d | 0.23 ± 0.002c | 0.24 ± 0.006b | 0.27 ± 0.001a |
| 2.5 | 0.36d ± 0.001d | 0.47 ± 0.009c | 0.49 ± 0.009b | 0.55 ± 0.007a | |
| 5 | 0.52 ± 0.01d | 0.71 ± 0.007b | 0.66 ± 0.008c | 0.84 ± 0.013a | |
| ORAC (µm Trolox) | 0.01 | 10.26 ± 2.26ab | 10.34 ± 1.55ab | 4.45 ± 2.18b | 11.66 ± 0.74a |
| 0.05 | 25.24 ± 0.52c | 37.09 ± 0.35b | 23.78 ± 0.10d | 65.45 ± 0.59a | |
| 0.1 | 45.10 ± 0.11c | 65.20 ± 0.21b | 41.35 ± 0.08d | 99.00 ± 1.12a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, M.W.; Choi, H.R.; Solomon, T.; Jeong, C.S.; Lee, O.-H.; Tilahun, S. Preharvest Methyl Jasmonate Treatment Increased the Antioxidant Activity and Glucosinolate Contents of Hydroponically Grown Pak Choi. Antioxidants 2021, 10, 131. https://doi.org/10.3390/antiox10010131
Baek MW, Choi HR, Solomon T, Jeong CS, Lee O-H, Tilahun S. Preharvest Methyl Jasmonate Treatment Increased the Antioxidant Activity and Glucosinolate Contents of Hydroponically Grown Pak Choi. Antioxidants. 2021; 10(1):131. https://doi.org/10.3390/antiox10010131
Chicago/Turabian StyleBaek, Min Woo, Han Ryul Choi, Tifsehit Solomon, Cheon Soon Jeong, Ok-Hwan Lee, and Shimeles Tilahun. 2021. "Preharvest Methyl Jasmonate Treatment Increased the Antioxidant Activity and Glucosinolate Contents of Hydroponically Grown Pak Choi" Antioxidants 10, no. 1: 131. https://doi.org/10.3390/antiox10010131
APA StyleBaek, M. W., Choi, H. R., Solomon, T., Jeong, C. S., Lee, O.-H., & Tilahun, S. (2021). Preharvest Methyl Jasmonate Treatment Increased the Antioxidant Activity and Glucosinolate Contents of Hydroponically Grown Pak Choi. Antioxidants, 10(1), 131. https://doi.org/10.3390/antiox10010131






