Antioxidant Activity and Biocompatibility of Fructo-Polysaccharides Extracted from a Wild Species of Ornithogalum from Lebanon
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials and Reagents
2.2. Extraction
2.3. Characterization of the Extracts
2.3.1. Total Carbohydrates Content (TCC)
2.3.2. Protein Content
2.3.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.4. Size-Exclusion Chromatography (SEC)
2.3.5. Monosaccharide Composition and Linkage Type Analysis
2.3.6. Thermogravimetric Analysis (TGA)
2.3.7. Differential Scanning Calorimetry (DSC)
2.4. Evaluation of Antioxidant Activities
2.4.1. ABTS Radical Cation Decolorization Assay
2.4.2. β-Carotene-Linoleic Acid Assay
2.4.3. Ferrous Ion-Chelating Activity
2.4.4. Total Antioxidant Activity Assay
2.5. Cytotoxic Assay on Human Cells
2.6. Hemolytic Activity Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield of Polysaccharides and Composition of the Extract
3.2. FTIR
3.3. Size-Exclusion Chromatography
3.4. GC–FID of TMS Derivatives Analysis
3.5. GC–MS of PMAA Derivatives Analysis
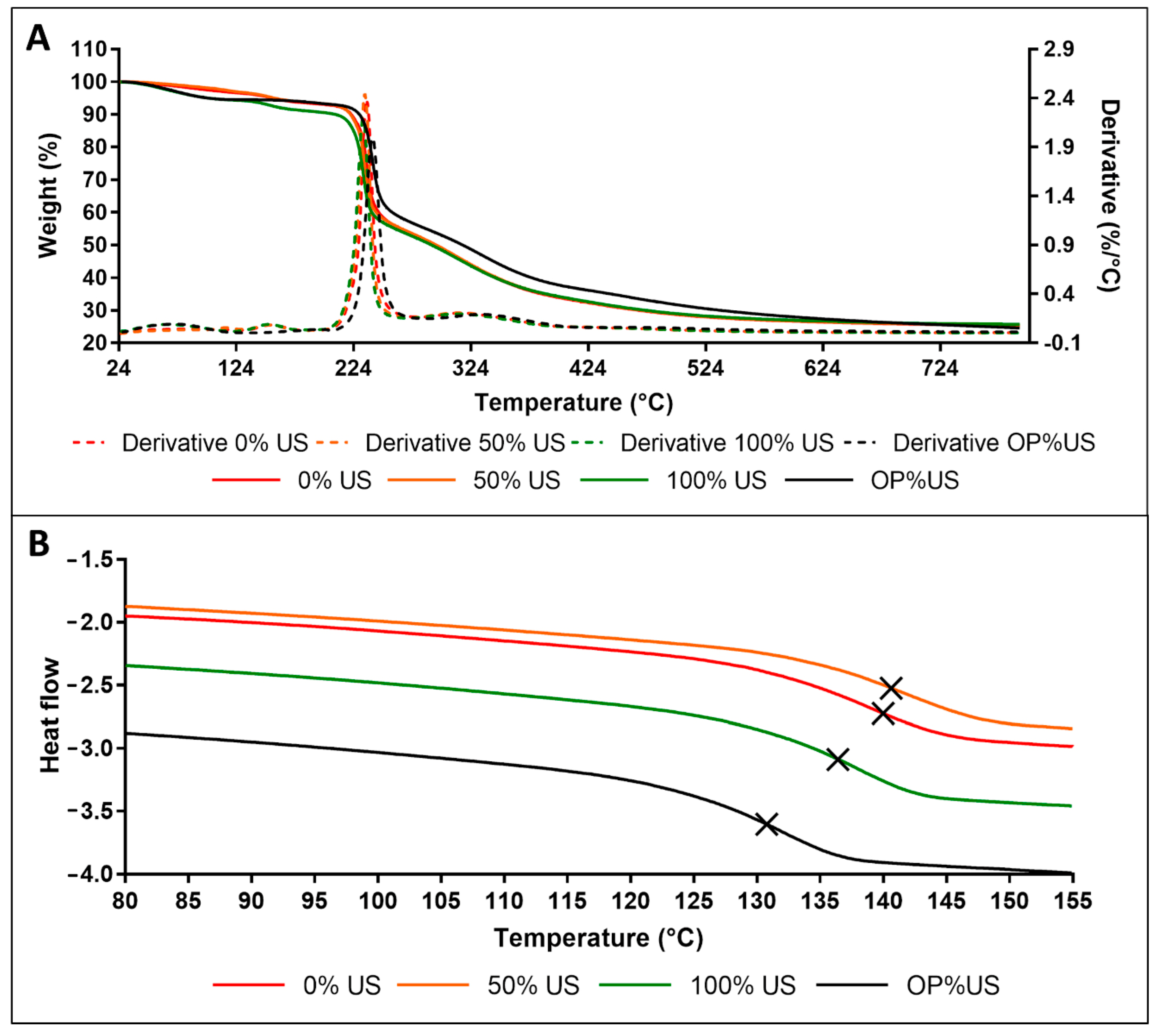
3.6. Thermal Analysis
3.7. Antioxidant Activities
3.7.1. Evaluation of Total Antioxidant Capacity
3.7.2. β-Carotene Bleaching Inhibition Assay
3.7.3. ABTS Radical Scavenging Activity
3.7.4. Ferrous Ion-Chelating Activity
3.8. Biocompatibility Evaluation
3.9. Evaluation of Safety Factor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Southorn, P.A.; Powis, G. Free Radicals in Medicine. II. Involvement in Human Disease. Mayo Clin. Proc. 1988, 63, 390–408. [Google Scholar] [CrossRef] [Green Version]
- Muriel, P.; Rivera-Espinoza, Y. Beneficial drugs for liver diseases. J. Appl. Toxicol. 2008, 28, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta ) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Meng, F.; Liu, Z.; Chen, R.; Zhang, M. Antitumor activities of different fractions of polysaccharide purified from Ornithogalum caudatum Ait. Carbohydr. Polym. 2010, 80, 845–851. [Google Scholar] [CrossRef]
- Dahech, I.; Belghith, K.S.; Hamden, K.; Feki, A.; Belghith, H.; Mejdoub, H. Antidiabetic activity of levan polysaccharide in alloxan-induced diabetic rats. Int. J. Biol. Macromol. 2011, 49, 742–746. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, H.; Liu, C.; Wang, Y.; Fan, S. Medicinal Chemistry Extraction of water-soluble polysaccharide and the antioxidant activity from Ginkgo biloba leaves. Med. Chem. Res. 2010, 262–270. [Google Scholar] [CrossRef]
- Qu, C.; Yu, S.; Luo, L.; Zhao, Y.; Huang, Y. Optimization of ultrasonic extraction of polysaccharides from Ziziphus jujubaMill. by response surface methodology. Chem. Cent. J. 2013, 7, 160. [Google Scholar] [CrossRef] [Green Version]
- Wahlström, R.M.; Suurnäkki, A. Enzymatic hydrolysis of lignocellulosic polysaccharides in the presence of ionic liquids. Green Chem. 2015, 17, 694–714. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.Q.; Yang, X.Q.; Qi, B.; Li, L.H.; Deng, J.C.; Hu, X. Study of Ultrasonic-freeze-thaw-cycle assisted extraction of polysaccharide and phycobiliprotein from Gracilaria lemaneiformis. Adv. Mater. Res. 2013, 781–784, 1818–1824. [Google Scholar] [CrossRef]
- Chen, R.; Li, Y.; Dong, H.; Liu, Z.; Li, S.; Yang, S.; Li, X. Optimization of ultrasonic extraction process of polysaccharides from Ornithogalum Caudatum Ait and evaluation of its biological activities. Ultrason. Sonochem. 2012, 19, 1160–1168. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Tiwari, B.; Sweeney, T.; O’Doherty, J. Extraction and Yield Optimisation of Fucose, Glucans and Associated Antioxidant Activities from Laminaria digitata by Applying Response Surface Methodology to High Intensity Ultrasound-Assisted Extraction. Mar. Drugs 2018, 16, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, H.-M.; Zhou, H.-Z.; Yang, J.-Y.; Li, R.; Song, H.; Wu, H.-X. In Vitro and In Vivo antioxidant activities of inulin. PLoS ONE 2018, 13, e0192273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-J.; Chung, B.H. Antioxidant activity of levan coated cerium oxide nanoparticles. Carbohydr. Polym. 2016, 150, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Medlej, M.K.; Cherri, B.; Nasser, G.; Zaviska, F.; Hijazi, A.; Li, S.; Pochat-Bohatier, C. Optimization of polysaccharides extraction from a wild species of Ornithogalum combining ultrasound and maceration and their anti-oxidant properties. Int. J. Biol. Macromol. 2020, 161, 958–968. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Montreuil, J.; Bouquelet, S.; Debray, H.; Fournet, B.; Spik, G.; Strecker, G. Carbohydrate Analysis: A Practical Approach, 2nd ed.; Chaplin, M.F., Kennedy, J.F., Eds.; A IRL Press Publication: Washington, DC, USA, 1986; pp. 143–204. ISBN 9780199634491. [Google Scholar]
- Hakomori, S.I. A Rapid Permethylation of Glycolipid, and Polysaccharide Catalyzed by Methylsulfinyl Carbanion in Dimethyl Sulfoxide. J. Biochem. 1964, 55, 205–208. [Google Scholar] [CrossRef]
- Tang, D.; Yu, S.; Ho, Y.; Huang, B.; Tsai, G.; Hsieh, H. Food Hydrocolloids Characterization of tea catechins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocoll. 2013, 30, 33–41. [Google Scholar] [CrossRef]
- Jridi, M.; Mezhoudi, M.; Abdelhedi, O.; Boughriba, S.; Elfalleh, W.; Souissi, N.; Nasri, R.; Nasri, M. Bioactive potential and structural characterization of sulfated polysaccharides from Bullet tuna (Auxis Rochei) by-products. Carbohydr. Polym. 2018. [Google Scholar] [CrossRef]
- Khadhri, A. Composes phenoliques et activites antioxydantes de deux extraits de chardon a glu: Atractylis gummifera composes phenoliques et activites antioxydantes de deux extraits de chardon a glu: Atractylis gummifera. Revue Soc. Sci. Nat. de Tunisie 2013, 39, 44–52. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E 1. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Razafimanjato, H.; Garmy, N.; Guo, X.J.; Varini, K.; Di Scala, C.; Di Pasquale, E.; Taïeb, N.; Maresca, M. The food-associated fungal neurotoxin ochratoxin A inhibits the absorption of glutamate by astrocytes through a decrease in cell surface expression of the excitatory amino-acid transporters GLAST and GLT-1. Neurotoxicology 2010, 31, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Olleik, H.; Baydoun, E.; Perrier, J.; Hijazi, A.; Raymond, J.; Manzoni, M.; Dupuis, L.; Pauleau, G.; Goudard, Y.; de La Villéon, B.; et al. Temporin-SHa and its analogs as potential candidates for the treatment of helicobacter pylori. Biomolecules 2019, 9, 598. [Google Scholar] [CrossRef] [Green Version]
- Benkhaled, B.T.; Hadiouch, S.; Olleik, H.; Perrier, J.; Ysacco, C.; Guillaneuf, Y.; Gigmes, D.; Maresca, M.; Lefay, C. Elaboration of antimicrobial polymeric materials by dispersion of well-defined amphiphilic methacrylic SG1-based copolymers. Polym. Chem. 2018, 9, 3127–3141. [Google Scholar] [CrossRef]
- Olleik, H.; Nicoletti, C.; Lafond, M.; Courvoisier-Dezord, E.; Xue, P.; Hijazi, A.; Baydoun, E.; Perrier, J.; Maresca, M. Comparative Structure–Activity Analysis of the Antimicrobial Activity, Cytotoxicity, and Mechanism of Action of the Fungal Cyclohexadepsipeptides Enniatins and Beauvericin. Toxins 2019, 11, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, C.; Guo, M. Effects of ultrasound treatment on extraction and rheological properties of polysaccharides from Auricularia cornea var. Li. Molecules 2019, 24, 939. [Google Scholar] [CrossRef] [Green Version]
- Ebringerová, A.; Hromádková, Z. An overview on the application of ultrasound in extraction, separation and purification of plant polysaccharides. Cent. Eur. J. Chem. 2010, 8, 243–257. [Google Scholar] [CrossRef]
- Hou, F.; Wu, Y.; Kan, L.; Li, Q.; Xie, S.; Ouyang, J. Effects of Ultrasound on the Physicochemical Properties and Antioxidant Activities of Chestnut Polysaccharide. Int. J. Food Eng. 2016, 12, 439–449. [Google Scholar] [CrossRef]
- Guerrero, P.; Kerry, J.P.; De La Caba, K. FTIR characterization of protein-polysaccharide interactions in extruded blends. Carbohydr. Polym. 2014, 111, 598–605. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Mao, F.; Liu, Y.; Wei, X. Purification, characterization and biological activities in vitro of polysaccharides extracted from tea seeds. Int. J. Biol. Macromol. 2013, 62, 508–513. [Google Scholar] [CrossRef]
- Dean, A.P.; Nicholson, J.M.; Sigee, D.C. Impact of phosphorus quota and growth phase on carbon allocation in Chlamydomonas reinhardtii: An FTIR microspectroscopy study. Eur. J. Phycol. 2008, 43, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Liu, Q.; Tao, Y.; Zhang, H.; Zhang, L.; Ding, K. Structure and chain conformation of a (1→6)-a-D -glucan from the root of Pueraria lobata (Willd.) Ohwi and the antioxidant activity of its sulfated derivative. Carbohydr. Polym. 2008, 74, 771–778. [Google Scholar] [CrossRef]
- Benkeblia, N. Fructooligosaccharides and fructans analysis in plants and food crops. J. Chromatogr. A 2013, 1313, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, C.K.; Kim, S.I. Characterization of the water state of hyaluronic acid and poly(vinyl alcohol) interpenetrating polymer networks. J. Appl. Polym. Sci. 2004, 92, 1467–1472. [Google Scholar] [CrossRef]
- Srikanth, R.; Siddartha, G.; Sundhar Reddy, C.H.S.S.; Harish, B.S.; Janaki Ramaiah, M.; Uppuluri, K.B. Antioxidant and anti-inflammatory levan produced from Acetobacter xylinum NCIM2526 and its statistical optimization. Carbohydr. Polym. 2015, 123, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.Y.; Poh, C.S.; Voon, C.H.; Salmah, H. Rheological and Thermal Study of Chitosan Filled Thermoplastic Elastomer Composites. Appl. Mech. Mater. 2015, 754–755, 34–38. [Google Scholar] [CrossRef]
- Van Tran, K.; Le, H.T.; Le, S.L.; Ho, V.X.A.; Trinh, K.T.; Tran, T.T. Van effect of temperatures extraction on in vitro antioxidant activities of polysaccharides from ophiocordyceps sobolifera. Hue Univ. J. Sci. Nat. Sci. 2019, 128, 17–21. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; He, W.; Shi, L.; Pan, H.; Fan, L. Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohydr. Polym. 2013, 98, 1524–1531. [Google Scholar] [CrossRef]
- Hu, J.; Gao, J.; Zhao, Z.; Yang, X. Response surface optimization of polysaccharide extraction from Galla Chinensis and determination of its antioxidant activity in vitro. Food Sci. Technol. 2020, 2061, 1–7. [Google Scholar] [CrossRef]
- Hernandez-Marin, E.; Martínez, A. Carbohydrates and Their Free Radical Scavenging Capability: A Theoretical Study. J. Phys. Chem. B 2012, 116, 9668–9675. [Google Scholar] [CrossRef]
- Peshev, D.; Vergauwen, R.; Moglia, A.; Hideg, É.; Van den Ende, W. Towards understanding vacuolar antioxidant mechanisms: A role for fructans? J. Exp. Bot. 2013, 64, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Vismara, E.; Pierini, M.; Mascellani, G.; Liverani, L.; Lima, M.; Guerrini, M.; Torri, G. Low-molecular-weight heparin from Cu2+ and Fe2+ Fenton type depolymerisation processes. Thromb. Haemost. 2010, 103, 613–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xing, R.; Liu, S.; Guo, Z.; Yu, H.; Zhong, Z.; Ji, X.; Li, P. Relevance of molecular weight of chitosan-N-2-hydroxypropyl trimethyl ammonium chloride and their antioxidant activities. Eur. J. Med. Chem. 2008, 43, 336–340. [Google Scholar] [CrossRef]
- Tang, W.; Lin, L.; Xie, J.; Wang, Z.; Wang, H.; Dong, Y.; Shen, M.; Xie, M. Effect of ultrasonic treatment on the physicochemical properties and antioxidant activities of polysaccharide from Cyclocarya paliurus. Carbohydr. Polym. 2016, 151, 305–312. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Li, C.; Huang, Q.; You, L.-J.; Chen, C.; Fu, X.; Liu, R.H. Comparative study on the physicochemical properties and bioactivities of polysaccharide fractions extracted from Fructus Mori at different temperatures. Food Funct. 2019, 10, 410–421. [Google Scholar] [CrossRef]
- Hu, Y.-N.; Sung, T.-J.; Chou, C.-H.; Liu, K.-L.; Hsieh, L.-P.; Hsieh, C.-W. Characterization and Antioxidant Activities of Yellow Strain Flammulina velutipes (Jinhua Mushroom) Polysaccharides and Their Effects on ROS Content in L929 Cell. Antioxidants 2019, 8, 298. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Kasimu, R.; Xie, X.; Zheng, Y.; Ding, W. Optimised extraction of Erythronium sibiricum bulb polysaccharides and evaluation of their bioactivities. Int. J. Biol. Macromol. 2016, 82, 898–904. [Google Scholar] [CrossRef]
- Surin, S.; You, S.; Seesuriyachan, P.; Muangrat, R.; Wangtueai, S.; Jambrak, A.R.; Phongthai, S.; Jantanasakulwong, K.; Chaiyaso, T.; Phimolsiripol, Y. Optimization of ultrasonic-assisted extraction of polysaccharides from purple glutinous rice bran (Oryza sativa L.) and their antioxidant activities. Sci. Rep. 2020, 10, 10410. [Google Scholar] [CrossRef]





| Extracts | Extraction Yield (%) | Total Carbohydrates Content (%) | Proteins Content (%) | Water Content (%) | Mw | Ð |
|---|---|---|---|---|---|---|
| 0% US | 25.5 ± 4.3 | 58.2 ±1.1 | 2.4 ± 0.1 | 3.4 | 3140 | 1.452 |
| 50% US | 45.5 ± 3.2 | 80.2 ± 1.0 | 4.8 ± 0.2 | 3.0 | 3235 | 1.467 |
| 100% US | 37.9 ± 2.7 | 66.1 ± 0.3 | 7.7 ± 0.3 | 5.6 | 3145 | 1.482 |
| OP%US | 85.7 ± 0.2% | 83.0 ± 0.1% | 2.1 ± 0.1 | - | - | - |
| Test | 0% US | 50% US | 100% US | OP%US |
|---|---|---|---|---|
| RBC | >20 | >20 | 1.3 ± 0.1 | >20 |
| HaCaT | >20 | >20 | 15.2 ± 1.1 | >20 |
| HUVEC | >20 | 14.3 ± 2.0 | 0.9 ± 0.1 | >20 |
| IMR90 | >20 | >20 | >20 | >20 |
| SF | 0% US | 50% US | 100% US | OP%US |
|---|---|---|---|---|
| HC50 RBC/IC50β | >20.62 | >37.04 | 1.63 | >152.7 |
| IC50 HaCaT/IC50β | >20.62 | >37.04 | 18.86 | >152.7 |
| IC50 HUVEC/IC50β | >20.62 | 26.54 | 1.11 | >152.7 |
| IC50 IMR90/IC50β | >20.62 | >37.04 | >24.75 | >152.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medlej, M.K.; Batoul, C.; Olleik, H.; Li, S.; Hijazi, A.; Nasser, G.; Maresca, M.; Pochat-Bohatier, C. Antioxidant Activity and Biocompatibility of Fructo-Polysaccharides Extracted from a Wild Species of Ornithogalum from Lebanon. Antioxidants 2021, 10, 68. https://doi.org/10.3390/antiox10010068
Medlej MK, Batoul C, Olleik H, Li S, Hijazi A, Nasser G, Maresca M, Pochat-Bohatier C. Antioxidant Activity and Biocompatibility of Fructo-Polysaccharides Extracted from a Wild Species of Ornithogalum from Lebanon. Antioxidants. 2021; 10(1):68. https://doi.org/10.3390/antiox10010068
Chicago/Turabian StyleMedlej, Mohammad Kazem, Cherri Batoul, Hamza Olleik, Suming Li, Akram Hijazi, Ghassan Nasser, Marc Maresca, and Céline Pochat-Bohatier. 2021. "Antioxidant Activity and Biocompatibility of Fructo-Polysaccharides Extracted from a Wild Species of Ornithogalum from Lebanon" Antioxidants 10, no. 1: 68. https://doi.org/10.3390/antiox10010068
APA StyleMedlej, M. K., Batoul, C., Olleik, H., Li, S., Hijazi, A., Nasser, G., Maresca, M., & Pochat-Bohatier, C. (2021). Antioxidant Activity and Biocompatibility of Fructo-Polysaccharides Extracted from a Wild Species of Ornithogalum from Lebanon. Antioxidants, 10(1), 68. https://doi.org/10.3390/antiox10010068







