Association of Urinary and Dietary Selenium and of Serum Selenium Species with Serum Alanine Aminotransferase in a Healthy Italian Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population of the Study
2.2. Laboratory Determinations
2.3. Statistical Analysis
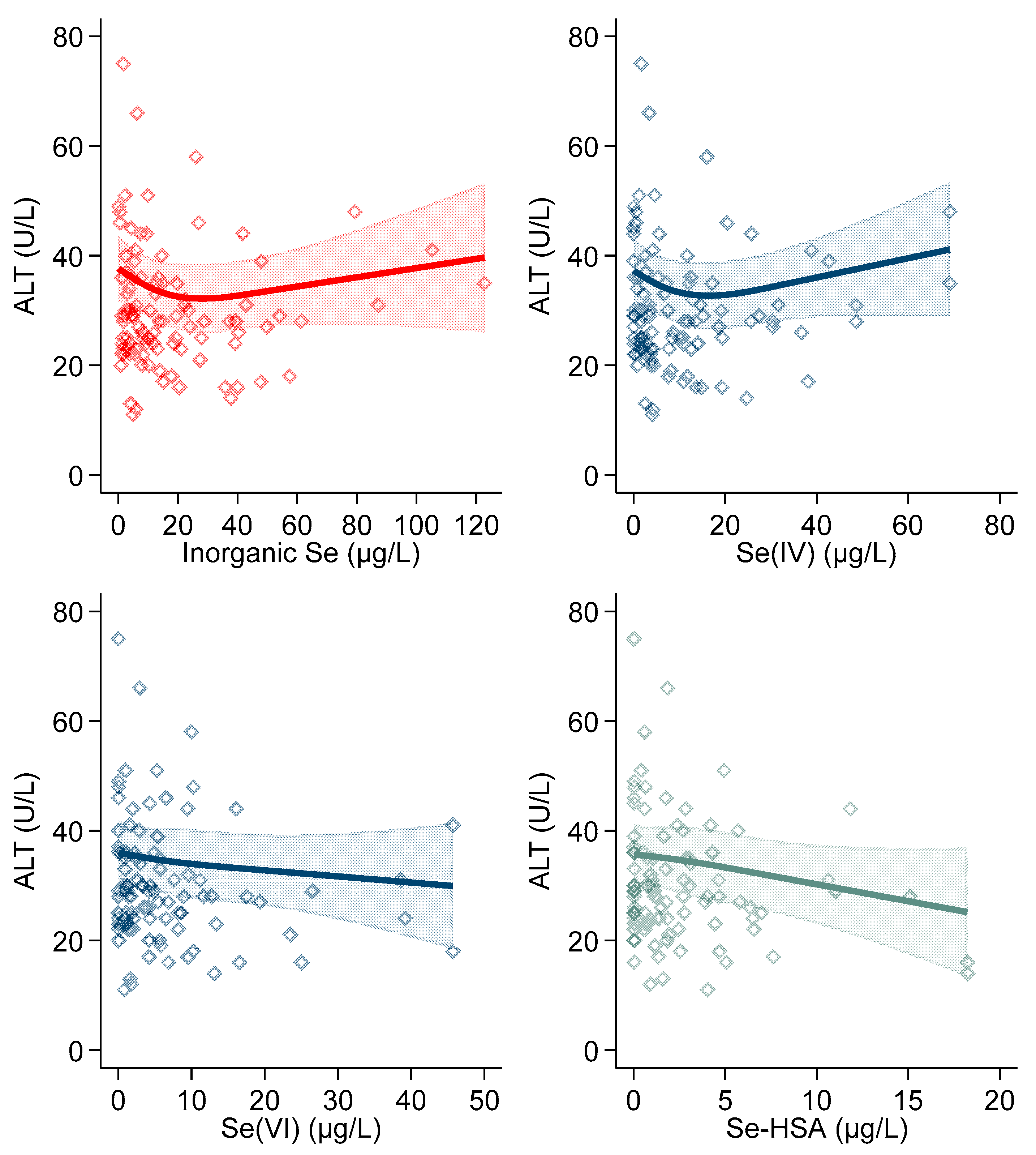
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinceti, M.; Filippini, T.; Wise, L.A. Environmental selenium and human health: An update. Curr. Environ. Health Rep. 2018, 5, 464–485. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Michalke, B.; Wise, L.A.; Malagoli, C.; Malavolti, M.; Vescovi, L.; Salvia, C.; Bargellini, A.; Sieri, S.; Krogh, V.; et al. Diet composition and serum levels of selenium species: A cross-sectional study. Food Chem. Toxicol. 2018, 115, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.; Vinceti, M. Selenium and its compunds. In Hamilton & Hardy’s Industrial Toxicology, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Heck, J.E.; Park, A.S.; Qiu, J.; Cockburn, M.; Ritz, B. Risk of leukemia in relation to exposure to ambient air toxics in pregnancy and early childhood. Int. J. Hyg. Environ. Health 2014, 217, 662–668. [Google Scholar] [CrossRef] [Green Version]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Bargellini, A.; Vergoni, A.V.; Tsatsakis, A.; Ferrante, M. Health risk assessment of environmental selenium: Emerging evidence and challenges. Mol. Med. Rep. 2017, 15, 3323–3335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinceti, M.; Grill, P.; Malagoli, C.; Filippini, T.; Storani, S.; Malavolti, M.; Michalke, B. Selenium speciation in human serum and its implications for epidemiologic research: A cross-sectional study. J. Trace Elem. Med. Biol. 2015, 31, 1–10. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium(-)fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [Green Version]
- Weekley, C.M.; Harris, H.H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef]
- Michalke, B.; Willkommen, D.; Drobyshev, E.; Solovyev, N. The importance of speciation analysis in neurodegeneration research. Trends Anal. Chem. 2018, 104, 160–170. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific opinion on dietary reference values for selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- Michalke, B. Element speciation definitions, analytical methodology, and some examples. Ecotoxicol. Environ. Saf. 2003, 56, 122–139. [Google Scholar] [CrossRef]
- Hawkes, W.C.; Alkan, Z. Regulation of redox signaling by selenoproteins. Biol. Trace Elem. Res. 2010, 134, 235–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallenberg, M.; Olm, E.; Hebert, C.; Bjornstedt, M.; Fernandes, A.P. Selenium compounds are substrates for glutaredoxins: A novel pathway for selenium metabolism and a potential mechanism for selenium-mediated cytotoxicity. Biochem. J. 2010, 429, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Roh, Y.J.; Han, S.J.; Park, I.; Lee, H.M.; Ok, Y.S.; Lee, B.C.; Lee, S.R. Role of selenoproteins in redox regulation of signaling and the antioxidant system: A review. Antioxidants 2020, 9, 383. [Google Scholar] [CrossRef]
- Jablonska, E.; Vinceti, M. Selenium and human health: Witnessing a Copernican revolution? J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 328–368. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Yoo, M.H.; Carlson, B.A.; Gladyshev, V.N. Selenoproteins that function in cancer prevention and promotion. Biochim. Biophys. Acta 2009, 1790, 1541–1545. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y. Selenoprotein P as an in vivo redox regulator: Disorders related to its deficiency and excess. J. Clin. Biochem. Nutr. 2020, 66, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Vinceti, M.; Crespi, C.M.; Malagoli, C.; Del Giovane, C.; Krogh, V. Friend or foe? The current epidemiologic evidence on selenium and human cancer risk. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2013, 31, 305–341. [Google Scholar] [CrossRef] [Green Version]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Selenium and diabetes: More bad news for supplements. Ann. Intern. Med. 2007, 147, 271–272. [Google Scholar] [CrossRef] [Green Version]
- Vinceti, M.; Filippini, T.; Rothman, K.J. Selenium exposure and the risk of type 2 diabetes: A systematic review and meta-analysis. Eur. J. Epidemiol. 2018, 33, 789–810. [Google Scholar] [CrossRef]
- Smith, M.I.; Franke, K.W.; Westfall, B.B. Further field studies on the selenium problem in relation to public health. Public Health Rep. 1937, 52, 1375–1384. [Google Scholar] [CrossRef]
- Smith, M.I.; Franke, K.W.; Westfall, B.B. The selenium problem in relation to public health: A preliminary survey to determine the possibility of selenium intoxication in the rural population living on seleniferous soil. Public Health Rep. 1936, 51, 1496–1505. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, C.; Yang, Z.; Li, X.; Lei, G.; Xie, D.; Wang, Y.; Wei, J.; Yang, T. Association between dietary selenium intake and the prevalence of nonalcoholic fatty liver disease: A cross-sectional study. J. Am. Coll. Nutr. 2020, 39, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Wei, E.T.; Malagoli, C.; Bergomi, M.; Vivoli, G. Adverse health effects of selenium in humans. Rev. Environ. Health 2001, 16, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Filippini, T.; Chawla, R.; Chaudhary, R.; Cilloni, S.; Datt, C.; Singh, S.; Dhillon, K.S.; Vinceti, M. Exposure to a high selenium environment in Punjab, India: Effects on blood chemistry. Sci. Total Environ. 2020, 716, 135347. [Google Scholar] [CrossRef] [PubMed]
- Coudray, C.; Hida, H.; Boucher, F.; Tirard, V.; de Leiris, J.; Favier, A. Effect of selenium supplementation on biological constants and antioxidant status in rats. J. Trace Elem. Med. Biol. 1996, 10, 12–19. [Google Scholar] [CrossRef]
- Senior, J.R. Alanine aminotransferase: A clinical and regulatory tool for detecting liver injury-past, present, and future. Clin. Pharmacol. Ther. 2012, 92, 332–339. [Google Scholar] [CrossRef]
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG clinical guideline: Evaluation of abnormal liver chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef]
- Kim, W.R.; Flamm, S.L.; Di Bisceglie, A.M.; Bodenheimer, H.C. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008, 47, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Fustinoni, S.; Campo, L.; Polledri, E.; Mercadante, R.; Erspamer, L.; Ranzi, A.; Lauriola, P.; Alberto Goldoni, C.; Bertazzi, P. A validated method for urinary cotinine quantification used to classify active and environmental tobacco smoke exposure. Curr. Anal. Chem. 2013, 9, 447–456. [Google Scholar] [CrossRef]
- Filippini, T.; Cilloni, S.; Malavolti, M.; Violi, F.; Malagoli, C.; Tesauro, M.; Bottecchi, I.; Ferrari, A.; Vescovi, L.; Vinceti, M. Dietary intake of cadmium, chromium, copper, manganese, selenium and zinc in a Northern Italy community. J. Trace Elem. Med. Biol. 2018, 50, 508–517. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Cilloni, S.; Wise, L.A.; Violi, F.; Malagoli, C.; Vescovi, L.; Vinceti, M. Intake of arsenic and mercury from fish and seafood in a Northern Italy community. Food Chem. Toxicol. 2018, 116 Pt B, 20–26. [Google Scholar] [CrossRef]
- Filippini, T.; Tancredi, S.; Malagoli, C.; Malavolti, M.; Bargellini, A.; Vescovi, L.; Nicolini, F.; Vinceti, M. Dietary estimated intake of trace elements: Risk assessment in an Italian population. Expo. Health 2020, 12, 641–655. [Google Scholar] [CrossRef] [Green Version]
- Malavolti, M.; Fairweather-Tait, S.J.; Malagoli, C.; Vescovi, L.; Vinceti, M.; Filippini, T. Lead exposure in an Italian population: Food content, dietary intake and risk assessment. Food Res. Int. 2020, 137, 109370. [Google Scholar] [CrossRef] [PubMed]
- Michalke, B.; Berthele, A. Contribution to selenium speciation in cerebrospinal fluid samples. J. Anal. Atom. Spectrom. 2011, 26, 165–170. [Google Scholar] [CrossRef]
- Solovyev, N.; Berthele, A.; Michalke, B. Selenium speciation in paired serum and cerebrospinal fluid samples. Anal. Bioanal. Chem. 2013, 405, 1875–1884. [Google Scholar] [CrossRef]
- Shigeta, K.; Sato, K.; Furuta, N. Determination of selenoprotein P in submicrolitre samples of human plasma using micro-affinity chromatography coupled with low flow ICP-MS. J. Anal. Atom. Spectrom. 2007, 22, 911–916. [Google Scholar] [CrossRef]
- Vinceti, M.; Solovyev, N.; Mandrioli, J.; Crespi, C.M.; Bonvicini, F.; Arcolin, E.; Georgoulopoulou, E.; Michalke, B. Cerebrospinal fluid of newly diagnosed amyotrophic lateral sclerosis patients exhibits abnormal levels of selenium species including elevated selenite. Neurotoxicology 2013, 38, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Yang, L.M.; Wang, Q.Q. Quantification of selenium-tagged proteins in human plasma using species-unspecific isotope dilution ICP-DRC-qMS coupled on-line with anion exchange chromatography. J. Anal. Atom. Spectrom. 2008, 23, 1545–1549. [Google Scholar] [CrossRef] [Green Version]
- Vinceti, M.; Chiari, A.; Eichmuller, M.; Rothman, K.J.; Filippini, T.; Malagoli, C.; Weuve, J.; Tondelli, M.; Zamboni, G.; Nichelli, P.F.; et al. A selenium species in cerebrospinal fluid predicts conversion to Alzheimer′s dementia in persons with mild cognitive impairment. Alzheimers Res. Ther. 2017, 9, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbano, T.; Filippini, T.; Lasagni, D.; De Luca, T.; Sucato, S.; Polledri, E.; Bruzziches, F.; Malavolti, M.; Baraldi, C.; Santachiara, A.; et al. Associations between urinary and dietary selenium and blood metabolic parameters in a healthy northern Italy population. Antioxidants 2021, 10, 1193. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Albanes, D.; Till, C.; Klein, E.A.; Goodman, P.J.; Mondul, A.M.; Weinstein, S.J.; Taylor, P.R.; Parnes, H.L.; Gaziano, J.M.; Song, X.; et al. Plasma tocopherols and risk of prostate cancer in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Cancer Prev. Res. 2014, 7, 886–895. [Google Scholar] [CrossRef] [Green Version]
- Kristal, A.R.; Darke, A.K.; Morris, J.S.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L., Jr.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J. Natl. Cancer Inst. 2014, 106, djt456. [Google Scholar] [CrossRef] [Green Version]
- Martinez, E.E.; Darke, A.K.; Tangen, C.M.; Goodman, P.J.; Fowke, J.H.; Klein, E.A.; Abdulkadir, S.A. A functional variant in NKX3.1 associated with prostate cancer risk in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Cancer Prev. Res. 2014, 7, 950–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinceti, M.; Filippini, T.; Wise, L.A.; Rothman, K.J. A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ. Res. 2021, 197, 111210. [Google Scholar] [CrossRef]
- Longnecker, M.P.; Taylor, P.R.; Levander, O.A.; Howe, M.; Veillon, C.; McAdam, P.A.; Patterson, K.Y.; Holden, J.M.; Stampfer, M.J.; Morris, J.S. Selenium in diet, blood, and toenails in relation to human health in a seleniferous area. Am. J. Clin. Nutr. 1991, 53, 1288–1294. [Google Scholar] [CrossRef]
- Wang, X.; Seo, Y.A.; Park, S.K. Serum selenium and non-alcoholic fatty liver disease (NAFLD) in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2011–2016. Environ. Res. 2021, 197, 111190. [Google Scholar] [CrossRef]
- Ashton, K.; Hooper, L.; Harvey, L.J.; Hurst, R.; Casgrain, A.; Fairweather-Tait, S.J. Methods of assessment of selenium status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2025S–2039S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Karp, D.D.; Lee, S.J.; Keller, S.M.; Wright, G.S.; Aisner, S.; Belinsky, S.A.; Johnson, D.H.; Johnston, M.R.; Goodman, G.; Clamon, G.; et al. Randomized, double-blind, placebo-controlled, phase III chemoprevention trial of selenium supplementation in patients with resected stage I non-small-cell lung cancer: ECOG 5597. J. Clin. Oncol. 2013, 31, 4179–4187. [Google Scholar] [CrossRef] [Green Version]
- Haldimann, M.; Venner, T.Y.; Zimmerli, B. Determination of selenium in the serum of healthy Swiss adults and correlation to dietary intake. J. Trace Elem. Med. Biol. 1996, 10, 31–45. [Google Scholar] [CrossRef]
- Vinceti, M.; Bonaccio, M.; Filippini, T.; Costanzo, S.; Wise, L.A.; Di Castelnuovo, A.; Ruggiero, E.; Persichillo, M.; Cerletti, C.; Donati, M.B.; et al. Dietary selenium intake and risk of hospitalization for type 2 diabetes in the Moli-sani study cohort. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1738–1746. [Google Scholar] [CrossRef]
- Cetindagli, I.; Kara, M.; Tanoglu, A.; Ozalper, V.; Aribal, S.; Hancerli, Y.; Unal, M.; Ozari, O.; Hira, S.; Kaplan, M.; et al. Evaluation of endothelial dysfunction in patients with nonalcoholic fatty liver disease: Association of selenoprotein P with carotid intima-media thickness and endothelium-dependent vasodilation. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 516–524. [Google Scholar] [CrossRef]
- Choi, H.Y.; Hwang, S.Y.; Lee, C.H.; Hong, H.C.; Yang, S.J.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Increased selenoprotein P levels in subjects with visceral obesity and nonalcoholic fatty liver disease. Diabetes Metab. J. 2013, 37, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Di Giuseppe, R.; Koch, M.; Schlesinger, S.; Borggrefe, J.; Both, M.; Müller, H.-P.; Kassubek, J.; Jacobs, G.; Nöthlings, U.; Lieb, W. Circulating selenoprotein P levels in relation to MRI-derived body fat volumes, liver fat content, and metabolic disorders. Obesity 2017, 25, 1128–1135. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mavrouli, M.; Katsinelos, P.; Doulberis, M.; Gavana, E.; Duntas, L. Selenoprotein P in patients with nonalcoholic fatty liver disease. Exp. Clin. Endocrinol. Diabetes 2019, 127, 598–602. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Rosso, C.; Armandi, A.; Gaggini, M.; Carli, F.; Abate, M.L.; Olivero, A.; Ribaldone, D.G.; Saracco, G.M.; Gastaldelli, A.; et al. Interplay between oxidative stress and metabolic derangements in non-alcoholic fatty liver disease: The role of selenoprotein P. Int. J. Mol. Sci. 2020, 21, 8838. [Google Scholar] [CrossRef]
- Papp, L.V.; Holmgren, A.; Khanna, K.K. Selenium and selenoproteins in health and disease. Antioxid. Redox Signal. 2010, 12, 793–795. [Google Scholar] [CrossRef]
- Schrauzer, G.N. The nutritional significance, metabolism and toxicology of selenomethionine. Adv. Food Nutr. Res. 2003, 47, 73–112. [Google Scholar] [CrossRef]
- Stadtman, T.C. Selenium biochemistry. Annu. Rev. Biochem. 1990, 59, 111–127. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Gladyshev, V.N. The outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) reveals the need for better understanding of selenium biology. Mol. Interv. 2009, 9, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Song, N. Modeling of selenite toxicity to wheat root elongation using biotic ligand model: Considering the effects of pH and phosphate anion. Environ. Pollut. 2021, 272, 115935. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Yan, L.; Cao, J.; Li, D.; Huang, G.Y.; Shi, W.J.; Dong, W.; Zha, J.; Ying, G.G.; et al. Subchronic effects of dietary selenium yeast and selenite on growth performance and the immune and antioxidant systems in Nile tilapia Oreochromis niloticus. Fish Shellfish Immunol. 2020, 97, 283–293. [Google Scholar] [CrossRef]
- Tarze, A.; Dauplais, M.; Grigoras, I.; Lazard, M.; Ha-Duong, N.T.; Barbier, F.; Blanquet, S.; Plateau, P. Extracellular production of hydrogen selenide accounts for thiol-assisted toxicity of selenite against Saccharomyces cerevisiae. J. Biol. Chem. 2007, 282, 8759–8767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondaparthi, P.; Deore, M.; Naqvi, S.; Flora, S.J.S. Dose-dependent hepatic toxicity and oxidative stress on exposure to nano and bulk selenium in mice. Environ. Sci. Pollut. Res. Int. 2021, 1–11. [Google Scholar] [CrossRef]
- EEFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Scientific opinion on the safety of selenite triglycerides as a source of selenium added for nutritional purposes to food supplements. EFSA J. 2020, 18, e06134. [Google Scholar] [CrossRef] [PubMed]
- IoM, Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; Institute of Medicine: Washington, DC, USA, 2000.
- WHO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- NNR Working Group. Nordic Nutrition Recommendations 2012, 5th ed.; Nordic Council of Ministers: Copenhagen, Denmark, 2014. [Google Scholar]
- Dauplais, M.; Mahou, P.; Plateau, P.; Lazard, M. Exposure to the methylselenol precursor dimethyldiselenide induces a reductive endoplasmic reticulum stress in Saccharomyces cerevisiae. Int. J. Mol. Sci. 2021, 22, 5467. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Lazard, M.; Dauplais, M.; Blanquet, S.; Plateau, P. Recent advances in the mechanism of selenoamino acids toxicity in eukaryotic cells. Biomol. Concepts 2017, 8, 93–104. [Google Scholar] [CrossRef]
- Vamanu, E.; Rai, S.N. The link between obesity, microbiota dysbiosis, and neurodegenerative pathogenesis. Diseases 2021, 9, 45. [Google Scholar] [CrossRef]
- Pereira, A.M.; Pinna, C.; Biagi, G.; Stefanelli, C.; Maia, M.R.G.; Matos, E.; Segundo, M.A.; Fonseca, A.J.M.; Cabrita, A.R.J. Supplemental selenium source on gut health: Insights on fecal microbiome and fermentation products of growing puppies. FEMS Microbiol. Ecol. 2020, 96, fiaa212. [Google Scholar] [CrossRef]
- Shen, Y.; Laue, H.; Shrubsole, M.; Wu, H.; Bloomquist, T.; Larouche, A.; Zhao, K.; Gao, F.; Boivin, A.; Prada, D.; et al. Association of childhood and perinatal blood metals with children gut microbiome in a Canadian gestation cohort. In International Society of Environmental Epidemiology (ISEE 2021) Abstract Book; Environmental Health Perspectives 2021. Available online: https://ehp.niehs.nih.gov/doi/10.1289/isee.2021.O-LT-045 (accessed on 16 September 2021).


| Characteristics | All | Men | Women | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | Urinary Se (µg/L) | Dietary Se (µg/day) | Serum Se (µg/day) | N | % | Urinary Se (µg/L) | Dietary Se (µg/day) | Serum Se (µg/day) | N | % | Urinary Se (µg/L) | Dietary Se (µg/day) | Serum Se (µg/day) | |
| Overall | 137 | 100 | 26.77 | 84.09 | 117.44 | 62 | 45.3 | 29.00 | 89.97 | 119.22 | 75 | 54.7 | 24.92 | 79.23 | 115.80 |
| Age | |||||||||||||||
| <50 years | 80 | 58.4 | 27.23 | 86.13 | 116.75 | 39 | 62.9 | 30.17 | 90.94 | 119.03 | 41 | 54.7 | 24.44 | 81.55 | 114.47 |
| ≥50 years | 57 | 41.6 | 26.12 | 81.24 | 118.39 | 23 | 37.1 | 27.04 | 88.32 | 119.50 | 34 | 45.3 | 25.50 | 76.44 | 117.46 |
| Body Mass Index | |||||||||||||||
| <25 kg/m2 | 74 | 54.0 | 25.59 | 82.21 | 116.50 | 32 | 51.6 | 28.47 | 91.10 | 116.92 | 42 | 56.0 | 23.39 | 75.44 | 116.13 |
| ≥25 kg/m2–<30 kg/m2 | 50 | 36.5 | 28.58 | 84.22 | 119.85 | 27 | 43.6 | 29.49 | 87.04 | 122.14 | 23 | 30.7 | 27.52 | 80.92 | 117.06 |
| ≥30 kg/m2 | 13 | 9.5 | 26.52 | 94.31 | 112.00 | 3 | 4.8 | 30.40 | 104.27 | 117.00 | 10 | 13.3 | 25.36 | 91.32 | 110.33 |
| Smoking habits | |||||||||||||||
| Never | 101 | 73.7 | 26.10 | 83.95 | 117.49 | 45 | 72.6 | 28.77 | 88.60 | 118.94 | 56 | 74.7 | 23.95 | 80.21 | 116.24 |
| Former | 36 | 26.3 | 28.66 | 84.50 | 117.32 | 17 | 27.4 | 29.64 | 93.60 | 119.87 | 19 | 25.3 | 27.79 | 76.36 | 114.38 |
| Selenium supplement users | |||||||||||||||
| No | 94 | 68.6 | 25.06 | 87.56 | 117.27 | 46 | 74.2 | 29.80 | 94.65 | 119.03 | 48 | 64.0 | 22.46 | 80.76 | 115.42 |
| Yes | 23 | 16.8 | 29.08 | 80.14 | 118.69 | 6 | 9.7 | 27.75 | 72.37 | 122.00 | 17 | 22.7 | 29.55 | 82.88 | 117.22 |
| Former | 20 | 14.6 | 27.48 | 72.34 | 117.23 | 10 | 16.1 | 26.11 | 78.97 | 118.67 | 10 | 13.3 | 28.85 | 65.71 | 116.00 |
| Marital status | |||||||||||||||
| Married/unmarried partner | 97 | 70.8 | 26.78 | 83.10 | 116.68 | 44 | 71.0 | 29.72 | 87.69 | 117.77 | 53 | 70.7 | 24.34 | 79.28 | 115.71 |
| Single | 26 | 19.0 | 27.65 | 87.72 | 119.25 | 12 | 19.4 | 28.80 | 104.28 | 121.80 | 14 | 18.7 | 26.66 | 73.52 | 116.70 |
| Separated/divorced | 14 | 10.2 | 25.07 | 84.26 | 119.00 | 6 | 9.6 | 24.20 | 78.05 | 123.17 | 8 | 10.7 | 25.73 | 88.91 | 114.83 |
| Educational level | |||||||||||||||
| Elementary school | 2 | 1.5 | 37.26 | 146.96 | 131.50 | 2 | 3.2 | 37.26 | 146.96 | 131.50 | - | - | - | - | - |
| Middle school | 20 | 14.6 | 25.99 | 84.79 | 120.07 | 8 | 12.9 | 29.46 | 80.08 | 126.14 | 12 | 16.0 | 23.67 | 87.92 | 114.75 |
| High school | 66 | 48.2 | 23.87 | 82.73 | 116.20 | 28 | 45.2 | 24.98 | 90.92 | 114.20 | 38 | 50.7 | 23.05 | 76.70 | 117.93 |
| College or more | 49 | 35.8 | 30.57 | 83.07 | 117.43 | 24 | 38.7 | 32.87 | 87.40 | 122.50 | 25 | 33.3 | 28.37 | 78.92 | 112.65 |
| Occupation | |||||||||||||||
| Managers | 9 | 6.6 | 21.30 | 84.83 | 118.58 | 6 | 9.7 | 21.42 | 80.45 | 117.51 | 3 | 4.0 | 21.04 | 93.60 | 120.00 |
| Professionals | 26 | 19.0 | 30.16 | 91.07 | 119.21 | 12 | 19.4 | 33.81 | 104.05 | 130.11 | 14 | 18.7 | 27.03 | 79.93 | 109.40 |
| Technicians/associate professionals | 21 | 15.3 | 25.96 | 82.74 | 115.00 | 11 | 17.7 | 23.41 | 89.03 | 116.89 | 10 | 13.3 | 28.75 | 75.82 | 112.57 |
| Clerical support workers | 43 | 31.4 | 25.78 | 79.34 | 115.48 | 12 | 19.4 | 32.10 | 82.18 | 104.50 | 31 | 41.3 | 22.99 | 78.24 | 119.00 |
| Service and sales workers | 11 | 8.0 | 26.65 | 71.98 | 111.33 | 2 | 3.2 | 22.71 | 81.07 | 108.00 | 9 | 12.0 | 27.52 | 69.96 | 113.00 |
| Craft and related trade workers | 10 | 7.3 | 22.16 | 80.73 | 114.60 | 8 | 12.9 | 24.80 | 82.40 | 114.63 | 2 | 2.7 | 11.61 | 74.09 | 114.50 |
| Plant and machine operators | 11 | 8.0 | 35.63 | 94.40 | 122.63 | 8 | 12.9 | 36.22 | 100.51 | 126.29 | 3 | 4.0 | 34.05 | 78.12 | 97.00 |
| Elementary occupations | 6 | 4.4 | 21.92 | 100.44 | 134.60 | 3 | 4.8 | 25.78 | 85.25 | 138.33 | 3 | 4.0 | 18.07 | 115.63 | 129.00 |
| All (n = 137) | Men (n = 62) | Women (n = 75) | ||||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| ALT (U/L) | 27 | 22–35 | 30 | 26–43 | 24 | 20–29 |
| Dietary Se intake (µg/day) | 78.74 | 62.62–101.48 | 88.37 | 69.77–108.28 | 71.06 | 54.77–91.68 |
| Urinary Se concentration (µg/L) | 22.02 | 14.64–37.15 | 24.21 | 16.72–39.20 | 21.30 | 13.30–34.66 |
| Total serum Se concentration (µg/L) 1 | 116.50 | 106.00–128.00 | 118.00 | 109.00–132.00 | 115.00 | 105.00–125.00 |
| All (n = 104) | Men (n = 50) | Women (n = 54) | ||||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| Organic Se (µg/L) | 102.24 | 84.53–113.86 | 100.56 | 79.28–117.28 | 102.58 | 87.24–112.13 |
| SELENOP (µg/L) | 84.81 | 64.23–104.05 | 79.44 | 59.88–105.03 | 86.02 | 72.48–101.65 |
| Se-GPX (µg/L) | 5.46 | 2.12–8.88 | 3.72 | 1.47–9.03 | 5.74 | 2.45–8.16 |
| Se-TXNRD (µg/L) | 0.40 | 0.03–1.51 | 0.46 | 0.03–1.66 | 0.12 | 0.03–1.31 |
| Se-Met (µg/L) | 2.74 | 1.41–5.23 | 2.81 | 1.63–5.10 | 2.55 | 1.23–5.67 |
| Se-Cys (µg/L) | 1.91 | 0.45–3.61 | 1.93 | 0.42–4.06 | 1.91 | 0.49–3.43 |
| Inorganic Se (µg/L) | 9.67 | 3.73–22.94 | 10.02 | 3.66–28.70 | 8.74 | 3.74–19.28 |
| Se (IV) (µg/L) | 4.37 | 1.57–13.83 | 4.38 | 1.21–19.09 | 4.37 | 1.77–11.95 |
| Se (VI) (µg/L) | 3.30 | 1.01–8.17 | 3.85 | 0.97–9.46 | 3.07 | 1.04–7.61 |
| Se-HSA (µg/L) | 1.12 | 0.03–3.06 | 1.06 | 0.03–4.02 | 1.24 | 0.03–2.85 |
| Selenium Biomarkers | Crude | Adjusted | ||
|---|---|---|---|---|
| β | (95% CI) | β | (95% CI) | |
| Urinary Se concentration (µg/L) | 0.14 | (0.03, 0.26) | 0.11 | (0.003, 0.21) |
| Dietary Se intake (µg/day) | 0.07 | (0.004, 0.13) | 0.03 | (−0.02, 0.09) |
| Total serum Se concentration (µg/L) 1 | −0.04 | (−0.16, 0.09) | −0.04 | (−0.16, 0.08) |
| Organic Se (µg/L) | −0.02 | (−0.09, 0.06) | 0.01 | (−0.06, 0.08) |
| SELENOP (µg/L) | −0.02 | (−0.09, 0.05) | 0.01 | (−0.06, 0.07) |
| Se-GPX (µg/L) | 0.06 | (−0.33, 0.45) | 0.09 | (−0.29, 0.47) |
| Se-TXNRD (µg/L) 2 | 0.17 | (−1.22, 1.57) | −0.24 | (−1.55, 1.07) |
| Se-Met (µg/L) 2 | 0.03 | (−0.46, 0.51) | 0.04 | (−0.41, 0.49) |
| Se-Cys (µg/L) 2 | 0.30 | (−0.57, 1.18) | 0.27 | (−0.55, 1.10) |
| Inorganic Se (µg/L) | −0.001 | (−0.10, 0.10) | −0.02 | (−0.11, 0.07) |
| Se (IV) (µg/L) 2 | 0.03 | (−0.12, 0.19) | −0.002 | (−0.15, 0.14) |
| Se (VI) (µg/L) 2 | −0.14 | (−0.37, 0.10) | −0.14 | (−0.36, 0.08) |
| Se-HSA (µg/L) 2 | −0.54 | (−1.13, 0.06) | −0.56 | (−1.11, −0.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbano, T.; Filippini, T.; Lasagni, D.; De Luca, T.; Grill, P.; Sucato, S.; Polledri, E.; Djeukeu Noumbi, G.; Malavolti, M.; Santachiara, A.; et al. Association of Urinary and Dietary Selenium and of Serum Selenium Species with Serum Alanine Aminotransferase in a Healthy Italian Population. Antioxidants 2021, 10, 1516. https://doi.org/10.3390/antiox10101516
Urbano T, Filippini T, Lasagni D, De Luca T, Grill P, Sucato S, Polledri E, Djeukeu Noumbi G, Malavolti M, Santachiara A, et al. Association of Urinary and Dietary Selenium and of Serum Selenium Species with Serum Alanine Aminotransferase in a Healthy Italian Population. Antioxidants. 2021; 10(10):1516. https://doi.org/10.3390/antiox10101516
Chicago/Turabian StyleUrbano, Teresa, Tommaso Filippini, Daniela Lasagni, Tiziana De Luca, Peter Grill, Sabrina Sucato, Elisa Polledri, Guy Djeukeu Noumbi, Marcella Malavolti, Annalisa Santachiara, and et al. 2021. "Association of Urinary and Dietary Selenium and of Serum Selenium Species with Serum Alanine Aminotransferase in a Healthy Italian Population" Antioxidants 10, no. 10: 1516. https://doi.org/10.3390/antiox10101516
APA StyleUrbano, T., Filippini, T., Lasagni, D., De Luca, T., Grill, P., Sucato, S., Polledri, E., Djeukeu Noumbi, G., Malavolti, M., Santachiara, A., Pertinhez, T. A., Baricchi, R., Fustinoni, S., Michalke, B., & Vinceti, M. (2021). Association of Urinary and Dietary Selenium and of Serum Selenium Species with Serum Alanine Aminotransferase in a Healthy Italian Population. Antioxidants, 10(10), 1516. https://doi.org/10.3390/antiox10101516








