Spermidine Induces Expression of Stress Associated Proteins (SAPs) Genes and Protects Rice Seed from Heat Stress-Induced Damage during Grain-Filling
Abstract
:1. Introduction
2. Materials and Methods
2.1. High-Temperature Treatment during Rice Development
2.2. OsSAP5 Genetic Transformation
2.3. Function Verification of OsSAP5 and Spd
2.4. Seed Germination Assay
2.5. Polyamines Measurement
2.6. Malondialdehyde Content
2.7. RNA Isolation and qRT-PCR
2.8. Statistical Analysis
3. Results
3.1. Effects of Exogenous Spermidine on Rice Seed Quality under High Temperature during Early Seed Filling Stage
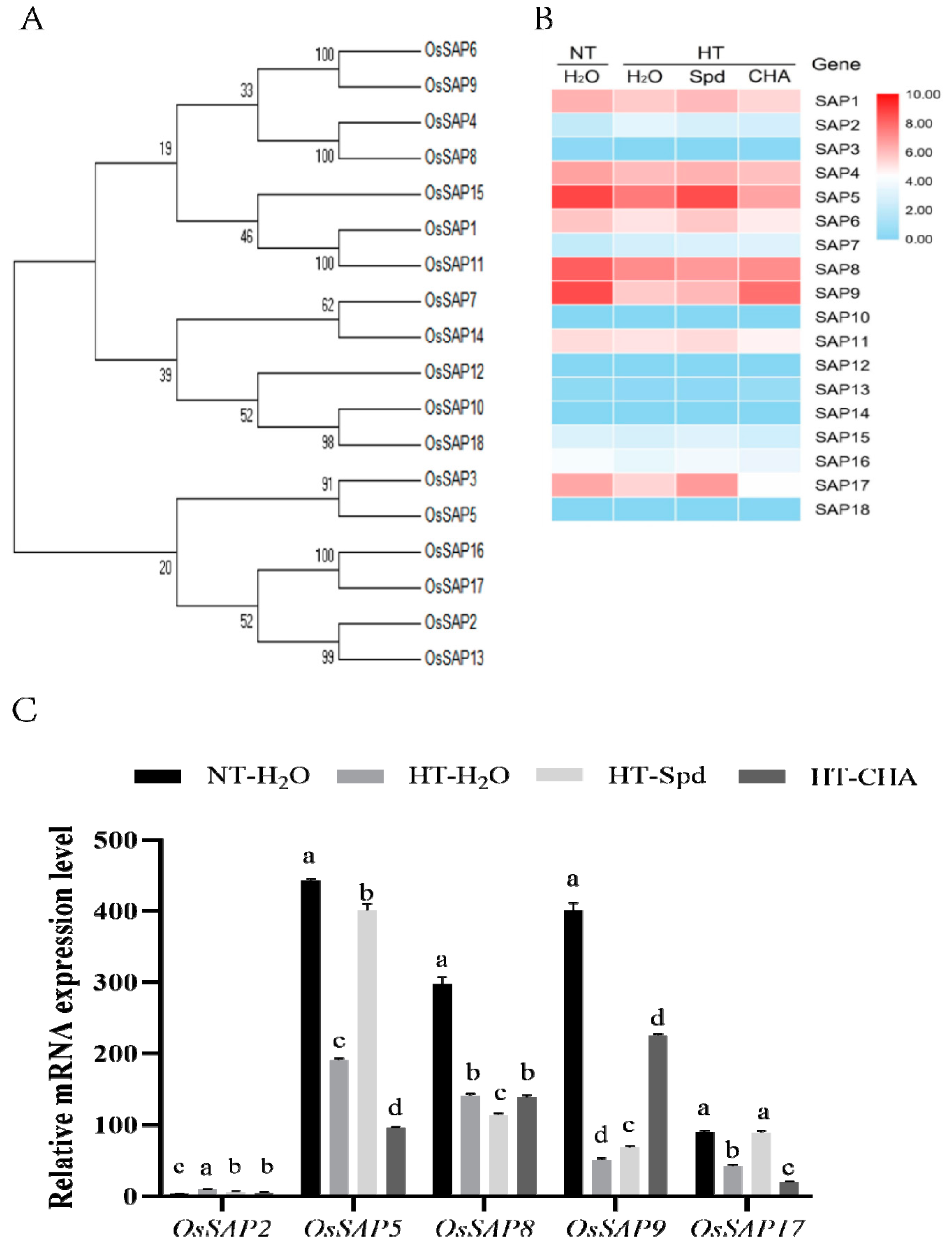
3.2. Expression Analysis of SAP Gene Family in Rice Treated by Spd under High Temperature during the Grain Early Filling Stage
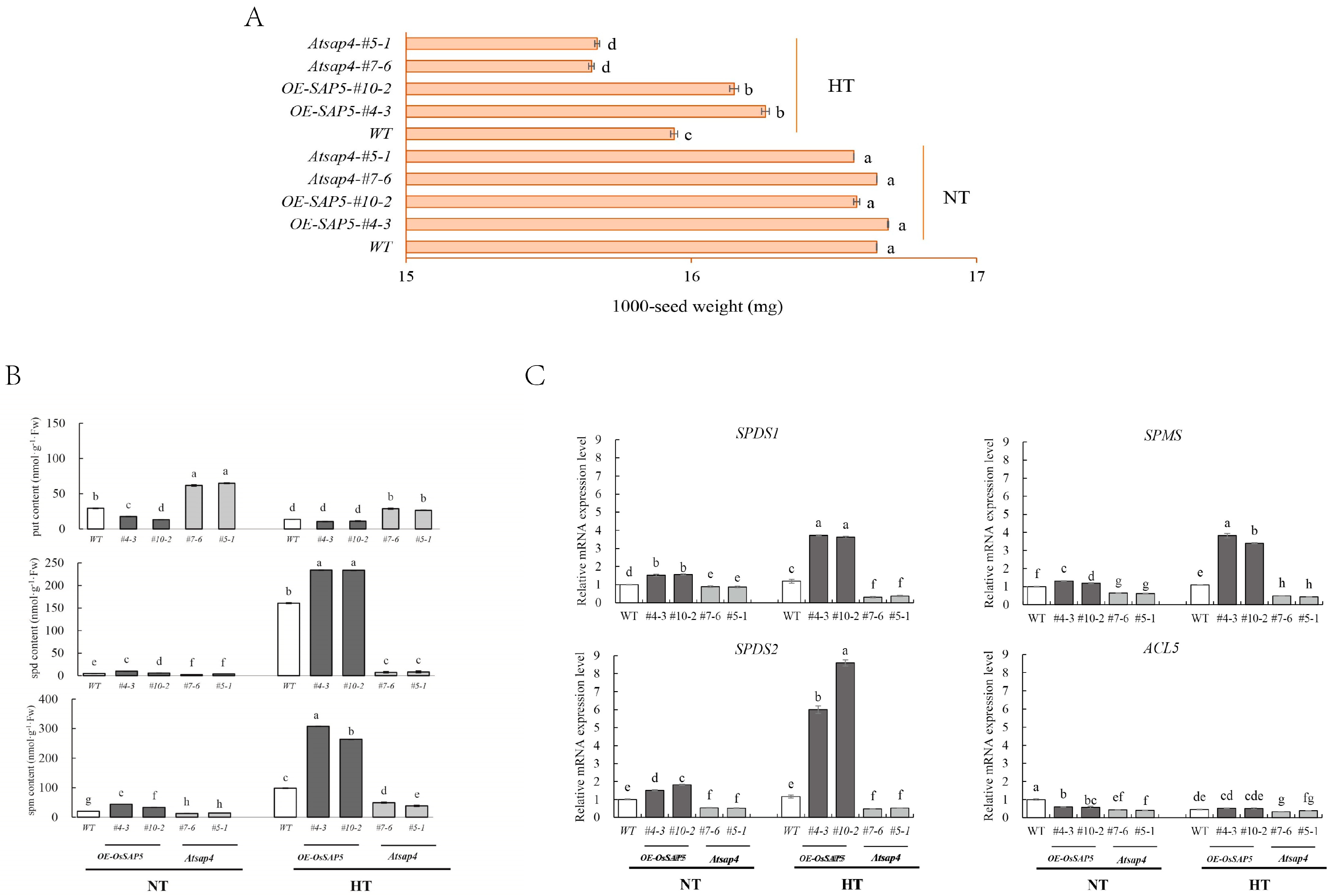
3.3. Over-Expression of OsSAP5 Enhanced the Heat Resistance of Arabidopsis Thaliana Seeds during Development
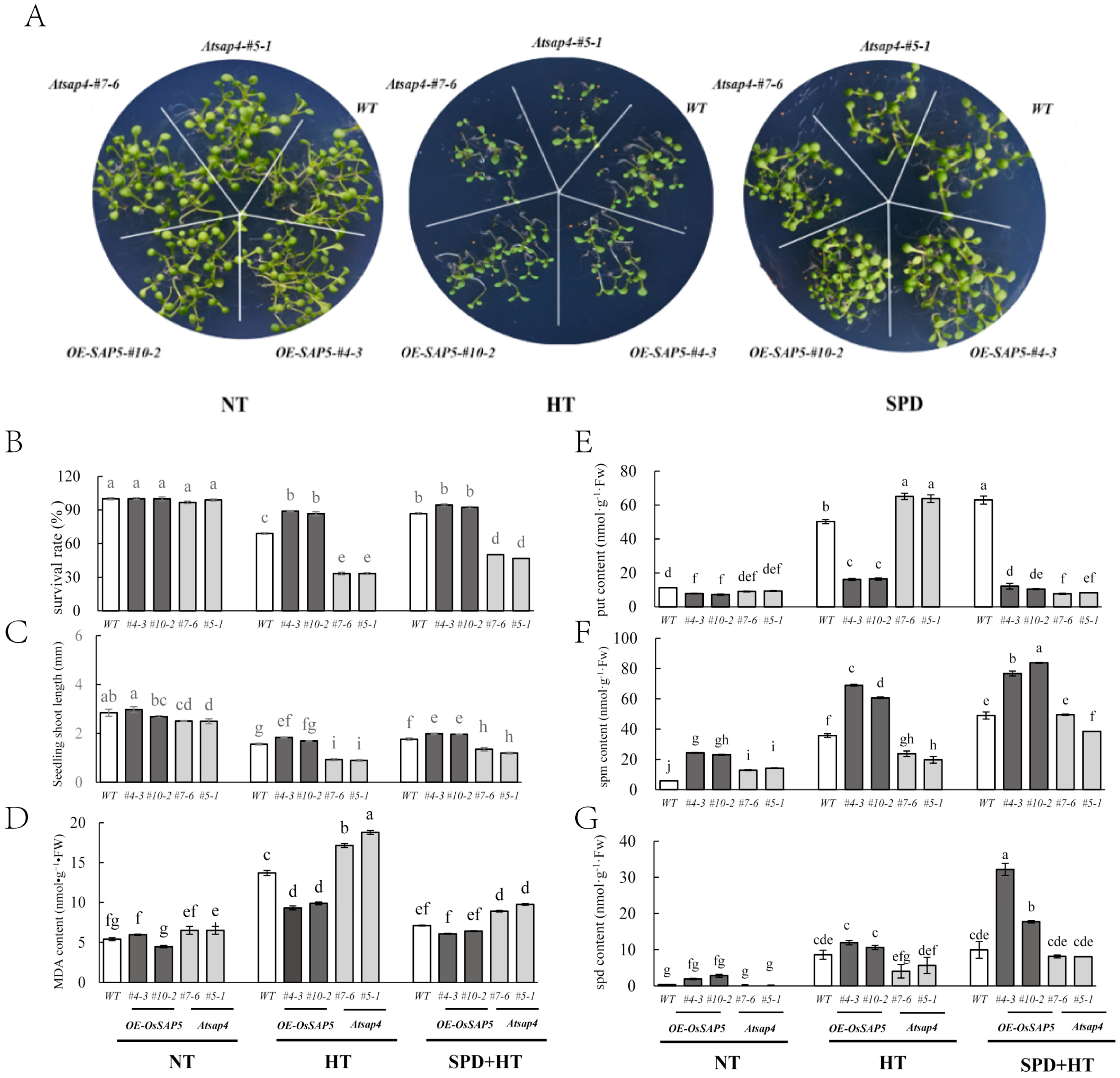
3.4. Spd Ameliorated the Heat Damage during the Germination Period of OE-SAP5 and Atsap4
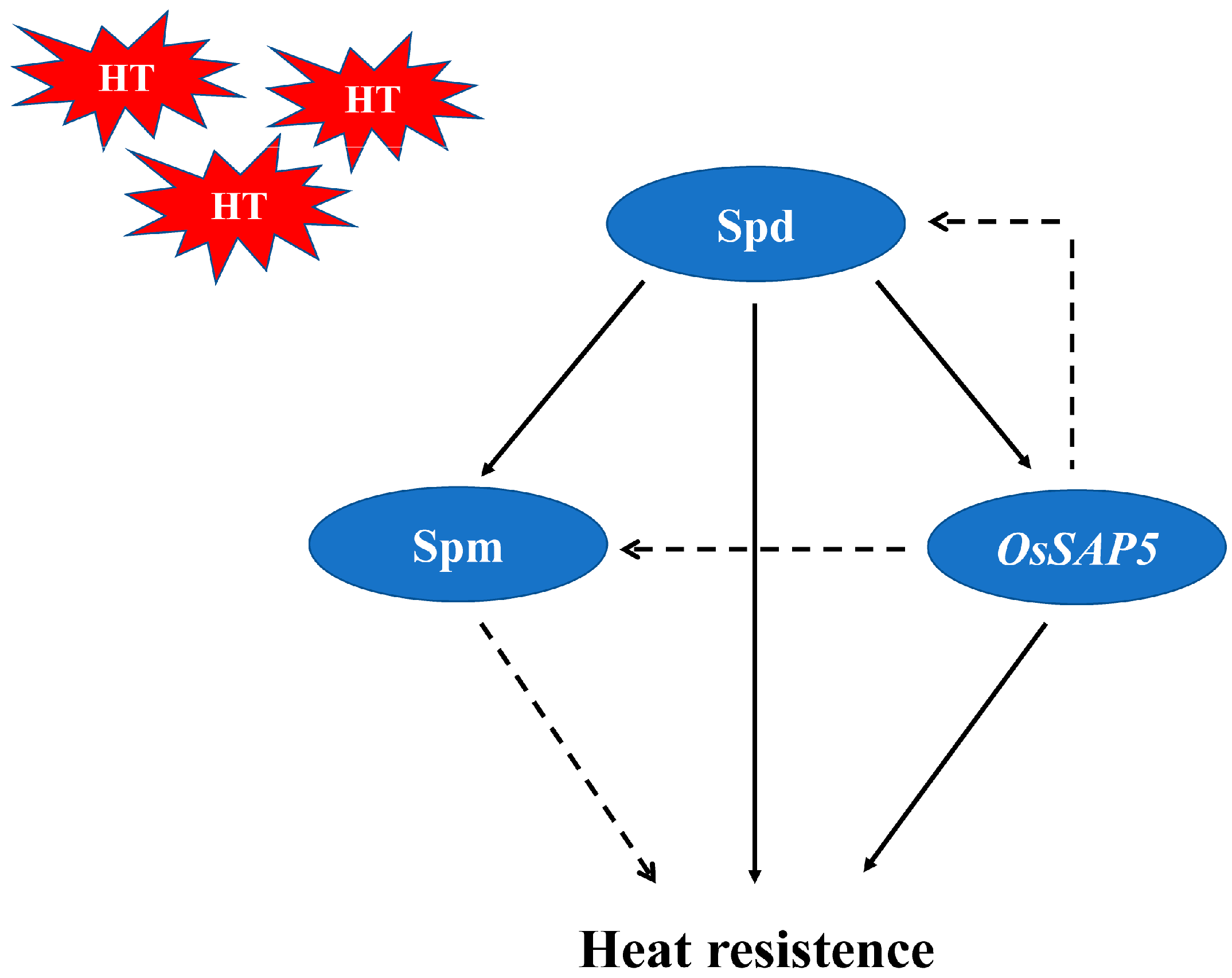
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.M.; Chao, D.Y.; Wu, Y.; Huang, X.H.; Chen, K.; Cui, L.G.; Su, L.; Ye, W.W.; Chen, H.; Chen, H.C.; et al. Natural alleles of a proteasome alpha 2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat. Genet. 2015, 47, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Butardo, V.M.; Misra, G.; Cuevas, R.P.; Anacleto, R.; Kishor, P.B.K. Designing climate-resilient rice with ideal grain quality suited for high-temperature stress. J. Exp. Bot. 2015, 66, 1737–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Storme, N.; Geelen, D. The impact of environmental stress on male reproductive development in plants: Biological processes and molecular mechanisms. Plant. Cell. Environ. 2014, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, I.F. Interaction between drought and chronic high temperature during kernel filling in wheat in a controlled environment. Ann. Bot. 2002, 90, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altenbach, S.B.; DuPont, F.M.; Kothari, K.M.; Chan, R.; Johnson, E.L.; Lieu, D. Temperature, water and fertilizer influence the timing of key events during grain development in a US spring wheat. J. Cereal. Sci. 2003, 37, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G.J.P.N.A.S.U.S.A. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.Y.; Gu, Q.Q.; Dong, Q.; Zhang, Z.H.; Lin, C.; Hu, W.M.; Pan, R.H.; Guan, Y.J.; Hu, J. Spermidine enhances heat tolerance of rice seeds by modulating endogenous starch and polyamine metabolism. Molecules 2019, 24, 1395–1409. [Google Scholar] [CrossRef] [Green Version]
- Sagor, G.H.M.; Berberich, T.; Takahashi, Y.; Niitsu, M.; Kusano, T. The polyamine spermine protects Arabidopsis from heat stress-induced damage by increasing expression of heat shock-related genes. Transgenic Res. 2013, 22, 595–605. [Google Scholar] [CrossRef]
- Jing, J.G.; Guo, S.Y.; Li, Y.F.; Li, W.H. The alleviating effect of exogenous polyamines on heat stress susceptibility of different heat resistant wheat (Triticum aestivum L.) varieties. Sci. Rep. 2020, 10, 7467. [Google Scholar] [CrossRef]
- Luo, L.; Li, Z.; Tang, M.Y.; Cheng, B.Z.; Zeng, W.H.; Peng, Y.; Nie, G.; Zhang, X.Q. Metabolic regulation of polyamines and gamma-aminobutyric acid in relation to spermidine-induced heat tolerance in white clover. Plant. Biol. 2020, 22, 794–804. [Google Scholar] [CrossRef]
- Zhou, R.; Hu, Q.J.; Pu, Q.; Chen, M.X.; Zhu, X.R.; Gao, C.; Zhou, G.X.; Liu, L.J.; Wang, Z.Q.; Yang, J.C.; et al. Spermidine enhanced free polyamine levels and expression of polyamine biosynthesis enzyme gene in rice spikelets under heat tolerance before heading. Sci. Rep. 2020, 10, 8976. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Lin, C.; He, F.; Li, Z.; Guan, Y.J.; Hu, Q.J.; Hu, J. Exogenous spermidine improves seed germination of sweet corn via involvement in phytohormone interactions, H2O2 and relevant gene expression. BMC Plant. Biol. 2017, 17, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.C.; Zhu, Q.; Wang, Z.; Cao, X.Z. Polyamines in rice grains and their relations with grain filling. Chin. Rice Res. Newsl. 1996, 4, 4–5. [Google Scholar]
- Cao, D.D.; Hu, J.; Zhu, S.J.; Hu, W.M.; Knapp, A. Relationship between changes in endogenous polyamines and seed quality during development of sh2 sweet corn (Zea mays L.) seed. Sci. Hortic. 2010, 123, 301–307. [Google Scholar] [CrossRef]
- Imai, A.; Matsuyama, T.; Hanzawa, Y.; Akiyama, T.; Tamaoki, M.; Saji, H.; Shirano, Y.; Kato, T.; Hayashi, H.; Shibata, D.; et al. Spermidine synthase genes are essential for survival of Arabidopsis. Plant. Physiol. 2004, 135, 1565–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choubey, A.; Rajam, M.V. RNAi-mediated silencing of spermidine synthase gene results in reduced reproductive potential in tobacco. Physiol. Mol. Biol. 2018, 24, 1069–1081. [Google Scholar] [CrossRef]
- Li, C.J.; Han, Y.Y.; Hao, J.H.; Qin, X.X.; Liu, C.J.; Fan, S.X. Effects of exogenous spermidine on antioxidants and glyoxalase system of lettuce seedlings under high temperature. Plant. Signal. Behav. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Vij, S.; Tyagi, A.K. Genome-wide analysis of the stress associated protein (SAP) gene family containing A20/AN1 zinc-finger(s) in rice and their phylogenetic relationship with Arabidopsis. Mol. Genet. Genom. 2006, 276, 565–575. [Google Scholar] [CrossRef]
- He, X.; Xie, S.; Xie, P.; Yao, M.; Liu, W.; Qin, L.W.; Liu, Z.S.; Zheng, M.; Liu, H.F.; Guan, M.; et al. Genome-wide identification of stress-associated proteins (SAP) with A20/AN1 zinc finger domains associated with abiotic stresses responses in Brassica napus. Environ. Exp. Bot. 2019, 165, 108–119. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Vij, S.; Tyagi, A.K. Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proc. Natl. Acad. Sci. USA 2004, 101, 6309–6314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozain, M.; Abdelmageed, H.; Lee, J.; Kang, M.; Fokar, M.; Allen, R.D.; Holaday, A.S. Expression of AtSAP5 in cotton up-regulates putative stress-responsive genes and improves the tolerance to rapidly developing water deficit and moderate heat stress. J. Plant. Physiol. 2012, 169, 1261–1270. [Google Scholar] [CrossRef]
- Kim, G.D.; Cho, Y.H.; Yoo, S.D. Regulatory functions of evolutionarily conserved AN1/A20-like Zinc finger family proteins in Arabidopsis stress responses under high temperature. Biochem. Bioph. Res. 2015, 457, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Charrier, A.; Planchet, E.; Cerveau, D.; Gimeno-Gilles, C.; Verdu, I.; Limami, A.M.; Lelièvre, E.J.P. Overexpression of a Medicago truncatula stress-associated protein gene (MtSAP1) leads to nitric oxide accumulation and confers osmotic and salt stress tolerance in transgenic tobacco. Planta 2012, 236, 567–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.Y.; Fokar, M.; Abdelmageed, H.; Allen, R.D. Arabidopsis SAP5 functions as a positive regulator of stress responses and exhibits E3 ubiquitin ligase activity. Plant. Mol. Biol. 2011, 75, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yin, Y.J.; Liu, X.Y.; Tong, S.M.; Xing, J.W.; Zhang, Y.; Pudake, R.N.; Izquierdo, E.M.; Peng, H.R.; Xin, M.M.; et al. The E3 ligase TaSAP5 alters drought stress responses by promoting the degradation of DRIP proteins. Plant. Physiol. 2017, 175, 1878–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben, S.R.; Ben, R.W.; Zouari, N.; Azaza, J.; Mieulet, D.; Verdeil, J.L.; Guiderdoni, E.; Hassairi, A. Promoter of the AlSAP gene from the halophyte grass Aeluropus littoralis directs developmental-regulated, stress-inducible, and organ-specific gene expression in transgenic tobacco. Transgenic Res. 2011, 20, 1003–1018. [Google Scholar] [CrossRef]
- Hanfrey, C.; Sommer, S.; Mayer, M.J.; Burtin, D.; Michael, A.J. Arabidopsis polyamine biosynthesis: Absence of ornithine decarboxylase and the mechanism of arginine decarboxylase activity. Plant. J. 2001, 27, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Tun, N.N.; Santa-Catarina, C.; Begum, T.; Silveira, V.; Handro, W.; Floh, E.I.; Scherer, G.F. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 2006, 47, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Cohen, M.F. NO signal at the crossroads: Polyamine-induced nitric oxide synthesis in plants? Trends Plant Sci. 2006, 11, 522–524. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Jiang, M.Y.; Zhang, J.H.; Ding, H.D.; Xu, S.C.; Hu, X.L.; Tan, M.P. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol. 2007, 175, 36–50. [Google Scholar] [CrossRef]
- Yang, W.B.; Li, Y.; Yin, Y.P.; Jiang, W.W.; Peng, D.L.; Cui, Z.Y.; Yang, D.Q.; Wang, Z.L. Ethylene and spermidine in wheat grains in relation to starch content and granule size distribution under water deficit. J. Integr. Agric. 2014, 13, 2141–2153. [Google Scholar] [CrossRef] [Green Version]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant. J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.J.; Fu, Y.Y.; Guan, Y.J.; Lin, C.; Cao, D.D.; Hu, W.M.; Sheteiwy, M.; Hu, J. Inhibitory effect of chemical combinations on seed germination and pre-harvest sprouting in hybrid rice. Plant. Growth Regul. 2016, 80, 281–289. [Google Scholar] [CrossRef]
- Gao, C.H.; Hu, J.; Zhang, S.; Zheng, Y.Y.; Knapp, A. Association of polyamines in governing the chilling sensitivity of maize genotypes. Plant. Growth Regul. 2009, 57, 31–38. [Google Scholar] [CrossRef]
- Tyagi, H.; Jha, S.; Sharma, M.; Giri, J.; Tyagi, A.K. Rice SAPs are responsive to multiple biotic stresses and overexpression of OsSAP1, an A20/AN1 zinc-finger protein, enhances the basal resistance against pathogen infection in tobacco. Plant. Sci. 2014, 225, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ubaidillah, M.; Kim, K.A.; Kim, Y.H.; Lee, I.J.; Yun, B.W.; Kim, D.H.; Loake, G.J.; Kim, K.M. Identification of a drought-induced rice gene, OsSAP, that suppresses Bax-induced cell death in yeast. Mol. Biol. Rep. 2013, 40, 6113–6121. [Google Scholar] [CrossRef]
- Kanneganti, V.; Gupta, A.K. Overexpression of OsiSAP8, a member of stress associated protein (SAP) gene family of rice confers tolerance to salt, drought and cold stress in transgenic tobacco and rice. Plant. Mol. Biol. 2008, 66, 445–462. [Google Scholar] [CrossRef]
- Cui, K.; Wang, H.Y.; Li, K.; Liao, S.X.; Li, L.; Zhang, C.H. Physiological and biochemical effects of ultra-dry storage on barbados nut seeds. Crop. Sci. 2014, 54, 1748–1755. [Google Scholar] [CrossRef]
- Diao, Q.N.; Song, Y.J.; Qi, H.Y. Exogenous spermidine enhances chilling tolerance of tomato (Solanum lycopersicum L.) seedlings via involvement in polyamines metabolism and physiological parameter levels. Acta. Physiol. Plant. 2015, 37, 1–15. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M.; Medicine, P. Radical chemistry off flavonoid antioxidants. Adv. Exp. Med. Biol. 1990, 264, 165–170. [Google Scholar]
- Zhang, L.; Hu, T.; Amombo, E.; Wang, G.Y.; Xie, Y.; Fu, J.M. The alleviation of heat damage to photosystem II and enzymatic antioxidants by exogenous spermidine in tall fescue. Front. Plant. Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Wi, S.J.; Kim, W.T.; Park, K.Y. Overexpression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant. Cell. Rep. 2006, 25, 1111–1121. [Google Scholar] [CrossRef]
- Wu, J.Q.; Shu, S.; Li, C.C.; Sun, J.; Guo, S.R. Spermidine-mediated hydrogen peroxide signaling enhances the antioxidant capacity of salt-stressed cucumber roots. Plant. Physiol. Biochem. 2018, 128, 152–162. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, M.; Hidalgo-Castellanos, J.; Muñoz-Sánchez, J.R.; Marin-pena, A.J.; Lluch, C.; Herrera-Cerbera, J.A. Polyamines contribute to salinity tolerance in the symbiosis Medicago truncatula-Sinorhizobium meliloti by preventing oxidative damage. Plant. Physiol. Biochem. 2017, 116, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Villanueva, V.R. Inhibition of polyamine biosynthesis and seed-germination in Picea-abies. Phytochemistry 1992, 31, 3353–3356. [Google Scholar] [CrossRef]
- Saleethong, P.; Sanitchon, J.; Kong-Ngern, K.; Theerakulpisut, P. Effects of exogenous spermidine (Spd) on yield, yield-related parameters and mineral composition of rice (Oryza Sativa L. Ssp. Indica) grains under salt stress. Australian. J. Crop. Sci. 2013, 7, 1293–1301. [Google Scholar]
- Goyal, M.; Asthir, B. Polyamine catabolism influences antioxidative defense mechanism in shoots and roots of five wheat genotypes under high temperature stress. Plant. Growth Regul. 2010, 60, 13–25. [Google Scholar] [CrossRef]
- Lim, S.D.; Cho, H.Y.; Park, Y.C.; Ham, D.J.; Lee, J.K.; Jang, C.S. The rice RING finger E3 ligase, OsHCI1, drives nuclear export of multiple substrate proteins and its heterogeneous overexpression enhances acquired thermotolerance. J. Exp. Bot. 2013, 64, 2899–2914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xu, J.; Liu, X.Y.; Liang, W.X.; Xin, M.M.; Du, J.K.; Hu, Z.R.; Peng, H.R.; Guo, W.L.; Ni, Z.F.; et al. Identification of HSP90C as a substrate of E3 ligase TaSAP5 through ubiquitylome profiling. Plant. Sci. 2019, 287, 1–6. [Google Scholar] [CrossRef]
- Nong, T.H.; Tran, H.T.; Phan, T.; Nakamura, J.; Iwata, T.; Harano, K.; Ishibashi, Y.; Yuasa, T.; Iwayainoue, M.J.A.S. Hsp90 and reactive oxygen species regulate thermotolerance of rice seedlings via induction of heat shock factor A2 (OsHSFA2) and galactinol synthase 1 (OsGolS1). Agric. Sci. 2013, 4, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Scharf, K.D.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Bba-Gene. Regul. Mech. 2012, 1819, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.T.; Tan, D.X.; Reiter, R.J.; Ye, T.T.; Yang, F.; Chan, Z.L. Melatonin induces class A1 heat-shock factors (HSFA1s) and their possible involvement of thermotolerance in Arabidopsis. J. Pineal Res. 2015, 58, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Liao, H.T.; Charng, Y.Y. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant. Cell. Environ. 2011, 34, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.L.; Guo, Q.L.; Li, Q.; Gai, J.Y.; Yang, S.P. Comparative transcriptomics analysis and functional study reveal important role of high-temperature stress response gene GmHSFA2 during flower bud development of CMS-based F1 in soybean. Front. Plant. Sci. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Schramm, F.; Larkindale, J.; Kiehlmann, E.; Ganguli, A.; Englich, G.; Vierling, E.; Koskull-Doring, P. A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant. J. 2008, 53, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.D.; Zhang, M.; Gao, D.J.; Zhou, K.; Tang, S.J.; Zhou, B.; Lv, Y.M. Rice OsHSFA3 gene improves drought tolerance by modulating polyamine biosynthesis depending on abscisic acid and ROS levels. Int. J. Mol. Sci. 2020, 21, 1857. [Google Scholar] [CrossRef] [Green Version]

| Temperature | Treatment | GP (%) | GI | VI | 1000 Grain Weight (g) |
|---|---|---|---|---|---|
| NT | H2O | 85 ± 1.8 a | 6.45 ± 0.32 a | 0.64 ± 0.031 a | 21.23 ± 0.04 a |
| HT | H2O | 59 ± 0.7 b | 3.82 ± 0.09 b | 0.20 ± 0.011 c | 16.78 ± 0.03 c |
| Spd | 87 ± 0.7 a | 6.06 ± 0.12 a | 0.38 ± 0.006 b | 19.38 ± 0.08 b | |
| CHA | 43 ± 0.7 c | 2.48 ± 0.15 c | 0.11 ± 0.008 d | 16.08 ± 0.01 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Fu, Y.; Mou, Q.; An, J.; Zhu, X.; Ahmed, T.; Zhang, S.; Basit, F.; Hu, J.; Guan, Y. Spermidine Induces Expression of Stress Associated Proteins (SAPs) Genes and Protects Rice Seed from Heat Stress-Induced Damage during Grain-Filling. Antioxidants 2021, 10, 1544. https://doi.org/10.3390/antiox10101544
Chen M, Fu Y, Mou Q, An J, Zhu X, Ahmed T, Zhang S, Basit F, Hu J, Guan Y. Spermidine Induces Expression of Stress Associated Proteins (SAPs) Genes and Protects Rice Seed from Heat Stress-Induced Damage during Grain-Filling. Antioxidants. 2021; 10(10):1544. https://doi.org/10.3390/antiox10101544
Chicago/Turabian StyleChen, Min, Yuying Fu, Qingshan Mou, Jianyu An, Xiaobo Zhu, Temoor Ahmed, Sheng Zhang, Farwa Basit, Jin Hu, and Yajing Guan. 2021. "Spermidine Induces Expression of Stress Associated Proteins (SAPs) Genes and Protects Rice Seed from Heat Stress-Induced Damage during Grain-Filling" Antioxidants 10, no. 10: 1544. https://doi.org/10.3390/antiox10101544







