Metabolic and Transcriptional Adaptations Improve Physical Performance of Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Maintenance
2.2. Swim Tunnel
2.3. Experiment Setup
2.4. Feeding
2.5. Determination of Basic Characters
2.6. Training Protocol
2.7. Determination of Blood Glucose
2.8. Glycogen Measurement
2.9. Metabolomics Profile
2.10. Statistics
2.11. RNA-Seq Analysis
3. Results
3.1. Maximum Speed Correlated with Body Length
3.2. Swim Training Improves the Physical Performance of Zebrafish
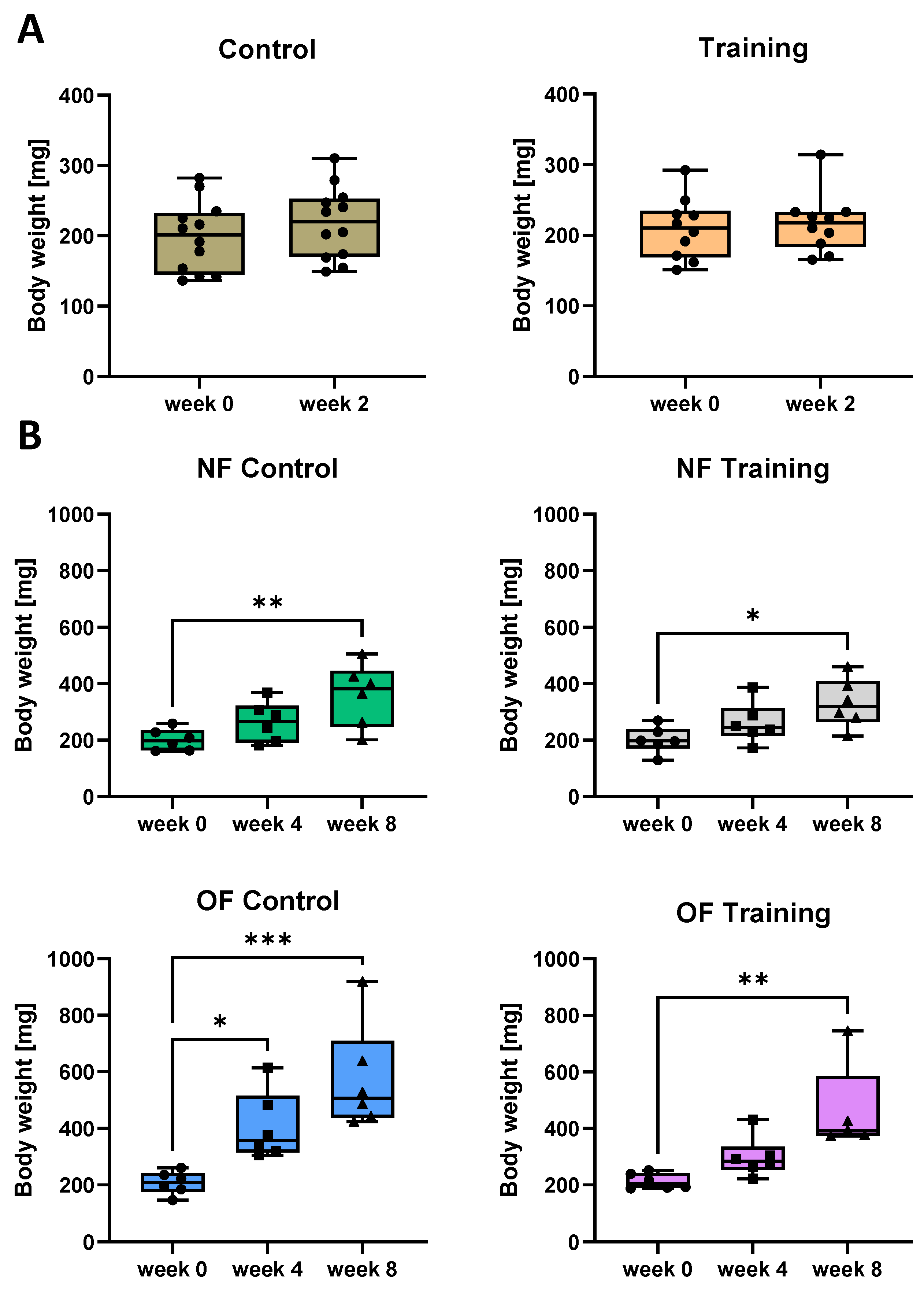
3.3. Swim Training Only Moderately Attenuates Weight Gain in Overfed Zebrafish
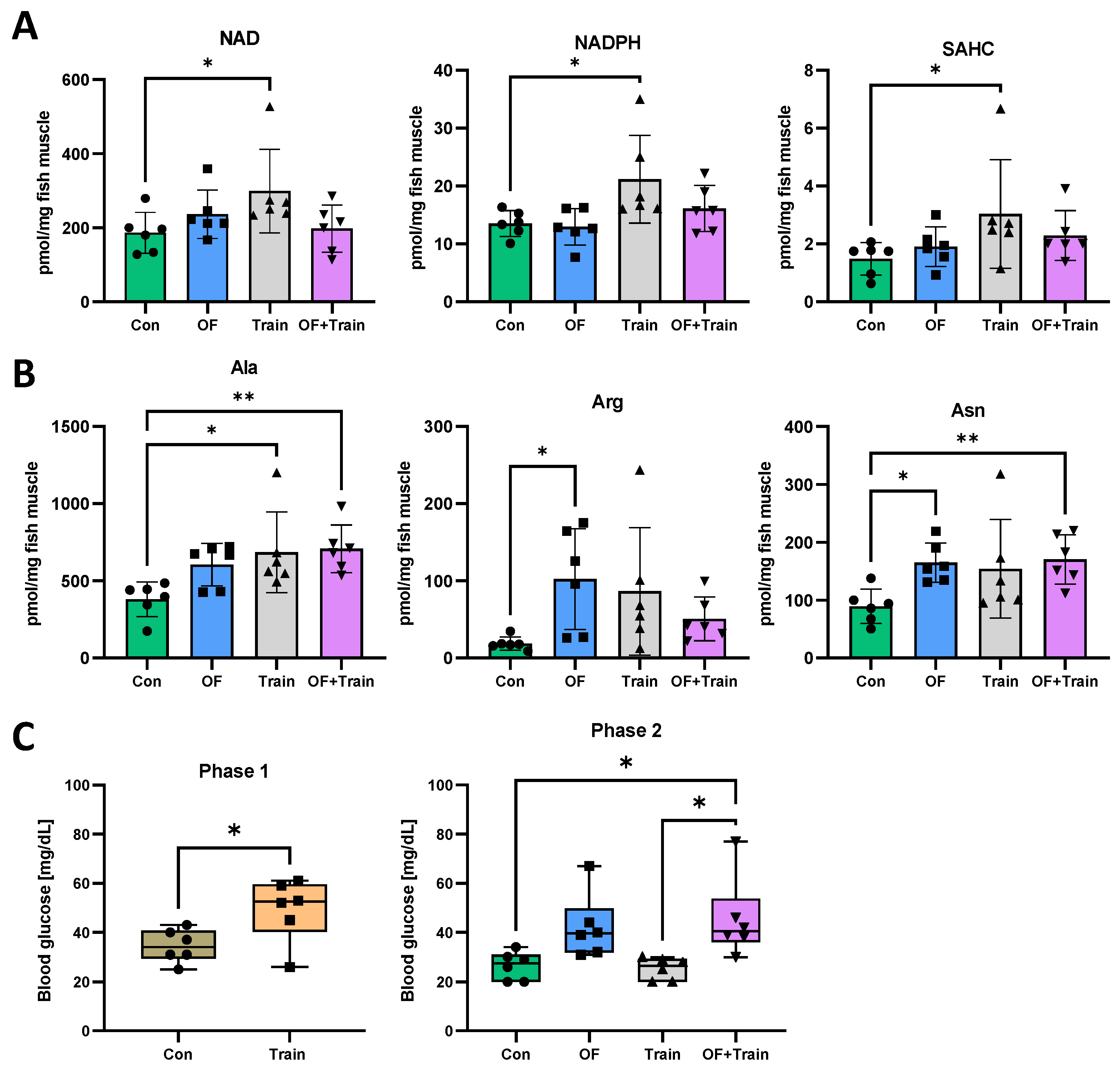
3.4. Swim Training Alters the Metabolome
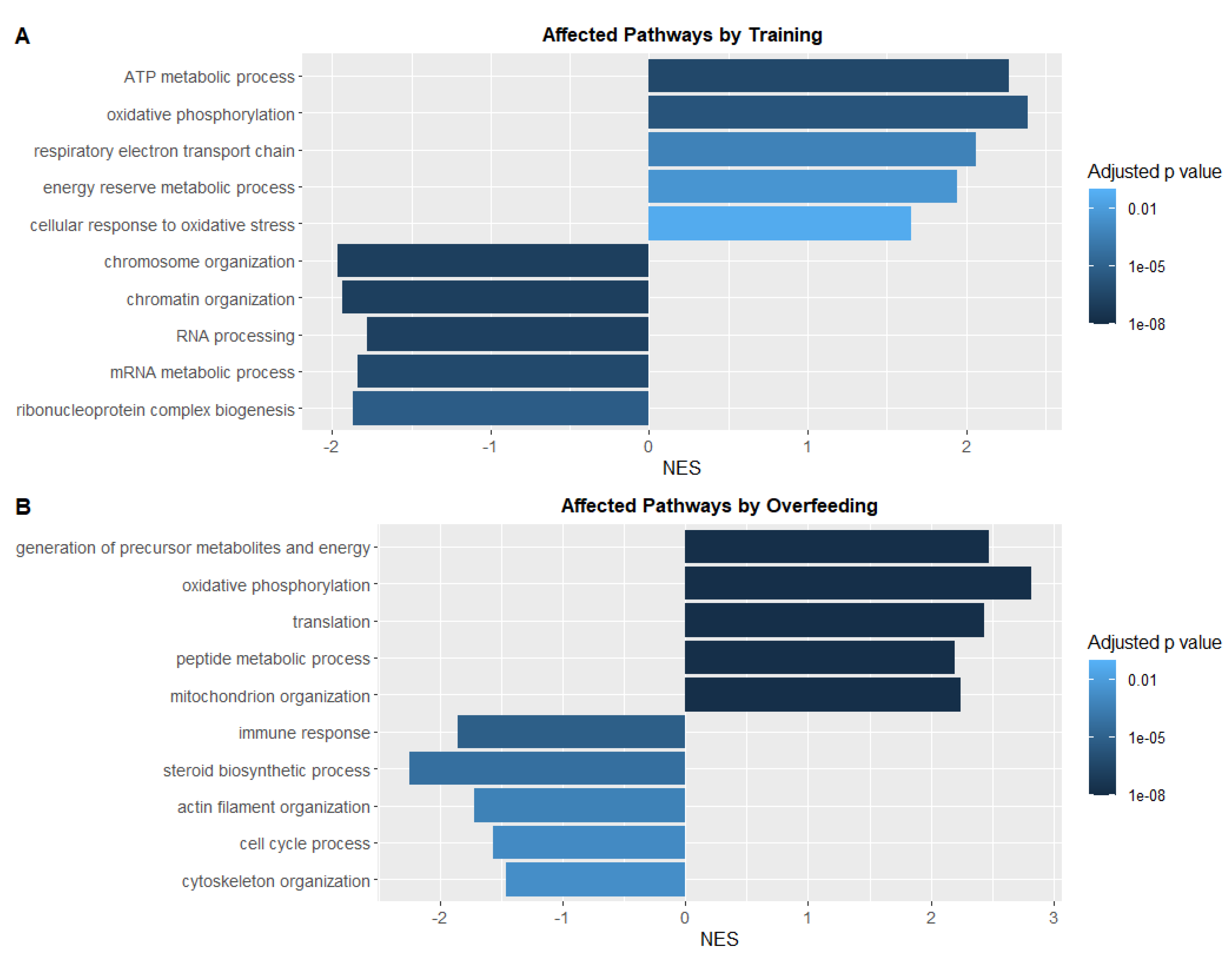
3.5. Swim Training Alters the Transcriptional Profile Linked to Energy Metabolism and Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, W.P.T. The epidemiology of obesity: The size of the problem. J. Intern. Med. 2008, 263, 336–352. [Google Scholar] [CrossRef] [PubMed]
- OECD. Overweight and obesity among adults. In Health at a Glance 2017; OECD Publishing: Paris, France, 2017. [Google Scholar]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Lee, J. Effects of Exercise Interventions on Weight, Body Mass Index, Lean Body Mass and Accumulated Visceral Fat in Overweight and Obese Individuals: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 2635. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, R.; Pierce, N.; Koppe, S. Obesity and nonalcoholic fatty liver disease: Current perspectives. Diabetes Metab. Syndr. Obes. 2018, 11, 533–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherer, P.E.; Hill, J.A. Obesity, Diabetes, and Cardiovascular Diseases: A Compendium. Circ. Res. 2016, 118, 1703–1705. [Google Scholar] [CrossRef] [Green Version]
- Bloomgarden, Z.T. American Diabetes Association Annual Meeting, 1999: Diabetes and obesity. Diabetes Care 2000, 23, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Jakicic, J.M.; Rogers, R.J.; Davis, K.K.; Collins, K.A. Role of Physical Activity and Exercise in Treating Patients with Overweight and Obesity. Clin. Chem. 2018, 64, 99–107. [Google Scholar] [CrossRef]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy Balance and Obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. 3), 1–72. [Google Scholar] [CrossRef] [Green Version]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef] [Green Version]
- Cardiovascular Diseases (CVDs). Available online: http://www.who.int/mediacentre/factsheets/fs317/en/index.html (accessed on 25 August 2021).
- Hannan, M.; Kringle, E.; Hwang, C.-L.; Laddu, D. Behavioral Medicine for Sedentary Behavior, Daily Physical Activity, and Exercise to Prevent Cardiovascular Disease: A Review. Curr. Atheroscler. Rep. 2021, 23, 1–11. [Google Scholar] [CrossRef]
- Brown, B.M.; Peiffer, J.J.; Martins, R.N. Multiple effects of physical activity on molecular and cognitive signs of brain aging: Can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol. Psychiatry 2013, 18, 864–874. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef] [Green Version]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heckler, K.; Kroll, J. Zebrafish as a Model for the Study of Microvascular Complications of Diabetes and Their Mechanisms. Int. J. Mol. Sci. 2017, 18, 2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a Model for Obesity and Diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Rosa, J.G.; Barcellos, H.H.; Idalencio, R.; Marqueze, A.; Fagundes, M.; Rossini, M.; Variani, C.; Balbinoti, F.; Tietböhl, T.M.; Rosemberg, D.B.; et al. Just Keep Swimming: Neuroendocrine, Metabolic, and Behavioral Changes After a Forced Swimming Test in Zebrafish. Zebrafish 2017, 14, 51–59. [Google Scholar] [CrossRef]
- Usui, T.; Noble, D.W.A.; O’Dea, R.E.; Fangmeier, M.L.; Lagisz, M.; Hesselson, D.; Nakagawa, S. The French press: A repeatable and high-throughput approach to exercising zebrafish (Danio rerio). PeerJ 2018, 6, e4292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakamatsu, Y.; Kashima, M.; Hirata, H. A Reproducible Protocol to Measure the Critical Swimming Speed of Adult Zebrafish. Bio-Protocol 2020, 10, e3712. [Google Scholar] [CrossRef] [PubMed]
- van der Meulen, T.; Schipper, H.; van den Boogaart, J.G.; Huising, M.O.; Kranenbarg, S.; van Leeuwen, J.L. Endurance exercise differentially stimulates heart and axial muscle development in zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1040–R1048. [Google Scholar] [CrossRef] [Green Version]
- Palstra, A.P.; Tudorache, C.; Rovira, M.; Brittijn, S.A.; Burgerhout, E.; van den Thillart, G.E.; Spaink, H.P.; Planas, J.V. Establishing zebrafish as a novel exercise model: Swimming economy, swimming-enhanced growth and muscle growth marker gene expression. PLoS ONE 2010, 5, e14483. [Google Scholar] [CrossRef] [Green Version]
- Wakamatsu, Y.; Ogino, K.; Hirata, H. Swimming capability of zebrafish is governed by water temperature, caudal fin length and genetic background. Sci. Rep. 2019, 9, 16307–16308. [Google Scholar] [CrossRef] [PubMed]
- Schmöhl, F.; Peters, V.; Schmitt, C.P.; Poschet, G.; Büttner, M.; Li, X.; Weigand, T.; Poth, T.; Volk, N.; Morgenstern, J.; et al. CNDP1 knockout in zebrafish alters the amino acid metabolism, restrains weight gain, but does not protect from diabetic complications. Cell Mol. Life Sci. 2019, 76, 4551–4568. [Google Scholar] [CrossRef] [PubMed]
- Backman, T.W.H.; Girke, T. systemPipeR: NGS workflow and report generation environment. BMC Bioinform. 2016, 17, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar]
- Carlson, M. org.Dr.eg.db: Genome Wide Annotation for Zebrafish. R Package Version 3.13.0. Available online: http://bioconductor.org/packages/release/data/annotation/html/org.Dr.eg.db.html (accessed on 9 September 2021).
- Kolnes, A.J.; Birk, J.B.; Eilertsen, E.; Stuenæs, J.T.; Wojtaszewski, J.F.; Jensen, J. Epinephrine-stimulated glycogen breakdown activates glycogen synthase and increases insulin-stimulated glucose uptake in epitrochlearis muscles. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E231–E240. [Google Scholar] [CrossRef] [Green Version]
- De Feo, P.; Di Loreto, C.; Lucidi, P.; Murdolo, G.; Parlanti, N.; De Cicco, A.; Piccioni, F.; Santeusanio, F. Metabolic response to exercise. J. Endocrinol. Investig. 2003, 26, 851–854. [Google Scholar] [CrossRef]
- Verdin, E. NAD⁺ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef]
- Murphy, M.P. Mitochondrial thiols in antioxidant protection and redox signaling: Distinct roles for glutathionylation and other thiol modifications. Antioxid. Redox Signal. 2012, 16, 476–495. [Google Scholar] [CrossRef]
- Stehling, O.; Lill, R. The role of mitochondria in cellular iron-sulfur protein biogenesis: Mechanisms, connected processes, and diseases. Cold Spring Harbor Perspect. Biol. 2013, 5, a011312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rennie, M.J.; Tipton, K.D. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu. Rev. Nutr. 2000, 20, 457–483. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Saltin, B.; Graham, T.; Pilegaard, H. Carbohydrate metabolism during prolonged exercise and recovery: Interactions between pyruvate dehydrogenase, fatty acids, and amino acids. J. Appl. Physiol. (1985) 2006, 100, 1822–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [Green Version]
- Srisawat, K.; Shepherd, S.O.; Lisboa, P.J.; Burniston, J.G. A Systematic Review and Meta-Analysis of Proteomics Literature on the Response of Human Skeletal Muscle to Obesity/Type 2 Diabetes Mellitus (T2DM) Versus Exercise Training. Proteomes 2017, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Civitarese, A.E.; Smith, S.R.; Ravussin, E. Diet, energy metabolism and mitochondrial biogenesis. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 679–687. [Google Scholar] [CrossRef]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinkele, F.J.; Lou, B.; Erben, V.; Bennewitz, K.; Poschet, G.; Sticht, C.; Kroll, J. Metabolic and Transcriptional Adaptations Improve Physical Performance of Zebrafish. Antioxidants 2021, 10, 1581. https://doi.org/10.3390/antiox10101581
Heinkele FJ, Lou B, Erben V, Bennewitz K, Poschet G, Sticht C, Kroll J. Metabolic and Transcriptional Adaptations Improve Physical Performance of Zebrafish. Antioxidants. 2021; 10(10):1581. https://doi.org/10.3390/antiox10101581
Chicago/Turabian StyleHeinkele, Franziska J., Bowen Lou, Vanessa Erben, Katrin Bennewitz, Gernot Poschet, Carsten Sticht, and Jens Kroll. 2021. "Metabolic and Transcriptional Adaptations Improve Physical Performance of Zebrafish" Antioxidants 10, no. 10: 1581. https://doi.org/10.3390/antiox10101581
APA StyleHeinkele, F. J., Lou, B., Erben, V., Bennewitz, K., Poschet, G., Sticht, C., & Kroll, J. (2021). Metabolic and Transcriptional Adaptations Improve Physical Performance of Zebrafish. Antioxidants, 10(10), 1581. https://doi.org/10.3390/antiox10101581





