Neuroprotective Activity of Melittin—The Main Component of Bee Venom—Against Oxidative Stress Induced by Aβ25–35 in In Vitro and In Vivo Models
Abstract
- Highlights
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Cell Culturing
2.3. Cell Viability Assay and Drug Administration
2.4. Measurements of In Vitro Cell Apoptosis, Cell ROS, SOD, MDA, LDH, and Protein Carbonyl
2.5. Preparation of Whole Cell, Cytosolic, and Nuclear Proteins
2.6. Animals
2.7. In Vivo Drug Administration
2.8. Morris Water Maze
2.9. Collection of In Vivo Animal Tissues
2.10. Doublecortin Immunofluorescence Staining
2.11. Western Blot Analysis
2.12. Measurements of ROS, NO, MDA, AchE, and Ach Levels in In Vivo Samples
2.13. Statistical Analysis
3. Results
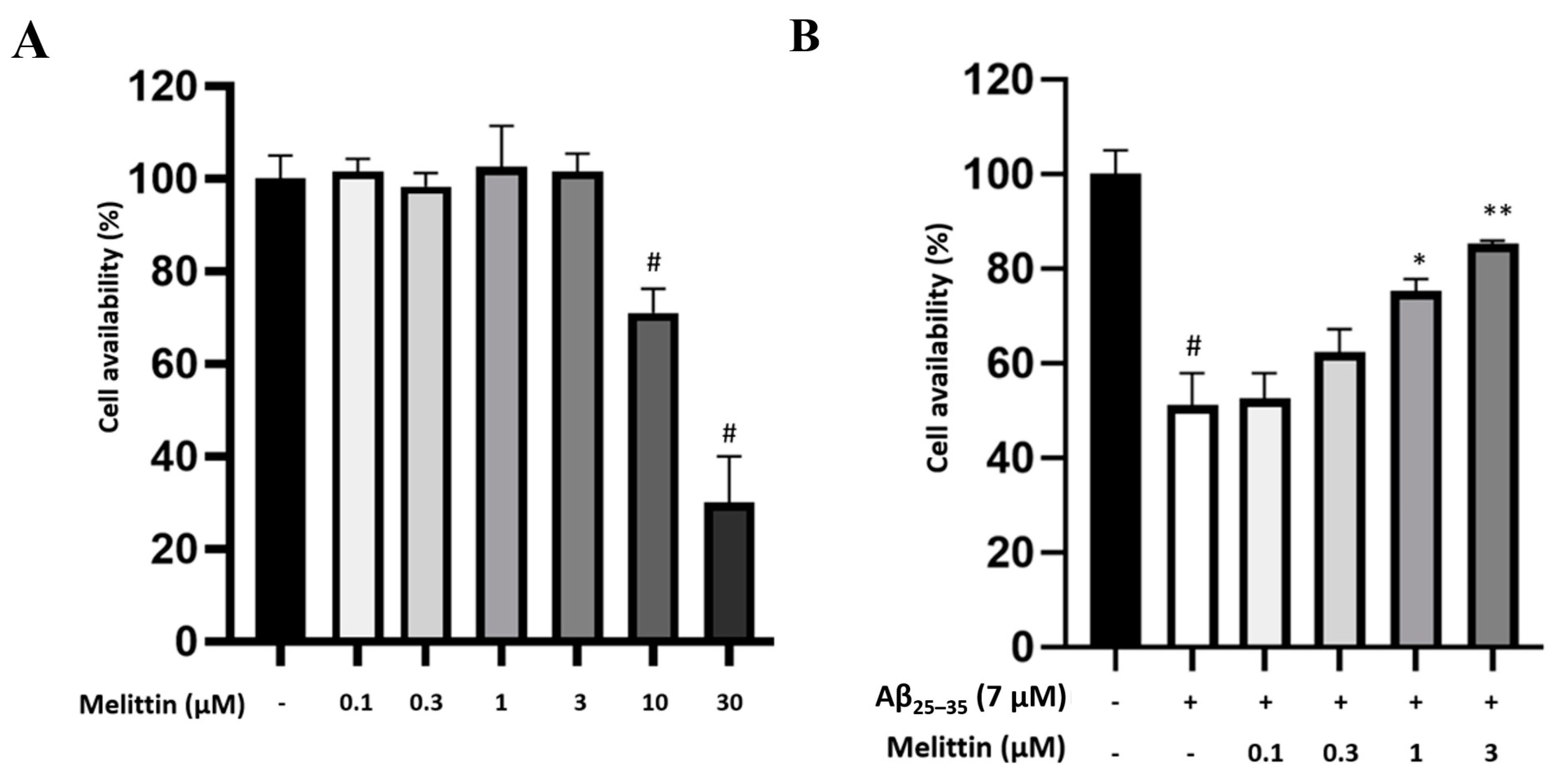
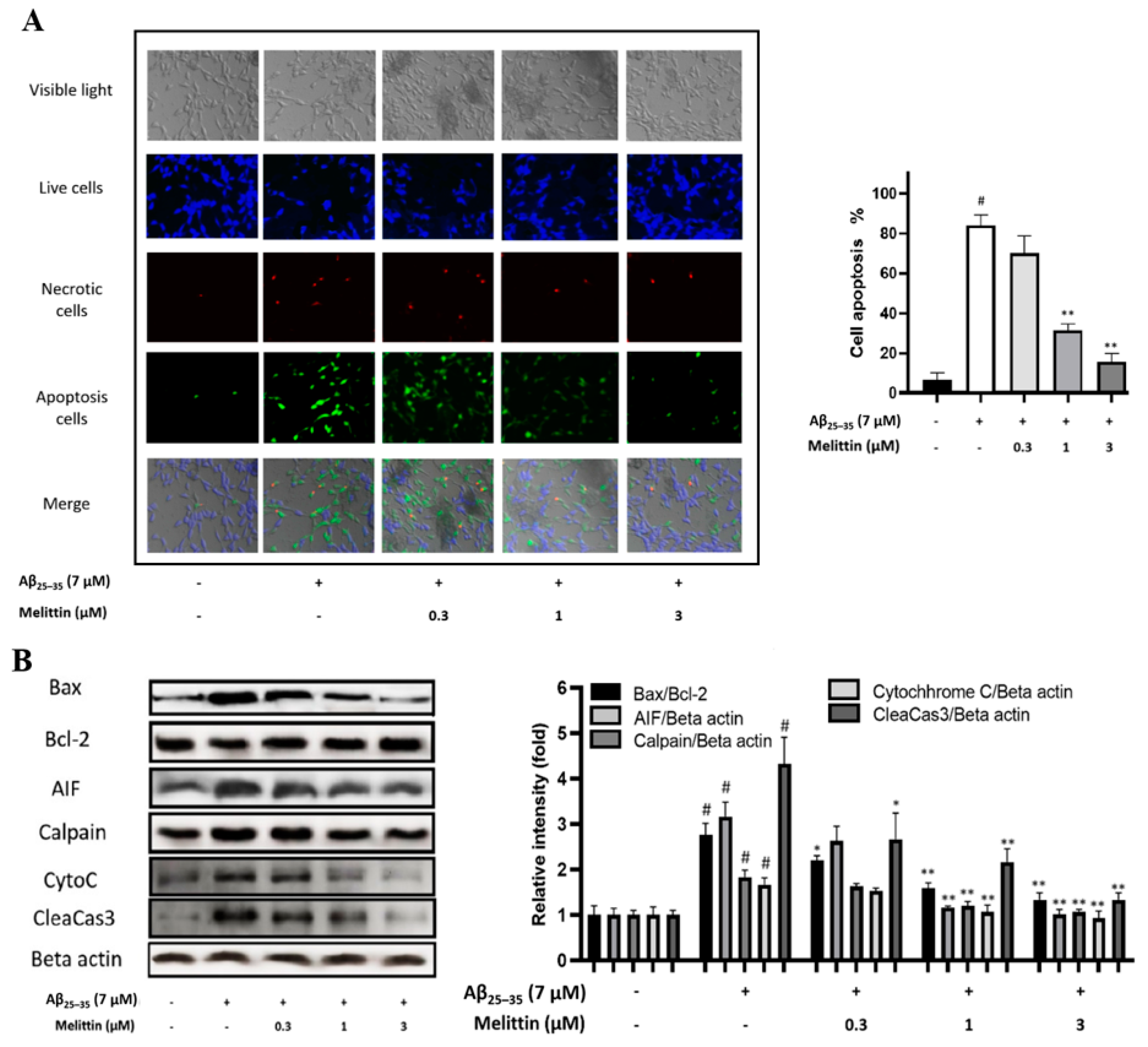
3.1. Protective Effect of Melittin on Oxidative Stress-Induced Apoptosis in HT-22 Cells
3.2. Effects of Melittin on Aβ25–35-Induced Oxidative Stress
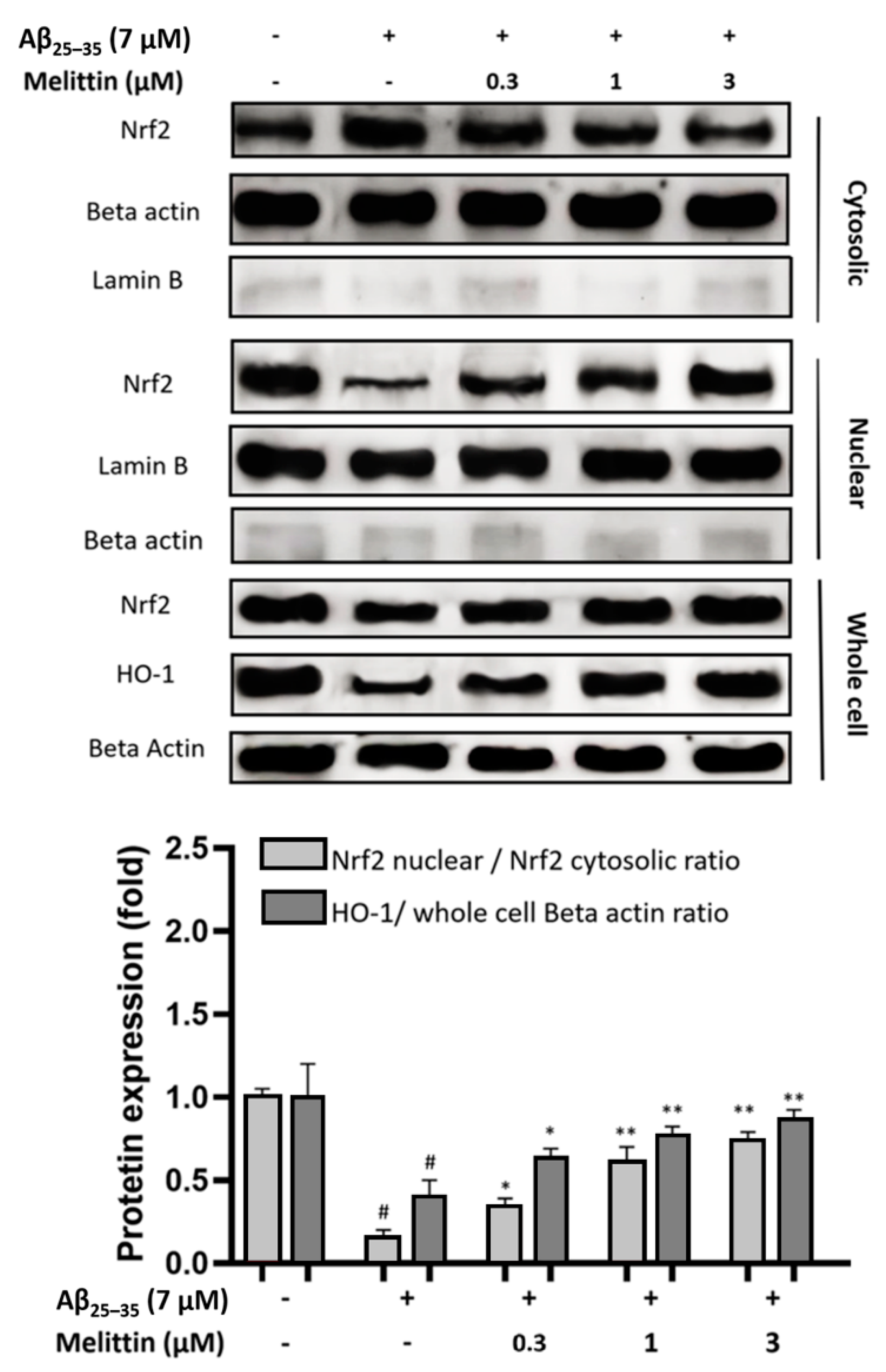
3.3. Effects of Melittin on Nuclear Translocation of Nrf2 and Expression of HO-1
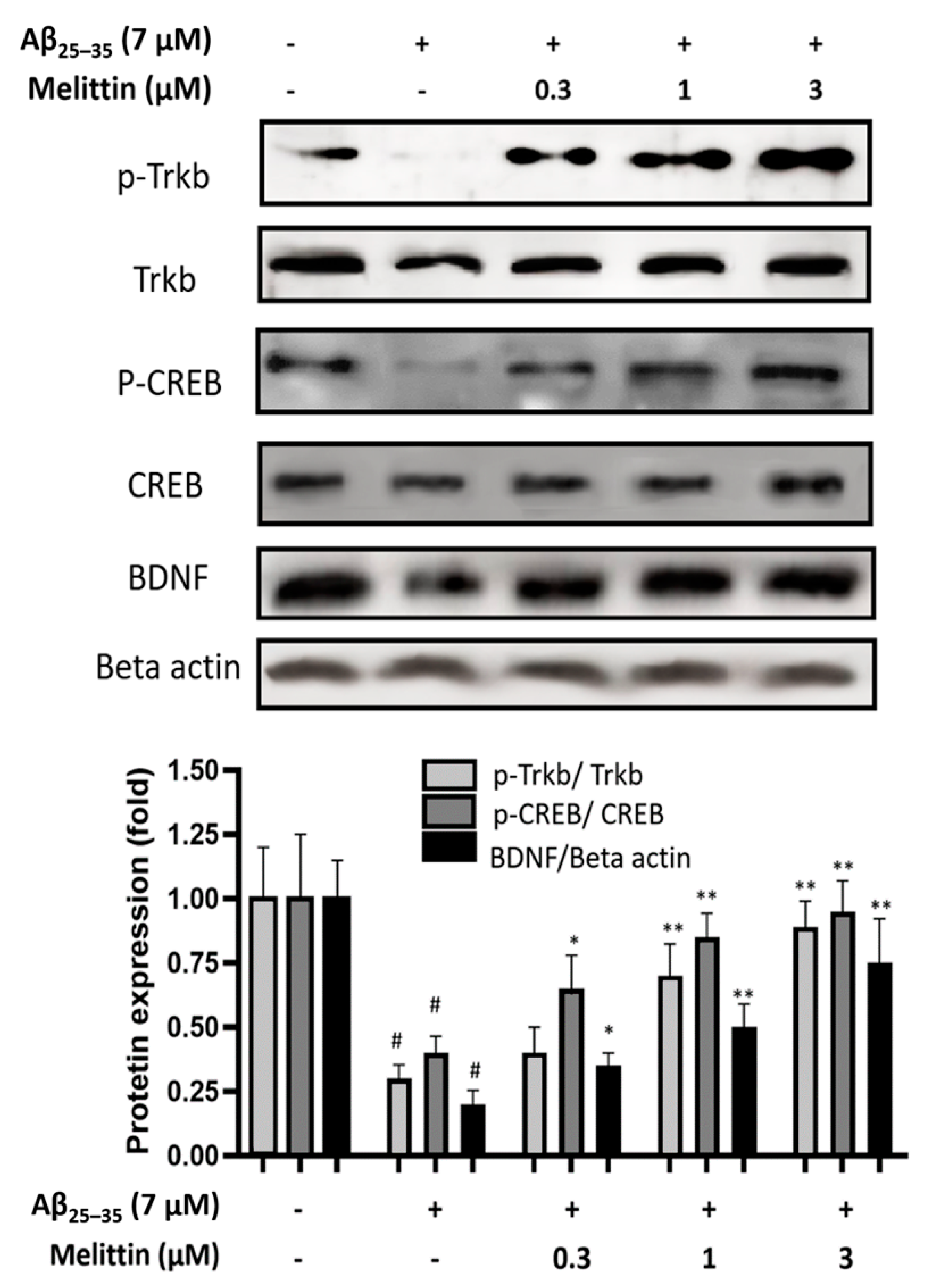
3.4. Effect of Melittin on the TrkB/CREB/BDNF Pathway
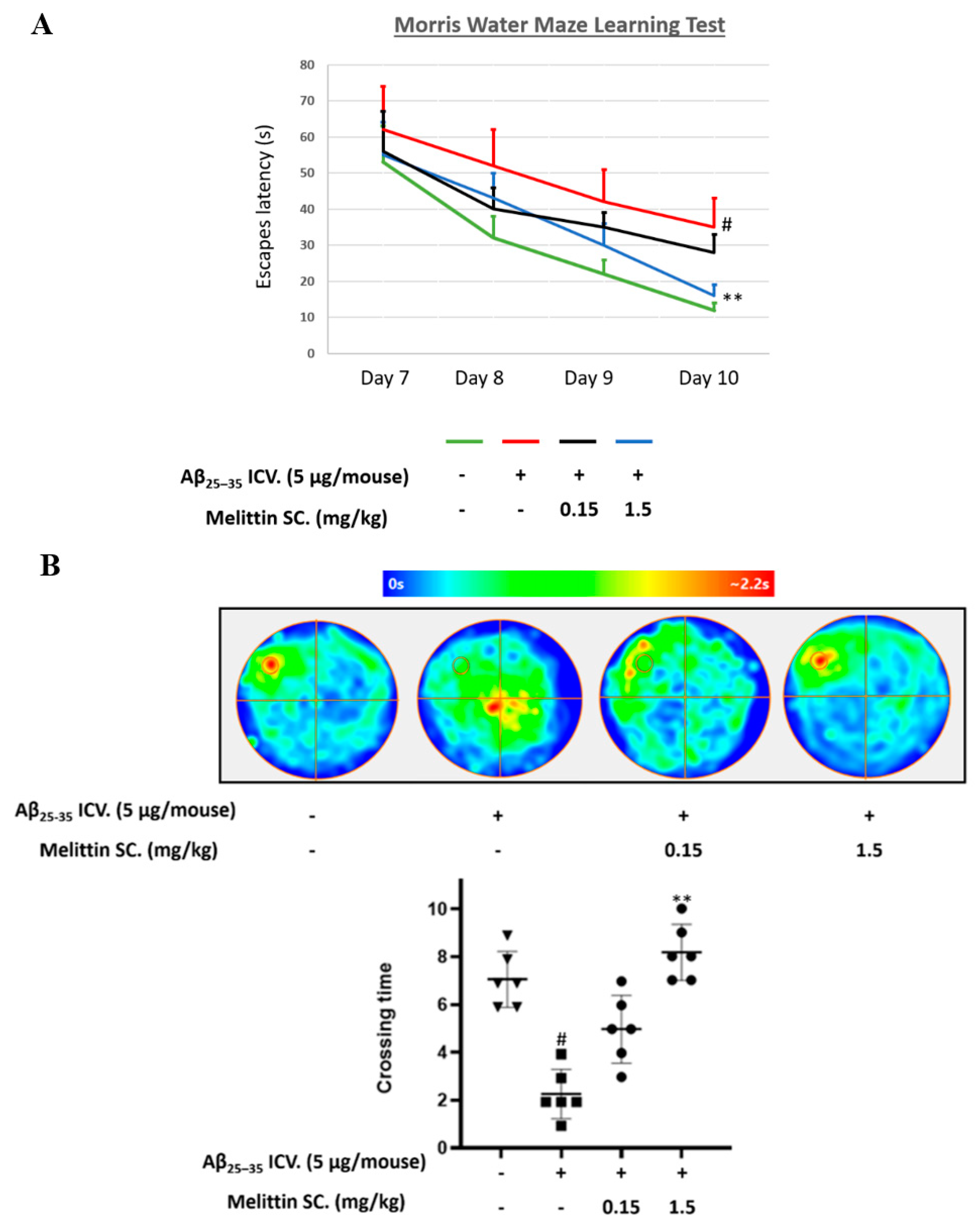
3.5. Improving the Effect of Melittin on Aβ25–35 Memory Impairment in Mice in a Water Maze Trial
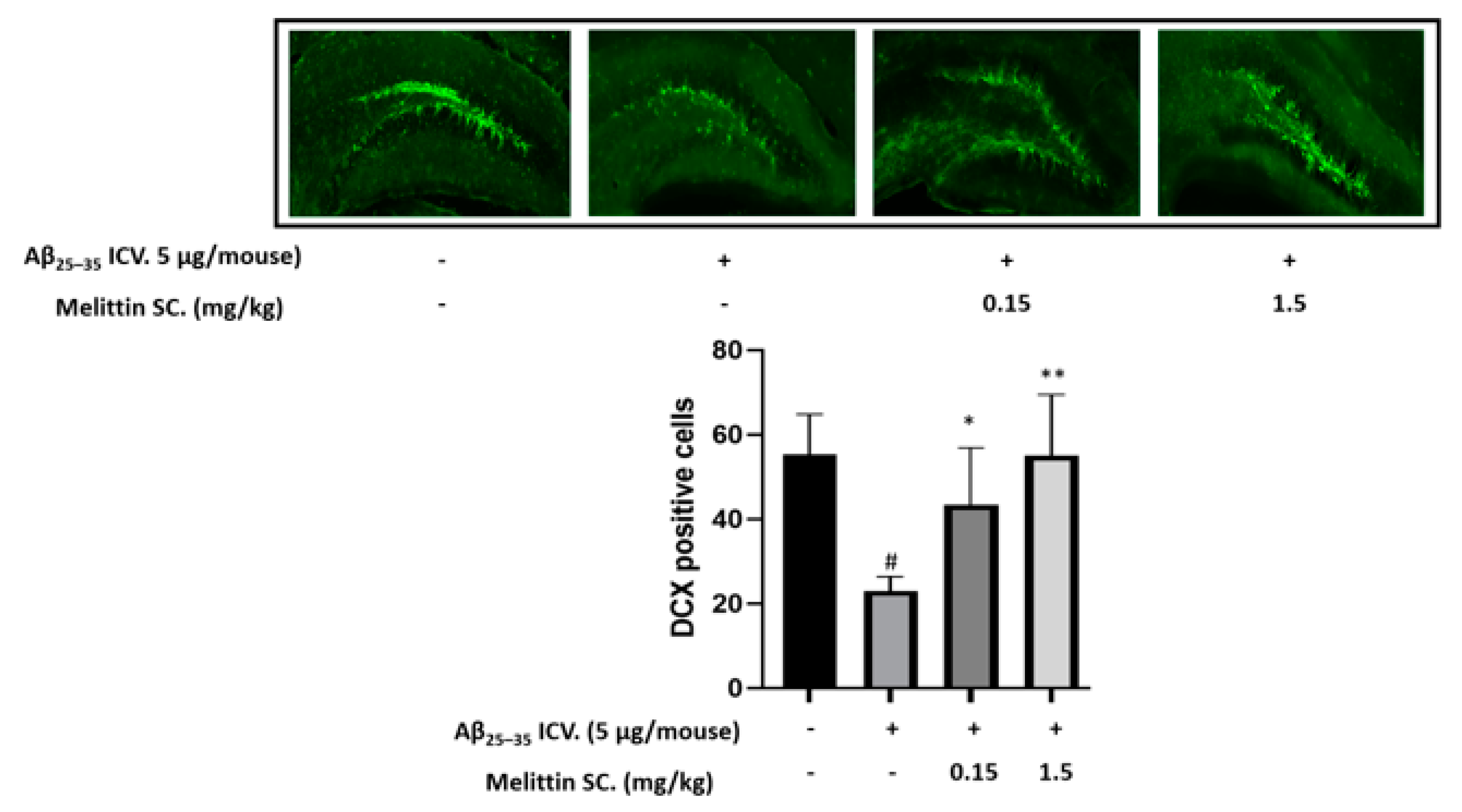
3.6. In Vivo Immunohistochemistry Study
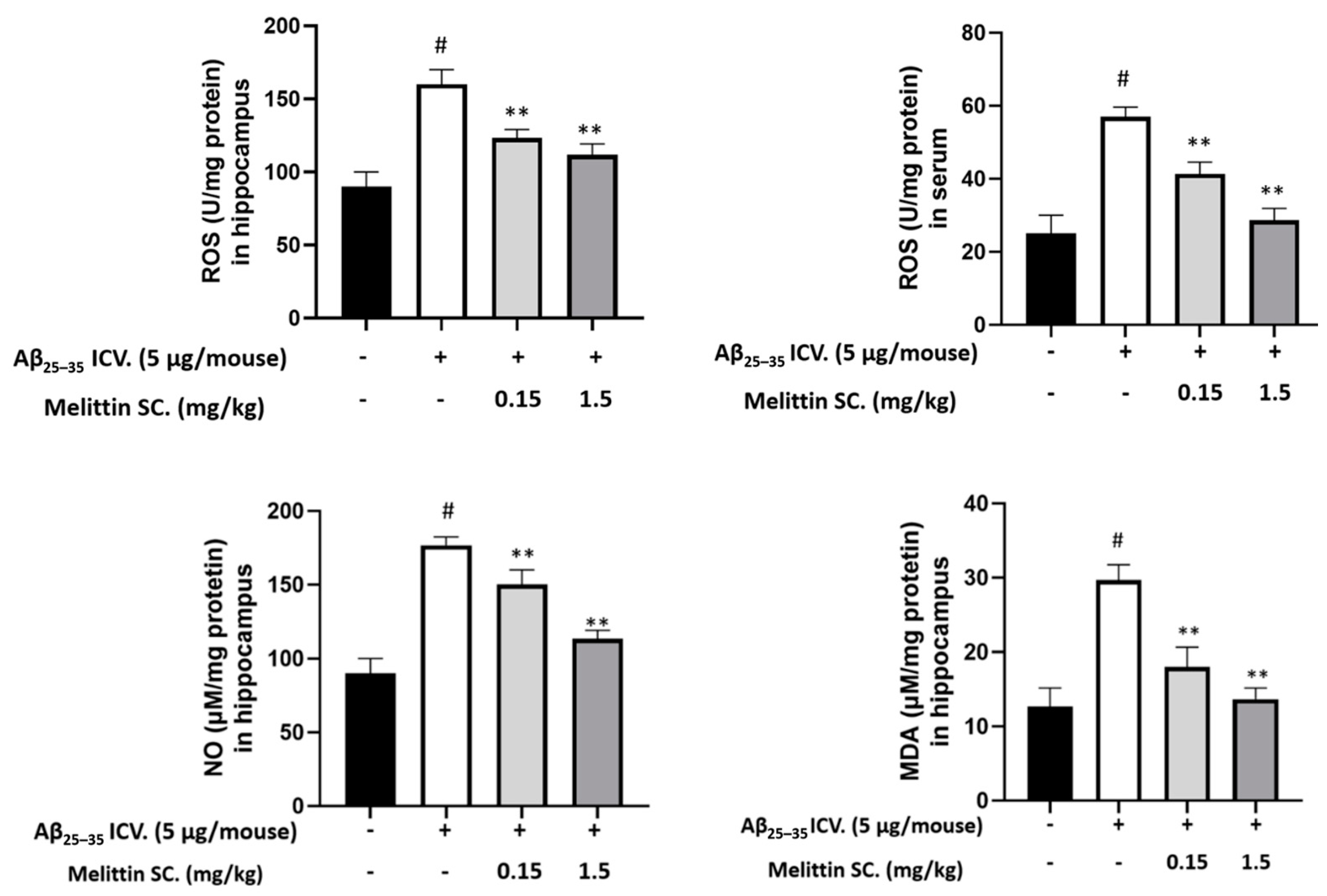
3.7. Oxidative and Inflammation Markers in Mouse Hippocampus and Serum
3.8. Study of In Vivo Key Marker Proteins
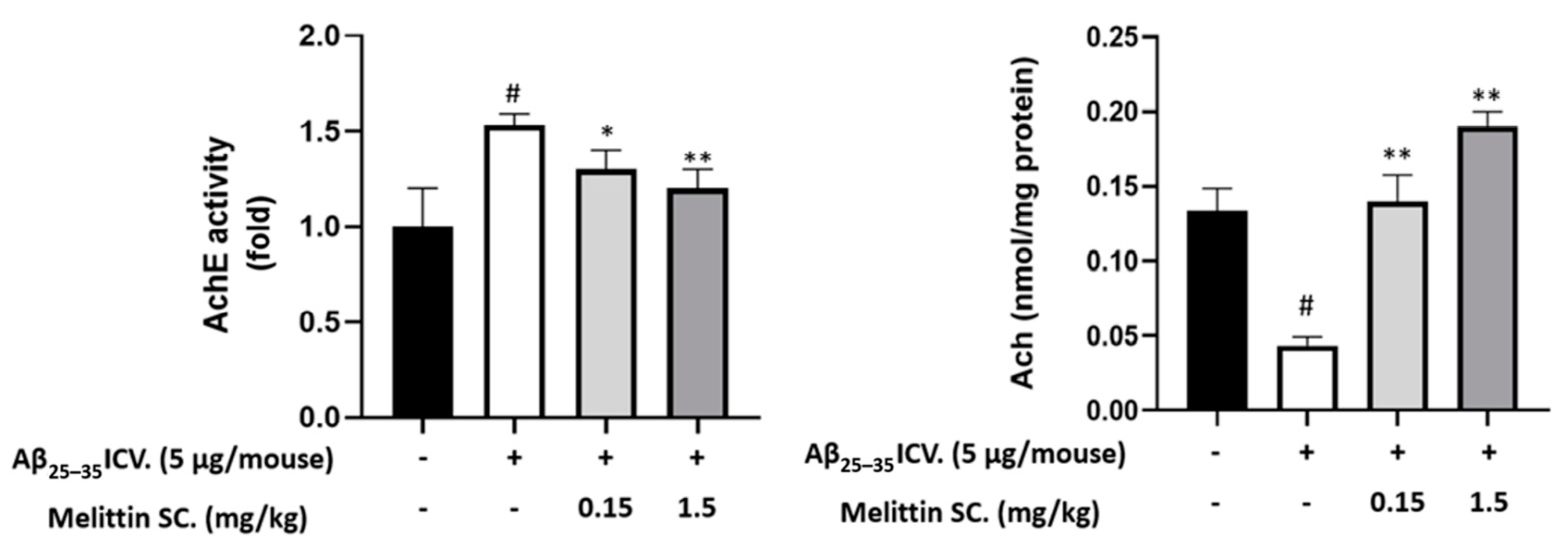
3.9. Study on In Vivo Neurotransmitter Alterations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hwang, D.S.; Kim, S.K.; Bae, H. Therapeutic effects of bee venom on immunological and neurological diseases. Toxins 2015, 7, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Frangieh, J.; Salma, Y.; Haddad, K.; Mattei, C.; Legros, C.; Fajloun, Z.; Obeid, D. El First characterization of the venom from apis mellifera syriaca, a honeybee from the middle east region. Toxins 2019, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Somwongin, S.; Chantawannakul, P.; Chaiyana, W. Antioxidant activity and irritation property of venoms from Apis species. Toxicon 2018, 145, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Park, S.Y.; Lee, K.J.; Heo, M.S.; Kim, K.C.; Kim, M.O.; Lee, J.D.; Choi, Y.H.; Kim, G.Y. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int. Immunopharmacol. 2007, 7, 1092–1101. [Google Scholar] [CrossRef]
- Han, S.M.; Kim, J.M.; Park, K.K.; Chang, Y.C.; Pak, S.C. Neuroprotective effects of melittin on hydrogen peroxide-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. BMC Complement. Altern. Med. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Atlante, A.; Bobba, A.; Petragallo, V.A.; Marra, E. Alzheimer’s proteins, oxidative stress, and mitochondrial dysfunction interplay in a neuronal model of Alzheimer’s disease. Int. J. Alzheimers. Dis. 2010, 2010. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-Peptide (1-42)-induced oxidative stress in alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Xiao, A.; Liu, W.; Shang, Y.; An, J. Melittin ameliorates CVB3-induced myocarditis via activation of the HDAC2-mediated GSK-3β/Nrf2/ARE signaling pathway. Biochem. Biophys. Res. Commun. 2016, 480, 126–131. [Google Scholar] [CrossRef]
- Kim, J.Y.; Leem, J.; Hong, H.L. Melittin Ameliorates Endotoxin-Induced Acute Kidney Injury by Inhibiting Inflammation, Oxidative Stress, and Cell Death in Mice. Oxid. Med. Cell. Longev. 2021, 2021, 8843051. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef]
- Yoo, J.M.; Lee, B.D.; Sok, D.E.; Ma, J.Y.; Kim, M.R. Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/Antioxidant enzyme in neuronal cells. Redox Biol. 2017, 11, 592–599. [Google Scholar] [CrossRef]
- Koike, H.; Iguchi, Y.; Sahashi, K.; Katsuno, M. Significance of Oligomeric and Fibrillar Species in Amyloidosis : Insights into Pathophysiology and Treatment. Molecules 2021, 26, 5091. [Google Scholar] [CrossRef]
- Koike, H.; Katsuno, M. The ultrastructure of tissue damage by amyloid fibrils. Molecules 2021, 26, 4611. [Google Scholar] [CrossRef] [PubMed]
- Pike, C.J.; Walencewicz-Wasserman, A.J.; Kosmoski, J.; Cribbs, D.H.; Glabe, C.G.; Cotman, C.W. Structure-Activity Analyses of β-Amyloid Peptides: Contributions of the β25–35 Region to Aggregation and Neurotoxicity. J. Neurochem. 1995, 64, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Zussy, C.; Brureau, A.; Keller, E.; Marchal, S.; Blayo, C.; Delair, B.; Ixart, G.; Maurice, T.; Givalois, L. Alzheimer’s Disease Related Markers, Cellular Toxicity and Behavioral Deficits Induced Six Weeks after Oligomeric Amyloid-β Peptide Injection in Rats. PLoS ONE 2013, 8, e0053117. [Google Scholar] [CrossRef]
- Chen, N.; Wang, J.; He, Y.; Xu, Y.; Zhang, Y.; Gong, Q.; Yu, C.; Gao, J. Trilobatin Protects Against Aβ25–35-Induced Hippocampal HT22 Cells Apoptosis Through Mediating ROS/p38/Caspase 3-Dependent Pathway. Front. Pharmacol. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Yang, S.J.; Kim, J.; Lee, S.E.; Ahn, J.Y.; Choi, S.Y.; Cho, S.W. Anti-inflammatory and anti-oxidative effects of 3-(naphthalen-2-yl(propoxy)methyl)azetidine hydrochloride on β-amyloid-induced microglial activation. BMB Rep. 2017, 50, 634–639. [Google Scholar] [CrossRef]
- Zhou, J.; Chao, G.; Li, Y.L.; Wu, M.; Zhong, S.Z.; Feng, Z.Y. Activation of NRF2/ARE by isosilybin alleviates Aβ25-35-induced oxidative stress injury in HT-22 cells. Neurosci. Lett. 2016, 632, 92–97. [Google Scholar] [CrossRef]
- Kim, N.; Choi, J.G.; Park, S.; Lee, J.K.; Oh, M.S. Butterbur leaves attenuate memory impairment and neuronal cell damage in amyloid beta-induced alzheimer’s disease models. Int. J. Mol. Sci. 2018, 19, 1644. [Google Scholar] [CrossRef] [PubMed]
- Stepanichev, M.Y.; Zdobnova, I.M.; Zarubenko, I.I.; Moiseeva, Y.V.; Lazareva, N.A.; Onufriev, M.V.; Gulyaeva, N.V. Amyloid-β(25-35)-induced memory impairments correlate with cell loss in rat hippocampus. Physiol. Behav. 2004, 80, 647–655. [Google Scholar] [CrossRef]
- Fang, F.; Liu, G.T. Protective effects of compound FLZ on β-amyloid peptide-(25-35)- induced mouse hippocampal injury and learning and memory impairment. Acta Pharmacol. Sin. 2006, 27, 651–658. [Google Scholar] [CrossRef]
- Mantha, A.K.; Moorthy, K.; Cowsik, S.M.; Baquer, N.Z. Neuroprotective role of Neurokinin B (NKB) on β-amyloid (25-35) induced toxicity in aging rat brain synaptosomes: Involvement in oxidative stress and excitotoxicity. Biogerontology 2006, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Naldi, M.; Fiori, J.; Pistolozzi, M.; Drake, A.F.; Bertucci, C.; Wu, R.; Mlynarczyk, K.; Filipek, S.; De Simone, A.; Andrisano, V. Amyloid β-peptide 25-35 self-assembly and its inhibition: A model undecapeptide system to gain atomistic and secondary structure details of the Alzheimers disease process and treatment. ACS Chem. Neurosci. 2012, 3, 952–962. [Google Scholar] [CrossRef]
- Furukawa-Hibi, Y.; Alkam, T.; Nitta, A.; Matsuyama, A.; Mizoguchi, H.; Suzuki, K.; Moussaoui, S.; Yu, Q.S.; Greig, N.H.; Nagai, T.; et al. Butyrylcholinesterase inhibitors ameliorate cognitive dysfunction induced by amyloid-β peptide in mice. Behav. Brain Res. 2011, 225, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Daniel, I.E.; Umoette, R. Toxic Effects and Safety of Bee Venom Protein [Melittin] in Mice: Search for Natural Vaccine Adjuvants. J. Nat. Prod. Resour. 2017, 3, 111–114. [Google Scholar]
- Lee, C.; Bae, S.J.S.; Joo, H.; Bae, H. Melittin suppresses tumor progression by regulating tumorassociated macrophages in a Lewis lung carcinoma mouse model. Oncotarget 2017, 8, 54951–54965. [Google Scholar] [CrossRef]
- Askari, P.; Namaei, M.H.; Ghazvini, K.; Hosseini, M. In vitro and in vivo toxicity and antibacterial efficacy of melittin against clinical extensively drug-resistant bacteria. BMC Pharmacol. Toxicol. 2021, 22, 1–12. [Google Scholar] [CrossRef]
- Li, S.P.; Wang, Y.W.; Qi, S.L.; Zhang, Y.P.; Deng, G.; Ding, W.G.; Ma, C.; Lin, Q.Y.; Guan, H.D.; Liu, W.; et al. Analogous β-Carboline Alkaloids Harmaline and Harmine Ameliorate Scopolamine-Induced Cognition Dysfunction by Attenuating Acetylcholinesterase Activity, Oxidative Stress, and Inflammation in Mice. Front Pharmacol. 2018, 9, 346. [Google Scholar] [CrossRef]
- Okamoto, S.; Yamauchi, K.; Sohn, J.; Takahashi, M. Exclusive labeling of direct and indirect pathway neurons in the mouse neostriatum by an adeno-associated virus vector with Cre/lox system. STAR Protoc. 2021, 2, 100230. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.G.; Lee, H.W.; Han, J.M.; Lee, S.K.; Kim, D.W.; Saravanakumar, A.; Son, C.G. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci. Rep. 2015, 5, 9651. [Google Scholar] [CrossRef]
- Cells, N.; Lee, B.D.; Yoo, J.; Baek, S.Y.; Li, F.Y.; Sok, D.; Kim, M.R. Antioxidant Enzyme Formation via TrkB/Akt Pathway Activation for Neuroprotection against Oxidative Stress-Induced Apoptosis in Hippocampal. Antioxidants 2019, 9, 3. [Google Scholar] [CrossRef]
- Seo, J.Y.; Pyo, E.; An, J.P.; Kim, J.; Sung, S.H.; Oh, W.K. Andrographolide Activates Keap1/Nrf2/ARE/HO-1 Pathway in HT22 Cells and Suppresses Microglial Activation by A β 42 through Nrf2-Related Inflammatory Response. Mediat. Inflamm. 2017, 2017. [Google Scholar] [CrossRef]
- Gutierrez-Merino, C.; Lopez-Sanchez, C.; Lagoa, R.K.; Samhan-Arias, A.; Bueno, C.; Garcia-Martinez, V. Neuroprotective Actions of Flavonoids. Curr. Med. Chem. 2011, 18, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Diaz, A.; Limon, D.; Chávez, R.; Zenteno, E.; Guevara, J. Aβ25-35 injection into the temporal cortex induces chronic inflammation that contributes to neurodegeneration and spatial memory impairment in rats. J. Alzheimer’s Dis. 2012, 30, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T. The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectr. 2005, 10, 6–9. [Google Scholar] [CrossRef]
- Hu, W.; Gray, N.W.; Brimijoin, S. Amyloid-beta increases acetylcholinesterase expression in neuroblastoma cells by reducing enzyme degradation. J. Neurochem. 2003, 86, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Cioffi, F.; Adam, R.H.I.; Broersen, K. Molecular Mechanisms and Genetics of Oxidative Stress in Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 72, 981–1017. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.H.; Arce, F.; Gillman, A.L.; Jang, H.; Kagan, B.L.; Nussinov, R.; Yang, J.; Lal, R. Amyloid β Ion Channels in a Membrane Comprising Brain Total Lipid Extracts. ACS Chem. Neurosci. 2017, 8, 1348–1357. [Google Scholar] [CrossRef]
- Zaretsky, D.V.; Zaretskaia, M.V. Flow cytometry method to quantify the formation of beta-amyloid membrane ion channels. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183506. [Google Scholar] [CrossRef]
- Ekinci, F.J.; Linsley, M.D.; Shea, T.B. β-Amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Mol. Brain Res. 2000, 76, 389–395. [Google Scholar] [CrossRef]
- Canevari, L.; Clark, J.B.; Bates, T.E. β-Amyloid fragment 25-35 selectively decreases complex IV activity in isolated mitochondria. FEBS Lett. 1999, 457, 131–134. [Google Scholar] [CrossRef]
- Lahmy, V.; Long, R.; Morin, D.; Villard, V.; Maurice, T. Mitochondrial protection by the mixed muscarinic/σ1 ligand ANAVEX2-73, a tetrahydrofuran derivative, in Aβ25–35 peptide-injected mice, a nontransgenic Alzheimer’s disease model. Front. Cell. Neurosci. 2015, 8, 463. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.E.; Marini, S.; Coletta, M.; Orsini, F.; Giardina, B.; Misiti, F. Aβ(31-35) and Aβ(25-35) fragments of amyloid beta-protein induce cellular death through apoptotic signals: Role of the redox state of methionine-35. FEBS Lett. 2005, 579, 2913–2918. [Google Scholar] [CrossRef]
- Luque-Contreras, D.; Carvajal, K.; Toral-Rios, D.; Franco-Bocanegra, D.; Campos-Peña, V. Oxidative stress and metabolic syndrome: Cause or consequence of Alzheimer’s disease? Oxid. Med. Cell. Longev. 2014, 2014, 497802. [Google Scholar] [CrossRef]
- Weitzel, K.; Chemie, F.; Rev, M.S.; Introduction, I.; Reference, C. Protein Carbonylation as a Major Hallmark of Oxidative Damage: Update of Analytical Strategies Maria. WHO Libr. Cat. Data 2014, 33, 79–97. [Google Scholar] [CrossRef]
- Hung, C.H.L.; Cheng, S.S.Y.; Cheung, Y.T.; Wuwongse, S.; Zhang, N.Q.; Ho, Y.S.; Lee, S.M.Y.; Chang, R.C.C. A reciprocal relationship between reactive oxygen species and mitochondrial dynamics in neurodegeneration. Redox Biol. 2018, 14, 7–19. [Google Scholar] [CrossRef]
- Misiti, F.; Martorana, G.E.; Nocca, G.; Di Stasio, E.; Giardina, B.; Clementi, M.E. Methionine 35 oxidation reduces toxic and pro-apoptotic effects of the amyloid β-protein fragment (31-35) on isolated brain mitochondria. Neuroscience 2004, 126, 297–303. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Kanski, J. Methionine residue 35 is critical for the oxidative stress and neurotoxic properties of Alzheimer’s amyloid β-peptide 1-42. Peptides 2002, 23, 1299–1309. [Google Scholar] [CrossRef]
- Clementi, M.E.; Martorana, G.E.; Pezzotti, M.; Giardina, B.; Misiti, F. Methionine 35 oxidation reduces toxic effects of the amyloid β-protein fragment (31-35) on human red blood cell. Int. J. Biochem. Cell Biol. 2004, 36, 2066–2076. [Google Scholar] [CrossRef]
- Kim, H.J.; Baek, S.Y.; Sok, D.E.; Lee, K.J.; Kim, Y.J.; Kim, M.R. Neuroprotective activity of polyphenol-rich ribes diacanthum pall against oxidative stress in glutamate-stimulated ht-22 cells and a scopolamine-induced amnesia animal model. Antioxidants 2020, 9, 895. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.Y.; Kim, M.R. Neuroprotective effect of carotenoid-rich enteromorpha prolifera extract via TrkB/Akt pathway against oxidative stress in hippocampal neuronal cells. Mar. Drugs 2020, 18, 372. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef]
- Bartkowska, K.; Paquin, A.; Gauthier, A.S.; Kaplan, D.R.; Miller, F.D. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development 2007, 134, 4369–4380. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Bernier, B.E.; Lacagnina, A.F.; Ayoub, A.; Shue, F.; Zemelman, B.V.; Krasne, F.B.; Drew, M.R. Dentate gyrus contributes to retrieval as well as encoding: Evidence from context fear conditioning, recall, and extinction. J. Neurosci. 2017, 37, 6359–6371. [Google Scholar] [CrossRef] [PubMed]
- Ledo, A.; Frade, J.; Barbosa, R.M.; Laranjinha, J. Nitric oxide in brain: Diffusion, targets and concentration dynamics in hippocampal subregions. Mol. Aspects Med. 2004, 25, 75–89. [Google Scholar] [CrossRef]
- Ledo, A.; Lourenço, C.F.; Cadenas, E.; Barbosa, R.M.; Laranjinha, J. The bioactivity of neuronal-derived nitric oxide in aging and neurodegeneration: Switching signaling to degeneration. Free Radic. Biol. Med. 2021, 162, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, R.; Zhang, Y.; Xia, Z.; Hu, Y. Role of CREB in the regulatory action of sarsasapogenin on muscarinic M1 receptor density during cell aging. FEBS Lett. 2010, 584, 1549–1552. [Google Scholar] [CrossRef]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.S.; Gan, S.H.; Khalil, M.I. Melittin, a Potential Natural Toxin of Crude Bee Venom: Probable Future Arsenal in the Treatment of Diabetes Mellitus. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Duffy, C.; Sorolla, A.; Wang, E.; Golden, E.; Woodward, E.; Davern, K.; Ho, D.; Johnstone, E.; Pfleger, K.; Redfern, A.; et al. Honeybee venom and melittin suppress growth factor receptor activation in HER2-enriched and triple-negative breast cancer. npj Precis. Oncol. 2020, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, C.D.; Lee, G. Neuroprotective Activity of Melittin—The Main Component of Bee Venom—Against Oxidative Stress Induced by Aβ25–35 in In Vitro and In Vivo Models. Antioxidants 2021, 10, 1654. https://doi.org/10.3390/antiox10111654
Nguyen CD, Lee G. Neuroprotective Activity of Melittin—The Main Component of Bee Venom—Against Oxidative Stress Induced by Aβ25–35 in In Vitro and In Vivo Models. Antioxidants. 2021; 10(11):1654. https://doi.org/10.3390/antiox10111654
Chicago/Turabian StyleNguyen, Cong Duc, and Gihyun Lee. 2021. "Neuroprotective Activity of Melittin—The Main Component of Bee Venom—Against Oxidative Stress Induced by Aβ25–35 in In Vitro and In Vivo Models" Antioxidants 10, no. 11: 1654. https://doi.org/10.3390/antiox10111654
APA StyleNguyen, C. D., & Lee, G. (2021). Neuroprotective Activity of Melittin—The Main Component of Bee Venom—Against Oxidative Stress Induced by Aβ25–35 in In Vitro and In Vivo Models. Antioxidants, 10(11), 1654. https://doi.org/10.3390/antiox10111654





