Mechanism, Prevention, and Treatment of Radiation-Induced Salivary Gland Injury Related to Oxidative Stress
Abstract
:1. Introduction
2. Mechanism of RISGI
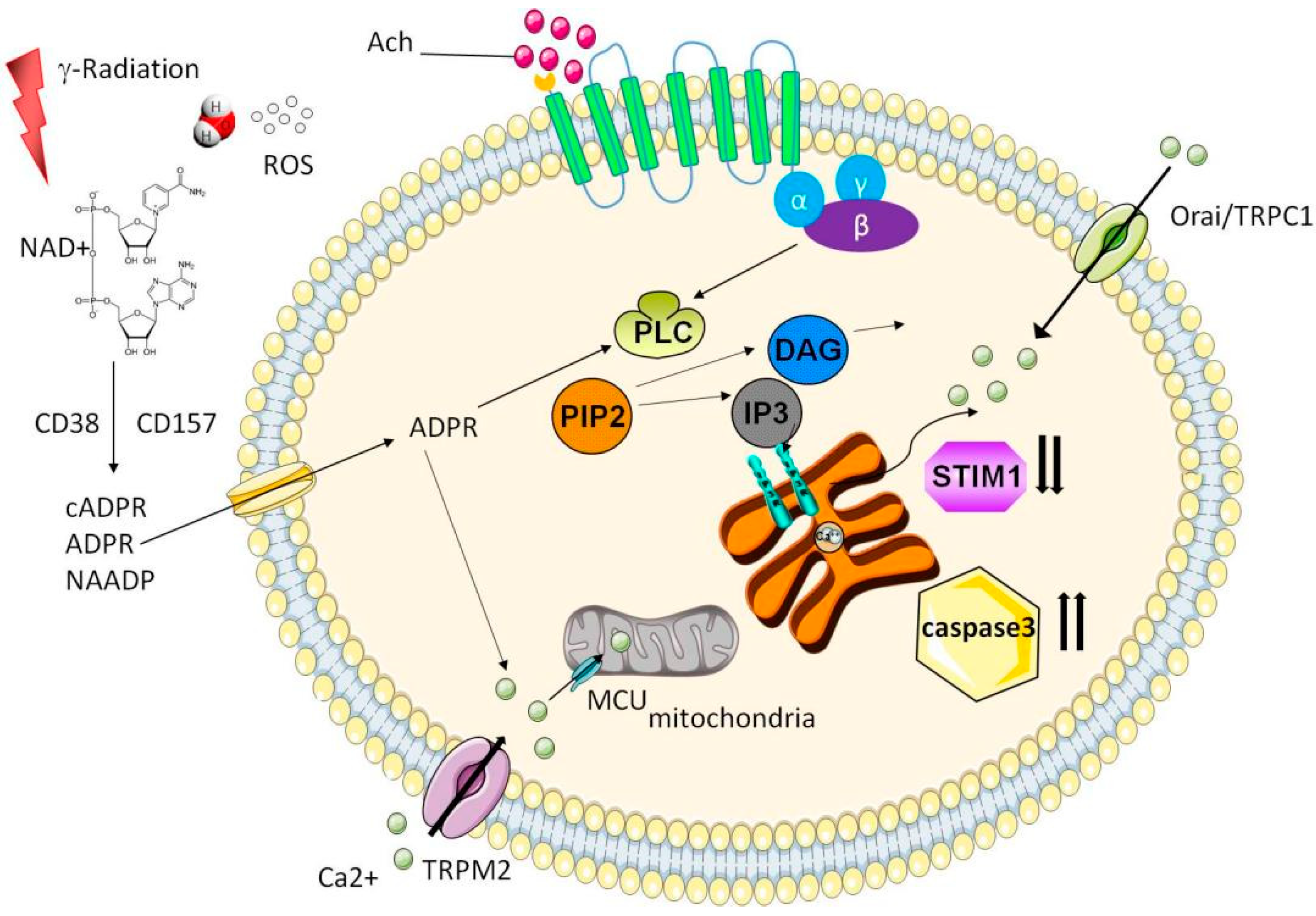
3. Calcium Signaling
4. Microvascular Injury
5. Decreased Parasympathetic Nerve Signals
6. Water Channel Hypothesis
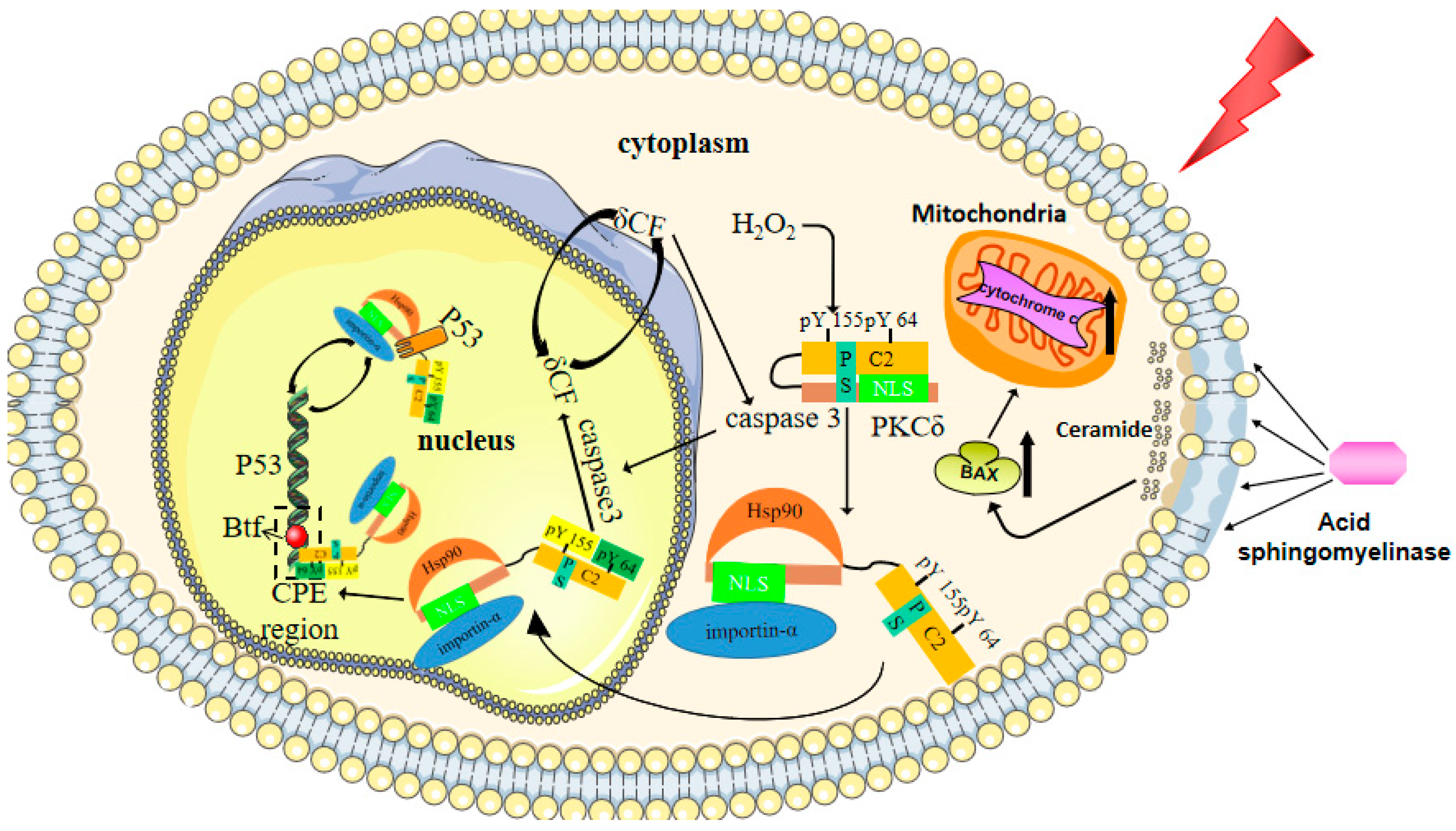
7. Cellular Senescence and Apoptosis
8. Treatment of RISGI
8.1. Amifostine
8.2. Antioxidant Stress Therapy
8.3. Growth Factor Therapy
8.4. Molecular Targeted Therapy
8.4.1. Targeted TGF-β Therapy
8.4.2. Targeted PKCδ Therapy
8.4.3. Targeting PI3K/AKT/mTOR Pathway
8.5. Stem Cell Therapy
8.5.1. Save SCs to Reduce IR Damage
8.5.2. Other Functioning SCs
8.6. Gene Transfer Therapy
Transferred Genes
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adelstein, D.; Gillison, M.L.; Pfister, D.G.; Spencer, S.; Adkins, D.; Brizel, D.; Burtness, B.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Eisbruch, A.; Kim, H.M.; Terrell, J.E.; Marsh, L.H.; Dawson, L.A.; Ship, J.A. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 695–704. [Google Scholar] [CrossRef]
- Eisbruch, A.; Ten Haken, R.K.; Kim, H.M.; Marsh, L.H.; Ship, J.A. Dose, volume, and function relationships in parotid salivary glands following conformal and intensi-ty-modulated irradiation of head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 577–587. [Google Scholar] [CrossRef]
- Tribius, S.; Haladyn, S.; Hanken, H.; Busch, C.J.; Krüll, A.; Petersen, C.; Bergelt, C. Parotid sparing and quality of life in long-term survivors of locally advanced head and neck cancer after intensi-ty-modulated radiation therapy. Strahlenther. Onkol. 2021, 197, 219–230. [Google Scholar] [CrossRef]
- Vries, S.A.G.D.; Tan, C.X.W.; Bouma, G.; Forouzanfar, T.; Brand, M.H.S.; de Boer, N. Salivary Function and Oral Health Problems in Crohn’s Disease Patients. Inflamm. Bowel Dis. 2018, 24, 1361–1367. [Google Scholar] [CrossRef]
- Hai, B.; Zhao, Q.; Deveau, M.A.; Liu, F. Delivery of Sonic Hedgehog Gene Repressed Irradiation-induced Cellular Senescence in Salivary Glands by Pro-moting DNA Repair and Reducing Oxidative Stress. Theranostics 2018, 8, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Henson, B.S.; Eisbruch, A.; d’Hondt, E.; Ship, J.A. Two-year longitudinal study of parotid salivary flow rates in head and neck cancer patients receiving unilat-eral neck parotid-sparing radiotherapy treatment. Oral Oncol. 1999, 35, 234–241. [Google Scholar] [CrossRef]
- Stephens, L.C.; King, G.K.; Peters, L.J.; Ang, K.K.; Schultheiss, T.; Jardine, J.H. Unique radiosensitivity of serous cells in rhesus monkey submandibular glands. Am. J. Pathol. 1986, 124, 479–487. [Google Scholar]
- Burlage, F.R.; Coppes, R.P.; Meertens, H.; Stokman, M.A.; Vissink, A. Parotid and submandibular/sublingual salivary flow during high dose radiotherapy. Radiother. Oncol. 2001, 61, 271–274. [Google Scholar] [CrossRef]
- Coppes, R.P.; Vissink, A.; Konings, A.W. Comparison of radiosensitivity of rat parotid and submandibular glands after different radiation schedules. Radiother. Oncol. 2002, 63, 321–328. [Google Scholar] [CrossRef]
- Dreizen, S.; Brown, L.R.; Handler, S.; Levy, B.M. Radiation-induced xerostomia in cancer patients.Effect on salivary and serum electrolytes. Cancer 1976, 38, 273–278. [Google Scholar] [CrossRef]
- Stephens, L.C.; Schultheiss, T.E.; Price, R.E.; Ang, K.K.; Peters, L.J. Radiation apoptosis of serous acinar cells of salivary and lacrimal glands. Cancer 1991, 67, 1539–1543. [Google Scholar] [CrossRef]
- Murdoch-Kinch, C.-A.; Kim, H.M.; Vineberg, K.A.; Ship, J.A.; Eisbruch, A. Dose-Effect Relationships for the Submandibular Salivary Glands and Implications for Their Sparing by Intensity Modulated Radiotherapy. Int. J. Radiat. Oncol. 2008, 72, 373–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.K.; Su, Y.; Jha, N.; Hong, M.H.; Mai, H.Q.; Fan, W.; Zeng, Z.Y.; Guo, Z.M. Submandibular salivary gland transfer for the prevention of radiation-induced xerostomia in patients with naso-pharyngeal carcinoma: 5-Year outcomes. Head Neck 2011, 33, 389–395. [Google Scholar] [PubMed]
- Grundmann, O.; Fillinger, J.L.; Victory, K.R.; Burd, R.; Limesand, K.H. Restoration of radiation therapy-induced salivary gland dysfunction in mice by post therapy IGF-1 ad-ministration. BMC Cancer 2010, 10, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagler, R.; Marmary, Y.; Fox, P.C.; Baum, B.J.; Har-El, R.; Chevion, M. Irradiation-induced damage to the salivary glands: The role of redox-active iron and copper. Radiat. Res. 1997, 147, 468. [Google Scholar] [CrossRef]
- Zeilstra, L.J.; Vissink, A.; Konings, A.W.; Coppes, R.P. Radiation induced cell loss in rat submandibular gland and its relation to gland function. Int. J. Radiat. Biol. 2000, 76, 419–429. [Google Scholar] [PubMed]
- Paardekooper, G.M.; Cammelli, S.; Zeilstra, L.J.; Coppes, R.P.; Konings, A.W. Radiation-induced apoptosis in relation to acute impairment of rat salivary gland function. Int. J. Radiat. Biol. 1998, 73, 641–648. [Google Scholar] [CrossRef]
- Ambudkar, I.S. Calcium signalling in salivary gland physiology and dysfunction. J. Physiol. 2016, 594, 2813–2824. [Google Scholar] [CrossRef] [Green Version]
- Ambudkar, I.S. Ca2+ signaling and regulation of fluid secretion in salivary gland acinar cells. Cell Calcium 2014, 55, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.H.; Li, Q.; Kim, M.S.; Shin, D.M.; Feske, S.; Birnbaumer, L.; Cheng, K.T.; Ambudkar, I.S.; Muallem, S. Polarized but Differential Localization and Recruitment of STIM1, Orai1 and TRPC Channels in Secretory Cells. Traffic 2010, 12, 232–245. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Cheng, K.T.; Bandyopadhyay, B.C.; Pani, B.; Dietrich, A.; Paria, B.C.; Swaim, W.D.; Beech, D.; Yildrim, E.; Singh, B.B.; et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(-/-) mice. Proc. Natl. Acad. Sci. USA 2007, 104, 17542–17547. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.T.; Liu, X.; Ong, H.L.; Swaim, W.; Ambudkar, I.S. Local Ca2+ Entry Via Orai1 Regulates Plasma Membrane Recruitment of TRPC1 and Controls Cytosolic Ca2+ Signals Required for Specific Cell Functions. PLoS Biol. 2011, 9, e1001025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, A.C.; Lillibridge, C.D.; Zheng, C.; Baum, B.J.; O’Connell, B.C.; Ambudkar, I.S. Gamma-irradiation-induced cell cycle arrest and cell death in a human submandibular gland cell line: Effect of E2F1 expression. J. Cell. Physiol. 1998, 177, 264–273. [Google Scholar] [CrossRef]
- O’Connell, A.C.; Redman, R.S.; Evans, R.L.; Ambudkar, I.S. Radiation-induced progressive decrease in fluid secretion in rat submandibular glands is related to de-creased acinar volume and not impaired calcium signaling. Radiat. Res. 1999, 151, 150–158. [Google Scholar] [CrossRef]
- Ogawa, N.; Kurokawa, T.; Mori, Y. Sensing of redox status by TRP channels. Cell Calcium 2016, 60, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Sumoza-Toledo, A.; Penner, R. TRPM2: A multifunctional ion channel for calcium signalling. J. Physiol. 2011, 589, 1515–1525. [Google Scholar] [CrossRef]
- Liu, X.; Cotrim, A.P.; Teos, L.Y.; Zheng, C.; Swaim, W.D.; Mitchell, J.B.; Mori, Y.; Ambudkar, I.S. Loss of TRPM2 function protects against irradiation-induced salivary gland dysfunction. Nat. Commun. 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Gong, B.; de Souza, L.B.; Ong, H.L.; Subedi, K.P.; Cheng, K.T.; Swaim, W.; Zheng, C.; Mori, Y.; Ambudkar, I.S. Radiation inhibits salivary gland function by promoting STIM1 cleavage by caspase-3 and loss of SOCE through a TRPM2-dependent pathway. Sci. Signal. 2017, 10, eaal4064. [Google Scholar] [CrossRef] [Green Version]
- Coppes, R.P.; Meter, A.; Latumalea, S.P.; Roffel, A.F.; Kampinga, H.H. Defects in muscarinic receptor-coupled signal transduction in isolated parotid gland cells after in vivo irradi-ation: Evidence for a non-DNA target of radiation. Br. J. Cancer 2005, 92, 539–546. [Google Scholar] [CrossRef] [Green Version]
- Cotrim, A.P.; Sowers, A.; Mitchell, J.B.; Baum, B.J. Prevention of Irradiation-induced Salivary Hypofunction by Microvessel Protection in Mouse Salivary Glands. Mol. Ther. 2007, 15, 2101–2106. [Google Scholar] [CrossRef]
- Mizrachi, A.; Cotrim, A.P.; Katabi, N.; Mitchell, J.B.; Verheij, M.; Haimovitz-Friedman, A. Radiation-Induced Microvascular Injury as a Mechanism of Salivary Gland Hypofunction and Potential Tar-get for Radioprotectors. Radiat. Res. 2016, 186, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yan, X.; Gao, R.; Mao, L.; Cotrim, A.P.; Zheng, C.; Zhang, C.; Baum, B.J.; Wang, S. Effect of Irradiation on Microvascular Endothelial Cells of Parotid Glands in the Miniature Pig. Int. J. Radiat. Oncol. 2010, 78, 897–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Fuks, Z.; Kolesnick, R. Ceramide mediates radiation-induced death of endothelium. Crit. Care Med. 2000, 28, N87–N93. [Google Scholar] [CrossRef]
- Lee, H.; Rotolo, J.A.; Mesicek, J.; Penate-Medina, T.; Rimner, A.; Liao, W.-C.; Yin, X.; Ragupathi, G.; Ehleiter, D.; Gulbins, E.; et al. Mitochondrial Ceramide-Rich Macrodomains Functionalize Bax upon Irradiation. PLoS ONE 2011, 6, e19783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.S.; Coppens, I.; Saorin, A.; Brady, N.R.; Hamacher-Brady, A. Endolysosomal Targeting of Mitochondria Is Integral to BAX-Mediated Mitochondrial Permeabilization during Apoptosis Signaling. Dev. Cell 2020, 53, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.M.; Lombaert, I.M.A.; Reed, X.; Vitale-Cross, L.; Gutkind, J.S.; Hoffman, M.P. Parasympathetic Innervation Maintains Epithelial Progenitor Cells During Salivary Organogenesis. Sci. 2010, 329, 1645–1647. [Google Scholar] [CrossRef] [Green Version]
- Tanida, S.; Kataoka, H.; Mizoshita, T.; Shimura, T.; Kamiya, T.; Joh, T. Intranuclear Translocation Signaling of HB-EGF Carboxy-Terminal Fragment and Mucosal Defense through Cell Proliferation and Migration in Digestive Tracts. Digestion 2010, 82, 145–149. [Google Scholar] [CrossRef]
- Emmerson, E.; May, A.J.; Berthoin, L.; Cruz-Pacheco, N.; Nathan, S.; Mattingly, A.J.; Chang, J.L.; Ryan, W.R.; Tward, A.D.; Knox, S.M. Salivary glands regenerate after radiation injury through SOX2-mediated secretory cell replacement. EMBO Mol. Med. 2018, 10, e8051. [Google Scholar] [CrossRef]
- Knox, S.; Lombaert, I.M.A.; Haddox, C.; Abrams, S.R.; Cotrim, A.P.; Wilson, A.J.; Hoffman, M.P. Parasympathetic stimulation improves epithelial organ regeneration. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Jeong, B.K.; Jang, S.J.; Yun, J.W.; Jung, M.H.; Kang, K.M.; Kim, T.G.; Woo, S.H. Alpha-Lipoic Acid Ameliorates Radiation-Induced Salivary Gland Injury by Preserving Parasympathetic Inner-vation in Rats. Int. J. Mol. Sci. 2020, 21, 2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Liu, Y.; Bakkar, M.; Elkashty, O.; El-Hakim, M.; Seuntjens, J.; Tran, S. Labial Stem Cell Extract Mitigates Injury to Irradiated Salivary Glands. J. Dent. Res. 2020, 99, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Smith, B.L.; Christensen, E.I.; Agre, P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. USA 1993, 90, 7275–7279. [Google Scholar] [CrossRef] [Green Version]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of Water Channels in Xenopus Oocytes Expressing Red Cell CHIP28 Protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [Green Version]
- Knepper, M.A. The aquaporin family of molecular water channels. Proc. Natl. Acad. Sci. USA 1994, 91, 6255–6258. [Google Scholar] [CrossRef] [Green Version]
- Saito, E.; Watari, I.; Mizumachi-Kubono, M.; Hsu-Hayashi, S.; Ono, T. Occlusional Modifications Reversibly Alter Aquaporin 5 Expression and Localization in Rat Salivary Glands. Front. Physiol. 2020, 11, 528. [Google Scholar] [CrossRef]
- Delporte, C.; O’Connell, A.C.; He, X.; Lancaster, H.E.; Agre, P.; Baum, B.J. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc. Natl. Acad. Sci. USA 1997, 94, 3268–3273. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, A.C.; Baccaglini, L.; Fox, P.C.; O’Connell, B.C.; Kenshalo, D.; Oweisy, H.; Hoque, A.T.M.S.; Sun, D.; Herscher, L.L.; Braddon, V.R.; et al. Safety and efficacy of adenovirus-mediated transfer of the human aquaporin-1 cDNA to irradiated parotid glands of non-human primates. Cancer Gene Ther. 1999, 6, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Baum, B.J.; Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; Burbelo, P.D.; Citrin, D.E.; Mitchell, J.B. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypo-function. Proc. Natl. Acad. Sci. USA 2012, 109, 19403–19407. [Google Scholar] [CrossRef] [Green Version]
- Asari, T.; Maruyama, K.; Kusama, H. Salivation triggered by pilocarpine involves aquaporin-5 in normal rats but not in irradiated rats. Clin. Exp. Pharmacol. Physiol. 2009, 36, 531–538. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, D.; Gong, B.; Xu, Y.; Sun, H.; Yang, B.; Zhao, X. Decreased Saliva Secretion and Down-Regulation of AQP5 in Submandibular Gland in Irradiated Rats. Radiat. Res. 2006, 165, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Yamaguchi, K.; Sakurai, T.; Asari, T.; Hashimoto, K.; Terakawa, S. Secretion of saliva in X-irradiated rat submandibular glands. Radiat. Res. 2003, 159, 351–360. [Google Scholar] [CrossRef]
- Li, X.; Azlina, A.; Karabasil, M.R.; Purwanti, N.; Hasegawa, T.; Yao, C.; Akamatsu, T.; Hosoi, K. Degradation of submandibular gland AQP5 by parasympathetic denervation of chorda tympani and its recovery by cevimeline, an M3 muscarinic receptor agonist. Am. J. Physiol. Liver Physiol. 2008, 295, G112–G123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azlina, A.; Javkhlan, P.; Hiroshima, Y.; Hasegawa, T.; Yao, C.; Akamatsu, T.; Hosoi, K. Roles of lysosomal proteolytic systems in AQP5 degradation in the submandibular gland of rats following chor-da tympani parasympathetic denervation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1106–G1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose-John, S.; Scheller, J.; Elson, G.; Jones, S.A. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: Role in inflammation and cancer. J. Leukoc. Biol. 2006, 80, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Marmary, Y.; Adar, R.; Gaska, S.; Wygoda, A.; Maly, A.; Cohen, J.; Eliashar, R.; Mizrachi, L.; Orfaig-Geva, C.; Baum, B.J.; et al. Radiation-Induced Loss of Salivary Gland Function Is Driven by Cellular Senescence and Prevented by IL6 Modulation. Cancer Res. 2016, 76, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Passos, J.F.; Von Zglinicki, T. Oxygen free radicals in cell senescence: Are they signal transducers? Free. Radic. Res. 2006, 40, 1277–1283. [Google Scholar] [CrossRef]
- Avila, J.L.; Grundmann, O.; Burd, R.; Limesand, K.H. Radiation-Induced Salivary Gland Dysfunction Results From p53-Dependent Apoptosis. Int. J. Radiat. Oncol. 2009, 73, 523–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeVries, T.A.; Neville, M.C.; Reyland, M.E. Nuclear import of PKCdelta is required for apoptosis: Identification of a novel nuclear import sequence. EMBO J. 2002, 21, 6050–6060. [Google Scholar] [CrossRef] [Green Version]
- Limesand, K.H.; Schwertfeger, K.L.; Anderson, S.M. MDM2 Is Required for Suppression of Apoptosis by Activated Akt1 in Salivary Acinar Cells. Mol. Cell. Biol. 2006, 26, 8840–8856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adwan, T.S.; Ohm, A.M.; Jones, D.N.; Humphries, M.J.; Reyland, M.E. Regulated binding of importin-α to protein kinase Cδ in response to apoptotic signals facilitates nuclear import. J. Biol. Chem. 2011, 286, 35716–35724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wie, S.M.; Adwan, T.S.; DeGregori, J.; Anderson, S.M.; Reyland, M.E. Inhibiting Tyrosine Phosphorylation of Protein Kinase Cδ (PKCδ) Protects the Salivary Gland from Radiation Damage. J. Biol. Chem. 2014, 289, 10900–10908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeVries-Seimon, T.A.; Ohm, A.M.; Humphries, M.J.; Reyland, M.E. Induction of apoptosis is driven by nuclear retention of protein kinase C delta. J. Biol. Chem. 2007, 282, 22307–22314. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Lu, Z.G.; Miki, Y.; Yoshida, K. Protein kinase C delta induces transcription of the TP53 tumor suppressor gene by controlling death-promoting factor Btf in the apoptotic response to DNA damage. Mol. Cell. Biol. 2007, 27, 8480–8491. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Miki, Y.; Yoshida, K. Protein kinase C delta activates IkappaB-kinase alpha to induce the p53 tumor suppres-sor in response to oxidative stress. Cell Signal. 2007, 19, 2088–2097. [Google Scholar] [CrossRef]
- Dashzeveg, N.; Yogosawa, S.; Yoshida, K. Transcriptional induction of protein kinase C delta by p53 tumor suppressor in the apoptotic response to DNA damage. Cancer Lett. 2016, 374, 167–174. [Google Scholar] [CrossRef]
- Reyland, M.E. Protein kinase Cdelta and apoptosis. Biochem. Soc. Trans. 2007, 35 Pt 5, 1001–1004. [Google Scholar] [CrossRef]
- Humphries, M.J.; Ohm, A.M.; Schaack, J.; Adwan, T.S.; Reyland, M.E. Tyrosine phosphorylation regulates nuclear translocation of PKCdelta. Oncogene 2008, 27, 3045–3053. [Google Scholar] [CrossRef] [Green Version]
- Mell, L.K.; Movsas, B. Pharmacologic normal tissue protection in clinical radiation oncology: Focus on amifostine. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1341–1350. [Google Scholar] [CrossRef]
- Anné, P.R.; Machtay, M.; Rosenthal, D.I.; Brizel, D.M.; Morrison, W.H.; Irwin, D.H.; Chougule, P.B.; Estopinal, N.C.; Berson, A.; Curran, W.J. A Phase II trial of subcutaneous amifostine and radiation therapy in patients with head-and-neck cancer. Int. J. Radiat. Oncol. 2007, 67, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Rudat, V.; Meyer, J.; Momm, F.; Bendel, M.; Henke, M.; Strnad, V.; Grötz, K.; Schulte, A. Protective effect of amifostine on dental health after radiotherapy of the head and neck. Int. J. Radiat. Oncol. 2000, 48, 1339–1343. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Sotiropoulou-Lontou, A.; Velegraki, A.; Pissakas, G.; Kolitsi, G.; Kyprianou, K.; Kouloulias, V.; Papanikolaou, I.; Yiotakis, I.; Dardoufas, K. Oral candidiasis in head and neck cancer patients receiving radiotherapy with amifostine cytoprotection. Oral Oncol. 2003, 39, 397–401. [Google Scholar] [CrossRef]
- Vacha, P.; Fehlauer, F.; Mahlmann, B.; Marx, M.; Hinke, A.; Sommer, K.; Richter, E.; Feyerabend, T. Randomized phase III trial of postoperative radiochemotherapy +/-amifostine in head and neck cancer. Is there evidence for radioprotection? Strahlenther. Onkol. 2003, 179, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Jellema, A.P.; Slotman, B.J.; Muller, M.J.; Leemans, C.R.; Smeele, L.E.; Hoekman, K.; Aaronson, N.K.; Langendijk, J.A. Radiotherapy alone, versus radiotherapy with amifostine 3 times weekly, versus radiotherapy with amifostine 5 times weekly: A prospective randomized study in squamous cell head and neck cancer. Cancer 2006, 107, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, T.H.; Brizel, D.; Henke, M.; Monnier, A.; Eschwege, F.; Sauer, R.; Strnad, V. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and- neck cancer: 2-year follow-up of a prospective, randomized, phase III trial. Int. J. Radiat. Oncol. 2005, 63, 985–990. [Google Scholar] [CrossRef]
- Büntzel, J.; Küttner, K.; Fröhlich, D.; Glatzel, M. Selective cytoprotection with amifostine in concurrent radiochemotherapy for head and neck cancer. Ann. Oncol. 1998, 9, 505–509. [Google Scholar] [CrossRef]
- Antonadou, D.; Pepelassi, M.; Synodinou, M.; Puglisi, M.; Throuvalas, N. Prophylactic use of amifostine to prevent radiochemotherapy-induced mucositis and xerostomia in head-and-neck cancer. Int. J. Radiat. Oncol. 2002, 52, 739–747. [Google Scholar] [CrossRef]
- Brizel, D.M.; Wasserman, T.H.; Henke, M.; Strnad, V.; Rudat, V.; Monnier, A.; Eschwege, F.; Zhang, J.; Russell, L.; Oster, W.; et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J. Clin. Oncol. 2000, 18, 3339–3345. [Google Scholar] [CrossRef]
- Buentzel, J.; Micke, O.; Adamietz, I.A.; Monnier, A.; Glatzel, M.; de Vries, A. Intravenous amifostine during chemoradiotherapy for head-and-neck cancer: A randomized placebo-controlled phase III study. Int. J. Radiat. Oncol. 2006, 64, 684–691. [Google Scholar] [CrossRef]
- Rades, D.; Fehlauer, F.; Bajrovic, A.; Mahlmann, B.; Richter, E.; Alberti, W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother. Oncol. 2004, 70, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Thorstad, W.L.; Chao, K.S.; Haughey, B. Toxicity and compliance of subcutaneous amifostine in patients undergoing postop-erative intensity-modulated radiation therapy for head and neck cancer. Semin. Oncol. 2004, 31 (Suppl. 18), 8–12. [Google Scholar] [CrossRef] [PubMed]
- Bardet, E.; Martin, L.; Calais, G.; Alfonsi, M.; Feham, N.E.; Tuchais, C.; Boisselier, P.; Dessard-Diana, B.; Seng, S.-H.; Garaud, P.; et al. Subcutaneous Compared With Intravenous Administration of Amifostine in Patients With Head and Neck Cancer Receiving Radiotherapy: Final Results of the GORTEC2000-02 Phase III Randomized Trial. J. Clin. Oncol. 2011, 29, 127–133. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Kyrias, G.; Kakolyris, S.; Kouroussis, C.; Frangiadaki, C.; Giatromanolaki, A.; Retalis, G.; Georgoulias, V. Subcutaneous Administration of Amifostine During Fractionated Radiotherapy: A Randomized Phase II Study. J. Clin. Oncol. 2000, 18, 2226–2233. [Google Scholar] [CrossRef]
- Lee, M.G.; Freeman, A.R.; Roos, D.E.; Milner, A.D.; Borg, M.F. Randomized double-blind trial of amifostine versus placebo for radiation-induced xerostomia in patients with head and neck cancer. J. Med. Imaging Radiat. Oncol. 2019, 63, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, R.; Sonis, S.; Posner, M.; Wirth, L.; Costello, R.; Braschayko, P.; Allen, A.; Mahadevan, A.; Flynn, J.; Burke, E.; et al. Randomized phase 2 study of concomitant chemoradiotherapy using weekly carboplatin/paclitaxel with or without daily subcutaneous amifostine in patients with locally advanced head and neck cancer. Cancer 2009, 115, 4514–4523. [Google Scholar] [CrossRef]
- Tong, Q.; Weaver, M.R.; Kosmacek, E.A.; O’Connor, B.P.; Harmacek, L.; Venkataraman, S.; Oberley-Deegan, R.E. MnTE-2-PyP reduces prostate cancer growth and metastasis by suppressing p300 activity and p300/HIF-1/CREB binding to the promoter region of the PAI-1 gene. Free Radic. Biol. Med. 2016, 94, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Tse, H.M.; Milton, M.J.; Piganelli, J.D. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: Implication for their use in targeting oxidation–reduction reactions in innate immunity. Free Radic. Biol. Med. 2004, 36, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Carroll, D.; You, Y.; Chaiswing, L.; Wen, R.; Batinic-Haberle, I.; Bondada, S.; Liang, Y.; Clair, D.S. A novel redox regulator, MnTnBuOE-2-PyP5+, enhances normal hematopoietic stem/progenitor cell function. Redox Biol. 2017, 12, 129–138. [Google Scholar] [CrossRef]
- Gauter-Fleckenstein, B.; Fleckenstein, K.; Owzar, K.; Jiang, C.; Batinic-Haberle, I.; Vujaskovic, Z. Comparison of two Mn porphyrin-based mimics of superoxide dismutase in pulmonary radioprotection. Free Radic. Biol. Med. 2008, 44, 982–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sishc, B.J.; Ding, L.; Nam, T.-K.; Heer, C.D.; Rodman, S.N.; Schoenfeld, J.D.; Fath, M.A.; Saha, D.; Pulliam, C.F.; Langen, B.; et al. Avasopasem manganese synergizes with hypofractionated radiation to ablate tumors through the generation of hydrogen peroxide. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.K.; Fey, E.G.; Watkins, B.A.; Wong, V.; Rothstein, D.; Sonis, S.T. Efficacy of superoxide dismutase mimetic M40403 in attenuating radiation-induced oral mucositis in hamsters. Clin. Cancer Res. 2008, 14, 4292–4297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sishc, B.; Saha, D.; Story, M. Avasopasem Manganese Protects Against Radiation Induced Oral Mucositis and Enhances the Response of Squamous Cell Carcinoma of the Head and Neck to Ionizing Radiation and Radioimmune Therapy. Int. J. Radiat. Oncol. 2020, 108, S159. [Google Scholar] [CrossRef]
- Anderson, C.M.; Sonis, S.T.; Lee, C.M.; Adkins, D.; Allen, B.G.; Sun, W.; Agarwala, S.S.; Venigalla, M.L.; Chen, Y.; Zhen, W.; et al. Phase 1b/2a Trial of the Superoxide Dismutase Mimetic GC4419 to Reduce Chemoradiotherapy-Induced Oral Mucositis in Patients With Oral Cavity or Oropharyngeal Carcinoma. Int. J. Radiat. Oncol. 2018, 100, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.M.; Lee, C.M.; Saunders, D.P.; Curtis, A.; Dunlap, N.; Nangia, C.; Lee, A.S.; Gordon, S.M.; Kovoor, P.; Arevalo-Araujo, R.; et al. Phase IIb, Randomized, Double-Blind Trial of GC4419 Versus Placebo to Reduce Severe Oral Mucositis Due to Concurrent Radiotherapy and Cisplatin For Head and Neck Cancer. J. Clin. Oncol. 2019, 37, 3256–3265. [Google Scholar] [CrossRef]
- El-Mahdy, M.A.; Alzarie, Y.A.; Hemann, C.; Badary, O.A.; Nofal, S.; Zweier, J.L. The novel SOD mimetic GC4419 increases cancer cell killing with sensitization to ionizing radiation while protecting normal cells. Free Radic. Biol. Med. 2020, 160, 630–642. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Boss, M.K.; Tovmasyan, A.; Choudhury, K.R.; Fontanella, A.N.; Young, K.H.; Palmer, G.M.; Birer, S.R.; Landon, C.D.; Park, W.; et al. Novel Manganese-Porphyrin Superoxide Dismutase-Mimetic Widens the Therapeutic Margin in a Preclin-ical Head and Neck Cancer Model. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Birer, S.R.; Lee, C.-T.; Choudhury, K.R.; Young, K.H.; Spasojevic, I.; Batinic-Haberle, I.; Crapo, J.D.; Dewhirst, M.W.; Ashcraft, K.A.; Young, K.H. Inhibition of the Continuum of Radiation-Induced Normal Tissue Injury by a Redox-Active Mn Porphyrin. Radiat. Res. 2017, 188, 94–104. [Google Scholar] [CrossRef]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.-I.; Simone, N.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, S.M.; Tochner, Z.; Krishna, C.M.; Glass, J.; Wilson, L.; Samuni, A.; Sprague, M.; Venzon, D.; Glatstein, E.; Mitchell, J.B. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992, 52, 1750–1753. [Google Scholar]
- Citrin, D.; Cotrim, A.P.; Hyodo, F.; Baum, B.J.; Krishna, M.C.; Mitchell, J.B. Radioprotectors and Mitigators of Radiation-Induced Normal Tissue Injury. Oncologist 2010, 15, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, A.P.; Sowers, A.L.; Lodde, B.M.; Vitolo, J.M.; Kingman, A.; Russo, A.; Mitchell, J.B.; Baum, B.J. Kinetics of Tempol for Prevention of Xerostomia Following Head and Neck Irradiation in a Mouse Model. Clin. Cancer Res. 2005, 11, 7564–7568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotrim, A.P.; Hyodo, F.; Matsumoto, K.I.; Sowers, A.L.; Cook, J.A.; Baum, B.J.; Krishna, M.C.; Mitchell, J.B. Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assess-ment of Tempol by magnetic resonance imaging. Clin. Cancer Res. 2007, 13, 4928–4933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Wang, Y.; Cotrim, A.; Zhu, Z.; Gao, R.; Zheng, C.; Goldsmith, C.; Jin, L.; Zhang, C.; Mitchell, J.; et al. Effect of Tempol on the prevention of irradiation-induced mucositis in miniature pigs. Oral Dis. 2017, 23, 801–808. [Google Scholar] [CrossRef]
- Dickinson, D.; DeRossi, S.; Yu, H.; Thomas, C.; Kragor, C.; Paquin, B.; Hahn, E.; Ohno, S.; Yamamoto, T.; Hsu, S. Epigallocatechin-3-gallate modulates anti-oxidant defense enzyme expression in murine submandibular and pancreatic exocrine gland cells and human HSG cells. Autoimmunity 2014, 47, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Staples, J.; Wataha, J.; Lewis, J.; Lockwood, P.; Schoenlein, P.; Rao, S.; Osaki, T.; Dickinson, D.; Kamatani, T.; et al. Protective effects of EGCG on salivary gland cells treated with gamma-radiation or cis-platinum(II)diammine dichloride. Anticancer Res. 2004, 24, 3065–3073. [Google Scholar] [PubMed]
- Sulistiyani, E.; Brimson, J.; Chansaenroj, A.; Sariya, L.; Urkasemsin, G.; Oonsiri, S.; Tencomnao, T.; Vacharaksa, A.; Chaisuparat, R.; Ferreira, J. Epigallocatechin-3-Gallate Protects Pro-Acinar Epithelia Against Salivary Gland Radiation Injury. Int. J. Mol. Sci. 2021, 22, 3162. [Google Scholar] [CrossRef]
- Jeong, B.K.; Song, J.H.; Jeong, H.; Choi, H.S.; Jung, J.H.; Hahm, J.R.; Woo, S.H.; Jung, M.H.; Choi, B.-H.; Kim, J.H.; et al. Effect of alpha-lipoic acid on radiation-induced small intestine injury in mice. Oncotarget 2016, 7, 15105–15117. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.H.; Jung, J.; Kim, S.K.; Woo, S.H.; Kang, K.M.; Jeong, B.K.; Jung, M.H.; Kim, J.H.; Hahm, J.R. Alpha lipoic acid attenuates radiation-induced thyroid injury in rats. PLoS ONE 2014, 9, e112253. [Google Scholar]
- Kim, J.H.; Kim, K.M.; Jung, M.H.; Jung, J.H.; Kang, K.M.; Jeong, B.K.; Kim, J.P.; Park, J.J.; Woo, S.H. Protective effects of alpha lipoic acid on radiation-induced salivary gland injury in rats. Oncotarget 2016, 7, 29143–29153. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, M.H.; Kim, J.P.; Kim, H.J.; Jung, J.H.; Hahm, J.R.; Kang, K.M.; Jeong, B.-K.; Woo, S.H. Alpha lipoic acid attenuates radiation-induced oral mucositis in rats. Oncotarget 2017, 8, 72739–72747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akyuz, M.; Taysi, S.; Baysal, E.; Demir, E.; Alkis, H.; Akan, M.; Binici, H.; Karatas, Z.A. Radioprotective effect of thymoquinone on salivary gland of rats exposed to total cranial irradiation. Head Neck 2017, 39, 2027–2035. [Google Scholar] [CrossRef] [PubMed]
- Abedi, S.M.; Yarmand, F.; Motallebnejad, M.; Seyedmajidi, M.; Moslemi, D.; Ashrafpour, M.; Bijani, A.; Moghadamnia, A.A.; Mardanshahi, A.; Hosseinimehr, S.J. Vitamin E protects salivary glands dysfunction induced by ionizing radiation in rats. Arch. Oral Biol. 2015, 60, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Karaer, I.C.; Simsek, G.; Yildiz, A.; Vardi, N.; Polat, A.; Tanbek, K.; Gurocak, S.; Parlakpinar, H. Melatonin’s protective effect on the salivary gland against ionized radiation damage in rats. J. Oral Pathol. Med. 2016, 45, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, X.; Cai, J.; Ma, J.; Cheng, H.; Zhao, K.; Yang, L.; Cao, Y.; Qin, Q.; Zhang, C.; et al. Resveratrol attenuates radiation-induced salivary gland dysfunction in mice. Laryngoscope 2013, 123, E23–E29. [Google Scholar] [CrossRef]
- Chung, M.K.; Kim, D.H.; Ahn, Y.C.; Choi, J.Y.; Kim, E.H.; Son, Y.-I. Randomized Trial of Vitamin C/E Complex for Prevention of Radiation-Induced Xerostomia in Patients with Head and Neck Cancer. Otolaryngol. Neck Surg. 2016, 155, 423–430. [Google Scholar] [CrossRef]
- Shin, H.-S.; Lee, S.; Kim, Y.-M.; Lim, J.-Y. Hypoxia-Activated Adipose Mesenchymal Stem Cells Prevents Irradiation-Induced Salivary Hypofunction by Enhanced Paracrine Effect Through Fibroblast Growth Factor 10. Stem Cells 2018, 36, 1020–1032. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Gao, R.; Xu, J.; Jin, L.; Cotrim, A.P.; Yan, X.; Zheng, C.; Goldsmith, C.M.; Shan, Z.; Hai, B.; et al. AdLTR2EF1α-FGF2-mediated prevention of fractionated irradiation-induced salivary hypofunction in swine. Gene Ther. 2014, 21, 866–873. [Google Scholar] [CrossRef]
- Zheng, C.; Cotrim, A.P.; Rowzee, A.; Swaim, W.; Sowers, A.; Mitchell, J.B.; Baum, B.J. Prevention of Radiation-Induced Salivary Hypofunction Following hKGF Gene Delivery to Murine Submandibular Glands. Clin. Cancer Res. 2011, 17, 2842–2851. [Google Scholar] [CrossRef] [Green Version]
- Meyer, S.; Chibly, A.; Burd, R.; Limesand, K. Insulin-Like Growth Factor-1–Mediated DNA Repair in Irradiated Salivary Glands Is Sirtuin-1 Dependent. J. Dent. Res. 2017, 96, 225–232. [Google Scholar] [CrossRef]
- Cho, J.; Yoon, Y.; Lee, S.; Kim, D.; Choi, D.; Kim, J.; Lim, J. Retroductal Delivery of Epidermal Growth Factor Protects Salivary Progenitors after Irradiation. J. Dent. Res. 2021, 100, 883–890. [Google Scholar] [CrossRef]
- Mitchell, G.C.; Fillinger, J.L.; Sittadjody, S.; Avila, J.L.; Burd, R.; Limesand, K.H. IGF1 activates cell cycle arrest following irradiation by reducing binding of ΔNp63 to the p21 promoter. Cell Death Dis. 2010, 1, e50. [Google Scholar] [CrossRef] [Green Version]
- Martin, K.L.; Hill, G.A.; Klein, R.R.; Arnett, D.G.; Burd, R.; Limesand, K.H. Prevention of Radiation-Induced Salivary Gland Dysfunction Utilizing a CDK Inhibitor in a Mouse Model. PLoS ONE 2012, 7, e51363. [Google Scholar] [CrossRef] [Green Version]
- Victory, K.; Burd, R.; Fribley, A.; Sittadjody, S.; Arnett, D.K.; Klein, R.; Limesand, K.H. Head and Neck Tumor Cell Radiation Response Occurs in the Presence of IGF1. J. Dent. Res. 2010, 90, 347–352. [Google Scholar] [CrossRef]
- Chibly, A.M.; Wong, W.Y.; Pier, M.; Cheng, H.; Mu, Y.; Chen, J.; Ghosh, S.; Limesand, K.H. aPKCζ-dependent Repression of Yap is Necessary for Functional Restoration of Irradiated Salivary Glands with IGF-1. Sci. Rep. 2018, 8, 6347. [Google Scholar] [CrossRef]
- Sun, X.; Yang, X.; Chen, J.; Ge, X.-L.; Qin, Q.; Zhu, H.; Zhang, C.; Xu, L. Simvastatin attenuates radiation-induced salivary gland dysfunction in mice. Drug Des. Dev. Ther. 2016, 10, 2271–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegelberg, L.; Swagemakers, S.M.A.; Van Ijcken, W.F.J.; Oole, E.; Wolvius, E.B.; Essers, J.; Braks, J.A.M. Gene Expression Analysis Reveals Inhibition of Radiation-Induced TGFβ-Signaling by Hyperbaric Oxygen Therapy in Mouse Salivary Glands. Mol. Med. 2014, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Wie, S.M.; Wellberg, E.; Karam, S.D.; Reyland, M.E. Tyrosine Kinase Inhibitors Protect the Salivary Gland from Radiation Damage by Inhibiting Activation of Pro-tein Kinase C-δ. Mol. Cancer Ther. 2017, 16, 1989–1998. [Google Scholar] [CrossRef] [Green Version]
- Affandi, T.; Ohm, A.M.; Gaillard, D.; Haas, A.; Reyland, M.E. Tyrosine kinase inhibitors protect the salivary gland from radiation damage by increasing DNA double-strand break repair. J. Biol. Chem. 2021, 296, 100401. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Pang, B.; Iglesias-Bartolome, R.; Wu, X.; Hu, L.; Zhang, C.; Wang, J.; Gutkind, J.S.; Wang, S. Prevention of irradiation-induced salivary hypofunction by rapamycin in swine parotid glands. Oncotarget 2016, 7, 20271–20281. [Google Scholar] [CrossRef]
- Hai, B.; Qin, L.; Yang, Z.; Zhao, Q.; Shangguan, L.; Ti, X.; Zhao, Y.; Kim, S.; Rangaraj, D.; Liu, F. Transient Activation of Hedgehog Pathway Rescued Irradiation-Induced Hyposalivation by Preserving Salivary Stem/Progenitor Cells and Parasympathetic Innervation. Clin. Cancer Res. 2014, 20, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Pringle, S.; Van Os, R.; Coppes, R.P. Concise Review: Adult Salivary Gland Stem Cells and a Potential Therapy for Xerostomia. Stem Cells 2013, 31, 613–619. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, L.; Xu, M.; Zheng, Y. Restoring the Secretory Function of Irradiation-Damaged Salivary Gland by Administrating Deferoxamine in Mice. PLoS ONE 2014, 9, e113721. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Varendi, K.; Maimets, M.; Andressoo, J.-O.; Coppes, R.P. Role of glial-cell-derived neurotrophic factor in salivary gland stem cell response to irradiation. Radiother. Oncol. 2017, 124, 448–454. [Google Scholar] [CrossRef]
- Xiao, N.; Lin, Y.; Cao, H.; Sirjani, D.; Giaccia, A.J.; Koong, A.; Kong, C.S.; Diehn, M.; Le, Q.-T. Neurotrophic factor GDNF promotes survival of salivary stem cells. J. Clin. Investig. 2014, 124, 3364–3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maimets, M.; Rocchi, C.; Bron, R.; Pringle, S.; Kuipers, J.; Giepmans, B.; Vries, R.G.; Clevers, H.; de Haan, G.; van Os, R.; et al. Long-Term In Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals. Stem Cell Rep. 2016, 6, 150–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hai, B.; Yang, Z.; Shangguan, L.; Zhao, Y.; Boyer, A.; Liu, F. Concurrent Transient Activation of Wnt/β-Catenin Pathway Prevents Radiation Damage to Salivary Glands. Int. J. Radiat. Oncol. 2012, 83, e109–e116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.-F.; Liu, C.; Zhang, Q.; Huang, G.-H. Research progress in the radioprotective effect of the canonical Wnt pathway. Cancer Biol. Med. 2013, 10, 61–71. [Google Scholar] [CrossRef]
- Feng, J.; van der Zwaag, M.; Stokman, M.A.; van Os, R.; Coppes, R.P. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother. Oncol. 2009, 92, 466–471. [Google Scholar] [CrossRef]
- Nanduri, L.S.; Lombaert, I.M.; van der Zwaag, M.; Faber, H.; Brunsting, J.F.; van Os, R.P.; Coppes, R.P. Salisphere derived c-Kit+ cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother. Oncol. 2013, 108, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Maimets, M.; Bron, R.; de Haan, G.; van Os, R.; Coppes, R.P. Similar ex vivo expansion and post-irradiation regenerative potential of juvenile and aged salivary gland stem cells. Radiother. Oncol. 2015, 116, 443–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombaert, I.M.A.; Brunsting, J.F.; Wierenga, P.K.; Kampinga, H.; de Haan, G.; Coppes, R.P. Cytokine Treatment Improves Parenchymal and Vascular Damage of Salivary Glands after Irradiation. Clin. Cancer Res. 2008, 14, 7741–7750. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Fang, D.; Liu, Y.; Ruan, G.; Seuntjens, J.; Kinsella, J.; Tran, S. Lyophilized bone marrow cell extract functionally restores irradiation-injured salivary glands. Oral Dis. 2018, 24, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Su, X.; Liu, Y.; Lee, J.C.; Seuntjens, J.; Tran, S.D. Cell extracts from spleen and adipose tissues restore function to irradiation-injured salivary glands. J. Tissue Eng. Regen. Med. 2017, 12, e1289–e1296. [Google Scholar] [CrossRef] [PubMed]
- Saiki, J.P.; Cao, H.; Van Wassenhove, L.D.; Viswanathan, V.; Bloomstein, J.; Nambiar, D.K.; Mattingly, A.J.; Jiang, D.; Chen, C.-H.; Stevens, M.C.; et al. Aldehyde dehydrogenase 3A1 activation prevents radiation-induced xerostomia by protecting salivary stem cells from toxic aldehydes. Proc. Natl. Acad. Sci. USA 2018, 115, 6279–6284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, D.; Shang, S.; Liu, Y.; Bakkar, M.; Sumita, Y.; Seuntjens, J.; Tran, S.D. Optimal timing and frequency of bone marrow soup therapy for functional restoration of salivary glands injured by single-dose or fractionated irradiation. J. Tissue Eng. Regen. Med. 2018, 12, e1195–e1205. [Google Scholar] [CrossRef]
- Mishima, K.; Inoue, H.; Nishiyama, T.; Mabuchi, Y.; Amano, Y.; Ide, F.; Matsui, M.; Yamada, H.; Yamamoto, G.; Tanaka, J.; et al. Transplantation of side population cells restores the function of damaged exocrine glands through clusterin. Stem Cells 2012, 30, 1925–1937. [Google Scholar] [CrossRef]
- Abughanam, G.; Elkashty, O.A.; Liu, Y.; Bakkar, M.O.; Tran, S.D. Mesenchymal Stem Cells Extract (MSCsE)-Based Therapy Alleviates Xerostomia and Keratoconjunctivitis Sicca in Sjogren’s Syndrome-Like Disease. Int. J. Mol. Sci. 2019, 20, 4750. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.-Y.; Yi, T.; Choi, J.-S.; Jang, Y.H.; Lee, S.; Kim, H.J.; Song, S.U.; Kim, Y.-M. Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage. Oral Oncol. 2013, 49, 136–143. [Google Scholar] [CrossRef]
- Sumita, Y.; Liu, Y.; Khalili, S.; Maria, O.M.; Xia, D.; Key, S.; Cotrim, A.P.; Mezey, E.; Tran, S.D. Bone marrow-derived cells rescue salivary gland function in mice with head and neck irradiation. Int. J. Biochem. Cell Biol. 2011, 43, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Yan, X.; Zheng, C.; Goldsmith, C.M.; Afione, S.; Hai, B.; Xu, J.; Zhou, J.; Zhang, C.; Chiorini, J.A.; et al. AAV2-mediated transfer of the human aquaporin-1 cDNA restores fluid secretion from irradiated miniature pig parotid glands. Gene Ther. 2010, 18, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Di Pasquale, G.; Riveros, P.P.; Tora, M.; Sheikh, T.; Son, A.; Teos, L.; Grewe, B.; Swaim, W.D.; Afione, S.; Zheng, C.; et al. Transduction of Salivary Gland Acinar Cells with a Novel AAV Vector 44.9. Mol. Ther. Methods Clin. Dev. 2020, 19, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Teos, L.Y.; Zheng, C.-Y.; Liu, X.; Swaim, W.D.; Goldsmith, C.M.; Cotrim, A.P.; Baum, B.J.; Ambudkar, I.S. Adenovirus-mediated hAQP1 expression in irradiated mouse salivary glands causes recovery of saliva secretion by enhancing acinar cell volume decrease. Gene Ther. 2016, 23, 572–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, C.; Baum, B.J.; Liu, X.; Goldsmith, C.M.; Perez, P.; Jang, S.-I.; Cotrim, A.P.; Mccullagh, L.; Ambudkar, I.S.; Alevizos, I. Persistence of hAQP1 expression in human salivary gland cells following AdhAQP1 transduction is associated with a lack of methylation of hCMV promoter. Gene Ther. 2015, 22, 758–766. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Zhu, Z.; Hai, B.; Chang, S.; Ma, L.; Xu, Y.; Li, X.; Feng, X.; Wu, X.; Zhao, Q.; et al. Intragland Shh gene delivery mitigated irradiation-induced hyposalivation in a miniature pig model. Theranostics 2018, 8, 4321–4331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, L.; Hai, B.; Wang, J.; Baetge, C.L.; Deveau, M.A.; Kapler, G.M.; Feng, J.Q.; Liu, F. Transient Activation of the Hedgehog-Gli Pathway Rescues Radiotherapy-Induced Dry Mouth via Recovering Salivary Gland Resident Macrophages. Cancer Res. 2020, 80, 5531–5542. [Google Scholar] [CrossRef] [PubMed]
- Verneret, M.; Tacnet-Delorme, P.; Osman, R.; Awad, R.; Grichine, A.; Kleman, J.-P.; Frachet, P. Relative contribution of c1q and apoptotic cell-surface calreticulin to macrophage phagocytosis. J. Innate Immun. 2014, 6, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Benoit, M.E.; Clarke, E.; Morgado, P.; Fraser, D.A.; Tenner, A.J. Complement Protein C1q Directs Macrophage Polarization and Limits Inflammasome Activity during the Uptake of Apoptotic Cells. J. Immunol. 2012, 188, 5682–5693. [Google Scholar] [CrossRef] [Green Version]
- Hai, B.; Zhao, Q.; Qin, L.; Rangaraj, D.; Gutti, V.R.; Liu, F. Rescue Effects and Underlying Mechanisms of Intragland Shh Gene Delivery on Irradiation-Induced Hyposaliva-tion. Hum. Gene Ther. 2016, 27, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Lombaert, I.M.; Patel, V.N.; Jones, C.E.; Villier, D.C.; Canada, A.E.; Moore, M.R.; Berenstein, E.; Zheng, C.; Goldsmith, C.M.; Chorini, J.A.; et al. CERE-120 Prevents Irradiation-Induced Hypofunction and Restores Immune Homeostasis in Porcine Salivary Glands. Mol. Ther. Methods Clin. Dev. 2020, 18, 839–855. [Google Scholar] [CrossRef]
- Li, S.-S.; Wu, C.-Z.; Zhang, B.-W.; Qiu, L.; Chen, W.; Yuan, Y.-H.; Liu, X.-C.; Li, C.-J.; Li, L.-J. Nerve growth factor protects salivary glands from irradiation-induced damage. Life Sci. 2021, 265, 118748. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.J.; Zheng, C.; Alevizos, I.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; McDermott, N.; Chiorini, J.A.; Nikolov, N.P.; et al. Development of a gene transfer-based treatment for radiation-induced salivary hypofunction. Oral Oncol. 2010, 46, 4–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hai, B.; Yan, X.; Voutetakis, A.; Zheng, C.; Cotrim, A.P.; Shan, Z.; Ding, G.; Zhang, C.; Xu, J.; Goldsmith, C.M.; et al. Long-term transduction of miniature pig parotid glands using serotype 2 adeno-associated viral vectors. J. Gene Med. 2009, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zourelias, L.; Wu, C.; Edwards, P.C.; Trombetta, M.; Passineau, M.J. Ultrasound-assisted nonviral gene transfer of AQP1 to the irradiated minipig parotid gland restores fluid secretion. Gene Ther. 2015, 22, 739–749. [Google Scholar] [CrossRef] [Green Version]
| Treatment | Radiation Dose | Medicine | Drug Dosage | Mode of Administration | Routes of Administration | Mechanism | Conclusion | Reference |
|---|---|---|---|---|---|---|---|---|
| IGF1 | 5 Gy | Roscovitine | 25 mg/kg 100 mg/kg | Intraperitoneal injection | Female FVB mice | A cyclin-dependent kinase inhibitor that acts to transiently inhibit cell cycle progression and allow for DNA repair in damaged tissues. | Induction of transient G2/M cell cycle arrest by roscovitine allows for the suppression of apoptosis, thus preserving normal salivary function following targeted head and neck irradiation. | [123] |
| 5 Gy | IGF1 | 5 μg/mouse | Tail-vein injection | FVB, C57BL/6 J, and Prkcz−/− mice | Administration of IGF1 post-radiation maintains the activation of aPKCζ and partially rescues Yap’s cellular localization in label-retaining cells while restoring salivary function. | aPKCζ is required to restore the function of irradiated SGs using IGF1. This restoration process involves the maintenance of aPKCζ phosphorylation and the modulation of nuclear translocation of Yap in acinar LRCs in an aPKCζ-dependent fashion. | [125] | |
| 2 or 5 Gy | IGF1 | 10 ng/mL | Intravenous injection | Human UMSCC1, UMSCC23, CAL 27, A-253, and FaDU cells | Not stated | Head and neck squamous carcinoma cell xenografts treated with concurrent radiation and IGF1 also exhibit significant tumor growth delay. | [124] | |
| 5 Gy | IGF1 | 5 μg | Vehicle injection | Female FVB mice | Not stated | Post-therapeutic IGF1 treatment restores SG function, potentially through the normalization of cell proliferation and the improved expression of amylase. | [16] | |
| 5 Gy | Intravenous recombinant human IGF1 | 5 μg | Intravenous | FVB females | In parotid glands of irradiated mice pretreated with IGF1, reduced ΔNp63 protein facilitates a p53-mediated increase in p21 expression that leads to G2/M arrest. | Radiation-induced increases in ΔNp63 protein correspond with enhanced binding to the p21 promoter and decreased p21 transcription in irradiated parotid glands after 8 h compared to parotid glands pretreated with IGF1. | [122] | |
| Targeted therapy | 15 Gy | HBOT | Once a day for five consecutive days a week | - | Female C3H mice | Not stated | HBOT can inhibit the TGFβ-pathway in irradiated SGs and restrain consequential radiation-induced tissue injury. | [127] |
| 15 Gy | SIM | 10 mg/kg | IP injection | Male ICR mice | The protective benefits of SIM may be attributed to scavenging malondialdehyde, remitting collagen deposition, and reducing and delaying the elevation of TGFβ1 expression induced by radiation. | SIM remitted the reduction of saliva secretion and restored salivary amylase activity. | [126] | |
| 10 or 15 Gy | Imatinib dasatinib | Imatinib (50 mg/kg) and dasatinib (20 mg/kg) | Not stated | The ParC5 cell line HNSCC cell lines | PKCδ is required for IR-induced apoptosis in the SG and that blocking activation of PKCδ with TKIs suppresses apoptosis. | Dasatinib and imatinib provide the profound and durable protection of SG function in vivo when delivered in conjunction with a single or fractionated doses of IR. | [128] | |
| Not stated | Imatinib dasatinib | Dasatinib (20 mg/kg) | Not stated | ParC5 cell line 293T cells | TKIs effective against c-Src and c-Abl are able to block multiple key regulatory steps necessary for PKCδ nuclear localization, leading to suppression of apoptosis both in vitro and in vivo. | TKIs is useful for the protection of nontumor tissues in patients undergoing radiotherapy of the head and neck. | [63] |
| Study | Cells | Radiation Dose | Treatment | Detection Method | Pathway to Restore SG Function | Treatment Effect | Conclusion | Reference |
|---|---|---|---|---|---|---|---|---|
| Martti Maimets et al. | SG ductal EpCAM and cells | 15 Gy | - | SFR | Wnt signals | Nuclear β-catenin. | Stimulating self-renewal and long-term expansion of SG organoids, containing all differentiated SG cell types. | [136] |
| Bo Hai et al. | Mouse SGs and cultured human salivary epithelial cells | 15 Gy | Sonic hedgehog (Shh) transgene or Smoothened Agonist in mouse salivary glands, adenovirus encoding Gli1 in human salivary epithelial cells | Detection of Ptch1-lacZ reporter gene and endogenous Hedgehog target gene expression | Transient activation of Hedgehog pathway | Preservation of salivary stem/progenitor cells, the Bmi1 signaling pathway, parasympathetic innervation, Chrm1/HB-EGF signaling, and expression of neurotrophic factors after IR by transient Hh activation. | Transient Shh overexpression activated the Hedgehog pathway in ductal epithelia, thus rescuing salivary function in male mice, which was related to the preservation of functional SSPCs and parasympathetic innervation. | [131] |
| Yoshinori Sumita et al. | Salivary epithelial cells | 18 Gy | BMDCs | The expression of stem cell markers (Sca-1 or c-kit) | Direct differentiation of donor BMDCs into salivary epithelial cells | An increased ratio of acinar-cell area and approximately 9% of Y-chromosome-positive (donor-derived) salivary epithelial cells in BMDC-treated mice. | A cell-therapy approach, the transplantation of BMDCs via intravenous injections, can regenerate radiation-damaged tissue and rescue SG functions. | [150] |
| Xiaohong Peng et al. | SG sphere derived cells of Gdnf hypermorphic (Gdnfwt/hyper) and wild type mice (Gdnfwt/wt) | 0, 1, 2, 4, and 8 Gy | - | QPCR and immunofluorescence | GDNF–RET signaling pathway | MSGSC of Gdnfwt/hyper mice showed high sphere-forming efficiency upon replating. | GDNF does not protect mSGSCs against irradiation but seems to promote mSGSC proliferation through the GDNF–RET signaling pathway. | [134] |
| Julie P Saiki et al. | Adult WT and Aldh3a1−/− murine SMGs | 15 and 30 Gy | D-limonene | PAS staining annexin V PI | ALDH3A1 plays an important role in protecting SSPCs from IR-induced injury by increasing aldehyde scavenging. | ALDH3A1 activation with d-Limonene reduces aldehydic load, improves sphere growth, and reduces apoptosis in SMGs. | d-limonene may be a good clinical candidate for mitigating xerostomia in patients with head and neck cancer receiving radiation therapy. | [145] |
| Nan Xiao et al. | C57BL/6 mice C57BL/6-Tg(UBC-GFP)30Scha/J mice | 15 Gy |
Lin–CD24+ c-Kit+Sca1+ stem cells (GDNF) | PAS staining revealed more functional and intact acini in GDNF-treated SMGs than in saline glands | Not stated | Administration of GDNF improved saliva production and enriched the number of functional acini in submandibular glands of irradiated animals, as well as enhancing salisphere formation in cultured salivary stem cells, but it did not accelerate growth of head and neck cancer cells. | GDNF pathway may have potential therapeutic benefit for the management of radiation-induced xerostomia. | [135] |
| Bo Hai et al. | C57BL/6 mice | 15 Gy | Adenoviral vector encoding GFP or rat Shh | Detection of SA-β-gal activity | mRNA levels of Chek1, Egfr, and survivin were significantly upregulated by the transfer of Shh inhibition of GDF15 upregulation | Shh gene transfer represses IR-induced cellular senescence by promoting DNA repair, decreasing oxidative stress (which is mediated through upregulating expression of genes related to DNA repair such as survivin and miR-21), and repressing expression of the pro-senescence gene Gdf15 likely downstream of miR-21. | Repressing cellular senescence contributes to the rescue of IR-induced hyposalivation by the transient activation of Hh signaling, which is related to enhanced DNA repair and decreased oxidative stress in SMGs. | [7] |
| Junye Zhang et al. | C57BL/6 mice | 18 Gy | DFO | SFR | DFO improved angiogenesis via activating HIF–1α–VEGF Pathway | In addition to the restoration of salivary function, DFO administration also increased angiogenesis and the number of stem/progenitor cells while reducing the apoptosis of acinar cells. | DFO could prevent the radiation-induced dysfunction of SGs in mice. | [133] |
| Scheme | Radiation Dose | Vector | Gene | Model | Detection Method | Pathway | Treatment Effect | Conclusion | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Runtao Gao et al. | 20 Gy | Serotype 2 and AAV2 vector | hAQP1 cDNA | Salivary hypofunction in minipigs | SFR | Not stated | In glands treated with the AAV2hAQP1 vector, a steady increase in parotid SFR was seen, such that by week 8, they were on average 35% of pre-IR values—nearly 1 mL/10 min. | The AAV2hAQP1 vector could be useful for targeting IR-surviving duct cells in previously irradiated head and neck cancer patients and providing them with a more extended (months) means of increasing salivary flow versus the AdhAQP1 vector (days). | [151] |
| C Zheng et al. | Not stated | hCMVp | hAQP1 cDNA | Female C3H mice and male Wistar rats | PCR assays Measurement of cell volume | Not stated | Not stated | The hCMVp in AdhAQP1was probably not methylated in transduced human SG cells of responding subjects, resulting in an unexpectedly longer functional expression of hAQP1. | [154] |
| L Guo et al. | 7.5 or 9 Gy | Hybrid serotype 5 adenoviral vector | FGF2 cDNA | Parotid glands of minipigs | Local blood flow rate measurement | Not stated | Compared to the IR and AdLacZ and IR groups, the salivary flow rates of the AdLTR2EF1α-FGF2 and IR group were significantly higher (p < 0.001) and only slightly changed from that of naive animals. | Pre-administration of AdLTR2EF1α-FGF2 prevented an IR-induced reduction in MVD in minipig parotid glands within 24 h after IR. Pre-administration of AdLTR2EF1α-FGF2 also led to significantly higher levels of salivary secretion than those seen with untreated but irradiated minipigs and with minipigs that were irradiated and administered a control vector. | [118] |
| Chang yu Zheng et al. | 15 Gy | AdLTR2EF1α-hKGF | KGF | Female C3H mice | Salivary flow | Not stated | The SFR from 2 groups (Mice receiving 15 Gy and AdControl) were also significantly lower than those of irradiated mice treated with AdLTR2EF1α-hKGF (p < 0.001). In contrast, the salivary flow rates from the no-IR and AdLTR2EF1α-hKGF plus IR groups were not significantly different (p = 0.065). | The hKGF gene transfer had no effect on the growth or radiation sensitivity of a model SCC. Transfer of the hKGF gene to SGs prior to both fractionated and single-dose IR substantially prevents salivary hypofunction. | [119] |
| Bo Hai et al. | 15 Gy | Adenoviral vector | Shh delivery | B6;129-Ptch1tm1Mps/J (Ptch1-lacZ) mice and wild-type C57BL/6 mice | X-Gal staining and qRT-PCR analysis saliva flow rate | Hedgehog/Gli | Shh gene transfer is a feasible approach to restore SG function after radiotherapy, which functions through ameliorations of IR damage to the microvasculature and parasympathetic innervation by the upregulation of paracrine factors. | Transient activation of the Hedgehog pathway by gene delivery is promising to rescue salivary function after irradiation in both sexes. | [159] |
| Liang Hu et al. | 20 Gy | Adenoviral vector | GFP or Shh | Healthy littermate BA–MA male miniature pigs | IHC qRT-PCR analysis | Hedgehog/Gli | Shh gene delivery 4 weeks after irradiation significantly improved stimulated saliva secretion and local blood supply up to 20 weeks; preserved saliva-producing acinar cells, parasympathetic innervation and microvessels as found in mouse models; and activated autophagy and inhibited fibrogenesis in irradiated glands. | The translational potential of transient activation of the Hedgehog pathway to preserve salivary function following irradiation. | [155] |
| Bo Hai et al. | 15 Gy | Adenoviral vector | human Gli1 or GFP | Ptch1-lacZ mice | qRT-PCR analysis and X-gal staining | Hedgehog/Gli | Transient Shh overexpression activated the Hedgehog pathway in ductal epithelia; and this, after irradiation, rescued salivary function in male mice, which was related to the preservation of functional SSPCs and parasympathetic innervation. | Transient activation of the Hedgehog pathway has the potential to restore irradiation-induced SG dysfunction. | [131] |
| Bo Hai et al. | Not stated | Serotype 2 AAV2 vectors | hEpo | Miniature pig | Enzyme-linked immunosorbent assay | - | AAV2 vectors mediate extended gene transfer to miniature pig parotid glands and should be useful for testing pre-clinical gene therapy strategies aiming to correct SG radiation damage. | AAV2 vectors mediate extended gene transfer to miniature pig parotid glands and should be useful for testing pre-clinical gene therapy strategies aiming to correct SG radiation damage. | [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Dong, L.; Zheng, Z.; Liu, S.; Gong, S.; Meng, L.; Xin, Y.; Jiang, X. Mechanism, Prevention, and Treatment of Radiation-Induced Salivary Gland Injury Related to Oxidative Stress. Antioxidants 2021, 10, 1666. https://doi.org/10.3390/antiox10111666
Liu Z, Dong L, Zheng Z, Liu S, Gong S, Meng L, Xin Y, Jiang X. Mechanism, Prevention, and Treatment of Radiation-Induced Salivary Gland Injury Related to Oxidative Stress. Antioxidants. 2021; 10(11):1666. https://doi.org/10.3390/antiox10111666
Chicago/Turabian StyleLiu, Zijing, Lihua Dong, Zhuangzhuang Zheng, Shiyu Liu, Shouliang Gong, Lingbin Meng, Ying Xin, and Xin Jiang. 2021. "Mechanism, Prevention, and Treatment of Radiation-Induced Salivary Gland Injury Related to Oxidative Stress" Antioxidants 10, no. 11: 1666. https://doi.org/10.3390/antiox10111666
APA StyleLiu, Z., Dong, L., Zheng, Z., Liu, S., Gong, S., Meng, L., Xin, Y., & Jiang, X. (2021). Mechanism, Prevention, and Treatment of Radiation-Induced Salivary Gland Injury Related to Oxidative Stress. Antioxidants, 10(11), 1666. https://doi.org/10.3390/antiox10111666








