A Study on Myogenesis by Regulation of Reactive Oxygen Species and Cytotoxic Activity by Selenium Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Selenium Nanoparticles (SeNPs)
2.3. Characterizations of SeNPs
2.4. Peroxidase Activity of SeNPs
2.5. Cell Culture
2.6. Cell Viability
2.7. ROS-Staining
2.8. qRT–PCR
2.9. Myotube Formation and Immunocytochemistry
2.10. Western Blot
2.11. Statistical Analysis
3. Results and Discussion
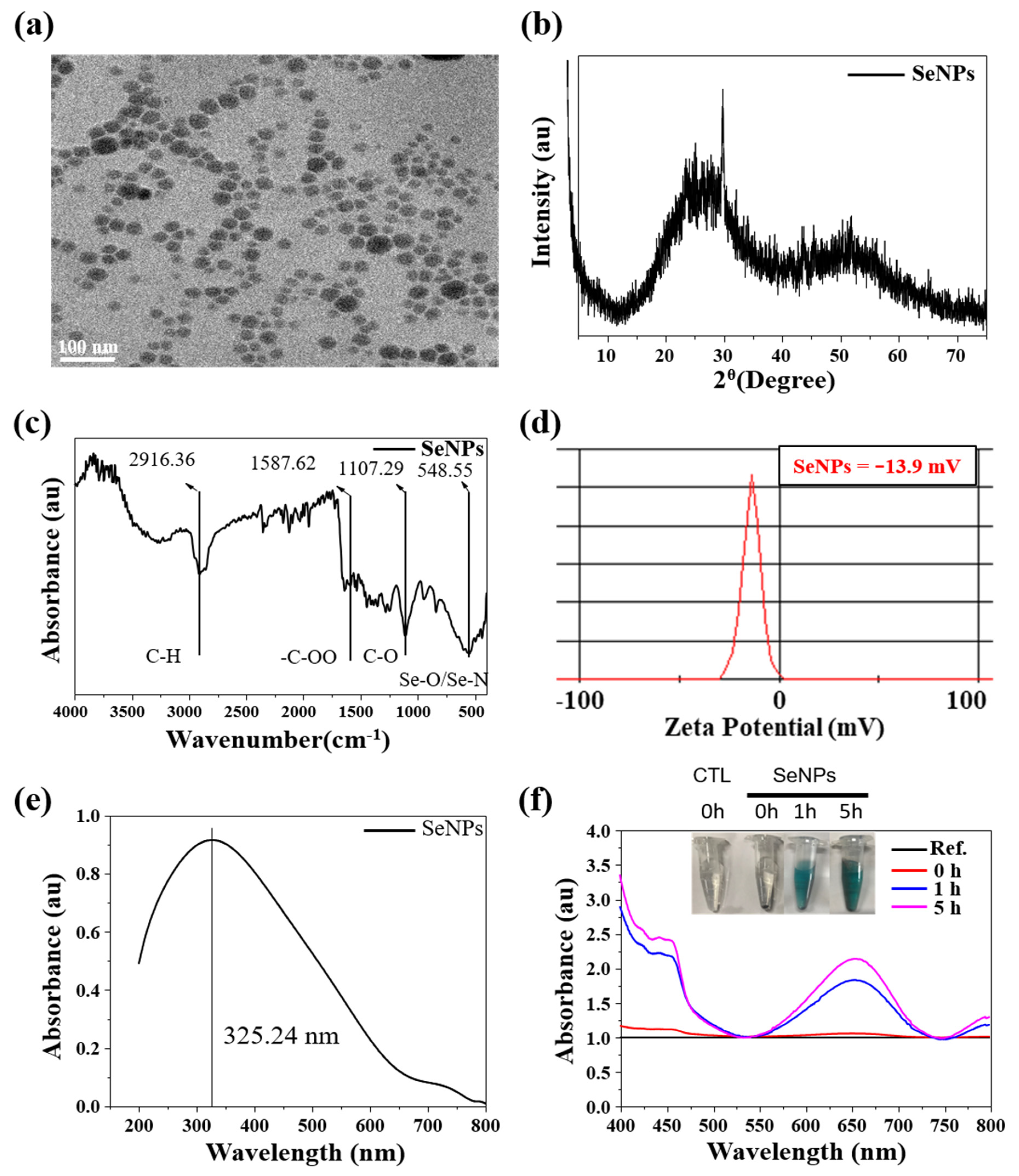
3.1. Characterization of SeNPs
3.2. TMB-Based Oxidase-like Activity
3.3. Effect of Selenium Nanoparticles on Cell Viability in C2C12-Induced ROS Conditions
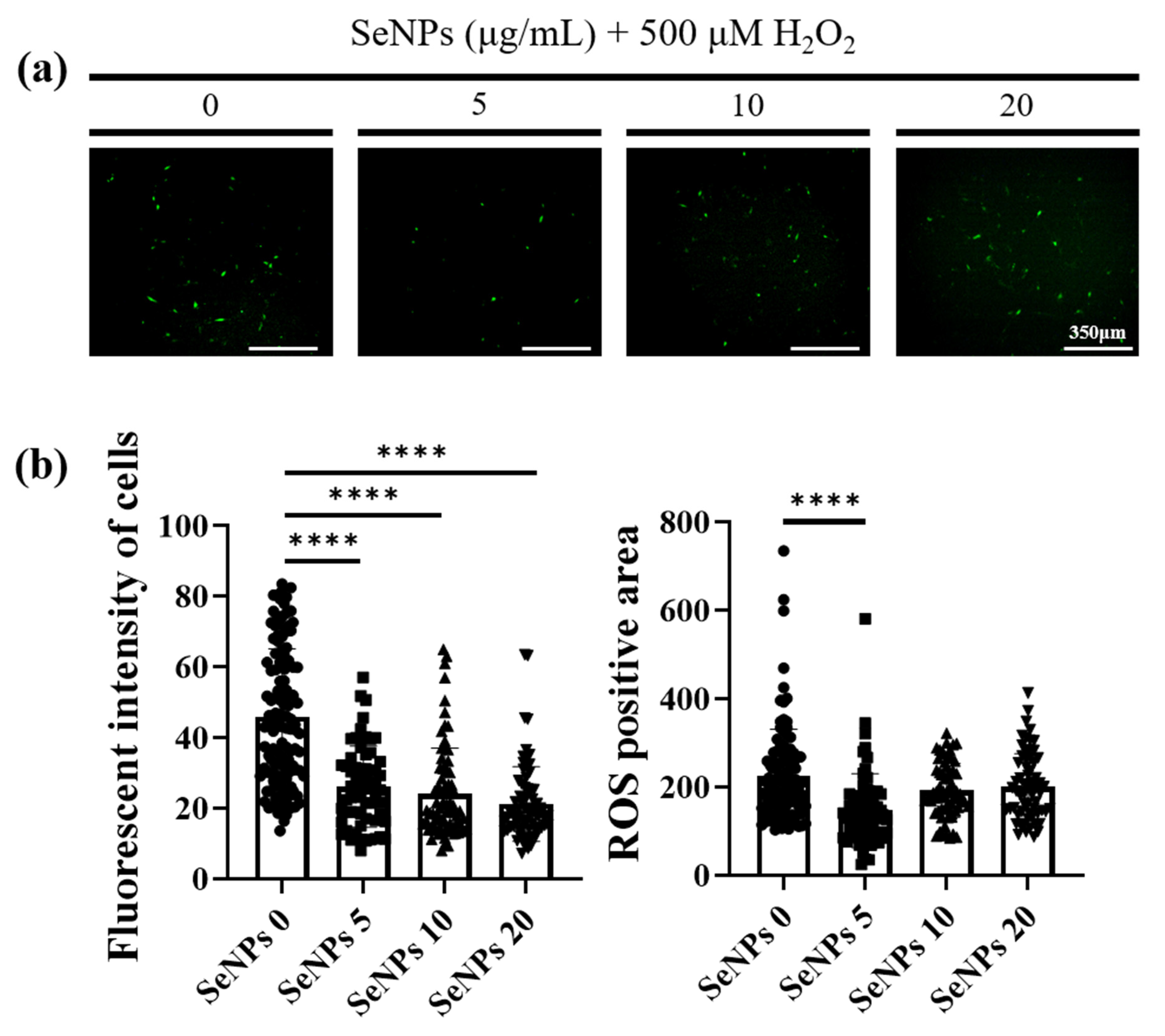
3.4. ROS-Staining
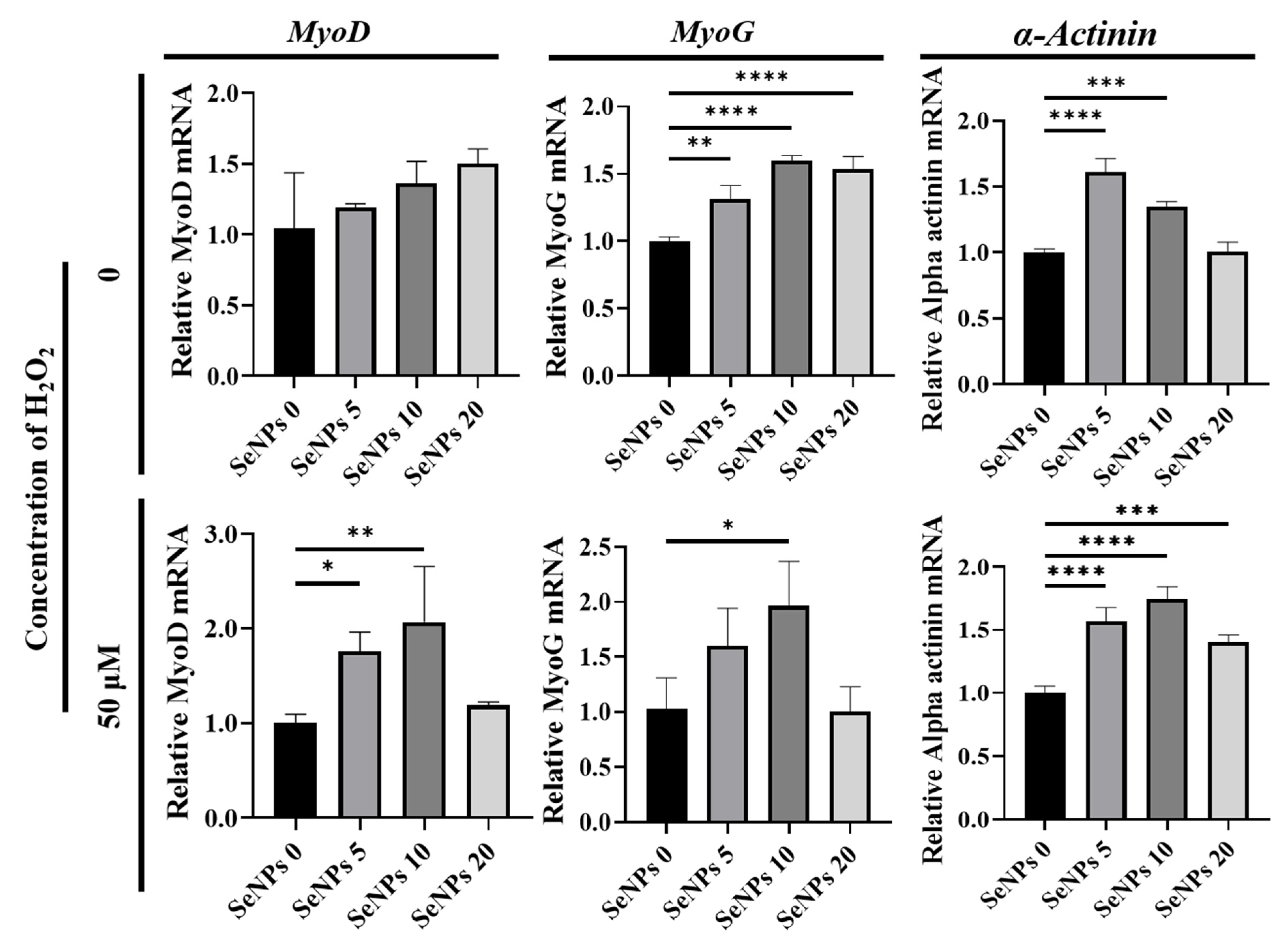
3.5. Effect of Selenium Nanoparticles on the Expression of Myogenic Genes Determined by qRT–PCR
3.6. Effect of Selenium Nanoparticles on Myotube Formation
3.7. Western Blot
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Yan, K.; Zhou, J.; Xu, T.; Xu, M.; Lin, J.; Bai, J.; Ge, G.; Hu, D.; Si, W.; et al. Myogenic differentiation of human amniotic mesenchymal cells and its tissue repair capacity on volumetric muscle loss. J. Tissue Eng. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Carleton, M.M.; Sefton, M.V. Promoting endogenous repair of skeletal muscle using regenerative biomaterials. J. Biomed. Mater. Res. Part A 2021, 109, 2720–2739. [Google Scholar] [CrossRef]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal muscle extracellular matrix–what do we know about its composition, regulation, and physiological roles? A narrative review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasekaran, N.S.; Shelar, S.B.; Jones, D.P.; Hoidal, J.R. Reductive stress impairs myogenic differentiation. Redox Biol. 2020, 34, 101492. [Google Scholar] [CrossRef]
- Laumonier, T.; Menetrey, J. Muscle injuries and strategies for improving their repair. J. Exp. Orthop. 2016, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Shan, H.; Gao, X.; Zhang, M.; Huang, M.; Fang, X.; Chen, H.; Tian, B.; Wang, C.; Zhou, C.; Bai, J.; et al. Injectable ROS-scavenging hydrogel with MSCs promoted the regeneration of damaged skeletal muscle. J. Tissue Eng. 2021, 12. [Google Scholar] [CrossRef]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Prather, E.R.; Garrison, D.E.; Zuo, L. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. Int. J. Mol. Sci. 2018, 19, 417. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Saul, D.; Böker, K.O.; Ernst, J.; Lehman, W.; Schilling, A.F. Current methods for skeletal muscle tissue repair and regeneration. BioMed Res. Int. 2018, 2018, 1984879. [Google Scholar] [CrossRef]
- Singh, R.K.; Knowles, J.C.; Kim, H.-W. Advances in nanoparticle development for improved therapeutics delivery: Nanoscale topographical aspect. J. Tissue Eng. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Qin, D.; Zhang, H.; Zhang, H.; Sun, T.; Zhao, H.; Lee, W.H. Anti-osteoporosis effects of osteoking via reducing reactive oxygen species. J. Ethnopharmacol. 2019, 244, 112045. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, L. Commercializing nanotechnology. Nat. Biotechnol. 2003, 21, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Paull, R.; Wolfe, J.; Hebert, P.; Sinkula, M. Investing in nanotechnology. Nat. Biotechnol. 2003, 21, 1144–1147. [Google Scholar] [CrossRef]
- Jo, S.B.; Erdenebileg, U.; Dashnyam, K.; Jin, G.Z.; Cha, J.R.; El-Fiqi, A.; Knowles, J.C.; Patel, K.D.; Lee, H.H.; Lee, J.H.; et al. Nano-graphene oxide/polyurethane nanofibers: Mechanically flexible and myogenic stimulating matrix for skeletal tissue engineering. J. Tissue Eng. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liu, H.; Luo, W.; Cai, T.; Li, Z.; Liu, Y.; Gao, W.; Wan, Q.; Wang, X.; Wang, J.; et al. Regeneration of skeletal system with genipin crosslinked biomaterials. J. Tissue Eng. 2020, 11. [Google Scholar] [CrossRef]
- Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials 2012, 33, 2916–2925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corona, B.T.; Ward, C.L.; Baker, H.B.; Walters, T.J.; Christ, G.J. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Eng. Part A 2014, 20, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Perniconi, B.; Costa, A.; Aulino, P.; Teodori, L.; Adamo, S.; Coletti, D. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials 2011, 32, 7870–7882. [Google Scholar] [CrossRef]
- Burns, K.E.; Uhrig, R.F.; Jewett, M.E.; Bourbon, M.F.; Krupa, K.A. Characterizing the Role of Biologically Relevant Fluid Dynamics on Silver Nanoparticle Dependent Oxidative Stress in Adherent and Suspension In Vitro Models. Antioxidants 2021, 10, 832. [Google Scholar] [CrossRef]
- Kestell, A.E.; DeLorey, G.T. Nanoparticles: Properties, Classification, Characterization, and Fabrication; Nova Science Publishers: New York, NY, USA, 2010. [Google Scholar]
- Wahab, R.; Dwivedi, S.; Khan, F.; Mishra, Y.K.; Hwang, I.H.; Shin, H.S.; Musarrat, J.; Al-Khedhairy, A.A. Statistical analysis of gold nanoparticle-induced oxidative stress and apoptosis in myoblast (C2C12) cells. Colloids Surf. B Biointerfaces 2014, 123, 664–672. [Google Scholar] [CrossRef]
- Seo, J.J.; Mandakhbayar, N.; Kang, M.S.; Yoon, J.-Y.; Lee, N.-H.; Ahn, J.; Lee, H.-H.; Lee, J.-H.; Kim, H.-W. Antibacterial, proangiogenic, and osteopromotive nanoglass paste coordinates regenerative process following bacterial infection in hard tissue. Biomaterials 2021, 268, 120593. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.H.; Kang, M.S.; Kim, T.H.; Yoon, D.S.; Mandakhbayar, N.; Jo, S.B.; Kim, H.S.; Knowles, J.C.; Lee, J.H.; Kim, H.W. Dual actions of osteoclastic-inhibition and osteogenic-stimulation through strontium-releasing bioactive nanoscale cement imply biomaterial-enabled osteoporosis therapy. Biomaterials 2021, 276, 121025. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Lee, N.H.; Patel, K.D.; Jang, T.S.; Knowles, J.C.; Kim, H.W.; Lee, H.H.; Lee, J.H. The Effect of Selenium Nanoparticles on the Osteogenic Differentiation of MC3T3-E1 Cells. Nanomaterials 2021, 11, 557. [Google Scholar] [CrossRef]
- Daems, N.; Penninckx, S.; Nelissen, I.; Van Hoecke, K.; Cardinaels, T.; Baatout, S.; Michiels, C.; Lucas, S.; Aerts, A. Gold nanoparticles affect the antioxidant status in selected normal human cells. Int. J. Nanomed. 2019, 14, 4991. [Google Scholar] [CrossRef] [Green Version]
- Karahaliloglu, Z.; Kilicay, E. In vitro evaluation of bone cements impregnated with selenium nanoparticles stabilized by phosphatidylcholine (PC) for application in bone. J. Biomater. Appl. 2020, 35, 385–404. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerin, C. New Antioxidant Multilayer Packaging with Nanoselenium to Enhance the Shelf-Life of Market Food Products. Nanomaterials 2018, 8, 837. [Google Scholar] [CrossRef] [Green Version]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorazilova, J.; Muchova, J.; Smerkova, K.; Kociova, S.; Divis, P.; Kopel, P.; Vesely, R.; Pavlinakova, V.; Adam, V.; Vojtova, L. Synergistic Effect of Chitosan and Selenium Nanoparticles on Biodegradation and Antibacterial Properties of Collagenous Scaffolds Designed for Infected Burn Wounds. Nanomaterials 2020, 10, 1971. [Google Scholar] [CrossRef] [PubMed]
- Arteel, G.E.; Sies, H. The biochemistry of selenium and the glutathione system. Environ. Toxicol. Pharmacol. 2001, 10, 153–158. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Stolzoff, M.; Webster, T.J. Reducing bone cancer cell functions using selenium nanocomposites. J. Biomed. Mater. Res. Part A 2016, 104, 476–482. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Roberts, B.R.; Bush, A.I.; Hare, D.J. Selenium, selenoproteins and neurodegenerative diseases. Metallomics 2015, 7, 1213–1228. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Webster, T.J.; Roy, A.K. Cytoprotective effects of cerium and selenium nanoparticles on heat-shocked human dermal fibroblasts: An in vitro evaluation. Int. J. Nanomed. 2016, 11, 1427–1433. [Google Scholar] [CrossRef] [Green Version]
- Schroterova, L.; Kralova, V.; Voracova, A.; Haskova, P.; Rudolf, E.; Cervinka, M. Antiproliferative effects of selenium compounds in colon cancer cells: Comparison of different cytotoxicity assays. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2009, 23, 1406–1411. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B.; Blazejak, S. Application of Sodium Selenite in the Prevention and Treatment of Cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badr, D.M.; Hafez, H.F.; Agha, A.M.; Shouman, S.A. The Combination of α-Tocopheryl Succinate and Sodium Selenite on Breast Cancer: A Merit or a Demerit? Oxidative Med. Cell. Longev. 2016, 2016, 4741694. [Google Scholar] [CrossRef] [Green Version]
- Pang, K.L.; Chin, K.Y. Emerging Anticancer Potentials of Selenium on Osteosarcoma. Int. J. Mol. Sci. 2019, 20, 5318. [Google Scholar] [CrossRef] [Green Version]
- Berggren, M.; Sittadjody, S.; Song, Z.; Samira, J.L.; Burd, R.; Meuillet, E.J. Sodium selenite increases the activity of the tumor suppressor protein, PTEN, in DU-145 prostate cancer cells. Nutr. Cancer 2009, 61, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Fatima, S.; Alfrayh, R.; Alrashed, M.; Alsobaie, S.; Ahmad, R.; Mahmood, A. Selenium Nanoparticles by Moderating Oxidative Stress Promote Differentiation of Mesenchymal Stem Cells to Osteoblasts. Int. J. Nanomed. 2021, 16, 331–343. [Google Scholar] [CrossRef]
- Guo, L.; Xiao, J.; Liu, H.; Liu, H. Selenium nanoparticles alleviate hyperlipidemia and vascular injury in ApoE-deficient mice by regulating cholesterol metabolism and reducing oxidative stress. Metallomics 2020, 12, 204–217. [Google Scholar] [CrossRef]
- Tugarova, A.V.; Mamchenkova, P.V.; Dyatlova, Y.A.; Kamnev, A.A. FTIR and Raman spectroscopic studies of selenium nanoparticles synthesised by the bacterium Azospirillum thiophilum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of Selenium Nanoparticles From Emblica officinalis Fruit Extract and Exploring Its Biopotential Applications: Antioxidant, Antimicrobial, and Biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef] [Green Version]
- Tapiero, H.; Townsend, D.; Tew, K. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 2003, 57, 134–144. [Google Scholar] [CrossRef]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Huang, K.; Liu, H. Biocompatibility selenium nanoparticles with an intrinsic oxidase-like activity. J. Nanoparticle Res. 2016, 18, 74. [Google Scholar] [CrossRef]
- Tang, J.; He, A.; Yan, H.; Jia, G.; Liu, G.; Chen, X.; Cai, J.; Tian, G.; Shang, H.; Zhao, H. Damage to the myogenic differentiation of C2C12 cells by heat stress is associated with up-regulation of several selenoproteins. Sci. Rep. 2018, 8, 10601. [Google Scholar] [CrossRef] [PubMed]
- Poussard, S.; Decossas, M.; Le Bihan, O.; Mornet, S.; Naudin, G.; Lambert, O. Internalization and fate of silica nanoparticles in C2C12 skeletal muscle cells: Evidence of a beneficial effect on myoblast fusion. Int. J. Nanomed. 2015, 10, 1479–1492. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biphys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Park, I.S.; Mahapatra, C.; Park, J.S.; Dashnyam, K.; Kim, J.W.; Ahn, J.C.; Chung, P.S.; Yoon, D.S.; Mandakhbayar, N.; Singh, R.K.; et al. Revascularization and limb salvage following critical limb ischemia by nanoceria-induced Ref-1/APE1-dependent angiogenesis. Biomaterials 2020, 242, 119919. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Torres, S.; Campos, V.; León, C.; Rodríguez-Llamazares, S.; Rojas, S.; Gonzalez, M.; Smith, C.; Mondaca, M. Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J. Nanoparticle Res. 2012, 14, 1236. [Google Scholar] [CrossRef]
- Bai, Y.; Qin, B.; Zhou, Y.; Wang, Y.; Wang, Z.; Zheng, W. Preparation and antioxidant capacity of element selenium nanoparticles sol-gel compounds. J. Nanosci. Nanotechnol. 2011, 11, 5012–5017. [Google Scholar] [CrossRef] [PubMed]
- Duran, B.O.S.; Goes, G.A.; Zanella, B.T.T.; Freire, P.P.; Valente, J.S.; Salomao, R.A.S.; Fernandes, A.; Mareco, E.A.; Carvalho, R.F.; Dal-Pai-Silva, M. Ascorbic acid stimulates the in vitro myoblast proliferation and migration of pacu (Piaractus mesopotamicus). Sci. Rep. 2019, 9, 2229. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, A.; Tang, J.; Shah, A.M.; Jia, G.; Liu, G.; Tian, G.; Chen, X.; Cai, J.; Kang, B.; et al. Selenium alleviates the negative effect of heat stress on myogenic differentiation of C2C12 cells with the response of selenogenome. J. Therm. Biol. 2021, 97, 102874. [Google Scholar] [CrossRef] [PubMed]
- Thaloor, D.; Miller, K.J.; Gephart, J.; Mitchell, P.O.; Pavlath, G.K. Systemic administration of the NF-kappaB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am. J. Physiol. 1999, 277, C320–C329. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-C.; Lee, N.-H.; Patel, K.D.; Jun, S.-K.; Park, J.-H.; Knowles, J.C.; Kim, H.-W.; Lee, H.-H.; Lee, J.-H. A Study on Myogenesis by Regulation of Reactive Oxygen Species and Cytotoxic Activity by Selenium Nanoparticles. Antioxidants 2021, 10, 1727. https://doi.org/10.3390/antiox10111727
Lee S-C, Lee N-H, Patel KD, Jun S-K, Park J-H, Knowles JC, Kim H-W, Lee H-H, Lee J-H. A Study on Myogenesis by Regulation of Reactive Oxygen Species and Cytotoxic Activity by Selenium Nanoparticles. Antioxidants. 2021; 10(11):1727. https://doi.org/10.3390/antiox10111727
Chicago/Turabian StyleLee, Sang-Cheol, Na-Hyun Lee, Kapil D. Patel, Soo-Kyung Jun, Jeong-Hui Park, Jonathan Campbell Knowles, Hae-Won Kim, Hae-Hyoung Lee, and Jung-Hwan Lee. 2021. "A Study on Myogenesis by Regulation of Reactive Oxygen Species and Cytotoxic Activity by Selenium Nanoparticles" Antioxidants 10, no. 11: 1727. https://doi.org/10.3390/antiox10111727
APA StyleLee, S.-C., Lee, N.-H., Patel, K. D., Jun, S.-K., Park, J.-H., Knowles, J. C., Kim, H.-W., Lee, H.-H., & Lee, J.-H. (2021). A Study on Myogenesis by Regulation of Reactive Oxygen Species and Cytotoxic Activity by Selenium Nanoparticles. Antioxidants, 10(11), 1727. https://doi.org/10.3390/antiox10111727







