Abstract
Mitochondria are the key organelles of Fe–S cluster synthesis. They contain the enzyme cysteine desulfurase, a scaffold protein, iron and electron donors, and specific chaperons all required for the formation of Fe–S clusters. The newly formed cluster can be utilized by mitochondrial Fe–S protein synthesis or undergo further transformation. Mitochondrial Fe–S cluster biogenesis components are required in the cytosolic iron–sulfur cluster assembly machinery for cytosolic and nuclear cluster supplies. Clusters that are the key components of Fe–S proteins are vulnerable and prone to degradation whenever exposed to oxidative stress. However, once degraded, the Fe–S cluster can be resynthesized or repaired. It has been proposed that sulfurtransferases, rhodanese, and 3-mercaptopyruvate sulfurtransferase, responsible for sulfur transfer from donor to nucleophilic acceptor, are involved in the Fe–S cluster formation, maturation, or reconstitution. In the present paper, we attempt to sum up our knowledge on the involvement of sulfurtransferases not only in sulfur administration but also in the Fe–S cluster formation in mammals and yeasts, and on reconstitution-damaged cluster or restoration of enzyme’s attenuated activity.
1. Introduction
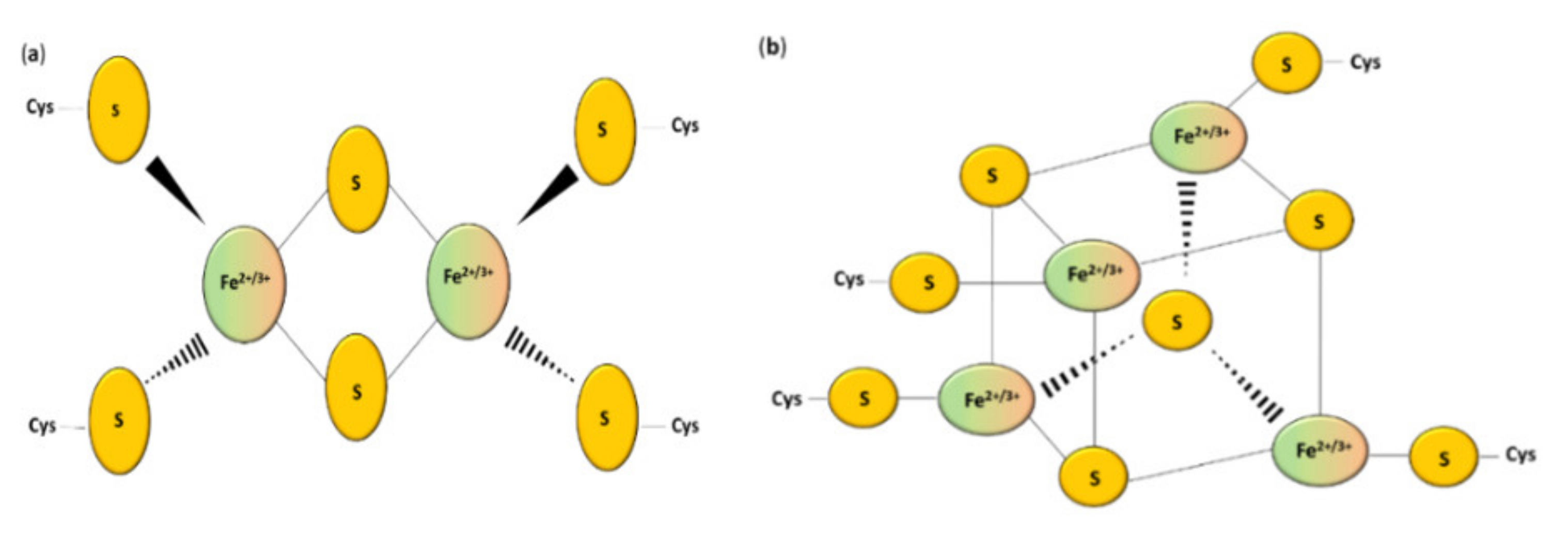
Sulfur is an important biological element. Various oxidation states of sulfur can occur, ranging from S2− (sulfide) to S6+ (sulfate) [1]. Sulfur can be incorporated into proteins, carbohydrates, and lipids participating in many cellular processes, including signaling and redox homeostasis [2]. The most important source of this chemical element are the sulfur-containing amino acids L-cysteine and L-methionine [3]. The sulfur pool can be divided into the stable form (for example L-cysteine and L-methionine) and the labile form. The labile form of sulfur can be further divided into sulfane sulfur (S0) and acid-labile sulfur [4]. Hydrogen sulfide (H2S) can be released from both labile pools of sulfur under specific conditions [5]. Sulfur, which is a part of metal–sulfur clusters, belongs to the acid-labile group [6]. However, sulfur is not likely to be present inside the cells in the “free” S2− form [7]. Non-heme iron ions can be connected with inorganic sulfur in the polymetallic clusters of proteins, which are called the iron–sulfur (Fe–S) proteins [8]. The iron ions are coordinated via the thiol groups of cysteinyl residues of this peptide [9] and bridged by inorganic sulfide [10] (Figure 1). It is possible that one or more coordinating ligands are changed from the original one to (1) other amino acids, (2) a non-sulfur-based ligand, or (3) another thiolate donating ligand [11].
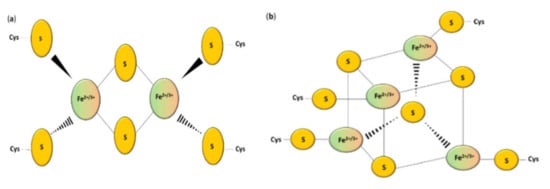
Figure 1.
Structure of the most common Fe–S clusters: (a) [2Fe–2S]; (b) [4Fe–4S] clusters [10].
2. Iron–Sulfur Clusters and Iron–Sulfur Proteins
2.1. Fe–S Clusters
Iron–sulfur clusters (Fe–S clusters) were firstly discovered around the 1950s [12]. Nowadays, Fe–S clusters can be spotted in every kingdom of life [13]. Moreover, elements of the cluster biogenesis machinery were detected not only in human mitochondria but also in plant mitochondria and even in mitosomes or hydrogenosomes of anaerobic organisms [14]. The primary task of iron ions results from their ability to change their oxidation state from Fe2+ to Fe3+, contrary to sulfur that is also a part of clusters and is always present in S2− oxidation state [15,16].
Iron–sulfur clusters can be divided into various geometric and stoichiometric forms in which the number of iron and sulfur atoms involved in clusters changes [17]. The simplest [Fe–S] cluster consists of one iron ion bound to a polypeptide by L-cysteine residues [10]. Rhombic [2Fe–2S] (Figure 1a) and cubane [4Fe–4S] clusters (Figure 1b) are the most prevalent groups [7]. Square-like cubane-type iron–sulfur clusters [4Fe–4S] can be produced from two units of [2Fe–2S] clusters [16], and this reaction can be reversed under specific conditions [18]. The loss of one of the atoms results in a non-symmetrical [3Fe–4S] or [8Fe–7S] structure [19,20]. Thus, the iron–sulfur clusters can undergo various transformation processes such as conversion, coordinating ligand exchange, and degradation in oxidative stress [21,22].
2.2. Fe–S Proteins
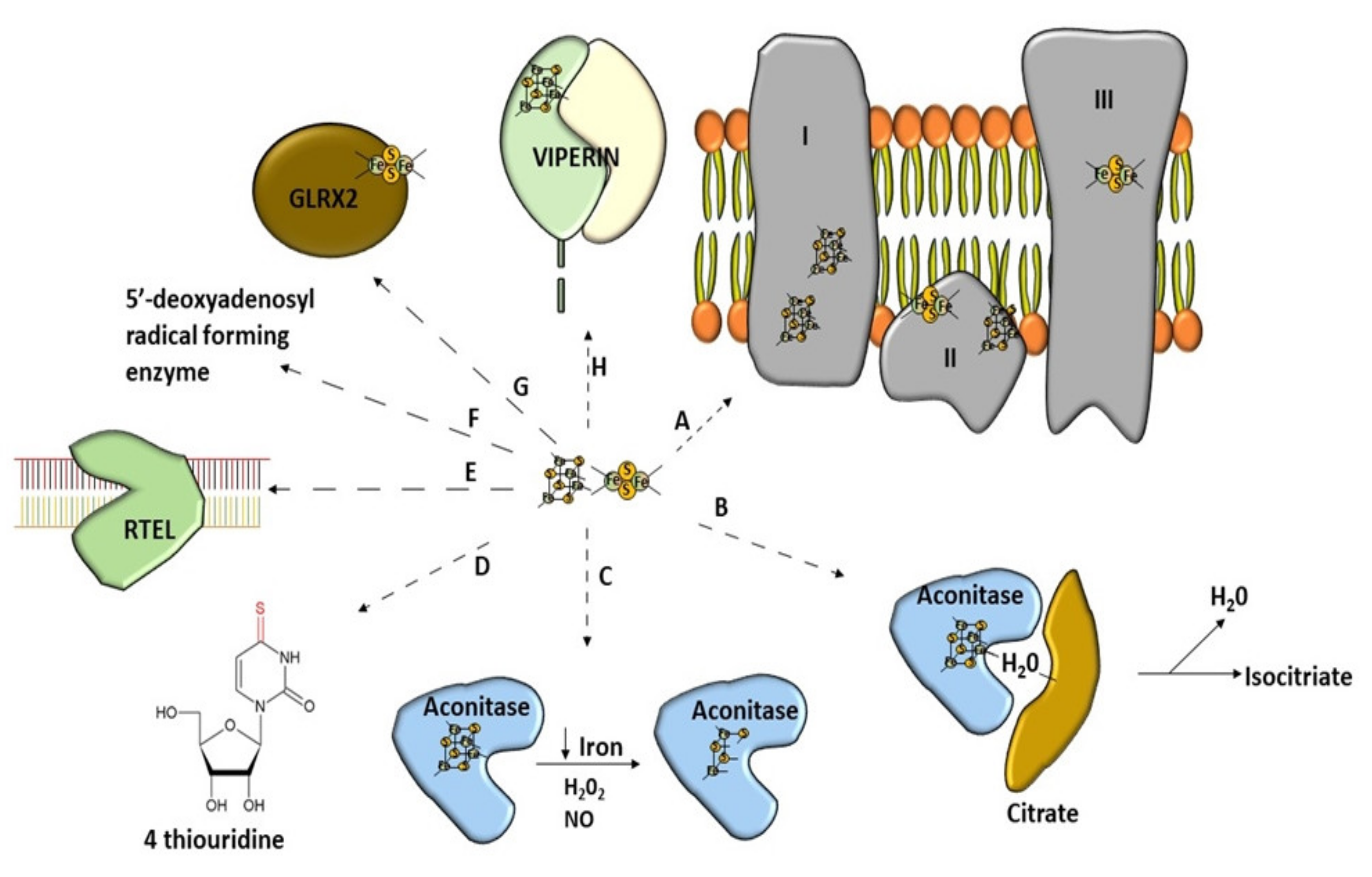
There is a growing list of enzymes that function as Fe–S proteins in various organisms from bacteria to eukaryotes [23,24] (Figure 2). Such proteins can be equipped with different kinds of clusters [25] or include more than one type [26]. All Fe–S proteins perform a wide range of tasks [27]. Human proteins, which include the Fe–S clusters in their structure, can be found mostly in mitochondria but also in the cytosol and even nucleus [28]. Firstly, they are playing the role of a carrier of electrons in the mitochondrial respiratory chain [9,29,30]. Furthermore, the Fe–S clusters are often components of the active site of proteins participating in non-redox catalysis [31]. The Fe–S proteins can also play a role as regulatory agents and protein stabilization factors [8]. They are highly vulnerable to oxidative stress and iron shortage [15]. Therefore, some regulatory proteins recruit the Fe–S clusters in order to better sense O2 or to respond to superoxide stress [15]. Furthermore, cells use proteins equipped with the Fe–S clusters to positively affect DNA transcription to regulate iron homeostasis [32,33]. Other peptides can also negatively affect DNA transcription when Fe–S cluster synthesis is sufficient [34]. The Fe–S clusters are cofactors of proteins involved in maintaining genome integrity [35]. DNA-binding proteins have the Fe–S clusters in their structure, and thus their activity is granted [19,36] (Figure 2). They are involved in tRNA modification [7,37]. The Fe–S clusters are also involved in the generation of 5-deoxyadenosyl radicals from S-adenosyl-L-methionine (SAM) [38]. Interestingly, this feature is useful in immunity [39]. Many glutaredoxins, which take part in the Fe–S cluster formation, contain the Fe–S clusters [40]. Most of them can coordinate at least one cluster [41]. The recently discovered activities in which iron–sulfur clusters may be participating are disulfide reduction and sulfur donation [16].
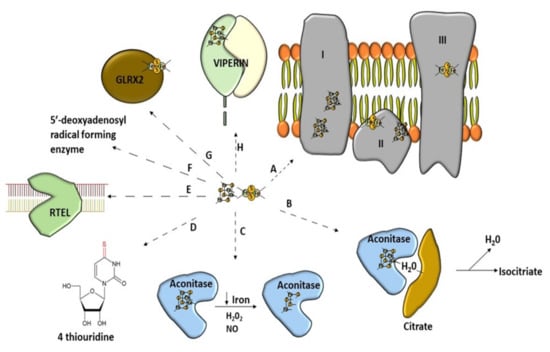
Figure 2.
Wide range of use of [Fe–S] clusters. (A) Mitochondrial respiratory chain–NADH dehydrogenase (complex I), succinate dehydrogenase (complex II), Cytochrome bc1 (complex III). (B) Non-redox catalysis (aconitase), (C) regulatory function of aconitase after oxidative stress, (D) involvement in tRNA modification, threonylcarbamoyladenosine tRNA methylthiotransferase (CDKAL1), (E) participation of Fe–S cluster in genome integrity; RTEL (helicase-nuclease DNA2 and regulator of telomere length 1), (F) molybdenum cofactor biosynthesis protein 1 (MOCS1A, a member of the S-adenosylmethionine (SAM)-dependent enzyme family) is an enzyme using the Fe–S cluster to generate a 5′-deoxyadenosyl radical, (G) glutaredoxins with Fe–S clusters are involved in Fe–S cluster synthesis, (H) antiviral proteins using Fe–S cluster to perform their activity (Viperin).
3. Iron–Sulfur Cluster Synthesis
L-cysteine is the source of sulfur for the Fe–S cluster synthesis in the overwhelming majority of cases, other sulfur species, such as sulfide are rarely involved [42]. The Fe–S cluster synthesis route is highly conserved among species and consists of a high number of specific proteins [43]. The cluster assembly can take place both in mitochondria and cytosol in Eucaryotes [44]. Fe–S cluster assembly pathways consist of two general events: (1) cluster assembly on the scaffold protein; (2) distribution and final insertion into apo-protein [45]. As far as mitochondrial synthesis and assembly of the Fe–S cluster are concerned, there are three separate systems named ISC (iron–sulfur cluster), SUF (sulfur utilization) [46], and NIF (nitrogen fixation) [47]. The most known system is ISC [10]; this route is the most important biogenesis pathway of the mitochondrial Fe–S clusters under normal conditions [48]. The SUF system is the most ancient of all the Fe–S assembly systems [49]. It is very similar to ISC in many ways [32]. It plays an essential role in viability in SUF-dependent organisms [49]. The components of this pathway (E. coli scaffold protein) seem to be more stable under severe conditions [32]. Moreover, the expression of components of this pathway is increased during iron depletion and oxidative stress [47]. Therefore, under adverse conditions the SUF machinery can cover all the Fe–S cluster demands [50]. The SUF system seems to be changing over time since it originated [49]. On the other hand, the NIF system is a dedicated machinery for producing the Fe–S clusters of the nitrogenase protein in Azotobacter nitrogen-fixing bacteria [47].
3.1. Mitochondrial Iron–Sulfur Cluster Synthesis
The ISC Fe–S cluster synthesis in mitochondria can be precisely divided into four steps detailed later in this manuscript [51]. Starting from the first, there is the [2Fe–2S] cluster de novo synthesis [35]. A main component of assembly system in mitochondria is a specific sulfur delivery enzyme named cysteine desulfurase (NFS1) [52,53]. A further, scaffold protein (ISCU2) [54,55]; a hypothetical iron donor called frataxin (FXN) [56]; an electron donor named ferredoxin (FDX1) [57]; glutaredoxin 5 monothiol (GLRX5)-transfer protein, a specific subset of the thioredoxin (Trx) superfamily [51,58]; and a specific chaperone/co-chaperone complex are required [59].
3.1.1. Early Step of Mitochondrial Fe–S Cluster Synthesis
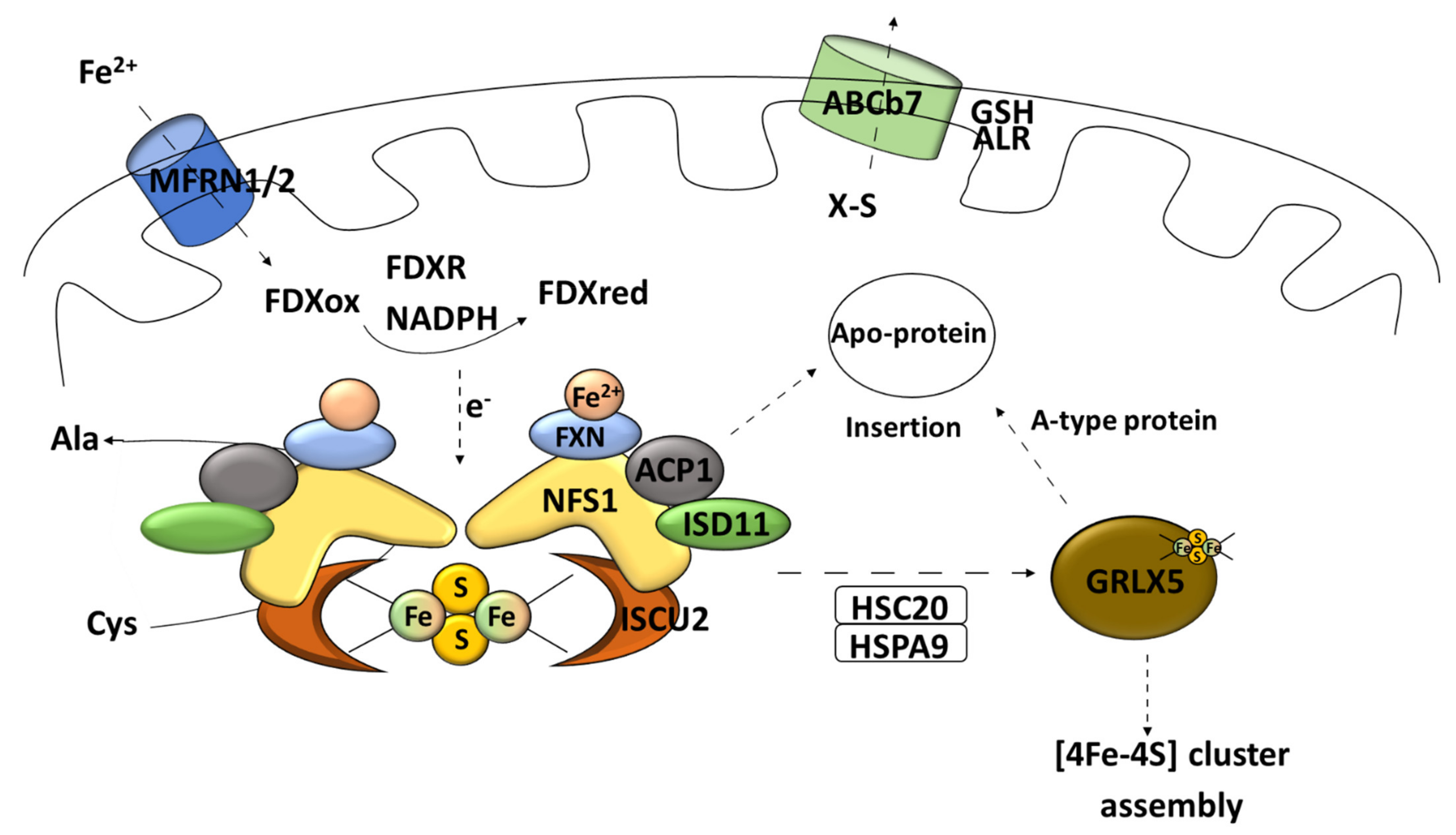
Cysteine desulfurase [EC 2.8.1.7] in the form of homodimers uses L-cysteine as the main source of sulfur for iron–sulfur cluster synthesis [60]. It is a pyridoxal phosphate (PLP)-dependent enzyme [15]. The catalyzed reaction leads to desulfuration of L-cysteine to alanine and sulfane sulfur via the formation of an enzyme-bound persulfide (-SSH) intermediate [61]. Cysteine desulfurases can provide sulfur not only for the Fe–S cluster formation but also for other metabolic pathways (thiamine biosynthesis, for example) [15]. Eukaryotic cysteine desulfurase NFS1 operates in the so-called SDA complex [53]. SDA consists of specific desulfurase, partner protein ISD11 (LYR protein family) [45], and acyl carrier protein (ACP1) [51]. ACP1, together with the above-mentioned core Fe–S cluster-forming components, take part in generation of transient persulfides [62]. Proteins from the LYR motif family are responsible for engaging the transferring complex for the maturation of proteins with the Fe–S clusters buried, for example, succinate dehydrogenase subunit B and Rieske protein [29]. Such a connection is empowered by the co-chaperone HSC20, which can bind the transiently formed LYR–scaffold complex [63]. Furthermore, proteins of the LYR family are often associated with the Fe–S cluster synthesis for proteins of the mitochondrial respiratory complex [64]. The process of combining iron and sulfur takes place on the scaffold protein dimer ISCU2 [45] (Figure 3).
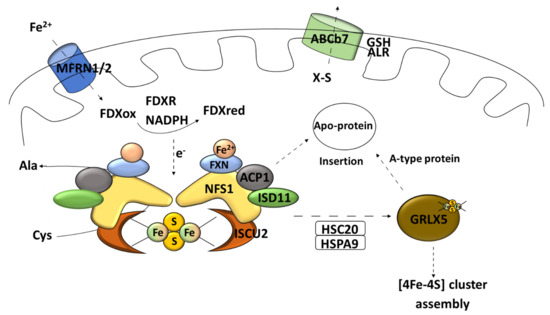
Figure 3.
Early stage of mitochondrial Fe–S synthesis. NFS1, cysteine desulfurase; ISD11, LYR protein; ACP1, acyl carrier protein; ISCU2, scaffold protein; MFRN1/2, carrier protein mitoferrin 1 and 2; FXN, frataxin; FDXR, mitochondrial ferredoxin reductase; Cys, L-cysteine; Ala, L-alanine; HSPA9, mortalin/mitochondrial 70 kDa heat shock protein; HSC20, iron–sulfur cluster co-chaperone protein HscB; GLRX5, monothiol glutaredoxin 5; GSH, reduced glutathione; ALR, mitochondrial FAD-linked sulfhydryl oxidase; ABCb7, ATP-binding cassette (ABC) transporter family.
The influx of iron ions to mitochondria is supported by the carrier protein mitoferrin 1 and 2 (MFRN1/2) [45] (Figure 3). Frataxin is an iron-binding protein that acts as an iron storage; it has also been postulated that this peptide can participate in vitro as an iron donor in the Fe–S cluster synthesis [65] (Figure 3). Moreover, recent data suggest that frataxin can change the conformation of the assembly complex based on an allosteric switch and, therefore, increase the cluster formation rate [55]. The data do not rule out both functions [66]. In electron delivery, ferredoxin needs to be paired with its reductase (mitochondrial ferredoxin reductase FDXR) and nicotinamide adenine dinucleotide phosphate (NADPH) to fulfil its task [37] (Figure 3).
This process called “early acting” leads to the formation of a [2Fe–2S] cluster intermediate that can be incorporated into the mitochondrial Fe–S protein (in cooperation with the chaperone/co-chaperone complex), sent outside of mitochondria (X–S compound), or undergo further modification (late step of mitochondrial Fe–S cluster biogenesis) [51]. Subsequently, the cluster transfer is organized by cooperating chaperons [37]. The chaperone complex composed of the HSPA9 chaperone and HSC20 co-chaperone allows for transferring the Fe–S cluster to the key transporter GLRX5 [45] (Figure 3). The homodimer complex of this glutaredoxin is known to be able to receive the Fe–S cluster directly from the cluster machinery complex by interacting with the HSPA9 chaperone [51]. It also requires two reduced glutathione (GSH) molecules [41] (Figure 4). What is interesting is that ISCU2 can donate a [2Fe–2S] cluster directly to the protein without the need of HSPA9/HSC20 [67] (Figure 4). The export of newly assembled clusters requires three different compounds: one membrane channel protein ABCb7 (yeast protein atm1, ATP-binding cassette (ABC) transporter family) [45], one sulfhydryl oxidase (FAD-linked sulfhydryl oxidase (ALR) [68], and one reducing factor (glutathione) [20].
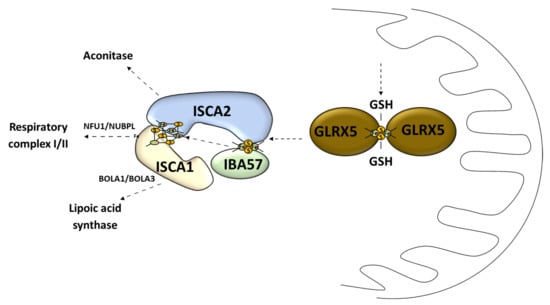
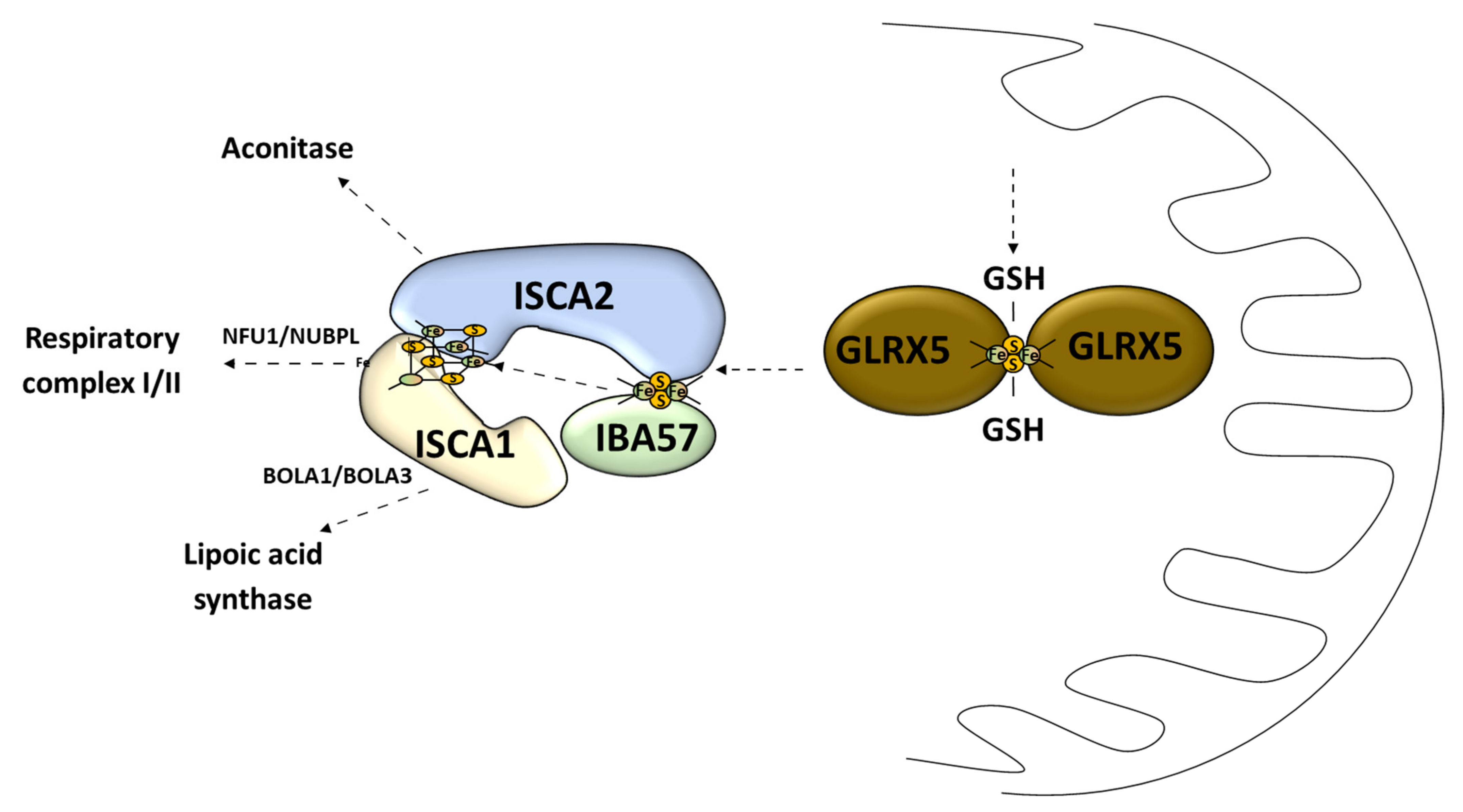
Figure 4.
Late stage of mitochondrial Fe–S cluster synthesis. GLRX5, monothiol glutaredoxin 5; GSH, reduced glutathione; IBA57, iron–sulfur cluster assembly factor IBA57; ISCA1, iron–sulfur cluster assembly 1; ISCA2, iron–sulfur cluster assembly 2; NFU1, iron–sulfur cluster scaffold NFU1; NUBPL, nucleotide binding protein like; BOLA1, bolA family member 1; BOLA3, bolA family member 3.
3.1.2. Late Step of Mitochondrial Fe–S Cluster Synthesis
The second stage of the Fe–S cluster formation takes place in the mitochondria where the [4Fe–4S] cluster is made of two [2Fe–2S] clusters [69]. This process is called the late-acting machinery [51]. It depends on the [2Fe–2S] cluster pass to form a working complex carried out by GLRX5 [51] (Figure 4). The late-step assembly machinery has been proposed to form the ISCA1–ISCA2–IBA57 complex that does not interfere with early stage components [51]. Recently it has been shown that this complex can convert the [2Fe–2S] cluster into the [4Fe–4S] cluster, which was demonstrated in vitro [70]. Sheftel et al. showed that the presence of all three proteins was crucial for optimal [4Fe–4S] cluster assembly and was conserved in its nature [71] (Figure 4). The association scheme of components of this pathway can be different under specific conditions, but it still remains unclear [72,73] (Figure 4). The late-acting machinery is essential in the production of the [4Fe–4S] clusters for aconitase-type protein [74], succinate dehydrogenase, radical SAM enzymes, and lipoic acid synthase [71]. The synthesis and delivery of the Fe–S cluster for specific proteins requires additional transporters: NFU1 and NUBPL in cluster formation for the respiratory complex 1; BOLA1 and BOLA3 in cluster delivery for lipoic acid synthase [75] (Figure 4).
4. Cytosolic Iron–Sulfur Cluster Synthesis
4.1. Cytosolic Fe–S Cluster Assembly Machinery
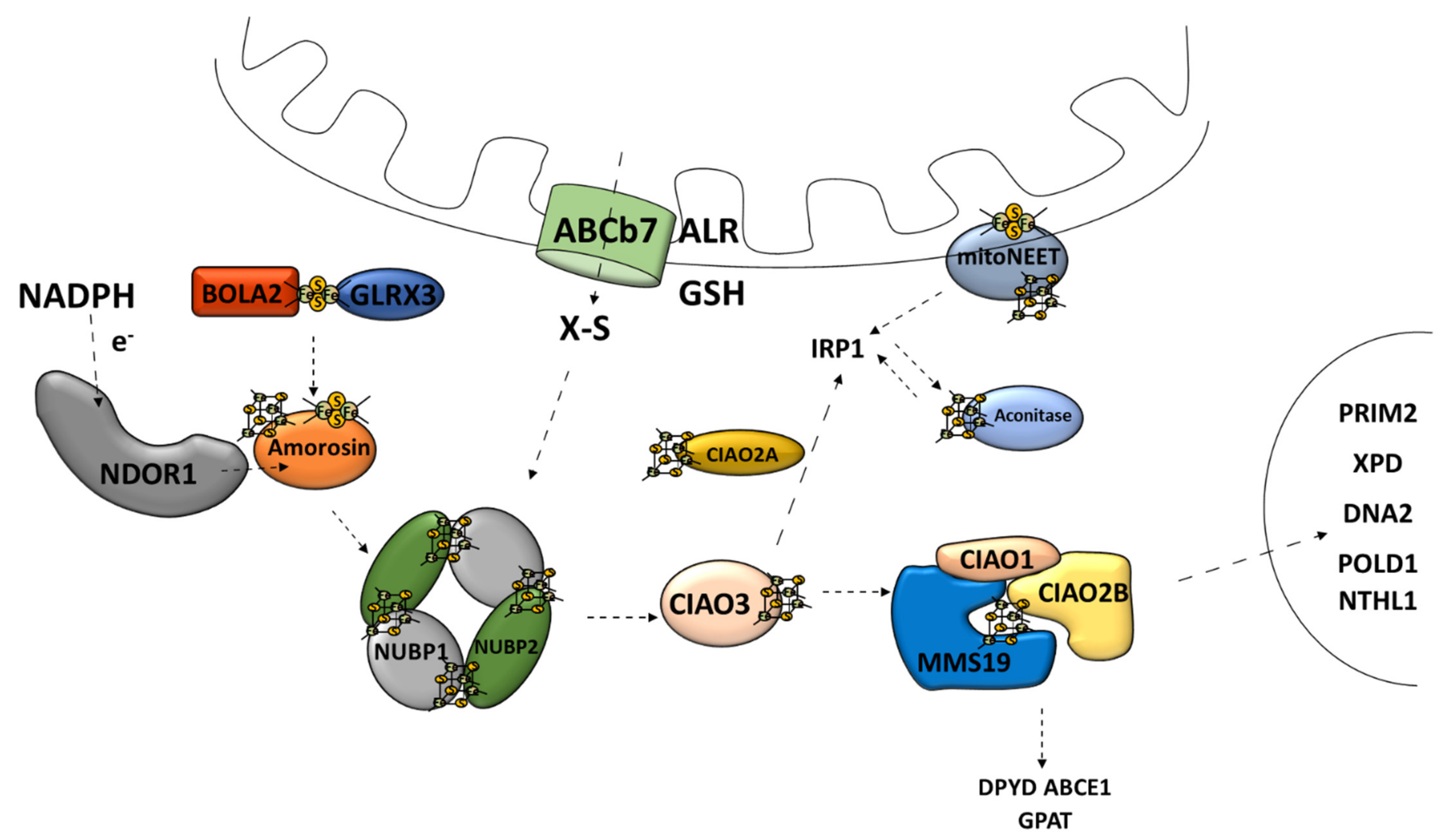
The second major route of the Fe–S cluster assembly pathways is the cytosolic Fe–S cluster assembly machinery, also referred to as the cytosolic iron–sulfur cluster assembly (CIA) [30]. CIA is responsible for the Fe–S cluster assembly for cytosolic and nucleic Fe–S proteins [9]. This is a multistage process consisting of at least two stages: the nascent cluster assembly upon the scaffold complex and delivery to apo-protein [76]. The scaffold complex consists of NUBP1–NUBP2 protein [45,77] (Figure 5). Such a complex has the ability to bind two [4Fe–4S] clusters on one NUBP1 monomer [78]. The assembly process requires electrons, which are provided by diflavin oxidoreductase (NDOR1) in cooperation with amorosin from NADPH [77,79] (Figure 5). A recent observation describes an interaction between the mitochondrial and cytosolic Fe–S protein assembly machinery linked by NEET proteins (mitoNEET) in which the Fe–S cluster is coordinated by three Cys residues and one His residue [80]. It is anchored into the outer membrane of the mitochondrion with one of its parts located in the cytosol [81]. Under oxidative conditions, various connections between the mitoNEET cluster allow for the transfer of this cluster via the protein–protein interaction into the apo-form of proteins, such as the bacterial FDX [82], IRP1 [83], or amorosin [84]. Protein BOLA2 forms a protective complex with glutaredoxin 3 (GLRX3) allowing for the transfer of the newly formed [2Fe–2S] cluster from the mitochondria through the cytosol and taking part in the GLRX3–BOLA2-dependent amorosin maturation pathway [41,85] (Figure 5).
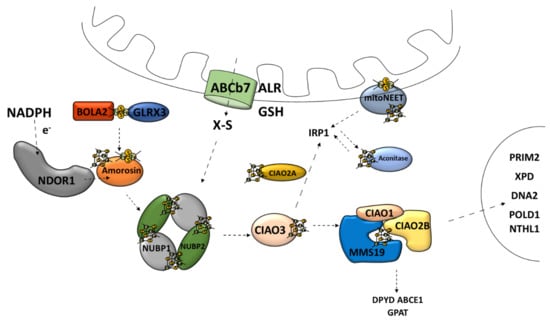
Figure 5.
Cytosolic [4Fe–4S] iron–sulfur cluster assembly and targeting machinery. ABCb7, ATP-binding cassette (ABC) transporter family; ALR, mitochondrial FAD-linked sulfhydryl oxidase; GSH, reduced glutathione; X–S, unidentified X–S compound; NDOR1, diflavin oxidoreductase 1; Amorosin, Fe–S cluster assembly protein DRE2 homolog; BOLA2, bolA family member 2; GLRX3, glutaredoxin 3; NUBP1, cytosolic Fe–S cluster assembly factor NUBP1; NUBP2, cytosolic Fe–S cluster assembly factor NUBP2; IOP1/CIAO3, cytosolic iron–sulfur assembly component 3; CIAO1, cytosolic iron–sulfur protein assembly protein CIAO1; CIAO2B, cytosolic iron–sulfur assembly component 2B; MMS19, cytosolic iron–sulfur assembly component MMS19; Aconitase/IRP1, aconitase 1; DPYD, dihydropyrimidine dehydrogenase; ABCE1, ATP binding cassette subfamily E member 1; GPAT, glutamine phosphoribosyl-pyrophosphate amidotransferase; POLD1, DNA polymerase δ; NTHL1, DNA base-excision repair enzyme; XPD, general transcription and DNA repair factor IIH helicase subunit XPD; RTEL1, regulator of telomere elongation helicase 1; DNA2, DNA replication helicase/nuclease 2; PRIM2, DNA primase subunit 2.
4.2. Cytosolic Fe–S Cluster Delivery Machinery
Transport and insertion into apo-protein of the fully assembled cytosolic Fe–S cluster are supported by the Fe–S protein named CIAO3 (human cytosolic iron–sulfur assembly component 3, also called IOP1) [86,87], in cooperation with the CIA-targeting complex (CTC) [77]. CTC is composed of the “transducing-like” protein CIAO1 [88], CIAO2B/FAM96B [77], and protein MMS19 [89]. The proteins involved in the CTC complex affect its ability to attach the Fe–S protein and provide stability to this complex [90]. The CTC complex is responsible for the maturation of many cytosolic proteins, such as dihydropyrimidine dehydrogenase (DPYD) and glutamine phosphoribosyl-pyrophosphate amidotransferase (GPAT) [77]. Furthermore, the CIA-targeting complex also transfers the Fe–S clusters to the nuclear Fe–S proteins, such as the catalytic subunit of DNA polymerase δ (POLD1), DNA base-excision repair enzyme (NTHL1), DNA helicase XPD, helicase-nuclease DNA2, and regulator of telomere length 1 (RTEL1) [19,77,91,92] (Figure 5). Moreover, CTC is responsible for the Fe–S cluster delivery for aconitase maturation [77,93].
Overall, the CIA system is very similar to ISC when it comes to the scaffold assembly process, chaperone-mediated release, and delivery pathway [37]. However, they are not evolutionarily related [77]. CIA relies on unknown sulfur-containing compounds, which have a mitochondrial origin [32]. The molecule that leaves the previously mentioned ABCb7 channel is an unidentified X–S compound [19] (Figure 5). It has been shown that mitochondria are capable of producing a specific form of intermediate Fe–S cluster (Fe–Sint) for the CIA assembly machinery [94].
5. Repair of Damaged Fe–S Clusters
Enzymes that rely on the Fe–S clusters activity are endangered not only by reactive oxygen species (ROS) [10] or nitric oxide [95] but also by heavy metals [96,97] and iron shortage [15]. Unfavorable conditions can also alter sulfur bioavailability and trafficking [49]. Both the first listed factors can react with each other creating even more toxic compounds such as peroxynitrite (ONOO−) [98]. This molecule can easily nitrate a tyrosine residue or oxidize the thiol residue of cysteine, alternately allowing them to be changed by S-glutathionylation [99]. This activity occurs rapidly in the enzyme active site, thereby changing its conformation [100]. Nitric oxide alone rapidly reacts with cluster-forming dinitrosyl iron complexes (DNICs) and even more complicated structures [101] or is responsible for thiol nitrosylation [102,103]. The radicals NO and ●OH can bind to Fe atoms of the Fe–S clusters [104]. ROS, mainly superoxide (O2−), can oxidize Fe–S clusters starting with an iron release, promoting cluster instability and slow degradation leading to irreversible damage to the protein backbone of a particular enzyme [105]. Therefore, enzymes that undergo prolonged exposure to oxidative stress show a poor prognosis for renewal [106]. The formation of hydroxyl radical via Fenton reaction significantly accelerates destruction [81]. Bruska et al. performed detailed studies of a different kind of ROS–Fe–S cluster interaction [104]. Most Fe–S proteins hide the vulnerable cluster inside the polypeptide chain; however, some of them require direct involvement of the cluster to perform their activity [105]. Such exposed clusters, mainly [4Fe–4S] forms, are more prone to oxidative stress [107]. Some enzymes containing [4Fe–4S] clusters, under oxidative stress, can keep [2Fe–2S] clusters and work with lower activity [108]. Oxidative stress can devastate the function of many enzymes [106]; however, some of them may return to correct function after such an event, and some of them are highly resistant to oxidation or undergo reversible modification [101]. It depends on the composition and solidity of the Fe–S cluster [104].
Fe–S Cluster Reconstitution Attempt
Stress oxidation agents often damage cysteine residue from the active site of important enzymes, which can be reduced in the process of repair [106]. It has been shown that inactive Fe–S clusters can be restored following the chemical or semi-enzymatic reconstitution protocol [64]. The cluster of bacterial NADH-cytochrome c reductase can be reconstituted in this way [109]. Superoxide dismutases (SODs) are enzymes actively participating in the frontline against ROS [110]. The SOD knockout E. coli dehydratases [4Fe–4S] cluster is rapidly damaged by ROS and undergoes reconstitution after the exposure is stopped [111]. Furthermore, the function of the aconitase [4Fe–4S] cluster, after being altered by nitric oxide, can be restored by iron, sulfide, and 1,4-dithiothreitol (DTT) after the NO influx is stopped [112]. Using the same compounds, restoration of peptide deformylase (PDF) and isopropylmalate isomerase (IPMI) of Bacteroides thetaiotaomicron can be performed [105,106]. The fumarate and nitrate reduction regulatory protein activity crucially depends on the process of switching forms of the cluster between [4Fe–4S] to [2Fe–2S] while sensing O2 and the reaction with IscS, L-cysteine, ferrous ions, and DTT can reverse it [113]. The activity of yeast proteins of the amino acids synthesis pathway: homoaconitase (Lys4p) and isopropylmalate dehydratase (Leu1p), which have the [4Fe–4S] cluster, can be restored under anaerobic conditions after superoxide-mediated inactivation [114].
The overall activity of cysteine desulfurase and its role in sulfur mobilization has been summarized [115]. Cysteine desulfurase may be involved in FNR apo-protein reparation after NO exposure [116,117]. The [4Fe–4S] cluster of endonuclease III can be reconstituted by using cysteine desulfurase (IscS), L-cysteine, ferrous ions, and DTT after exposure to NO by a new cluster assembly process in vitro [118]. On the other hand, some investigated bacteria without IscS protein were still able to repair clusters but at a slower rate (6-phosphogluconate dehydratase, fumarase A) [111]. Furthermore, cluster repair in the IscS mutant of E. coli with iron source/reducing agent (DTT) was unsuccessful in vitro [119]. Interestingly, the reconstitution of these clusters takes place without new protein synthesis de novo [111,112,114,118].
Other factors can play a crucial role in the recovery of the [Fe–S] clusters [120]. MitoNEET primarily affects the repair of the apo-form of aconitase (IRP1) and then its maturation [45]; it is also capable of donating this cluster to E. coli apo-ferredoxin [120]. Furthermore, di-iron proteins taking part in the Fe–S cluster synthesis are capable of fumarase A [4Fe–4S] cluster repair from E. coli [121], and two enzymes from yeast (aconitase B and fumarase A) [122]. Spinach apo-ferredoxin can be converted into a functional enzyme by using the E. coli RIC protein and IscS, DTT, and L-cysteine [123]. Furthermore, the activity of damaged clusters in cells without functioning RIC protein can be restored after RIC supplementation [124]. Interestingly, RIC proteins can be crucial factors that allow bacteria to survive in deep tissues after infection [98].
N-acetyl-L-cysteine and GSH were able to repair clusters to a much lower degree [125]. However, GSH failed to protect the E. coli [4Fe–4S] cluster of dihydroxyacid dehydratase from NO-mediated transformation [102].
6. Sulfurtransferases
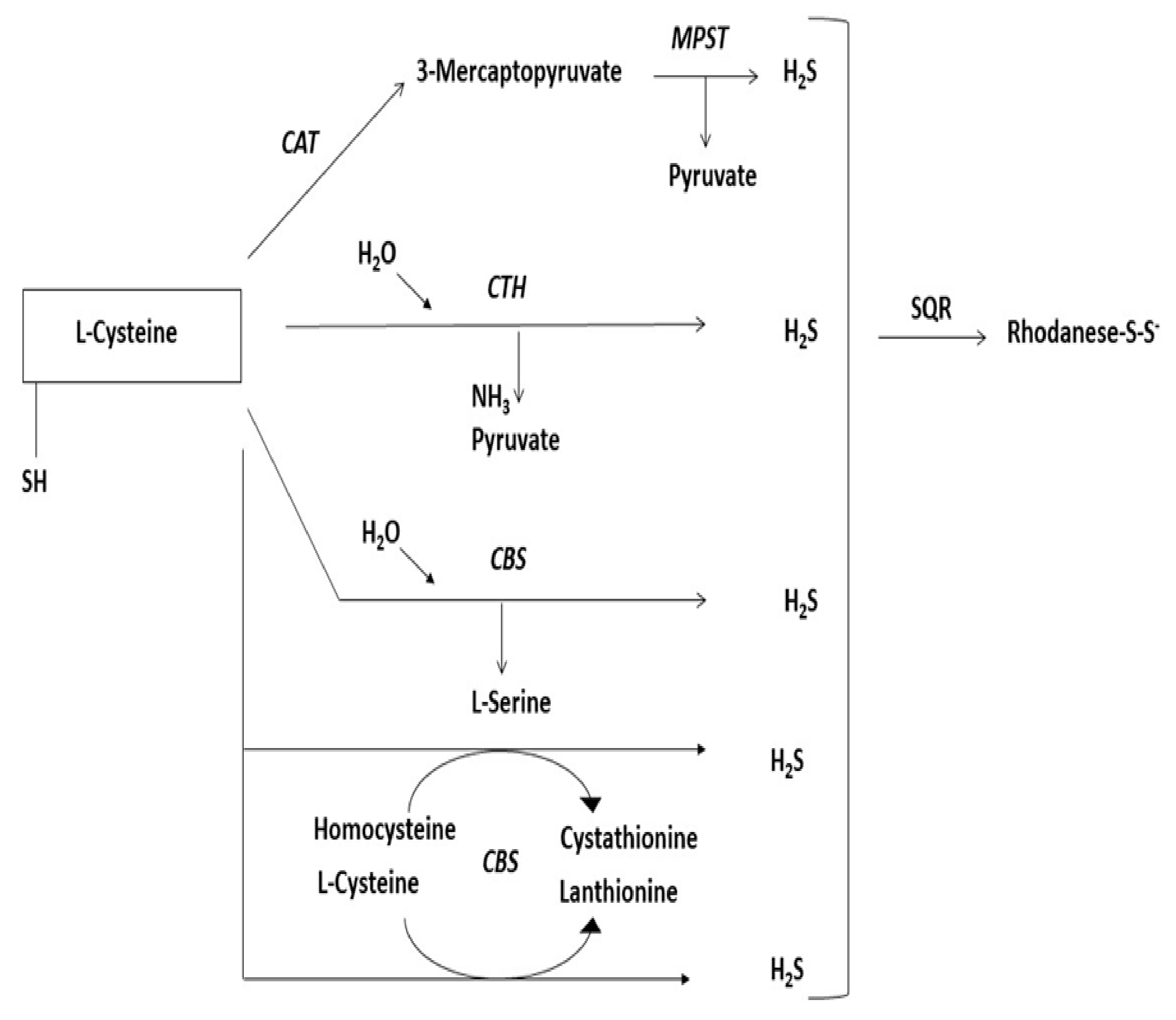
Sulfurtransferases are a widespread group of enzymes that can be found in archaea, bacteria, and eukaryotes [126]. They can catalyze sulfane sulfur atom transfer from a donor to a proper nucleophilic sulfur acceptor [127]. During such reactions, a persulfide-containing intermediate (enzyme-SSH) is created [128]. It is composed of at least a single catalytic rhodanese-like domain (RLD) [129], possibly two or even more [130], with specific conserved C-terminal-characteristic cysteine residue of the functional catalytic domain [131]. Furthermore, the structural localization of these domains is their specific feature [132]. Enzymes that possess such a catalytic cysteine (redox-active) play a critical role in many biological processes [133,134]. The group of enzymes involved in L-cysteine metabolism [135] are sulfurtransferases participating in the desulfuration pathway of L-cysteine, mainly 3-mercaptopyruvate sulfurtransferase (MPST, EC 2.8.1.2), thiosulfate sulfurtransferase (rhodanese or TST, EC 2.8.1.1), and lyases participating in the transsulfuration pathway of L-cysteine, mainly cystathionine γ-lyase (CTH, EC 4.4.1.1) and cystathionine-β-synthase (CBS, EC 4.2.1.22) [136] (Figure 6). CTH and CBS are PLP-dependent enzymes that belong to the group of lyases [135]. The predominant role of these enzymes is their key function in the transsulfuration pathway from L-methionine to L-cysteine [135]. On the other hand, these two enzymes take part in the further transformation of L-cysteine, whereby H2S production occurs [136]. Sulfurtransferases act in a two-step reaction in which sulfur is transferred to cysteine residue on the reactive site of the enzyme, where persulfide is created firstly from a suitable sulfur donor, and then sulfur is transferred to the nucleophilic acceptor [137,138]. During this process, particular complexes are formed: 1. Enzyme-SH-substrate at first, then 2. Enzyme-S-SH + product, so that ultimately 3. Enzyme-SH + S-product is formed [139]. This process is called double displacement [140]. Cysteine desulfurase (EC 2.8.1.7) shares the same mechanism of sulfur transfer [115]. According to their abilities MPST, CBS, and CTH affect the increase of and TST affects the decrease of the amount of sulfur in the sulfane sulfur pool [135]. Thus, participating in overall sulfur fraction equilibrium [141]. Malfunctions of these enzymes can result in various diseases [137,142,143,144]. Better understanding of multiple aspects of sulfurtransferases activity would give us the ability to control overall H2S production and its concentration-dependent action [145]. Sulfurtransferases have become promising targets for the development of new therapies [146]. Nowadays, advanced research aims at developing selective inhibitors for particular enzymes and reliable measurement tools of their overall activity [147].
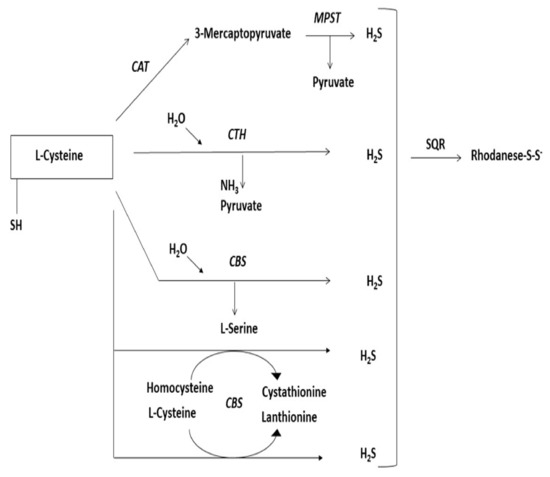
Figure 6.
Schematic diagram showing the L-cysteine desulfuration pathway resulting H2S release. CAT, cysteine aminotransferase, MPST, 3-mercaptopyruvate sulfurtransferase, CTH, cystathionine γ-lyase, CBS, cystathionine-β-synthase, SQR, sulfide quinone oxidoreductase.
6.1. Rhodanese and 3-Mercaptopyruvate Sulfurtransferase
Thiosulfate sulfurtransferase (rhodanese, TST, EC 2.8.1.1) and 3-mercaptopyruvate sulfurtransferase (MPST, EC 2.8.1.2) are important enzymes of the rhodanese/Cdc25 phosphatase superfamily [141]. These enzymes show high sequence similarity to each other [148]. TST is found in the mitochondria of eukaryotic cells [149]. This enzyme consists of a single polypeptide, about 32 kDa in molecular weight [139]. MPST shows both cytosolic and mitochondrial localization [150] with different isoforms (mitochondrial 35 kDa and mitochondrial/cytosol 33 kDa) existing [151]. Both proteins show specific occurrence in tissues [152,153]. Changes in this pattern are often linked with metabolic disease development and aging [138]. TST and MPST are composed of tandem rhodanese domains [128]. Cysteine localized in the enzyme active site (MPST- Cys248, TST- Cys247) is crucial for prime enzyme activity [154,155]. The sequence of amino acids located in the active site loop determines substrate specificity [155].
The best characterized bovine TST has maintained its double-domain structure and is considered to be TST sensu stricto [128]. The ability of TST that has been first discovered is to catalyze the reaction between thiosulfate and cyanide in which thiocyanate and sulfite are produced [156]. Interestingly, TST is involved in sulfane sulfur transformation into thiosulfate [157]. In this case, the initial source of sulfur is SQR acting in the process called mitochondrial sulfide oxidation, resulting in persulfide formation and electron donation on the respiratory chain [157]. This enzyme allows for incorporating H2S into the bound labile pool of sulfur inside the cell, thus participating in H2S clearance [158,159]. TST is capable of changing its conformation after oxidation, which grants this enzyme new features [160]. Moreover, phosphorylation of TST introduces a conformational change ability [161]. MPST can catalyze the reaction between cysteine-derived 3-mercaptopyruvate (3MP) and sulfur-acceptor substrate to yield pyruvate and enzyme-bound persulfide [162,163]. The enzyme is able to transfer this outer sulfur to an abundant number of small molecules or proteins [138]. Transferring sulfane sulfur onto its most suitable acceptor- thioredoxin (which ends up in the oxidized form), results in the enzyme’s active site restoration and H2S release [155]. 3-Mercaptopyruvate is produced from cysteine by cysteine aminotransferase (CAT; EC 2.6.1.3). Cooperation of MPST: CAT enzymatic system allows for transferring the sulfur from cysteine to active site cysteine and further to acceptor protein [164]. The transfer of sulfane sulfur is one of the possible mechanisms of persulfide species (Cys-SSH, GSSH, protein-SSH) formation [146]. Furthermore, MPST itself can generate H2Sn species (including H2S) [134]. In this case, the newly formed H2Sn can directly react with L-cysteine, GSH, or various proteins to create persulfide species [146]. Overall, Nagahara and colleagues summarized various reactions that can be catalyzed by MPST [154]. When MPST is unable to sustain its activity, TST expression is increased [165]. Proteins of the TST family are widespread in nature [166]. They are a heterogeneous group, differing from each other at various levels [130]. They form a diverse group of proteins called rhodanese-like proteins with identified examples divided into groups [128]. E. coli protein ThiI, which is a sulfurtransferase, has a similar sequence as TST [166]. The similarity also involves the amino acid sequence of the characteristic and catalytically critical “P loop” motif [167]. It seems to be only a temporary sulfur carrier between cysteine desulfurase IscS in the tRNA thiolation process [168]. Azotobacter vinelandii rhodanese-like protein (RhdA) can interact with E. coli cysteine desulfurase IscS with the use of L-cysteine, and another protein, RhdA-SSH, can carry sulfur to [2Fe–2S] holo adrenodoxin protein synthesis [129,169]. The carried sulfur is released after the apo-protein–RhdA complex formation and can be incorporated into the Fe–S cluster with iron ions in the presence of 2-mercaptoethanol [169]. Moreover, RhdA seems to modulate specific cysteine desulfurase (NifS or IscS) persulfide production, therefore, it cooperates with the enzyme during growth, increased cysteine concentration, and generally in sulfur administration [170]. However, deletion of the RhlA rhodanese-like protein from Streptomyces clavuligerus did not change the overall TST activity [171].
6.2. Participation of Rhodanese and 3-Mercaptopyruvate Sulfurtransferase in Fe–S Cluster Formation and Reconstitution
Sulfurtransferases have been proposed to be involved in the biogenesis of iron–sulfur clusters [172]. It has been shown that incorporating sulfur species into protein requires specific proteins and complex pathways [131]. Solvent-exposed clusters, which often serve as a Lewis-acid, are much more vulnerable to reactive oxygen species as H2O2, and in vivo analysis showed that only clusters undergo destruction, without damaging the specific peptide [111,173]. After a promising start, several unsuccessful attempts at recreating damaged clusters were made (using inorganic sulfide, iron, and 2-mercaptoethanol) [174]. Subsequent reports indicated that sulfurtransferases may be involved in the process of restoring the functional activity of apo-proteins [175]. TST was observed to restore the activity of succinate dehydrogenase (complex II) [175], NADH dehydrogenase [176], xanthine oxidase [177], ferredoxin of spinach [178], NADH: nitrate reductase [179], bacterial ferredoxin [180], and bacterial nitrogenase [181] (Table 1). Interestingly, the reaction rate when TST was employed was twice as fast as in the case of purely chemical synthesis [172]. More than 30% of all TST in bovine liver mitochondria is connected to its membrane where they form complexes with other compounds and may play a crucial role in the Fe–S cluster reconstitution [182], or somehow support it [175]. Moreover, MPST in cooperation with other components can also restore the activity of adrenal ferredoxin with an efficiency similar to that achieved in a chemical reaction [183]. The mechanism of such reconstitution depends on the phosphorylation/dephosphorylation continuum of TST where the dephosphorylated enzyme can insert sulfur into the newly forming Fe–S cluster [137]. Such a role is getting attention recently [184,185]. Sulfurtransferases participate in both the bound sulfane sulfur pool and acid-labile sulfur formation as well as H2S generating from these pools [186]. Increased H2S production is associated with the induction of antioxidative mechanisms [187]. H2S can be utilized by TST or spontaneously reacts with oxidized thiol residues, creating persulfide suitable for transfer [186]. More research is required to confirm the potential participation of the Fe–S cluster reconstitution in direct/indirect support.

Table 1.
List of enzymes that undergo a process of reconstitution of their activity with the participation of TST and MPST.
6.3. Antioxidant Properties of Sulfurtransferases Involved in Maintaining Fe–S Cluster Function
Evidence shows that cysteine desulfurase is a primary source of sulfane sulfur for biomolecules, including the Fe–S cluster formation [131]. Mitochondria are a major cellular compartment when reactive oxygen species are produced [188]. Enzymes that possess catalytic cysteine residue of the active site are exposed to a constant attack of hydrogen peroxide [189].
6.3.1. Involvement of Sulfurtransferases in Antioxidant Response
TST and rhodanese-like protein may play an antioxidant role in invertebrates [190]. Rhodanese-like protein or rhodanese homologue (MnRDH2) play an important role in maintaining the redox balance in invertebrates [148,191,192]. Both mammalian TST and MPST are involved in maintaining antioxidant defense [134,193]. Glutathione in the reduced (GSH) and oxidized (GSSG) forms plays a crucial role in maintaining redox homeostasis mainly by preserving sulfhydryl (-SH) group oxidation [188]. GSH in cooperation with glutathione peroxidase contributes to this process via ROS-scavenging (mainly H2O2 and lipid peroxides) [137,155]. TST takes part in the sulfide oxidation pathway inside mitochondria where sulfur is transferred from H2S to specific acceptors: sulfide quinone oxidoreductase (SQR) [1,162,194]. In cooperation with GSH, TST is also able to react with selenite (SeO3−2) to produce a stable intermediate (E-Se TST), and it takes part in selenium administration [195]. In addition, bovine liver TST [166] and MPST from Leishmania [149] have a higher affinity for the reduced form of thioredoxin than for its natural substrate, cyanide [166,170,196]. The presence of reduced thioredoxin (thioredoxin and Trx reductase system) is crucial to maintain the sulfur administration activity of TST [196] and MPST [197]. Nagahara has proposed a scheme of transformations of closely related elements (Grx, GSH, MPST) [197] and demonstrated in which way redox changes affect MPST activity step by step [154].
Interestingly, thioredoxin seems to be an important substrate for all TST proteins [198]. Moreover, thioredoxin can regulate the TST function by reducing propenyl sulfur protein (stereoisomer of S-allylcysteine, SAC) to restore the TST activity [199]. The thus formed glutathione persulfide can be used to reduce thioredoxin [137].
6.3.2. External Molecules with Ability to Modulate Sulfurtransferases Activity
Overall, external stress enhances H2S-related enzyme activity, such as TST [200]. However, Kruger et al. observed a reduced expression and translation of TST with a high production of superoxide in the mitochondria of monocytes, which could predict mortality [201]. Radiation causes an overall oxidative stress, and in the liver, it induces an antioxidative response [193]. Such a response is also generated along with rhodanese-domain-bearing proteins in ethanol-treated murine livers and other bacterial cells [170]. Depletion of any of ISC or CIA-conserved factors results in a lower survival rate of the human embryonic kidney 293 cells (HEK 293 cell line) in response to UV radiation or methyl-methane sulfonate (MMS) exposure [89]. It is noteworthy that, in mouse liver, a low-dose radiation exposure enhances the transcription of thioredoxin mRNA and a low dose rate, while long-term radiation exposure induces TST expression for a long period of time [202]. Besides radiation exposure, there are numerous factors that enhance TST: gene expression (resveratrol [203], enzyme activity-α-lipoic acid [204]) or protein expression (Phellinus linteus polysaccharide extracts) [205]. The activity of TST and MPST is increased after garlic-derived diallyl trisulfide (DATS) treatment [206]; moreover, N-acetyl-L-cysteine increases the MPST activity [207]. On the other hand, the level of TST and MPST proteins in the mitochondria is decreased after 4-hydroxybenzyl isothiocyanate (HBITC) (H2S donor) treatment [208]. The activity of all these factors is associated with the TST antioxidative activity [202]. Interestingly, another chemical compound of garlic, i.e., sodium 2-propenyl thiosulfate, can interact with the active site of TST, inhibiting its activity and expression [199].
Pagani et al. showed that lipoic acid and its reduced form (dihydrolipoic acid) affected TST activity [209] with a relatively low affinity [210]. The reduced form decreased the activity of TST after preincubation, but on the other hand, the oxidized form had no effect on the enzyme activity [209,210]. This is explained by the fact that dihydrolipoate is a sulfur acceptor from TST [210]. Moreover, the presence of thiosulfate inhibits decreasing TST activity [209]. Proteins that possess fully functional Fe–S clusters (holoproteins) may decrease rhodanese activity [181].
Oxidative stress is linked with many cancers [211]. Downregulation of TST expression is associated with many tumors [212,213,214]. Deficiency in TST activity is often reported in the case of malignant cells [215]. Some cancer cells can promote the expression of H2S-producing enzymes, such as MPST, to control oxidative stress [216]. Overexpressed protein Naf-1 with the labile Fe–S cluster is used by tumor cells to increase their aggressiveness by enhancing proliferation and tolerance to oxidative stress. This activity can be stopped, and it leads to ROS, iron, and oxidative stress accumulation [211]. The proliferation- and expansion-resistant cancer type can be stopped by induction and sensitization to oxidative stress [217]. In response to the generation of reactive oxygen/nitrogen/sulfur species (ROS/RNS/RSS), the protein cysteine thiols (R–SH) undergo a range of oxidative modifications, for example: nitrosylation (R–SNO), sulfenylation (R–SOH), and persulfidation (S-sulfuration, R–SSH) [218,219]. The enzymes that possess catalytic cysteine residues of the active site are exposed to a constant attack by hydrogen peroxide [189]. Nagahara showed that reactive oxygen species could oxidize the catalytic site Cys247 of MPST (Cys–SO−, Cys–SO2− and Cys–SO3−) and that the MPST activity decreased under oxidizing conditions and increased under reducing conditions [220]. Our previous studies [208] have demonstrated that the increased H2S and thiosulfate levels in HBITC-treated SH-SY5Y cells have been associated with downregulation of the level of TST and MPST, which suggest that the sulfhydryl groups of these enzymes can be modified by S-sulfuration (-SH to -SSH), or by oxidative stress (-SH to -SOH).
7. Conclusions
Sulfur is a very important microelement. We can distinguish different pools with specific abilities and functions. Acid-labile sulfur is a part of the iron–sulfur clusters, which are included in different proteins where they can perform various tasks. Mitochondria are the primary place where the Fe–S clusters are assembled. The source of sulfur is the cysteine desulfurase reaction. The mitochondrial process can be divided into two steps: the [2Fe–2S] cluster synthesis and [4Fe–4S] cluster synthesis. Interestingly, the mitochondrial machinery can produce an unidentified sulfur compound, X–S, which after being transferred to the cytoplasm may be a source of sulfur for the cytosolic Fe–S cluster formation machinery. Biological sulfur transfer via the persulfide group (containing sulfane sulfur atom) seems to be crucial for the Fe–S cluster formation. Many reports suggest the involvement of sulfurtransferases in the Fe–S cluster formation or repair/reconstitution, as well as sulfur-containing enzymes’ modification [221]. The exact mechanism is poorly understood. According to Freibert and colleagues, proven procedures were developed to reconstitute the Fe–S clusters by chemical reconstitution or de novo synthesis [64]. Many papers show that bacterial cysteine desulfurase (IscS) can repair damaged clusters; however, the repair occurs at a slower rate as compared to that in wild-type cells [111]. Agents of oxidative stress are often able to damage cysteine residues of the active site of enzymes. Reduction of oxidized cysteines can be performed by DTT or other agents, such as thioredoxin or glutaredoxin. The very first research addressing this topic has shown that it is unlikely that TST and MPST are responsible for the formation of the Fe–S clusters, but they cannot be excluded [183]. Fe–S clusters are oxidative-stress-sensitive structures. During cell life, oxidative stress is common, therefore the Fe–S clusters are often damaged. During the catalyzed reaction, both TST and cysteine desulfurase create cysteine persulfide, primarily in active sites, then they donate sulfane sulfur atoms to an acceptor molecule. Sulfurtransferases (MPST and TST) are involved in the antioxidative mechanism of cells. TST plays a protective role in oxidative stress induced by many factors, including radiation. Oxidative stress damages the Fe–S cluster and is often linked to cancer formation; therefore, it can be a potential target of treatment. MPST and TST, but mostly TST, seem to non-directly participate in Fe–S cluster formation; however, reports show possible direct participation in reconstitution/repair. The involvement of MPST and TST in oxidative stress could also provide an indirect mechanism of Fe–S cluster protection. Nevertheless, the synthesis, maturation of the Fe–S protein, repair of the Fe–S cluster, and antioxidative mechanism to protect clusters are not fully clarified.
Author Contributions
Conceptualization: M.W.; writing—literature collection and review: M.W., L.R. and H.J.; writing—original draft preparation: L.R.; writing—figure preparation: L.R.; writing—review, discussion, and editing: M.W. and H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Polish Ministry of Science and Higher Education, grant no. N41/DBS/000433 of the Jagiellonian University Medical College.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ehigie, L. Rhodanese: One of natures “sulphur” biotransformation machinery. Int. J. Sci. Res. 2018, 8, 838–845. [Google Scholar]
- Hou, N.; Yan, Z.; Fan, K.; Li, H.; Zhao, R.; Xia, Y.; Xun, L.; Liu, H. OxyR senses sulfane sulfur and activates the genes for its removal in Escherichia coli. Redox Biol. 2019, 26, 101293. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T. Assay methods and biological roles of labile sulfur in animal tissues. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 781, 227–249. [Google Scholar] [CrossRef]
- Kimura, H. Signaling Molecules: Hydrogen Sulfide and Polysulfide. Antioxid. Redox Signal. 2015, 22, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Predmore, B.L.; Lefer, D.J.; Gojon, G. Hydrogen Sulfide in Biochemistry and Medicine. Antioxid. Redox Signal. 2012, 17, 119–140. [Google Scholar] [CrossRef]
- Raulfs, E.C.; O’Carroll, I.P.; Dos Santos, P.C.; Unciuleac, M.-C.; Dean, D.R. In vivo iron–sulfur cluster formation. Proc. Natl. Acad. Sci. USA 2008, 105, 8591–8596. [Google Scholar] [CrossRef] [PubMed]
- Rouault, T.A.; Klausner, R.D. Iron–sulfur clusters as biosensors of oxidants and iron. Trends Biochem. Sci. 1996, 21, 174–177. [Google Scholar] [CrossRef]
- Couturier, J.; Touraine, B.; Briat, J.-F.; Gaymard, F.; Rouhier, N. The iron–sulfur cluster assembly machineries in plants: Current knowledge and open questions. Front. Plant Sci. 2013, 4, 259. [Google Scholar] [CrossRef]
- Imlay, J.A. Iron–sulphur clusters and the problem with oxygen. Mol. Microbiol. 2006, 59, 1073–1082. [Google Scholar] [CrossRef]
- Bak, D.; Elliott, S.J. Alternative FeS cluster ligands: Tuning redox potentials and chemistry. Curr. Opin. Chem. Biol. 2014, 19, 50–58. [Google Scholar] [CrossRef]
- Anand, B.N. Iron sulfur proteins and their synthetic analogues: Structure, reactivity and redox properties. Reson 1998, 3, 52–61. [Google Scholar] [CrossRef]
- Lill, R.; Mühlenhoff, U. Maturation of Iron–Sulfur Proteins in Eukaryotes: Mechanisms, Connected Processes, and Diseases. Annu. Rev. Biochem. 2008, 77, 669–700. [Google Scholar] [CrossRef]
- Goldberg, A.V.; Molik, S.; Tsaousis, A.D.; Neumann, K.; Kuhnke, G.; Delbac, F.; Vivares, C.P.; Hirt, R.; Lill, R.; Embley, T.M. Localization and functionality of microsporidian iron–sulphur cluster assembly proteins. Nat. Cell Biol. 2008, 452, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Py, B.; Barras, F. Building Fe–S proteins: Bacterial strategies. Nat. Rev. Microbiol. 2010, 8, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C.; Dean, D.R.; Smith, A.D.; Johnson, M.K. Structure, function, and formation of biological iron–sulfur clusters. Annu. Rev. Biochem. 2005, 74, 247–281. [Google Scholar] [CrossRef]
- DeBeer, S. Chapter fifteen—Advanced X-ray spectroscopic methods for studying iron–sulfur-containing proteins and model complexes. Methods Enzymol. 2018, 599, 427–450. [Google Scholar] [PubMed]
- Fu, W.; Morgan, T.; Mortenson, L.E.; Johnson, M.K. Resonance Raman studies of the [4Fe–4S] to [2Fe–2S] cluster conversion in the iron protein of nitrogenase. FEBS Lett. 1991, 284, 165–168. [Google Scholar] [CrossRef]
- Fuss, J.O.; Tsai, C.-L.; Ishida, J.P.; Tainer, J.A. Emerging critical roles of Fe–S clusters in DNA replication and repair. Biochim. Biophys. Acta Bioenerg. 2015, 1853, 1253–1271. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, S.; Teixeira, M. Resonance Raman spectroscopy of Fe–S proteins and their redox properties. J. Biol. Inorg. Chem. 2018, 23, 647–661. [Google Scholar] [CrossRef]
- Beinert, H.; Holm, R.H.; Münck, E. Iron–sulfur clusters: Nature’s modular, multipurpose structures. Science 1997, 277, 653–659. [Google Scholar] [CrossRef]
- Marquet, A.; Bui, B.T.S.; Smith, A.G.; Warren, M.J. Iron–sulfur proteins as initiators of radical chemistry. Nat. Prod. Rep. 2007, 24, 1027–1040. [Google Scholar] [CrossRef]
- Barton, J.K.; Silva, R.M.B.; O’Brien, E. Redox chemistry in the genome: Emergence of the [4Fe4S] cofactor in repair and replication. Annu. Rev. Biochem. 2019, 88, 163–190. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, B.J.; Clarkson, S.M.; Holden, J.F.; Lee, D.W.; Wu, C.H.; Pooleli, F.L.; Cotelesage, J.J.H.; Hackett, M.J.; Mohebbi, S.; Sun, J.; et al. Biological iron–sulfur storage in a thioferrate-protein nanoparticle. Nat. Commun. 2017, 8, 16110. [Google Scholar] [CrossRef]
- Lotierzo, M.; Bui, B.T.; Leech, H.K.; Warren, M.J.; Marquet, A.; Rigby, S.E. Iron–sulfur cluster dynamics in biotin synthase: A new [2Fe–2S]1+ cluster. Biochem. Biophys. Res. Commun. 2009, 381, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Ugulava, N.B.; Sacanell, C.J.; Jarrett, J.T. Spectroscopic Changes during a Single Turnover of Biotin Synthase: Destruction of a [2Fe–2S] Cluster Accompanies Sulfur Insertion. Biochemistry 2001, 40, 8352–8358. [Google Scholar] [CrossRef] [PubMed]
- Khoroshilova, N.; Beinert, H.; Kiley, P. Association of a polynuclear iron–sulfur center with a mutant FNR protein enhances DNA binding. Proc. Natl. Acad. Sci. USA 1995, 92, 2499–2503. [Google Scholar] [CrossRef] [PubMed]
- Biederbick, A.; Stehling, O.; Rösser, R.; Niggemeyer, B.; Nakai, Y.; Elsässer, H.-P.; Lill, R. Role of Human Mitochondrial Nfs1 in Cytosolic Iron–Sulfur Protein Biogenesis and Iron Regulation. Mol. Cell. Biol. 2006, 26, 5675–5687. [Google Scholar] [CrossRef] [PubMed]
- Rouault, T.A.; Maio, N. Biogenesis and functions of mammalian iron–sulfur proteins in the regulation of iron homeostasis and pivotal metabolic pathways. J. Biol. Chem. 2017, 292, 12744–12753. [Google Scholar] [CrossRef] [PubMed]
- Beilschmidt, L.K.; Puccio, H.M. Mammalian Fe–S cluster biogenesis and its implication in disease. Biochimie 2014, 100, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Barupala, D.P.; Dzul, S.P.; Riggs-Gelasco, P.J.; Stemmler, T.L. Synthesis, delivery and regulation of eukaryotic heme and Fe–S cluster cofactors. Arch. Biochem. Biophys. 2016, 592, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Rouault, T.A. Biogenesis of iron–sulfur clusters in mammalian cells: New insights and relevance to human disease. Dis. Model. Mech. 2012, 5, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ghosh, M.C.; Rouault, T.A. The physiological functions of iron regulatory proteins in iron homeostasis—An update. Front. Pharmacol. 2014, 5, 124. [Google Scholar] [CrossRef]
- Giel, J.L.; Rodionov, D.; Liu, M.; Blattner, F.R.; Kiley, P.J. IscR-dependent gene expression links iron–sulphur cluster assembly to the control of O2-regulated genes in Escherichia coli. Mol. Microbiol. 2006, 60, 1058–1075. [Google Scholar] [CrossRef]
- Lill, R.; Hoffmann, B.; Molik, S.; Pierik, A.J.; Rietzschel, N.; Stehling, O.; Uzarska, M.A.; Webert, H.; Wilbrecht, C.; Mühlenhoff, U. The role of mitochondria in cellular iron–sulfur protein biogenesis and iron metabolism. Biochim. Biophys. Acta 2012, 1823, 1491–1508. [Google Scholar] [CrossRef]
- Mariotti, L.; Wild, S.; Brunoldi, G.; Piceni, A.; Ceppi, I.; Kummer, S.; Lutz, R.E.; Cejka, P.; Gari, K. The iron–sulphur cluster in human DNA2 is required for all biochemical activities of DNA2. Commun. Biol. 2020, 3. [Google Scholar] [CrossRef] [PubMed]
- Pain, D.; Dancis, A. Roles of Fe–S proteins: From cofactor synthesis to iron homeostasis to protein synthesis. Curr. Opin. Genet. Dev. 2016, 38, 45–51. [Google Scholar] [CrossRef]
- Mueller, E.G. Trafficking in persulfides: Delivering sulfur in biosynthetic pathways. Nat. Chem. Biol. 2006, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Marsh, E.N.G. Viperin: An ancient radical SAM enzyme finds its place in modern cellular metabolism and innate immunity. J. Biol. Chem. 2020, 295, 11513–11528. [Google Scholar] [CrossRef] [PubMed]
- Rouhier, N.; Couturier, J.; Johnson, M.K.; Jacquot, J.-P. Glutaredoxins: Roles in iron homeostasis. Trends Biochem. Sci. 2010, 35, 43–52. [Google Scholar] [CrossRef]
- Banci, L.; Brancaccio, D.; Baffoni, S.C.; Del Conte, R.; Gadepalli, R.; Mikolajczyk, M.; Neri, S.; Piccioli, M.; Winkelmann, J. [2Fe–2S] cluster transfer in iron–sulfur protein biogenesis. Proc. Natl. Acad. Sci. USA 2014, 111, 6203–6208. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sieprawska-Lupa, M.; Whitman, W.B.; White, R.H. cysteine is not the sulfur source for iron–sulfur cluster and methionine biosynthesis in the methanogenic archaeon methanococcus maripaludis. J. Biol. Chem. 2010, 285, 31923–31929. [Google Scholar] [CrossRef] [PubMed]
- Gervason, S.; Larkem, D.; Mansour, A.B.; Botzanowski, T.; Müller, C.S.; Pecqueur, L.; Le Pavec, G.; Delaunay-Moisan, A.; Brun, O.; Agramunt, J.; et al. Physiologically relevant reconstitution of iron–sulfur cluster biosynthesis uncovers persulfide-processing functions of fer-redoxin-2 and frataxin. Nat. Commun. 2019, 10, 3566. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Rouault, T.A. Human Iron–Sulfur Cluster Assembly, Cellular Iron Homeostasis, and Disease. Biochemistry 2010, 49, 4945–4956. [Google Scholar] [CrossRef]
- Wachnowsky, C.; Fidai, I.; Cowan, J.A. Iron–sulfur cluster biosynthesis and trafficking—Impact on human disease conditions. Metallomics 2018, 10, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Tsaousis, A.D.; Gentekaki, E.; Eme, L.; Gaston, D.; Roger, A.J. Evolution of the Cytosolic Iron–Sulfur Cluster Assembly Machinery in Blastocystis Species and Other Microbial Eukaryotes. Eukaryot. Cell 2014, 13, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Tokumoto, U.; Kitamura, S.; Fukuyama, K.; Takahashi, Y. Interchangeability and distinct properties of bacterial Fe–S cluster assembly systems: Functional replacement of the isc and suf operons in Escherichia coli with the nifSU-like operon from Helicobacter pylori. J. Biochem. 2004, 136, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Kiley, P.J.; Beinert, H. The role of Fe–S proteins in sensing and regulation in bacteria. Curr. Opin. Microbiol. 2003, 6, 181–185. [Google Scholar] [CrossRef]
- Boyd, E.S.; Thomas, K.M.; Dai, Y.; Boyd, J.M.; Outten, F.W. Interplay between Oxygen and Fe–S Cluster Biogenesis: Insights from the Suf Pathway. Biochemie 2014, 53, 5834–5847. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, T.; Happe, T.; Lu, Y.; Sawyer, A. Iron–sulphur cluster biogenesis via the SUF pathway. Metallomics 2018, 10, 1038–1052. [Google Scholar] [CrossRef]
- Braymer, J.J.; Lill, R. Iron–sulfur cluster biogenesis and trafficking in mitochondria. J. Biol. Chem. 2017, 292, 12754–12763. [Google Scholar] [CrossRef]
- Shi, R.; Proteau, A.; Villarroya, M.; Moukadiri, I.; Zhang, L.; Trempe, J.F.; Matte, A.; Armengod, M.E.; Cygler, M. Structural basis for Fe–S cluster assembly and tRNA thiolation mediated by IscS protein-protein interactions. PLoS Biol. 2010, 8, 1000354. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Van Vranken, J.G.; Brignole, E.J.; Patra, S.; Winge, D.R.; Drennan, C.L.; Rutter, J.; Barondeau, D.P. Structure of human Fe–S assembly subcomplex reveals unexpected cysteine desulfurase architecture and acyl-ACP–ISD11 interactions. Proc. Natl. Acad. Sci. USA 2017, 114, E5325–E5334. [Google Scholar] [CrossRef]
- Rouault, T.A. The indispensable role of mammalian iron sulfur proteins in function and regulation of multiple diverse metabolic pathways. BioMetals 2019, 32, 343–353. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Barondeau, D.P. Human Frataxin Is an Allosteric Switch That Activates the Fe–S Cluster Biosynthetic Complex. Biochemistry 2010, 49, 9132–9139. [Google Scholar] [CrossRef]
- Amela, I.; Delicado, P.; Gómez, A.; Querol, E.; Cedano, J. A Dynamic Model of the Proteins that Form the Initial Iron–Sulfur Cluster Biogenesis Machinery in Yeast Mitochondria. Protein J. 2013, 32, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Tonelli, M.; Frederick, R.O.; Markley, J.L. Human Mitochondrial Ferredoxin 1 (FDX1) and Ferredoxin 2 (FDX2) Both Bind Cysteine Desulfurase and Donate Electrons for Iron–Sulfur Cluster Biosynthesis. Biochemistry 2017, 56, 487–499. [Google Scholar] [CrossRef]
- Dlouhy, A.C.; Li, H.; Albetel, A.-N.; Zhang, B.; Mapolelo, D.T.; Randeniya, S.; Holland, A.A.; Johnson, M.K.; Outten, C.E. The Escherichia coli BolA Protein IbaG Forms a Histidine-Ligated [2Fe–2S]-Bridged Complex with Grx4. Biochemistry 2016, 55, 6869–6879. [Google Scholar] [CrossRef] [PubMed]
- Voisine, C.; Cheng, Y.C.; Ohlson, M.; Schilke, B.; Hoff, K.; Beinert, H.; Marszalek, J.; Craig, E.A. Jac1, a mitochondrial J-type chaperone, is involved in the biogenesis of Fe/S clusters in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2001, 98, 1483–1488. [Google Scholar] [CrossRef]
- Godat, E.; Madalinski, G.; Muller, L.; Heilier, J.F.; Labarre, J.; Junot, C. Chapter 2—Mass spectrometry-based methods for the determination of sulfur and related metabolite concentrations in cell extracts. Methods Enzymol. 2010, 473, 41–76. [Google Scholar]
- Zheng, L.; Dean, D. Catalytic formation of a nitrogenase iron–sulfur cluster. J. Biol. Chem. 1994, 269, 18723–18726. [Google Scholar] [CrossRef]
- Van Vranken, J.G.; Jeong, M.-Y.; Wei, P.; Chen, Y.-C.; Gygi, S.P.; Winge, D.R.; Rutter, J. The mitochondrial acyl carrier protein (ACP) coordinates mitochondrial fatty acid synthesis with iron sulfur cluster biogenesis. eLife 2016, 5, e17828. [Google Scholar] [CrossRef]
- Maio, N.; Ghezzi, D.; Verrigni, D.; Rizza, T.; Bertini, E.; Martinelli, D.; Zeviani, M.; Singh, A.; Carrozzo, R.; Rouault, T.A. Disease-Causing SDHAF1 Mutations Impair Transfer of Fe–S Clusters to SDHB. Cell Metab. 2016, 23, 292–302. [Google Scholar] [CrossRef]
- Freibert, S.-A.; Weiler, B.D.; Bill, E.; Pierik, A.J.; Mühlenhoff, U.; Lill, R. Biochemical Reconstitution and Spectroscopic Analysis of Iron–Sulfur Proteins. Methods Enzymol. 2018, 599, 197–226. [Google Scholar] [CrossRef]
- Cavadini, P.; O’Neill, H.A.; Benada, O.; Isaya, G. Assembly and iron-binding properties of human frataxin, the protein deficient in Friedreich ataxia. Hum. Mol. Genet. 2002, 11, 217–227. [Google Scholar] [CrossRef]
- Webert, H.; Freibert, S.-A.; Gallo, A.; Heidenreich, T.; Linne, U.; Amlacher, S.; Hurt, E.; Mühlenhoff, U.; Banci, L.; Lill, R. Functional reconstitution of mitochondrial Fe/S cluster synthesis on Isu1 reveals the involvement of ferredoxin. Nat. Commun. 2014, 5, 5013. [Google Scholar] [CrossRef]
- Mendel, R.R.; Hercher, T.W.; Zupok, A.; Hasnat, M.A.; Leimkühler, S. The Requirement of Inorganic Fe–S Clusters for the Biosynthesis of the Organometallic Molybdenum Cofactor. Inorganics 2020, 8, 43. [Google Scholar] [CrossRef]
- Guo, P.-C.; Ma, J.-D.; Jiang, Y.; Wang, S.-J.; Bao, Z.-Z.; Yu, X.-J.; Chen, Y.; Zhou, C.-Z.; Yao, S.; Qian, M.; et al. Structure of Yeast Sulfhydryl Oxidase Erv1 Reveals Electron Transfer of the Disulfide Relay System in the Mitochondrial Intermembrane Space. J. Biol. Chem. 2012, 287, 34961–34969. [Google Scholar] [CrossRef] [PubMed]
- Melber, A.; Na, U.; Vashisht, A.; Weiler, B.D.; Lill, R.; Wohlschlegel, J.; Winge, D.R. Role of Nfu1 and Bol3 in iron–sulfur cluster transfer to mitochondrial clients. eLife 2016, 5, e15991. [Google Scholar] [CrossRef]
- Ciofi-Baffoni, S.; Nasta, V.; Banci, L. Protein networks in the maturation of human iron–sulfur proteins. Metallomics 2018, 10, 49–72. [Google Scholar] [CrossRef] [PubMed]
- Sheftel, A.D.; Wilbrecht, C.; Stehling, O.; Niggemeyer, B.; Elsässer, H.-P.; Mühlenhoff, U.; Lill, R. The human mitochondrial ISCA1, ISCA2, and IBA57 proteins are required for [4Fe–4S] protein maturation. Mol. Biol. Cell 2012, 23, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Gourdoupis, S.; Nasta, V.; Calderone, V.; Ciofi-Baffoni, S.; Banci, L. IBA57 Recruits ISCA2 to Form a [2Fe–2S] Cluster-Mediated Complex. J. Am. Chem. Soc. 2018, 140, 14401–14412. [Google Scholar] [CrossRef]
- Maio, N.; Rouault, T.A. Outlining the Complex Pathway of Mammalian Fe–S Cluster Biogenesis. Trends Biochem. Sci. 2020, 45, 411–426. [Google Scholar] [CrossRef]
- Mühlenhoff, U.; Richter, N.; Pines, O.; Pierik, A.J.; Lill, R. Specialized function of yeast Isa1 and Isa2 proteins in the maturation of mitochondrial [4Fe–4S] proteins. J. Biol. Chem. 2017, 292, 17979. [Google Scholar] [CrossRef] [PubMed]
- Uzarska, M.; Nasta, V.; Weiler, B.D.; Spantgar, F.; Ciofi-Baffoni, S.; Saviello, M.R.; Gonnelli, L.; Mühlenhoff, U.; Banci, L.; Lill, R. Mitochondrial Bol1 and Bol3 function as assembly factors for specific iron–sulfur proteins. eLife 2016, 5, e16673. [Google Scholar] [CrossRef]
- Maio, N.; Rouault, T.A. Iron–sulfur cluster biogenesis in mammalian cells: New insights into the molecular mechanisms of cluster delivery. Biochim. Biophys. Acta Bioenerg. 2015, 1853, 1493–1512. [Google Scholar] [CrossRef]
- Stehling, O.; Jeoung, J.-H.; Freibert, S.A.; Paul, V.D.; Bänfer, S.; Niggemeyer, B.; Rösser, R.; Dobbek, H.; Lill, R. Function and crystal structure of the dimeric P-loop ATPase CFD1 coordinating an exposed [4Fe–4S] cluster for transfer to apoproteins. Proc. Natl. Acad. Sci. USA 2018, 115, E9085–E9094. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, L.J.; Solodovnikova, N.; Sharma, A.K.; Walden, W.E. Interaction with Cfd1 Increases the Kinetic Lability of FeS on the Nbp35 Scaffold. J. Biol. Chem. 2013, 288, 23358–23367. [Google Scholar] [CrossRef]
- Camponeschi, F.; Ciofi-Baffoni, S.; Banci, L. Anamorsin/Ndor1 complex reduces [2Fe–2S]-mitoNEET via a transient protein–protein interaction. J. Am. Chem. Soc. 2017, 28, 9479–9482. [Google Scholar] [CrossRef]
- Tamir, S.; Paddock, M.L.; Darash-Yahana-Baram, M.; Holt, S.H.; Sohn, Y.S.; Agranat, L.; Michaeli, D.; Stofleth, J.; Lipper, C.H.; Morcos, F.; et al. Structure–function analysis of NEET proteins uncovers their role as key regulators of iron and ROS homeostasis in health and disease. Biochim. Biophys. Acta Bioenerg. 2015, 1853, 1294–1315. [Google Scholar] [CrossRef]
- Vernis, L.; El Banna, N.; Baïlle, D.; Hatem, E.; Heneman, A.; Huang, M.-E. Fe–S Clusters Emerging as Targets of Therapeutic Drugs. Oxid. Med. Cell. Longev. 2017, 2017, 3647657. [Google Scholar] [CrossRef] [PubMed]
- Zuris, J.A.; Harir, Y.; Conlan, A.R.; Shvartsman, M.; Michaeli, D.; Tamir, S.; Paddock, M.L.; Onuchic, J.N.; Mittler, R.; Cabantchik, Z.I.; et al. Facile transfer of [2Fe–2S] clusters from the diabetes drug target mitoNEET to an apo-acceptor protein. Proc. Natl. Acad. Sci. USA 2011, 108, 13047–13052. [Google Scholar] [CrossRef] [PubMed]
- Ferecatu, I.; Gonçalves, S.; Golinelli-Cohen, M.-P.; Clémancey, M.; Martelli, A.; Riquier, S.; Guittet, E.; Latour, J.-M.; Puccio, H.; Drapier, J.-C.; et al. The Diabetes Drug Target MitoNEET Governs a Novel Trafficking Pathway to Rebuild an Fe–S Cluster into Cytosolic Aconitase/Iron Regulatory Protein. J. Biol. Chem. 2014, 289, 28070–28086. [Google Scholar] [CrossRef] [PubMed]
- Lipper, C.H.; Paddock, M.L.; Onuchic, J.N.; Mittler, R.; Nechushtai, R.; Jennings, P.A. Cancer-Related NEET Proteins Transfer 2Fe–2S Clusters to Anamorsin, a Protein Required for Cytosolic Iron–Sulfur Cluster Biogenesis. PLoS ONE 2015, 10, e0139699. [Google Scholar] [CrossRef]
- Frey, A.G.; Palenchar, D.J.; Wildemann, J.D.; Philpott, C.C. A Glutaredoxin·BolA Complex Serves as an Iron–Sulfur Cluster Chaperone for the Cytosolic Cluster Assembly Machinery. J. Biol. Chem. 2016, 291, 22344–22356. [Google Scholar] [CrossRef]
- Netz, D.J.; Pierik, A.J.; Stümpfig, M.; Bill, E.; Sharma, A.K.; Pallesen, L.J.; Walden, W.E.; Lill, R. A Bridging [4Fe–4S] Cluster and Nucleotide Binding Are Essential for Function of the Cfd1-Nbp35 Complex as a Scaffold in Iron–Sulfur Protein Maturation. J. Biol. Chem. 2012, 287, 12365–12378. [Google Scholar] [CrossRef]
- Seki, M.; Takeda, Y.; Iwai, K.; Tanaka, K. IOP1 Protein Is an External Component of the Human Cytosolic Iron–Sulfur Cluster Assembly (CIA) Machinery and Functions in the MMS19 Protein-dependent CIA Pathway. J. Biol. Chem. 2013, 288, 16680–16689. [Google Scholar] [CrossRef]
- Srinivasan, V.; Netz, D.J.; Webert, H.; Mascarenhas, J.; Pierik, A.J.; Michel, H.; Lill, R. Structure of the Yeast WD40 Domain Protein Cia1, a Component Acting Late in Iron–Sulfur Protein Biogenesis. Structure 2007, 15, 1246–1257. [Google Scholar] [CrossRef]
- Stehling, O.; Vashisht, A.A.; Mascarenhas, J.; Jonsson, Z.O.; Sharma, T.; Netz, D.J.A.; Pierik, A.J.; Wohlschlegel, J.A.; Lill, R. MMS19 Assembles Iron–Sulfur Proteins Required for DNA Metabolism and Genomic Integrity. Science 2012, 337, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, D.C.; Gari, K. The CIA Targeting Complex Is Highly Regulated and Provides Two Distinct Binding Sites for Client Iron–Sulfur Proteins. Cell Rep. 2017, 18, 1434–1443. [Google Scholar] [CrossRef]
- Paul, V.D.; Lill, R. Biogenesis of cytosolic and nuclear iron–sulfur proteins and their role in genome stability. Biochim. Biophys. Acta Bioenerg. 2015, 1853, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- Vannier, J.-B.; Sarek, G.; Boulton, S.J. RTEL1: Functions of a disease-associated helicase. Trends Cell Biol. 2014, 24, 416–425. [Google Scholar] [CrossRef]
- Paul, V.D.; Mühlenhoff, U.; Stümpfig, M.; Seebacher, J.; Kugler, K.G.; Renicke, C.; Taxis, C.; Gavin, A.-C.; Pierik, A.J.; Lill, R. The deca-GX3 proteins Yae1-Lto1 function as adaptors recruiting the ABC protein Rli1 for iron–sulfur cluster insertion. eLife 2015, 4, e08231. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pain, J.; Dancis, A.; Pain, D. Mitochondria export iron–sulfur and sulfur intermediates to the cytoplasm for iron–sulfur cluster assembly and tRNA thiolation in yeast. J. Biol. Chem. 2019, 294, 9489–9502. [Google Scholar] [CrossRef]
- Crack, J.C.; Green, J.; Thomson, A.J.; Le Brun, N. Iron–Sulfur Clusters as Biological Sensors: The Chemistry of Reactions with Molecular Oxygen and Nitric Oxide. Acc. Chem. Res. 2014, 47, 3196–3205. [Google Scholar] [CrossRef]
- Xu, F.F.; Imlay, J.A. Silver(I), Mercury(II), Cadmium(II), and Zinc(II) Target Exposed Enzymic Iron–Sulfur Clusters when They Toxify Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 3614–3621. [Google Scholar] [CrossRef]
- Brancaccio, D.; Gallo, A.; Piccioli, M.; Novellino, E.; Ciofi-Baffoni, S.; Banci, L. [4Fe–4S] Cluster Assembly in Mitochondria and Its Impairment by Copper. J. Am. Chem. Soc. 2017, 139, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.M.; Krupp, J.; Clark, S.; Isberg, R.R. Iron–Sulfur Cluster Repair Contributes to Yersinia pseudotuberculosis Survival within Deep Tissues. Infect. Immun. 2019, 87, 533. [Google Scholar] [CrossRef]
- Musaogullari, A.; Chai, Y.-C. Redox Regulation by Protein S-Glutathionylation: From Molecular Mechanisms to Implications in Health and Disease. Int. J. Mol. Sci. 2020, 21, 8113. [Google Scholar] [CrossRef] [PubMed]
- Serrano, P.N.; Wang, H.; Crack, J.C.; Prior, C.; Hutchings, M.I.; Thomson, A.J.; Kamali, S.; Yoda, Y.; Zhao, J.; Hu, M.Y.; et al. Nitrosylation of Nitric-Oxide-Sensing Regulatory Proteins Containing [4Fe–4S] Clusters Gives Rise to Multiple Iron-Nitrosyl Complexes. Angew. Chem. 2016, 128, 14795–14799. [Google Scholar] [CrossRef]
- Pearce, L.L.; Martinez-Bosch, S.; Manzano, E.L.; Winnica, D.E.; Epperly, M.W.; Peterson, J. The resistance of electron-transport chain Fe–S clusters to oxidative damage during the reaction of peroxynitrite with mitochondrial complex II and rat-heart pericardium. Nitric Oxide 2009, 20, 135–142. [Google Scholar] [CrossRef]
- Duan, X.; Yang, J.; Ren, B.; Tan, G.; Ding, H. Reactivity of nitric oxide with the [4Fe–4S] cluster of dihydroxyacid dehydratase from Escherichia coli. Biochem. J. 2009, 417, 783–789. [Google Scholar] [CrossRef]
- Wang, Z. Protein S-nitrosylation and cancer. Cancer Lett. 2012, 320, 123–129. [Google Scholar] [CrossRef]
- Bruska, M.K.; Stiebritz, M.T.; Reiher, M. Binding of Reactive Oxygen Species at Fe–S Cubane Clusters. Chemistry 2015, 21, 19081–19089. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Imlay, J.A. A conserved motif liganding the [4Fe–4S] cluster in [4Fe–4S] fumarases prevents irreversible inactivation of the enzyme during hydrogen peroxide stress. Redox Biol. 2019, 26, 101296. [Google Scholar] [CrossRef]
- Anjem, A.; Imlay, J.A. Mononuclear Iron Enzymes Are Primary Targets of Hydrogen Peroxide Stress. J. Biol. Chem. 2012, 287, 15544–15556. [Google Scholar] [CrossRef]
- Mettert, E.L.; Kiley, P.J. How Is Fe–S Cluster Formation Regulated? Annu. Rev. Microbiol. 2015, 69, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Where in the world do bacteria experience oxidative stress? Environ. Microbiol. 2019, 21, 521–530. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Fujisawa, H. Reconstitution of iron–sulfur cluster of NADH-cytochrome c reductase, a component of benzoate 1,2-dioxygenase system from Pseudomonas arvilla C-1. J. Biol. Chem. 1981, 256, 6783–6787. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2) and EC-SOD (SOD3) gene structures evolution and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Djaman, O.; Outten, F.; Imlay, J.A. Repair of Oxidized Iron–Sulfur Clusters in Escherichia coli. J. Biol. Chem. 2004, 279, 44590–44599. [Google Scholar] [CrossRef] [PubMed]
- Bouton, C.; Chauveau, M.-J.; Lazereg, S.; Drapier, J.-C. Recycling of RNA Binding Iron Regulatory Protein 1 into an Aconitase after Nitric Oxide Removal Depends on Mitochondrial ATP. J. Biol. Chem. 2002, 277, 31220–31227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Crack, J.C.; Subramanian, S.; Green, J.; Thomson, A.J.; Le Brun, N.; Johnson, M.K. Reversible cycling between cysteine persulfide-ligated [2Fe–2S] and cysteine-ligated [4Fe–4S] clusters in the FNR regulatory protein. Proc. Natl. Acad. Sci. USA 2012, 109, 15734–15739. [Google Scholar] [CrossRef]
- Wallace, M.A.; Liou, L.-L.; Martins, J.; Clement, M.H.S.; Bailey, S.; Longo, V.D.; Valentine, J.S.; Gralla, E.B. Superoxide Inhibits 4Fe–4S Cluster Enzymes Involved in Amino Acid Biosynthesis: Cross-compartment protection by CuZn-superoxide dismutase. J. Biol. Chem. 2004, 279, 32055–32062. [Google Scholar] [CrossRef]
- Black, K.A.; Dos Santos, P.C. Shared-intermediates in the biosynthesis of thio-cofactors: Mechanism and functions of cysteine desulfurases and sulfur acceptors. Biochim. Biophys. Acta Bioenerg. 2015, 1853, 1470–1480. [Google Scholar] [CrossRef]
- Vasil’Eva, S.V.; Strel’Tsova, D.; Vlaskina, A.V.; Mikoian, V.D.; Vanin, A.F. The sources of inorganic sulfur in the process of cluster protein Fnr[4Fe–4S]2+ reconstruction in Escherichia coli cells cultivated with NO-donating agents. Biofizika 2012, 57, 247–252. [Google Scholar] [PubMed]
- Yang, W.; Rogers, P.A.; Ding, H. Repair of Nitric Oxide-modified Ferredoxin [2Fe–2S] Cluster by Cysteine Desulfurase (IscS). J. Biol. Chem. 2002, 277, 12868–12873. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.A.; Eide, L.; Klungland, A.; Ding, H. Reversible inactivation of E. coli endonuclease III via modification of its [4Fe–4S] cluster by nitric oxide. DNA Repair 2003, 2, 809–817. [Google Scholar] [CrossRef]
- Schwartz, C.J.; Djaman, O.; Imlay, J.A.; Kiley, P.J. The cysteine desulfurase, IscS, has a major role in in vivo Fe–S cluster formation in Escherichia coli. Proc. Natl. Acad. Sci. USA 2000, 97, 9009–9014. [Google Scholar] [CrossRef] [PubMed]
- Golinelli-Cohen, M.-P.; Lescop, E.; Mons, C.; Gonçalves, S.; Clémancey, M.; Santolini, J.; Guittet, E.; Blondin, G.; Latour, J.-M.; Bouton, C. Redox Control of the Human Iron–Sulfur Repair Protein MitoNEET Activity via Its Iron–Sulfur Cluster. J. Biol. Chem. 2016, 291, 7583–7593. [Google Scholar] [CrossRef] [PubMed]
- Overton, T.; Justino, M.C.; Li, Y.; Baptista, J.M.; Melo, A.P.; Cole, J.A.; Saraiva, L.M. Widespread Distribution in Pathogenic Bacteria of Di-Iron Proteins That Repair Oxidative and Nitrosative Damage to Iron–Sulfur Centers. J. Bacteriol. 2008, 190, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Justino, M.; Almeida, C.C.; Teixeira, M.; Saraiva, L. Escherichia coli Di-iron YtfE Protein Is Necessary for the Repair of Stress-damaged Iron–Sulfur Clusters. J. Biol. Chem. 2007, 282, 10352–10359. [Google Scholar] [CrossRef] [PubMed]
- Nobre, L.S.; García-Serres, R.; Todorović, S.; Hildebrandt, P.; Teixeira, M.; Latour, J.-M.; Saraiva, L.M. Escherichia coli RIC Is Able to Donate Iron to Iron–Sulfur Clusters. PLoS ONE 2014, 9, e95222. [Google Scholar] [CrossRef] [PubMed]
- Justino, M.C.; Baptista, J.M.; Saraiva, L.M. Di-iron proteins of the Ric family are involved in iron–sulfur cluster repair. BioMetals 2009, 22, 99–108. [Google Scholar] [CrossRef]
- Rogers, P.A.; Ding, H. l-Cysteine-mediated Destabilization of Dinitrosyl Iron Complexes in Proteins. J. Biol. Chem. 2001, 276, 30980–30986. [Google Scholar] [CrossRef] [PubMed]
- Mishanina, T.; Libiad, M.; Banerjee, R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat. Chem. Biol. 2015, 11, 457–464. [Google Scholar] [CrossRef]
- Acosta, M.; Beard, S.; Ponce, J.; Vera, M.; Mobarec, J.C.; Jerez, C.A. Identification of Putative Sulfur transferase Genes in the Extremophilic Acidithiobacillus ferrooxidans ATCC 23270 Genome: Structural and Functional Characterization of the Proteins. OMICS J. Integr. Biol. 2005, 9, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Bordo, D.; Bork, P. The rhodanese/Cdc25 phosphatase superfamily. EMBO Rep. 2002, 3, 741–746. [Google Scholar] [CrossRef]
- Forlani, F.; Cereda, A.; Freuer, A.; Nimtz, M.; Leimkühler, S.; Pagani, S. The cysteine-desulfurase IscS promotes the production of the rhodanese RhdA in the persulfurated form. FEBS Lett. 2005, 579, 6786–6790. [Google Scholar] [CrossRef] [PubMed]
- Hãnzelmann, P.; Dahl, J.U.; Kuper, J.; Urban, A.; Müller-Theissen, U.; Leimkühler, S.; Schindelin, H.; Mãller-Theissen, U.; Leimkãhler, S. Crystal structure of YnjE from Escherichia coli, a sulfurtransferase with three rhodanese domains. Protein Sci. 2009, 18, 2480–2491. [Google Scholar] [CrossRef]
- Leimkühler, S.; Bühning, M.; Beilschmidt, L. Shared Sulfur Mobilization Routes for tRNA Thiolation and Molybdenum Cofactor Biosynthesis in Prokaryotes and Eukaryotes. Biomolecules 2017, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Libiad, M.; Motl, N.; Akey, D.L.; Sakamoto, N.; Fearon, E.R.; Smith, J.L.; Banerjee, R. Thiosulfate sulfurtransferase-like do-main-containing 1 protein interacts with thioredoxin. J. Biol. Chem. 2018, 293, 2675–2686. [Google Scholar] [CrossRef]
- Nagahara, N. Catalytic Site Cysteines of Thiol Enzyme: Sulfurtransferases. J. Amino Acids 2011, 2011, 709404. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Koike, S.; Nirasawa, T.; Kimura, H.; Ogasawara, Y. Alternative pathway of H2S and polysulfides production from sulfurated catalytic-cysteine of reaction intermediates of 3-mercaptopyruvate sulfurtransferase. Biochem. Biophys. Res. Commun. 2018, 496, 648–653. [Google Scholar] [CrossRef]
- Saidu, Y. Physicochemical features of rhodanese: A review. Afr. J. Biotechnol. 2004, 3, 370–374. [Google Scholar] [CrossRef]
- Wróbel, M.; Sura, P.; Srebro, Z. Sulfurtransferases and the content of cysteine, glutathione and sulfane sulfur in tissues of the frog Rana temporaria. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 125, 211–217. [Google Scholar] [CrossRef]
- Kruithof, P.D.; Lunev, S.; Lozano, S.P.A.; Batista, F.D.A.; Al-Dahmani, Z.M.; Joles, J.A.; Dolga, A.; Groves, M.R.; van Goor, H. Unraveling the role of thiosulfate sulfurtransferase in metabolic diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165716. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Ogasawara, Y. Sulfur Atom in its Bound State Is a Unique Element Involved in Physiological Functions in Mammals. Molecules 2016, 21, 1753. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, C.; Shi, J.-P.; Ding, J.; Wan, X.; Chen, D.; Gao, J.; Li, C.; Zhang, J.; Lin, Y.; et al. Fatty acids promote fatty liver disease via the dysregulation of 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Gut 2017, 67, 2169–2180. [Google Scholar] [CrossRef]
- Ray, W.K.; Zeng, G.; Potters, M.B.; Mansuri, A.M.; Larson, T.J. Characterization of a 12-Kilodalton Rhodanese Encoded by glpE of Escherichia coli and Its Interaction with Thioredoxin. J. Bacteriol. 2000, 182, 2277–2284. [Google Scholar] [CrossRef]
- Poole, C.J.; Kind, P.R. Deficiency of thiosulphate sulphurtransferase (rhodanese) in Leber’s hereditary optic neuropathy. Br. Med. J. Clin. Res. Ed. 1986, 292, 1229–1230. [Google Scholar] [CrossRef]
- Arijs, I.; Vanhove, W.; Rutgeerts, P.; Schuit, F.; Verbeke, K.; De Preter, V. Decreased mucosal sulfide detoxification capacity in patients with Crohn’s disease. Inflamm. Bowel Dis. 2013, 19, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Hydrogen Sulfide, an Endogenous Stimulator of Mitochondrial Function in Cancer Cells. Cells 2021, 10, 220. [Google Scholar] [CrossRef]
- Takano, Y.; Echizen, H.; Hanaoka, K. Fluorescent Probes and Selective Inhibitors for Biological Studies of Hydrogen Sulfide- and Polysulfide-Mediated Signaling. Antioxid. Redox Signal. 2017, 27, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Ito, T.; Kitamura, H.; Nishino, T. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: Confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem. Cell Biol. 1998, 110, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Koike, S.; Shibuya, N.; Lefer, D.; Ogasawara, Y.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine- and glutathione-persulfide (Cys-SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S. Sci. Rep. 2017, 7, 10459. [Google Scholar] [CrossRef]
- Whitehouse, D.B.; Pilz, A.J.; Porta, G.; Hopkinson, D.A. Rhodanese isozymes in human tissues. Ann. Hum. Genet. 1988, 52, 1–10. [Google Scholar] [CrossRef]
- Tang, T.; Ji, C.; Yang, Z.; Liu, F.; Xie, S. Involvement of the Macrobrachium nipponense rhodanese homologue 2, MnRDH2 in innate immunity and antioxidant defense. Fish Shellfish. Immunol. 2017, 70, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Kelly, S.; Mottram, J.; Coombs, G.H. 3-Mercaptopyruvate Sulfurtransferase of LeishmaniaContains an Unusual C-terminal Extension and Is Involved in Thioredoxin and Antioxidant Metabolism. J. Biol. Chem. 2003, 278, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Augsburger, F.; Szabo, C. Potential role of the 3-mercaptopyruvate sulfurtransferase (3-MST)—hydrogen sulfide (H2S) pathway in cancer cells. Pharmacol. Res. 2020, 154, 104083. [Google Scholar] [CrossRef] [PubMed]
- Fräsdorf, B.; Radon, C.; Leimkühler, S. Characterization and Interaction Studies of Two Isoforms of the Dual Localized 3-Mercaptopyruvate Sulfurtransferase TUM1 from Humans. J. Biol. Chem. 2014, 289, 34543–34556. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.K.; Vitvitsky, V.; Carballal, S.; Seravalli, J.; Banerjee, R. Thioredoxin regulates human mercaptopyruvate sul-furtransferase at physiologically-relevant concentrations. J. Biol. Chem. 2020, 295, 6299–6311. [Google Scholar] [CrossRef]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Nagano, M.; Ito, T.; Suzuki, H. Chapter thirteen—Redox regulation of mammalian 3-mercaptopyruvate sul-furtransferase. Methods Enzymol. 2015, 554, 229–254. [Google Scholar] [PubMed]
- Pedre, B.; Dick, T.P. 3-Mercaptopyruvate sulfurtransferase: An enzyme at the crossroads of sulfane sulfur trafficking. Biol. Chem. 2021, 402, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Cipollone, R.; Frangipani, E.; Tiburzi, F.; Imperi, F.; Ascenzi, P.; Visca, P. Involvement of Pseudomonas aeruginosa Rhodanese in Protection from Cyanide Toxicity. Appl. Environ. Microbiol. 2007, 73, 390–398. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Grieshaber, M.K. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008, 275, 3352–3361. [Google Scholar] [CrossRef]
- Picton, R.; Eggo, M.C.; Merrill, G.; Langman, M.J.S.; Singh, S. Mucosal protection against sulphide: Importance of the enzyme rhodanese. Gut 2002, 50, 201–205. [Google Scholar] [CrossRef]
- Libiad, M.; Sriraman, A.; Banerjee, R. Polymorphic Variants of Human Rhodanese Exhibit Differences in Thermal Stability and Sulfur Transfer Kinetics. J. Biol. Chem. 2015, 290, 23579–23588. [Google Scholar] [CrossRef]
- Horowitz, P.M.; Bowman, S. Conformational changes accompany the oxidative inactivation of rhodanese by a variety of reagents. J. Biol. Chem. 1987, 262, 8728–8733. [Google Scholar] [CrossRef]
- Ogata, K.; Dai, X.; Volini, M. Bovine mitochondrial rhodanese is a phosphoprotein. J. Biol. Chem. 1989, 264, 2718–2725. [Google Scholar] [CrossRef]
- Nagahara, N.; Nagano, M.; Ito, T.; Shimamura, K.; Akimoto, T.; Suzuki, H. Antioxidant enzyme, 3-mercaptopyruvate sul-furtransferase-knockout mice exhibit increased anxiety-like behaviors: A model for human mercaptolactate-cysteine disul-fiduria. Sci. Rep. 2013, 3, 1986. [Google Scholar] [CrossRef]
- Yadav, P.K.; Yamada, K.; Chiku, T.; Koutmos, M.; Banerjee, R. Structure and Kinetic Analysis of H2S Production by Human Mercaptopyruvate Sulfurtransferase. J. Biol. Chem. 2013, 288, 20002–20013. [Google Scholar] [CrossRef] [PubMed]
- Hipólito, A.; Nunes, S.C.; Vicente, J.B.B.; Serpa, J. Cysteine Aminotransferase (CAT): A Pivotal Sponsor in Metabolic Remodeling and an Ally of 3-Mercaptopyruvate Sulfurtransferase (MST) in Cancer. Molecules 2020, 25, 3984. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Tanaka, M.; Tanaka, Y.; Ito, T. Novel characterization of antioxidant enzyme, 3-mercaptopyruvate sul-furtransferase-knockout mice: Overexpression of the evolutionarily related enzyme rhodanese. Antioxidants 2019, 8, 116. [Google Scholar] [CrossRef]
- Beinert, H. A tribute to sulfur. J. Biol. Inorg. Chem. 2000, 267, 5657–5664. [Google Scholar] [CrossRef] [PubMed]
- Palenchar, P.M.; Buck, C.J.; Cheng, H.; Larson, T.J.; Mueller, E.G. Evidence That ThiI, an Enzyme Shared between Thiamin and 4-Thiouridine Biosynthesis, May Be a Sulfurtransferase That Proceeds through a Persulfide Intermediate. J. Biol. Chem. 2000, 275, 8283–8286. [Google Scholar] [CrossRef]
- Hidese, R.; Mihara, H.; Esaki, N. Bacterial cysteine desulfurases: Versatile key players in biosynthetic pathways of sulfur-containing biofactors. Appl. Microbiol. Biotechnol. 2011, 91, 47–61. [Google Scholar] [CrossRef]
- Cereda, A.; Forlani, F.; Iametti, S.; Bernhardt, R.; Ferranti, P.; Picariello, G.; Pagani, S.; Bonomi, F. Molecular Recognition between Azotobacter vinelandii Rhodanese and a Sulfur Acceptor Protein. Biol. Chem. 2003, 384, 1473–1481. [Google Scholar] [CrossRef]
- Cartini, F.; Remelli, W.; Dos Santos, P.C.; Papenbrock, J.; Pagani, S.; Forlani, F. Mobilization of sulfane sulfur from cysteine desulfurases to the Azotobacter vinelandii sulfurtransferase RhdA. Amino Acids 2010, 41, 141–150. [Google Scholar] [CrossRef]
- Nárdiz, N.; Santamarta, I.; Lorenzana, L.M.; Martín, J.F.; Liras, P. A rhodanese-like protein is highly overrepresented in the mutant S. clavuligerus oppA2::aph: Effect on holomycin and other secondary metabolites production. Microb. Biotechnol. 2011, 4, 216–225. [Google Scholar] [CrossRef]
- Pagani, S.; Bonomi, F.; Cerletti, P. Enzymic synthesis of the iron–sulfur cluster of spinach ferredoxin. Eur. J. Biol. Inorg. Chem. 1984, 142, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Imlay, J.A. Micromolar Intracellular Hydrogen Peroxide Disrupts Metabolism by Damaging Iron–Sulfur Enzymes. J. Biol. Chem. 2007, 282, 929–937. [Google Scholar] [CrossRef]
- Malkin, R.; Rabinowitz, J.C. The reconstitution of clostridial ferredoxin. Biochem. Biophys. Res. Commun. 1966, 23, 822–827. [Google Scholar] [CrossRef]
- Bonomi, F.; Pagani, S.; Cerletti, P.; Cannella, C. Rhodanese-Mediated Sulfur Transfer to Succinate Dehydrogenase. Eur. J. Biol. Inorg. Chem. 1977, 72, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pagani, S.; Galante, Y.M. Interaction of rhodanese with mitochondrial NADH dehydrogenase. Biochim Biophys Acta. 1983, 742, 278–284. [Google Scholar] [CrossRef]
- Nishino, T.; Usami, C.; Tsushima, K. Reversible interconversion between sulfo and desulfo xanthine oxidase in a system containing rhodanese, thiosulfate, and sulfhydryl reagent. Proc. Natl. Acad. Sci. USA 1983, 80, 1826–1829. [Google Scholar] [CrossRef]
- Pagani, S.; Bonomi, F.; Cerletti, P. Sulfide insertion into spinach ferredoxin by rhodanese. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 1982, 700, 154–164. [Google Scholar] [CrossRef]
- Tomati, U.; Giovannozzi-Sermanni, G.; Duprè, S.; Cannella, C. NADH: Nitrate reductase activity restoration by rhodanese. Phytochemistry 1976, 15, 597–598. [Google Scholar] [CrossRef]
- Bonomi, F.; Pagani, S.; Kurtz, N.M. Enzymic synthesis of the 4Fe–4S clusters of Clostridium pasteurianum ferredoxin. Eur. J. Biol. Inorg. Chem. 1985, 148, 67–73. [Google Scholar] [CrossRef]
- Pagani, S.; Eldridge, M.; Eady, R.R. Nitrogenase of Klebsiella pneumoniae. Rhodanese-catalysed restoration of activity of the inactive 2Fe species of the Fe protein. Biochem. J. 1987, 244, 485–488. [Google Scholar] [CrossRef]
- Abdrakhmanova, A.; Dobrynin, K.; Zwicker, K.; Kerscher, S.; Brandt, U. Functional sulfurtransferase is associated with mitochondrial complex I from Yarrowia lipolytica, but is not required for assembly of its iron–sulfur clusters. FEBS Lett. 2005, 579, 6781–6785. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Kimura, T. Role of 3-mercaptopyruvate sulfurtransferase in the formation of the iron–sulfur chromophore of adrenal ferredoxin. Biochim. Biophys. Acta Enzym. 1974, 364, 284–295. [Google Scholar] [CrossRef]
- Dominic, P.; Ahmad, J.; Bhandari, R.; Pardue, S.; Solorzano, J.; Jaisingh, K.; Watts, M.; Bailey, S.R.; Orr, A.W.; Kevil, C.G.; et al. Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19. Redox Biol. 2021, 43, 101982. [Google Scholar] [CrossRef]
- Kasamatsu, S.; Nishimura, A.; Morita, M.; Matsunaga, T.; Hamid, H.A.; Akaike, T. Redox Signaling Regulated by Cysteine Persulfide and Protein Polysulfidation. Molecules 2016, 21, 1721. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Kevil, C.G. Reactive sulfur species: A new redox player in cardiovascular pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 874–884. [Google Scholar] [CrossRef]
- Yan, Q.; Mao, Z.; Hong, J.; Gao, K.; Niimi, M.; Mitsui, T.; Yao, J. Tanshinone IIA Stimulates Cystathionine γ-Lyase Expression and Protects Endothelial Cells from Oxidative Injury. Antioxidants 2021, 10, 1007. [Google Scholar] [CrossRef] [PubMed]
- Most, P.; Papenbrock, J. Possible Roles of Plant Sulfurtransferases in Detoxification of Cyanide, Reactive Oxygen Species, Selected Heavy Metals and Arsenate. Molecules 2015, 20, 1410–1423. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Katayama, A. Post-translational Regulation of Mercaptopyruvate Sulfurtransferase via a Low Redox Potential Cysteine-sulfenate in the Maintenance of Redox Homeostasis. J. Biol. Chem. 2005, 280, 34569–34576. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Sun, H.; Li, Y.; Chen, P.; Liu, F. MdRDH1, a HSP67B2-like rhodanese homologue plays a positive role in maintaining redox balance in Musca domestica. Mol. Immunol. 2019, 107, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Cereda, A.; Carpen, A.; Picariello, G.; Tedeschi, G.; Pagani, S. The lack of rhodanese RhdA affects the sensitivity of Azotobacter vinelandii to oxidative events. Biochem. J. 2009, 418, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Li, X.; Liu, X.; Wang, Y.; Ji, C.; Wang, Y.; Wang, X.; Xie, S.; Liu, F.; Wang, J. A single-domain rhodanese homologue MnRDH1 helps to maintain redox balance in Macrobrachium nipponense. Dev. Comp. Immunol. 2017, 78, 160–168. [Google Scholar] [CrossRef]
- Nakajima, T. Roles of Sulfur Metabolism and Rhodanese in Detoxification and Anti-Oxidative Stress Functions in the Liver: Responses to Radiation Exposure. Med. Sci. Monit. 2015, 21, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Libiad, M.; Yadav, P.K.; Vitvitsky, V.; Martinov, M.; Banerjee, R. Organization of the Human Mitochondrial Hydrogen Sulfide Oxidation Pathway. J. Biol. Chem. 2014, 289, 30901–30910. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, Y.; Lacourciere, G.; Stadtman, T.C. Formation of a selenium-substituted rhodanese by reaction with selenite and glutathione: Possible role of a protein perselenide in a selenium delivery system. Proc. Natl. Acad. Sci. USA 2001, 98, 9494–9498. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.L.; Horowitz, P.M.; Westley, J. Rhodanese as a thioredoxin oxidase. Int. J. Biochem. Cell Biol. 2000, 32, 465–473. [Google Scholar] [CrossRef]
- Nagahara, N. Activation of 3-Mercaptopyruvate Sulfurtransferase by Glutaredoxin Reducing System. Biomolecules 2020, 10, 826. [Google Scholar] [CrossRef]
- Westrop, G.D.; Georg, I.; Coombs, G.H. The Mercaptopyruvate Sulfurtransferase of Trichomonas vaginalis Links Cysteine Catabolism to the Production of Thioredoxin Persulfide. J. Biol. Chem. 2009, 284, 33485–33494. [Google Scholar] [CrossRef]
- Sabelli, R.; Iorio, E.; De Martino, A.; Podo, F.; Ricci, A.; Viticchiè, G.; Rotilio, G.; Paci, M.; Melino, S. Rhodanese-thioredoxin system and allyl sulfur compounds. FEBS J. 2008, 275, 3884–3899. [Google Scholar] [CrossRef]
- Pavlovskiy, Y.; Yashchenko, A.; Zayachkivska, O. H2S donors reverse age-related gastric malfunction impaired due to fruc-tose-induced injury via CBS, CSE, and TST expression. Front. Pharmacol. 2020, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.; Koch, K.; Jühling, A.; Tepel, M.; Scholze, A. Low expression of thiosulfate sulfurtransferase (rhodanese) predicts mortality in hemodialysis patients. Clin. Biochem. 2010, 43, 95–101. [Google Scholar] [CrossRef]
- Nakajima, T.; Taki, K.; Wang, B.; Ono, T.; Matsumoto, T.; Oghiso, Y.; Tanaka, K.; Ichinohe, K.; Nakamura, S.; Tanaka, S.; et al. Induction of rhodanese, a detoxification enzyme, in livers from mice after long-term irradiation with low-dose-rate gamma-rays. J. Radiat. Res. 2008, 49, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Hebbar, V.; Shen, G.; Hu, R.; Kim, B.-R.; Chen, C.; Korytko, P.J.; Crowell, J.A.; Levine, B.S.; Kong, A.-N.T. Toxicogenomics of resveratrol in rat liver. Life Sci. 2005, 76, 2299–2314. [Google Scholar] [CrossRef] [PubMed]
- Bilska, A.; Dudek, M.; Iciek, M.; Kwiecień, I.; Sokołowska-Jezewicz, M.; Filipek, B.; Włodek, L. Biological actions of lipoic acid associated with sulfane sulfur metabolism. Pharmacol. Rep. 2008, 60, 225–232. [Google Scholar]
- Wang, H.; Wu, G.; Park, H.J.; Jiang, P.P.; Sit, W.H.; van Griensven, L.J.; Wan, J.M. Protective effect of Phellinus linteus poly-saccharide extracts against thioacetamide-induced liver fibrosis in rats, a proteomics analysis. Chin. Med. 2012, 7, 23. [Google Scholar] [CrossRef]
- Jurkowska, H.; Wróbel, M.; Kaczor-Kamińska, M.; Jasek-Gajda, E. A possible mechanism of inhibition of U87MG and SH-SY5Y cancer cell proliferation by diallyl trisulfide and other aspects of its activity. Amino Acids 2017, 49, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Jurkowska, H.; Wróbel, M. Inhibition of human neuroblastoma cell proliferation by N-acetyl-L-cysteine as a result of increased sulfane sulfur level. Anticancer Res. 2018, 38, 5109–5113. [Google Scholar] [CrossRef] [PubMed]
- Jurkowska, H.; Wróbel, M.; Szlęzak, D.; Jasek-Gajda, E. New aspects of antiproliferative activity of 4-hydroxybenzyl isothi-ocyanate, a natural H2S-donor. Amino Acids 2018, 50, 699–709. [Google Scholar] [CrossRef]
- Pagani, S.; Bonomi, F.; Cerletti, P. The inhibition of rhodanese by lipoate and iron–sulfur proteins. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 1983, 742, 116–121. [Google Scholar] [CrossRef]
- Cianci, M.; Gliubich, F.; Zanotti, G.; Berni, R. Specific interaction of lipoate at the active site of rhodanese. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 2000, 1481, 103–108. [Google Scholar] [CrossRef]
- Darash-Yahana, M.; Pozniak, Y.; Lu, M.; Sohn, Y.-S.; Karmi, O.; Tamir, S.; Bai, F.; Song, L.; Jennings, P.A.; Pikarsky, E.; et al. Breast cancer tumorigenicity is dependent on high expression levels of NAF-1 and the lability of its Fe–S clusters. Proc. Natl. Acad. Sci. USA 2016, 113, 10890–10895. [Google Scholar] [CrossRef]
- Birkenkamp-Demtroder, K.; Christensen, L.L.; Olesen, S.H.; Frederiksen, C.M.; Laiho, P.; Aaltonen, L.A.; Laurberg, S.; Sørensen, F.B.; Hagemann, R.; Ørntoft, T.F. Gene expression in colorectal cancer. Cancer Res. 2002, 62, 4352–4363. [Google Scholar] [PubMed]
- Birkenkamp-Demtroder, K.; Olesen, S.H.; Sørensen, F.B.; Laurberg, S.; Laiho, P.; Aaltonen, L.; Ørntoft, T.F. Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut 2005, 54, 374–384. [Google Scholar] [CrossRef]
- Wróbel, M.; Czubak, J.; Bronowicka-Adamska, P.; Jurkowska, H.; Adamek, D.; Papla, B. Is Development of High-Grade Gliomas Sulfur-Dependent? Molecules 2014, 19, 21350–21362. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, V.; Palanivelu, J. Effects of the Gut microbiota on Amygdalin and its use as an anti-cancer therapy: Substantial review on the key components involved in altering dose efficacy and toxicity. Biochem. Biophys. Rep. 2018, 14, 125–132. [Google Scholar] [CrossRef]
- Ostrakhovitch, E.A.; Akakura, S.; Sanokawa-Akakura, R.; Goodwin, S.; Tabibzadeh, S. Dedifferentiation of cancer cells fol-lowing recovery from a potentially lethal damage is mediated by H2S-Nampt. Exp. Cell Res. 2015, 330, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.M.; Al Awaida, W.; Alkhateeb, H.H.; Abu-Ayyad, A.N. Antitumor Action of Amygdalin on Human Breast Cancer Cells by Selective Sensitization to Oxidative Stress. Nutr. Cancer 2018, 71, 483–490. [Google Scholar] [CrossRef]
- Benhar, M. Oxidants, Antioxidants and Thiol Redox Switches in the Control of Regulated Cell Death Pathways. Antioxidants 2020, 9, 309. [Google Scholar] [CrossRef]
- Zuhra, K.; Tomé, C.S.; Forte, E.; Vicente, J.B.; Giuffrè, A. The multifaceted roles of sulfane sulfur species in cancer-associated processes. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148338. [Google Scholar] [CrossRef]
- Nagahara, N. Multiple role of 3-mercaptopyruvate sulfurtransferase: Antioxidative function, H2S and polysulfide production and possible SOx production. Br. J. Pharmacol. 2018, 175, 577–589. [Google Scholar] [CrossRef]
- Koj, A.; Frendo, J. The activity of cysteine desulphhydrase and rhodanase in animal tissues. Acta Biochim. Pol. 1962, 9, 373–379. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).