Oxysterols and Retinal Microvascular Dysfunction as Early Risk Markers for Cardiovascular Disease in Normal, Ageing Individuals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. General Investigations
2.3. Dynamic Retinal Vessel Analysis
2.4. Biomarkers Assays
2.5. Measurement of Glutathione (GSH) and Oxidized Glutathione (GSSG)
2.6. Measurement of Nitric Oxide
2.7. Measurement of Interleukin-6 (IL-6)
2.8. Extraction and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) of Plasma Oxysterols
2.9. Sample Size and Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Retinal Microvascular Function
3.3. Oxidative Stress and Inflammatory Markers
3.4. Correlations between Vascular and Systemic Circulatory Parameters
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gariano, R.F.; Gardner, T.W. Retinal angiogenesis in development and disease. Nature 2005, 438, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Alam, T.A.; Seifalian, A.M.; Baker, D. A Review of Methods Currently Used for Assessment of In vivo Endothelial Function. Eur. J. Vasc. Endovasc. Surg. 2005, 29, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Karimzad, S.E.; Shokr, H.; Gherghel, D. Retinal and peripheral vascular function in healthy individuals with low cardiovascular risk. Microvasc. Res. 2019, 126, 103908. [Google Scholar] [CrossRef] [PubMed]
- Shokr, H.; Gherghel, D. European Society of Cardiology/European Society of Hypertension versus the American College of Cardiology/American Heart Association guidelines on the cut-off values for early hypertension: A microvascular perspective. Sci. Rep. 2021, 11, 3473. [Google Scholar] [CrossRef]
- Heitmar, R.; Blann, A.D.; Cubbidge, R.P.; Lip, G.Y.H.; Gherghel, D. Continuous retinal vessel diameter measurements: The future in retinal vessel assessment? Investig. Ophthalmol. Vis. Sci. 2010, 51, 5833–5839. [Google Scholar] [CrossRef] [Green Version]
- Seshadri, S.; Ekart, A.; Gherghel, D. Ageing effect on flicker-induced diameter changes in retinal microvessels of healthy individuals. Acta Ophthalmol. 2016, 94, e35–e42. [Google Scholar] [CrossRef]
- Shokr, H.; Dias, I.H.K.; Gherghel, D. Microvascular function and oxidative stress in adult individuals with early onset of cardiovascular disease. Sci. Rep. 2020, 10, 4881. [Google Scholar] [CrossRef]
- Seshadri, S.; Mroczkowska, S.; Qin, L.; Patel, S.; Ekart, A.; Gherghel, D. Systemic circulatory influences on retinal microvascular function in middle-age individuals with low to moderate cardiovascular risk. Acta Ophthalmol. 2015, 93, e266–e274. [Google Scholar] [CrossRef] [Green Version]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2021, 18, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M.; Boutouyrie, P.; Cunha, P.; Kotsis, V.; Narkiewicz, K.; Parati, G.; Rietzschel, E.; Scuteri, A.; Laurent, S. Early vascular ageing in translation: From laboratory investigations to clinical applications in cardiovascular prevention. J. Hypertens. 2013, 31, 1517–1526. [Google Scholar] [CrossRef]
- Glavic, M.M.; Blagus, L.; Bosnjak, V.; Frkanec, S.; Katic, L.; Domislovic, V.; Matasin, M.; Kos, J.; Vrkic, T.Z.; Pecin, I.; et al. Characteristics Of Healthy Vascular Ageing (Hva) and Early Vascular Ageing (Eva) in General Population. Eh-Uh Study (Croatian Scientific Foundation). J. Hypertens. 2021, 39, e67. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Safar, M.E.; Dzau, V. The Cardiovascular Continuum extended: Aging effects on the aorta and microvasculature. Vasc. Med. 2010, 15, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, 2181–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samman Tahhan, A.; Sandesara, P.B.; Hayek, S.S.; Alkhoder, A.; Chivukula, K.; Hammadah, M.; Mohamed-Kelli, H.; O’Neal, W.T.; Topel, M.; Ghasemzadeh, N.; et al. Association between oxidative stress and atrial fibrillation. Heart Rhythm 2017, 14, 1849–1855. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Rafieian-Kopaei, M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J. Res. Med. Sci. 2014, 19, 358. [Google Scholar]
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2008, 113, 234–258. [Google Scholar] [CrossRef]
- Chen, C.-A.; Wang, T.-Y.; Varadharaj, S.; Reyes, L.A.; Hemann, C.; Talukder, M.A.H.; Chen, Y.-R.; Druhan, L.J.; Zweier, J.L. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 2010, 468, 1115–1118. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Dikalov, S.; Griendling, K.K.; Harrison, D.G. Detection of and nitric oxide in vascular cells and tissues. InVasc. Biol. Protoc. 2007, 18, 293–311. [Google Scholar]
- Patel, S.R.; Bellary, S.; Qin, L.; Gill, P.S.; Taheri, S.; Heitmar, R.; Gibson, J.M.; Gherghel, D. Abnormal retinal vascular function and lipid levels in a sample of healthy UK South Asians. Br. J. Ophthalmol. 2011, 95, 1573–1576. [Google Scholar] [CrossRef]
- Shokr, H.; Wolffsohn, J.S.; Trave Huarte, S.; Scarpello, E.; Gherghel, D. Dry eye disease is associated with retinal microvascular dysfunction and possible risk for cardiovascular disease. Acta Ophthalmol. 2021, 99, e1236–e1242. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [Green Version]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Jessup, W. Oxysterols and atherosclerosis. Atherosclerosis 1999, 142, 1–28. [Google Scholar] [CrossRef]
- Kummerow, F.A. Interaction between sphingomyelin and oxysterols contributes to atherosclerosis and sudden death. Am. J. Cardiovasc. Dis. 2013, 3, 17–26. [Google Scholar] [PubMed]
- Griffiths, W.J.; Wang, Y. Oxysterol research: A brief review. Biochem. Soc. Trans. 2019, 47, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Terao, J. Cholesterol hydroperoxides and their degradation mechanism. Subcell. Biochem. 2014, 77, 83–91. [Google Scholar] [CrossRef]
- Khatib, S.; Vaya, J. Oxysterols and symptomatic versus asymptomatic human atherosclerotic plaque. Biochem. Biophys. Res. Commun. 2014, 446, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, S.; Gamba, P.; Testa, G.; Leonarduzzi, G.; Poli, G. The role of oxysterols in vascular ageing. J. Physiol. 2016, 594, 2095–2113. [Google Scholar] [CrossRef] [Green Version]
- Gordiyenko, N.; Campos, M.; Lee, J.W.; Fariss, R.N.; Sztein, J.; Rodriguez, I.R. RPE Cells Internalize Low-Density Lipoprotein (LDL) and Oxidized LDL (oxLDL) in Large Quantities In Vitro and In Vivo. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2822–2829. [Google Scholar] [CrossRef]
- Uptake of Cholesterol by the Retina Occurs Primarily via a Low Density Lipoprotein Receptor-Mediated Process. Available online: https://pubmed.ncbi.nlm.nih.gov/17110914/#:~:text=Conclusions%3A The retina is capable, and uptake by the retina (accessed on 31 August 2021).
- Nagel, E.; Vilser, W.; Lanzl, I. Age, blood pressure, and vessel diameter as factors influencing the arterial retinal flicker response. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Karimzad, S.E.; Shokr, H.; Gherghel, D. Retinal vascular function in asymptomatic individuals with a positive family history of cardiovascular disease. Acta Ophthalmol. 2018, 96, e956–e962. [Google Scholar] [CrossRef] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Jones, D.P.; Carlson, J.L.; Samiec, P.S.; Sternberg, P.; Mody, V.C.; Reed, R.L.; Brown, L.A.S. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin. Chim. Acta 1998, 275, 175–184. [Google Scholar] [CrossRef]
- Gherghel, D.; Mroczkowska, S.; Qin, L. Reduction in blood glutathione levels occurs similarly in patients with primary-open angle or normal tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3333–3339. [Google Scholar] [CrossRef]
- Dias, I.H.K.; Milic, I.; Lip, G.Y.H.; Devitt, A.; Polidori, M.C.; Griffiths, H.R. Simvastatin reduces circulating oxysterol levels in men with hypercholesterolaemia. Redox Biol. 2018, 16, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Pemp, B.; Garhofer, G.; Weigert, G.; Karl, K.; Resch, H.; Wolzt, M.; Schmetterer, L. Reduced retinal vessel response to flicker stimulation but not to exogenous nitric oxide in type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4029–4032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, E.A.; Rees, D.; Banerjee, T.; Cazzola, R.; Lewis, S.; Wood, R.; Oates, R.; Tallant, A.; Cestaro, B.; Yaqoob, P.; et al. Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis 2008, 196, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [Green Version]
- Schleicher, E.; Friess, U. Oxidative stress, AGE, and atherosclerosis. Kidney Int. 2007, 72, S17–S26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, A.; Campo, A.; Fulton, E.; Corwin, A.; Jerome, W.G.; O’Connor, M.S. 7-Ketocholesterol in disease and aging. Redox Biol. 2020, 29, 101380. [Google Scholar] [CrossRef]
- Van den Kommer, T.N.; Dik, M.G.; Comijs, H.C.; Fassbender, K.; Lütjohann, D.; Jonker, C. Total cholesterol and oxysterols: Early markers for cognitive decline in elderly? Neurobiol. Aging 2009, 30, 534–545. [Google Scholar] [CrossRef]
- Zarrouk, A.; Vejux, A.; Mackrill, J.; O’Callaghan, Y.; Hammami, M.; O’Brien, N.; Lizard, G. Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res. Rev. 2014, 18, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Félétou, M. Endothelium-Dependent Regulation of Vascular Tone; Morgan & Claypool Life Sciences Publishers: San Rafael, CA, USA, 2011; Volume 1, pp. 43–137. [Google Scholar]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Marce, M.; Okuyama, H.; Wesley, J.B.; Sarkar, K.; Kimura, H.; Liu, Y.V.; Zhang, H.; Strazza, M.; Rey, S.; Savino, L.; et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ. Res. 2007, 101, 1310–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, D.S.; Ali, B.R. Pathological Crosstalk Between Oxidized LDL and ER Stress in Human Diseases: A Comprehensive Review. Front. Cell Dev. Biol. 2021, 26, 1276. [Google Scholar]
- Dias, H.I.; Brown, C.L.; Polidori, M.C.; Lip, G.Y.; Griffiths, H.R. LDL-lipids from patients with hypercholesterolaemia and Alzheimer’s disease are inflammatory to microvascular endothelial cells: Mitigation by statin intervention. Clin. Sci. 2015, 129, 1195–2063. [Google Scholar] [CrossRef] [Green Version]
- Cohn, J.N. Identifying the Risk and Preventing the Consequences of Cardiovascular Disease. Heart Lung Circ. 2013, 22, 512–516. [Google Scholar] [CrossRef] [PubMed]


| Variable | (19–30) Year | (31–50) Year | (51–70) Year | p-Value | Post-Hoc |
|---|---|---|---|---|---|
| Number | 16 10M:6F | 16 8M:8F | 10 4M:6F | - | - |
| Age (years) | 24.54 (1.74) | 37.81 (1.57) | 60.75 (2.22) | 0.0001 * | 1 < 2 < 3 |
| SBP (mmHg) | 115.27 (3.80) | 114.7 (3.95) | 118.5 (5.10) | 0.82946 | - |
| DBP (mmHg) | 64.64 (2.54) | 70.9 (2.67) | 72.5 (3.44) | 0.13002 | - |
| HR (bpm) | 63.17 (3.29) | 68.1 (2.55) | 66.18 (2.43) | 0.50522 | - |
| IOP (mmHg) | 12.93 (0.57) | 13.23 (0.60) | 14.83 (0.80) | 0.14723 | - |
| BMI (kg/m2) | 23.34 (1.13) | 26.02 (1.19) | 26.59 (1.53) | 0.16039 | - |
| Glucose (mmol/L) | 4.41 (0.21) | 4.99 (0.28) | 4.68 (0.28) | 0.26740 | - |
| TG (mmol/L) | 0.83 (0.065) | 1.0 (0.081) | 0.96 (0.088) | 0.22376 | - |
| CHOL (mmol/L) | 3.95 (0.17) | 4.14 (0.21) | 4.93 (0.24) | 0.0071 * | 1 < 2 < 3 |
| HDL-C (mmol/L) | 1.33 (0.08) | 1.44 (0.10) | 1.25 (0.12) | 0.50590 | - |
| LDL-C (mmol/L) | 2.28 (0.17) | 2.60 (0.21) | 3.01 (0.24) | 0.01116 * | 1 < 2 < 3 |
| LDL-C/HDL-C | 1.64 (0.13) | 1.85 (0.2) | 2.37 (0.19) | 0.0658 | 1 = 2 3 > 1,2 |
| Variable | (19–30) Year | (31–50) Year | (51–70) Year | p-Value Anova/Ancova | Post-Hoc |
|---|---|---|---|---|---|
| Baseline | 117.44 (4.24) | 116.60 (4.45) | 113.41(5.74) | 0.61902 | |
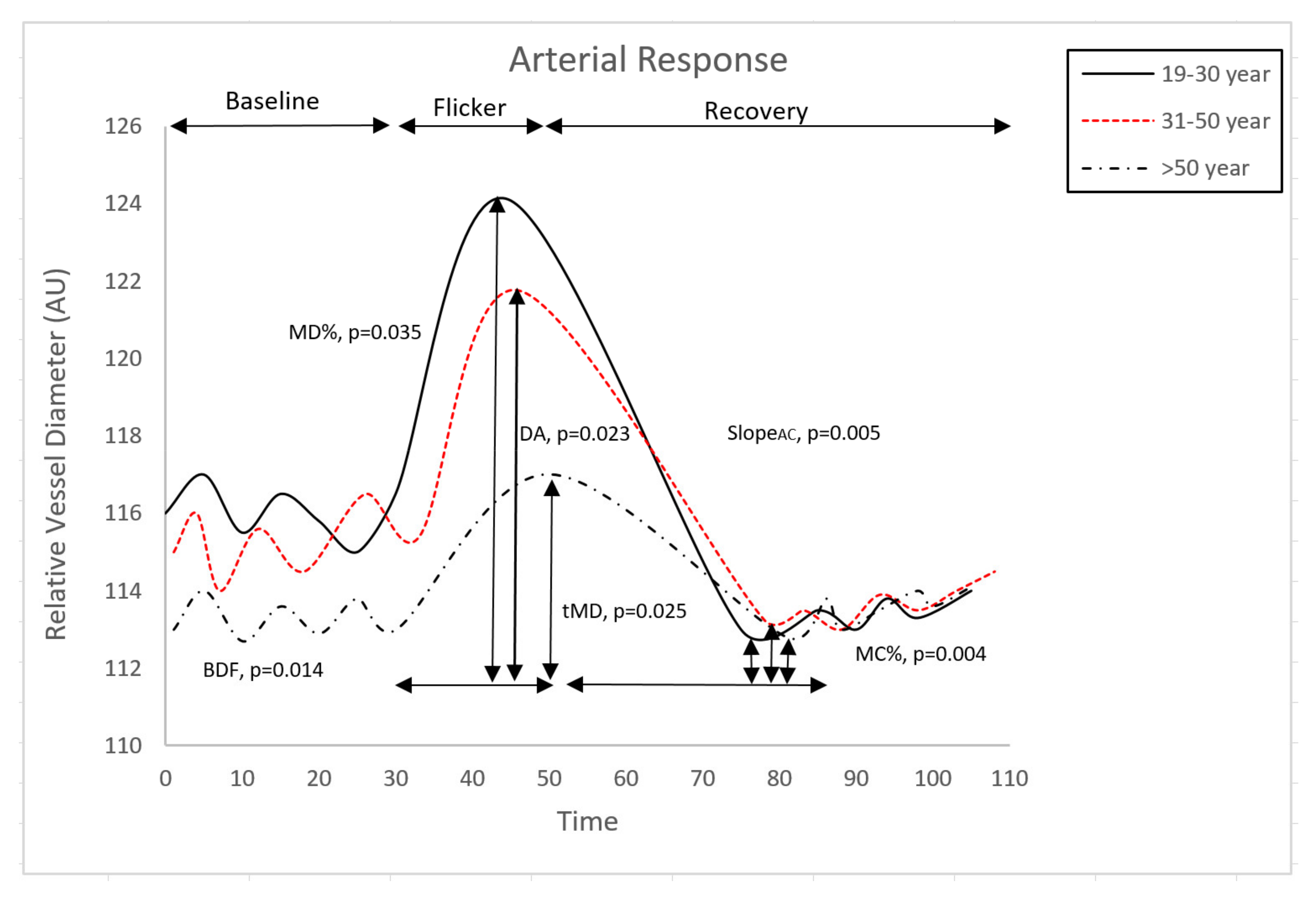
| BDF | 7.06 (0.65) | 5.92 (0.70) | 3.49 (0.88) | 0.0140 * | 1 > 2 > 3 |
| BCFR | 4.90 (0.72) | 4.81 (0.80) | 2.54 (0.90) | 0.1058 | - |
| MD | 124.94 (4.13) | 121.76 (4.60) | 117.02 (5.20) | 0.4986 | - |
| tMD | 14.67 (0.77) | 16.15 (0.73) | 19.29 (0.83) | 0.0254 * | 1 < 2 < 3 |
| MD % | 5.64 (0.63) | 4.50 (0.70) | 3.24 (0.79) | 0.0360 * | 1 > 2 > 3 |
| MC | 112.99 (4.32) | 113.24 (4.78) | 112.80 (5.42) | 0.5985 | - |
| tMC | 25.39 (2.34) | 28.93 (2.60) | 27.523 (2.93) | 0.5974 | - |
| MC% | −3.85 (0.36) | −2.43 (0.4) | −1.70 (0.46) | 0.0040 * | 1 > 2 > 3 |
| DA | 11.95 (1.27) | 9.74 (1.40) | 6.04 (1.60) | 0.0230 * | 1 > 2 > 3 |
| SlopeAD | 0.52 (0.11) | 0.51 (0.12) | 0.26 (0.14) | 0.3030 | - |
| SlopeAC | −0.62 (0.062) | −0.38 (0.07) | −0.30 (0.08) | 0.0055 * | 1 > 2, 2 = 3, 1 > 3 |
| Variable | (19–30) Year | (31–50) Year | (51–70) Year | p-Value Anova/Ancova | Post-Hoc |
|---|---|---|---|---|---|
| Baseline | 142.47 (4.25) | 143.55 (4.70) | 131.39 (5.33) | 0.19389 | - |
| BDF | 5.45 (0.75) | 5.02 (0.84) | 5.61 (0.95) | 0.88379 | - |
| BCFR | 3.00 (0.85) | 4.87 (0.95) | 3.14 (1.07) | 0.30859 | - |
| MD | 149.09 (4.44) | 150.26 (4.91) | 138.19 (5.60) | 0.22687 | - |
| tMD | 23.76 (1.47) | 19.37 (1.63) | 21.95 (1.85) | 0.15744 | - |
| MD % | 4.66 (0.66) | 4.75 (0.74) | 5.15 (0.83) | 0.89666 | - |
| MC | 140.64 (4.24) | 140.37 (4.69) | 129.44 (5.32) | 0.22113 | - |
| tMC | 32.12 (2.16) | 34.41 (2.40) | 33.71 (2.71) | 0.76678 | - |
| MC% | −1.30 (0.45) | −2.20 (0.49) | −1.50 (0.56) | 0.38622 | - |
| DA | 8.45 (1.36) | 9.90 (1.50) | 8.75 (1.70) | 0.76728 | - |
| SlopeVD | 0.33 (0.046) | 0.36 (0.051) | 0.35 (0.06) | 0.86531 | - |
| SlopeVC | −0.60 (0.12) | −0.31 (0.13) | −0.39 (0.14) | 0.22394 | - |
| Variable | (19–30) Year | (31–50) Year | (51–70) Year | p-Value Anova/Ancova | Post-Hoc |
|---|---|---|---|---|---|
| 7-KC (nmol/mmol) | 10.49 (1.71) | 13.28 (1.17) | 17.26 (1.17) | 0.0051 * | 1 < 2 < 3 |
| 25-OHC (nmol/mmol) | 16.42 (3.86) | 20.86 (2.65) | 30.56 (2.65) | 0.0067 * | 1 < 2 < 3 |
| 27-OHC (nmol/mmol) | 16.61 (3.05) | 20.55 (2.09) | 26.79 (2.09) | 0.0192 * | 1 < 2 < 3 |
| 4β-OHC (nmol/mmol) | 1.02 (0.35) | 0.81 (0.24) | 1.14 (0.24) | 0.6054 | - |
| 7β-OHC (nmol/mmol) | 6.65 (1.70) | 9.86 (1.17) | 12.74 (1.17) | 0.0168 * | 1 < 2 < 3 |
| 7,27-OHC (nmol/mmol) | 8.45 (2.30) | 8.64 (1.57) | 10.65 (1.57) | 0.5986 | - |
| 7,25-OHC (nmol/mmol) | 5.60 (2.60) | 8.89 (1.78) | 11.41 (1.80) | 0.0113 * | 1 < 2 < 3 |
| NO (uM) | 46.45 (4.37) | 43.02 (3.39) | 39.73 (3.10) | 0.4495 | |
| IL-6 (pg/mL) | 3.23 (0.45) | 3.24 (0.31) | 3.09 (0.31) | 0.9424 | - |
| GSH/GSSG | 27.25(7.95) | 20.84 (7.95) | 17.05 (8.60) | 0.0279 * | 1 > 2 > 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokr, H.; Dias, I.H.; Gherghel, D. Oxysterols and Retinal Microvascular Dysfunction as Early Risk Markers for Cardiovascular Disease in Normal, Ageing Individuals. Antioxidants 2021, 10, 1756. https://doi.org/10.3390/antiox10111756
Shokr H, Dias IH, Gherghel D. Oxysterols and Retinal Microvascular Dysfunction as Early Risk Markers for Cardiovascular Disease in Normal, Ageing Individuals. Antioxidants. 2021; 10(11):1756. https://doi.org/10.3390/antiox10111756
Chicago/Turabian StyleShokr, Hala, Irundika HK Dias, and Doina Gherghel. 2021. "Oxysterols and Retinal Microvascular Dysfunction as Early Risk Markers for Cardiovascular Disease in Normal, Ageing Individuals" Antioxidants 10, no. 11: 1756. https://doi.org/10.3390/antiox10111756
APA StyleShokr, H., Dias, I. H., & Gherghel, D. (2021). Oxysterols and Retinal Microvascular Dysfunction as Early Risk Markers for Cardiovascular Disease in Normal, Ageing Individuals. Antioxidants, 10(11), 1756. https://doi.org/10.3390/antiox10111756






