Novel Photoinduced Squalene Cyclic Peroxide Identified, Detected, and Quantified in Human Skin Surface Lipids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SQ-OOH Isomers
2.3. Photooxidation of SQ-OOH Isomers
2.4. Structural Elucidation of the Purified Secondary Oxidation Product and Oxidation of Individual SQ-OOH Isomers
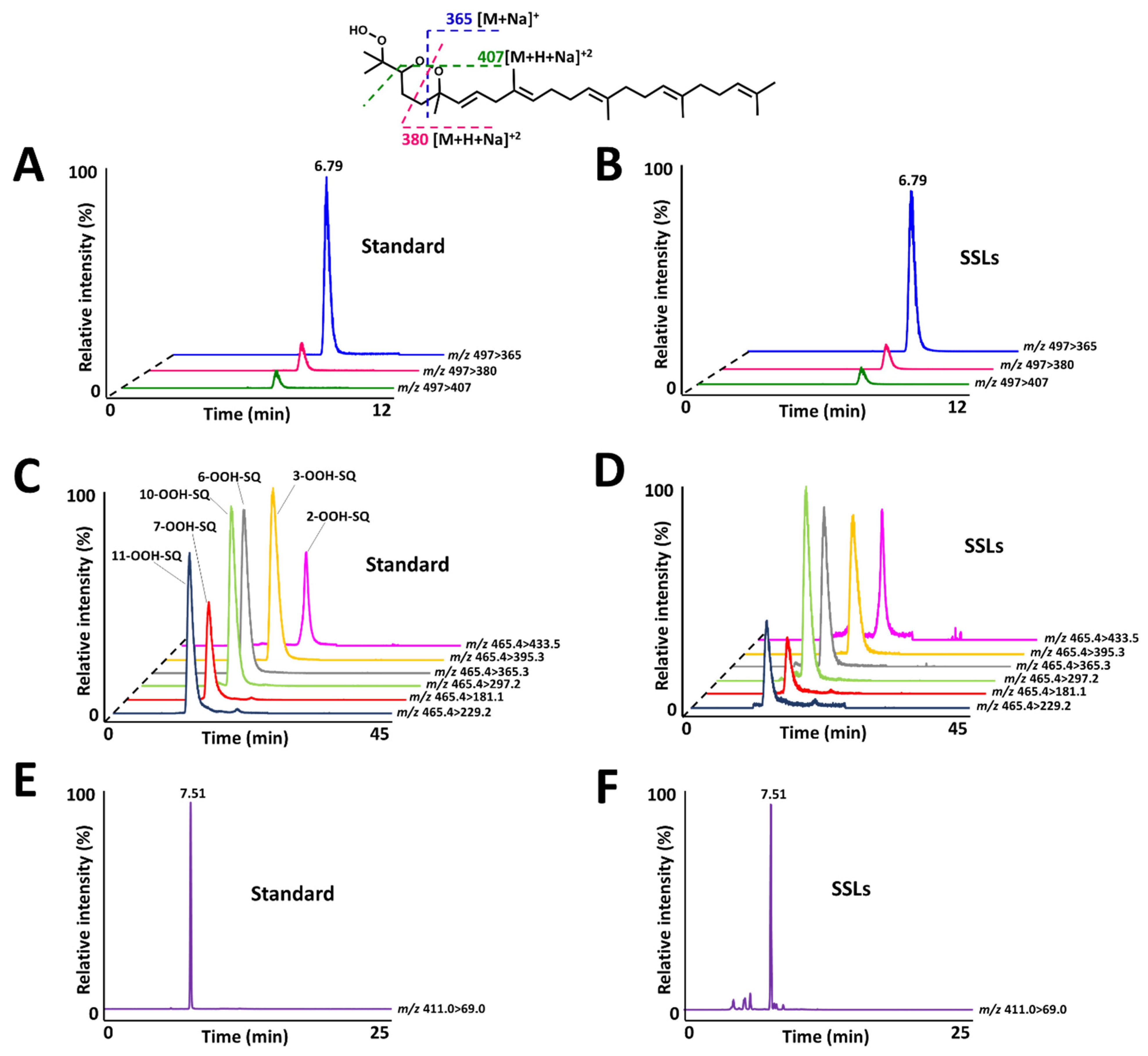
2.5. Analysis of the Identified Compound from Human SSLs
3. Results and Discussion
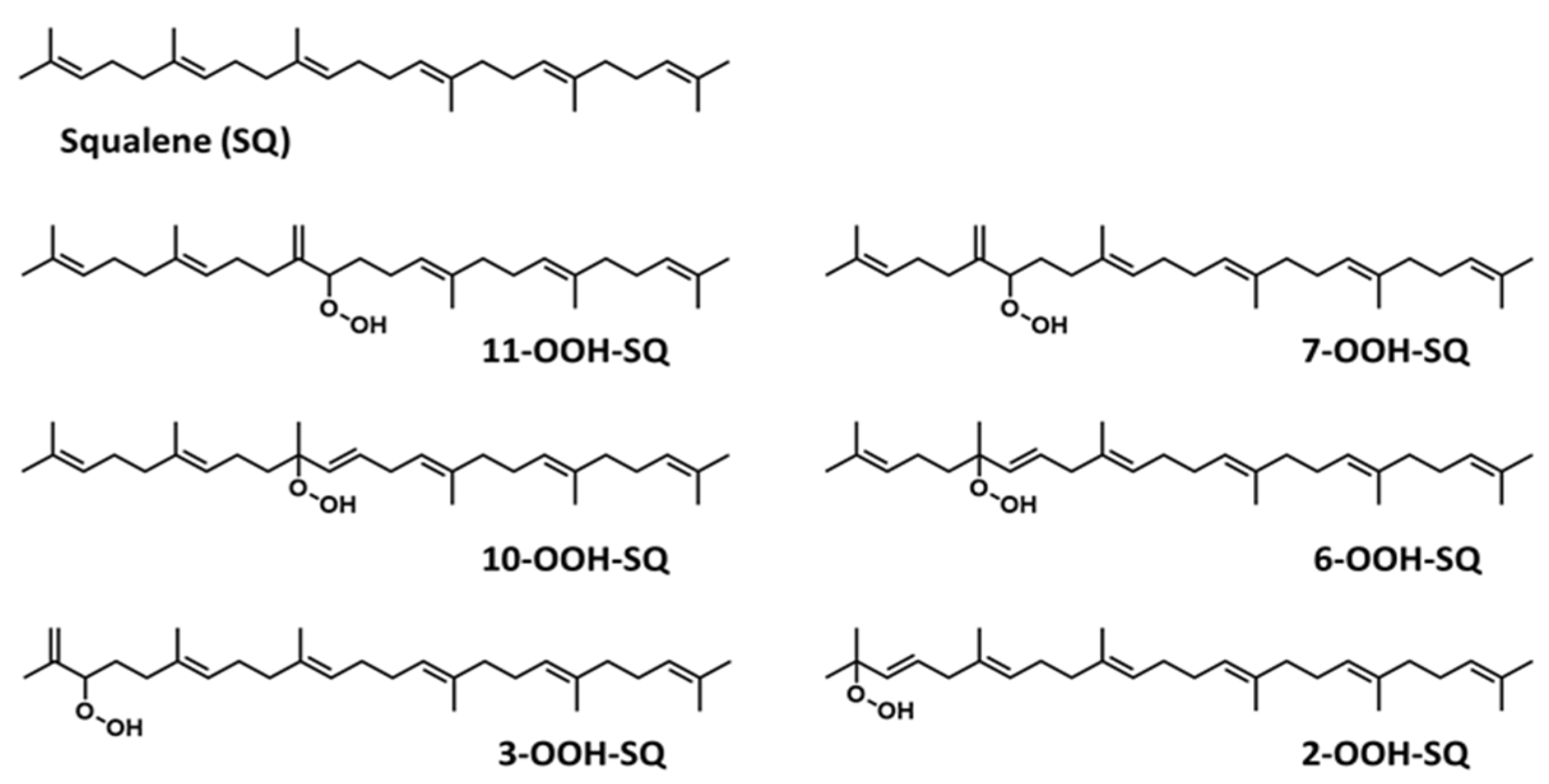
3.1. Preparation of Total and Individual SQ-OOH Isomers
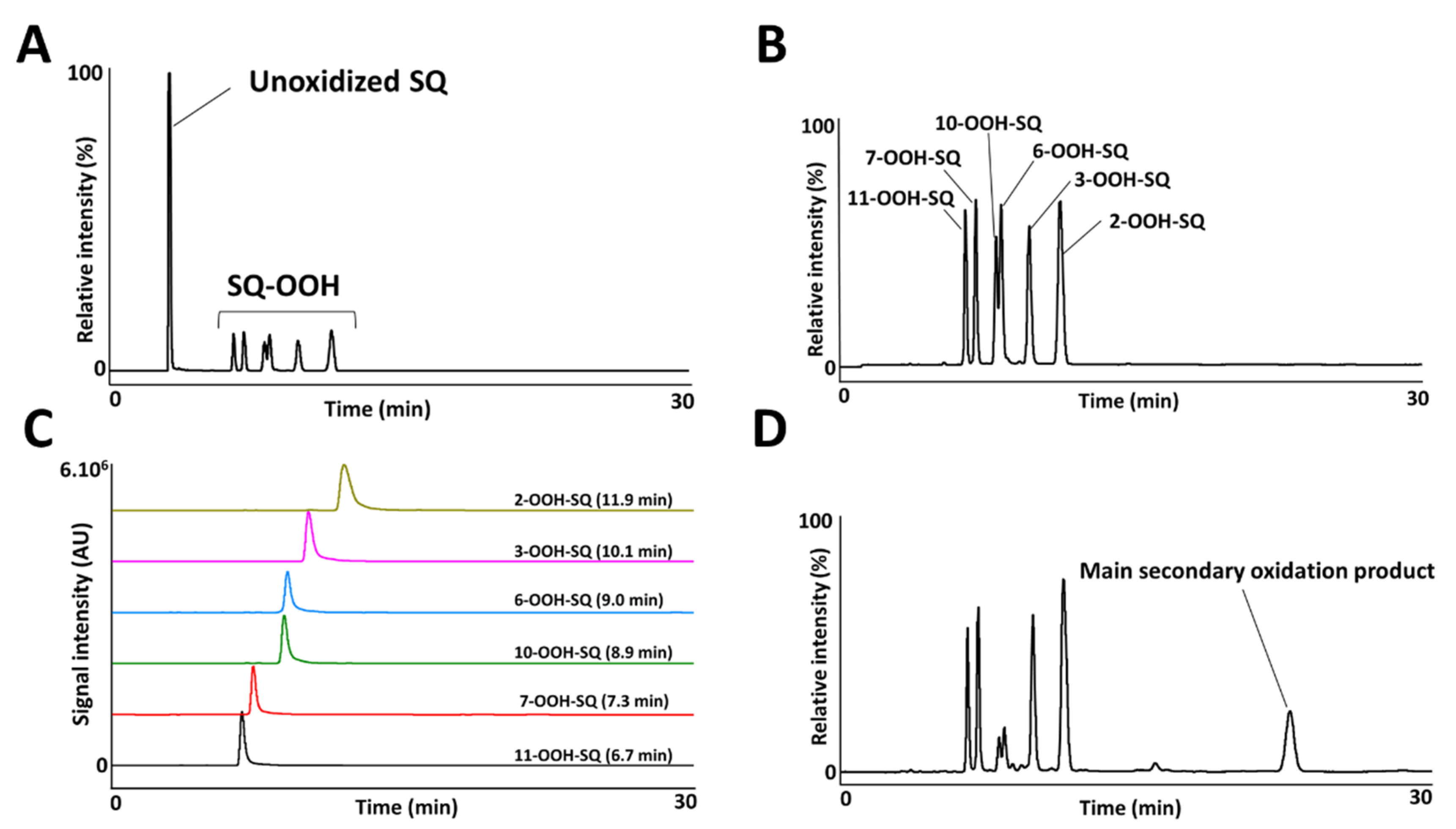
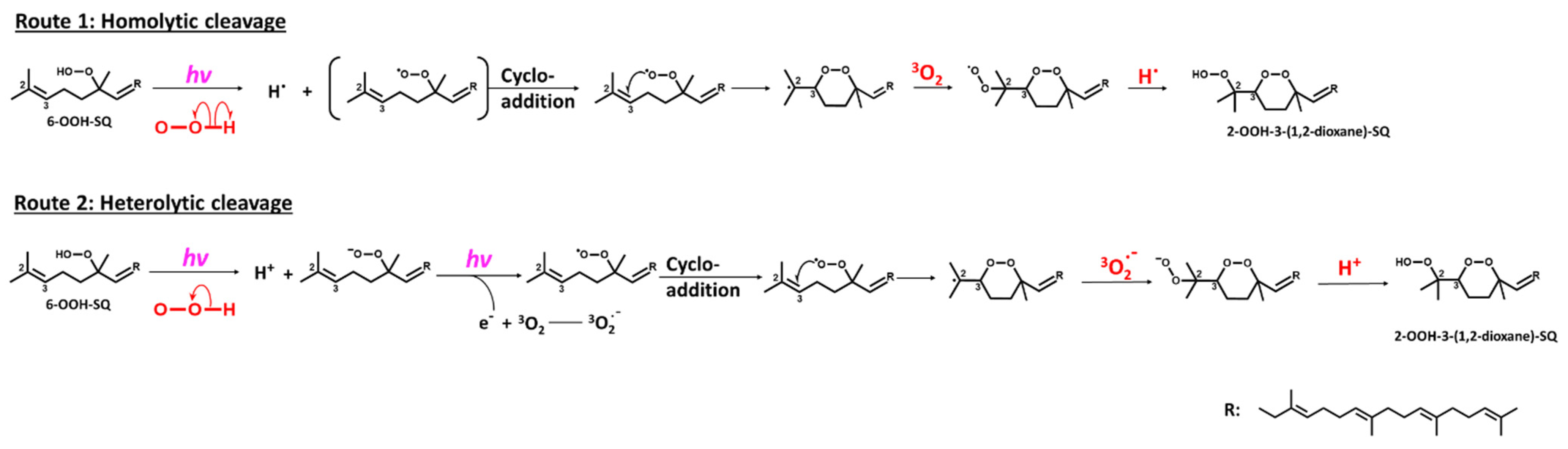
3.2. Photooxidation of SQ-OOH Isomers
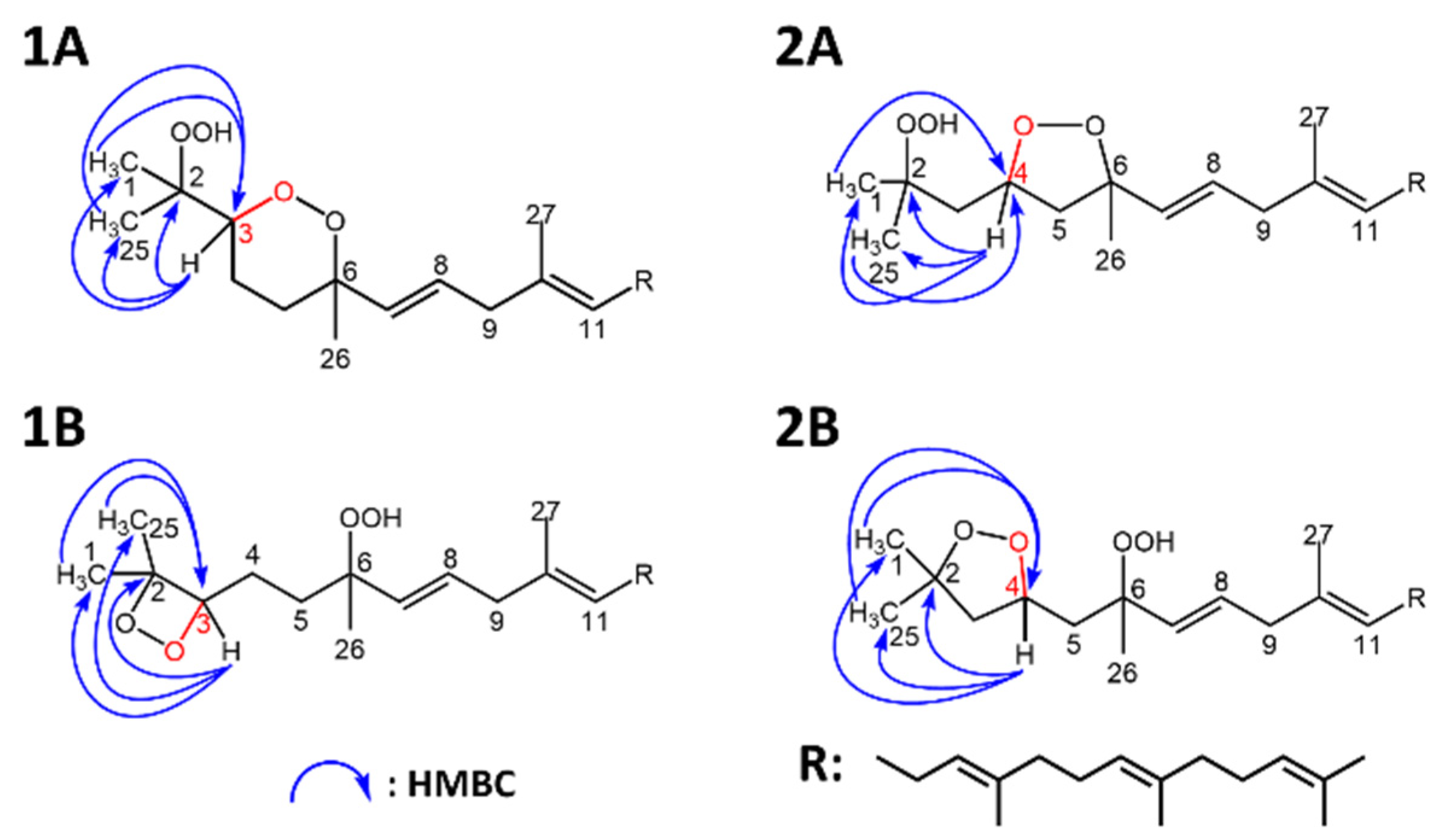
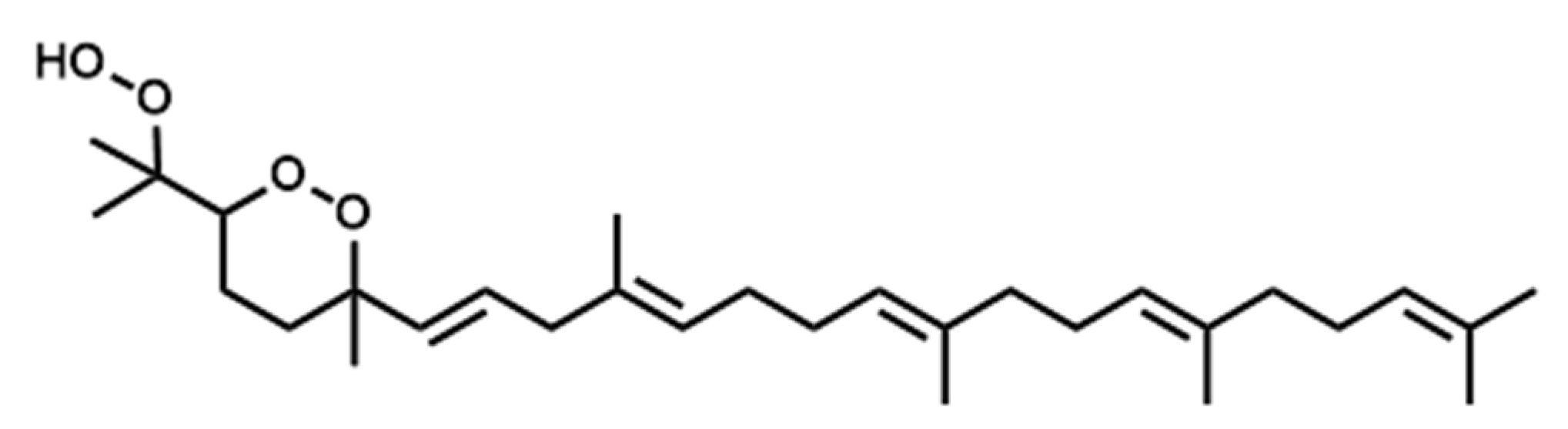
3.3. Structural Elucidation of the Purified Secondary Oxidation Product
3.4. Oxidation of Individual SQ-OOH Isomers
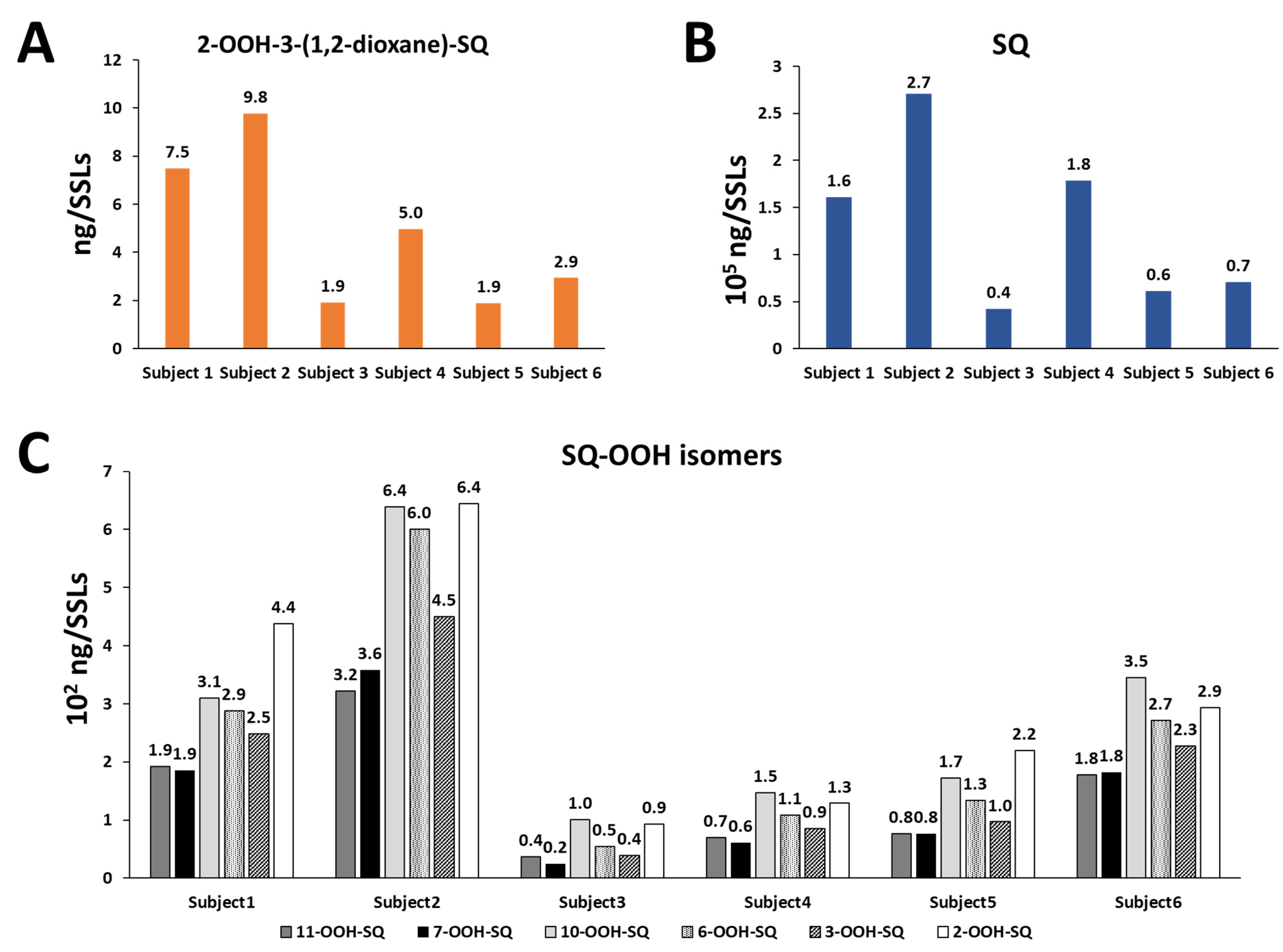
3.5. Analysis of the Identified Compound from Human SSLs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Luca, C.; Valacchi, G. Surface Lipids as Multifunctional Mediators of Skin Responses to Environmental Stimuli. Mediat. Inflamm. 2010, 2010, 1–11. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Sander, C.S.; Hamm, F.; Elsner, P.; Thiele, J.J. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br. J. Dermatol. 2003, 148, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Okayama, Y. Oxidative Stress in Allergic and Inflammatory Skin Diseases. Curr. Drug Target Inflamm. Allergy 2005, 4, 517–519. [Google Scholar] [CrossRef]
- Wang, S.; Yi, X.; Wu, Z.; Guo, S.; Dai, W.; Wang, H.; Shi, Q.; Zeng, K.; Guo, W.; Li, C. CAMKK2 Defines Ferroptosis Sensitivity of Melanoma Cells by Regulating AMPK‒NRF2 Pathway. J. Investig. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.V.; Domenichiello, A.F.; Dey, A.K.; Yuan, Z.-X.; Goyal, A.; Rose, S.M.; Playford, M.P.; Ramsden, C.E.; Mehta, N.N. Bioactive Lipid Mediator Profiles in Human Psoriasis Skin and Blood. J. Investig. Dermatol. 2018, 138, 1518–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, R.S.; Downing, D.T.; Pochi, P.E.; Strauss, J.S. Anatomical Variation in the Amount and Composition of Human Skin Surface Lipid. J. Investig. Dermatol. 1970, 54, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolaides, N. Skin Lipids: Their Biochemical Uniqueness. Science 1974, 186, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Thoma, R.; Schulz-Gasch, T.; D’Arcy, B.; Benz, J.; Aebi, J.D.; Dehmlow, H.; Hennig, M.; Stihle, M.; Ruf, A. Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase. Nature 2004, 432, 118–122. [Google Scholar] [CrossRef]
- Kohno, Y.; Egawa, Y.; Itoh, S.; Nagaoka, S.-I.; Takahashi, M.; Mukai, K. Kinetic study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n-butanol. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1995, 1256, 52–56. [Google Scholar] [CrossRef]
- Boussouira, B.; Pham, D.M. Squalene and Skin Barrier Function: From Molecular Target to Biomarker of Environmental Exposure. In Skin Stress Response Pathways; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 29–48. [Google Scholar]
- Lovászi, M.; Mattii, M.; Eyerich, S.; Gácsi, A.; Csányi, E.; Kovács, D.; Rühl, R.; Szegedi, A.; Kemény, L.; Ståhle, M.; et al. Sebum lipids influence macrophage polarization and activation. Br. J. Dermatol. 2017, 177, 1671–1682. [Google Scholar] [CrossRef]
- De Jong, A.; Cheng, T.-Y.; Huang, S.; Gras, S.; Birkinshaw, R.W.; Kasmar, A.G.; Van Rhijn, I.; Pena-Cruz, V.; Ruan, D.T.; Altman, J.D.; et al. CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens. Nat. Immunol. 2013, 15, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Bakes, M.J.; Nichols, P.D. Lipid, fatty acid and squalene composition of liver oil from six species of deep-sea sharks collected in southern australian waters. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 110, 267–275. [Google Scholar] [CrossRef]
- Khallouki, F.; Younos, C.; Soulimani, R.; Oster, T.; Charrouf, Z.; Spiegelhalder, B.; Bartsch, H.; Owen, R.W. Consumption of argan oil (Morocco) with its unique profile of fatty acids, tocopherols, squalene, sterols and phenolic compounds should confer valuable cancer chemopreventive effects. Eur. J. Cancer Prev. 2003, 12, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R. Squalene: A natural antioxidant? Eur. J. Lipid Sci. Technol. 2009, 111, 411–412. [Google Scholar] [CrossRef]
- Brusini, R.; Dormont, F.; Cailleau, C.; Nicolas, V.; Peramo, A.; Varna, M.; Couvreur, P. Squalene-based nanoparticles for the targeting of atherosclerotic lesions. Int. J. Pharm. 2020, 581, 119282. [Google Scholar] [CrossRef] [PubMed]
- Dormont, F.; Brusini, R.; Cailleau, C.; Reynaud, F.; Peramo, A.; Gendron, A.; Mougin, J.; Gaudin, F.; Varna, M.; Couvreur, P. Squalene-based multidrug nanoparticles for improved mitigation of uncontrolled inflammation in rodents. Sci. Adv. 2020, 6, eaaz5466. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.C. Squalene and Squalane Emulsions as Adjuvants. Methods 1999, 19, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Garçon, N.; Vaughn, D.W.; Didierlaurent, A.M. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev. Vaccines 2012, 11, 349–366. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, J.; van Dijken, H.; de Heij, F.; Spijkers, S.; Mouthaan, J.; de Jong, R.; Roholl, P.; Adami, E.A.; Akamatsu, M.A.; Ho, P.L.; et al. H7N9 influenza split vaccine with SWE oil-in-water adjuvant greatly enhances cross-reactive humoral immunity and protection against severe pneumonia in ferrets. NPJ Vaccines 2020, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Newmark, H.L. Squalene, olive oil, and cancer risk. Review and hypothesis. Ann. N. Y. Acad. Sci. 1999, 889, 193–203. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Spiteller, G. Lipid peroxidation in aging and age-dependent diseases. Exp. Gerontol. 2001, 36, 1425–1457. [Google Scholar] [CrossRef]
- Miró, Ò.; Casademont, J.; Casals, E.; Perea, M.; Urbano-Márquez, Á.; Rustin, P.; Cardellach, F. Aging is associated with increased lipid peroxidation in human hearts, but not with mitochondrial respiratory chain enzyme defects. Cardiovasc. Res. 2000, 47, 624–631. [Google Scholar] [CrossRef]
- Shimizu, N.; Ito, J.; Kato, S.; Eitsuka, T.; Saito, T.; Nishida, H.; Miyazawa, T.; Nakagawa, K. Evaluation of squalene oxidation mechanisms in human skin surface lipids and shark liver oil supplements. Ann. N. Y. Acad. Sci. 2019, 1457, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Ibusuki, D.; Suzuki, Y.; Yamashita, S.; Higuchi, O.; Oikawa, S.; Miyazawa, T. Ion-trap tandem mass spectrometric analysis of squalene monohydroperoxide isomers in sunlight-exposed human skin. J. Lipid Res. 2007, 48, 2779–2787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, A.; Arakane, K.; Koide, C.; Arai, H.; Nagano, T. Squalene as a Target Molecule in Skin Hyperpigmentation Caused by Singlet Oxygen. Biol. Pharm. Bull. 2009, 32, 1504–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, K.; Shibata, A.; Maruko, T.; Sookwong, P.; Tsuduki, T.; Kawakami, K.; Nishida, H.; Miyazawa, T. γ-Tocotrienol Reduces Squalene Hydroperoxide-Induced Inflammatory Responses in HaCaT Keratinocytes. Lipids 2010, 45, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Kawakami, K.; Sone, T.; Onoue, M. Characteristics of Skin Wrinkling and Dermal Changes Induced by Repeated Application of Squalene Monohydroperoxide to Hairless Mouse Skin. Skin Pharmacol. Physiol. 2003, 16, 242–251. [Google Scholar] [CrossRef]
- Chiba, K.; Yoshizawa, K.; Makino, I.; Kawakami, K.; Onoue, M. Comedogenicity of squalene monohydroperoxide in the skin after topical application. J. Toxicol. Sci. 2000, 25, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Petrick, L.; Dubowski, Y. Heterogeneous oxidation of squalene film by ozone under various indoor conditions. Indoor Air 2009, 19, 381–391. [Google Scholar] [CrossRef]
- Arata, C.; Heine, N.; Wang, N.; Misztal, P.K.; Wargocki, P.; Bekö, G.; Williams, J.; Nazaroff, W.W.; Wilson, K.R.; Goldstein, A.H. Heterogeneous Ozonolysis of Squalene: Gas-Phase Products Depend on Water Vapor Concentration. Environ. Sci. Technol. 2019, 53, 14441–14448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; Mekic, M.; Xu, X.; Loisel, G.; Zhou, Z.; Gligorovski, S.; Li, X. A Novel Insight into the Ozone–Skin Lipid Oxidation Products Observed by Secondary Electrospray Ionization High-Resolution Mass Spectrometry. Environ. Sci. Technol. 2020, 54, 13478–13487. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Bersabe, H.; Ito, J.; Kato, S.; Towada, R.; Eitsuka, T.; Kuwahara, S.; Miyazawa, T.; Nakagawa, K. Mass Spectrometric Discrimination of Squalene Monohydroperoxide Isomers. J. Oleo Sci. 2017, 66, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Nakagawa, K.; Suzuki, Y.; Asai, A.; Nagao, M.; Nagashima, K.; Oikawa, S.; Miyazawa, T. Liquid chromatography–tandem mass spectrometry determination of human plasma 1-palmitoyl-2-hydroperoxyoctadecadienoyl-phosphatidylcholine isomers via promotion of sodium adduct formation. Anal. Biochem. 2015, 471, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Otoki, Y.; Nakagawa, K.; Kato, S.; Miyazawa, T. MS/MS and LC-MS/MS analysis of choline/ethanolamine plasmalogens via promotion of alkali metal adduct formation. J. Chromatogr. B 2015, 1004, 85–92. [Google Scholar] [CrossRef]
- Ito, J.; Mizuochi, S.; Nakagawa, K.; Kato, S.; Miyazawa, T. Tandem Mass Spectrometry Analysis of Linoleic and Arachidonic Acid Hydroperoxides via Promotion of Alkali Metal Adduct Formation. Anal. Chem. 2015, 87, 4980–4987. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Tian, X.-Y.; Li, Z.-W.; Peng, X.-S.; Wong, H.N.C. Total Synthesis of Plakortide E and Biomimetic Synthesis of Plakortone B. Chem. Eur. J. 2011, 17, 5874–5880. [Google Scholar] [CrossRef] [PubMed]
- Hehre, W.; Klunzinger, P.; Deppmeier, B.; Driessen, A.; Uchida, N.; Hashimoto, M.; Fukushi, E.; Takata, Y. Efficient Protocol for Accurately Calculating 13C Chemical Shifts of Conformationally Flexible Natural Products: Scope, Assessment, and Limitations. J. Nat. Prod. 2019, 82, 2299–2306. [Google Scholar] [CrossRef]
- Wada, Y.; Kikuchi, A.; Kaga, A.; Shimizu, N.; Ito, J.; Onuma, R.; Fujishima, F.; Totsune, E.; Sato, R.; Niihori, T.; et al. Metabolic and pathologic profiles of human LSS deficiency recapitulated in mice. PLoS Genet. 2020, 16, e1008628. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, N.; Ito, J.; Kato, S.; Otoki, Y.; Goto, M.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Oxidation of squalene by singlet oxygen and free radicals results in different compositions of squalene monohydroperoxide isomers. Sci. Rep. 2018, 8, 9116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.-Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Gay, C.; Collins, J.; Gebicki, J.M. Hydroperoxide Assay with the Ferric–Xylenol Orange Complex. Anal. Biochem. 1999, 273, 149–155. [Google Scholar] [CrossRef]
- Tanno, R.; Kato, S.; Shimizu, N.; Ito, J.; Sato, S.; Ogura, Y.; Sakaino, M.; Sano, T.; Eitsuka, T.; Kuwahara, S.; et al. Analysis of oxidation products of α-tocopherol in extra virgin olive oil using liquid chromatography–tandem mass spectrometry. Food Chem. 2020, 306, 125582. [Google Scholar] [CrossRef]
- Martini, S.; Cavalchi, M.; Conte-Junior, C.; Tagliazucchi, D. The paradoxical effect of extra-virgin olive oil on oxidative phenomena during in vitro co-digestion with meat. Food Res. Int. 2018, 109, 82–90. [Google Scholar] [CrossRef]
- Hosoda, S.; Sato, T.; Takahashi, M.; Tanuma, I.; Yamada, R. Chemiluminescence studies on the photooxidation of isotactic polypropylene. Polym. Degrad. Stab. 2021, 188, 109575. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, X.; Chen, Q.; Zhang, H.; Sun, H.; Zhang, W.-M.; Li, C. Oxidative stabilities of olive and camellia oils: Possible mechanism of aldehydes formation in oleic acid triglyceride at high temperature. LWT 2019, 118, 108858. [Google Scholar] [CrossRef]
- Miotto, G.; Rossetto, M.; Di Paolo, M.L.; Orian, L.; Venerando, R.; Roveri, A.; Vučković, A.-M.; Travain, V.B.; Zaccarin, M.; Zennaro, L.; et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 2020, 28, 101328. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Neff, W.E.; Selke, E. Analysis of autoxidized fats by gas chromatography-mass spectrometry. IX. Homolytic vs. Heterolytic cleavage of primary and secondary oxidation products. Lipids 1984, 19, 790–800. [Google Scholar] [CrossRef]
- Frankel, E.N.; Neff, W.E.; Miyashita, K. Autoxidation of polyunsaturated triacylglycerols. II. Trilinolenoylglycerol. Lipids 1990, 25, 40–47. [Google Scholar] [CrossRef]
- Frankel, E.N.; Neff, W.E.; Weisleder, D. Formation of hydroperoxy bis-epidioxides in sensitized photo-oxidized methyl linolenate. J. Chem. Soc. Chem. Commun. 1982, 599–600. [Google Scholar] [CrossRef]
- Neff, W.E.; Frankel, E.N.; Selke, E.; Weisleder, D. Photosensitized oxidation of methyl linoleate monohydroperoxides: Hydroperoxy cyclic peroxides, dihydroperoxides, keto esters and volatile thermal decomposition products. Lipids 1983, 18, 868–876. [Google Scholar] [CrossRef]
- Porter, N.A.; Funk, M.O.; Gilmore, D.; Isaac, R.; Nixon, J. The formation of cyclic peroxides from unsaturated hydroperoxides: Models for prostaglandin biosynthesis. J. Am. Chem. Soc. 1976, 98, 6000–6005. [Google Scholar] [CrossRef] [PubMed]
- Bielfeldt, S.; Jung, K.; Laing, S.; Moga, A.; Wilhelm, K. Anti-pollution effects of two antioxidants and a chelator—Ex vivo electron spin resonance and in vivo cigarette smoke model assessments in human skin. Skin Res. Technol. 2021, 13068. [Google Scholar] [CrossRef] [PubMed]


 Cis 1A |  Trans 1A |  Cis 2A |  Trans 2A |  R 1B | 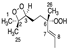 S 1B | 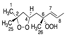 R 2B | 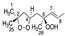 S 2B | |
|---|---|---|---|---|---|---|---|---|
| RMS | 0.96 | 1.85 | 9.55 | 10.05 | 4.23 | 4.31 | 10.72 | 10.35 |
| Max absolute | 1.80 | 3.40 | 21.00 | 22.00 | 7.20 | 5.90 | 31.60 | 30.20 |
| Mean absolute | 0.75 | 1.58 | 6.90 | 7.51 | 3.65 | 3.61 | 6.62 | 6.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, S.; Enomoto, M.; Kato, S.; Nakagawa, K. Novel Photoinduced Squalene Cyclic Peroxide Identified, Detected, and Quantified in Human Skin Surface Lipids. Antioxidants 2021, 10, 1760. https://doi.org/10.3390/antiox10111760
Khalifa S, Enomoto M, Kato S, Nakagawa K. Novel Photoinduced Squalene Cyclic Peroxide Identified, Detected, and Quantified in Human Skin Surface Lipids. Antioxidants. 2021; 10(11):1760. https://doi.org/10.3390/antiox10111760
Chicago/Turabian StyleKhalifa, Saoussane, Masaru Enomoto, Shunji Kato, and Kiyotaka Nakagawa. 2021. "Novel Photoinduced Squalene Cyclic Peroxide Identified, Detected, and Quantified in Human Skin Surface Lipids" Antioxidants 10, no. 11: 1760. https://doi.org/10.3390/antiox10111760
APA StyleKhalifa, S., Enomoto, M., Kato, S., & Nakagawa, K. (2021). Novel Photoinduced Squalene Cyclic Peroxide Identified, Detected, and Quantified in Human Skin Surface Lipids. Antioxidants, 10(11), 1760. https://doi.org/10.3390/antiox10111760







