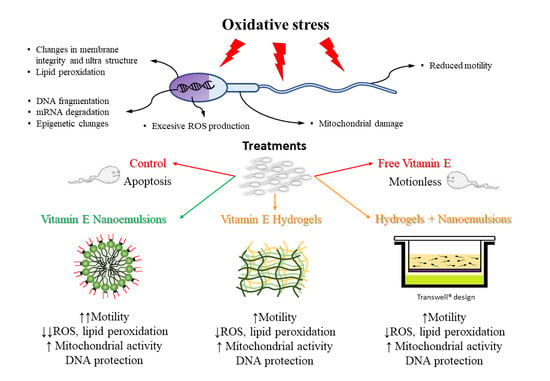
Vitamin E Delivery Systems Increase Resistance to Oxidative Stress in Red Deer Sperm Cells: Hydrogel and Nanoemulsion Carriers
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Media
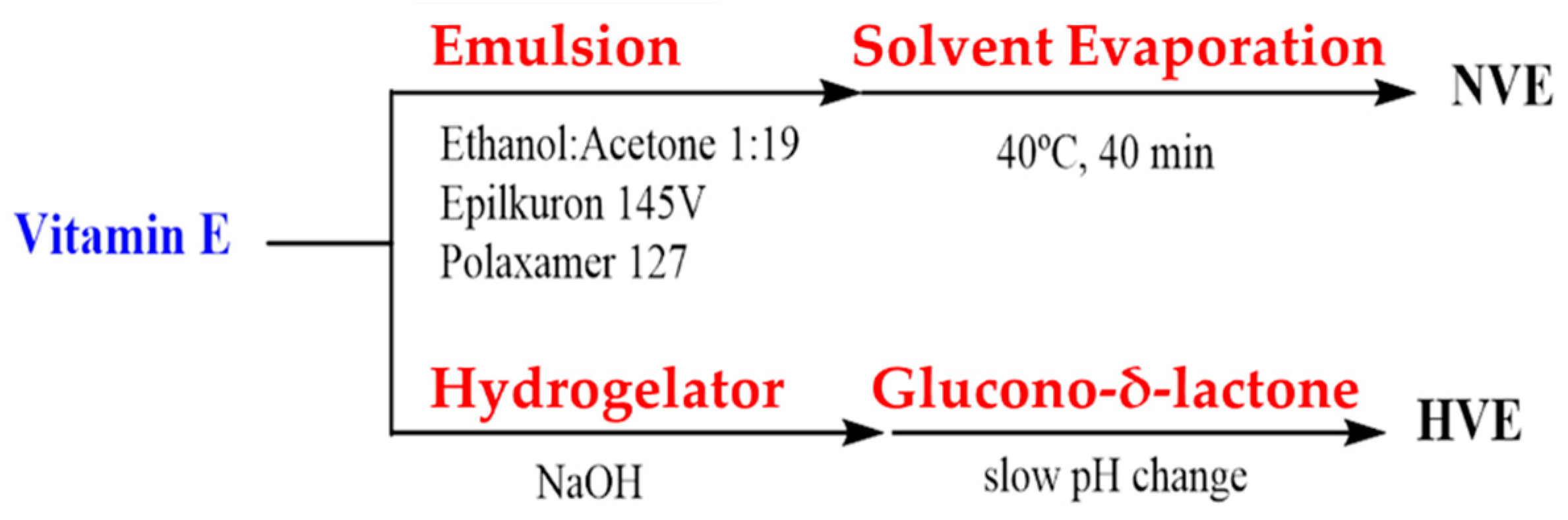
2.2. Vitamin E Nanoemulsion Formulation
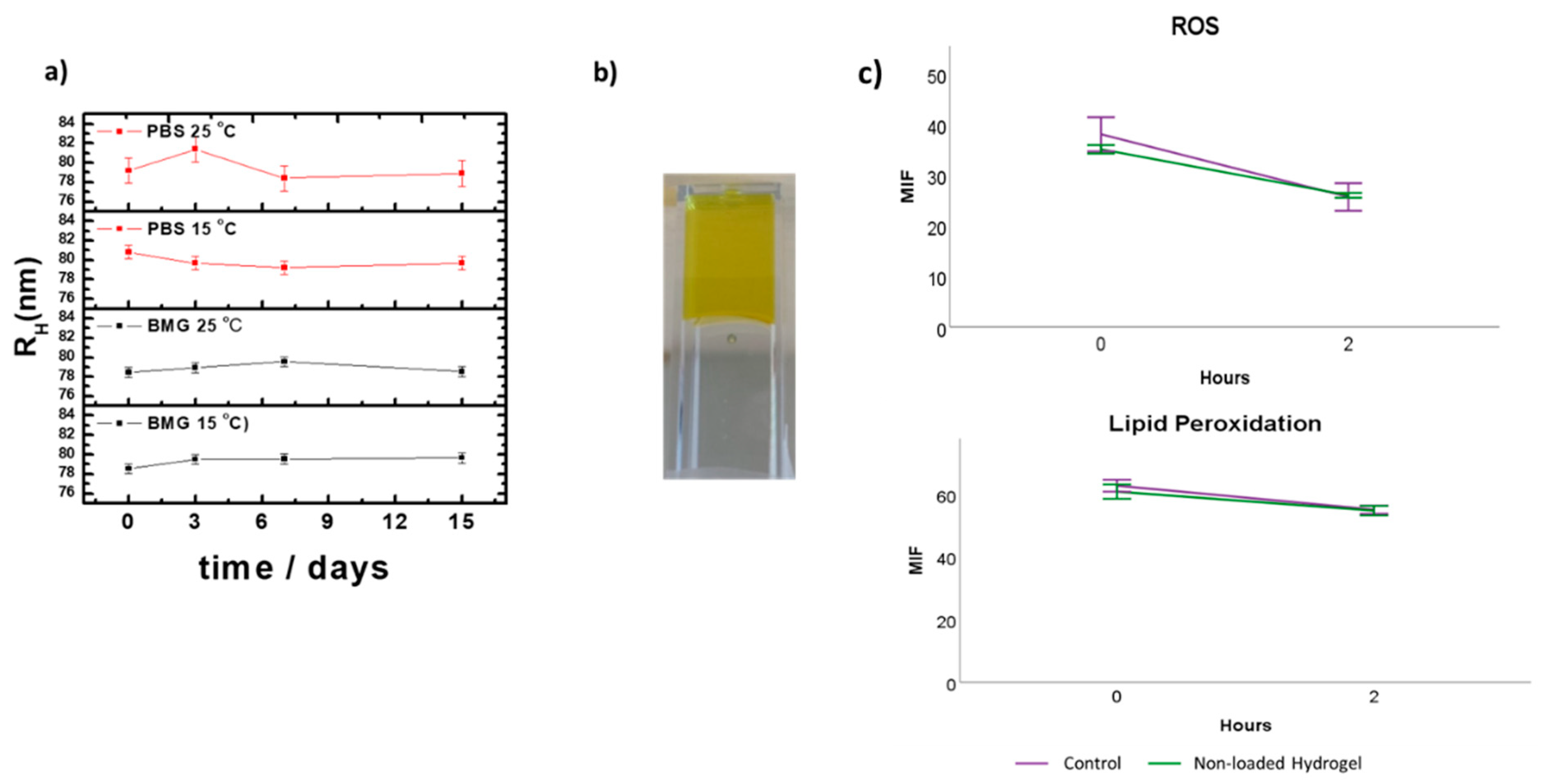
2.3. Stability Studies of the Vitamin E Nanoemulsions
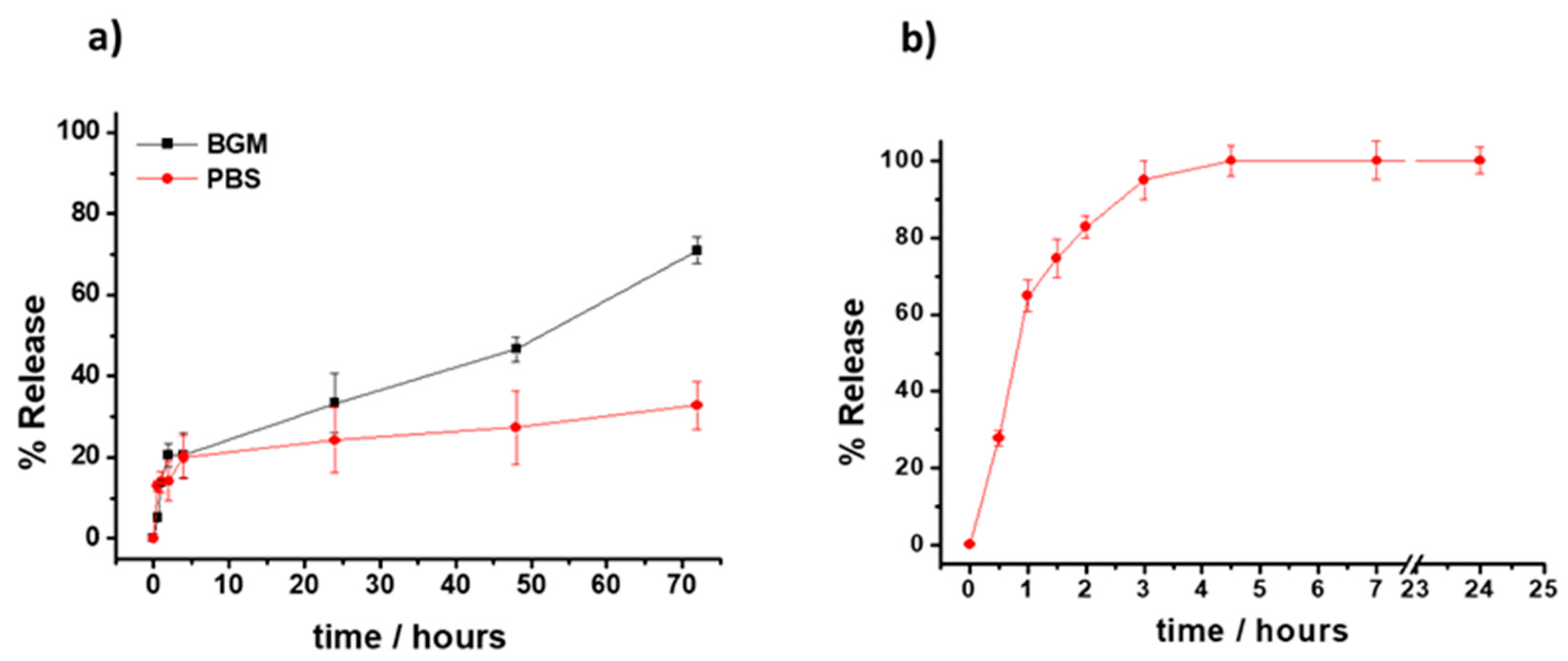
2.4. Release Studies
2.4.1. Vitamin E Nanoemulsions
2.4.2. Vitamin E-Loaded Hydrogels
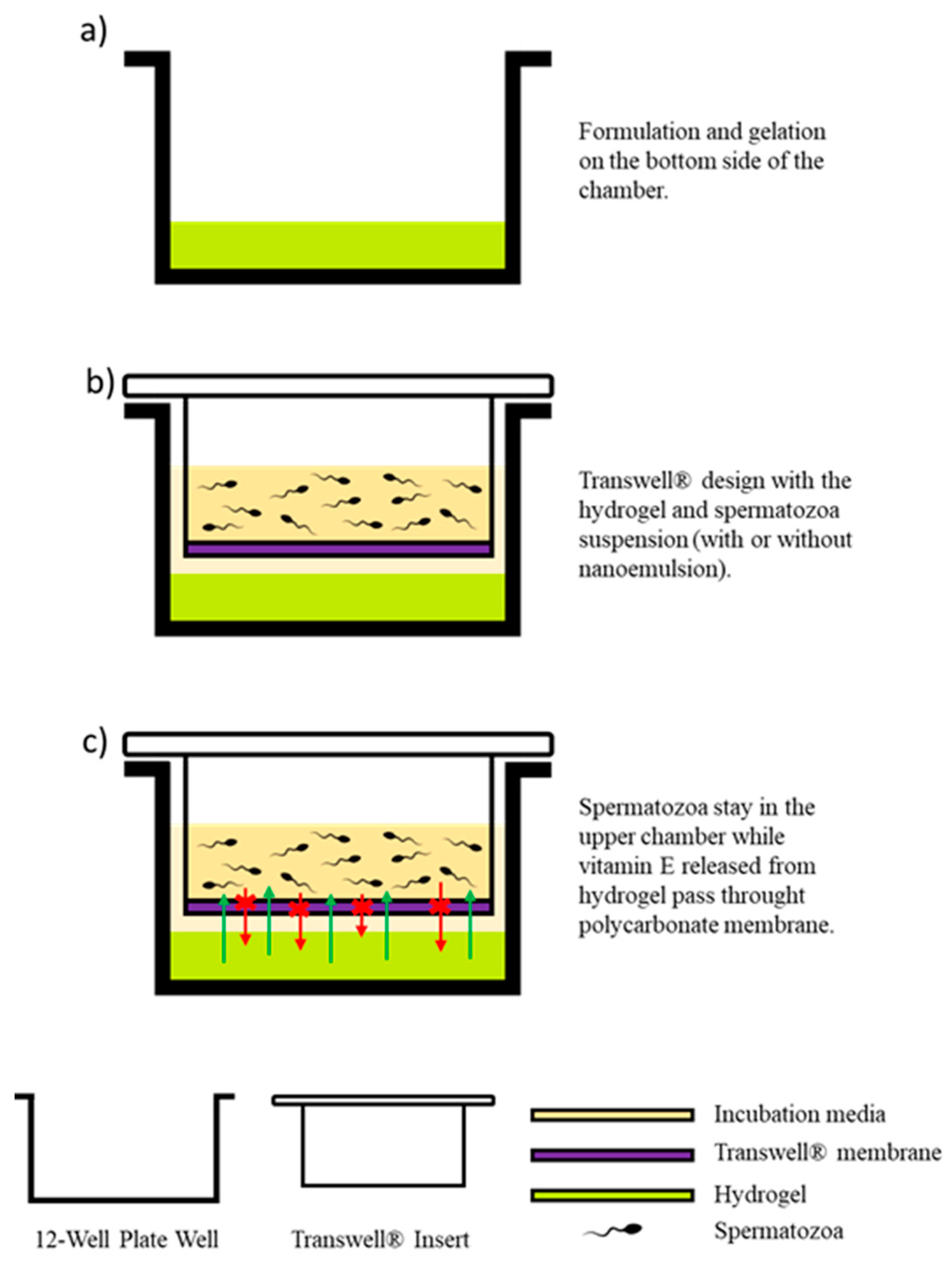
2.5. Transwell® Design and Vitamin E Hydrogel Formulation
2.6. Animals, Sperm Collection, and Cryopreservation
2.7. Experimental Design
2.8. Sperm Motility Analysis
2.9. Fluorescence Probes for Sperm Analysis
2.9.1. Sperm Viability and Apoptosis-like Changes
2.9.2. Mitochondrial Activity Assessment
2.9.3. Lipid Peroxidation Assessment
2.9.4. Reactive Oxygen Species Production
2.9.5. Sperm Chromatin Structure Assay (SCSA®)
2.10. Flow Cytometry Analysis
2.11. Statistical Analysis
3. Results
3.1. Formulation and Characterization of the VE-Loaded Devices
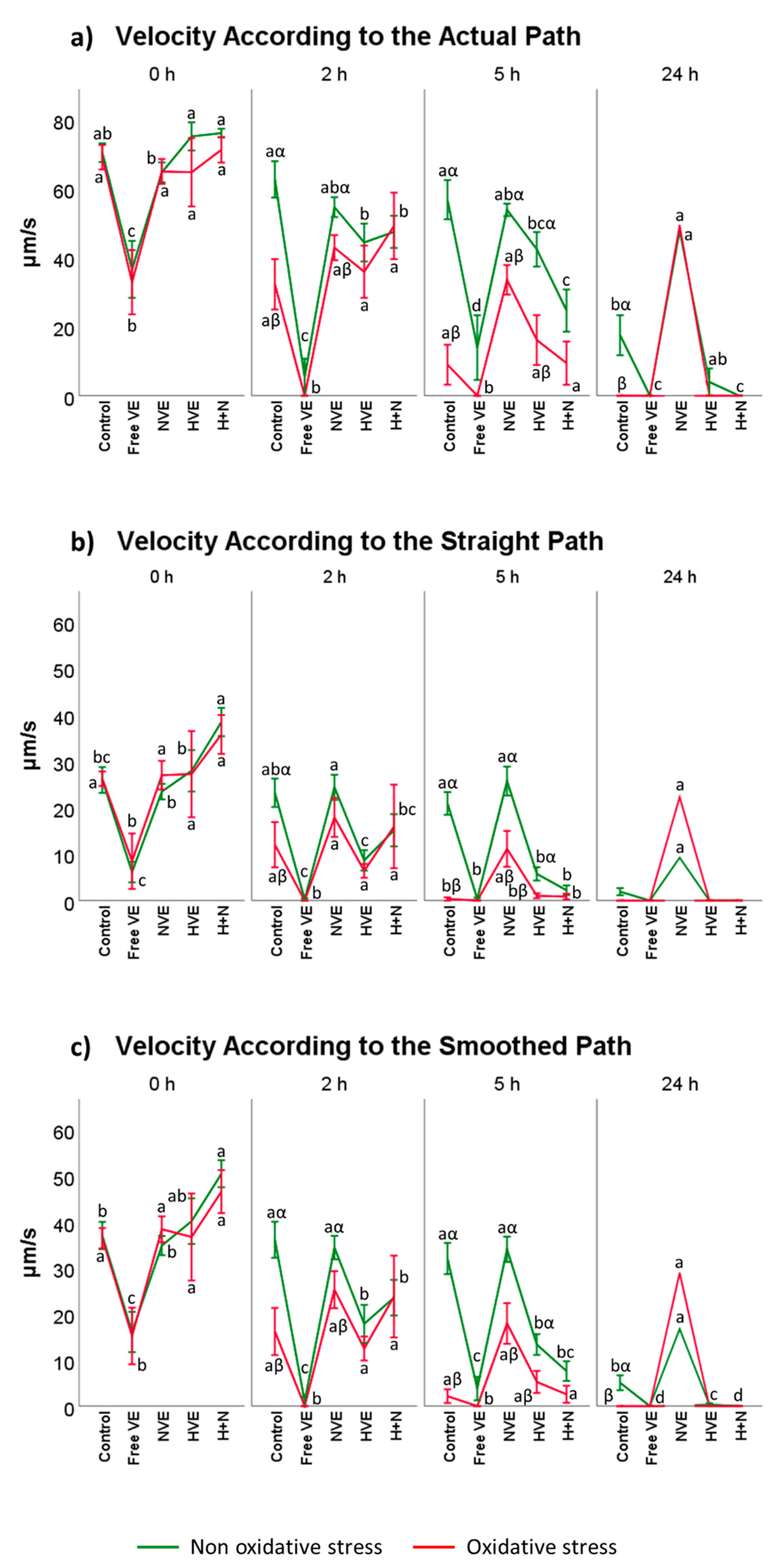
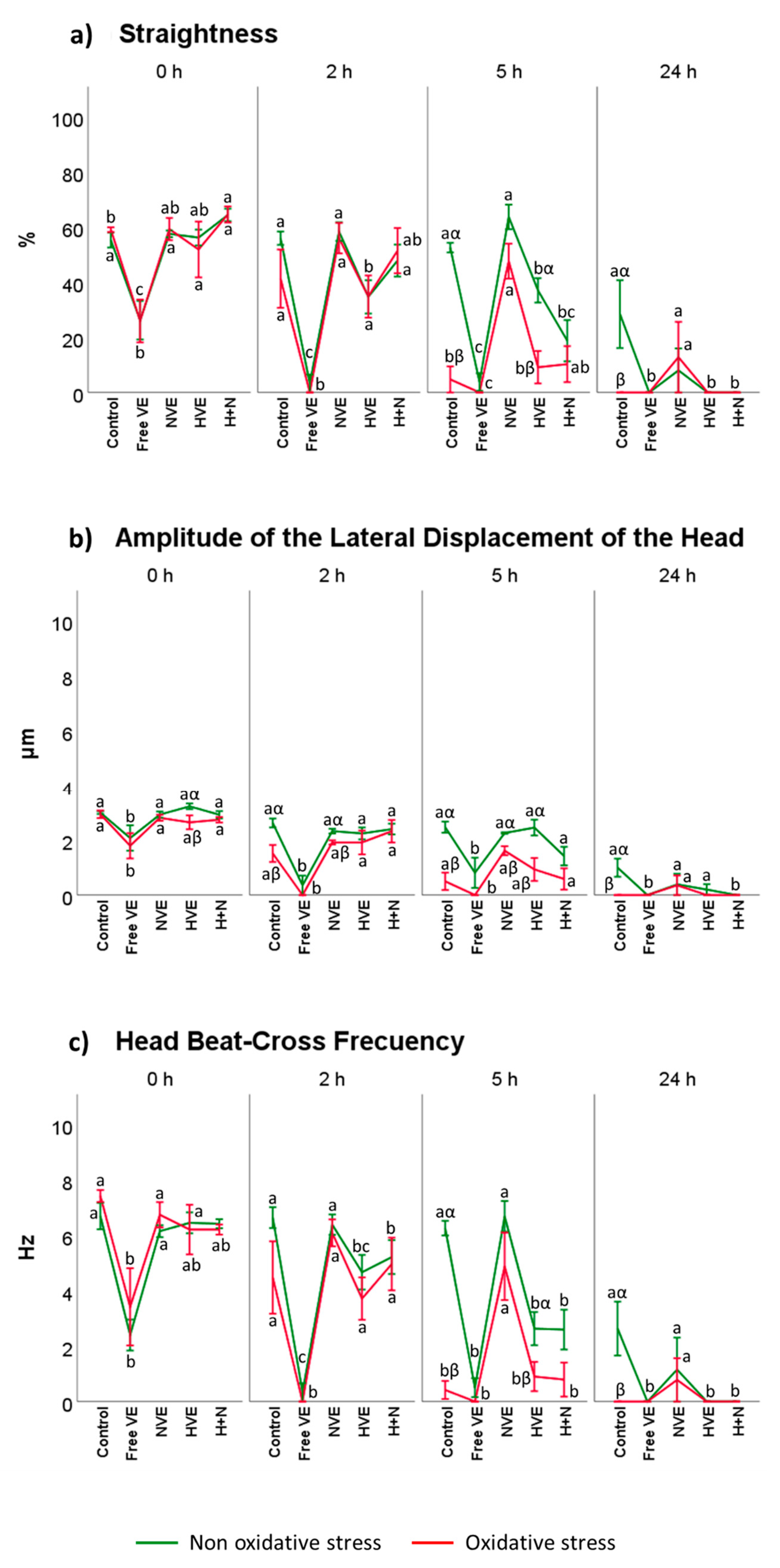
3.2. Vitamin E Formulation Effects on Sperm Motility Assessed Using CASA®
3.3. Vitamin E Formulation Effects on Sperm Physiology
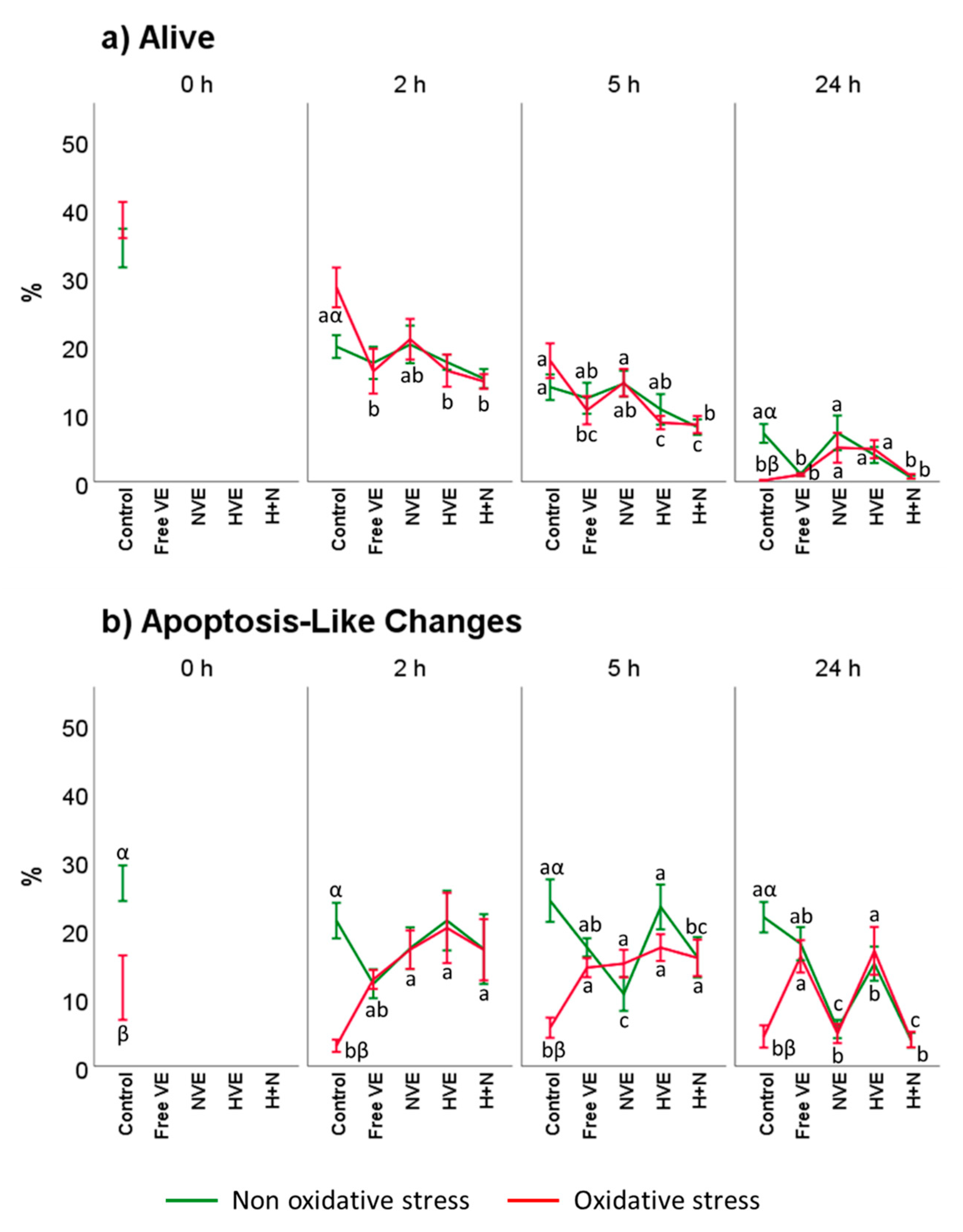
3.3.1. Sperm Viability
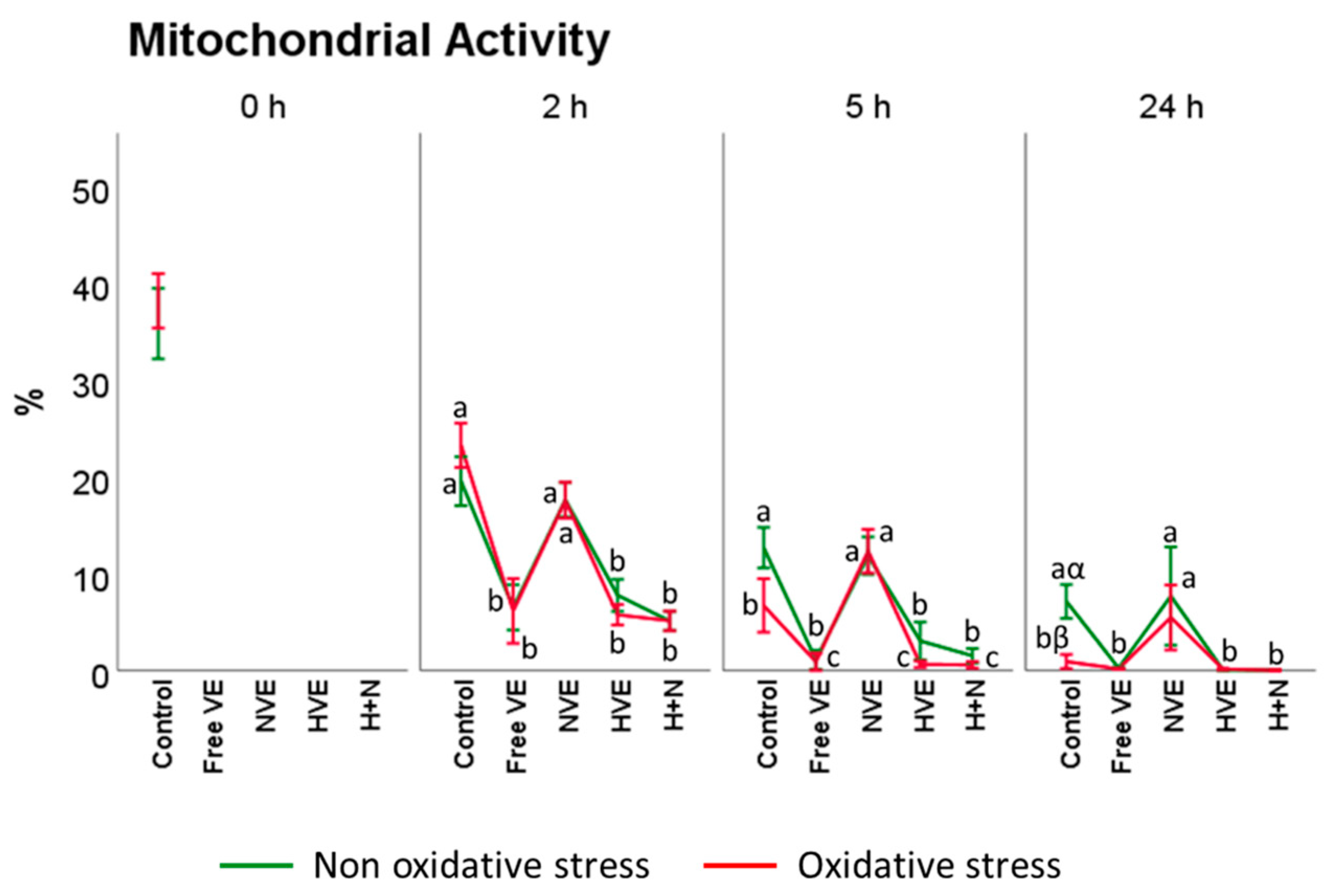
3.3.2. Mitochondrial Activity Assessment
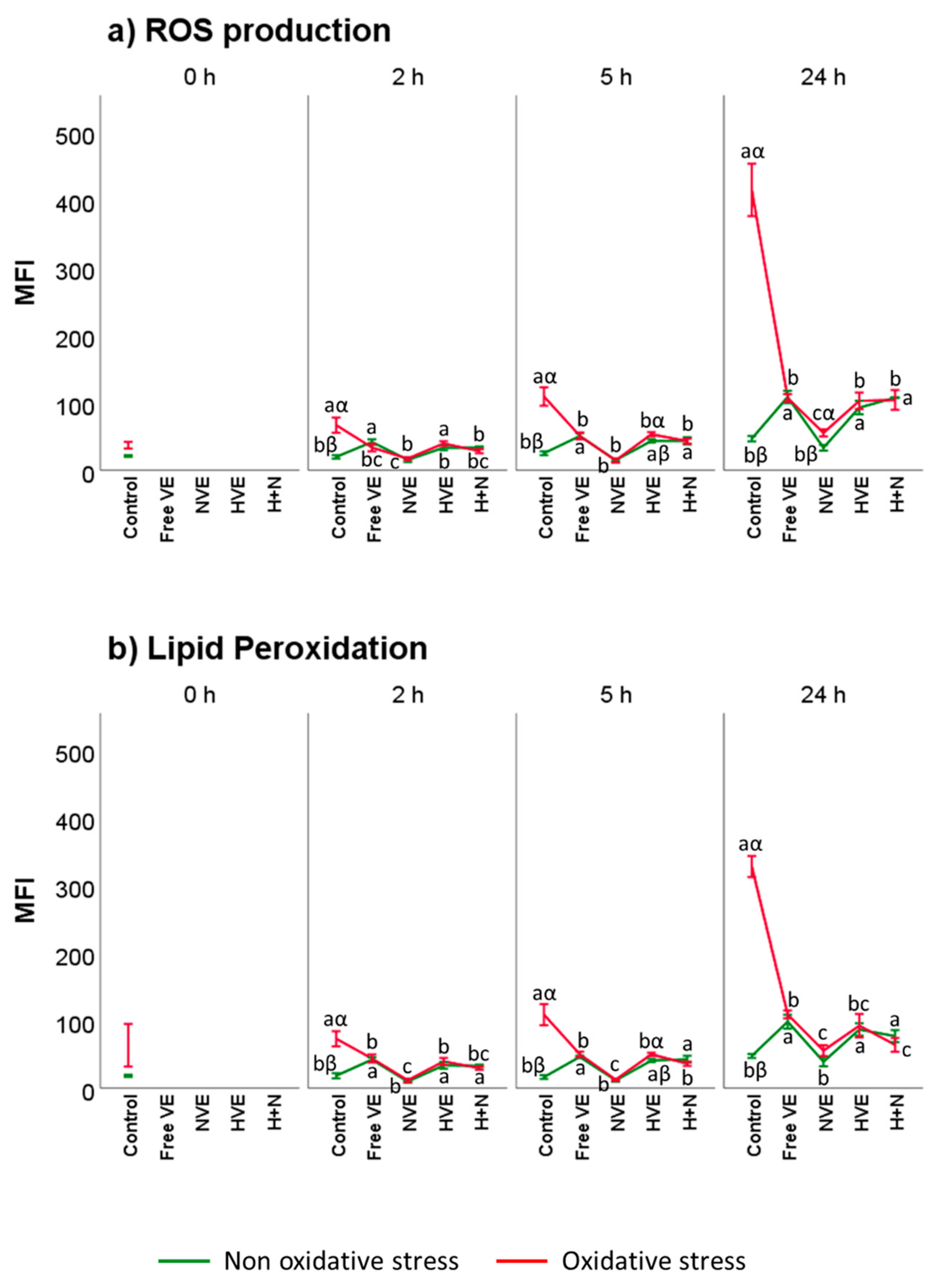
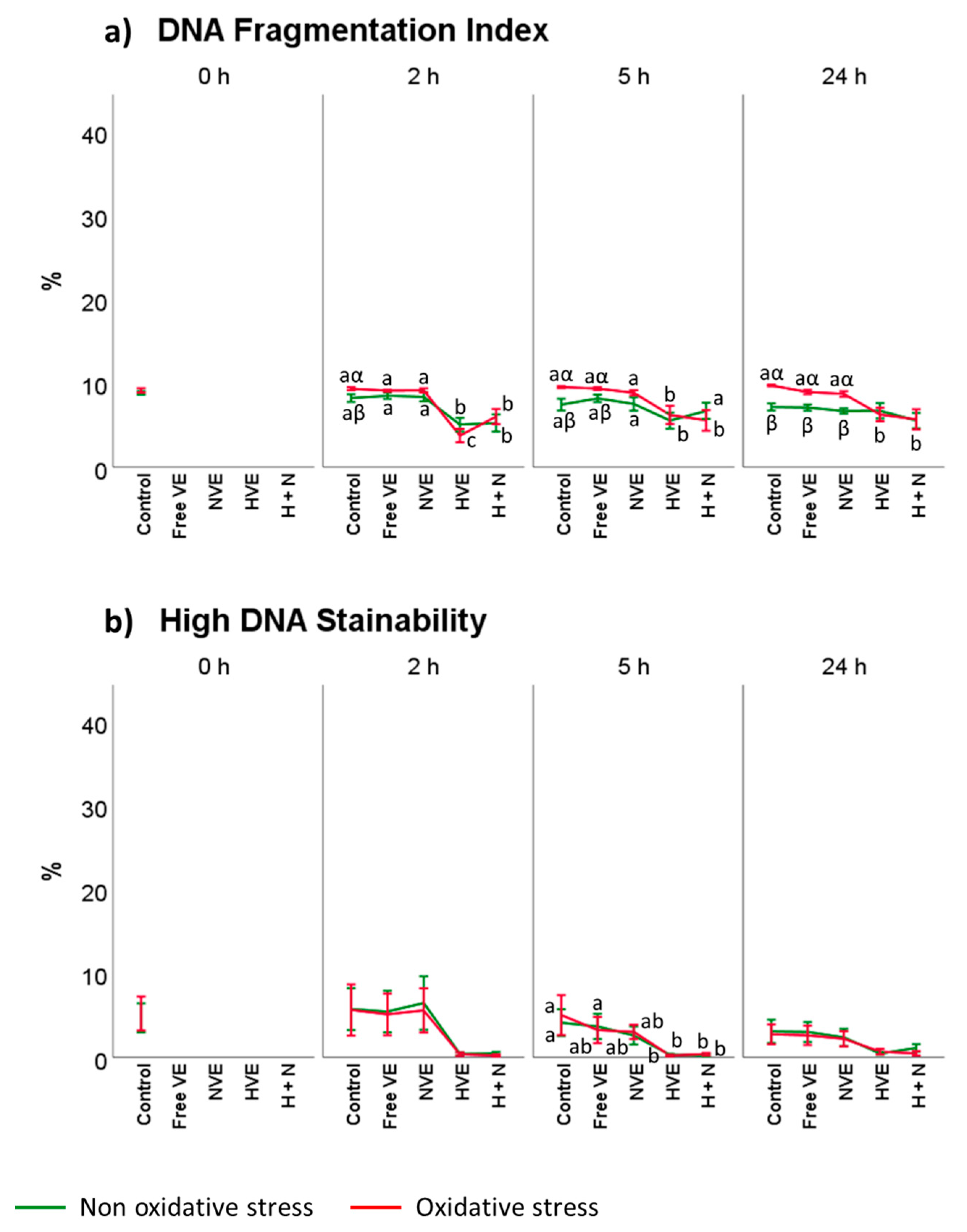
3.4. Vitamin E Formulation Effects on Lipid Peroxidation, Intracellular ROS, and DNA Damage of Thawed Spermatozoa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griveau, J.E.; Renard, P.; Le Lannou, D. An in vitro promoting role for hydrogen peroxide in human sperm capacitation. Int. J. Androl. 1994, 17, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Storey, B.T. Mammalian sperm metabolism: Oxygen and sugar, friend and foe. Int. J. Dev. Biol. 2008, 52, 427–437. [Google Scholar] [CrossRef]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef]
- Hallap, T.; Nagy, S.; Jaakma, Ü.; Johannisson, A.; Rodriguez-Martinez, H. Mitochondrial activity of frozen-thawed spermatozoa assessed by MitoTracker Deep Red 633. Theriogenology 2005, 63, 2311–2322. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gordon, E.L.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative Impact of Oxidative Stress on the Functional Competence and Genomic Integrity of Human Spermatozoa1. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef]
- Soria-Meneses, P.J.; Jurado-Campos, A.; Montoro, V.; Soler, A.J.; Garde, J.J.; Fernández-Santos, M.D.R. Ovine sperm DNA oxidation quantification using an 8-OHdG immunodetection assay. Reprod. Domest. Anim. 2019, 54, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Anel-López, L.; García-Álvarez, O.; Maroto-Morales, A.; Iniesta-Cuerda, M.; Ramón, M.; Soler, A.J.; Fernández-Santos, M.R.; Garde, J.J. Reduced glutathione addition improves both the kinematics and physiological quality of post-thawed red deer sperm. Anim. Reprod. Sci. 2015, 162, 73–79. [Google Scholar] [CrossRef]
- Domínguez-Rebolledo, A.E.; Fernández-Santos, M.R.; Bisbal, A.; Ros-Santaella, J.L.; Ramón, M.; Carmona, M.; Martínez-Pastor, F.; Garde, J.J. Improving the effect of incubation and oxidative stress on thawed spermatozoa from red deer by using different antioxidant treatments. Reprod. Fertil. Dev. 2010, 22, 856–870. [Google Scholar] [CrossRef]
- Domínguez-Rebolledo, A.E.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Garde, J.J.; Martínez-Pastor, F. Washing increases the susceptibility to exogenous oxidative stress in red deer spermatozoa. Theriogenology 2009, 72, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Santos, M.R.; Martínez-Pastor, F.; García-Macías, V.; Esteso, M.C.; Soler, A.J.; Paz, P.; Anel, L.; Garde, J.J. Sperm char-acteristics and DNA integrity of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa frozen in the presence of enzymatic and nonenzymatic antioxidants. J. Androl. 2007, 28, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Mata-Campuzano, M.; Álvarez-Rodríguez, M.; del Olmo, E.; Fernández-Santos, M.R.; Garde, J.J.; Martínez-Pastor, F. Quality, oxidative markers and DNA damage (DNA) fragmentation of red deer thawed spermatozoa after incubation at 37 degrees C in presence of several antioxidants. Theriogenology 2012, 78, 1005–1019. [Google Scholar] [CrossRef]
- Sánchez-Rubio, F.; Soria-Meneses, P.J.; Jurado-Campos, A.; Bartolomé-García, J.; Gómez-Rubio, V.; Soler, A.J.; Arroyo-Jimenez, M.M.; Santander-Ortega, M.J.; Plaza-Oliver, M.; Lozano, M.V.; et al. Nanotechnology in re-production: Vitamin E nanoemulsions for reducing oxidative stress in sperm cells. Free Radic. Biol. Med. 2020, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Anel-López, L.; García-Álvarez, O.; Parrilla, I.; del Olmo, D.; Fernández-Santos, M.R.; Soler, A.J.; Maroto-Morales, A.; Ortiz, J.A.; Alkmin, D.V.; Tarantini, T.; et al. The Effect of Oxidative Stress on Thawed Bulk-Sorted Red Deer Sperm. Reprod. Domest. Anim. 2016, 51, 407–414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anel-López, L.; Martínez-Rodríguez, C.; Soler, A.J.; Fernández-Santos, M.R.; Garde, J.J.; Morrell, J.M. Use of Androcoll-S after thawing improves the quality of electroejaculated and epididymal sperm samples from red deer. Anim. Reprod. Sci. 2015, 158, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rebolledo, A.E.; Martínez-Pastor, F.; Bisbal, A.F.; Ros-Santaella, J.L.; García-Álvarez, O.; Maroto-Morales, A.; Soler, A.J.; Garde, J.J.; Fernández-Santos, M.R. Response of thawed epididymal red deer spermatozoa to increasing concentrations of hydrogen peroxide, and importance of individual male variability. Reprod. Domest. Anim. 2011, 46, 393–403. [Google Scholar] [CrossRef]
- Fernández-Santos, M.R.; Domínguez-Rebolledo, A.E.; Esteso, M.C.; Garde, J.J.; Martínez-Pastor, F. Refrigerated storage of red deer epididymal spermatozoa in the epididymis, diluted and with vitamin C supplementation. Reprod. Domest. Anim. 2009, 44, 212–220. [Google Scholar] [CrossRef]
- Sánchez-Rubio, F.; Fernández-Santos, M.R.; Castro-Vázquez, L.; García-Álvarez, O.; Maroto-Morales, A.; Soler, A.J.; Mar-tínez-Pastor, F.; Garde, J.J. Cinnamtannin B-1, a novel antioxidant for sperm in red deer. Anim. Reprod. Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- García-Álvarez, O.; Maroto-Morales, A.; Martínez-Pastor, F.; Garde, J.J.; Ramón, M.; Fernández-Santos, M.R.; Esteso, M.C.; Pérez-Guzmán, M.D.; Soler, A.J. Sperm characteristics and in vitro fertilization ability of thawed spermatozoa from Black Manchega ram: Electroejaculation and postmortem collection. Theriogenology 2009, 72, 160–168. [Google Scholar] [CrossRef]
- Fernández-Santos, M.R.; Esteso, M.C.; Soler, A.J.; Montoro, V.; Garde, J.J. The effects of different cryoprotectants and the tem-perature of addition on the survival of red deer epididymal spermatozoa. Cryo. Lett. 2005, 26, 25–32. [Google Scholar]
- Rahimipour, M.; Talebi, A.R.; Anvari, M.; Sarcheshmeh, A.A.; Omidi, M. Effects of different doses of ethanol on sperm parameters, chromatin structure and apoptosis in adult mice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 423–428. [Google Scholar] [CrossRef]
- Lozano, M.V.; Torrecilla, D.; Torres, D.; Vidal, A.; Dominguez, F.; Alonso, M.J. Highly Efficient System to Deliver Taxanes into Tumor Cells: Docetaxel-Loaded Chitosan Oligomer Colloidal Carriers. Biomacromolecules 2008, 9, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Wong, W.-T.; Huang, M.; Wu, R.; Lai, W.-F. Self-healing properties of hydrogels based on natural polymers. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–245. [Google Scholar] [CrossRef]
- Bastiancich, C.; Danhier, P.; Préat, V. Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. J. Control. Release 2016, 243, 29–42. [Google Scholar] [CrossRef]
- Niza, E.; Castro-Osma, J.A.; Posadas, I.; Alonso-Moreno, C.; Bravo, I.; Garzón, A.; Canales-Vázquez, J.; Ceña, V.; Lara-Sánchez, A.; Albaladejo, J.; et al. Assessment of doxorubicin delivery devices based on tailored bare polycaprolactone against glioblastoma. Int. J. Pharm. 2019, 558, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech. 2014, 5, 123–127. [Google Scholar] [CrossRef]
- Ridzewski, C.; Li, M.; Dong, B.; Magdanz, V. Gelatin Microcartridges for Onboard Activation and Antioxidant Protection of Sperm. ACS Appl. Bio. Mater. 2020, 3, 1616–1627. [Google Scholar] [CrossRef]
- Rodríguez-Llansola, F.; Escuder, B.; Miravet, J.F.; Hermida-Merino, D.; Hamley, I.W.; Cardin, C.J.; Hayes, W. Selective and highly efficient dye scavenging by a pH-responsive molecular hydrogelator. Chem. Commun. 2010, 46, 7960–7962. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Martínez-Pastor, F.; Aisen, E.; Fernández-Santos, M.R.; Esteso, M.C.; Maroto-Morales, A.; García-Álvarez, O.; Garde, J.J. Reactive oxygen species generators affect quality parameters and apoptosis markers differently in red deer spermatozoa. Reproduction 2009, 137, 225–235. [Google Scholar] [CrossRef]
- del Olmo, E.; Bisbal, A.; García-Álvarez, O.; Maroto-Morales, A.; Ramón, M.; Jiménez-Rabadán, P.; Anel-López, L.; Soler, A.J.; Garde, J.J.; Fernández-Santos, M.R. Free-radical production after post-thaw incubation of ram spermatozoa is related to decreased in vivo fertility. Reprod. Fertil. Dev. 2015, 27, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rebolledo, A.E.; Martínez-Pastor, F.; Fernández-Santos, M.R.; del Olmo, E.; Bisbal, A.; Ros-Santaella, J.L.; Garde, J.J. Comparison of the TBARS assay and BODIPY C11 probes for assessing lipid peroxidation in red deer spermatozoa. Reprod. Domest. Anim. 2010, 45, e360–e368. [Google Scholar] [CrossRef] [PubMed]
- Evenson, D.P. The Sperm Chromatin Structure Assay (SCSA®) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim. Reprod. Sci. 2016, 169, 56–75. [Google Scholar] [CrossRef] [PubMed]
- Evenson, D.P.; Wixon, R. Clinical aspects of sperm DNA fragmentation detection and male infertility. Theriogenology 2006, 65, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, R.; Abhilash, P.A.; Das, S.S.; Prathibha, P.; Rejitha, S.; John, F.; Kavitha, S.; Indira, M. Protective effect of ascorbic acid against ethanol-induced re-productive toxicity in male guinea pigs. Br. J. Nutr. 2013, 110, 689–698. [Google Scholar] [CrossRef]
- Swathy, S.S.; Panicker, S.; Indira, M. Effect of exogenous selenium on the testicular toxicity induced by ethanol in rats. Indian J. Physiol. Pharmacol. 2006, 50, 215–224. [Google Scholar] [PubMed]
- Vuthiphandchai, V.; Chomphuthawach, S.; Nimrat, S. Cryopreservation of red snapper (Lutjanus argenti-maculatus) sperm: Effect of cryoprotectants and cooling rates on sperm motility, sperm viability, and fertilization capacity. Theriogenology 2009, 72, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Gagnon, C. Human sperm hyperactivation in whole semen and its association with low superoxide scavenging capacity in seminal plasma. Fertil. Steril. 1993, 59, 1291–1295. [Google Scholar] [CrossRef]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Jiang, H.; Zini, A.; Kodama, H.; Gagnon, C. Reactive oxygen species and sperm physiology. Rev. Reprod. 1997, 2, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Polzien, L.; Baljuls, A.; Rennefahrt, U.E.E.; Fischer, A.; Schmitz, W.; Zahedi, R.P.; Sickmann, A.; Metz, R.; Albert, S.; Benz, R.; et al. Identification of novel in vivo phosphorylation sites of the human proapoptotic protein BAD: Pore-forming activity of BAD is regulated by phosphorylation. J. Biol. Chem. 2009, 284, 28004–28020. [Google Scholar] [CrossRef]
- Sawyer, D.E.; Mercer, B.G.; Wiklendt, A.M.; Aitken, R.J. Quantitative analysis of gene-specific DNA damage in human spermatozoa. Mutat. Res. 2003, 529, 21–34. [Google Scholar] [CrossRef]
- Koppers, A.J.; Mitchell, L.A.; Wang, P.; Lin, M.; Aitken, R.J. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem. J. 2011, 436, 687–698. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Gagnon, C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int. J. Androl. 1993, 16, 21–25. [Google Scholar] [CrossRef]
- Ferreira, G.; Costa, C.; Bassaizteguy, V.; Santos, M.; Cardozo, R.; Montes, J.; Settineri, R.; Nicolson, G.L. Incubation of human sperm with micelles made from glycerophospholipid mixtures increases sperm motility and resistance to oxidative stress. PLoS ONE 2018, 13, e0197897. [Google Scholar] [CrossRef] [PubMed]
- Isaac, A.V.; Kumari, S.; Nair, R.; Urs, D.R.; Salian, S.R.; Kalthur, G.; Adiga, S.K.; Manikkath, J.; Mutalik, S.; Sachdev, D.; et al. Supplementing zinc oxide nanoparticles to cryopreservation medium minimizes the freeze-thaw-induced damage to spermatozoa. Biochem. Biophys. Res. Commun. 2017, 494, 656–662. [Google Scholar] [CrossRef]
- Falchi, L.; Bogliolo, L.; Galleri, G.; Ariu, F.; Zedda, M.T.; Pinna, A.; Malfatti, L.; Innocenzi, P.; Ledda, S. Cerium dioxide nanoparticles did not alter the functional and morphologic characteristics of ram sperm during short-term exposure. Theriogenology 2016, 85, 1274–1281. [Google Scholar] [CrossRef]
- Falchi, L.; Galleri, G.; Dore, G.M.; Zedda, M.T.; Pau, S.; Bogliolo, L.; Ariu, F.; Pinna, A.; Nieddu, S.; Innocenzi, P.; et al. Effect of exposure to CeO2 nanoparticles on ram spermatozoa during storage at 4 degrees C for 96 hours. Reprod. Biol. Endocrinol. 2018, 16, 19. [Google Scholar] [CrossRef]
- Fernández-Serrano, P.; Casares-Crespo, L.; Viudes-De-Castro, M. Chitosan-dextran sulphate nanoparticles for GnRH release in rabbit insemination extenders. Reprod. Domest. Anim. 2017, 52, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.D.C.J.; Remirão, M.H.; Lucas, C.G.; Domingues, W.B.; Silveira, T.; Paschoal, J.D.; Jornada, D.S.; Corcine, C.D.; Junior, A.S.V.; Prado, W.A.; et al. Effects of chitosan-coated lipid-core nanocapsules on bovine sperm cells. Toxicol. Vitr. 2017, 40, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Falchi, L.; Khalil, W.; Hassan, M.; Marei, W.F. Perspectives of nanotechnology in male fertility and sperm function. Int. J. Veter- Sci. Med. 2018, 6, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Yu, Y.; Li, Y.; Li, Y.B.; Yu, Y.B.; Zhou, X.Q.; Sun, Z.W. Exposure to silica nanoparticles causes reversible damage of the spermatogenic process in mice. PLoS ONE 2014, 9, e101572. [Google Scholar] [CrossRef]
- Talebi, A.R.; Khorsandi, L.; Moridian, M. The effect of zinc oxide nanoparticles on mouse spermatogenesis. J. Assist. Reprod. Genet. 2013, 30, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Si, W.; Zhao, X.; Wang, L.; Zhou, Y.; Chen, M.; Ge, Y.; Zhang, Q.; Wang, Y.; Zhang, J. TiO2 Nanoparticle Exposure Decreases Spermatogenesis via Biochemical Dysfunctions in the Testis of Male Mice. J. Agric. Food Chem. 2015, 63, 7084–7092. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurado-Campos, A.; Soria-Meneses, P.J.; Sánchez-Rubio, F.; Niza, E.; Bravo, I.; Alonso-Moreno, C.; Arenas-Moreira, M.; García-Álvarez, O.; Soler, A.J.; Garde, J.J.; et al. Vitamin E Delivery Systems Increase Resistance to Oxidative Stress in Red Deer Sperm Cells: Hydrogel and Nanoemulsion Carriers. Antioxidants 2021, 10, 1780. https://doi.org/10.3390/antiox10111780
Jurado-Campos A, Soria-Meneses PJ, Sánchez-Rubio F, Niza E, Bravo I, Alonso-Moreno C, Arenas-Moreira M, García-Álvarez O, Soler AJ, Garde JJ, et al. Vitamin E Delivery Systems Increase Resistance to Oxidative Stress in Red Deer Sperm Cells: Hydrogel and Nanoemulsion Carriers. Antioxidants. 2021; 10(11):1780. https://doi.org/10.3390/antiox10111780
Chicago/Turabian StyleJurado-Campos, Alejandro, Pedro Javier Soria-Meneses, Francisca Sánchez-Rubio, Enrique Niza, Iván Bravo, Carlos Alonso-Moreno, María Arenas-Moreira, Olga García-Álvarez, Ana Josefa Soler, José Julián Garde, and et al. 2021. "Vitamin E Delivery Systems Increase Resistance to Oxidative Stress in Red Deer Sperm Cells: Hydrogel and Nanoemulsion Carriers" Antioxidants 10, no. 11: 1780. https://doi.org/10.3390/antiox10111780
APA StyleJurado-Campos, A., Soria-Meneses, P. J., Sánchez-Rubio, F., Niza, E., Bravo, I., Alonso-Moreno, C., Arenas-Moreira, M., García-Álvarez, O., Soler, A. J., Garde, J. J., & Fernández-Santos, M. d. R. (2021). Vitamin E Delivery Systems Increase Resistance to Oxidative Stress in Red Deer Sperm Cells: Hydrogel and Nanoemulsion Carriers. Antioxidants, 10(11), 1780. https://doi.org/10.3390/antiox10111780