Calcium Dobesilate Modulates PKCδ-NADPH Oxidase- MAPK-NF-κB Signaling Pathway to Reduce CD14, TLR4, and MMP9 Expression during Monocyte-to-Macrophage Differentiation: Potential Therapeutic Implications for Atherosclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. THP-1 Cells Culture, Differentiation, and Treatment
2.3. Primary Monocyte Differentiation and Treatment
2.4. Assays of Cell Viability and Adhesion
2.5. Quantitative RT-PCR Analysis
2.6. Cytokine Production
2.7. Determination of Intracellular ROS
2.8. MMP9 Gelatin Zymography
2.9. Immunoblotting
2.10. Statistical Analysis
3. Results
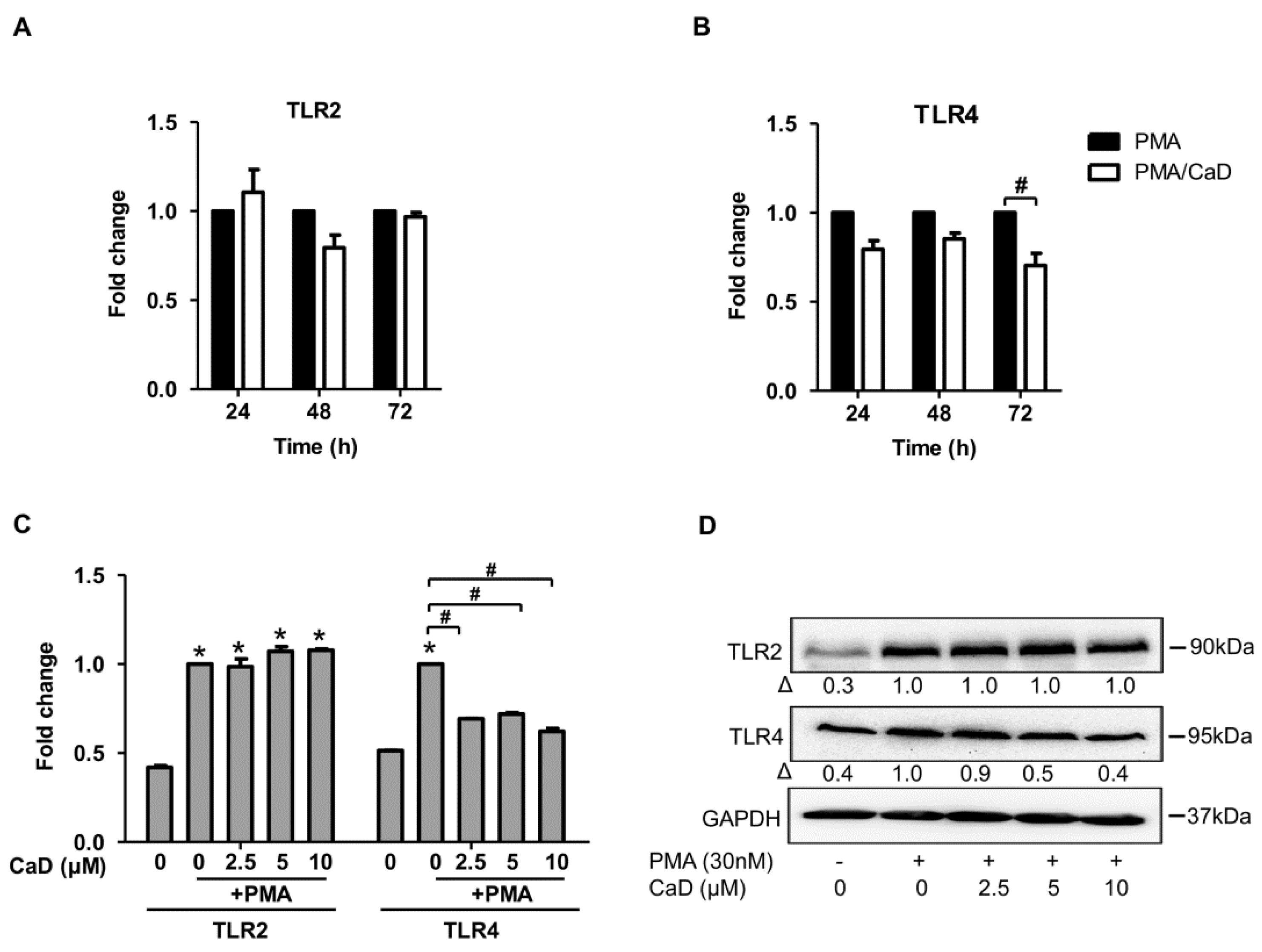
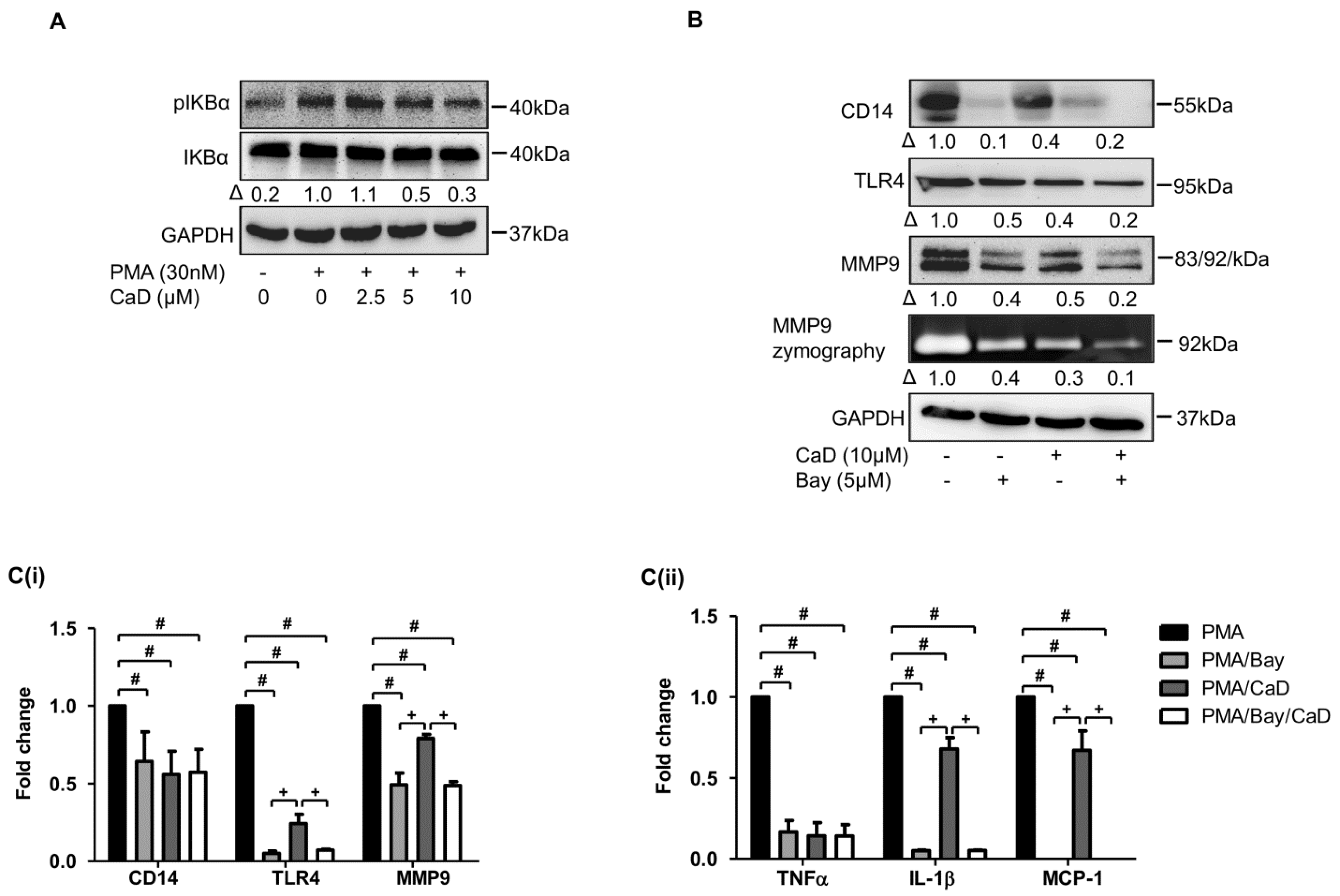
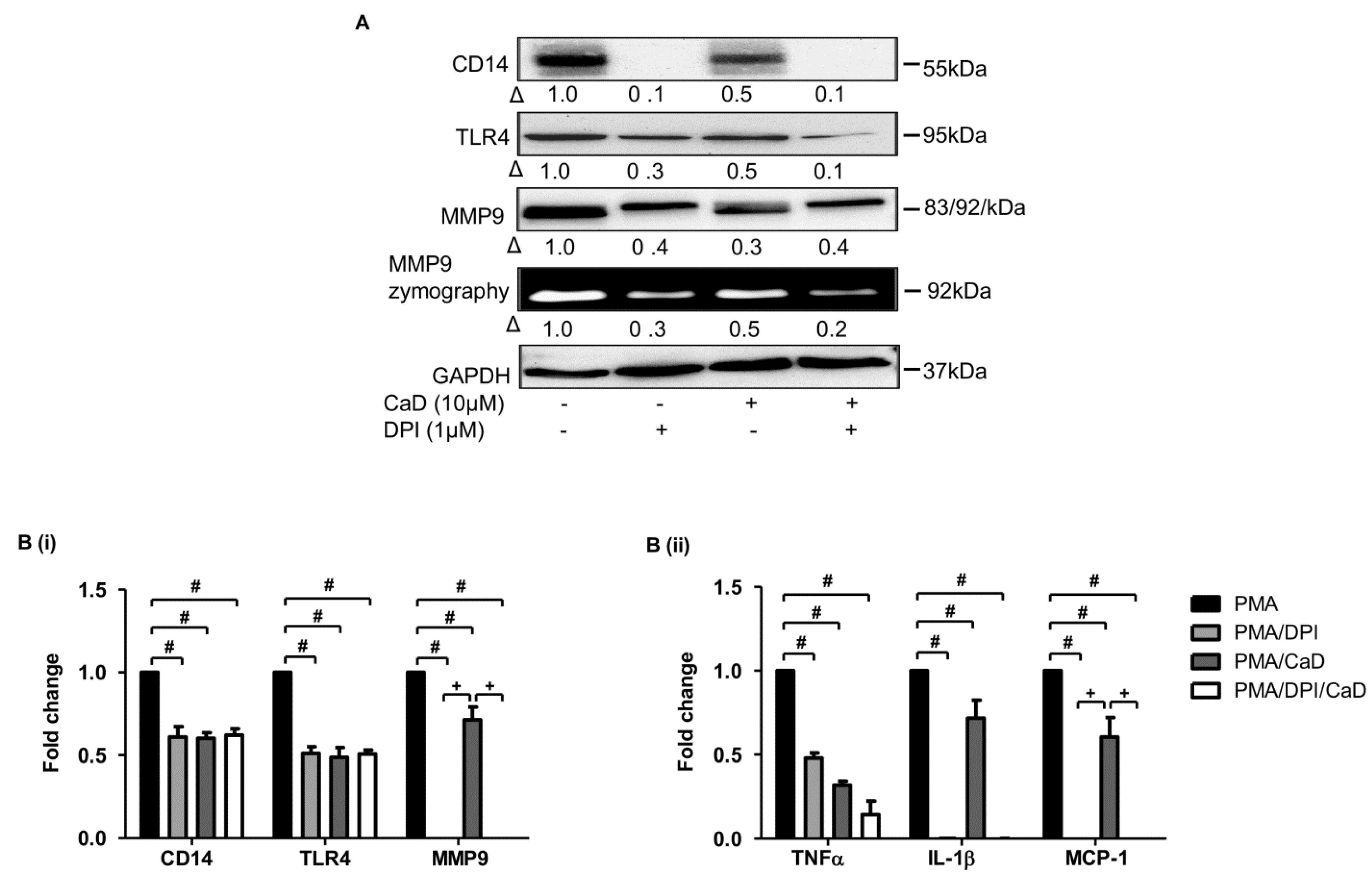
3.1. Calcium Dobesilate (CaD) Inhibits CD14 and TLR4 Expression during Monocyte-to-Macrophage Differentiation
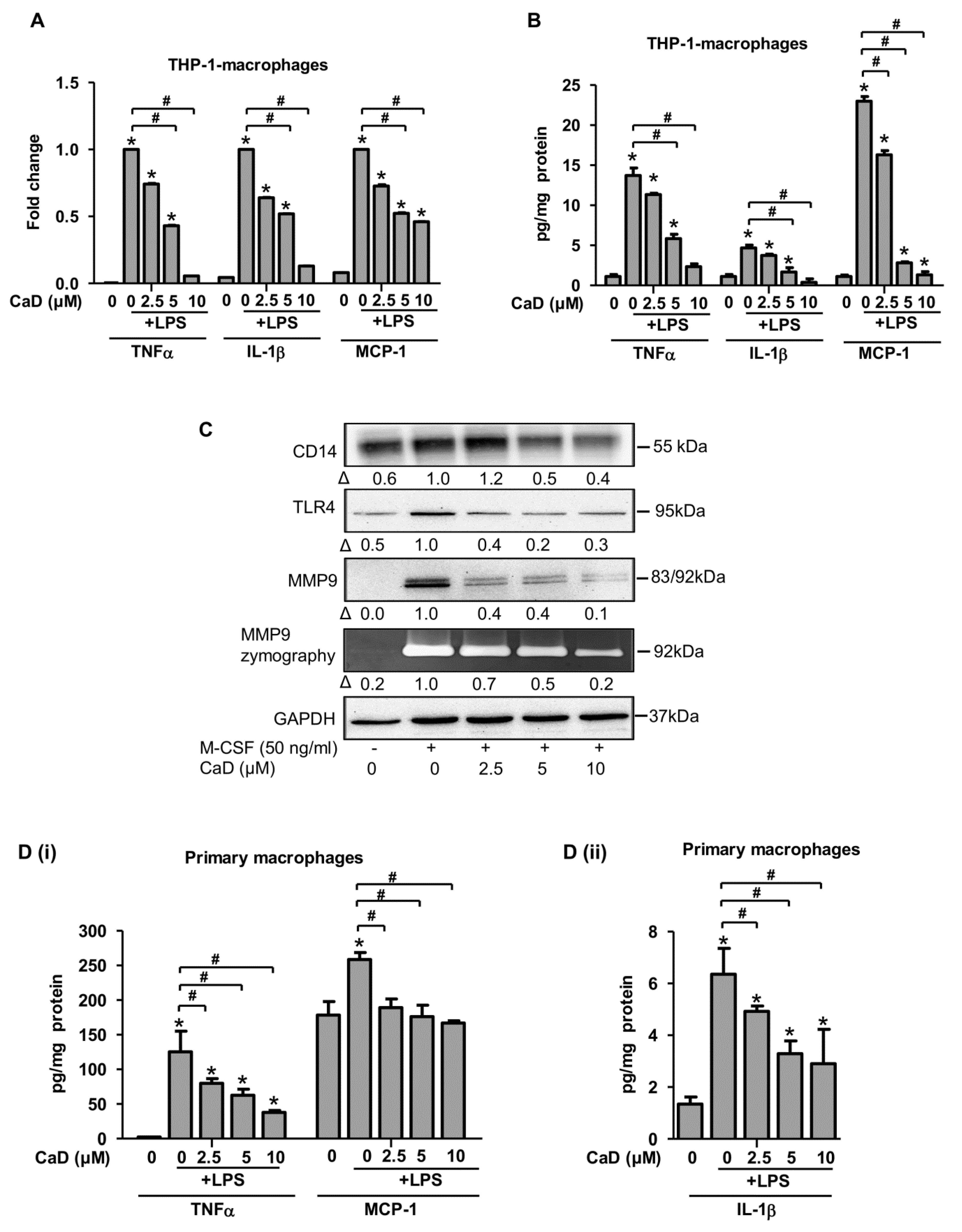
3.2. CaD Inhibits Inflammation during Monocyte-to-Macrophage Differentiation
3.3. The Effect of CaD Is Not Limited to PMA-Induced Monocyte-to-Macrophage Differentiation and Inflammation: Effects of LPS and High Glucose Stimulation
3.4. CaD Modulates Primary Human Monocyte-to-Macrophage Differentiation and Inflammation
3.5. Signaling Pathways Affected by CaD during Monocyte-to-Macrophage Differentiation
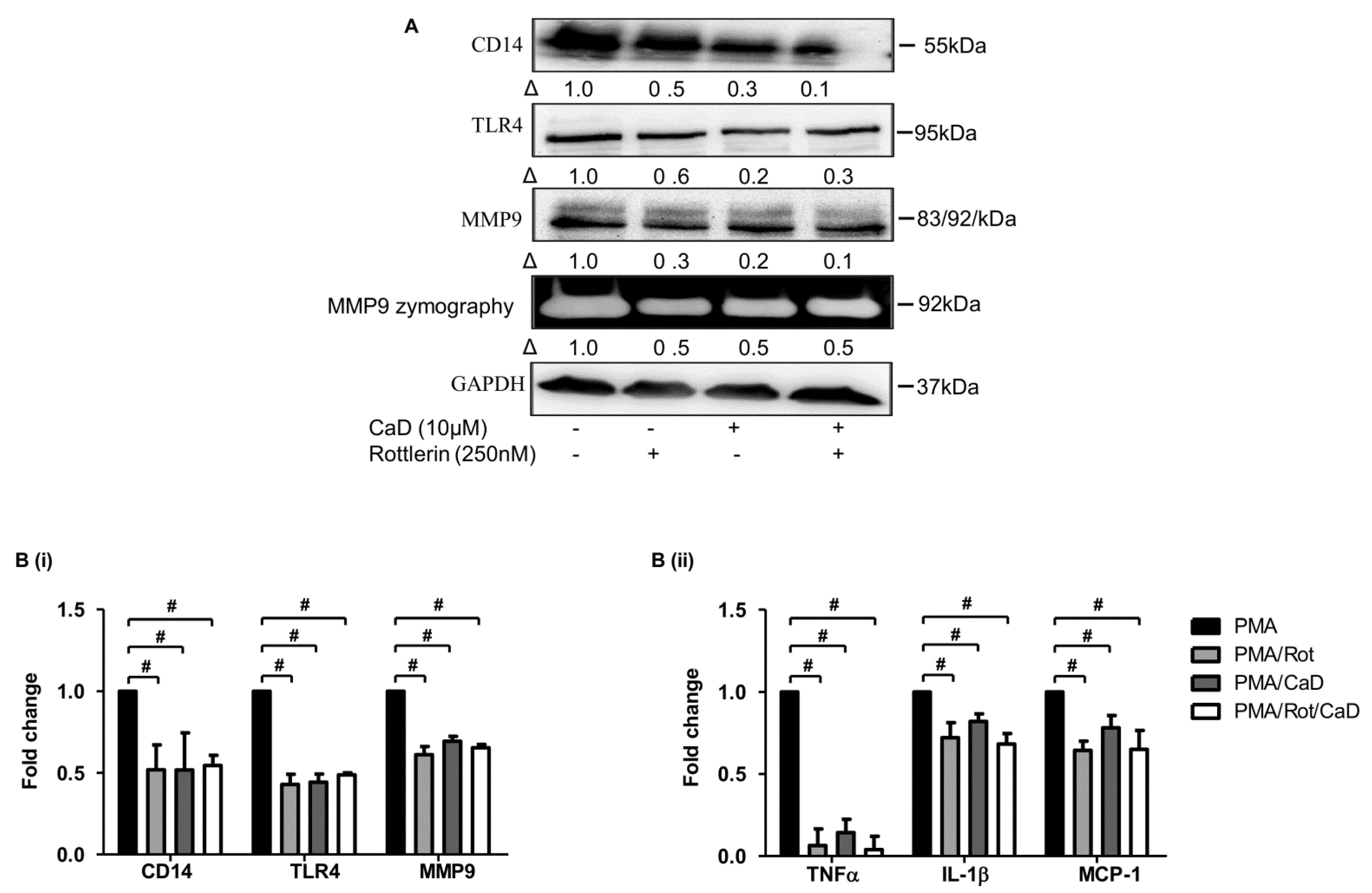
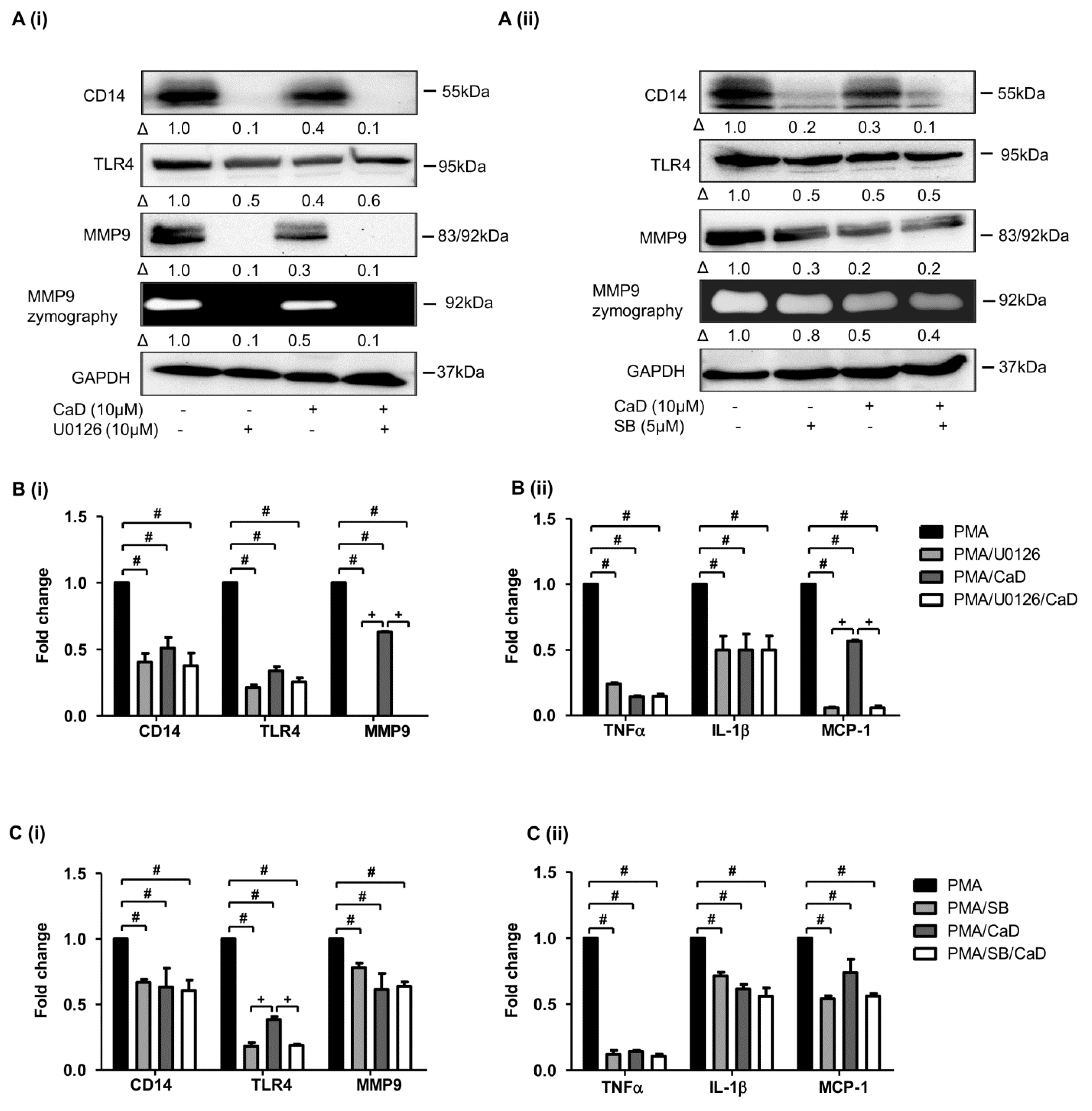
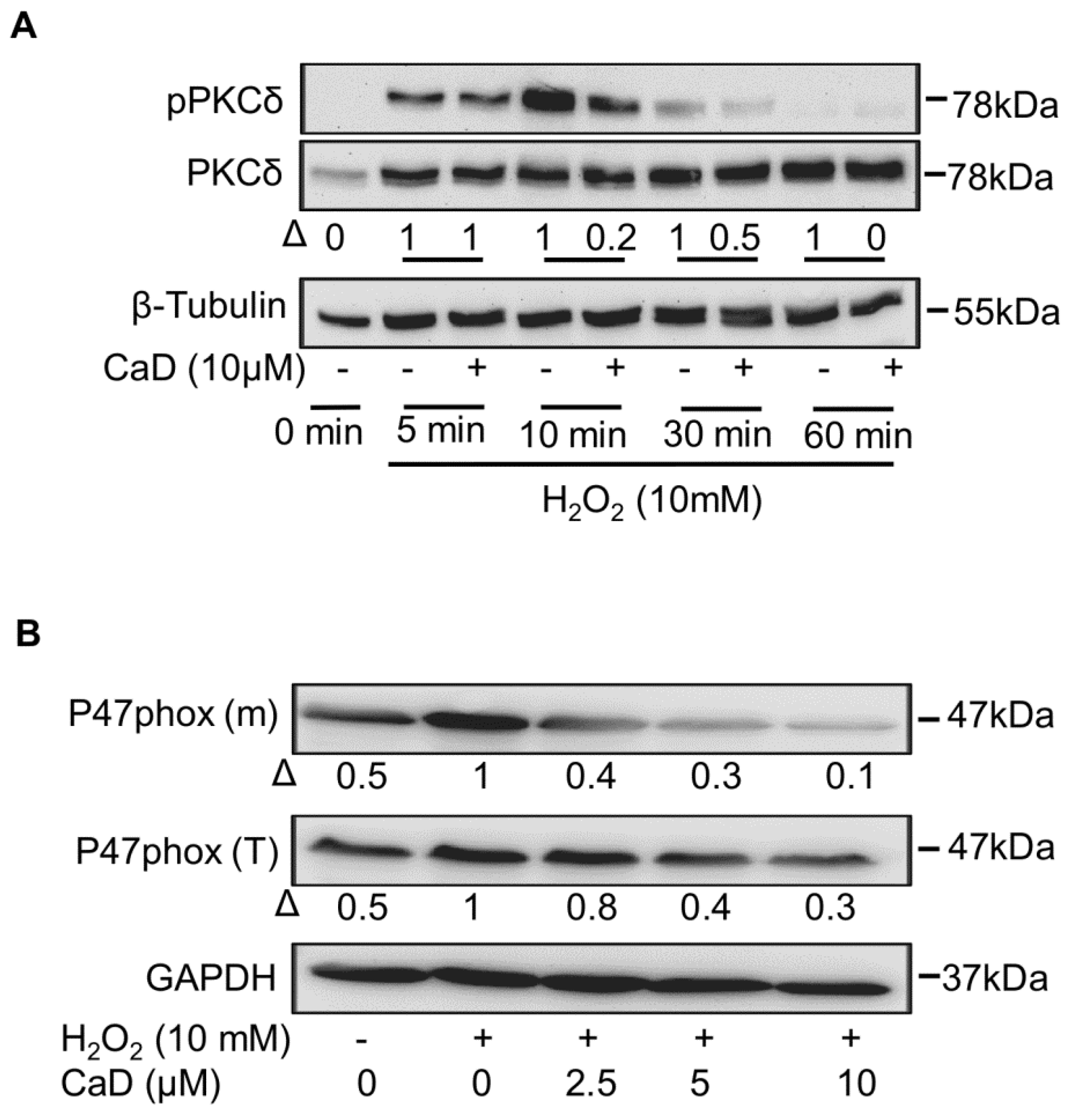
3.6. CaD Inhibits Monocyte-to-Macrophage Differentiation and Inflammation via PKCδ, MAPK Pathway
3.7. NF-kB Is Involved in CaD-Mediated Inhibition of Monocyte-to-Macrophage Differentiation and Inflammation
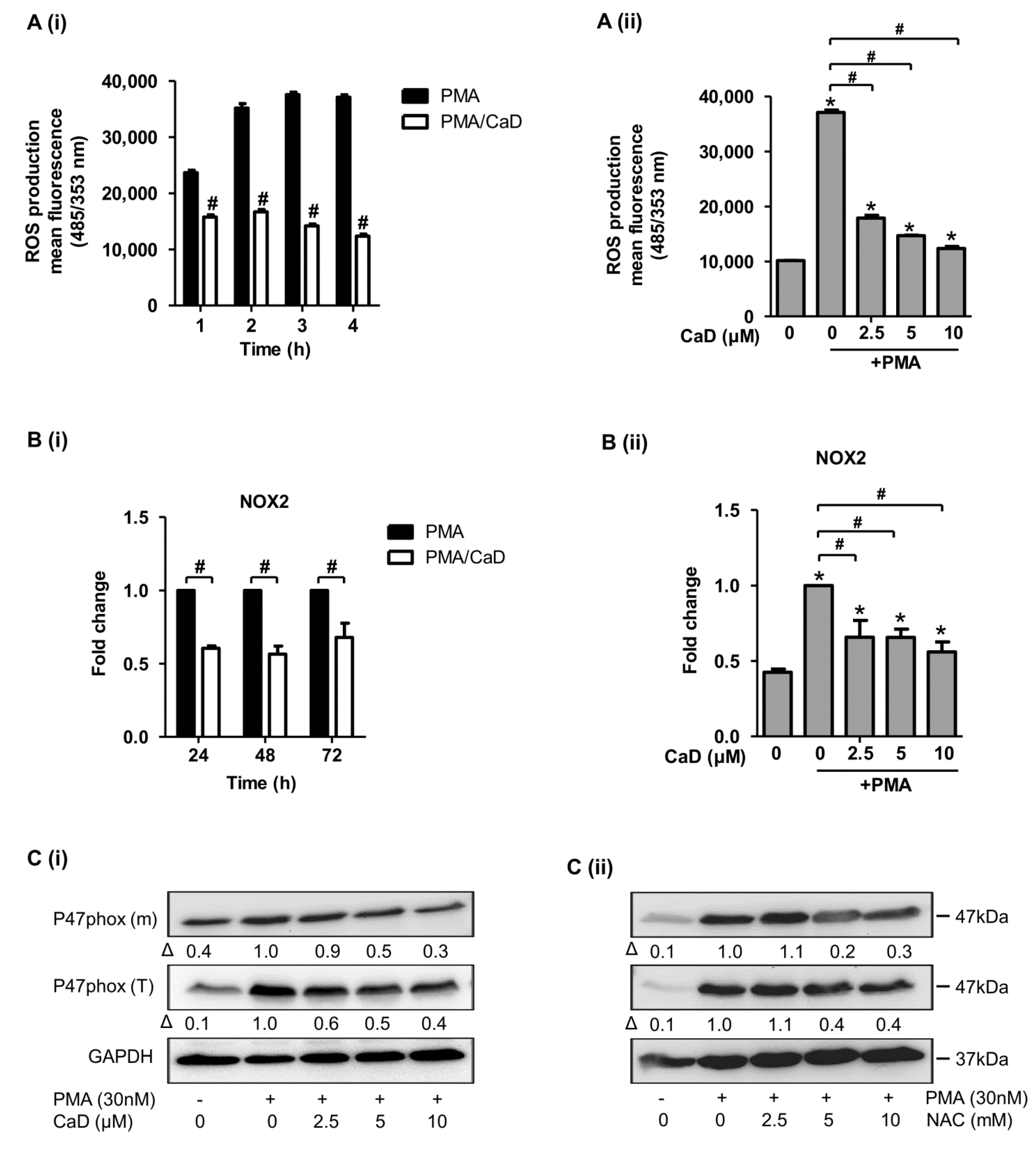
3.8. Effect of CaD on NADPH Oxidase Activation
3.9. Proximal Signaling Mediator under CaD Treatment
3.10. CaD Inhibits PKCδ via Its Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermansson, C.; Lundqvist, A.; Magnusson, L.U.; Ullström, C.; Bergström, G.; Hultén, L.M. Macrophage CD14 expression in human carotid plaques is associated with complicated lesions, correlates with thrombosis, and is reduced by angiotensin receptor blocker treatment. Int. Immunopharmacol. 2014, 22, 318–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Xia, Y.; Hu, B. Infection and atherosclerosis: TLR-dependent pathways. Cell. Mol. Life Sci. 2020, 77, 2751–2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, S.; Zheng, F.; Zhang, W.; Wang, D.; Xing, Q. Relationship between toll-like receptor 4 levels in aorta and severity of atherosclerosis. J. Int. Med. Res. 2014, 42, 958–965. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Choksi, S.; Qu, J.; Jang, J.; Choe, M.; Banfi, B.; Engelhardt, J.; Liu, Z.-G. NADPH Oxidases Are Essential for Macrophage Differentiation. J. Biol. Chem. 2016, 291, 20030–20041. [Google Scholar] [CrossRef] [Green Version]
- Moss, J.W.; Ramji, D.P. Cytokines: Roles in atherosclerosis disease progression and potential therapeutic targets. Future Med. Chem. 2016, 8, 1317–1330. [Google Scholar] [CrossRef] [Green Version]
- Dollery, C.M.; Libby, P. Atherosclerosis and proteinase activation. Cardiovasc. Res. 2006, 69, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Ezhov, M.; Safarova, M.; Afanasieva, O.; Mitroshkin, M.; Matchin, Y.; Pokrovsky, S. Matrix Metalloproteinase 9 as a Predictor of Coronary Atherosclerotic Plaque Instability in Stable Coronary Heart Disease Patients with Elevated Lipoprotein(a) Levels. Biomolecules 2019, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Quesada, I.; Lucero, A.; Amaya, C.; Meijles, D.; Cifuentes, M.; Pagano, P.; Castro, C. Selective inactivation of NADPH oxidase 2 causes regression of vascularization and the size and stability of atherosclerotic plaques. Atherosclerosis 2015, 242, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Cannizzo, B.; Quesada, I.; Militello, R.; Amaya, C.; Miatello, R.; Cruzado, M.; Castro, C. Tempol attenuates atherosclerosis associated with metabolic syndrome via decreased vascular inflammation and NADPH-2 oxidase expression. Free Radic. Res. 2014, 48, 526–533. [Google Scholar] [CrossRef]
- Leal, E.C.; Martins, J.; Voabil, P.; Liberal, J.; Chiavaroli, C.; Bauer, J.; Cunha-Vaz, J.; Ambrósio, A.F. Calcium Dobesilate Inhibits the Alterations in Tight Junction Proteins and Leukocyte Adhesion to Retinal Endothelial Cells Induced by Diabetes. Diabetes 2010, 59, 2637–2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voabil, P.; Liberal, J.; Leal, E.C.; Bauer, J.; Cunha-Vaz, J.; Santiago, A.R.; Ambrósio, A.F. Calcium Dobesilate Is Protective against Inflammation and Oxidative/Nitrosative Stress in the Retina of a Type 1 Diabetic Rat Model. Ophthalmic Res. 2017, 58, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, P.; Solà-Adell, C.; Hernández, C.; García-Ramírez, M.; Sampedro, J.; Simó-Servat, O.; Valeri, M.; Pasquali, C.; Simó, R. Calcium dobesilate prevents the oxidative stress and inflammation induced by diabetes in the retina of db/db mice. J. Diabetes Its Complicat. 2017, 31, 1481–1490. [Google Scholar] [CrossRef]
- Njau, F.; Shushakova, N.; Schenk, H.; Wulfmeyer, V.C.; Bollin, R.; Menne, J.; Haller, H. Calcium dobesilate reduces VEGF signaling by interfering with heparan sulfate binding site and protects from vascular complications in diabetic mice. PLoS ONE 2020, 15, e0218494. [Google Scholar] [CrossRef] [Green Version]
- Graber, R.; Farine, J.-C.; Losa, G.A. Calcium Dobesilate protects human peripheral blood mononuclear cells from oxidation and apoptosis. Apoptosis 1998, 3, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Piller, N.B. Assessment of the anti-inflammatory action of calcium dobesilate. Effect on macrophages attaching to subcutaneously implanted coverslips in guinea pigs. Arzneimittelforschung 1990, 40, 698–700. [Google Scholar] [PubMed]
- He, Z.; Chen, J.; Zhu, X.; An, S.; Dong, X.; Yu, J.; Zhang, S.; Wu, Y.; Li, G.; Zhang, Y.; et al. NLRP3 Inflammasome Activation Mediates Zika Virus–Associated Inflammation. J. Infect. Dis. 2018, 217, 1942–1951. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, E.-J.; Suk, K.; Lee, W.-H. Stimulation of FasL Induces Production of Proinflammatory Mediators Through Activation of Mitogen-Activated Protein Kinases and Nuclear Factor-κB in THP-1 Cells. Inflammation 2012, 35, 1–10. [Google Scholar] [CrossRef]
- Bai, X.; Feldman, N.E.; Chmura, K.; Ovrutsky, A.R.; Su, W.-L.; Griffin, L.; Pyeon, D.; McGibney, M.T.; Strand, M.; Numata, M.; et al. Inhibition of Nuclear Factor-Kappa B Activation Decreases Survival of Mycobacterium tuberculosis in Human Macrophages. PLoS ONE 2013, 8, e61925. [Google Scholar] [CrossRef] [Green Version]
- Hattori, T.; Watanabe-Takahashi, M.; Ohoka, N.; Hamabata, T.; Furukawa, K.; Nishikawa, K.; Naito, M. Proteasome inhibitors prevent cell death and prolong survival of mice challenged by Shiga toxin. FEBS Open Biol. 2015, 5, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Malinin, N.; Meller, J.; Ma, Y.; West, X.Z.; Bledzka, K.; Qin, J.; Podrez, E.A.; Byzova, T.V. Regulation of Cell Adhesion and Migration by Kindlin-3 Cleavage by Calpain. J. Biol. Chem. 2012, 287, 40012–40020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Lee, S.W.; Kim, H.Y.; Lee, S.Y.; Lee, W.S.; Hong, K.W.; Kim, C.D. SIRT1 inhibits differentiation of monocytes to macrophages: Amelioration of synovial inflammation in rheumatoid arthritis. J. Mol. Med. 2016, 94, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Niki, Y.; Kawasaki, T.; Takeda, Y.; Ikegami, H.; Toyama, Y.; Miyamoto, T. IL-32-PAR2 axis is an innate immunity sensor providing alternative signaling for LPS-TRIF axis. Sci. Rep. 2013, 3, 2960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasu, M.R.; Devaraj, S.; Zhao, L.; Hwang, D.H.; Jialal, I. High Glucose Induces Toll-Like Receptor Expression in Human Monocytes: Mechanism of Activation. Diabetes 2008, 57, 3090–3098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sindhu, S.; Akhter, N.; Kochumon, S.; Thomas, R.; Wilson, A.; Shenouda, S.; Tuomilehto, J.; Ahmad, R. Increased Expression of the Innate Immune Receptor TLR10 in Obesity and Type-2 Diabetes: Association with ROS-Mediated Oxidative Stress. Cell. Physiol. Biochem. 2018, 45, 572–590. [Google Scholar] [CrossRef]
- Toth, M.; Sohail, A.; Fridman, R. Assessment of Gelatinases (MMP-2 and MMP-9) by Gelatin Zymography. In Metastasis Research Protocols; Humana Press: Totowa, NJ, USA, 2012; pp. 163–174. [Google Scholar]
- Schwende, H.; Fitzke, E.; Ambs, P.; Dieter, P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukoc. Biol. 1996, 59, 555–561. [Google Scholar] [CrossRef]
- Ding, Y.; Subramanian, S.; Montes, V.N.; Goodspeed, L.; Wang, S.; Han, C.; TeresaIII, A.S.; Kim, J.; O’Brien, K.D.; Chait, A. Toll-Like Receptor 4 Deficiency Decreases Atherosclerosis But Does Not Protect Against Inflammation in Obese Low-Density Lipoprotein Receptor–Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1596–1604. [Google Scholar] [CrossRef] [Green Version]
- Perera, P.Y.; Vogel, S.N.; Detore, G.R.; Haziot, A.; Goyert, S. CD14-dependent and CD14-independent signaling pathways in murine macrophages from normal and CD14 knockout mice stimulated with lipopolysaccharide or taxol. J. Immunol. 1997, 158, 158. [Google Scholar]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential Roles of TLR2 and TLR4 in Recognition of Gram-Negative and Gram-Positive Bacterial Cell Wall Components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar]
- Angulo, J.; Cuevas, P.; Cuevas, B.; El Youssef, M.; Fernández, A.; Martínez-Salamanca, E.; González-Corrochano, R.; Giménez-Gallego, G. Diacetyloxyl derivatization of the fibroblast growth factor inhibitor dobesilate enhances its anti-inflammatory, anti-angiogenic and anti-tumoral activities. J. Transl. Med. 2015, 13, 48. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yan, D.; Zou, Y.; Zhang, T.; Lin, G.; Xiao, J. A herbal extract treats type 2 diabetes mellitus effectively by down-regulating expression of CD14. Int. J. Clin. Exp. Med. 2019, 2, 1535–1544. [Google Scholar]
- Richter, E.; Ventz, K.; Harms, M.; Mostertz, J.; Hochgräfe, F. Induction of Macrophage Function in Human THP-1 Cells Is Associated with Rewiring of MAPK Signaling and Activation of MAP3K7 (TAK1) Protein Kinase. Front. Cell Dev. Biol. 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontny, E.; Kurowska, M.; Szczepańska, K.; Maśliński, W. Rottlerin, a PKC isozyme-selective inhibitor, affects signaling events and cytokine production in human monocytes. J. Leukoc. Biol. 2000, 67, 249–258. [Google Scholar] [CrossRef]
- Noh, K.T.; Son, K.H.; Jung, I.D.; Kang, H.K.; Hwang, S.A.; Lee, W.S.; You, J.C.; Park, Y.-M. Protein Kinase C δ (PKCδ)-Extracellular Signal-regulated Kinase 1/2 (ERK1/2) Signaling Cascade Regulates Glycogen Synthase Kinase-3 (GSK-3) Inhibition-mediated Interleukin-10 (IL-10) Expression in Lipopolysaccharide (LPS)-induced Endotoxemia. J. Biol. Chem. 2012, 287, 14226–14233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solà-Adell, C.; Bogdanov, P.; Hernández, C.; Sampedro, J.; Valeri, M.; Garcia-Ramírez, M.; Pasquali, C.; Simó, R. Calcium Dobesilate Prevents Neurodegeneration and Vascular Leakage in Experimental Diabetes. Curr. Eye Res. 2017, 42, 1273–1286. [Google Scholar] [CrossRef]
- Dasu, M.R.; Devaraj, S.; Jialal, I. High glucose induces IL-1β expression in human monocytes: Mechanistic insights. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E337–E346. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Han, Z.; Tian, L.; Chen, K.; Fan, Y.; Ye, B.; Huang, W.; Wang, C.; Huang, Z. Curcumin inhibits EMMPRIN and MMP-9 expression through AMPK-MAPK and PKC signaling in PMA induced macrophages. J. Transl. Med. 2014, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Peroval, M.Y.; Boyd, A.C.; Young, J.R.; Smith, A.L. A Critical Role for MAPK Signalling Pathways in the Transcriptional Regulation of Toll Like Receptors. PLoS ONE 2013, 8, e51243. [Google Scholar] [CrossRef] [Green Version]
- Tong, W.; Chen, X.; Song, X.; Chen, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, L.; He, C.; Liang, X.; et al. Resveratrol inhibits LPS-induced inflammation through suppressing the signaling cascades of TLR4-NF-κB/MAPKs/IRF3. Exp. Ther. Med. 2019, 19, 1824–1834. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Qi, C.; Li, S.; Shao, X.; Ni, Z. Investigation of the Mechanism Underlying Calcium Dobesilate-Mediated Improvement of Endothelial Dysfunction and Inflammation Caused by High Glucose. Mediat. Inflamm. 2019, 2019, 9893682. [Google Scholar] [CrossRef] [PubMed]
- Traore, K.; Trush, M.A.; George, M.; Spannhake, E.W.; Anderson, W.; Asseffa, A. Signal transduction of phorbol 12-myristate 13-acetate (PMA)-induced growth inhibition of human monocytic leukemia THP-1 cells is reactive oxygen dependent. Leuk. Res. 2005, 29, 863–879. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-Y.; Wen, M.-H. Lipopolysaccharide-mediated Reactive Oxygen Species and Signal Transduction in the Regulation of Interleukin-1 Gene Expression. J. Biol. Chem. 2002, 277, 22131–22139. [Google Scholar] [CrossRef] [Green Version]
- Qiao, H.; May, J.M. Macrophage differentiation increases expression of the ascorbate transporter (SVCT2). Free Radic. Biol. Med. 2009, 46, 1221–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.-S.; Lin, Y.-W.; Huang, C.-Y.; Tsai, Y.-T.; Tsao, N.-W.; Lin, C.-S.; Jeng, H.; Lin, F.-Y.; Shih, C.-M.; Shih, C.-C. Thrombomodulin regulates monocye differentiation via PKCδ and ERK1/2 pathway in vitro and in atherosclerotic artery. Sci. Rep. 2016, 6, 38421. [Google Scholar] [CrossRef] [Green Version]
- Newton, A.C. Protein kinase C as a tumor suppressor. Semin. Cancer Biol. 2018, 48, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, D.; Hornia, A.; Devonish, W.; Pagano, M.; Foster, D.A. Activation of Protein Kinase C Triggers Its Ubiquitination and Degradation. Mol. Cell. Biol. 1998, 18, 839–845. [Google Scholar] [CrossRef] [Green Version]
- Hong, D.-H.; Huan, J.; Ou, B.-R.; Yeh, J.-Y.; Saido, T.C.; Cheeke, P.; Forsberg, N.E. Protein kinase C isoforms in muscle cells and their regulation by phorbol ester and calpain. Biochim. Biophys. Acta Mol. Cell Res. 1995, 1267, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Konishi, H.; Yamauchi, E.; Taniguchi, H.; Yamamoto, T.; Matsuzaki, H.; Takemura, Y.; Ohmae, K.; Kikkawa, U.; Nishizuka, Y. Phosphorylation sites of protein kinase C δ in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc. Natl. Acad. Sci. USA 2001, 98, 6587–6592. [Google Scholar] [CrossRef] [Green Version]
- Akhter, N.; Madhoun, A.; Arefanian, H.; Wilson, A.; Kochumon, S.; Thomas, R.; Shenouda, S.; Al-Mulla, F.; Ahmad, R.; Sindhu, S. Oxidative Stress Induces Expression of the Toll-Like Receptors (TLRs) 2 and 4 in the Human Peripheral Blood Mononuclear Cells: Implications for Metabolic Inflammation. Cell. Physiol. Biochem. 2019, 53, 1–18. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Z.; Lei, Z.; Lei, P. CD14: Biology and role in the pathogenesis of disease. Cytokine Growth Factor Rev. 2019, 48, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Sun, D. Calcium Dobesilate and Micro-vascular diseases. Life Sci. 2019, 221, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Szmitko, P.E.; Wang, C.H.; Weisel, R.D.; Jeffries, G.A.; Anderson, T.J.; Verma, S. Biomarkers of Vascular Disease Linking Inflammation to Endothelial Activation Part II. Circulation 2003, 108, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Luttun, A.; Lutgens, E.; Manderveld, A.; Maris, K.; Collen, D.; Carmeliet, P.; Moons, L. Loss of Matrix Metalloproteinase-9 or Matrix Metalloproteinase-12 Protects Apolipoprotein E–Deficient Mice Against Atherosclerotic Media Destruction but Differentially Affects Plaque Growth. Circulation 2004, 109, 1408–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, C.; Zhu, F.; Jin, X. The effect of calcium dobesilate on the formation of atherosclerotic plaques and its mechanism. J. Wezhou Med. Univ. 2020, 50, 387–390. [Google Scholar]
- Phorbol Ester-Induced Myeloid Differentiation Is Mediated by Protein Kinase C-Alpha and -Delta and Not by Protein Kinase C-Beta II, -Epsilon, -Zeta, and -Eta—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/8376369/ (accessed on 25 October 2021).
- Activation of Beta-Isozyme of Protein Kinase C (PKC Beta) Is Necessary and Sufficient for Phorbol Ester-Induced Differentiation of HL-60 Promyelocytes. Studies with PKC Beta-Defective PET mutant—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/8308000/ (accessed on 25 October 2021).
- Chen, B.-C.; Lin, W.W. PKC- and ERK-dependent activation of IκB kinase by lipopolysaccharide in macrophages: Enhancement by P2Y receptor-mediated CaMK activation. Br. J. Pharmacol. 2001, 134, 1055–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, F.S.; Peters, R.T.; Dang, L.C.; Maniatis, T. MEKK1 activates both IkappaB kinase alpha and IkappaB kinase beta. Proc. Natl. Acad. Sci. USA 1998, 95, 9319–9324. [Google Scholar] [CrossRef] [Green Version]
- Das, J.; Ramani, R.; Suraju, M.O. Polyphenol compounds and PKC signaling. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 2107–2121. [Google Scholar] [CrossRef] [Green Version]
- Kyaw, M.; Yoshizumi, M.; Tsuchiya, K.; Kirima, K.; Tamaki, T. Antioxidants Inhibit JNK and p38 MAPK Activation but not ERK 1/2 Activation by Angiotensin II in Rat Aortic Smooth Muscle Cells. Hypertens. Res. 2001, 24, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Kubo-Murai, M.; Hazeki, K.; Sukenobu, N.; Yoshikawa, K.; Nigorikawa, K.; Inoue, K.; Yamamoto, T.; Matsumoto, M.; Seya, T.; Inoue, N.; et al. Protein kinase Cδ binds TIRAP/Mal to participate in TLR signaling. Mol. Immunol. 2007, 44, 2257–2264. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.-H.; Lim, J.H.; Kim, Y.-H.; Suh, S.-I.; Min, D.S.; Chang, J.-S.; Lee, Y.H.; Park, J.-W.; Kwon, T.K. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC δ signal transduction. Oncogene 2004, 23, 1845–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-G.; Oh, G.-T. The role of peroxidases in the pathogenesis of atherosclerosis. BMB Rep. 2011, 44, 497–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Li, L.; Sawamura, T.; Renier, G. Glucose Enhances Human Macrophage LOX-1 Expression. Circ. Res. 2004, 94, 892–901. [Google Scholar] [CrossRef]
- Brunet, J.; Farine, J.C.; Garay, R.P.; Hannaert, P. In vitro antioxidant properties of calcium dobesilate. Fundam. Clin. Pharmacol. 1998, 12, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Farine, J.-C.; Garay, R.P.; Hannaert, P. Angioprotective action of calcium dobesilate against reactive oxygen species-induced capillary permeability in the rat. Eur. J. Pharmacol. 1998, 358, 213–220. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, J.-G.; Cho, W.-S.; Cho, K.-H.; Sakong, J.; Kim, J.-R.; Chin, B.-R.; Baek, S.-H. Role of NADPH oxidase-2 in lipopolysaccharide-induced matrix metalloproteinase expression and cell migration. Immunol. Cell Biol. 2009, 88, 197–204. [Google Scholar] [CrossRef]
- Sorescu, D.; Weiss, D.; Lassègue, B.; Clempus, R.E.; Szöcs, K.; Sorescu, G.P.; Valppu, L.; Quinn, M.; Lambeth, J.D.; Vega, J.D.; et al. Superoxide Production and Expression of Nox Family Proteins in Human Atherosclerosis. Circulation 2002, 105, 1429–1435. [Google Scholar] [CrossRef] [Green Version]
- El Benna, J.; Faust, L.R.P.; Johnson, J.L.; Babior, B.M. Phosphorylation of the Respiratory Burst Oxidase Subunit p47phox as Determined by Two-dimensional Phosphopeptide Mapping: Phosphorylation by protein kinase C, protein kinase A, and a mitogen-activated protein kinase. J. Biol. Chem. 1996, 271, 6374–6378. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-L.; Chen, P.-Y.; Wu, M.-J.; Tai, M.-H.; Ho, C.-T.; Yen, J.-H. Curcuminoids Modulate the PKCδ/NADPH Oxidase/Reactive Oxygen Species Signaling Pathway and Suppress Matrix Invasion during Monocyte–Macrophage Differentiation. J. Agric. Food Chem. 2015, 63, 8838–8848. [Google Scholar] [CrossRef]
- Lee, I.-T.; Shih, R.-H.; Lin, C.-C.; Chen, J.-T.; Yang, C.-M. Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1 expression induced by lipopolysaccharide in human renal mesangial cells. Cell Commun. Signal. 2012, 10, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batchuluun, B.; Inoguchi, T.; Sonoda, N.; Sasaki, S.; Inoue, T.; Fujimura, Y.; Miura, D.; Takayanagi, R. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis 2014, 232, 156–164. [Google Scholar] [CrossRef]
- Vasamsetti, S.B.; Karnewar, S.; Kanugula, A.K.; Thatipalli, A.R.; Kumar, J.M.; Kotamraju, S. Metformin Inhibits Monocyte-to-Macrophage Differentiation via AMPK-Mediated Inhibition of STAT3 Activation: Potential Role in Atherosclerosis. Diabetes 2015, 64, 2028–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry-Lane, P.A.; Patterson, C.; Van Der Merwe, M.; Hu, Z.; Holland, S.M.; Yeh, E.T.; Runge, M.S. p47phox is required for atherosclerotic lesion progression in Apo−/− mice. J. Clin. Investig. 2001, 108, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-P.; Standiford, T.J.; Rahman, A.; Newstead, M.; Holland, S.M.; Dinauer, M.C.; Liu, Q.-H.; Malik, A.B. Role of NADPH Oxidase in the Mechanism of Lung Neutrophil Sequestration and Microvessel Injury Induced by Gram-Negative Sepsis: Studies in p47phox−/− and gp91phox−/− Mice. J. Immunol. 2002, 168, 3974–3982. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.C.; Fang, F.; Zhou, J.; Koulajian, K.; Yang, S.; Lam, L.; Reich, H.N.; John, R.; Herzenberg, A.M.; Giacca, A.; et al. Deletion of p47 phox attenuates the progression of diabetic nephropathy and reduces the severity of diabetes in the Akita mouse. Diabetologia 2012, 55, 2522–2532. [Google Scholar] [CrossRef] [Green Version]
- Bey, E.A.; Xu, B.; Bhattacharjee, A.; Oldfield, C.M.; Zhao, X.; Li, Q.; Subbulakshmi, V.; Feldman, G.M.; Wientjes, F.B.; Cathcart, M.K. Protein Kinase Cδ Is Required for p47phox Phosphorylation and Translocation in Activated Human Monocytes. J. Immunol. 2004, 173, 5730–5738. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.B.; Yu, M.R.; Song, J.S.; Ha, H. Reactive oxygen species amplify protein kinase C signaling in high glucose-induced fibronectin expression by human peritoneal mesothelial cells. Kidney Int. 2004, 65, 1170–1179. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.-S.; Tsai, R.K.; Chang, C.H.; Wang, S.; Wu, J.-R.; Chang, Y.-X. Reactive Oxygen Species Mediated Sustained Activation of Protein Kinase C α and Extracellular Signal-Regulated Kinase for Migration of Human Hepatoma Cell Hepg2. Mol. Cancer Res. 2006, 4, 747–758. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-L.; Chan, P.-C.; Wang, S.-H.; Pan, Y.-R.; Chen, H.-C. Elevated expression of protein kinase Cδ induces cell scattering upon serum deprivation. J. Cell Sci. 2010, 123, 2901–2913. [Google Scholar] [CrossRef] [Green Version]
- Lien, C.-F.; Chen, S.-J.; Tsai, M.-C.; Lin, C.-S. Potential Role of Protein Kinase C in the Pathophysiology of Diabetes-Associated Atherosclerosis. Front. Pharmacol. 2021, 12, 1674. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Cho, M.; Burrows, J.; Polverini, P.; Leibovich, S.J. Inhibition of production of monocyte/macrophage-derived angiogenic activity by oxygen free-radical scavengers. Cell Biol. Int. Rep. 1992, 16, 415–425. [Google Scholar] [CrossRef]
- Nespereira, B.; Pérez-Ilzarbe, M.; Fernández, P.; Fuentes, A.M.; Páramo, J.A.; Rodríguez, J.A. Vitamins C and E downregulate vascular VEGF and VEGFR-2 expression in apolipoprotein-E-deficient mice. Atherosclerosis 2003, 171, 67–73. [Google Scholar] [CrossRef]
- Oak, M.-H.; El Bedoui, J.; Schini-Kerth, V.B. Antiangiogenic properties of natural polyphenols from red wine and green tea. J. Nutr. Biochem. 2005, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Njau, F.; Haller, H. Calcium Dobesilate Modulates PKCδ-NADPH Oxidase- MAPK-NF-κB Signaling Pathway to Reduce CD14, TLR4, and MMP9 Expression during Monocyte-to-Macrophage Differentiation: Potential Therapeutic Implications for Atherosclerosis. Antioxidants 2021, 10, 1798. https://doi.org/10.3390/antiox10111798
Njau F, Haller H. Calcium Dobesilate Modulates PKCδ-NADPH Oxidase- MAPK-NF-κB Signaling Pathway to Reduce CD14, TLR4, and MMP9 Expression during Monocyte-to-Macrophage Differentiation: Potential Therapeutic Implications for Atherosclerosis. Antioxidants. 2021; 10(11):1798. https://doi.org/10.3390/antiox10111798
Chicago/Turabian StyleNjau, Florence, and Hermann Haller. 2021. "Calcium Dobesilate Modulates PKCδ-NADPH Oxidase- MAPK-NF-κB Signaling Pathway to Reduce CD14, TLR4, and MMP9 Expression during Monocyte-to-Macrophage Differentiation: Potential Therapeutic Implications for Atherosclerosis" Antioxidants 10, no. 11: 1798. https://doi.org/10.3390/antiox10111798




