Integration of Choline Chloride-Based Natural Deep Eutectic Solvents and Macroporous Resin for Green Production of Enriched Oil Palm Flavonoids as Natural Wound Healing Agents
Abstract
1. Introduction
2. Materials and Methods
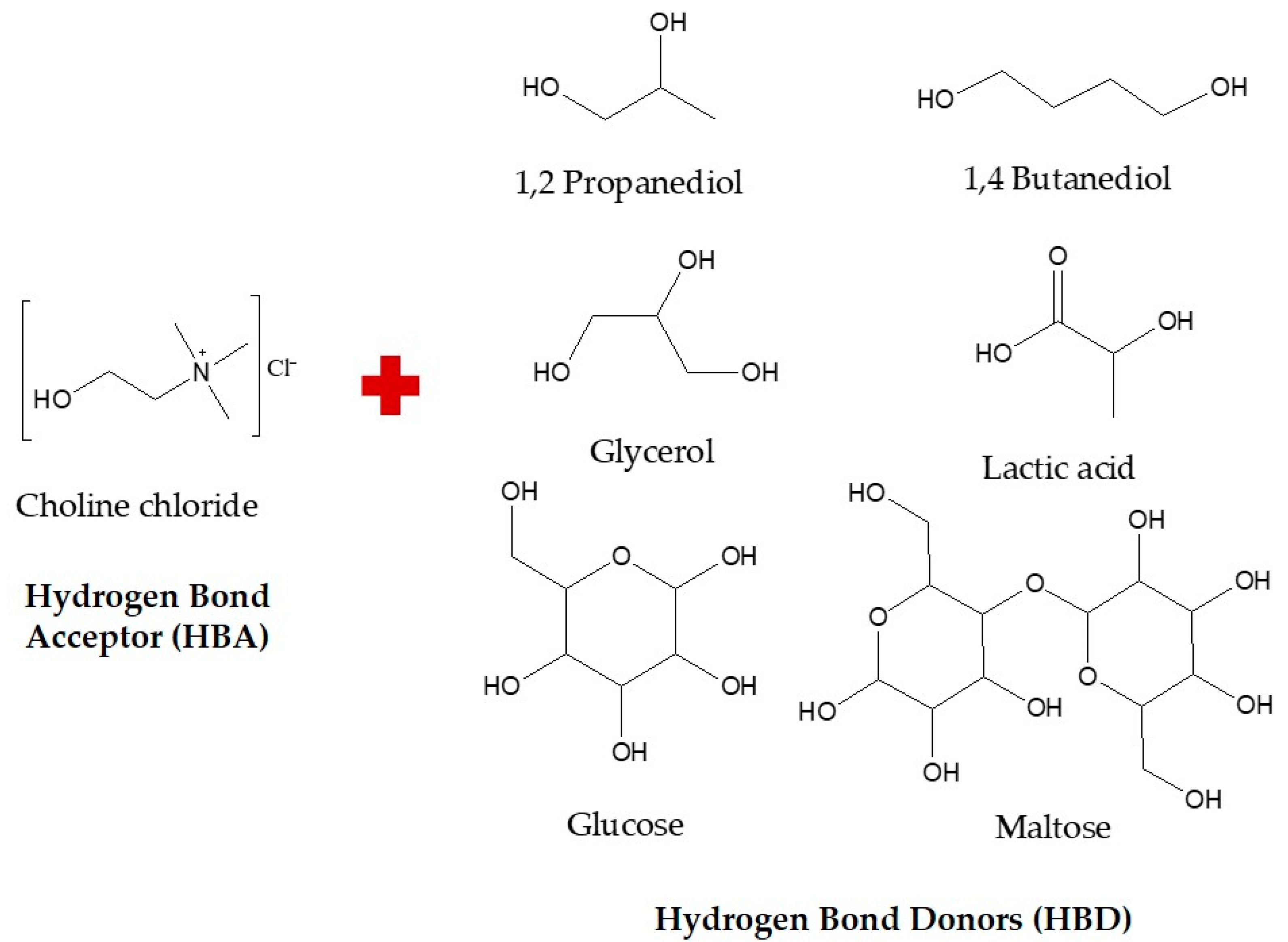
2.1. Chemicals, Reagents, and Equipment Used in This Study
2.2. Sample Preparation
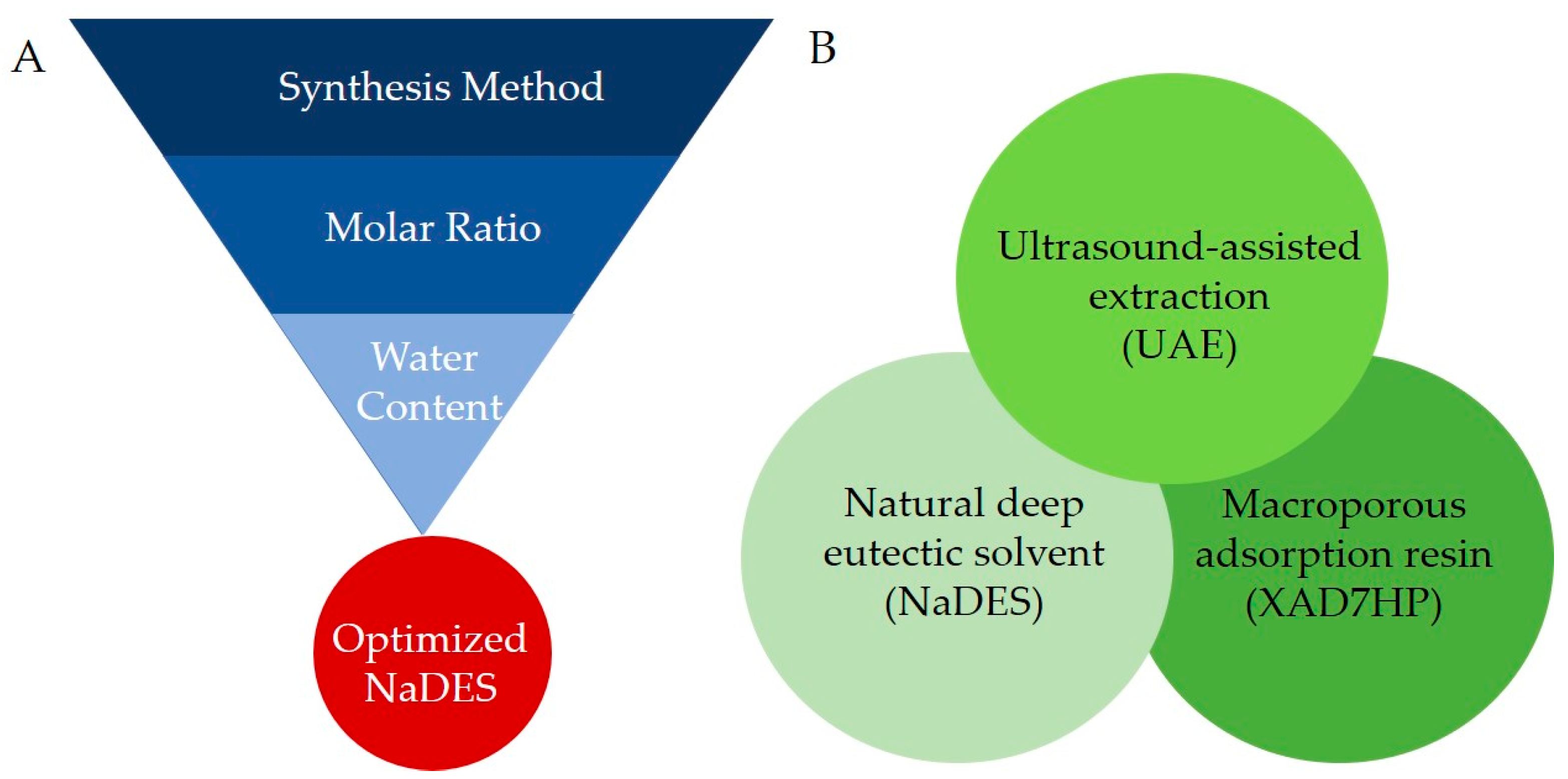
2.3. Preparation and Optimization of NaDES Formulation
2.3.1. Method of Synthesis
2.3.2. Molar Ratios
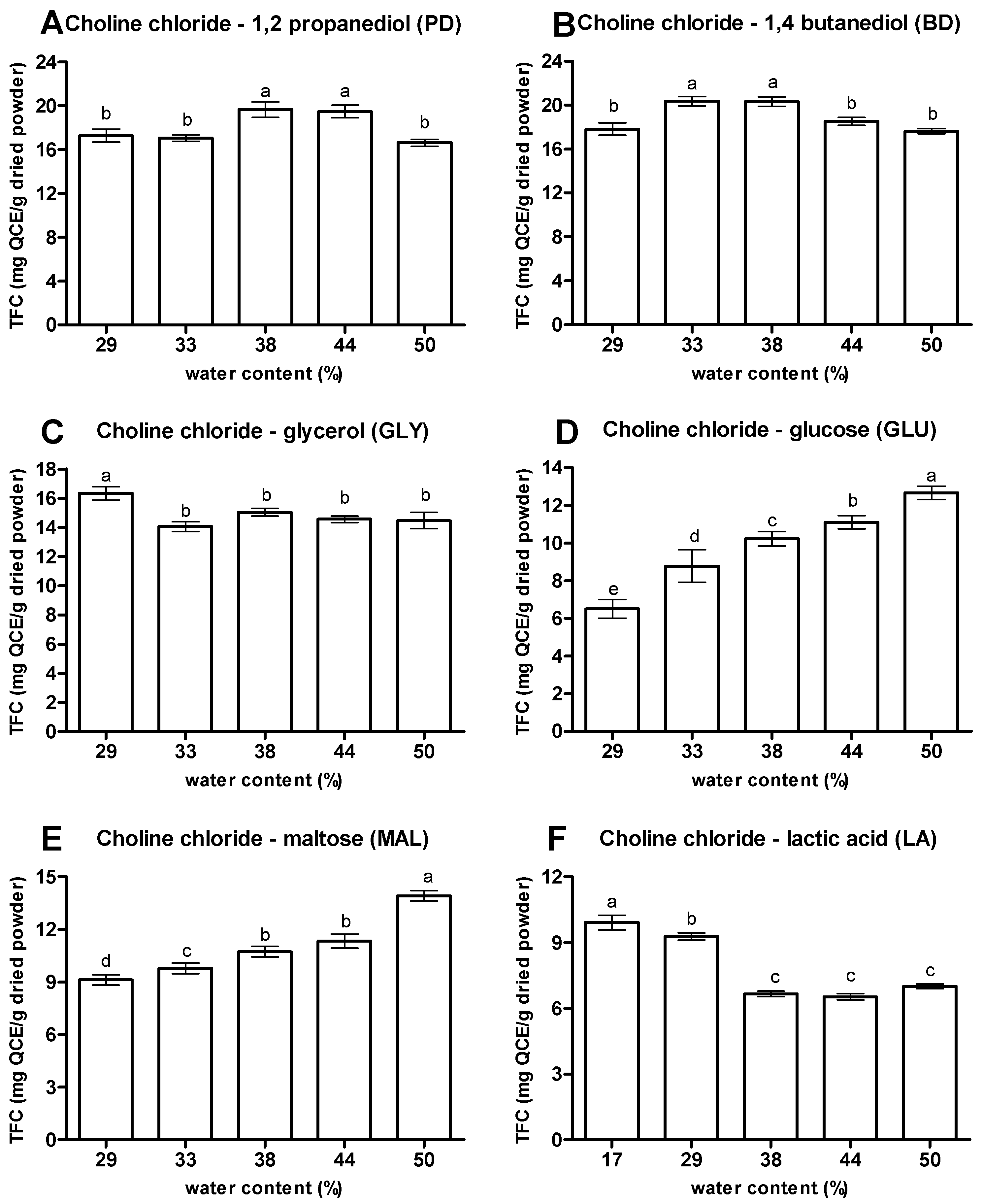
2.3.3. Water Contents
2.4. Ultrasound-Assisted Extraction of OPL with Optimized NaDES
2.5. Analysis of Compound Recovery from Macroporous Resin
2.6. Determination of Total Phenolic Content
2.7. Determination of Total Flavonoid Content
2.8. Determination of Antioxidant Free Radical Scavenging Activity
2.9. Determination of Cell Proliferation and Migration Activity
2.10. UHPLC-UV/PDA-MS/MS Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Choline Chloride-Based NaDES
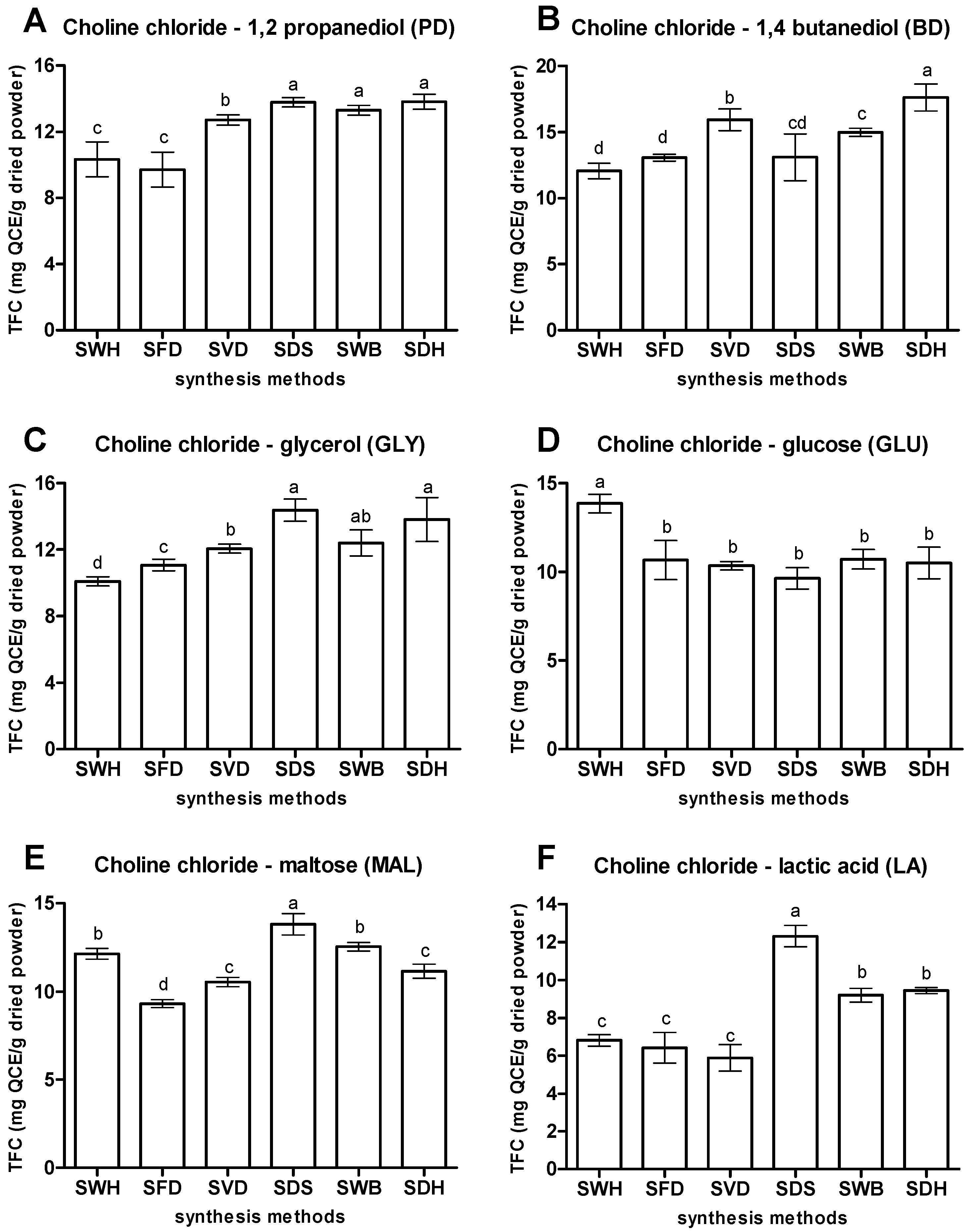
3.1.1. Method of Synthesis
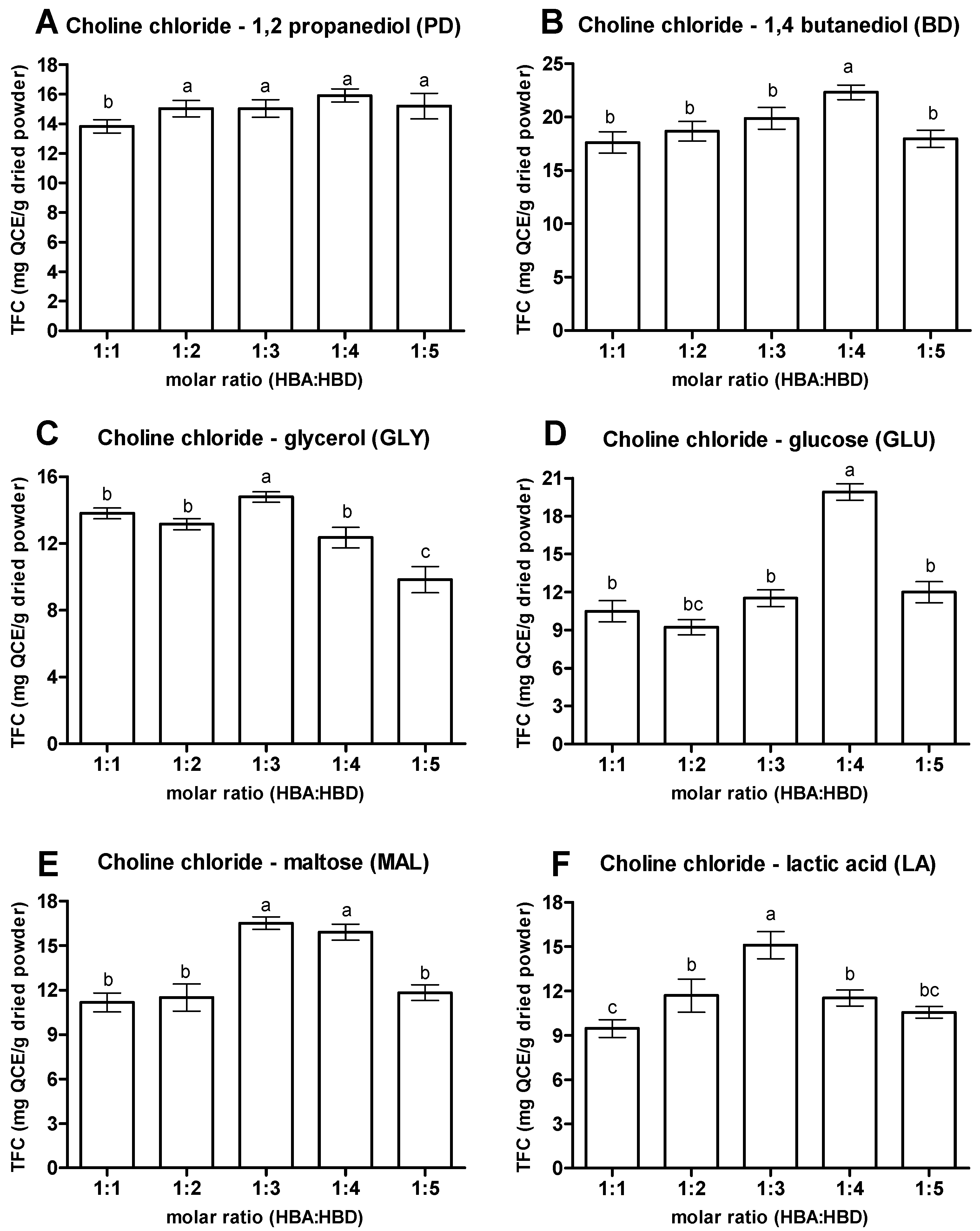
3.1.2. Molar Ratios
3.1.3. Water Contents
3.2. Identification and Quantification of Flavonoids in OPL–NaDES Extracts
3.3. Antioxidant Free Radical Scavenging Activities of NaDES Extracts
3.4. Cell Viablity of OPL–NaDES Extracts
3.5. Cell Proliferation and Migration Activity of OPL–NaDES Extracts
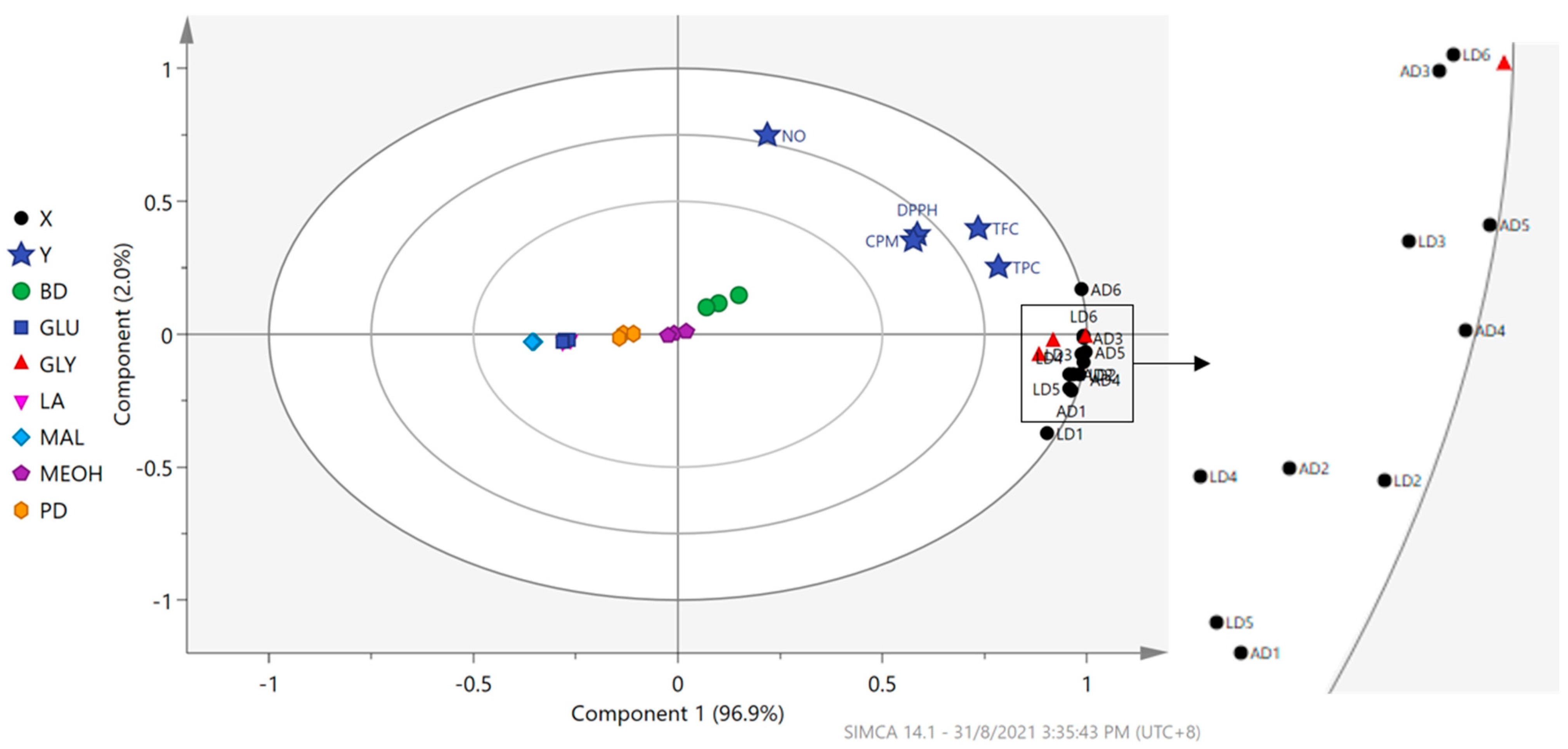
3.6. Correlation of Flavonoids in OPL–NaDES Extracts with Wound Healing Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Dai, Y.; Spronsen, J.; Van Witkamp, G. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Comptes Rendus Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Redovniković, I.R.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT—Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Bentley, J.; Olsen, E.K.; Moore, J.P.; Farrant, J.M. The phenolic profile extracted from the desiccation-tolerant medicinal shrub Myrothamnus flabellifolia using natural deep eutectic solvents varies according to the solvation conditions. Phytochemistry 2020, 173, 112323. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-assisted extraction and natural deep eutectic solvents combination: A green strategy to improve the recovery of phenolic compounds from Lavandula pedunculata subsp. lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef]
- Ahmad, I.; Syakfanaya, A.M.; Azminah, A.; Saputri, F.C.; Mun’im, A. Optimization of betaine-sorbitol natural deep eutectic solvent-based ultrasound-assisted extraction and pancreatic lipase inhibitory activity of chlorogenic acid and caffeine content from robusta green coffee beans. Heliyon 2021, 7, e07702. [Google Scholar] [CrossRef] [PubMed]
- Sakti, A.S.; Saputri, F.C.; Mun’im, A. Optimization of choline chloride-glycerol based natural deep eutectic solvent for extraction bioactive substances from Cinnamomum burmannii barks and Caesalpinia sappan heartwoods. Heliyon 2019, 5, e02915. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Development of a method based on natural deep eutectic solvents for extraction of flavonoids from food samples. Food Anal. Methods 2018, 11, 1330–1344. [Google Scholar] [CrossRef]
- Galván D’Alessandro, L.; Vauchel, P.; Przybylski, R.; Chataigné, G.; Nikov, I.; Dimitrov, K. Integrated process extraction-adsorption for selective recovery of antioxidant phenolics from Aronia melanocarpa berries. Sep. Purif. Technol. 2013, 120, 92–101. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, X.; Peng, X.; Wang, W.; Zhao, C. Fast and green extraction and separation of main bioactive flavonoids from Radix Scutellariae. Ind. Crop. Prod. 2015, 63, 175–181. [Google Scholar] [CrossRef]
- Che Zain, M.S.; Lee, S.Y.; Sarian, M.N.; Fakurazi, S.; Shaari, K. In vitro wound healing potential of flavonoid c-glycosides from oil palm (Elaeis guineensis Jacq.) leaves on 3t3 fibroblast cells. Antioxidants 2020, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Che Zain, M.S.; Lee, S.Y.; Teo, C.Y.; Shaari, K. Adsorption and Desorption properties of total flavonoids from oil palm (Elaeis guineensis Jacq.) mature leaf on macroporous adsorption resins. Molecules 2020, 25, 778. [Google Scholar] [CrossRef] [PubMed]
- Che Zain, M.S.; Lee, S.Y.; Teo, C.Y.; Shaari, K. Adsorption/desorption characteristics and simultaneous enrichment of orientin, isoorientin, vitexin and isovitexin from hydrolyzed oil palm leaf extract using macroporous resins. Processes 2021, 9, 659. [Google Scholar] [CrossRef]
- Che Zain, M.S.; Lee, S.Y.; Mad Nasir, N.; Fakurazi, S.; Shaari, K. Metabolite characterization and correlations with antioxidant and wound healing properties of oil palm (Elaeis guineensis Jacq.) leaflets via 1H-NMR-based metabolomics approach. Molecules 2020, 25, 5636. [Google Scholar] [CrossRef]
- Tahir, N.I.; Shaari, K.; Abas, F.; Parveez, G.K.A.; Ishak, Z.; Ramli, U.S. Characterization of apigenin and luteolin derivatives from oil palm (Elaeis guineensis Jacq.) leaf using LC-ESI-MS/MS. J. Agric. Food Chem. 2012, 60, 11201–11210. [Google Scholar] [CrossRef]
- Che Zain, M.S.; Edirisinghe, S.L.; Kim, C.-H.; De Zoysa, M.; Shaari, K. Nanoemulsion of flavonoid-enriched oil palm (Elaeis guineensis Jacq.) leaf extract enhances wound healing in zebrafish. Phytomedicine Plus 2021, 1, 100124. [Google Scholar] [CrossRef]
- Soundararajan, V.; Sreenivasan, S. Antioxidant activity of Elaeis guineensis leaf extract: An alternative nutraceutical approach in impeding aging. APCBEE Procedia 2012, 2, 153–159. [Google Scholar] [CrossRef]
- Sasidharan, S.; Logeswaran, S.; Latha, L.Y. Wound healing activity of Elaeis guineensis leaf extract ointment. Int. J. Mol. Sci. 2012, 13, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef]
- Wu, W.; Yan, C.; Li, L.; Liu, Z.; Liu, S. Studies on the flavones using liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2004, 1047, 213–220. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; Mcalpine, J.B.; Lankin, D.C.; Chen, S.; Pauli, G.F. Natural Deep eutectuc solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Cui, Q.; Peng, X.; Yao, X.; Wei, Z.; Luo, M.; Wang, W.; Zhao, C.-J.; Fu, Y.-J.; Zu, Y.-G. Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep. Purif. Technol. 2015, 150, 63–72. [Google Scholar] [CrossRef]
- Alanon, M.; Ivanovic, M.; Gomez-Caravaca, A.; Arraez-Roman, D. Choline chloride derivative-based deep eutectic liquids as novel green alternative solvents for extraction of phenolic compounds from olive leaf. Arab. J. Chem. 2018, 13, 1685–1701. [Google Scholar] [CrossRef]
- Santana, A.P.R.; Mora-Vargas, J.A.; Guimarães, T.G.S.; Amaral, C.D.B.; Oliveira, A.; Gonzalez, M.H. Sustainable synthesis of natural deep eutectic solvents (NADES) by different methods. J. Mol. Liq. 2019, 293, 111452. [Google Scholar] [CrossRef]
- Khezeli, T.; Daneshfar, A.; Sahraei, R. A green ultrasonic-assisted liquid-liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of ferulic, caffeic and cinnamic acid from olive, almond, sesame and cinnamon oil. Talanta 2016, 150, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Owczarek, K.; Szczepanska, N.; Rutkowska, M.; Shyshchak, O.; Bratychak, M.; Namiesnik, J. Natural Deep Eutectic Solvents in Extraction Process. Chem. Chem. Technol. 2016, 10, 602–606. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Ferrer, M.L.; Yuste, L.; Rojo, F.; Monte, F.D. Bacteria incorporation in deep-eutectic solvents through freezedrying. Angew Chem.—Int. Ed. 2010, 49, 2158–2162. [Google Scholar] [CrossRef]
- García, A.; Rodríguez-juan, E.; Rodríguez-gutiérrez, G.; Rios, J.J.; Fernández-bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents ( DESs ). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef]
- Ferreres, F.; Gil-Izquierdo, A.; Andrade, P.B.; Valentão, P.; Tomás-Barberán, F.A. Characterization of C-glycosyl flavones O-glycosylated by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1161, 214–223. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Distribution of C- and O-glycosyl flavonoids, (3-hydroxy-3-methylglutaryl)glycosyl flavanones and furocoumarins in Citrus aurantium L. juice. Food Chem. 2011, 124, 576–582. [Google Scholar] [CrossRef]
- Figueirinha, A.; Paranhos, A.; Pérez-Alonso, J.J.; Santos-Buelga, C.; Batista, M.T. Cymbopogon citratus leaves: Characterization of flavonoids by HPLC-PDA-ESI/MS/MS and an approach to their potential as a source of bioactive polyphenols. Food Chem. 2008, 110, 718–728. [Google Scholar] [CrossRef]
- Ferreres, F.; Silva, B.M.; Andrade, P.B.; Seabra, R.M.; Ferreira, M.A. Approach to the study of C-glycosyl flavones by ion trap HPLC-PAD-ESI/MS/MS: Application to seeds of quince (Cydonia oblonga). Phytochem. Anal 2003, 14, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Qimin, L.; van den Heuvel, H.; Delorenzo, O.; Corthout, J.; Pieters, L.A.C.; Vlietinck, A.J.; Claeys, M. Mass spectral characterization of C-glycosisidic flavonoids isolated from a medicinal plant (Passittora incarnata). J. Chromatogrpahy 1991, 562, 435–446. [Google Scholar] [CrossRef]
- Wei, Z.; Qi, X.; Li, T.; Luo, M.; Wang, W.; Zu, Y.; Fu, Y. Application of natural deep eutectic solvents for extraction and determination of phenolics in Cajanus cajan leaves by ultra performance liquid chromatography. Sep. Purif. Technol. 2015, 149, 237–244. [Google Scholar] [CrossRef]
- Koutsoukos, S.; Tsiaka, T.; Tzani, A.; Zoumpoulakis, P.; Detsi, A. Choline chloride and tartaric acid, a Natural Deep Eutectic Solvent for the efficient extraction of phenolic and carotenoid compounds. J. Clean. Prod. 2019, 241, 118384. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron processing wastes as a bioresource of high-value added compounds: Development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef]
- Figueiró, L.R.; Comerlato, L.C.; Da Silva, M.V.; Zuanazzi, J.Â.S.; Von Poser, G.L.; Ziulkoski, A.L. Toxicity of Glandularia selloi (Spreng.) Tronc. leave extract by MTT and neutral red assays: Influence of the test medium procedure. Interdiscip. Toxicol. 2017, 9, 25–29. [Google Scholar] [CrossRef][Green Version]
- Syarina, P.N.A.; Karthivashan, G.; Abas, F.; Arulselvan, P.; Fakurazi, S. Original article: Wound healing potential of Spirulina platensis. EXCI J. 2015, 14, 385–393. [Google Scholar]
- Gothai, S.; Arulselvan, P.; Tan, W.; Fakurazi, S. Wound healing properties of ethyl acetate fraction of Moringa oleifera in normal human dermal fibroblasts. J. Intercult. Ethnopharmacol. 2016, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.; Arulselvan, P.; Karthivashan, G.; Fakurazi, S. Moringa oleifera flower extract suppresses the activation of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 macrophages via NF- κ B pathway. Mediat. Inflamm 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Hajiaghaalipour, F.; Kanthimathi, M.S.; Sanusi, J.; Rajarajeswaran, J. White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chem. 2015, 169, 401–410. [Google Scholar] [CrossRef]
| Peak | tR (min) | λmax, (nm) | [M-H]− (m/z) | Formula | Key MS/MS Fragments (m/z) | Compound | Relative Quantification (µg/mg Dried Extract) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PD | BD | GLY | GLU | MAL | LA | MEOH | |||||||
| 1 | 3.17 | 272, 348 | 609.1411 | C27H30O16 | 519.1104 489.0998, 429.0786, 399.0696, 369.0585 | Luteolin-6,8-di-C-hexose (Isomer 1) | 1.55 ± 0.15 | 1.65 ± 0.06 | 1.19 ± 0.15 | 1.21 ± 0.11 | 1.12 ± 0.07 | 1.16 ± 0.06 | 1.88 ± 0.21 |
| 2 | 4.88 | 272, 336 | 593.1464 | C27H30O15 | 503.1155, 473.1051, 383.0739, 353.0638 | Apigenin-6,8-di-C-hexose | 8.36 ± 1.12 | 10.57 ± 1.26 | 7.33 ± 0.71 | 5.68 ± 0.64 | 4.10 ± 0.42 | 5.32 ± 0.73 | 10.90 ± 1.44 |
| 3 | 6.72 | 272, 346 | 609.1411 | C27H30O16 | 489.1001, 429.0789, 399.0679, 369.0604 | Luteolin-6,8-di-C-hexose (Isomer 2) | 1.11 ± 0.04 | 1.33 ± 0.07 | 1.21 ± 0.05 | 0.98 ± 0.04 | 0.91 ± 0.01 | 0.97 ± 0.02 | 1.19 ± 0.05 |
| 4 | 7.10 | 272, 334 | 563.1359 | C26H28O14 | 473.1053, 443.0949, 383.0742, 353.0639 | Apigenin-6-C-pentose-8-C-hexose (Isomer 1) | 2.10 ± 0.11 | 2.73 ± 0.64 | 2.36 ± 0.33 | 1.92 ± 0.02 | 1.65 ± 0.04 | 1.82 ± 0.13 | 2.47 ± 0.16 |
| 5 | 7.86 | 270, 348 | 447.0896 | C21H20O11 | 357.0588, 339.0480, 327.0483, 297.0379, 285.0381 | Luteolin-6-C-hexose (Isoorientin) | 7.18 ± 0.48 | 11.16 ± 0.83 | 9.63 ± 0.83 | 3.87 ± 0.15 | 2.35 ± 0.06 | 4.11 ± 0.21 | 10.32 ± 0.76 |
| 6 | 9.00 | 270, 350 | 447.0896 | C21H20O11 | 357.0587, 339.0476, 327.0485, 297.0378, 285.0380 | Luteolin-8-C-hexose (Orientin) | 4.57 ± 0.73 | 6.88 ± 1.50 | 6.09 ± 1.38 | 2.59 ± 0.49 | 1.81 ± 0.24 | 2.84 ± 0.38 | 6.26 ± 1.18 |
| 7 | 9.87 | 270, 348 | 593.1464 | C27H30O15 | 473.1049, 429.0792, 369.0590, 357.0589, 327.0485 | Luteolin-6-C-hexose- 8-C-deoxyhexose (Isomer 1) | 2.57 ± 0.26 | 3.42 ± 0.56 | 2.89 ± 0.38 | 1.69 ± 0.17 | 1.32 ± 0.11 | 1.69 ± 0.15 | 3.64 ± 0.42 |
| 8 | 11.22 | 274, 334 | 563.1359 | C26H28O14 | 503.1168, 473.1056, 443.0950, 383.0743, 353.0639 | Apigenin-6-C-pentose-8-C-hexose (Isomer 2) | 2.68 ± 0.22 | 3.57 ± 0.51 | 3.75 ± 0.61 | 2.01 ± 0.18 | 1.66 ± 0.06 | 2.06 ± 0.17 | 3.13 ± 0.52 |
| 9 | 11.60 | 272, 336 | 593.1464 | C27H30O15 | 473.1067, 413.0846, 369.0590, 357.0589, 293.0434 | Luteolin-6-C-hexose- 8-C-deoxyhexose (Isomer 2) | 2.36 ± 0.31 | 3.37 ± 0.29 | 3.60 ± 0.33 | 1.68 ± 0.12 | 1.22 ± 0.07 | 1.68 ± 0.13 | 2.87 ± 0.23 |
| 10 | 12.44 | 270, 338 | 431.0947 | C21H20O10 | 341.0639, 323.0529, 311.0536, 283.0589 | Apigenin-6-C-hexose (Vitexin) | 10.23 ± 0.44 | 14.46 ± 0.66 | 15.81 ± 0.78 | 6.24 ± 0.34 | 4.01 ± 0.17 | 6.68 ± 0.24 | 13.89 ± 0.32 |
| 11 | 13.85 | 270, 338 | 431.0947 | C21H20O10 | 341.0638, 323.0536, 311.0536, 283.0588 | Apigenin-8-C-hexose (Isovitexin) | 19.76 ± 0.33 | 29.43 ± 0.40 | 32.39 ± 1.97 | 11.43 ± 0.28 | 6.58 ± 0.17 | 11.76 ± 0.70 | 27.40 ± 0.91 |
| 12 | 17.19 | 270, 338 | 577.1306 | C27H30O14 | 457.1098, 413.0845, 353.0630, 341.0640, 311.0536, 293.0432 | Apigenin-6-C-hexose-8-C-deoxyhexose | 32.93 ± 3.70 | 52.77 ± 5.94 | 71.15 ± 8.24 | 16.12 ± 1.72 | 7.95 ± 0.77 | 16.15 ± 1.68 | 45.08 ± 4.77 |
| NaDES | Polyphenolic Contents | Antioxidant Activities (100 µg mL−1) | Wound Healing Properties at 7.81 µg mL−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TPC (mg GAE/g) | TFC (mg QCE/g) | TAC (µg VE/mg) | TLC (µg OE/mg) | DPPH (%) | NO (%) | Cell Viability (%) | Cell Proliferation and Migration (%) | ||
| 24 h | 48 h | ||||||||
| GLY | 145.81 ± 4.11 | 28.82 ± 0.39 | 132.79 ± 12.42 | 24.60 ± 2.33 | 93.46 ± 3.82 | 56.16 ± 2.82 | 231.65 ± 22.55 | 57.82 ± 2.52 | 95.52 ± 0.61 |
| BD | 105.21 ± 4.99 | 26.63 ± 2.26 | 113.54 ± 8.76 | 27.82 ± 2.59 | 94.15 ± 4.52 | 73.00 ± 7.53 | 225.53 ± 26.09 | 56.79 ± 1.48 | 95.02 ± 0.99 |
| PD | 102.32 ± 5.52 | 13.55 ± 2.16 | 76.06 ± 5.74 | 19.33 ± 1.24 | 93.46 ± 2.87 | 57.34 ± 3.12 | 278.66 ± 68.08 | 55.82 ± 5.17 | 95.43 ± 1.02 |
| LA | 33.27 ± 8.14 | 3.88 ± 0.22 | 43.8 ± 3.36 | 12.46 ± 0.67 | 67.75 ± 2.49 | 54.15 ± 5.49 | 247.24 ± 36.86 | 56.34 ± 5.04 | 91.11 ± 0.81 |
| MAL | 23.96 ± 2.48 | 2.24 ± 0.62 | 25.96 ± 1.39 | 8.73 ± 0.39 | 72.17 ± 1.78 | 50.62 ± 5.86 | 283.82 ± 14.39 | 51.70 ± 3.83 | 89.56 ± 2.40 |
| GLU | 23.30 ± 3.59 | 1.29 ± 0.09 | 43.4 ± 2.69 | 12.02 ± 0.78 | 69.26 ± 7.29 | 51.76 ± 5.99 | 254.38 ± 47.85 | 37.27 ± 4.59 | 84.72 ± 2.04 |
| MEOH | 129.64 ± 7.39 | 29.55 ± 0.31 | 102.87 ± 7.58 | 26.16 ± 1.80 | 94.37 ± 3.10 | 67.37 ± 1.25 | 306.72 ± 11.13 | 63.64 ± 4.58 | 93.14 ± 0.62 |
| Control | BD | PD | GLY | GLU | MAL | LA | MEOH | Allantoin | |
|---|---|---|---|---|---|---|---|---|---|
| 0 h |  |  |  |  |  |  |  |  | 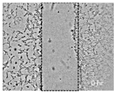 |
| 24 h |  |  | 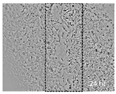 |  |  |  |  |  |  |
| 48 h |  |  |  |  |  |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che Zain, M.S.; Yeoh, J.X.; Lee, S.Y.; Afzan, A.; Shaari, K. Integration of Choline Chloride-Based Natural Deep Eutectic Solvents and Macroporous Resin for Green Production of Enriched Oil Palm Flavonoids as Natural Wound Healing Agents. Antioxidants 2021, 10, 1802. https://doi.org/10.3390/antiox10111802
Che Zain MS, Yeoh JX, Lee SY, Afzan A, Shaari K. Integration of Choline Chloride-Based Natural Deep Eutectic Solvents and Macroporous Resin for Green Production of Enriched Oil Palm Flavonoids as Natural Wound Healing Agents. Antioxidants. 2021; 10(11):1802. https://doi.org/10.3390/antiox10111802
Chicago/Turabian StyleChe Zain, Mohamad Shazeli, Jen Xen Yeoh, Soo Yee Lee, Adlin Afzan, and Khozirah Shaari. 2021. "Integration of Choline Chloride-Based Natural Deep Eutectic Solvents and Macroporous Resin for Green Production of Enriched Oil Palm Flavonoids as Natural Wound Healing Agents" Antioxidants 10, no. 11: 1802. https://doi.org/10.3390/antiox10111802
APA StyleChe Zain, M. S., Yeoh, J. X., Lee, S. Y., Afzan, A., & Shaari, K. (2021). Integration of Choline Chloride-Based Natural Deep Eutectic Solvents and Macroporous Resin for Green Production of Enriched Oil Palm Flavonoids as Natural Wound Healing Agents. Antioxidants, 10(11), 1802. https://doi.org/10.3390/antiox10111802








