Effects of Daily Melatonin Supplementation on Visual Loss, Circadian Rhythms, and Hepatic Oxidative Damage in a Rodent Model of Retinitis Pigmentosa
Abstract
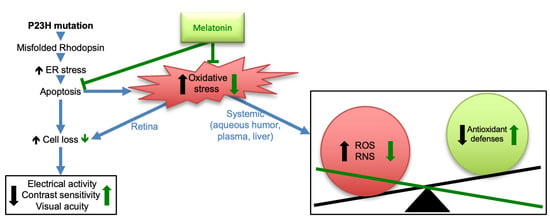
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
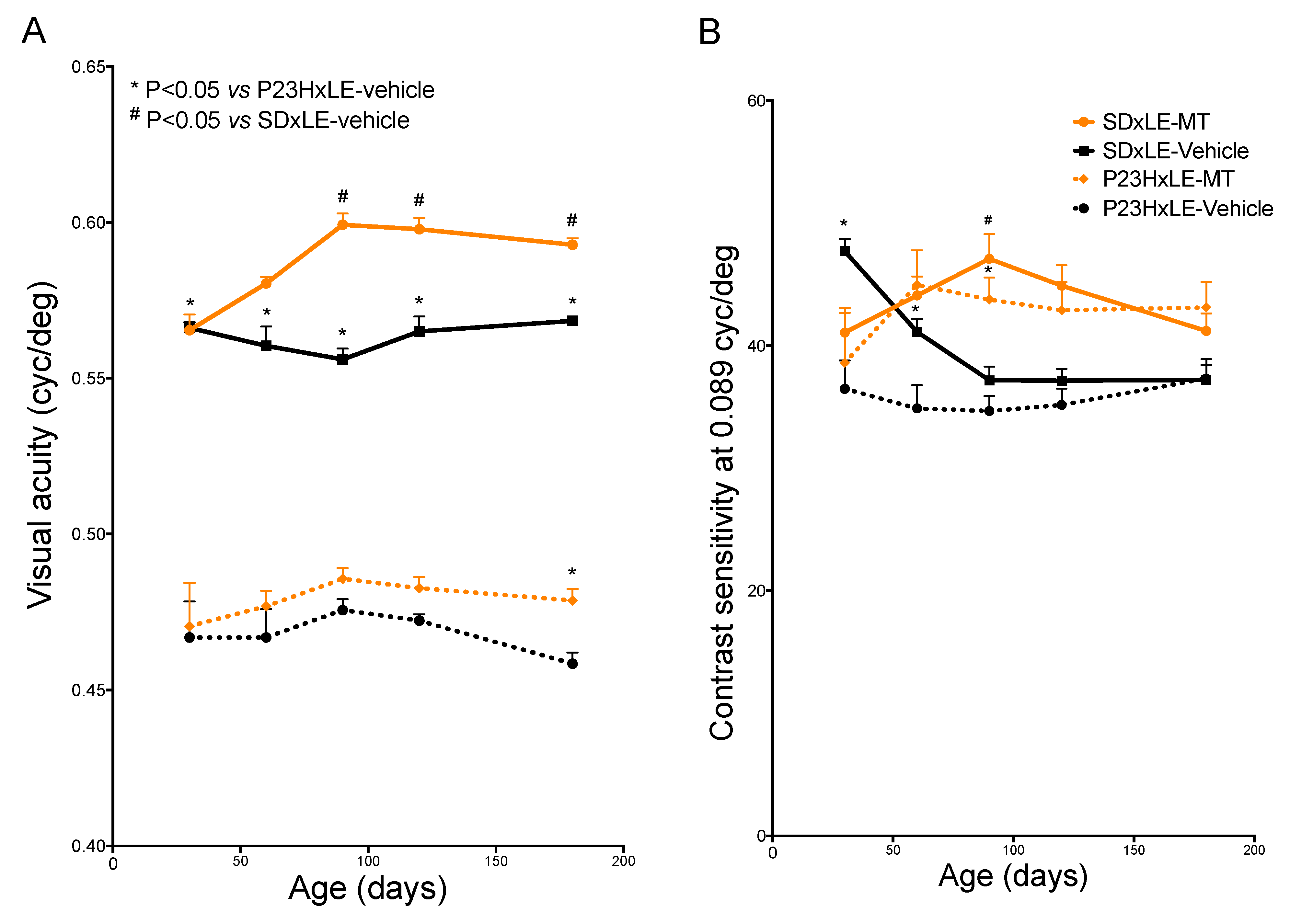
2.2. Visual Assessment: Visual Acuity and Contrast Sensitivity
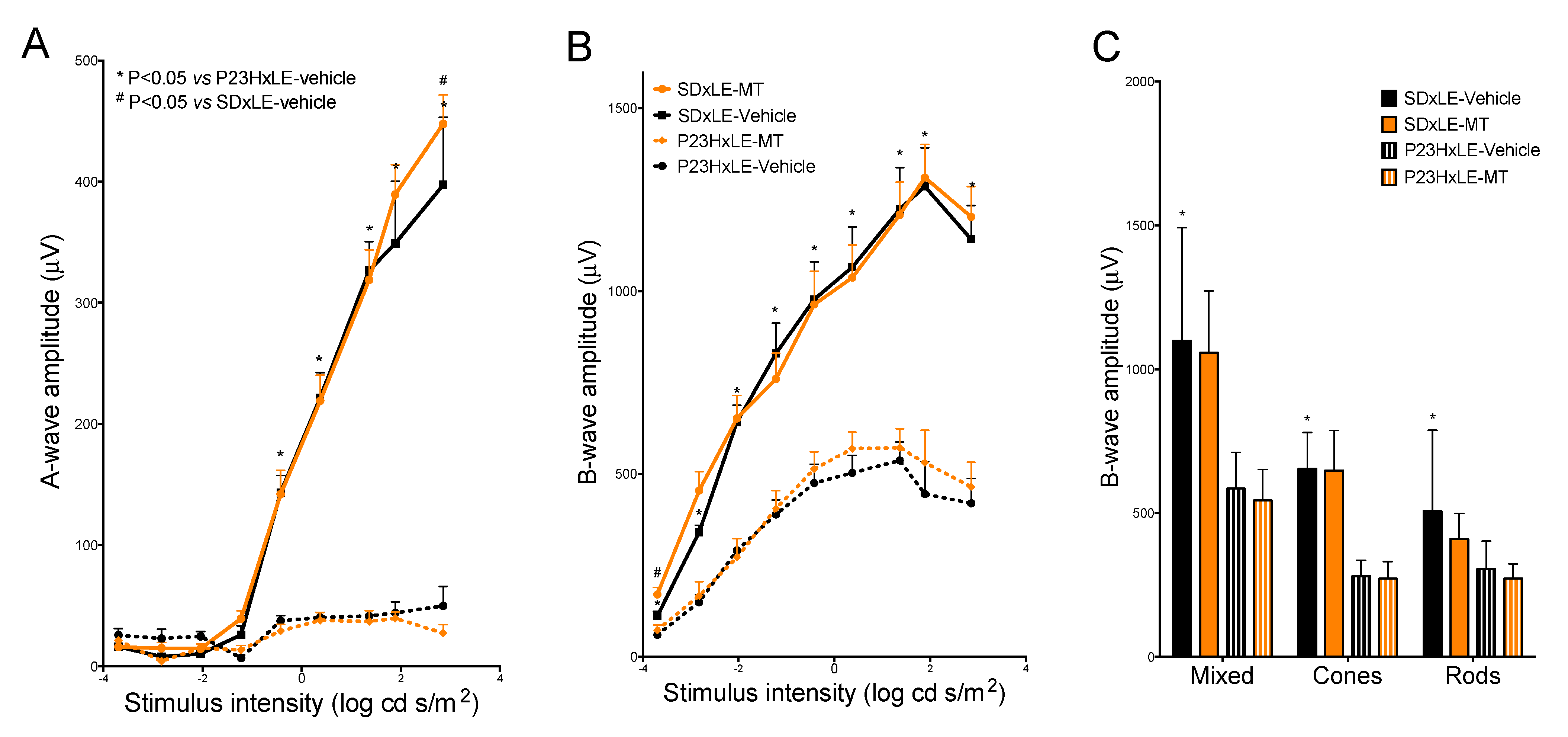
2.3. Electroretinogram Recordings
2.3.1. Mixed b-Wave
2.3.2. Double-Flash Protocol
2.4. Telemetry: Body Temperature and Locomotor Activity Recording
2.5. Sample Collection and Preparation
2.6. Measurement of Oxidative Stress Parameters
2.7. Determination of Antioxidant Status
2.8. Statistical Analysis
3. Results
3.1. Visual Parameters
3.2. Electroretinogram Results
3.3. Body Temperature and Locomotor Activity Rhythms
3.4. Oxidative Stress Parameters
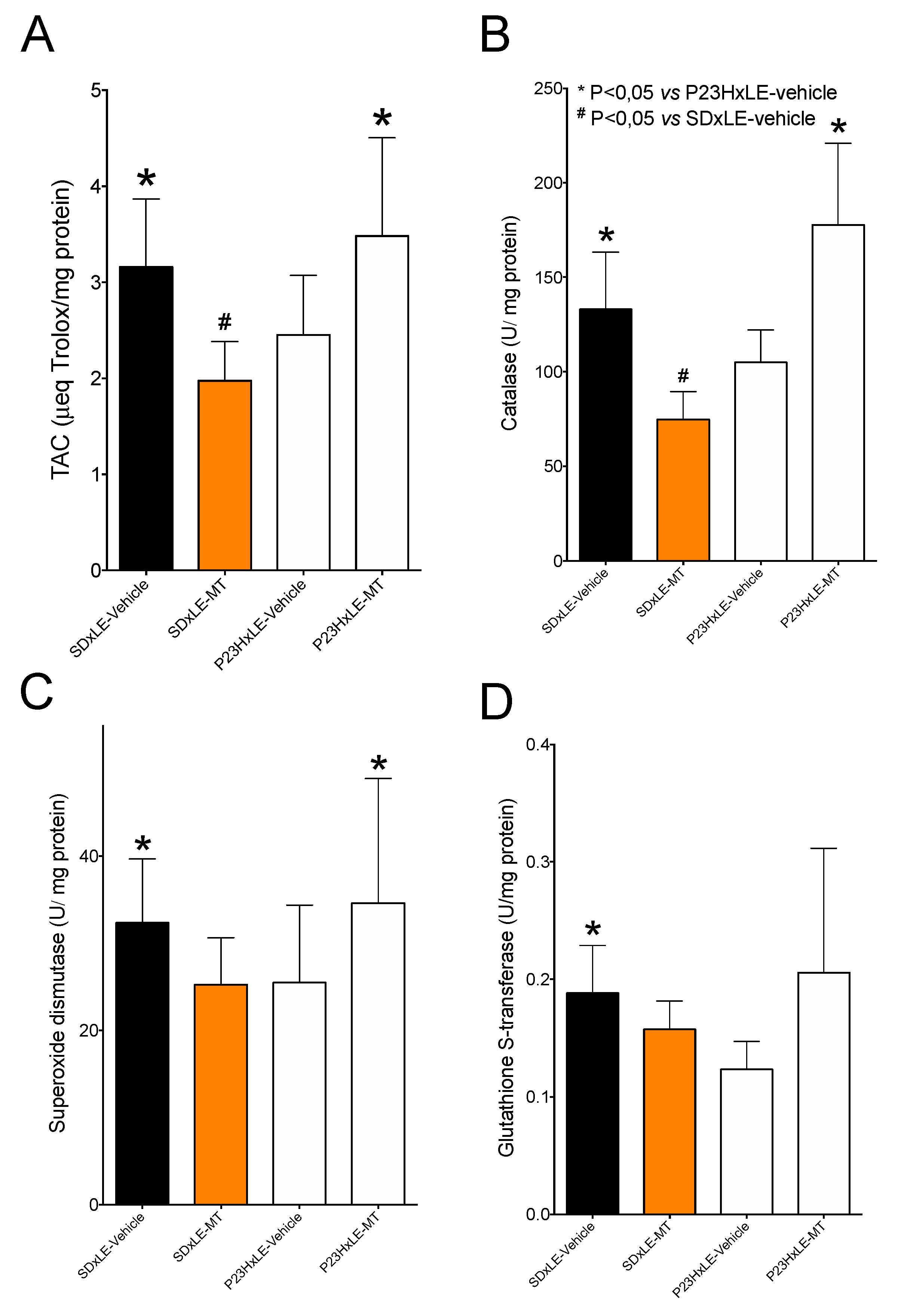
3.5. Antioxidant Defenses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinilla, I.; Fernandez-Sanchez, L.; Segura, F.J.; Sanchez-Cano, A.I.; Tamarit, J.M.; Fuentes-Broto, L.; Eells, J.T.; Lax, P.; Cuenca, N. Long time remodeling during retinal degeneration evaluated by optical coherence tomography, immunocytochemistry and fundus autofluorescence. Exp. Eye Res. 2016, 150, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Martinez-Fernandez de la Camara, C.; Salom, D.; Sequedo, M.D.; Hervas, D.; Marin-Lambies, C.; Aller, E.; Jaijo, T.; Diaz-Llopis, M.; Millan, J.M.; Rodrigo, R. Altered antioxidant-oxidant status in the aqueous humor and peripheral blood of patients with retinitis pigmentosa. PLoS ONE 2013, 8, e74223. [Google Scholar] [CrossRef]
- Perdices, L.; Fuentes-Broto, L.; Segura, F.; Ben Gdara, N.; Sanchez-Cano, A.I.; Insa, G.; Orduna, E.; Pinilla, I. Hepatic oxidative stress in pigmented P23H rhodopsin transgenic rats with progressive retinal degeneration. Free Radic Biol. Med. 2018, 124, 550–557. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Ma, T.; Deng, Z.; Gutierrez-Gamboa, G.; Ge, Q.; Xu, P.; Zhang, Q.; Zhang, J.; Meng, J.; Reiter, R.J.; et al. Plant-derived melatonin from food: A gift of nature. Food Funct. 2021, 12, 2829–2849. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H.B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef]
- Reiter, R.J.; Coto-Montes, A.; Boga, J.A.; Fuentes-Broto, L.; Rosales-Corral, S.; Tan, D.X. Melatonin: New applications in clinical and veterinary medicine, plant physiology and industry. Neuro Endocrinol. Lett. 2011, 32, 575–587. [Google Scholar]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Ghorbaninejad, P.; Sheikhhossein, F.; Djafari, F.; Tijani, A.J.; Mohammadpour, S.; Shab-Bidar, S. Effects of melatonin supplementation on oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Horm. Mol. Biol. Clin. Investig. 2020, 41. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin antioxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox Res. 2013, 23, 267–300. [Google Scholar] [CrossRef] [PubMed]
- Wiechmann, A.F.; Sherry, D.M. Role of melatonin and its receptors in the vertebrate retina. Int. Rev. Cell Mol. Biol. 2013, 300, 211–242. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Weng, S.-J.; Sun, X.-H.; Yang, X.-L. Neuromodulatory role of melatonin in retinal information processing. Prog. Retin. Eye Res. 2013, 32, 64–87. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.R.; Abbas, A.; Jefferson, L.S. Melatonin represses oxidative stress-induced activation of the MAP kinase and mTOR signaling pathways in H4IIE hepatoma cells through inhibition of Ras. J. Pineal Res. 2008, 44, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, J.; Wang, Z.; Yang, Y.; Wang, X.; Duan, Q. Melatonin protects N2a against ischemia/reperfusion injury through autophagy enhancement. J. Huazhong Univ. Sci. Technol. Med. Sci 2010, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vega-Naredo, I.; Caballero, B.; Sierra, V.; Garcia-Macia, M.; de Gonzalo-Calvo, D.; Oliveira, P.J.; Rodriguez-Colunga, M.J.; Coto-Montes, A. Melatonin modulates autophagy through a redox-mediated action in female Syrian hamster Harderian gland controlling cell types and gland activity. J. Pineal Res. 2012, 52, 80–92. [Google Scholar] [CrossRef]
- Xu, G.X.; Xiao, Z.Y.; Xie, M.S.; Feng, Y.L.; Guo, J.; Fu, L.X. Protective effects of melatonin on cultural human retinal pigment epithelial cells against oxidative damage in vitro. Zhonghua Yan Ke Za Zhi 2009, 45, 528–532. [Google Scholar]
- Osborne, N.N.; Nash, M.S.; Wood, J.P. Melatonin counteracts ischemia-induced apoptosis in human retinal pigment epithelial cells. Invest. Ophthalmol Vis. Sci. 1998, 39, 2374–2383. [Google Scholar]
- Fu, Y.; Tang, M.; Fan, Y.; Zou, H.; Sun, X.; Xu, X. Anti-apoptotic effects of melatonin in retinal pigment epithelial cells. Front. Biosci. 2012, 17, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Belforte, N.A.; Moreno, M.C.; de Zavalia, N.; Sande, P.H.; Chianelli, M.S.; Keller Sarmiento, M.I.; Rosenstein, R.E. Melatonin: A novel neuroprotectant for the treatment of glaucoma. J. Pineal Res. 2010, 48, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Patschan, D.; Hildebrandt, A.; Rinneburger, J.; Wessels, J.T.; Patschan, S.; Becker, J.U.; Henze, E.; Kruger, A.; Muller, G.A. The hormone melatonin stimulates renoprotective effects of “early outgrowth” endothelial progenitor cells in acute ischemic kidney injury. Am. J. Physiol. Ren. Physiol. 2012, 302, F1305–F1312. [Google Scholar] [CrossRef]
- Rastmanesh, R. Potential of melatonin to treat or prevent age-related macular degeneration through stimulation of telomerase activity. Med. Hypotheses 2011, 76, 79–85. [Google Scholar] [CrossRef]
- Segura, F.; Arines, J.; Sanchez-Cano, A.; Perdices, L.; Orduna-Hospital, E.; Fuentes-Broto, L.; Pinilla, I. Development of optokinetic tracking software for objective evaluation of visual function in rodents. Sci. Rep. 2018, 8, 10009. [Google Scholar] [CrossRef] [PubMed]
- Perdices, L.; Fuentes-Broto, L.; Segura, F.; Cavero, A.; Orduna-Hospital, E.; Insa-Sanchez, G.; Sanchez-Cano, A.I.; Fernandez-Sanchez, L.; Cuenca, N.; Pinilla, I. Systemic epigallocatechin gallate protects against retinal degeneration and hepatic oxidative stress in the P23H-1 rat. Neural Regen Res. 2022, 17, 625–631. [Google Scholar] [CrossRef]
- Pinilla, I.; Lund, R.; Sauve, Y. Contribution of rod and cone pathways to the dark-adapted electroretinogram (ERG) b-wave following retinal degeneration in RCS rats. Vis. Res. 2004, 44, 2467–2474. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lax, P.; Otalora, B.B.; Esquiva, G.; Rol Mde, L.; Madrid, J.A.; Cuenca, N. Circadian dysfunction in P23H rhodopsin transgenic rats: Effects of exogenous melatonin. J. Pineal Res. 2011, 50, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Tudela, E.; Martinez-Nicolas, A.; Campos, M.; Rol, M.Á.; Madrid, J.A. A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comput. Biol. 2010, 6, e1000996. [Google Scholar] [CrossRef]
- Perdices, L.; Fuentes-Broto, L.; Segura, F.; Cuenca, N.; Orduna-Hospital, E.; Pinilla, I. Epigallocatechin Gallate Slows Retinal Degeneration, Reduces Oxidative Damage, and Modifies Circadian Rhythms in P23H Rats. Antioxidants 2020, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- Green, L.C.; Ruiz de Luzuriaga, K.; Wagner, D.A.; Rand, W.; Istfan, N.; Young, V.R.; Tannenbaum, S.R. Nitrate biosynthesis in man. Proc. Natl. Acad. Sci. USA 1981, 78, 7764–7768. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Miller, N.J.; Paganga, G.; Wiseman, S.; Van Nielen, W.; Tijburg, L.; Chowienczyk, P.; Rice-Evans, C.A. Total antioxidant activity of low density lipoproteins and the relationship with alpha-tocopherol status. FEBS Lett. 1995, 365, 164–166. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. New insights into the role of melatonin in plants and animals. Chem. Biol. Interact. 2019, 299, 163–167. [Google Scholar] [CrossRef]
- Pevet, P.; Klosen, P.; Felder-Schmittbuhl, M.P. The hormone melatonin: Animal studies. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 547–559. [Google Scholar] [CrossRef]
- Crooke, A.; Huete-Toral, F.; Colligris, B.; Pintor, J. The role and therapeutic potential of melatonin in age-related ocular diseases. J. Pineal Res. 2017, 63. [Google Scholar] [CrossRef]
- Yi, C.; Pan, X.; Yan, H.; Guo, M.; Pierpaoli, W. Effects of melatonin in age-related macular degeneration. Ann. N. Y. Acad. Sci. 2005, 1057, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, N.; Fernandez-Sanchez, L.; Sauve, Y.; Segura, F.J.; Martinez-Navarrete, G.; Tamarit, J.M.; Fuentes-Broto, L.; Sanchez-Cano, A.; Pinilla, I. Correlation between SD-OCT, immunocytochemistry and functional findings in an animal model of retinal degeneration. Front. Neuroanat. 2014, 8, 151. [Google Scholar] [CrossRef]
- Segura, F.; Sánchez-Cano, A.; Jarabo, S.; López de la Fuente, C.; Cuenca, N.; Villegas-Pérez, M.P.; Pinilla, I. Assessment of Visual and Chromatic Functions in a Rodent Model of Retinal Degeneration. Invest. Ophthalmol. Vis. Sci 2015, 56, 6275–6283. [Google Scholar] [CrossRef][Green Version]
- Hanif, A.M.; Kim, M.K.; Thomas, J.G.; Ciavatta, V.T.; Chrenek, M.; Hetling, J.R.; Pardue, M.T. Whole-eye electrical stimulation therapy preserves visual function and structure in P23H-1 rats. Exp. Eye Res. 2016, 149, 75–83. [Google Scholar] [CrossRef]
- Rayapudi, S.; Schwartz, S.G.; Wang, X.; Chavis, P. Vitamin A and fish oils for retinitis pigmentosa. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Fernandez-Sanchez, L.; Bravo-Osuna, I.; Lax, P.; Arranz-Romera, A.; Maneu, V.; Esteban-Perez, S.; Pinilla, I.; Puebla-Gonzalez, M.D.M.; Herrero-Vanrell, R.; Cuenca, N. Controlled delivery of tauroursodeoxycholic acid from biodegradable microspheres slows retinal degeneration and vision loss in P23H rats. PLoS ONE 2017, 12, e0177998. [Google Scholar] [CrossRef]
- Tosini, G.; Baba, K.; Hwang, C.K.; Iuvone, P.M. Melatonin: An underappreciated player in retinal physiology and pathophysiology. Exp. Eye Res. 2012, 103, 82–89. [Google Scholar] [CrossRef]
- Gagne, A.M.; Danilenko, K.V.; Rosolen, S.G.; Hebert, M. Impact of oral melatonin on the electroretinogram cone response. J. Circadian Rhythm. 2009, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Pozdeyev, N.; Mazzoni, F.; Contreras-Alcantara, S.; Liu, C.; Kasamatsu, M.; Martinez-Merlos, T.; Strettoi, E.; Iuvone, P.M.; Tosini, G. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc. Natl. Acad. Sci. USA 2009, 106, 15043–15048. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, M.; Clesse, D.; Pévet, P.; Challet, E. From daily behavior to hormonal and neurotransmitters rhythms: Comparison between diurnal and nocturnal rat species. Horm. Behav. 2009, 55, 338–347. [Google Scholar] [CrossRef]
- Jagannath, A.; Taylor, L.; Wakaf, Z.; Vasudevan, S.R.; Foster, R.G. The genetics of circadian rhythms, sleep and health. Hum. Mol. Genet. 2017, 26, R128–R138. [Google Scholar] [CrossRef]
- Aubin, S.; Gacon, C.; Jennum, P.; Ptito, M.; Kupers, R. Altered sleep–wake patterns in blindness: A combined actigraphy and psychometric study. Sleep Med. 2016, 24, 100–108. [Google Scholar] [CrossRef]
- Lax, P.; Esquiva, G.; Fuentes-Broto, L.; Segura, F.; Sanchez-Cano, A.; Cuenca, N.; Pinilla, I. Age-related changes in photosensitive melanopsin-expressing retinal ganglion cells correlate with circadian rhythm impairments in sighted and blind rats. Chronobiol. Int. 2016, 33, 374–391. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Ung, L.; Pattamatta, U.; Carnt, N.; Wilkinson-Berka, J.L.; Liew, G.; White, A.J.R. Oxidative stress and reactive oxygen species: A review of their role in ocular disease. Clin. Sci. 2017, 131, 2865–2883. [Google Scholar] [CrossRef]
- Dehdashtian, E.; Mehrzadi, S.; Yousefi, B.; Hosseinzadeh, A.; Reiter, R.J.; Safa, M.; Ghaznavi, H.; Naseripour, M. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci. 2018, 193, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Manikonda, P.K.; Jagota, A. Melatonin administration differentially affects age-induced alterations in daily rhythms of lipid peroxidation and antioxidant enzymes in male rat liver. Biogerontology 2012, 13, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tipoe, G.L.; Fung, M.L. Melatonin attenuates intermittent hypoxia-induced lipid peroxidation and local inflammation in rat adrenal medulla. Int. J. Mol. Sci. 2014, 15, 18437–18452. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Reiter, R.J.; Qi, W.; Tan, D.X.; Cabrera, J. Melatonin prevents oxidative damage to protein and lipid induced by ascorbate-Fe(3+)-EDTA: Comparison with glutathione and alpha-tocopherol. Neuro. Endocrinol. Lett. 2000, 21, 269–276. [Google Scholar]
- Kolli, V.K.; Kanakasabapathy, I.; Faith, M.; Ramamoorthy, H.; Isaac, B.; Natarajan, K.; Abraham, P. A preclinical study on the protective effect of melatonin against methotrexate-induced small intestinal damage: Effect mediated by attenuation of nitrosative stress, protein tyrosine nitration, and PARP activation. Cancer Chemother Pharmacol. 2013, 71, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Blanco, S.; Hernández, R.; Franchelli, G.; Ramos-Álvarez, M.M.; Peinado, M.Á. Melatonin influences NO/NOS pathway and reduces oxidative and nitrosative stress in a model of hypoxic-ischemic brain damage. Nitric Oxide 2017, 62, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Lai, C.J.; Tsai, M.H.; Wu, Y.C.; Chen, K.T.; Jou, M.J.; Fu, P.I.; Wu, C.H.; Wei, I.H. Effects of melatonin on the nitric oxide system and protein nitration in the hypobaric hypoxic rat hippocampus. BMC Neurosci. 2015, 16, 61. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds. Molecules 2021, 26, 4105. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef]
- Kurhaluk, N.; Szarmach, A.; Zaitseva, O.V.; Sliuta, A.; Kyriienko, S.; Winklewski, P.J. Effects of melatonin on low dose lipopolysaccharide-induced oxidative stress in the mouse liver, muscle and kidney. Can. J. Physiol. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Siew-Keah, L.; Sundaram, A.; Sirajudeen, K.N.; Zakaria, R.; Singh, H.J. Effect of melatonin supplementation and cross-fostering on renal glutathione system and development of hypertension in spontaneously hypertensive rats. J. Physiol. Biochem. 2014, 70, 73–79. [Google Scholar] [CrossRef]
- Pinazo-Duran, M.D.; Gallego-Pinazo, R.; Garcia-Medina, J.J.; Zanon-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martinez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramirez, C.; Lopez-Galvez, M.I.; et al. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging 2014, 9, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Kubasik-Kladna, K.; Aboul-Enein, H.Y. The role oxidative stress in the pathogenesis of eye diseases: Current status and a dual role of physical activity. Mini Rev. Med. Chem. 2016, 16, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10, S18. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Ghosal, I.; Das, D.; Chakraborty, S.B. Melatonin ameliorates H. Biol. Res. 2018, 51, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Lu, C.; Li, T.; Wang, W.; Ye, W.; Zeng, R.; Ni, L.; Lai, Z.; Wang, X.; Liu, C. The protective effect of melatonin on brain ischemia and reperfusion in rats and humans: In vivo assessment and a randomized controlled trial. J. Pineal Res. 2018, 65, e12521. [Google Scholar] [CrossRef]
- Yamazaki, H.; Tanji, K.; Wakabayashi, K.; Matsuura, S.; Itoh, K. Role of the Keap1/Nrf2 pathway in neurodegenerative diseases. Pathol. Int. 2015, 65, 210–219. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. The Keap1-Nrf2-antioxidant response element pathway: A review of its regulation by melatonin and the proteasome. Mol. Cell Endocrinol. 2015, 401, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhu, J.; Mi, C.; Xu, B.; Jiao, C.; Li, Y.; Xu, D.; Liu, W.; Xu, Z. Melatonin antagonizes Mn-induced oxidative injury through the activation of keap1-Nrf2-ARE signaling pathway in the striatum of mice. Neurotox Res. 2015, 27, 156–171. [Google Scholar] [CrossRef]
- Barchas, J.; DaCosta, F.; Spector, S. Acute pharmacology of melatonin. Nature 1967, 214, 919–920. [Google Scholar] [CrossRef]
- Wasdell, M.B.; Jan, J.E.; Bomben, M.M.; Freeman, R.D.; Rietveld, W.J.; Tai, J.; Hamilton, D.; Weiss, M.D. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J. Pineal Res. 2008, 44, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Amstrup, A.K.; Sikjaer, T.; Mosekilde, L.; Rejnmark, L. The effect of melatonin treatment on postural stability, muscle strength, and quality of life and sleep in postmenopausal women: A randomized controlled trial. Nutr. J. 2015, 14, 102. [Google Scholar] [CrossRef]

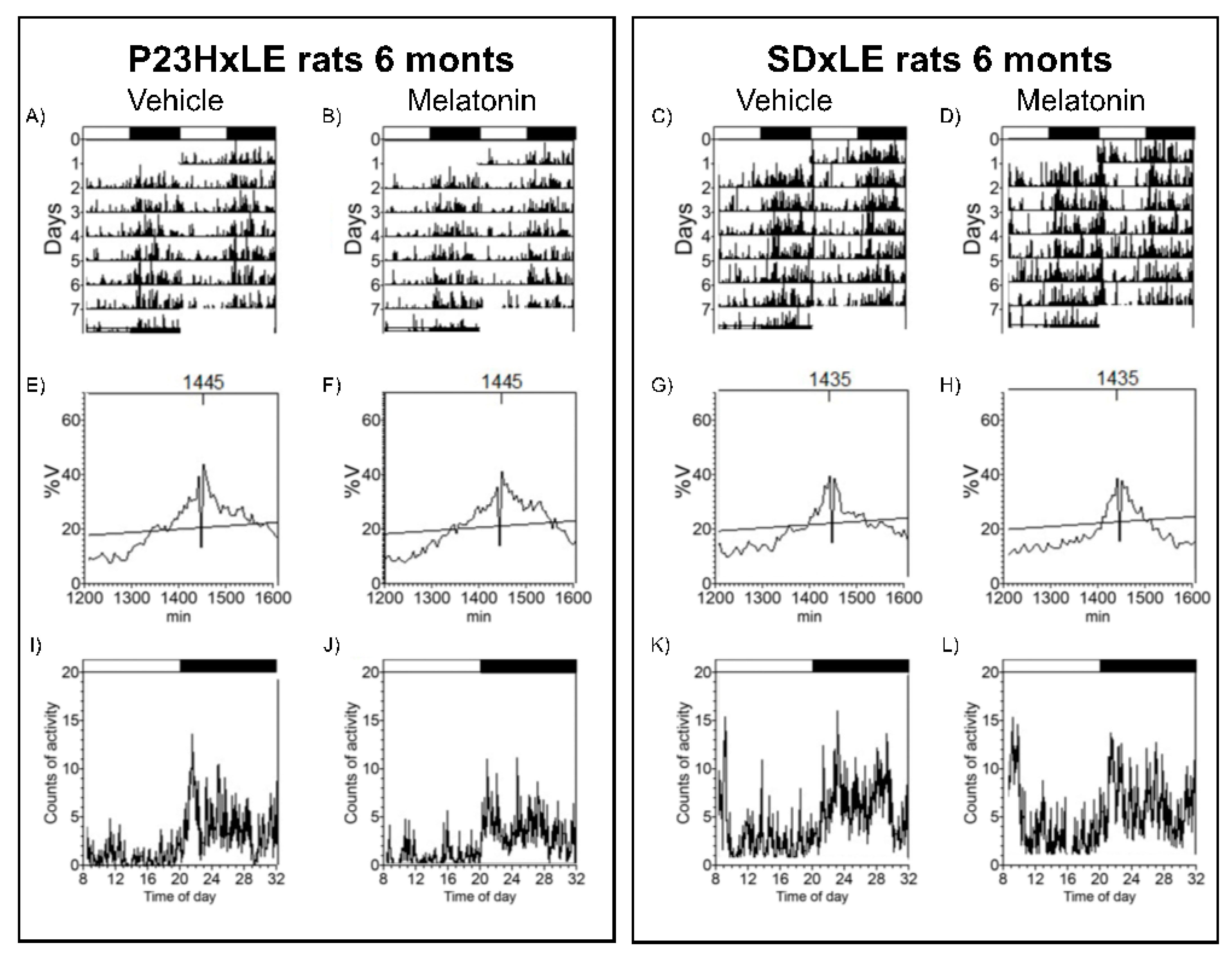

| SDxLE | P23HxLE | |||||||
|---|---|---|---|---|---|---|---|---|
| Temperature | Locomotor Activity | Temperature | Locomotor Activity | |||||
| Vehicle (n = 5) | MT (n = 5) | Vehicle (n = 5) | MT (n = 5) | Vehicle (n = 5) | MT (n = 5) | Vehicle (n = 5) | MT (n = 5) | |
| Rhythm Parameters | ||||||||
| Mesor (°C) | 36.96 ± 0.04 | 36.93 ± 0.06 | 4.47 ± 0.42 * | 4.45 ± 0.24 | 37.16 ± 0.13 | 37.00 ± 0.06 | 2.69 ± 0.28 | 3.09 ± 0.48 |
| Amplitude (°C) | 0.37 ± 0.01 * | 0.46 ± 0.03 # | 2.67 ± 0.35 | 2.44 ± 0.17 | 0.51 ± 0.06 | 0.57 ± 0.13 | 1.90 ± 0.17 | 1.70 ± 0.17 |
| Acrophase (min) | 1118.26 ± 23.66 | 1077.83 ± 15.82 | 1145.71 ± 35.07 | 1191.68 ± 27.01 | 1022.90 ± 30.69 | 1018.58 ± 4.63 | 1056.88 ± 21.28 | 980.50 ± 43.69 |
| Acrophase (h:min) | 2:43 ± 0:23 | 2:02 ± 0:15 | 3:10 ± 0:35 | 3:56 ± 0:27 | 1:07 ± 0:30 | 1:03 ± 0:04 | 1:41 ± 0:21 | 0:25 ± 0:43 |
| Variance (%) | 26.08 ± 1.076 * | 31.05 ± 4.165 | 11.77 ± 1.59 | 10.56 ± 1.98 | 38.03 ± 4.806 | 40.65 ± 9.102 | 13.24 ± 1.39 | 9.08 ± 2.75 |
| Period (min) | 1445.00 ± 20.00 | 1440.00 ± 5.00 | 1441.67 ± 3.33 | 1437.50 ± 2.50 | 1442.50 ± 2.50 | 1442.50 ± 2.50 | 1442.50 ± 2.50 | 1457.50 ± 13.15 |
| Non-Parametric Variables | ||||||||
| IS | 0.63 ± 0.03 | 0.63 ± 0.01 | 0.33 ± 0.02 | 0.34 ± 0.02 | 0.69 ± 0.04 | 0.69 ± 0.03 | 0.32 ± 0.03 | 0.26 ± 0.03 |
| IV | 0.25 ± 0.03 | 0.24 ± 0.03 | 0.96 ± 0.01 | 0.95 ± 0.03 | 0.18 ± 0.03 | 0.20 ± 0.04 | 1.08 ± 0.07 | 1.07 ± 0.01 |
| RA | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.50 ± 0.02 | 0.55 ± 0.03 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.60 ± 0.04 | 0.49 ± 0.07 |
| L2 (hh:mm) | 5:05 ± 2:30 * | 6:17 ± 0:32 | 6:31 ± 0:24 * | 5:12 ± 0:30 | 13:55 ± 1:24 | 10:02 ± 0:11 | 6:45 ± 0:20 | 6:55 ± 0:43 |
| VL2 (°C) | 37.53 ± 0.08 | 37.55 ± 0.08 | 4.67 ± 0.89 * | 4.54 ± 0.32 | 37.43 ± 0.07 | 37.30 ± 0.11 | 2.82 ± 0.13 | 2.74 ± 0.30 |
| Media (°C) | 37.43 ± 0.04 | 37.44 ± 0.04 | 4.87 ± 0.43 * | 4.85 ± 0.25 | 37.48 ± 0.03 | 37.48 ± 0.05 | 3.07 ± 0.29 | 3.48 ± 0.49 |
| CFI | 0.30 ± 0.00 | 0.29 ± 0.00 | 0.60 ± 0.01 | 0.61 ± 0.01 | 0.29 ± 0.01 | 0.30 ± 0.00 | 0.66 ± 0.03 | 0.61 ± 0.03 |
| DesynchroIndex L2 | - | - | 0.19 ± 0.14 | 0.09 ± 0.04 # | -- | - | 0.60 ± 0.11 | 0.26 ± 0.07 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes-Broto, L.; Perdices, L.; Segura, F.; Orduna-Hospital, E.; Insa-Sánchez, G.; Sánchez-Cano, A.I.; Cuenca, N.; Pinilla, I. Effects of Daily Melatonin Supplementation on Visual Loss, Circadian Rhythms, and Hepatic Oxidative Damage in a Rodent Model of Retinitis Pigmentosa. Antioxidants 2021, 10, 1853. https://doi.org/10.3390/antiox10111853
Fuentes-Broto L, Perdices L, Segura F, Orduna-Hospital E, Insa-Sánchez G, Sánchez-Cano AI, Cuenca N, Pinilla I. Effects of Daily Melatonin Supplementation on Visual Loss, Circadian Rhythms, and Hepatic Oxidative Damage in a Rodent Model of Retinitis Pigmentosa. Antioxidants. 2021; 10(11):1853. https://doi.org/10.3390/antiox10111853
Chicago/Turabian StyleFuentes-Broto, Lorena, Lorena Perdices, Francisco Segura, Elvira Orduna-Hospital, Gema Insa-Sánchez, Ana I. Sánchez-Cano, Nicolás Cuenca, and Isabel Pinilla. 2021. "Effects of Daily Melatonin Supplementation on Visual Loss, Circadian Rhythms, and Hepatic Oxidative Damage in a Rodent Model of Retinitis Pigmentosa" Antioxidants 10, no. 11: 1853. https://doi.org/10.3390/antiox10111853
APA StyleFuentes-Broto, L., Perdices, L., Segura, F., Orduna-Hospital, E., Insa-Sánchez, G., Sánchez-Cano, A. I., Cuenca, N., & Pinilla, I. (2021). Effects of Daily Melatonin Supplementation on Visual Loss, Circadian Rhythms, and Hepatic Oxidative Damage in a Rodent Model of Retinitis Pigmentosa. Antioxidants, 10(11), 1853. https://doi.org/10.3390/antiox10111853