Lutein and Zeaxanthin Distribution in the Healthy Macula and Its Association with Various Demographic Factors Examined in Pseudophakic Eyes
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Measurement of Macular Pigment Optical Density
2.3. Evaluation of Skin Carotenoid (SC) Status
2.4. Statistical Analyses
3. Results
3.1. Subject Characteristics
3.2. Macular Pigment Optical Density and Macular Pigment Optical Volume
3.3. Multivariate Analyses
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bone, R.A.; Landrum, J.T.; Hime, G.W.; Cains, A.; Zamor, J. Stereochemistry of the human macular carotenoids. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2033–2040. [Google Scholar]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar]
- Mortensen, A.; Skibsted, L.H.; Sampson, J.; Rice-Evans, C.; Everett, S.A. Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett. 1997, 418, 91–97. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Richer, S.; Stiles, W.; Statkute, L.; Pulido, J.; Frankowski, J.; Rudy, D.; Pei, K.; Tsipursky, M.; Nyland, J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: The Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optom. J. Am. Optom. Assoc. 2004, 75, 216–229. [Google Scholar] [CrossRef]
- Richer, S.; Devenport, J.; Lang, J.C. LAST II: Differential temporal responses of macular pigment optical density in patients with atrophic age-related macular degeneration to dietary supplementation with xanthophylls. Optom. J. Am. Optom. Assoc. 2007, 78, 213–219. [Google Scholar] [CrossRef]
- Tan, J.S.; Wang, J.J.; Flood, V.; Rochtchina, E.; Smith, W.; Mitchell, P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: The Blue Mountains Eye Study. Ophthalmology 2008, 115, 334–341. [Google Scholar] [CrossRef]
- Krishnadev, N.; Meleth, A.D.; Chew, E.Y. Nutritional supplements for age-related macular degeneration. Curr. Opin. Ophthalmol. 2010, 21, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Yan, S.-F.; Huang, Y.-M.; Lu, X.-R.; Qian, F.; Pang, H.-L.; Xu, X.-R.; Zou, Z.; Dong, P.-C.; Xiao, X.; et al. Effect of Lutein and Zeaxanthin on Macular Pigment and Visual Function in Patients with Early Age-related Macular Degeneration. Ophthalmology 2012, 119, 2290–2297. [Google Scholar] [CrossRef]
- The Carmis Study Group; Piermarocchi, S.; Saviano, S.; Parisi, V.; Tedeschi, M.; Panozzo, G.; Scarpa, G.; Boschi, G.; Giudice, G.L.; Sartore, M.; et al. Carotenoids in Age-Related Maculopathy Italian Study (CARMIS): Two-Year Results of a Randomized Study. Eur. J. Ophthalmol. 2012, 22, 216–225. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Mayne, S.T.; Gomez, C.M.; Tibor, S.E.; Twaroska, E.E. Macular pigment in donor eyes with and without AMD: A case-control study. Investig. Ophthalmol. Vis. Sci. 2001, 42, 235–240. [Google Scholar]
- Bernstein, P.S.; Zhao, D.-Y.; Wintch, S.W.; Ermakov, I.V.; McClane, R.W.; Gellermann, W. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology 2002, 109, 1780–1787. [Google Scholar] [CrossRef] [Green Version]
- Beatty, S.; Murray, I.J.; Henson, D.; Carden, D.; Koh, H.; Boulton, M.E. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Investig. Ophthalmol. Vis. Sci. 2001, 42, 439–446. [Google Scholar]
- Obana, A.; Hiramitsu, T.; Gohto, Y.; Ohira, A.; Mizuno, S.; Hirano, T.; Bernstein, P.S.; Fujii, H.; Iseki, K.; Tanito, M.; et al. Macular Carotenoid Levels of Normal Subjects and Age-Related Maculopathy Patients in a Japanese Population. Ophthalmology 2008, 115, 147–157. [Google Scholar] [CrossRef]
- Snodderly, D.M.; Auran, J.D.; Delori, F.C. The macular pigment. II. Spatial distribution in primate retinas. Investig. Ophthalmol. Vis. Sci. 1984, 25, 674–685. [Google Scholar]
- Obana, A.; Gohto, Y.; Sasano, H.; Gellermann, W.; Sharifzadeh, M.; Seto, T.; Bernstein, P.S. Spatial distribution of macular pigment estimated by autofluorescence imaging in elderly Japanese individuals. Jpn. J. Ophthalmol. 2020, 64, 160–170. [Google Scholar] [CrossRef]
- Gass, J.D. Muller cell cone, an overlooked part of the anatomy of the fovea centralis: Hypotheses concerning its role in the pathogenesis of macular hole and foveomacualr retinoschisis. Arch. Ophthalmol. 1999, 117, 821–823. [Google Scholar] [CrossRef] [Green Version]
- Helb, H.-M.; Issa, P.C.; VAN DER Veen, R.L.P.; Berendschot, T.T.J.M.; Scholl, H.P.N.; Holz, F.G. Abnormal macular pigment distribution in type 2 idiopathic macular telangiectasia. Retina 2008, 28, 808–816. [Google Scholar] [CrossRef]
- Zeimer, M.B.; Padge, B.; Heimes, B.; Pauleikhoff, D. Idiopathic macular telangiectasia type 2: Distribution of macular pigment and functional investigations. Retina 2010, 30, 586–595. [Google Scholar] [CrossRef]
- Powner, M.B.; Gillies, M.C.; Tretiach, M.; Scott, A.; Guymer, R.; Hageman, G.S.; Fruttiger, M. Perifoveal Müller Cell Depletion in a Case of Macular Telangiectasia Type 2. Ophthalmology 2010, 117, 2407–2416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obana, A.; Sasano, H.; Okazaki, S.; Otsuki, Y.; Seto, T.; Gohto, Y. Evidence of Carotenoid in Surgically Removed Lamellar Hole-Associated Epiretinal Proliferation. Investig. Opthalmol. Vis. Sci. 2017, 58, 5157–5163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gass, J.D.M. Reappraisal of Biomicroscopic Classification of Stages of Development of a Macular Hole. Am. J. Ophthalmol. 1995, 119, 752–759. [Google Scholar] [CrossRef]
- Obana, A.; Nakazawa, R.; Noma, S.; Sasano, H.; Gohto, Y. Macular Pigment in Eyes with Macular Hole Formation and Its Change After Surgery. Transl. Vis. Sci. Technol. 2020, 9, 28. [Google Scholar] [CrossRef]
- Li, B.; George, E.W.; Rognon, G.T.; Gorusupudi, A.; Ranganathan, A.; Chang, F.-Y.; Shi, L.; Frederick, J.M.; Bernstein, P.S. Imaging lutein and zeaxanthin in the human retina with confocal resonance Raman microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 12352–12358. [Google Scholar] [CrossRef]
- Delori, F.C. Autofluorescence method to measure macular pigment optical densities fluorometry and autofluorescence imaging. Arch. Biochem. Biophys. 2004, 430, 156–162. [Google Scholar] [CrossRef]
- Dennison, J.L.; Stack, J.; Beatty, S.; Nolan, J.M. Concordance of macular pigment measurements obtained using customized heterochromatic flicker photometry, dual-wavelength autofluorescence, and single-wavelength reflectance. Exp. Eye Res. 2013, 116, 190–198. [Google Scholar] [CrossRef]
- Creuzot-Garcher, C.; Koehrer, P.; Picot, C.; Aho, S.; Bron, A.M. Comparison of Two Methods to Measure Macular Pigment Optical Density in Healthy Subjects. Investig. Opthalmol. Vis. Sci. 2014, 55, 2941–2946. [Google Scholar] [CrossRef] [Green Version]
- Akuffo, K.O.; Beatty, S.; Stack, J.; Peto, T.; Leung, I.; Corcoran, L.; Power, R.; Nolan, J.M. Concordance of Macular Pigment Measurement Using Customized Heterochromatic Flicker Photometry and Fundus Autofluorescence in Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2015, 56, 8207–8214. [Google Scholar] [CrossRef] [Green Version]
- Obana, A.; Gellermann, W.; Gohto, Y.; Seto, T.; Sasano, H.; Tanito, M.; Okazaki, S. Reliability of a two-wavelength autofluorescence technique by Heidelberg Spectralis to measure macular pigment optical density in Asian subjects. Exp. Eye Res. 2018, 168, 100–106. [Google Scholar] [CrossRef]
- Trieschmann, M.; Heimes, B.; Hense, H.W.; Pauleikhoff, D. Macular pigment optical density measurement in autofluorescence imaging: Comparison of one- and two-wavelength methods. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 1565–1574. [Google Scholar] [CrossRef]
- You, Q.S.; Bartsch, D.-U.G.; Espina, M.; Alam, M.; Camacho, N.; Mendoza, N.; Freeman, W.R. Reproducibility of macular pigment optical density measurement by two-wavelength autofluorescence in a clinical setting. Retina 2016, 36, 1381–1387. [Google Scholar] [CrossRef] [Green Version]
- Sharifzadeh, M.; Obana, A.; Gohto, Y.; Seto, T.; Gellermann, W. Autofluorescence imaging of macular pigment: Influence and correction of ocular media opacities. J. Biomed. Opt. 2014, 19, 096010. [Google Scholar] [CrossRef]
- Akuffo, K.O.; Nolan, J.; Stack, J.; Power, R.; Kirwan, C.; Moran, R.; Corcoran, L.; Owens, N.; Beatty, S. The Impact of Cataract, and Its Surgical Removal, on Measures of Macular Pigment Using the Heidelberg Spectralis HRA+OCT MultiColor Device. Investig. Opthalmol. Vis. Sci. 2016, 57, 2552–2563. [Google Scholar] [CrossRef]
- Obana, A.; Gohto, Y.; Sasano, H.; Gellermann, W.; Sharifzadeh, M.; Seto, T.; Bernstein, P.S. Grade of Cataract and Its Influence on Measurement of Macular Pigment Optical Density Using Autofluorescence Imaging. Investig. Opthalmol. Vis. Sci. 2018, 59, 3011–3019. [Google Scholar] [CrossRef] [Green Version]
- Obana, A.; Ote, K.; Hashimoto, F.; Asaoka, R.; Gohto, Y.; Okazaki, S.; Yamada, H. Correction for the Influence of Cataract on Macular Pigment Measurement by Autofluorescence Technique Using Deep Learning. Transl. Vis. Sci. Technol. 2021, 10, 18. [Google Scholar] [CrossRef]
- Nolan, J.M.; Stringham, J.M.; Beatty, S.; Snodderly, D. Spatial Profile of Macular Pigment and Its Relationship to Foveal Architecture. Investig. Opthalmol. Vis. Sci. 2008, 49, 2134–2142. [Google Scholar] [CrossRef]
- Obana, A.; Gohto, Y.; Asaoka, R. Macular pigment changes after cataract surgery with yellow-tinted intraocular lens implantation. PLoS ONE 2021, 16, e0248506. [Google Scholar] [CrossRef]
- Obana, A.; Tanito, M.; Gohto, Y.; Gellermann, W.; Okazaki, S.; Ohira, A. Macular Pigment Changes in Pseudophakic Eyes Quantified with Resonance Raman Spectroscopy. Ophthalmology 2011, 118, 1852–1858. [Google Scholar] [CrossRef]
- Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991, 98, 786–806. [CrossRef]
- Gomez, M.G.; Bernstein, P.S.; Curcio, C.A.; Moran, R.; Roche, W.; Nolan, J.M. Standardizing the Assessment of Macular Pigment Using a Dual-Wavelength Autofluorescence Technique. Transl. Vis. Sci. Technol. 2019, 8, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriksen, B.S.; Chan, G.; Hoffman, R.O.; Sharifzadeh, M.; Ermakov, I.V.; Gellermann, W.; Bernstein, P.S. Interrelationships Between Maternal Carotenoid Status and Newborn Infant Macular Pigment Optical Density and Carotenoid Status. Investig. Opthalmol. Vis. Sci. 2013, 54, 5568–5578. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Sharifzadeh, M.; Liu, A.; Ermakov, I.; Nelson, K.; Sheng, X.; Panish, C.; Carlstrom, B.; Hoffman, R.O.; Gellermann, W. Blue-Light Reflectance Imaging of Macular Pigment in Infants and Children. Investig. Opthalmol. Vis. Sci. 2013, 54, 4034–4040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gellermann, W. Raman detection of carotenoids in human tissue. Carotenoids Retin. Mol. Asp. Health Issues 2005, 6, 86–114. [Google Scholar]
- Zidichouski, J.A.; Mastaloudis, A.; Poole, S.J.; Reading, J.C.; Smidt, C.R. Clinical Validation of a Noninvasive, Raman Spectroscopic Method to Assess Carotenoid Nutritional Status in Humans. J. Am. Coll. Nutr. 2009, 28, 687–693. [Google Scholar] [CrossRef]
- Nguyen, L.M.; Scherr, R.E.; Linnell, J.D.; Ermakov, I.V.; Gellermann, W.; Jahns, L.; Keen, C.L.; Miyamoto, S.; Steinberg, F.M.; Young, H.M.; et al. Evaluating the relationship between plasma and skin carotenoids and reported dietary intake in elementary school children to assess fruit and vegetable intake. Arch. Biochem. Biophys. 2015, 572, 73–80. [Google Scholar] [CrossRef]
- Jahns, L.; Johnson, L.K.; Mayne, S.T.; Cartmel, B.; Picklo, M.J.; Ermakov, I.V.; Gellermann, W.; Whigham, L. Skin and plasma carotenoid response to a provided intervention diet high in vegetables and fruit: Uptake and depletion kinetics. Am. J. Clin. Nutr. 2014, 100, 930–937. [Google Scholar] [CrossRef]
- Aguilar, S.S.; Wengreen, H.J.; Lefevre, M.; Madden, G.J.; Gast, J. Skin Carotenoids: A Biomarker of Fruit and Vegetable Intake in Children. J. Acad. Nutr. Diet. 2014, 114, 1174–1180. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Ermakova, M.R.; Bernstein, P.S.; Chan, G.M.; Gellermann, W. Resonance Raman based skin carotenoid measurements in newborns and infants. J Biophoton. 2013, 6, 793–802. [Google Scholar] [CrossRef] [Green Version]
- Mayne, S.T.; Cartmel, B.; Scarmo, S.; Lin, H.; Leffell, D.J.; Welch, E.; Ermakov, I.; Bhosale, P.; Bernstein, P.S.; Gellermann, W. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am. J. Clin. Nutr. 2010, 92, 794–800. [Google Scholar] [CrossRef] [Green Version]
- Ermakov, I.V.; Gellermann, W. Dermal carotenoid measurements via pressure mediated reflection spectroscopy. J. Biophoton. 2012, 5, 559–570. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtainingR2from generalized linear mixed-effects models. Methods Ecol. Evol. 2012, 4, 133–142. [Google Scholar] [CrossRef]
- Canovas, R.; Morini, C.; Prata, T.S.; Rosen, R.B.; Lima, V.C.; García, P. Comparison between Macular Pigment Optical Density Measurements Using Two-Wavelength Autofluorescence and Heterochromatic Flicker Photometry Techniques. Investig. Opthalmol. Vis. Sci. 2010, 51, 3152–3156. [Google Scholar] [CrossRef] [Green Version]
- Green-Gomez, M.; Moran, R.; Stringham, J.; Hernández-Alcaraz, C.; Mendoza-Herrera, K.; Fromow-Guerra, J.J.; Prado-Cabrero, A.; Nolan, J. Environmental and Nutritional Determinants of Macular Pigment in a Mexican Population. Investig. Opthalmol. Vis. Sci. 2021, 62, 18. [Google Scholar] [CrossRef]
- Howells, O.; Eperjesi, H.E.B.; Bartlett, H. Macular Pigment Optical Density in Young Adults of South Asian Origin. Investig. Opthalmol. Vis. Sci. 2013, 54, 2711–2719. [Google Scholar] [CrossRef] [Green Version]
- Huntjens, B.; Asaria, T.S.; Dhanani, S.; Konstantakopoulou, E.; Ctori, I. Macular Pigment Spatial Profiles in South Asian and White Subjects. Investig. Opthalmol. Vis. Sci. 2014, 55, 1440–1446. [Google Scholar] [CrossRef] [Green Version]
- Hammond, B.R.; Caruso-Avery, M. Macular pigment optical density in a Southwestern sample. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1492–1497. [Google Scholar]
- Berendschot, T.T.J.M.; Willemse-Assink, J.J.M.; Bastiaanse, M.; De Jong, P.T.V.M.; Van Norren, D. Macular pigment and melanin in age-related maculopathy in a general population. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1928–1932. [Google Scholar]
- Werner, J.S.; Donnelly, S.K.; Kliegl, R. Aging and human macular pigment density: Appended with translations from the work of Max Schultze and Ewald Hering. Vis. Res. 1987, 27, 257–268. [Google Scholar] [CrossRef]
- Broekmans, W.M.R.; Berendschot, T.T.; Klöpping-Ketelaars, I.A.; De Vries, A.J.; Goldbohm, R.A.; Tijburg, L.B.M.; Kardinaal, A.F.M.; Van Poppel, G. Macular pigment density in relation to serum and adipose tissue concentrations of lutein and serum concentrations of zeaxanthin. Am. J. Clin. Nutr. 2002, 76, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-F.; Chang, Y.; Wu, J.-C. The spatial distribution of macular pigment in humans. Curr. Eye Res. 2001, 23, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Berendschot, T.T.; van Norren, D. On the age dependency of the macular pigment optical density. Exp. Eye Res. 2005, 81, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Pipis, A.; Touliou, E.; Augustin, A.J. Macular Pigment Optical Density in a Central European Population. Ophthalmic Surg. Lasers Imaging Retin. 2013, 44, 260–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obana, A.; Gohto, Y.; Tanito, M.; Okazaki, S.; Gellermann, W.; Bernstein, P.S.; Ohira, A. Effect of age and other factors on macular pigment optical density measured with resonance Raman spectroscopy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subhi, Y.; Forshaw, T.; Sørensen, T.L. Macular thickness and volume in the elderly: A systematic review. Ageing Res. Rev. 2016, 29, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Hammond, B.; Yeum, K.-J.; Qin, J.; Wang, X.D.; Castaneda, C.; Snodderly, D.; Russell, R.M. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am. J. Clin. Nutr. 2000, 71, 1555–1562. [Google Scholar] [CrossRef] [Green Version]
- Andersen, L.F.; Jacobs, D.R.; Gross, M.D.; Schreiner, P.J.; Williams, O.D.; Lee, D.-H. Longitudinal associations between body mass index and serum carotenoids: The CARDIA study. Br. J. Nutr. 2006, 95, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.; O’Donovan, O.; Kavanagh, H.; Stack, J.; Harrison, M.; Muldoon, A.; Mellerio, J.; Beatty, S. Macular pigment and percentage of body fat. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3940–3950. [Google Scholar] [CrossRef]
- Obana, A.; Tanito, M.; Gohto, Y.; Okazaki, S.; Gellermann, W.; Bernstein, P.S. Changes in Macular Pigment Optical Density and Serum Lutein Concentration in Japanese Subjects Taking Two Different Lutein Supplements. PLoS ONE 2015, 10, e0139257. [Google Scholar] [CrossRef]
- Fujimura, S.; Ueda, K.; Nomura, Y.; Yanagi, Y. Preliminary analysis of the relationship between serum lutein and zeaxanthin levels and macular pigment optical density. Clin. Ophthalmol. 2016, 10, 2149–2155. [Google Scholar] [CrossRef] [Green Version]
- Obana, A.; Gohto, Y.; Nakazawa, R.; Moriyama, T.; Gellermann, W.; Bernstein, P.S. Effect of an antioxidant supplement containing high dose lutein and zeaxanthin on macular pigment and skin carotenoid levels. Sci. Rep. 2020, 10, 10262. [Google Scholar] [CrossRef]
- Beatty, S.; Nolan, J.; Kavanagh, H.; Donovan, O.O. Macular pigment optical density and its relationship with serum and dietary levels of lutein and zeaxanthin. Arch. Biochem. Biophys. 2004, 430, 70–76. [Google Scholar] [CrossRef]
- Nolan, J.M.; Stack, J.; Beatty, S.; O’Connell, E. The Relationships between Macular Pigment Optical Density and Its Constituent Carotenoids in Diet and Serum. Investig. Opthalmol. Vis. Sci. 2007, 48, 571–582. [Google Scholar] [CrossRef] [Green Version]
- Loane, E.; Nolan, J.M.; Beatty, S. The Respective Relationships between Lipoprotein Profile, Macular Pigment Optical Density, and Serum Concentrations of Lutein and Zeaxanthin. Investig. Opthalmol. Vis. Sci. 2010, 51, 5897–5905. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.M.; Stack, J.; Mellerio, J.; Godhinio, M.; Donovan, O.O.; Neelam, K.; Beatty, S. Monthly Consistency of Macular Pigment Optical Density and Serum Concentrations of Lutein and Zeaxanthin. Curr. Eye Res. 2006, 31, 199–213. [Google Scholar] [CrossRef]
- Trieschmann, M.; Beatty, S.; Nolan, J.; Hense, H.W.; Heimes, B.; Austermann, U.; Fobker, M.; Pauleikhoff, D. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: The LUNA study. Exp. Eye Res. 2007, 84, 718–728. [Google Scholar] [CrossRef]
- Hammond, B.R., Jr.; Wooten, B.R.; Snodderly, D. Cigarette Smoking and Retinal Carotenoids: Implications for Age-related Macular Degeneration. Vis. Res. 1996, 36, 3003–3009. [Google Scholar] [CrossRef] [Green Version]
- Iannaccone, A.; Mura, M.; Gallaher, K.T.; Johnson, E.J.; Todd, W.A.; Kenyon, E.; Harris, T.L.; Harris, T.; Satterfield, S.; Johnson, K.C.; et al. Macular Pigment Optical Density in the Elderly: Findings in a Large Biracial Midsouth Population Sample. Investig. Opthalmol. Vis. Sci. 2007, 48, 1458–1465. [Google Scholar] [CrossRef]
- Yu, J.; Johnson, E.J.; Shang, F.; Lim, A.; Zhou, H.; Cui, L.; Xu, J.; Snellingen, T.; Liu, X.; Wang, N.; et al. Measurement of Macular Pigment Optical Density in a Healthy Chinese Population Sample. Investig. Opthalmol. Vis. Sci. 2012, 53, 2106–2111. [Google Scholar] [CrossRef]
- Abell, R.G.; Hewitt, A.W.; Andric, M.; Allen, P.L.; Verma, N. The use of heterochromatic flicker photometry to determine macular pigment optical density in a healthy Australian population. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 417–421. [Google Scholar] [CrossRef]


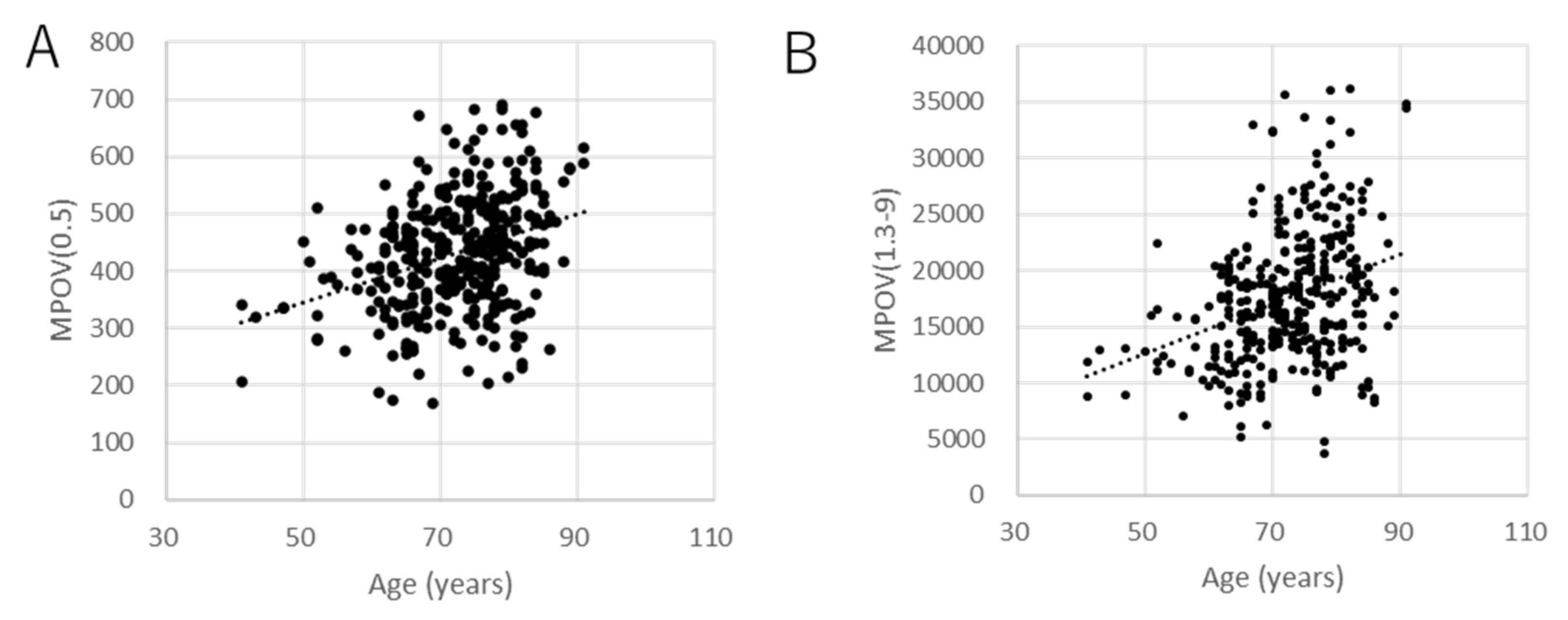



| Characteristics | Number of Patients |
|---|---|
| Enrolled eyes | One eye, 132, Both eyes, 110 |
| Sex | Men, 108, women, 134 |
| Age (years) | Range, 41–91, mean, 72.3 ± 8.6 (standard deviation), median, 74 |
| Body mass index (kg/m2) | Range, 12.5–39.9, mean, 22.7 ± 3.3 (standard deviation) |
| Tobacco | No, 170, Yes, 70 (Past 52/Current18), unknown, 2 |
| Lutein supplement | No, 222, Yes, 18, unknown, 2 |
| Diabetes | No, 205, Yes, 37, unknown, 3 |
| Characteristics | |
|---|---|
| Axial length (mm) | Range, 20.8–28.9, mean, 23.9 ± 1.4 (standard deviation) |
| Central retinal thickness, CRT (µm) | Range, 214–381, mean, 267.7 ± 22.7 |
| Central retinal volume, CRV (mm3) | Range, 0.17–0.30, mean, 0.20 ± 0.02 |
| Paracentral retinal volume, PRV (mm3) | Range, 6.82–9.53, mean, 8.26 ± 0.44 |
| Total retinal volume, TRV (mm3) | Range, 7.02–9.79, mean, 8.47 ± 0.45 |
| Glaucoma | No, 328, Yes, 25 |
| BCVA at the MPOD measurement | 1.2 (logMAR −0.08), 283 1.0 (logMAR 0.00), 39 0.9 (logMAR 0.05), 10 0.8 (logMAR 0.10), 13 0.7 (logMAR 0.15), 4 0.6 (logMAR 0.22), 2 0.5 (logMAR 0.30), 1 |
| Ecentricity | Local MPOD | MPOV |
|---|---|---|
| 0.23° | 0.24–1.24 0.78 ± 0.18 | |
| 0.5° | 0.25–1.19 0.73 ± 0.18 | 169–692 432 ±101 |
| 1° | 0.22–1.17 0.69 ± 0.16 | 519–2358 1490 ± 331 |
| 1.33° | 0.15–1.05 0.55 ± 0.15 | 832–5179 2542 ± 600 |
| 2° | 0.08–0.74 0.35 ± 0.11 | 1289–8244 44,462 ± 1125 |
| 9° | - | 5100–40,224 20,121 ± 6293 |
| Zone A | Zone B | Zone C | ||||||
|---|---|---|---|---|---|---|---|---|
| MPOD(0.23) | MPOD(0.5) | MPOV(0.5) | MPOD(1) | MPOV(1.3) | MPOD(2) | MPOV(1.3–9) | ||
| mR2 | 0.24 | 0.25 | 0.26 | 0.24 | 0.27 | 0.24 | 0.20 | |
| Age (years) | B | 6.5 × 10−3 | 6.8 × 10−3 | 4.0 | 7.9 × 10−3 | 7.3 × 10−3 | 4.6 × 10−3 | 2.4 × 102 |
| β | 5.7 × 10−3 | 6.0 × 10−2 | 3.5 × 101 | 6.9 × 10−2 | 6.3 × 10−2 | 4.0 × 10−2 | 2.1 × 103 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Sex (0 woman, 1 man) | B | 2.1 × 10−2 | 1.0 × 10−2 | 1.5 × 101 | 7.9 × 10−3 | 1.4 × 10−2 | 4.1 × 10−3 | 5.5 × 101 |
| p | 0.444 | 0.707 | 0.487 | 0.747 | 0.548 | 0.814 | 0.956 | |
| BMI (kg/m2) | B | −5.2 × 10−3 | −4.8 × 10−3 | −2.9 | −7.6 × 10−3 | −6.7 × 10−3 | −5.3 × 10−3 | −3.3 × 102 |
| β | −1.6 × 10−2 | −1.5 × 10−2 | −9.1 | −2.3 × 10−2 | −2.1 × 10−2 | −1.6 × 10−2 | −1.0 × 103 | |
| p | 0.133 | 0.159 | 0.128 | 0.017 | 0.023 | 0.020 | 0.010 | |
| Supplement (0 no, I yes) | B | −8.1 × 10−4 | −4.3 × 10−2 | −5.0 | 5.3·10−2 | 6.3 × 10−2 | 6.0 × 10−2 | 3.8 × 103 |
| p | 0.984 | 0.277 | 0.824 | 0.112 | 0.040 | 0.010 | 0.004 | |
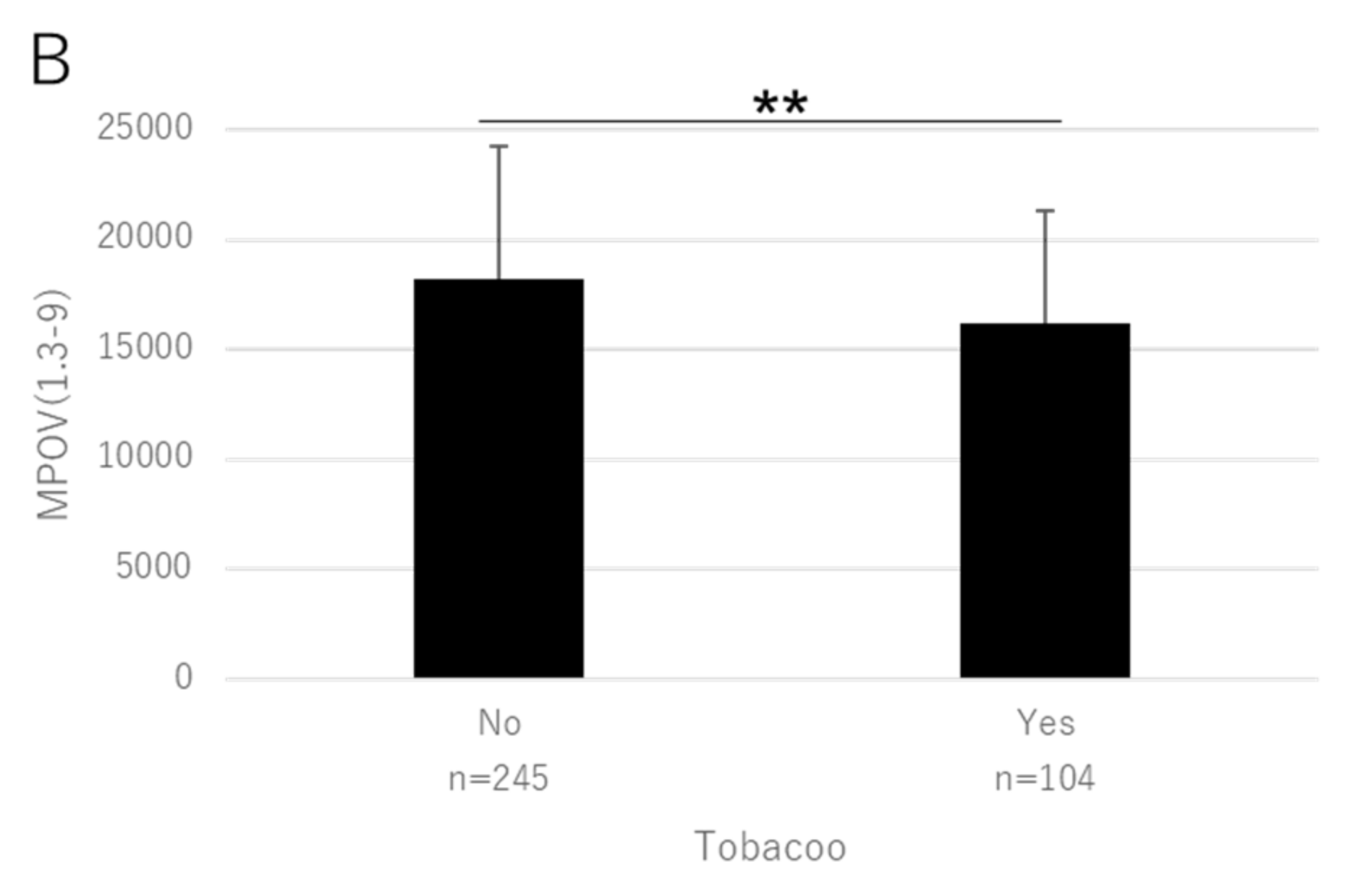
| Smoking (0 no, I yes) | B | −5.5 × 10−2 | −6.4 × 10−2 | −3.6 × 101 | −4.4 × 10−2 | −3.6 × 10−2 | −3.0 × 10−2 | −1.3 × 103 |
| p | 0.045 | 0.020 | 0.020 | 0.079 | 0.128 | 0.100 | 0.207 | |
| SC levels | B | 7.4 × 10−5 | 1.4 × 10−4 | 4.9 × 10−2 | 1.1 × 10−4 | 1.0 × 10−4 | 7.6 × 10−5 | 5.1 |
| β | 1.1 × 10−2 | 2.0 × 10−2 | 7.0 | 1.5 × 10−2 | 1.5 × 10−2 | 1.1 × 10−2 | 7.3 × 102 | |
| p | 0.331 | 0.072 | 0.252 | 0.115 | 0.098 | 0.112 | 0.061 | |
| Glaucoma (0 no, I yes) | B | −7.7 × 10−2 | −6.4 × 10−2 | −4.4 × 101 | −3.1 × 10−2 | −2.1 × 10−2 | −5.2 × 10−3 | 1.5 × 102 |
| p | 0.064 | 0.120 | 0.059 | 0.415 | 0.551 | 0.848 | 0.919 | |
| Diabetes (0 no, I yes) | B | −1.9 × 10−2 | −1.4 × 10−2 | −9.1 | −2.0 × 10−3 | 6.4 × 10−3 | 1.3 × 10−2 | 2.2 × 102 |
| p | 0.513 | 0.636 | 0.585 | 0.941 | 0.803 | 0.517 | 0.844 | |
| Axial length (mm) | B | −6.7 × 10−3 | 6.5 × 10−3 | −6.9 × 10−2 | 5.1 × 10−3 | −5.2 × 10−3 | −3.3 × 10−3 | −1.2 × 102 |
| β | −9.5 × 10−3 | 9.2 × 10−3 | −9.8 × 10−2 | 7.2 × 10−3 | −7.3 × 10−3 | −4.6 × 10−3 | −1.7 × 102 | |
| p | 0.480 | 0.487 | 0.990 | 0.530 | 0.491 | 0.563 | 0.698 | |
| CRT (µm) | B | 3.4 × 10−3 | 2.3 × 10−3 | 1.70 | −1.0 × 10−4 | −1.5 × 10−3 | −7.2 × 10−4 | −3.2 × 101 |
| β | 7.7 × 10−2 | 5.1 × 10−2 | 3.8 × 101 | −2.3 × 10−3 | −3.4 × 10−2 | −1.6 × 10−2 | −7.3 × 102 | |
| p | 0.080 | 0.195 | 0.110 | 0.933 | 0.161 | 0.353 | 0.434 | |
| CRV (µm3) | B | −4.4 × 10−1 | 2.9·10−1 | −3.3 × 101 | 6.5 × 10−1 | 1.5 | 6.9 × 10−1 | 3.2 × 104 |
| β | −8.0 × 10−3 | 5.1·10−3 | −5.9 × 10−1 | 1.2 × 10−2 | 2.5 10−2 | 1.2 × 10−2 | 5.7 × 102 | |
| p | 0.846 | 0.888 | 0.979 | 0.634 | 0.226 | 0.427 | 0.489 | |
| PRV (µm3) | B | −2.1 × 10−1 | −3.5 × 10−1 | −1.8 × 102 | 2.8 × 10−1 | 5.2 × 10−1 | 2.9 × 10−1 | 1.6 × 104 |
| β | −9.2 × 10−2 | −1.5 × 10−1 | −8.1 × 102 | 1.2 × 10−1 | 2.3 × 10−1 | 1.3 × 10−1 | 7.1 × 103 | |
| p | 0.787 | 0.610 | 0.662 | 0.554 | 0.197 | 0.327 | 0.304 | |
| TRV (µm3) | B | 9.5 × 10−2 | 2.4 × 10−1 | 1.2 × 102 | −3.3 × 10−1 | −5.5 × 10−1 | −3.2 × 10−1 | −1.6 × 104 |
| β | 4.3 × 10−2 | 1.1 × 10−1 | 5.3 × 101 | −1.5 × 10−1 | −2.5 × 10−1 | −1.4 × 10−1 | −7.2 × 103 | |
| p | 0.902 | 0.730 | 0.779 | 0.484 | 0.178 | 0.280 | 0.308 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obana, A.; Gohto, Y.; Asaoka, R.; Gellermann, W.; Bernstein, P.S. Lutein and Zeaxanthin Distribution in the Healthy Macula and Its Association with Various Demographic Factors Examined in Pseudophakic Eyes. Antioxidants 2021, 10, 1857. https://doi.org/10.3390/antiox10121857
Obana A, Gohto Y, Asaoka R, Gellermann W, Bernstein PS. Lutein and Zeaxanthin Distribution in the Healthy Macula and Its Association with Various Demographic Factors Examined in Pseudophakic Eyes. Antioxidants. 2021; 10(12):1857. https://doi.org/10.3390/antiox10121857
Chicago/Turabian StyleObana, Akira, Yuko Gohto, Ryo Asaoka, Werner Gellermann, and Paul S. Bernstein. 2021. "Lutein and Zeaxanthin Distribution in the Healthy Macula and Its Association with Various Demographic Factors Examined in Pseudophakic Eyes" Antioxidants 10, no. 12: 1857. https://doi.org/10.3390/antiox10121857
APA StyleObana, A., Gohto, Y., Asaoka, R., Gellermann, W., & Bernstein, P. S. (2021). Lutein and Zeaxanthin Distribution in the Healthy Macula and Its Association with Various Demographic Factors Examined in Pseudophakic Eyes. Antioxidants, 10(12), 1857. https://doi.org/10.3390/antiox10121857







