Antioxidant Activity of Mushroom Extracts/Polysaccharides—Their Antiviral Properties and Plausible AntiCOVID-19 Properties
Abstract
:1. Introduction
2. A Snapshot of the Biological Activities of Mushrooms
3. Antioxidant Activity of Mushrooms
3.1. Bioactivity of Mushroom Extracts and Their Polysaccharides
3.1.1. Antioxidant Mushroom Extracts and Polysaccharide Applications
3.1.2. Mechanism of Antioxidant Mushroom Polysaccharides
4. Antiviral Activity of Mushrooms/Mushroom Polysaccharides
AntiCOVID-19 Activity of Mushroom Polysaccharides
| Mushroom | Bioactive Component | Antiviral Activity against | IC50/CC50 Values | Reference |
|---|---|---|---|---|
| Lentinus edodes | Mannoglucan, polysaccharide–protein complex, glucan, lentinan | HSV-1; HNV | IC50: 26.69 mg·mL−1 to 35.12 mg·mL−1 | [295] |
| Grifola frondosa | Proteoglycan, glucan, galatomannan, heteroglycan, and grifolan | Enterovirus 71, HSV-1 | Unspecified | [283] |
| Flammulina velutipes | Glucan-protein complex, glycoprotein | Antitumor, anti-inflammatory, antiviral, immunomodulating | Unspecified | [40] |
| Coriolus versicolor | Polysaccharides PSK and PSP | Antiviral effect on HIV and cytomegalovirus in vitro and anticancer | 6.25–150 μg mL−1 | [330] |
| Daedaleopsis confragosa, Datronia mollis, Ganoderma valesiacum, Irpex lacteus, Ischnoderma benzoinum, Laricifomes officinalis, Lenzites betulina, Phellinus concha-tus, Piptoporus betulinus, Trametes gibbosa, and Trametes versicolor | Mushroom extracts | A/chicken/kurgan/05/2005 (H5N1) bird virus and the A/Aichi/2/68 (H3N2)human virus | Unspecified | [277] |
| Pleurotus pulmonarius | Mushroom water extracts | A/California/07/09 (H1N1pdm) | CC50: 1.7–8 | [278] |
| Pleurotus tuber-regium | β-glucans | Herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2), respiratory syncytial virus (RSV) and influenza A virus (Flu A) | IC50: 3.3–6.8 μg mL−1 | [279] |
| Boletus edulis, Lentinus edodes and Pleurotus ostreatus | Water and methanolic mushroom extracts | Herpes simplex type 1 (HSV-1) | IC50: 26.69 mg mL−1 to 35.12 mg·mL−1 | [280] |
| Agaricus brasiliensis | Polysaccharide | Type 1 poliovirus | IC50: 97.2–922.9 μg mL−1 | [281] |
| Grifola frondosa | Mushroom extracts | Enterovirus 71 and HSV-1 | IC50: 4.1 μg/mL | [282,283] |
| Inonotus obliquus | Mushroom extracts | Hepatitis C virus | TCD50: 6.0 lg/mL | [284] |
| Pleurotus sp. and Lentinus sp. | Methanolic mushroom extracts | Cytomegalovirus (HCMV) | IC50: 180 μg/mL and 160 μg/mL | [285] |
| Pleurotus ostreatus | β-glucans | Influenza virus | IC50: 26.69 mg·mL−1 to 35.12 mg·mL−1 | [280] |
| Ganoderma lucidum | Ganolucidic acid A, 3β-5α-Dihydroxy-6β-Methoxyergosta-7,22-Diene,ganoderic acid A–C, Ganoderic acid β, Ganodermanondiol, Ganodermanontriol and Lucidumol B | Inhibits HIV-1 protease activity | IC50: 0.17–0.23 mM | [287,288,289] |
| Ganoderma colosum | Colossolactones, ganomycin I, and ganomycin B | Anti-HIV-1 protease activity | IC50: 5–39 µg/mL | [290,291] |
| Ganoderma sinnense | Ganoderic acid GS-2, 20-hydroxylucidenic acid N, 20(21)-dehydrolucidenicacid N and ganoderiol F | Anti-HIV-1 protease activity | IC50: 22–116 μM | [290,291] |
| Lignosus rhinocerus | Crude mushroom extracts | Anti-HIV-1 protease activity | Unspecified | [292] |
| Auricularia polytricha | Ergosterol, linoleic acid and two triacylglycerols | Anti-HIV-1 protease activity | IC50: 0.80 ± 0.08 mg/mL | [292] |
| Cordycep militaris | Arabinogalactan (APS) | Anti-HIV-1 protease activity | Unspecified | [297] |
| Russula paludosa | 4.5 kDa protein | Anti-HIV-1 protease activity | IC50 = 0.25 mg/mL | [293] |
| Cordycep sinensis and Cordycep militaris | cordycepin | Anti-HIV-1 | Unspecified | [296,297] |
| Cordycep militaris | cordycepin | DBA/2 mice infected with H1N1 | Unspecified | [232] |
| Cordycep militaris | Acidic polysaccharides (APS) | Anti-influenza | Unspecified | [324] |
| Cordycep militaris | Cordycepin | Anti-Hepatitis C Virus | Unspecified | [294] |
| Grifola frondosa | β-glucan | Inhibit in vitro replication of HSV type 1 | 4.1 μg/ml | [306] |
| Grifola frondosa | Protein extract | Hepatitis B virus | 0.59 mg/mL and 1399 IU/ml | [306] |
| Pleurotus columbinus, Pleurotus sajor-caju, and Agaricus bisporus | Mushroom extracts | Ad7 and HSV2 viruses | Unspecified | [307] |
| Grifola frondosa | D-fraction from Grifola frondosa (GF-D) | Anti HIV | 0.59 mg/mL and 1399 IU/ml | [301] |
| Lentinus edodes | Lentinan | Hematopoietic necrosis virus (IHNV) | Unspecified | [314] |
| Pan cyanescens, Pan natalensis, Pan cubensis and Pan A+ strain | Hot water mushroom extracts | Anti Cox sackievirus (COX-2) | Unspecified | [303,304,305,306] |
| Inonotus obliquus | Aqueous extract | HSV | 3.82 μg/mL | [319] |
| Lentinus edodes | Lentinan | SARS-CoV-2 | Unspecified | [324] |
| Lentinus edodes | Lentinan | Anti-COVID-19 | Unspecified | [329] |
| Inonotus obliquus | Polysaccharides | SARS-CoV-2 virus | Unspecified | [324] |
| Cordycep militaris | Cordycepin | Anti SARS/Anti-COVID-19 | Unspecified | [323,324] |
| Cordycep sinensis | Cordycepin | Anti SARS/Anti-COVID-19 | Unspecified | [323,324] |
5. What Is and What Is to Be
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, S.-T.; Miles, P.G. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ergönül, P.G.; Akata, I.; Kalyoncu, F.; Ergönül, B. Fatty acid compositions of six wild edible mushroom species. Sci. World J. 2013, 2013, 163964. [Google Scholar] [CrossRef] [Green Version]
- Kosanić, M.; Ranković, B.; Rančić, A.; Stanojković, T. Evaluation of metal concentration and antioxidant, antimicrobial, and anticancer potentials of two edible mushrooms Lactarius deliciosus and Macrolepiota procera. J. Food Drug Anal. 2016, 24, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Ruthes, A.C.; Smiderle, F.R.; Iacomini, M. Mushroom heteropolysaccharides: A review on their sources, structure and biological effects. Carbohydr. Polym. 2016, 136, 358–375. [Google Scholar] [CrossRef]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.M.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediat. Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Thomas, P.A.; Sheu, J.R.; Geraldine, P. In-vitro and in-vivo antioxidant effects of the oyster mushroom Pleurotus ostreatus. Food Res. Int. 2011, 44, 851–861. [Google Scholar] [CrossRef]
- Aida, F.M.N.A.; Shuhaimi, M.; Yazid, M.; Maaruf, A.G. Mushroom as a potential source of prebiotics: A review. Trends Food Sci. Technol. 2009, 20, 567–575. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Recent developments in mushrooms as anticancer therapeutics: A review. 3 Biotech 2012, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef] [Green Version]
- Mattila, P.; Konkö, K.; Eurola, M.; Eurola, M.; Pihlava, J.-M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; et al. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Correia, D.M.; Casal, S.; Oliveira, B.; Ferreira, I.C.F.R. Fatty acid and sugar compositions, and nutritional value of fve wild edible mushrooms from Northeast Portugal. Food Chem. 2007, 105, 140–145. [Google Scholar] [CrossRef]
- Barros, L.; Correia, D.M.; Ferreira, I.C.F.R.; Baptista, P.; Santos-Buelga, C. Optimization of the determination of tocopherols in Agaricus sp. edible mushrooms by a normal phase liquid chromatographic method. Food Chem. 2008, 110, 1046–1050. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R.M.V. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, E.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Towards chemical and nutritional inventory of Portuguese wild edible mushrooms in different habitats. Food Chem. 2012, 130, 394–403. [Google Scholar] [CrossRef] [Green Version]
- Vaz, J.A.; Heleno, S.A.; Martins, A.; Almeida, G.M.; Vasconcelos, M.H.; Ferreira, I.C.F.R. Wild mushrooms Clitocybe alexandri and Lepista inversa: In vitro antioxidant activity and growth inhibition of human tumour cell lines. Food Chem. Toxicol. 2010, 48, 2881–2884. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Vaz, J.A.; Vasconcelos, M.H.; Martins, A. Compounds from wild mushrooms with antitumor potential. Anti-Cancer Agents Med. Chem. 2010, 10, 424–436. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.-T.; Wasser, S.P. The role of culinary-medicinal mushrooms on human welfare with a pyramid model for human health. Int. J. Med. Mushrooms 2012, 14, 95–134. [Google Scholar] [CrossRef] [PubMed]
- Finimundy, T.; Gambato, G.; Fontana, R.; Camassola, M.; Salvador, M.; Moura, S.; Hess, J.; Henriques, J.; Dillon, A.; Roesch-Ely, M. Aqueous extracts of Lentinula edodes and Pleurotus sajor-caju exhibit high antioxidant capability and promising in vitro antitumor activity. Nutr. Res. 2013, 33, 76–84. [Google Scholar] [CrossRef]
- Yu, S.; Weaver, V.; Martin, K.; Cantorna, M.T. Te effects of whole mushrooms during inflammation. BMC Immunol. 2009, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Fan, C.; Liu, S.; Zang, Z.; Jiao, L. Chemical composition and antitumor activity of polysaccharide from Inonotus obliquus. J. Med. Plants Res. 2011, 5, 1251–1260. [Google Scholar]
- Chen, J.; Seviour, R. Medicinal importance of fungal β-(1 → 3), (1 → 6 )-glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef]
- Brown, A.C.; Waslien, C.I. Stress and nutrition. In Encyclopedia of Food Sciences and Nutrition; Trugo, L., Finglas, P.M., Eds.; Academic Press: London, UK, 2003. [Google Scholar]
- Carneiro, A.A.J.; Ferreira, I.C.F.R.; Dueñas, M.; Barros, L.; da Silva, R.; Gomes, E.; Santos-Buelga, C. Chemical composition and antioxidant activity of dried powder formulations of Agaricus blazei and Lentinus edodes. Food Chem. 2013, 138, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Yoon, D.H.; Lee, W.H.; Han, S.K.; Shrestha, B.; Kim, C.H.; Lim, M.H.; Chang, W.; Lim, S.; Choi, S.; et al. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-κB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J. Ethnopharmacol. 2007, 114, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Tepe, B.; Yamaç, M. Evaluation of the antioxidant activity of four edible mushrooms from the Central Anatolia, Eskisehir—Turkey: Lactarius deterrimus, Suillus collitinus, Boletus edulis, Xerocomus chrysenteron. Bioresour. Technol. 2008, 99, 6651–6655. [Google Scholar] [CrossRef]
- Synytsya, A.; Mícková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, D.; Liang, Z. Structure of polysaccharides from the fruiting body of Hericium erinaceus Pers. Carbohydr. Polym. 2004, 57, 241–247. [Google Scholar] [CrossRef]
- Flegg, P.B.; Maw, G. Mushrooms and their possible contribution to the world. Mushroom J. 1997, 48, 395–403. [Google Scholar]
- Gruen, F.H.; Wong, M.W. Distribution of cellular amino acids, proteins and total nitrogen during fruit body development in Flammuling velutipes. Can. J. Bot. 1982, 160, 1339–1341. [Google Scholar]
- Kalac, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Mattila, P.; Salo-Väänänen, P.; Könkö, K.; Aro, H.; Jalava, T. Basic composition and amino acid contents of mushrooms cultivated in Finland. J. Agric. Food Chem. 2002, 50, 6419–6422. [Google Scholar] [CrossRef]
- Mdachi, S.J.M.; Nkunya, M.H.H.; Nyigo, V.A.; Urasa, I.T. Amino acid composition of some Tanzanian wild mushrooms. Food Chem. 2004, 86, 179–182. [Google Scholar] [CrossRef]
- Ouzouni, P.K.; Petridis, D.; Koller, W.-D.; Riganakos, K.A. Nutritional value and metal content of wild edible mushrooms collected from West Macedonia and Epirus, Greece. Food Chem. 2009, 115, 1575–1580. [Google Scholar] [CrossRef]
- Ribeiro, B.; de Pinho, P.G.; Andrade, P.; Baptista, P.; Valentão, P. Fatty acid composition of wild edible mushrooms species: A comparative study. Microchem. J. 2009, 93, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Erjavec, J.; Kos, J.; Ravnikar, M.; Dreo, T.; Sabotic, J. Proteins of higher fungi—From forest to application. Trends Biotechnol. 2012, 30, 259–273. [Google Scholar] [CrossRef]
- Wang, X.-M.; Zhang, J.; Wu, L.-H.; Zhao, Y.-L.; Li, T.; Li, J.-Q.; Wang, Y.-Z.; Liu, H.-G. A mini-review of chemical composition and nutritional value of edible wildgrown mushroom from China. Food Chem. 2014, 151, 279–285. [Google Scholar] [CrossRef]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic, polysaccharidic, and lipidic fractions of mushrooms from northeastern portugal: Chemical compounds with antioxidant properties. J. Agric. Food Chem. 2012, 60, 4634–4640. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar]
- Klis, F.M.; De Groot, P.; Hellingwerf, K. Molecular organization of the cell wall of Candida albicans. Med. Mycol. 2001, 39, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Fortes, R.C.; Recôva, V.L.; Melo, A.L.; Novaes, M.R.C.G. Effects of dietary supplementation with medicinal fungus in fasting glycemia levels of patients with colorectal cancer: A randomized, double-blind, placebo-controlled clinical study. Nutr. Hosp. 2008, 23, 591–598. [Google Scholar]
- Shaffique, S.; Kang, S.-M.; Kim, A.-Y.; Imran, M.; Khan, M.A.; Lee, I.-J. Current knowledge of medicinal mushrooms related to anti-oxidant properties. Sustainability 2021, 13, 7948. [Google Scholar] [CrossRef]
- Ren, L.; Perera, C.; Hemar, Y. Antitumor activity of mushroom polysaccharides: A review. Food Funct. 2012, 3, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Fortes, R.C.; Novaes, M.R.C.G. The effects of Agaricus sylvaticus fungi dietary supplementation on the metabolism and blood pressure of patients with colorectal cancer during post surgical phase. Nutr. Hosp. 2011, 26, 176–186. [Google Scholar]
- Deng, G.; Lin, H.; Seidman, A.; Fornier, M.; D’Andrea, G.; Wesa, K.; Yeung, S.; Cunningham-Rundles, S.; Vickers, A.J.; Cassileth, B. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: Immunological effects. J. Cancer Res. Clin. Oncol. 2009, 135, 1215–1221. [Google Scholar] [CrossRef] [Green Version]
- Okuno, K.; Uno, K. Efficacy of orally administered Lentinula edodes mycelia extract for advanced gastrointestinal cancer patients undergoing cancer chemotherapy: A pilot study. Asian Pac. J. Cancer Prev. 2011, 12, 1671–1674. [Google Scholar] [PubMed]
- Tanigawa, K.; Ito, Y.; Sakai, M.; Kobayashi, Y. Evaluation of quality of life and immune function in cancer patients receiving combined immunotherapy and oral administration of lentinula edodes mycelia extract. Gan Kagaku Ryoho 2012, 39, 1779–1781. [Google Scholar]
- Yamaguchi, Y.; Miyahara, E.; Hihara, J. Efficacy and safety of orally administered lentinula edodes mycelia extract for patients undergoing cancer chemotherapy: A pilot study. Am. J. Chin. Med. 2011, 39, 451–459. [Google Scholar] [CrossRef]
- Suzuki, N.; Takimoto, Y.; Suzuki, R.; Arai, T.; Uebaba, K.; Nakai, M.; Strong, J.M.; Tokuda, H. Efficacy of oral administration of Lentinula eododes mycelia extract for breast cancer patients undergoing postoperative hormone therapy. Asian Pac. J. Cancer Prev. 2013, 14, 3469–3472. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, Y.; Maeda, N.; Yamamoto, S.; Yoshino, S.; Oka, M. Evaluation of host quality of life and immune function in breast cancer patients treated with combination of adjuvant chemotherapy and oral administration of Lentinula edodes mycelia extract. OncoTargets Ther. 2013, 6, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Ina, K.; Kataoka, T.; Ando, T. The use of lentinan for treating gastric cancer. Anticancer Agents Med. Chem. 2013, 13, 681–688. [Google Scholar] [CrossRef] [Green Version]
- Valadares, F.; Novaes, M.R.C.G.; Cañete, R. Effect of Agaricus sylvaticus supplementation on nutritional status and adverse events of chemotherapy of breast cancer: A randomized, placebo-controlled, double-blind clinical trial. Indian J. Pharmacol. 2013, 45, 217–222. [Google Scholar] [CrossRef]
- Li, J.; Zou, L.; Chen, W.; Zhu, B.; Shen, N.; Ke, J.; Lou, J.; Song, R.; Zhong, R.; Miao, X. Dietary mushroom intake may reduce the risk of breast cancer: Evidence from a meta-analysis of observational studies. PLoS ONE 2014, 9, e93437. [Google Scholar] [CrossRef]
- Schwartz, B.; Hadar, Y. Possible mechanisms of action of mushroom-derived glucans on inflammatory bowel disease and associated cancer. Ann. Transl. Med. 2014, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Twardowski, P.; Kanaya, N.; Frankel, P.; Synold, T.; Ms, C.R.; Pal, S.K.; Rn, M.J.; Rn, M.P.; Moore, T.; Tryon, P.; et al. A phase I trial of mushroom powder in patients with biochemically recurrent prostate cancer: Roles of cytokines and myeloid-derived suppressor cells for Agaricus bisporus-induced prostate-specific antigen responses. Cancer 2015, 121, 2942–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.H.; Niu, Y.B.; Sun, Y.; Zhang, F.; Liu, C.X.; Fan, L.; Mei, Q.B. Role of phytochemicals in colorectal cancer prevention. World J. Gastroenterol. 2015, 21, 9262–9272. [Google Scholar] [CrossRef]
- Friedman, M. Mushroom polysaccharides: Chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, D. Obesity: Medicinal mushroom reduces obesity by modulating microbiota. Nat. Rev. Endocrinol. 2015, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Tseng, S.-F.; Wu, T.-R.; Chen, Y.-Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.; Førland, D.T.; Sætre, L.; Bernardshaw, S.V.; Lyberg, T.; Hetland, G. Effect of an extract based on the medicinal mushroom agaricus blazei murill on release of cytokines, chemokines and leukocyte growth factors in human blood ex vivo and in vivo. Scand. J. Immun. 2009, 69, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.-S.; Halpern, G.M.; Jones, K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis Part I. J. Altern. Complement. Med. Fall 1998, 4, 289–303. [Google Scholar] [CrossRef]
- Jordan, J.; Sullivan, A.; Lee, T. Immune activation by a sterile aqueous extract of cordyceps sinensis: Mechanism of action. Immunopharmacol. Immunotoxicol. 2008, 30, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Lull, C.; Wichers, H.J.; Savelkoul, H.F.J. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediat. Inflamm. 2005, 2005, 895272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradali, M.-F.; Mostafavi, H.; Ghods, S.; Hedjaroude, G.-A. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). Int. Immunopharmacol. 2007, 7, 701–724. [Google Scholar] [CrossRef]
- Enshasy, H.; Hatti-Kaul, R. Mushroom immunomodulators: Unique molecules with unlimited applications. Trends Biotechnol. 2013, 31, 668–677. [Google Scholar] [CrossRef]
- Jeong, Y.-T.; Yang, B.-K.; Jeong, S.-C.; Kim, S.-M.; Song, C.-H. Ganoderma applanatum: A promising mushroom for antitumor and immunomodulating activity. Phytother. Res. 2008, 22, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Ren, W.; Zhou, Y.; Ma, J.; Ruan, Y.; Wen, C.-N. Triterpenoids from the spores of Ganoderma lucidum. N. Am. J. Med. Sci. 2011, 3, 495–498. [Google Scholar] [CrossRef]
- Su, H.-G.; Peng, X.-R.; Shi, Q.-Q.; Huang, Y.-J.; Zhou, L.; Qiu, M.-H. Lanostane triterpenoids with anti-inflammatory activities from Ganoderma lucidum. Phytochemistry 2020, 173, 112256. [Google Scholar] [CrossRef]
- Li, Q.-Z.; Zheng, Y.-Z.; Zhou, X.-W. Fungal immunomodulatory proteins: Characteristic, potential antitumor activities and their molecular mechanisms. Drug Discov. Today 2019, 24, 307–314. [Google Scholar] [CrossRef]
- Sonawane, H.; Bhosle, H.; Bapat, G.; Vikram, G. Pharmaceutical metabolites with potent bioactivity from mushrooms. J. Pharm. Res. 2014, 8, 969–972. [Google Scholar]
- Sze, S.; Ho, J.; Liu, W. Volvariella volvacea lectin activates mouse T lymphocytes by a calcium dependent pathway. J. Cell. Biochem. 2004, 92, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, A.G.; Wright, K.M.; Zwickey, H.L. Immune modulation from five majorMushrooms: Application to integrative oncology. Integr. Med. (Encinitas Calif.) 2014, 13, 32–44. [Google Scholar]
- Suwannarach, N.; Kumla, J.; Sujarit, K.; Pattananandecha, T.; Saenjum, C.; Lumyong, S. Natural bioactive compounds from fungi as potential candidates for protease inhibitors and immunomodulators to apply for coronaviruses. Molecules 2020, 25, 1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunde-Cimerman, N.; Plemenitaš, A.; Cimerman, A. Pleurotus fungi produce mevinolin, an inhibitor of HMG CoA reductase. FEMS Microbiol. Lett. 1993, 111, 333–337. [Google Scholar] [CrossRef]
- Hossain, S.; Hashimoto, M.; Choudhury, E.K.; Alam, N.; Hussain, S.; Hasan, M.; Choudhury, S.K.; Mahmud, I. Dietary mushroom (Pleurotus ostreatus) ameliorates atherogenic lipid in hypercholesterolaemic rats. Clin. Exp. Pharmacol. Physiol. 2003, 30, 470–475. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M.M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef] [Green Version]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; Van Griensven, L. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozarski, M.; Klaus, A.; Vunduk, J.; Zizak, Z.; Niksic, M.; Jakovljevic, D.; Vrvic, M.M.; Van Griensven, L.J.L.D. Nutraceutical properties of the methanolic extract of edible mushroom Cantharellus cibarius (Fries): Primary mechanisms. Food Funct. 2015, 6, 1875–1886. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-Y.; Ho, K.-J.; Hsieh, Y.-J.; Wang, L.-T.; Mau, J.-L. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT Food Sci. Technol. 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Niksic, M.; Jakovljevic, D.; Todorovic, N.; Van Griensven, L.J.L.D. Antioxidative activities and chemical characterization of polysaccharides extracted from the basidiomycete Schizophyllum commune. LWT Food Sci. Technol. 2011, 44, 2005–2011. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Niksic, M.; Jakovljevic, D.; Todorovic, N.; van Griensven, L.J.L.D.; Stefanoska, I. The edible mushroom Laetiporus sulphureus as potential source of natural antioxidants. Int. J. Food Sci. Nutr. 2013, 64, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Nikšić, M.; Vrvic, M.; Todorović, N.; Jakovljević, D.; Van Griensven, L.J.L.D. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljević, D.; Helsper, J.P.; Van Griensven, L.J. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Vunduk, J.; Todorovic, N.; Jakovljević, D.; Zizak, Z.; Pavlović, V.; Levic, S.; Niksic, M.; Van Griensven, L.J. Biological potential of extracts of the wild edible Basidiomycete mushroom Grifola frondosa. Food Res. Int. 2015, 67, 272–283. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljević, D.; Todorovic, N.; Niksic, M.; Vrvic, M.; van Griensven, L.J. Dietary polysaccharide extracts of Agaricus brasiliensis fruiting bodies: Chemical characterization and bioactivities at different levels of purification. Food Res. Int. 2014, 64, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Glamočlija, J.; Ciric, A.; Nikolic, M.; Fernandes, Â.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R.; Sokovic, M.; van Griensven, L.J.L.D. Chemical characterization and biological activity of Chaga (Inonotus obliquus), a medicinal “mushroom”. J. Ethnopharmacol. 2015, 162, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Debnath, T.; Park, D.K.; Lee, B.R.; Jin, H.L.; Lee, S.Y.; Samad, N.B.; Lim, B.O. Antioxidant activity of Inonotus obliquus grown on germinated brown rice extracts. J. Biochem. 2013, 37, 456–464. [Google Scholar]
- Nakajima, Y.; Sato, Y.; Konishi, T. Antioxidant small phenolic ingredients in Inonotus obliquus (persoon) Pilat (Chaga). Chem. Pharm. Bull. 2007, 55, 1222–1226. [Google Scholar] [CrossRef] [Green Version]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Stojković, D.; Reis, F.S.; Glamočlija, J.; Ćirić, A.; Barros, L.; Van Griensven, L.J.L.D.; Ferreira, I.C.F.R.; Soković, M. Cultivated strains of Agaricus bisporus and A. brasiliensis: Chemical characterization and evaluation of antioxidant and antimicrobial properties for the final healthy product—Natural preservatives in yoghurt. Food Funct. 2014, 5, 1602–1612. [Google Scholar] [CrossRef]
- Ker, Y.-B.; Chen, K.-C.; Chyau, C.-C.; Chen, C.-C.; Guo, J.-H.; Hsieh, C.-L.; Wang, H.-E.; Peng, C.-C.; Chang, C.-H.; Peng, R.Y. Antioxidant capability of polysaccharides fractionated from submerge-cultured agaricus blazei mycelia. J. Agric. Food Chem. 2005, 53, 7052–7058. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.C.T.; Chang, C.A.; Chiuc, K.H.; Tsayd, P.K.; Jena, J.F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Heleno, S.A.; Stojkovic, D.; Barros, L.; Glamočlija, J.; Sokovic, M.; Martins, A.; Queiroz, M.J.; Ferreira, I. A comparative study of chemical composition, antioxidant and antimicrobial properties of Morchella esculenta (L.) Pers. from Portugal and Serbia. Food Res. Int. 2013, 51, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-J.; Nie, S.-P.; Liu, X.-Z.; Zhang, H.; Yang, Y.; Yu, Q.; Xie, M.-Y. Antimicrobial properties, antioxidant activity and cytotoxicity of ethanol-soluble acidic components from Ganoderma atrum. Food Chem. Toxicol. 2012, 50, 689–694. [Google Scholar] [CrossRef]
- Yeh, J.-Y.; Hsieh, L.-H.; Wu, K.-T.; Tsai, C.-F. Antioxidant properties and antioxidant compounds of various extracts from the edible basidiomycete Grifola frondosa (Maitake). Molecules 2011, 16, 3197–3211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajith, T.A.; Janardhanan, K.K. Indian medicinal mushrooms as a source of antioxidant and antitumor agents. J. Clin. Biochem. Nutr. 2007, 40, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.; Gao, X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Mau, J.-L.; Chao, G.-R.; Wu, K.-T. Antioxidant properties of methanolic extracts from several ear mushrooms. J. Agric. Food Chem. 2001, 49, 5461–5467. [Google Scholar] [CrossRef] [PubMed]
- Puttaraju, N.G.; Venkateshaiah, S.U.; Dharmesh, S.M.; Urs, S.M.N.; Somasundaram, R. Antioxidant activity of indigenous edible mushrooms. J. Agric. Food Chem. 2006, 54, 9764–9772. [Google Scholar] [CrossRef]
- Shin, K.H.; Lim, S.S.; Lee, S.H.; Lee, Y.S.; Cho, S.Y. Antioxidant and immunostimulating activities of the fruiting bodies of Paecilomyces japonica, a new type of Cordyceps sp. Ann. N. Y. Acad. Sci. 2006, 928, 261–273. [Google Scholar] [CrossRef]
- Song, W.; van Griensven, L.J.L.D. Pro- and antioxidative properties of medicinal mushroom extracts. Int. J. Med. Mushrooms 2008, 10, 315–324. [Google Scholar]
- Tseng, Y.-H.; Yang, J.-H.; Mau, J.-L. Antioxidant properties of polysaccharides from Ganoderma tsugae. Food Chem. 2008, 107, 732–738. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lin, H.-C.; Mau, J.-L. Antioxidant properties of several commercial mushrooms. Food Chem. 2002, 77, 229–235. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.; Vasconcelos, M.H.; Sokovic, M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, L.; Hemar, Y.; Perera, C.; Lewis, G.; Krissansen, G.W.; Buchanan, P.K. Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioact. Carbohydr. Diet. Fibre 2014, 3, 41–51. [Google Scholar] [CrossRef]
- Yu, Y.; Guzha, N.; Ying, T. Extraction of polysaccharide from Ganoderma lucidum assisted ultrafiltration and optimization of free radical scavenging capacity. J. Chin. Inst. Food Sci. Technol. 2014, 34, 40–46. [Google Scholar]
- Siu, K.-C.; Chen, X.; Wu, J.-Y. Constituents actually responsible for the antioxidant activities of crude polysaccharides isolated from mushrooms. J. Funct. Foods 2014, 11, 548–556. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Wang, W.-D. Optimization of ultrasonic-assisted extraction and in vitro antioxidant activities of polysaccharides from Trametes orientalis. Carbohydr. Polym. 2014, 111, 315–323. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, G.; Yan, H.; Geng, X.; Cao, Q.; Wang, H.; Ng, T.B. Characterization of polysaccharides with antioxidant and hepatoprotective activities from the wild edible mushroom Russula vinosa Lindblad. J. Agric. Food Chem. 2014, 62, 8858–8866. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Pei, J.-J.; Ma, H.-L.; Cai, P.-F.; Yan, J.-K. Effect of extraction media on preliminary characterizations and antioxidant activities of Phellinus linteus polysaccharides. Carbohydr. Polym. 2014, 109, 49–55. [Google Scholar] [CrossRef]
- Fu, H.-Y.; Shieh, D.-E.; Ho, C.-T. Antioxidant and free radical scavenging activities of edible mushrooms. J. Food Lipids 2002, 9, 35–43. [Google Scholar] [CrossRef]
- Elmastas, M.; Isildak, O.; Turkekul, I.; Temur, N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J. Food Compos. Anal. 2007, 20, 337–345. [Google Scholar] [CrossRef]
- Bao, H.N.; Osako, K.; Ohshima, T. Value-added use of mushroom ergothioneine as a colour stabilizer in processed fish meats. J. Sci. Food Agric. 2010, 90, 1634–1641. [Google Scholar] [CrossRef]
- Cheung, L.; Cheung, P.C.; Ooi, V.E. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.; Santos-Buelga, C.; Ferreira, I.C. Fruiting body, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: A comparative study of the antioxidant potential of phenolic and polysaccharidic extracts. Food Res. Int. 2012, 46, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.Y.; Ho, Y.L.; Sheu, M.J.; Lin, Y.H.; Tseng, M.C.; Wu, S.H.; Huang, G.J.; Chang, Y.S. Antioxidant and free radical scavenging activities of Phellinus merrillii extracts. Bot. Stud. 2007, 48, 407–417. [Google Scholar]
- Guo, C.Y.; Ji, S.Z.; Ping, C.X. Modulatory effect of Ganoderma lucidum polysaccharides on serum antioxidant enzymes activities in ovarian cancer rats. Carbohydr. Polym. 2009, 78, 258–262. [Google Scholar]
- Ping, C.X.; Yan, C.; Bing, L.S.; Guo, C.Y.; Yun, L.J.; Ping, L.L. Free radical scavenging of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes and immunity activities in cervical carcinoma rats. Carbohydr. Polym. 2009, 77, 389–393. [Google Scholar]
- Jia, J.; Zhang, X.; Hu, Y.-S.; Wu, Y.; Wang, Q.-Z.; Li, N.-N.; Guo, Q.-C.; Dong, X.-C. Evaluation of in vivo antioxidant activities of Ganoderma lucidum polysaccharides in STZ-diabetic rats. Food Chem. 2009, 115, 32–36. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, S.; Yu, L.; Ma, L. Evaluation of antioxidant property and quality of breads containing Auricularia auricula polysaccharide flour. Food Chem. 2007, 101, 1158–1163. [Google Scholar] [CrossRef]
- Lee, Y.L.; Jian, S.Y.; Lian, P.Y.; Mau, J.L. Antioxidant properties of extracts from a white mutant of the mushroom Hypsizigus marmoreus. J. Food Comp. Anal. 2008, 21, 116–124. [Google Scholar] [CrossRef]
- Mau, J.-L.; Lin, H.-C.; Song, S.-F. Antioxidant properties of several specialty mushrooms. Food Res. Int. 2002, 35, 519–526. [Google Scholar] [CrossRef]
- Murcia, M.A.; Martínez-Tomé, M.; Jiménez, A.M.; Vera, A.M.; Honrubia, M.; Parras, P. Antioxidant activity of edible fungi (truffles and mushrooms): Losses during industrial processing. J. Food Prot. 2002, 65, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Kim, S.-H.; Sa, J.-H.; Jin, C.; Lim, C.-J.; Park, E.-H. Anti-angiogenic, antioxidant and xanthine oxidase inhibition activities of the mushroom Phellinus linteus. J. Ethnopharmacol. 2003, 88, 113–116. [Google Scholar] [CrossRef]
- Acharya, K.; Samui, K.; Rai, M.; Dutta, B.B.; Acharya, R. Antioxidant and nitric oxide synthase activation properties of Auricularia auricula. Indian J. Exp. Biol. 2004, 42, 538–540. [Google Scholar]
- Mau, J.-L.; Chang, C.-N.; Huang, S.-J.; Chen, C.-C. Antioxidant properties of methanolic extracts from Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia. Food Chem. 2004, 87, 111–118. [Google Scholar] [CrossRef]
- Acharya, K.; Yonzone, P.; Rai, M.; Rupa, A. Antioxidant and nitric oxide synthase activation properties of Ganoderma applanatum. Indian J. Exp. Biol. 2005, 43, 926–929. [Google Scholar]
- Cheung, L.; Cheung, P.C. Mushroom extracts with antioxidant activity against lipid peroxidation. Food Chem. 2005, 89, 403–409. [Google Scholar] [CrossRef]
- Lo, K.; Cheung, P.C. Antioxidant activity of extracts from the fruiting bodies of Agrocybe aegerita var. alba. Food Chem. 2005, 89, 533–539. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Chun, J.; Lee, H.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Ribeiro, B.; Rangel, J.; Valentão, P.; Baptista, P.; Seabra, R.M.; Andrade, P.B. Contents of carboxylic acids and two phenolics and antioxidant activity of dried portuguese wild edible mushrooms. J. Agric. Food Chem. 2006, 54, 8530–8537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.H.; Liang, Z.C.; Chia, Y.C.; Lien, J.L.; Chen, K.S.; Lee, M.Y.; Wang, J.C. Antihyperlipidemic and antioxidant effects of extracts from Pleurotus citrinopileatus. J. Agric. Food Chem. 2006, 54, 2103–2110. [Google Scholar] [CrossRef]
- Barros, L.; Ferreira, M.-J.; Queirós, B.; Ferreira, I.; Baptista, P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Ferreira, I.C. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol. 2007, 45, 1731–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dore, C.M.P.G.; Azevedo, T.C.G.; de Souza, M.C.R.; Rego, L.A.; de Dantas, J.C.M.; Silva, F.R.F.; Rocha, H.A.O.; Basela, I.G.; Leite, E.L. Antiinflammatory, antioxidant and cytotoxic actions of beta-glucan-rich extract from Geastrum saecatum mushroom. Int. Immunopharmacol. 2007, 7, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Kitzberger, C.S.G.; Smânia, A.; Pedrosa, R.C.; Ferreira, S.R.S. Antioxidant and antimicrobial activities of shiitake (Lentinula edodes) extracts obtained by organic solvents and supercritical fluids. J. Food Eng. 2007, 80, 631–638. [Google Scholar] [CrossRef]
- Ng, L.T.; Wu, S.J.; Tsai, J.Y.; Lai, M.N. Antioxidant activities of cultured Armillariella mellea. Appl. Biochem. Microbiol. 2007, 43, 495–500. [Google Scholar] [CrossRef]
- Oliveira, O.; Vellosa, J.C.R.; Fernandes, A.; Buffa-Filho, W.; Hakime-Silva, R.; Furlan, M.; Brunetti, I.L. Antioxidant activity of Agaricus blazei. Fitoterapia 2007, 78, 263–264. [Google Scholar] [CrossRef]
- Barros, L.; Falcão, S.I.; Baptista, P.; Freire, C.; Vilas-Boas, M.; Ferreira, I.C. Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem. 2008, 111, 61–66. [Google Scholar] [CrossRef]
- Soares, A.A.; De Souza, C.G.M.; Daniel, F.M.; Ferrari, G.P.; Da Costa, S.M.G.; Peralta, R.M. Antioxidant activity and total phenolic content of Agaricus brasiliensis (Agaricus blazei Murril) in two stages of maturity. Food Chem. 2009, 112, 775–781. [Google Scholar] [CrossRef]
- Obodai, M.; Ferreira, I.C.; Fernandes, A.; Barros, L.; Mensah, D.L.N.; Dzomeku, M.; Urben, A.F.; Prempeh, J.; Takli, R.K. Evaluation of the chemical and antioxidant properties of wild and cultivated mushrooms of ghana. Molecules 2014, 19, 19532–19548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaszek, M.; Osińska-Jaroszuk, M.; Janusz, G.; Matuszewska, A.; Stefaniuk, D.; Sulej, J.; Polak, J.; Ruminowicz, M.; Grzywnowicz, K.; Jarosz-Wilkolazka, A. New bioactive fungal molecules with high antioxidant and antimicrobial capacity isolated from Cerrena unicolor idiophasic cultures. BioMed Res. Int. 2013, 2013, 497492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigand-Heller, J.; Kris-Etherton, P.M.; Beelman, R.B. The bioavailability of ergothioneine from mushrooms (Agaricus bisporus) and the acute effects on antioxidant capacity and biomarkers of inflammation. Prev. Med. 2012, 54, S75–S78. [Google Scholar] [CrossRef] [PubMed]
- Encarnacion, A.B.; Fagutao, F.; Jintasataporn, O.; Worawattanamateekul, W.; Hirono, I.; Ohshima, T. Application of ergothioneine-rich extract from an edible mushroom Flammulina velutipes for melanosis prevention in shrimp, Penaeus monodon and Litopenaeus vannamei. Food Res. Int. 2012, 45, 232–237. [Google Scholar] [CrossRef]
- Encarnacion, A.B.; Fagutao, F.; Hirono, I.; Ushio, H.; Ohshima, T. Effects of ergothioneine from mushrooms (Flammulina velutipes) on melanosis and lipid oxidation of kuruma shrimp (Marsupenaeus japonicus). J. Agric. Food Chem. 2010, 58, 2577–2585. [Google Scholar] [CrossRef]
- Bao, H.N.D.; Ushio, H.; Ohshima, T. Antioxidative Activity and antidiscoloration efficacy of ergothioneine in mushroom (Flammulina velutipes) extract added to beef and fish meats. J. Agric. Food Chem. 2008, 56, 10032–10040. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.; Baptista, P.; Santos-Buelga, C. Phenolic acids determination by HPLC–DAD–ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem. Toxicol. 2008, 47, 1076–1079. [Google Scholar] [CrossRef]
- Vunduk, J.; Klaus, A.; Kozarski, M.; Petrovic, P.; Zizak, Z.; Niksic, M.; van Griensven, L.J.L.D. Did the “Iceman” know better? Screening of the medicinal properties of Piptoporus betulinus. Int. J. Med. Mushrooms 2015, 17, 1113–1125. [Google Scholar] [CrossRef]
- Wei, S.; Helsper, J.P.F.G.; Van Griensven, L.J.L.D. Phenolic compounds present in medicinal mushroom extracts generate reactive oxygen species in human cells in vitro. Int. J. Med. Mushrooms 2008, 10, 1–13. [Google Scholar] [CrossRef]
- Dubost, N.J.; Beelman, R.B.; Peterson, D.; Royse, D.J. Identification and quantification of ergothioneine in cultivated mushrooms by liquid chromatography-mass spectroscopy. Int. J. Med. Mushrooms 2006, 8, 215–222. [Google Scholar] [CrossRef]
- Muszynska, B.; Sulkowska-Ziaja, K.; Ekiert, H. Phenolic acids in selected edible basidiomycota species: Armillaria mellea, Boletus badius, Boletus edulis, Cantharellus cibarius, Lactarius deliciosus and Pleurotus ostreatus. Acta Sci. Pol. Hortorum Cultus 2013, 12, 107–116. [Google Scholar]
- Cheung, Y.-C.; Siu, K.-C.; Liu, Y.-S.; Wu, J.-Y. Molecular properties and antioxidant activities of polysaccharideprotein complexes from selected mushrooms by ultrasound-assisted extraction. Process. Biochem. 2012, 47, 892–895. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.-Y.; Nie, S.-P.; Li, C.; Wang, Y.-X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Barros, L.; Venturini, B.A.; Baptista, P.; Estevinho, M.L.M.F.; Ferreira, I.C.F.R. Chemical composition and biological properties of portuguese wild mushrooms: A comprehensive study. J. Agric. Food Chem. 2008, 56, 3856–3862. [Google Scholar] [CrossRef]
- Suabjakyong, P.; Saiki, P.; Van Griensven, L.J.L.D.; Higashi, K.; Nishimura, K.; Igarashi, K.; Toida, T. Polyphenol extract from phellinus igniarius protects against acrolein toxicity in vitro and provides protection in a mouse stroke model. PLoS ONE 2015, 10, e0122733. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sun, J.; Luo, Z.-Y.; Rao, S.-Q.; Su, Y.-J.; Xu, R.-R.; Yang, Y.-J. Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem. Toxicol. 2012, 50, 1238–1244. [Google Scholar] [CrossRef]
- Bai, M.-S.; Wang, C.; Zong, S.-C.; Lei, M.; Gao, J.-M. Antioxidant polyketide phenolic metabolites from the edible mushroom Cortinarius purpurascens. Food Chem. 2013, 141, 3424–3427. [Google Scholar] [CrossRef]
- Liu, K.; Wang, J.; Zhao, L.; Wang, Q. Anticancer, antioxidant and antibiotic activities of mushroom Ramaria flava. Food Chem. Toxicol. 2013, 58, 375–380. [Google Scholar] [CrossRef]
- Liu, J.; Jia, L.; Kan, J.; Jin, C.-H. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [CrossRef]
- Barbosa, J.R.; dos Santos Freitas, M.M.; da Silva Martins, L.H.; de Carvalho, R.N. Polysaccharides of mushroom Pleurotus spp.: New extraction techniques, biological activities and development of new technologies. Carbohydr. Polym. 2020, 229, 115550. [Google Scholar] [CrossRef]
- Barbosa, J.R.; Freitas, M.M.S.; Oliveira, L.C.; Martins, L.H.S.; Almada-Vilhena, A.O.; Oliveira, R.M.; Pieczarka, J.C.; Brasil, D.D.S.B.; Carvalho Junior, R.N. Obtaining extracts rich in antioxidant polysaccharides from the edible mushroom Pleurotus ostreatus using binary system with hot water and supercritical CO2. Food Chem. 2020, 330, 127173. [Google Scholar] [CrossRef]
- Chaiyama, V.; Keawsompong, S.; LeBlanc, J.G.; De LeBlanc, A.D.M.; Chatel, J.M.; Chanput, W. Action modes of the immune modulating activities of crude mushroom polysaccharide from Phallus atrovolvatus. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100216. [Google Scholar] [CrossRef]
- Mingyi, Y.; Belwal, T.; Devkota, H.P.; Li, L.; Luo, Z. Trends of utilizing mushroom polysaccharides (MPs) as potent nutraceutical components in food and medicine: A comprehensive review. Trends Food Sci. Technol. 2019, 92, 94–110. [Google Scholar] [CrossRef]
- Weis, A.L.; Wasser, S.P. Therapeutic effects of substances occurring in higher basidiomycetes mushrooms: A modern perspective. Crit. Rev. Immunol. 1999, 19, 32–96. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushroom science: History, current status, future trends, and unsolved problems. Int. J. Med. Mushrooms 2010, 12, 1–16. [Google Scholar] [CrossRef]
- Batbayar, S.; Lee, D.-H.; Kim, H.-W. Immunomodulation of fungal β-Glucan in host defense signaling by dectin-1. Biomol. Ther. 2012, 20, 433–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipriani, T.R.; Mellinger, C.G.; de Souza, L.M.; Baggio, C.H.; Freitas, C.S.; Marques, M.C.A.; Gorin, P.A.J.; Sassaki, G.L.; Iacomini, M. A polysaccharide from a tea (infusion) of Maytenus ilicifolia leaves with anti-ulcer protective effects. J. Nat. Prod. 2006, 69, 1018–1021. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Babior, B.M.; Woodman, R.C. Chronic granulomatous disease. Semin. Hematol. 1990, 27, 247–259. [Google Scholar] [PubMed]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, F.; Jia, S.; Ren, H.; Gong, G.; Wang, Y.; Lv, Z.; Liu, Y. Drying effects on the antioxidant properties of polysaccharides obtained from Agaricus blazei Murrill. Carbohydr. Polym. 2014, 103, 414–417. [Google Scholar] [CrossRef]
- Mao, G.; Feng, W.; Xiao, H.; Zhao, T.; Li, F.; Zou, Y.; Ren, Y.; Zhu, Y.; Yang, L.; Wu, X. Purification, characterization, and antioxidant activities of selenium-containing proteins and polysaccharides in royalsun mushroom, Agaricus brasiliensis (higher Basidiomycetes). Int. J. Med. Mushrooms 2014, 16, 463–475. [Google Scholar] [CrossRef]
- Salvador, C.; Martins, M.R.; Caldeira, A.T. Microanalysis characterization of bioactive protein-bound polysaccharides produced by Amanita ponderosa cultures. Microsc. Microanal. 2015, 21, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.Y.; Zhang, J.Y.; Chen, L.J.; Liu, X.C.; Liu, Y.; Wang, W.X.; Zhang, Y.M. Comparative evaluation of polysaccharides isolated from Astragalus, oyster mushroom, and yacon as inhibitors of α-glucosidase. Chin. J. Nat. Med. 2014, 12, 290–293. [Google Scholar] [CrossRef]
- Zhao, S.; Rong, C.; Liu, Y.; Xu, F.; Wang, S.; Duan, C.; Chen, J.; Wu, X. Extraction of a soluble polysaccharide from Auricularia polytricha and evaluation of its anti-hypercholesterolemic effect in rats. Carbohydr. Polym. 2015, 122, 39–45. [Google Scholar] [CrossRef]
- Wang, D.; Sun, S.-Q.; Wu, W.-Z.; Yang, S.-L.; Tan, M.-J. Characterization of a water-soluble polysaccharide from Boletus edulis and its antitumor and immunomodulatory activities on renal cancer in mice. Carbohydr. Polym. 2014, 105, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.B.; Ruthes, A.C.; Baggio, C.H.; Vilaplana, F.; Komura, D.L.; Iacomini, M. Structure and antinociceptive effects of β-D-glucans from Cookeina tricholoma. Carbohydr. Polym. 2016, 141, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Zhu, J.; Liu, T.; Bi, S.; Hu, X.; Chen, Z.; Song, L.; Lv, W.; Yu, R. Structural characterization and biological activities of a novel polysaccharide from cultured Cordyceps militaris and its sulfated derivative. J. Agric. Food Chem. 2015, 63, 3464–3471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Lv, G.Y.; Jiang, X.; Cheng, J.H.; Fan, L.F. Extraction optimization and biological properties of a polysaccharide isolated from Gleoestereum incarnatum. Carbohydr. Polym. 2015, 117, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, G.; Huang, F.; Wu, X.; Yang, H. Improved production, purification and bioactivity of apolysaccharide from submerged cultured Ganoderma lucidum. Arch. Pharm. Res. 2014, 37, 1530–1537. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, F.; Chen, Y.; Zhang, Y.; Hou, L.; Cao, X.; Wang, C. A polysaccharide from Grifola frondosarelieves insulin resistance of HepG2 cell by Akt-GSK-3 pathway. Glycoconj. J. 2014, 31, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.H.; Ren, Y.; Feng, W.W.; Li, Q.; Wu, H.Y.; Jin, D.; Zhao, T.; Xu, C.Q.; Yang, L.Q.; Wu, X.Y. Antitumorand immunomodulatory activity of a water-soluble polysaccharide from Grifola frondosa. Carbohydr. Polym. 2015, 134, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L. Effect of extraction method on structure and antioxidant activity of Hohenbuehelia serotinapolysaccharides. Int. J. Biol. Macromol. 2016, 83, 270–276. [Google Scholar] [CrossRef]
- Yan, P.S.; Cao, L.X.; Zhang, B.Z. Efficient purification of antiproliferative polysaccharides from Hypsizigus marmoreus with radial flow chromatography. Biotechnol. Prog. 2014, 30, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Edwards, P.J.; Perera, C.O.; Hemar, Y. Structural features of a novel polysaccharide isolated from a New Zealand Maori mushroom Iliodiction cibarium. Carbohydr. Res. 2015, 406, 19–26. [Google Scholar] [CrossRef]
- Villares, A. Isolation and characterization of a glucan-type polysaccharide from the red pine mushroom, Lactarius deliciosus (higher Basidiomycetes). Int. J. Med. Mushrooms 2013, 15, 583–589. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Gao, X.; Xu, N.; Lin, L.; Zhao, H.; Jia, S.; Jia, L. Purification, characterization andanti-aging capacity of mycelia zinc polysaccharide by Lentinus edodes SD-08. BMC Complement. Altern. Med. 2015, 15, 111. [Google Scholar] [CrossRef] [Green Version]
- Samanta, S.; Nandi, A.K.; Sen, I.K.; Maity, P.; Pattanayak, M.; Devi, K.S.P.; Khatua, S.; Maiti, T.K.; Acharya, K.; Islam, S.S. Studies on antioxidative and immunostimulating fucogalactan of the edible mushroom Macrolepiota dolichaula. Carbohydr. Res. 2015, 413, 22–29. [Google Scholar] [CrossRef]
- Cao, X.Y.; Liu, J.L.; Yang, W.; Hou, X.; Li, Q.J. Antitumor activity of polysaccharide extracted from Pleurotus ostreatus mycelia against gastric cancer in vitro and in vivo. Mol. Med. Rep. 2015, 12, 2383–2389. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Liao, Z.; Wu, X.; Liu, Y.; Liu, X.; Lin, Z.; Huang, Y.; Liu, B. Isolation, purification, and structuralfeatures of a polysaccharide from Phellinus linteus and its hypoglycemic effect in alloxan-induced diabeticmice. J. Food Sci. 2014, 79, H1002–H1010. [Google Scholar] [CrossRef]
- Yang, K.; Jin, Y.; Xing, C.; Hu, J.; Wang, R.; Sun, P. Chemical characterization and in vitro antioxidantactivity evaluation of polysaccharides from the fruiting bodies of the red heart mushroom Phellinus pini (higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, H.; Ng, T.B. Isolation and purification of polysaccharides with anti-tumor activity from Pholiota adiposa (Batsch) P. Kumm. (higher Basidiomycetes). Int. J. Med. Mushrooms 2012, 14, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, N.; Wang, G.; Zhao, H.; Lin, L.; Jia, M.; Jia, L. In vitro and in vivo antioxidant effects of polysaccharides from Nameko medicinal mushroom, Pholiota nameko SW-01 (higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Yang, W.; Mariga, A.M.; Fang, Y.; Ma, N.; Pei, F.; Hu, Q. Purification, characterization and antitumoractivity of polysaccharides from Pleurotus eryngii residue. Carbohydr. Polym. 2014, 114, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, T.; Zhou, H.; Zhang, Y.; Jin, G.; Yang, Y. Antidiabetic effect of polysaccharides from Pleurotus ostreatus in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2016, 83, 126–132. [Google Scholar] [CrossRef]
- Manna, D.K.; Nandi, A.K.; Pattanayak, M.; Maity, P.; Tripathy, S.; Mandal, A.K.; Roy, S.; Tripathy, S.S.; Gupta, N.; Islam, S.S. A water soluble β-glucan of an edible mushroom Termitomyces heimii: Structural and biological investigation. Carbohydr. Polym. 2015, 134, 375–384. [Google Scholar] [CrossRef]
- Cheng, H.; Jia, Y.; Wang, L.; Liu, X.; Liu, G.; Li, L.; He, C. Isolation and structural elucidation of a novel homogenous polysaccharide from Tricholoma matsutake. Nat. Prod. Res. 2016, 30, 58–64. [Google Scholar] [CrossRef]
- You, Q.; Yin, X.; Zhao, Y.; Jiang, Z. Extraction and purification of polysaccharides from pine medicinal mushroom, Tricholoma matsutake (higher Basidiomycetes) fruit bodies. Int. J. Med. Mushrooms 2014, 16, 149–160. [Google Scholar] [CrossRef]
- Yin, X.; You, Q.; Su, X. A comparison study on extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr. Polym. 2014, 102, 419–422. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Song, J.H.; Wang, J.; Yang, J.M.; Wang, Z.B.; Liu, Y.H. Optimization of cellulase-assisted extraction process and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai. J. Sci. Food Agric. 2016, 96, 4484–4491. [Google Scholar] [CrossRef]
- Masuda, Y.; Nawa, D.; Nakayama, Y.; Konishi, M.; Nanba, H. Soluble β-glucan from Grifola frondosa induces tumor regression in synergy with TLR9 agonist via dendritic cell-mediated immunity. J. Leukoc. Biol. 2015, 98, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Han, Y.; Huang, J.; Yu, X.; Jiao, C.; Yang, X.; Dhaliwal, P.; Xie, Y.; Yang, B.B. Purification and identification of a polysaccharide from medicinal mushroom Amauroderma rude with immunomodulatory activity and inhibitory effect on tumor growth. Oncotarget 2015, 6, 17777–17791. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.S.; Kuo, H.P.; Chang, K.L.; Kong, Z.L. Apoptosis of hepatocellular carcinoma cells induced by nanoencapsulated polysaccharides extracted from Antrodia camphorata. PLoS ONE 2015, 10, e0136782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.P.; Nam, S.H.; Friedman, M. Correction to Hericium erinaceus (Lion’s Mane) Mushroom Extracts Inhibit Metastasis of Cancer Cells to the Lung in CT-26 Colon Cancer-Transplanted Mice. J. Agric. Food Chem. 2013, 61, 5411. [Google Scholar] [CrossRef]
- Gaullier, J.M.; Sleboda, J.; Øfjord, E.S.; Ulvestad, E.; Nurminiemi, M.; Moe, C.; Tor, A.; Gudmundsen, O. Supplementation with a soluble-glucan exported from Shiitake medicinal mushroom, Lentinus edodes (Berk.) singer mycelium: A crossover, placebo-controlled study in healthy elderly. Int. J. Med. Mushrooms 2011, 13, 319–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef]
- Yan, J.K.; Pei, J.J.; Ma, H.L.; Wang, Z.B.; Liu, Y.S. Advances in antitumor polysaccharides from Phellinus sensu lato: Production, isolation, structure, antitumor activity, and mechanisms. Crit. Rev. Food Sci. Nutr. 2015, 57, 1256–1269. [Google Scholar] [CrossRef]
- Bergendiova, K.; Tibenska, E.; Majtan, J. Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur. J. Appl. Physiol. 2011, 111, 2033–2040. [Google Scholar] [CrossRef]
- Sen, I.K.; Mandal, A.K.; Chakraborti, S.; Dey, B.; Chakraborty, R.; Islam, S.S. Green synthesis of silver nanoparticles using glucan from mushroom and study of antibacterial activity. Int. J. Biol. Macromol. 2013, 62, 439–449. [Google Scholar] [CrossRef]
- Cui, H.-Y.; Wang, C.-L.; Wang, Y.-R.; Li, Z.-J.; Chen, M.-H.; Li, F.-J.; Sun, Y.-P. Pleurotus nebrodensis polysaccharide (PN-S) enhances the immunity of immunosuppressed mice. Chin. J. Nat. Med. 2015, 13, 760–766. [Google Scholar] [CrossRef]
- Hu, S.-H.; Cheung, P.C.K.; Hung, R.-P.; Chen, Y.-K.; Wang, J.-C.; Chang, S.-J. Antitumor and immunomodulating activities of exopolysaccharide produced by big cup culinary-medicinal mushroom Clitocybe maxima (higher Basidiomycetes) in liquid submerged culture. Int. J. Med. Mushrooms 2015, 17, 891–901. [Google Scholar] [CrossRef]
- Kim, S.P.; Park, S.O.; Lee, S.J.; Nam, S.H.; Friedman, M. A polysaccharide isolated from the liquid culture of Lentinus edodes (Shiitake) mushroom mycelia containing black rice bran protects mice against a Salmonella lipopolysaccharide-induced endotoxemia. J. Agric. Food Chem. 2013, 61, 10987–10994. [Google Scholar] [CrossRef]
- Kim, S.P.; Moon, E.; Nam, S.H.; Friedman, M. Hericium erinaceus mushroom extracts protect infected mice against Salmonella Typhimurium-induced liver damage and mortality by stimulation of innate immune cells. J. Agric. Food Chem. 2012, 60, 5590–5596. [Google Scholar] [CrossRef] [PubMed]
- Signoretto, C.; Marchi, A.; Bertoncelli, A.; Burlacchini, G.; Papetti, A.; Pruzzo, C.; Zaura, E.; Lingström, P.; Ofek, I.; Pratten, J.; et al. The anti-adhesive mode of action of a purified mushroom (Lentinus edodes) extract with anticaries and antigingivitis properties in two oral bacterial phatogens. BMC Complement. Altern. Med. 2014, 14, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, M.; Lin, Y.; Luo, Y.L.; Liang, H.H.; Sun, P.L. Extraction, antimicrobial, and antioxidant activities of crude polysaccharides from the Wood Ear medicinal mushroom Auricularia auricula-judae (higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shah, N.P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275. Food Chem. 2014, 165, 262–270. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Nutrition, and health-promoting properties of Hericium erinaceus (Lion’s Mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef]
- Chen, P.; Yong, Y.; Gu, Y.; Wang, Z.; Zhang, S.; Lu, L. Comparison of antioxidant and antiproliferation activities of polysaccharides from eight species of medicinal mushrooms. Int. J. Med. Mushrooms 2015, 17, 287–295. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Li, Q.; Zhao, T.; Zhang, M.; Feng, W.; Takase, M.; Wu, X.; Zhou, Z.; Yang, L.; et al. Preparation, characterization, and anti-Helicobacter pylori activity of Bi3+-Hericium erinaceus polysaccharide complex. Carbohydr. Polym. 2014, 110, 231–237. [Google Scholar] [CrossRef]
- Huang, H.Y.; Korivi, M.; Chaing, Y.Y.; Chien, T.Y.; Tsai, Y.C. Pleurotus tuber-regium polysaccharides attenuate hyper-glycemia and oxidative stress in experimental diabetic rats. Evid. Based Complement. Altern. Med. 2012, 2012, 856381. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.Y.; Korivi, M.; Yang, H.T.; Huang, C.C.; Chaing, Y.Y.; Tsai, Y.C. Effect of Pleurotus tuber-regium polysaccha-rides supplementation on the progression of diabetes complications in obese-diabetic rats. Chin. J. Physiol. 2014, 57, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Berven, L.; Karppinen, P.; Hetland, G.; Samuelsen, A.B.C. The polar high molecular weight fraction of the Agaricus blazei Murill extract, AndoSan, reduces the activity of the tumor-associated protease, legumain, in RAW 264.7 cells. J. Med. Food 2015, 18, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Johnson, E.; Lyberg, T.; Kvalheim, G. The mushroom Agaricus blazei Murill elicits medicinal effects on tumor, infection, allergy, and inflammation through Its modulation of innate immunity and amelioration of Th1/Th2 imbalance and inflammation. Adv. Pharmacol. Sci. 2011, 2011, 157015. [Google Scholar] [PubMed]
- Tangen, J.-M.; Tierens, A.; Caers, J.; Binsfeld, M.; Olstad, O.K.; Trøseid, A.-M.S.; Wang, J.; Tjønnfjord, G.E.; Hetland, G. Immunomodulatory effects of the Agaricus blazei Murrill-based mushroom extract AndoSan in patients with multiple myeloma undergoing high dose chemotherapy and autologous stem cell transplantation: A randomized, double blinded clinical study. BioMed Res. Int. 2015, 2015, 718539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Shao, H. Extract from Agaricus blazei Murill can enhance immune responses elicited by DNA vaccine against foot-and-mouth disease. Vet. Immunol. Immunopathol. 2006, 109, 177–182. [Google Scholar] [CrossRef]
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.-J.; Han, S.K.; Park, J.H.; Park, H.; Sung, J.-M.; et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-κB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 2006, 545, 192–199. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; He, Y.; Li, T.; Wang, W.; Zhang, J.; Wei, J.; Deng, Y.; Lin, R. Cordycepin alleviates airway hyperreactivity in a murine model of asthma by attenuating the inflammatory process. Int. Immunopharmacol. 2015, 26, 401–408. [Google Scholar] [CrossRef]
- Mueller, W.E.G.; Weiler, B.E.; Charubala, R.; Pfleiderer, W.; Leserman, L.; Sobol, R.W.; Suhadolnik, R.J.; Schroeder, H.C. Cordycepin analogs of 2′,5′-oligoadenylate inhibit human immunodeficiency virus infection via inhibition of reverse transcriptase. Biochemistry 1991, 30, 2027–2033. [Google Scholar] [CrossRef]
- Jiang, Y.; Wong, J.H.; Fu, M.; Ng, T.B.; Liu, Z.K.; Wang, C.R.; Li, N.; Qiao, W.T.; Wen, T.Y.; Liu, F. Isolation of adenosine, iso-sinensetin and dimethylguanosine with antioxidant and HIV-1 protease inhibiting activities from fruiting bodies of Cordyceps militaris. Phytomedicine 2011, 18, 189–193. [Google Scholar] [CrossRef]
- Lee, H.H.; Park, H.; Sung, G.-H.; Lee, K.; Lee, T.; Lee, I.; Park, M.-S.; Jung, Y.W.; Shin, Y.S.; Kang, H.; et al. Anti-influenza effect of Cordyceps militaris through immunomodulation in a DBA/2 mouse model. J. Microbiol. 2014, 52, 696–701. [Google Scholar] [CrossRef]
- Ohta, Y.; Lee, J.-B.; Hayashi, K.; Fujita, A.; Park, D.K.; Hayashi, T. In vivo anti-influenza virus activity of an immunomodulato-ry acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. J. Agric. Food Chem. 2007, 55, 10194–10199. [Google Scholar] [CrossRef]
- Badalyan, S.M. Edible and medicinal higher basidiomycetes mushrooms as a source of natural antioxidants. Int. J. Med. Mushrooms 2003, 5, 153–162. [Google Scholar] [CrossRef]
- Mau, J.-L.; Lin, H.-C.; Chen, C.-C. Antioxidant properties of several medicinal mushrooms. J. Agric. Food Chem. 2002, 50, 6072–6077. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Seguin, P.; Ahn, J.-K.; Kim, J.-J.; Chun, S.-C.; Kim, E.-H.; Seo, S.-H.; Kang, E.-Y.; Kim, S.-L.; Park, Y.-J.; et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Sulkowska-Ziaja, K.; Muszynska, B.; Motyl, P.; Pasko, P.; Ekiert, H. Phenolic compounds and antioxidant activity in some species of polyporoid mushrooms from Poland. Int. J. Med. Mushrooms 2012, 14, 385–393. [Google Scholar] [CrossRef]
- Aziz, T.; Mehmet, E.D.; Nazime, M.; Ibrahim, K.; Kudret, G. Antimicrobial and antioxidant activities of mycelia of 10 wild mushroom species. J. Med. Food 2010, 13, 415–419. [Google Scholar]
- Lee, I.-K.; Kim, Y.-S.; Jang, Y.-W.; Jung, J.-Y.; Yun, B.-S. New antioxidant polyphenols from the medicinal mushroom Inonotus obliquus. Bioorg. Med. Chem. Lett. 2007, 17, 6678–6681. [Google Scholar] [CrossRef]
- Song, X.; Cai, W.; Ren, Z.; Jia, L.; Zhang, J. Antioxidant and hepatoprotective effects of acidic-hydrolysis residue polysaccharides from shiitake culinary-medicinal mushroom Lentinus edodes (Agaricomycetes) in mice. Int. J. Med. Mushrooms 2021, 23, 85–96. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Nabavi, S.M.; Nabavi, S.F.; Eslami, S. Antioxidant and free radical scavenging activities of culinary-medicinal mushrooms, golden chanterelle Cantharellus cibarius and angel’s wings Pleurotus porrigens. Int. J. Med. Mushrooms 2010, 12, 265–272. [Google Scholar] [CrossRef]
- Woldegiorgis, A.Z.; Abate, D.; Haki, G.D.; Ziegler, G. Antioxidant property of edible mushrooms collected from Ethiopia. Food Chem. 2014, 157, 30–36. [Google Scholar] [CrossRef]
- Liang, C.-H.; Ho, K.-J.; Huang, L.-Y.; Tsai, C.-H.; Lin, S.-Y.; Mau, J.-L. Antioxidant properties of fruiting bodies, mycelia, and fermented products of the culinary-medicinal king oyster mushroom, Pleurotus eryngii (higher Basidiomycetes), with high ergothioneine content. Int. J. Med. Mushrooms 2013, 15, 267–275. [Google Scholar] [CrossRef]
- Nhi, N.; Hung, P. Nutritional composition and antioxidant capacity of several edible mushrooms grown in the Southern Vietnam. Int. Food Res. J. 2012, 19, 611–615. [Google Scholar]
- Mau, J.-L.; Tsai, S.-Y.; Tseng, Y.-H.; Huang, S.-J. Antioxidant properties of hot water extracts from Ganoderma tsugae Murrill. LWT 2005, 38, 589–597. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Estevinho, L.; Ferreira, I.; Estevinho, M.L.M.F. Bioactive properties of the medicinal mushroom Leucopaxillus giganteus mycelium obtained in the presence of different nitrogen sources. Food Chem. 2007, 105, 179–186. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Huang, S.-J.; Lo, S.-H.; Wu, T.-P.; Lian, P.-Y.; Mau, J.-L. Flavour components and antioxidant properties of several cultivated mushrooms. Food Chem. 2009, 113, 578–584. [Google Scholar] [CrossRef]
- Wong, J.Y.; Chye, F.Y. Antioxidant properties of selected tropical wild edible mushrooms. J. Food Compos. Anal. 2009, 22, 269–277. [Google Scholar] [CrossRef]
- Kalač, P. (Ed.) Edible Mushrooms: Chemical Composition and Nutritional Value; Elsevier Academic Press: Cambridge, CA, USA, 2016. [Google Scholar]
- Masterson, C.H.; Murphy, E.J.; Gonzalez, H.; Major, I.; Mccarthy, S.D.; O’Toole, D.; Rowan, N.J. Purified β-glucans from the Shiitake mushroomameliorates antibiotic-resistant Klebsiella pneumoniae-induced pulmonary sepsis. Lett. Appl. Microbiol. 2020, 71, 405–412. [Google Scholar] [PubMed]
- Lee, J.S.; Hong, E.K. Immunostimulating activity of the polysaccharides isolated from Cordyceps militaris. Int. Immunopharmacol. 2011, 11, 1226–1233. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, Q.; Zhou, S.; Liu, Y.; Zhang, Z.; Gao, X.; Wang, S.; Wang, Z. Isolation and purification of apolysaccharide from the caterpillar medicinal mushroom Cordyceps militaris (Ascomycetes) fruit bodies and its immunomodulation of RAW 264.7 macrophages. Int. J. Med. Mushrooms 2014, 16, 247–257. [Google Scholar] [CrossRef]
- Mu, H.; Zhang, A.; Zhang, W.; Cui, G.; Wang, S.; Duan, J. Antioxidative properties of crude polysaccharides from Inonotus obliquus. Int. J. Mol. Sci. 2012, 13, 9194–9206. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Lv, G.; Pan, H.; Pandey, A.; He, W.; Fan, L. Antioxidant and hepatoprotective potential of endo-polysaccharides from Hericium erinaceus grown on tofu whey. Int. J. Biol. Macromol. 2012, 51, 1140–1146. [Google Scholar] [CrossRef]
- Nandi, A.K.; Samanta, S.; Maity, S.; Sen, I.K.; Khatua, S.; Devi, K.S.P.; Acharya, K.; Maiti, T.K.; Islam, S.S. Antioxidant and immunostimulant β-glucan from edible mushroom Russula albonigra (Krombh.) Fr. Carbohydr. Polym. 2014, 99, 774–782. [Google Scholar] [CrossRef]
- Cheng, J.H.; Tsai, C.L.; Lien, Y.Y.; Lee, M.S.; Sheu, S.C. High molecular weight of polysaccharides from Hericium erinaceus against amyloid β-induced neurotoxicity. BMC Complement. Altern. Med. 2016, 16, 170. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Zhang, Y.; Xie, J.; Wang, L.; Zhang, H.; Wei, W.; Li, Y. Royal Sun medicinal mushroom, Agaricus brasiliensis (Agaricomycetidae), derived polysaccharides rxert immunomodulatory activities in vitro and in vivo. Int. J. Med. Mushrooms 2016, 18, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; You, Q.; Zhou, X. Complex enzyme-assisted extraction, purification, and antioxidant activity of polysaccharides from the button mushroom, Agaricus bisporus (higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Alzorqi, I.; Sudheer, S.; Lu, T.J.; Manickam, S. Ultrasonically extracted β-D-glucan from artificially cultivated mushroom, characteristic properties and antioxidant activity. Ultrason. Sonochem. 2016, 35, 531–540. [Google Scholar] [CrossRef]
- Maity, P.; Sen, I.K.; Maji, P.K.; Paloi, S.; Devi, K.S.P.; Acharya, K.; Maiti, T.K.; Islam, S.S. Structural, immunological, and antioxidant studies of β-glucan from edible mushroom Entoloma lividoalbum. Carbohydr. Polym. 2015, 123, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.T.; Santos, M.N.; de Souza, L.A.; Pinheiro, T.S.; Paiva, A.A.; Dore, C.M.; Costa, M.S.; Santos, N.D.; Baseia, Y.G.; Araujo, R.M.; et al. Chemical characteristics of a heteropolysaccharide from Tylopilus ballouiimushroom and its antioxidant and anti-inflammatory activities. Carbohydr. Polym. 2016, 144, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Wang, L.; Walid, E.; Zhang, H. In vitro antioxidant and anti-proliferation activities of polysaccharides from various extracts of different mushrooms. Int. J. Mol. Sci. 2012, 13, 5801–5817. [Google Scholar] [CrossRef] [Green Version]
- Khatua, S.; Paul, S.; Acharya, K. Mushroom as the potential source of new generation of antioxidant: A review. Res. J. Pharm. Technol. 2013, 6, 496–505. [Google Scholar]
- Mohsin, M.; Negi, P.S.; Ahmed, Z. Determination of the antioxidant activity and polyphenol contents of wild Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt. Fr.) P. Karst. (higher Basidiomycetes) from central Himalayan hills of India. Int. J. Med. Mushrooms 2011, 13, 535–544. [Google Scholar] [CrossRef]
- Mathew, J.; Sudheesh, N.P.; Rony, K.A.; Smina, T.P.; Janardhanan, K.K. Antioxidant and antitumor activities of cultured mycelium of culinary-medicinal paddy straw mushroom Volvariella volvacea (Bull.: Fr.) singer (agaricomycetideae). Int. J. Med. Mushrooms 2008, 10, 139–147. [Google Scholar] [CrossRef]
- Turkoglu, A.; Kivrak, I.; Mercan, N.; Duru, M.E.; Gezer, K.; Turkoglu, H. Antioxidant and antimicrobial activities of Morchella conica Pers. Afr. J. Biotechnol. 2006, 5, 1146–1150. [Google Scholar]
- Mau, J.-L.; Tsai, S.-Y.; Tseng, Y.-H.; Huang, S.-J. Antioxidant properties of methanolic extracts from Ganoderma tsugae. Food Chem. 2005, 93, 641–649. [Google Scholar] [CrossRef]
- Xiao, J.-H.; Xiao, D.-M.; Chen, D.-X.; Xiao, Y.; Liang, Z.-Q.; Zhong, J.-J. Polysaccharides from the medicinal mushroom cordyceps taii show antioxidant and immunoenhancing activities in a D-Galactose-induced aging mouse model. Evid. Based Complement. Altern. Med. 2012, 2012, 273435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozarski, M.; Klaus, A.; Niksic, M.; Van Griensven, L.J.L.D.; Vrvic, M.; Jakovljevic, D. Polysaccharides of higher fungi: Biological role, structure, and antioxidative activity. Chem. Ind. 2014, 68, 305–320. [Google Scholar] [CrossRef]
- Lindequist, U.; Niedermeyer, T.; Jülich, W.-D. The pharmacological potential of mushrooms. Evid. Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Tsiapali, E.; Whaley, S.; Kalbfleisch, J.; Ensley, H.E.; Browder, I.; Williams, D.L. Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radic. Biol. Med. 2001, 30, 393–402. [Google Scholar] [CrossRef]
- Kishk, Y.F.M.; Al-Sayed, H.M.A. Free-radical scavenging and antioxidative activities of some polysaccharides in emulsions. LWT Food Sci. Technol. 2007, 40, 270–277. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Ge, Q.; Mao, J.-W.; Zhang, A.-Q.; Wang, Y.-J.; Sun, P.-L. Purification, chemical characterization, and antioxidant activity of a polysaccharide from the fruiting bodies of sanghuang mushroom (Phellinus baumii Pilát). Food Sci. Biotechnol. 2013, 22, 301–307. [Google Scholar] [CrossRef]
- Li, N.; Li, L.; Fang, J.C.; Wong, J.H.; Ng, T.B.; Jiang, Y.; Wang, C.R.; Zhang, N.Y.; Wen, T.Y.; Qu, L.Y.; et al. Isolation and identification of a novel polysaccharide-peptide complex with antioxidant, anti-proliferative and hypoglycaemic activities from the abalone mushroom. Biosci. Rep. 2012, 32, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.T.; Zeng, H.L.; Xu, Z.B.; Zheng, B.D.; Lin, Y.X.; Gan, C.J.; Lo, Y.M. Ultrasonic-assisted extraction and antioxidant activity of polysaccharides recovered from white button mushroom (Agaricus bisporus). Carbohydr. Polym. 2012, 88, 522–529. [Google Scholar] [CrossRef]
- Teplyakova, T.V.; Psurtseva, N.V.; Kosogova, T.A.; Mazurkova, N.A.; Khanin, V.A.; Vlasenko, V.A. Antiviral activity of polyporoid mushrooms (higher Basidiomycetes) from altai mountains (Russia). Int. J. Med. 2012, 14, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ilyicheva, T.N.; Teplyakova, T.V.; Svyatchenko, S.V.; Asbaganov, S.V.; Zmitrovich, I.V.; Vlasenko, A.V. Antiviral activity of total polysaccharide fraction of water and ethanol extracts of pleurotus pulmonarius against the influenza a virus. Curr. Res. Environ. Appl. Mycol. J. Fungal Biol. 2020, 10, 224–235. [Google Scholar]
- Zhang, M.; Cheung, P.C.; Ooi, V.E.; Zhang, L. Evaluation of sulfatedfungal β-glucans from the sclerotium of pleurotus tuber-regium as a potential water-solubleanti-viral agent. Carbohydr. Res. 2004, 339, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, S.; Ramírez-Anguiano, A.C.; Aldars-García, L.; Reglero, G.; Soler-Rivas, C. Antiviral activities of boletus edulis, pleurotus ostreatus and lentinus edodes extracts and polysaccharide fractions against herpes simplex virus type 1. J. Food Nutr. Res. 2012, 51, 225–235. [Google Scholar]
- Faccin, L.C.; Benati, F.; Rincao, V.P.; Mantovani, M.S.; Soares, S.A.; Gonzaga, M.L.; Carvalho Linhares, R.E. Antiviral activity of aqueous and ethanol extracts and of an isolated polysaccharide from agaricus brasiliensis against poliovirus type 1. Lett. Appl. Microbiol. 2007, 45, 24–28. [Google Scholar] [CrossRef]
- Gu, C.-Q.; Li, J.-W.; Chao, F.; Jin, M.; Wang, X.-W.; Shen, Z.-Q. Isolation, identification and function of a novel anti-HSV-1 protein from Grifola frondosa. Antivir. Res. 2007, 75, 250–257. [Google Scholar] [CrossRef]
- Zhao, C.; Gao, L.; Wang, C.; Liu, B.; Jin, Y.; Xing, Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from grifolafrondosa against enterovirus 71. Carbohydr. Polym. 2016, 144, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Shibnev, V.A.; Mishin, D.V.; Garaev, T.M.; Finogenova, N.P.; Botikov, A.G.; Deryabin, P.G. Antiviral activity of inonotus obliquus fungus extract towards infection caused by hepatitis c virus in cell cultures. Bull. Exp. Biol. Med. 2011, 151, 612–614. [Google Scholar] [CrossRef]
- Roy, D.; Ansari, S.; Chatterjee, A.; Luganini, A.; Ghosh, S.K.; Chakraborty, N. In vitro search for antiviral activity against humancytomegalovirus from medicinal mushrooms Pleurotus sp. and Lentinus sp. J. Antivir. Antiretrovir. 2020, 12, 201. [Google Scholar]
- Stovall, M.; Smith, S.A.; Langholz, B.M.; Boice, J.D., Jr.; Shore, R.E.; Andersson, M.; Malone, K.E. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Elmekkawy, S.; Meselhy, M.R.; Nakamura, N.; Tezuka, Y.; Hattori, M.; Kakiuchi, N.; Shimotohno, K.; Kawahata, T.; Otake, T. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma Lucidum. Phytochemistry 1998, 49, 1651–1657. [Google Scholar] [CrossRef]
- Min, B.S.; Nakamura, N.; Miyashiro, H.; Bae, K.W.; Hattori, M. Triterpenes from the spores of Ganodermalucidum and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 1998, 46, 1607–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Montemayor, M.; Ling, T.; Suárez-Arroyo, I.J.; Ortiz-Soto, G.; Santiago-Negrón, C.L.; Lacourt-Ventura, M.Y.; Valentín-Acevedo, A.; Lang, W.H.; Rivas, F. Identification of biologically Active Ganoderma lucidum compounds and synthesis of improved derivatives That confer anti-cancer activities in vitro. Front. Pharmacol. 2019, 10, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Dine, R.S.; Halawany, A.M.E.; Ma, C.M.; Hattori, M. Anti-HIV1- protease activity of lanostane triterpenes from the Vienamese mushroom Ganoderma colossum. J. Nat. Prod. 2008, 71, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- El Dine, R.S.; El-Halawany, A.; Ma, C.-M.; Hattori, M. Inhibition of the dimerization and active site of HIV-1 protease by secondary metabolites from the vietnamese mushroom ganoderma colossum. J. Nat. Prod. 2009, 72, 2019–2023. [Google Scholar] [CrossRef]
- Sillapachaiyaporn, C.; Nilkhet, S.; Ung, A.T.; Chuchawankul, S. Anti-HIV-1 protease activity of the crudeextracts and isolated compounds from Auricularia polytricha. BMC Complement. Altern. Med. 2019, 19, 351. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, H.; Ng, T. A peptide with HIV-1 reverse transcriptase inhibitory activity from the medicinal mushroom Russula paludosa. Peptides 2007, 28, 560–565. [Google Scholar] [CrossRef]
- Ueda, Y.; Mori, K.; Satoh, S.; Dansako, H.; Ikeda, M.; Kato, N. Anti-HCV activity of the Chinese medicinal fungus Cordyceps militaris. Biochem. Biophys. Res. Commun. 2014, 447, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.-M.; Pan, G.-F.; Guo, J.-Y. Anti-inflammatory and antinociceptive effects of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis. Nat. Prod. Res. 2012, 26, 2358–2362. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Sun, H.-L.; Sheu, J.-N.; Ku, M.-S.; Hu, C.-M.; Chan, Y.; Lue, K.H. Effects of the immunomodulatory agent Cordyceps militaris on airway inflammation in a mouse asthma model. Pediatr. Neonatol. 2008, 49, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Kelly, E.A.; Jarjour, N.N. Role of matrix metalloproteinases in asthma. Curr. Opin. Pulm. Med. 2003, 9, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-K.; Shen, W. Inhibitive effect of cordyceps sinensis on experimental hepatic fibrosis and its possible mechanism. World J. Gastroenterol. 2003, 9, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Kaymakci, M.A.; Guler, E.M. Promising potential pharmaceuticals from the genus cordyceps for COVID-19 treatment: A review study. Bezmialem Sci. 2020, 8, 140–144. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Hao, C.; Zeng, P.; Zhang, M.; Liu, Y.; Chang, Y.; Zhang, L. Grifola frondosa polysaccharide: A review of antitumor and other biological activity studies in China. Discov. Med. 2018, 25, 159–176. [Google Scholar]
- Gu, C.-Q.; Li, J.W.; Chao, F.-H. Inhibition of hepatitis B virus by D-fraction from Grifola frondosa: Synergistic effect of combination with interferon-α in HepG2 2.2. Antivir. Res. 2006, 72, 162–165. [Google Scholar] [CrossRef]
- Nanba, H.; Kodama, N.; Schar, D.; Turner, D. Effects of maitake (Grifola frondosa) glucan in HIV-infected patients. Mycoscience 2000, 41, 293–295. [Google Scholar] [CrossRef]
- Baraf, H.S. Efficacy of the newest COX-2 selective inhibitors in rheumatic disease. Curr. Pharm. Des. 2007, 13, 2228–2236. [Google Scholar] [CrossRef]
- Bustillos, R.G.; Dulay, R.M.R.; Bauto, J.J.; Pascual, F.; Baltazar, K.; Bunag, H.W.; Macatula, A.; Nicolas, M.A.; Torres, M.A.A.; Nillosa, J.C.; et al. Mycochemical profle of mycelia and fruiting body of Panaeolus cyanescens and its optimal submerged culture conditions for antioxidant properties. Int. J. Pure Appl. Biosci. 2014, 2, 175–181. [Google Scholar]
- Dhanasekaran, D.; Latha, S.; Suganya, P.; Panneerselvam, A.; Kumar, T.S.; Alharbi, N.S.; Arunachalam, C.; Alharbi, S.A.; Thajuddin, N. Taxonomic identification and bioactive compounds characterization of Psilocybe cubensis DPT1 to probe its antibacterial and mosquito larvicidal competency. Microb. Pathog. 2020, 143, 104138. [Google Scholar] [CrossRef] [PubMed]
- Nkadimeng, S.M.; Nabatanzi, A.; Steinmann, C.M.; Eloff, J.N. Phytochemical, cytotoxicity, antioxidant and anti-inflammatory effects of Psilocybe Natalensis magic mushroom. Plants 2020, 9, 1127. [Google Scholar] [CrossRef] [PubMed]
- Nkadimeng, S.M.; Steinmann, C.M.L.; Eloff, J.N. Effects and safety of Psilocybe cubensis and Panaeolus cyanescens magic mushroom extracts on endothelin-1-induced hypertrophy and cell injury in cardiomyocytes. Sci. Rep. 2020, 10, 22314. [Google Scholar] [CrossRef]
- Elhusseiny, S.; El-Mahdy, T.; Awad, M.; Elleboudy, N.; Farag, M.; Aboshanab, K.; Yassien, M. Antiviral, cytotoxic, and antioxidant activities of three edible agaricomycetes mushrooms: Pleurotus columbinus, Pleurotus sajor-caju, and Agaricus bisporus. J. Fungi 2021, 7, 645. [Google Scholar] [CrossRef]
- Singh, N.K.; Rao, G.N. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 2019, 73, 28–45. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020, 20, 400–402. [Google Scholar] [CrossRef]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The antiviral, anti-inflammatory effects of natural medicinal herbs and mushrooms and SARS-CoV-2 infection. Nutrients 2020, 12, 2573. [Google Scholar] [CrossRef]
- Vilček, J.; Le, J. Interferon γ. In Encyclopedia of Immunology, 2nd ed.; Delves, P.J., Ed.; Elsevier: Oxford, UK, 1998. [Google Scholar]
- Ren, G.; Xu, L.; Lu, T.; Yin, J. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int. J. Biol. Macromol. 2018, 115, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Abu-serie, M.M.; Habashy, N.H.; Attia, W.E. In vitro evaluation of the synergistic antioxidant andanti-inflammatory activities of the combined extracts from Malaysian Ganoderma lucidum and Egyptian Chlorella vulgaris. BMC Complement. Altern. Med. 2018, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.; Jeong, S.; Lee, D.; Park, J.; Lee, J. Isolation and characterization of a novel platelet aggregation inhibitory peptide from the medicinal mushroom, Inonotus obliquus. Peptides 2006, 27, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.-H.; Yu, X.-T.; Li, T.; Wu, H.-L.; Jiao, C.-W.; Cai, M.-H.; Li, X.-M.; Xie, Y.-Z.; Wang, Y.; Peng, T. Aqueous extract from a chaga medicinal mushroom, Inonotus obliquus (higher Basidiomyetes), prevents HerpesSimplex virus entry through inhibition of viral-induced membrane fusion. Int. J. Med. Mushrooms 2013, 15, 29–38. [Google Scholar] [CrossRef]
- Lemieszek, M.; Langner, E.; Kaczor, J.; Kandefer-Szersze’n, M.; Sanecka, B.; Mazurkiewicz, W.; Rzeski, W. Anticancer effects of fraction isolated from fruiting bodies of chaga medicinal mushroom, Inonotus obliquus (Pers.: Fr.) Pilát (Aphyllophoromycetideae): In vitro studies. Int. J. Med. Mushrooms 2011, 13, 131–143. [Google Scholar] [CrossRef]
- Filippova, E.I.; Mazurkova, N.A.; Kabanov, A.S.; Teplyakova, T.V.; Ibragimova, Z.B.; Makarevich, E.V.; Mazurkov, O.Y.; Shishkina, L.N. Antiviral properties of aqueous extracts isolated from higher basidiomycetesas respect to pandemic influenza virus. Mod. Probl. Sci. Educ. 2009, 5, 1–9. [Google Scholar]
- Verma, A.K. Cordycepin: A bioactive metabolite of Cordyceps militaris and polyadenylation inhibitor with therapeutic potential against COVID-19. J. Biomol. Struct. Dyn. 2020, 1–8. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Lin, Y.; Liu, R.; Gu, Y.; Liao, D. Effectiveness of cultured Cordyceps sinensis combined with glucocorticosteroid on pulmonary fibrosis induced by bleomycin in rats. Zhongguo Zhong Yao Za Zhi 2011, 36, 2265–2270. [Google Scholar]
- Dharsono, T.; Rudnicka, K.; Wilhelm, M.; Schoen, C. Effects of yeast (1,3)-(1,6)-beta-glucan on severity of upper respiratory tract infections: A double-blind, randomized, placebo-controlled study in healthy subjects. J. Am. Coll. Nutr. 2019, 38, 40–50. [Google Scholar] [CrossRef]
- Geller, A.; Yan, J. Could the Induction of Trained Immunity by β-Glucan Serve as a Defense Against COVID-19? Front. Immunol. 2020, 11, 1782. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [CrossRef] [Green Version]
- Lao, E.J.; Dimoso, N.; Raymond, J.; Mbega, E.R. The prebiotic potential of brewers’ spent grain on livestock’s health: A review. Trop. Anim. Health Prod. 2020, 52, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Johnson, E.; Bernardshaw, S.V.; Grinde, B. Can medicinal mushrooms have prophylactic or therapeutic effect against COVID-19 and its pneumonic superinfection and complicating inflammation? Scand. J. Immunol. 2021, 93, e12937. [Google Scholar] [CrossRef]
- Popa, M.; Oancea, S. Studies on bioactive compounds of mushrooms and their potential antiviral effects against COVID-19. In Proceedings of the 44th Conference for Students of Agriculture and Veterinary Medicine with International Participatio, Novi Sad, Serbia, 15 December 2020. [Google Scholar]
- Rahi, D.K.; Malik, D. Diversity of mushrooms and their metabolites of nutraceutical and therapeutic significance. J. Mycol. 2016, 2016, 7654123. [Google Scholar] [CrossRef]
- Sakagami, H.; Takeda, M. Diverse biological activity of PSK (Krestin), a protein-bound polysaccharide from Coriolus versicolor (Fr.) Quel. In Mushroom Biology and Mushroom Products; Chang, S.T., Ed.; The Chinese University Press: Hong Kong, China, 1993; pp. 237–245. [Google Scholar]
- Sagar, A.; Gautam, C.; Sehgal, A.K. Studies on some medicinal mushrooms of Himachal Pradesh. Indian J. Mushrooms 2007, 25, 8–14. [Google Scholar]
- Chai, R.; Qiu, C.; Liu, D.; Qi, Y.; Gao, Y.; Shen, J.; Qiu, L. β-Glucan synthase gene overexpression and β-glucans over-production in Pleurotus ostreatus using promoter swapping. PLoS ONE 2013, 8, e61693. [Google Scholar]
- Ji, S.L.; Liu, R.; Ren, M.F.; Li, H.J.; Xu, J.W. Enhanced production of polysaccharide through the overexpression of homologous uridine diphosphate glucose pyrophosphorylase gene in a submerged culture of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Torres, J.V.; Mata, G.; Martinez-Carrera, D.; Garibay-Orijel, C.; Garibay-Orijel, R. Primers for (1,3)-β-glucan synthase gene amplification and partial characterization of the enzyme in Ganoderma lucidum. Rev. Iberoam. Micol. 2013, 30, 267–270. [Google Scholar] [CrossRef]
- Khan, A.A.; Gani, A.; Shah, A.; Masoodi, F.A.; Hussain, P.R.; Wani, I.A.; Khanday, F.A. Effect of γ-irradiation on structural, functional and antioxidant properties of β-glucan extracted from button mushroom (Agaricus bisporus). Innov. Food Sci. Emerg. Technol. 2015, 31, 123–130. [Google Scholar] [CrossRef]


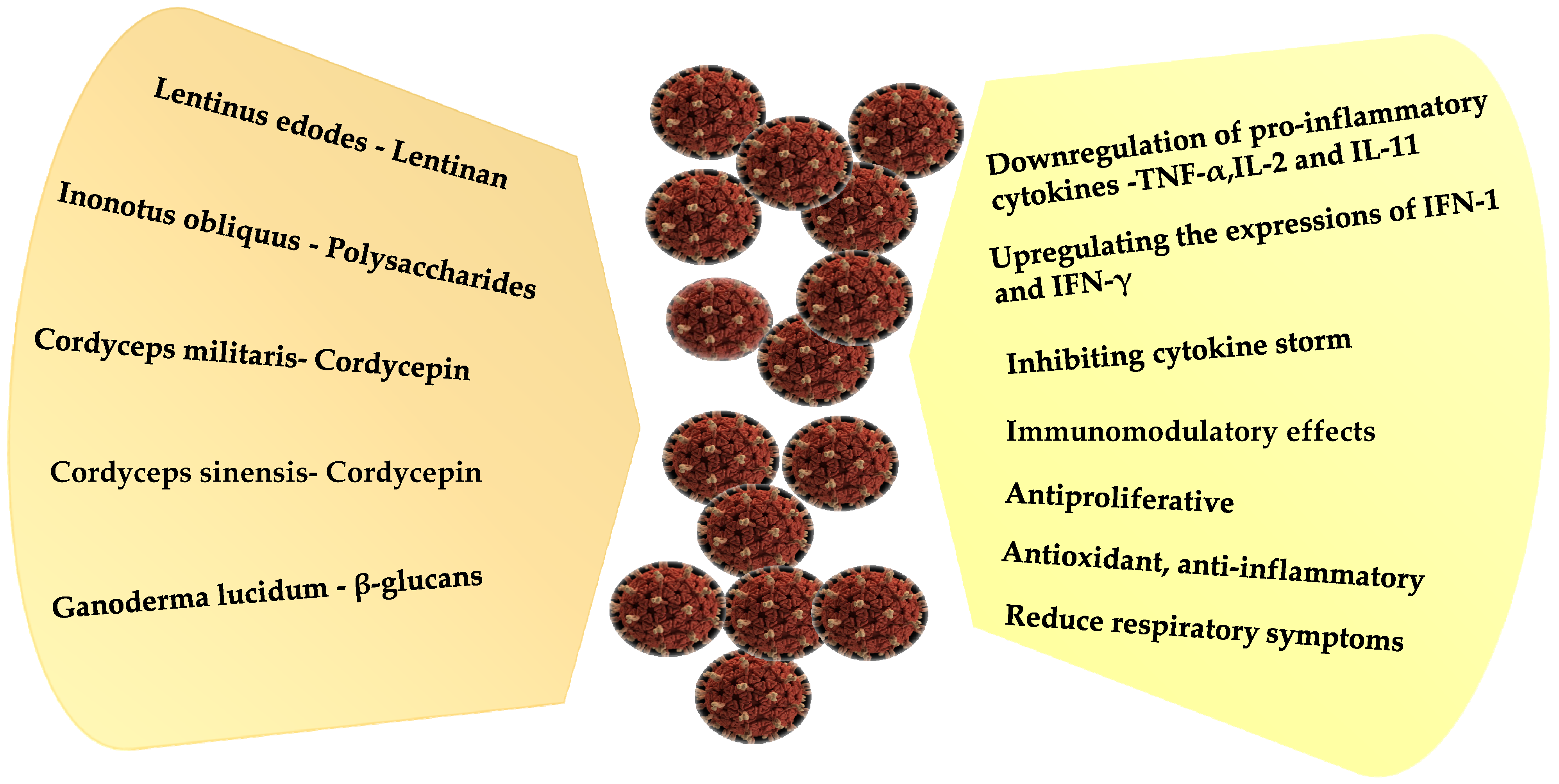
| Source Mushroom | Bioactive Component | Antioxidant Activity | References |
|---|---|---|---|
| Agaricus brasiliensis | Crude Se polysaccharide | Scavenging of DPPH and hydroxyl radicals | [272] |
| Phellinus xiaobaumii | Homogenous water soluble polysaccharide | Hydroxyl, superoxide and DPPH radical scavenging | [274] |
| Pleurotus abalonus | Polysaccharide–peptide complex LB-1b | Antioxidant activity in erythrocyte haemolysis | [275] |
| Cordyceps taii | Polysaccharides | DPPH, hydroxyl, and superoxide anion radical scavenging | [268] |
| Agaricus bisporus | Polysaccharides | Free radical scavangers enhancement of antioxidant enzymes in sera, liver, and heart of mice | [276] |
| Ganoderma lucidum | Heteroglycan, mannoglucan, glycopeptide | Antioxidant | [34] |
| Pleurotus ostreatus | Glycoprotein | Antitumor, hyperglycemia, antioxidant | [34] |
| Cordiceps militaris | Polysaccharides | Antioxidant activity suppression of in vivo growth of melanoma in mouse models | [251,252] |
| Inonotus oblique | Crude polysaccharide | Used as an antioxidant in Russian folk medicine | [253] |
| Hericium erinaceus | Unique polysaccharide EP-1 | Strong in vitro antioxidant activity in mice | [254] |
| Macrolepiota dolichaula | Fucogalactan | Antioxidant and immunostimulating properties in vitro | [189] |
| Russula albonigra | β-glucan | Antioxidant and immunostimulating properties in vitro | [255] |
| Tricholoma mongolicum | Folysaccharides | In vitro antioxidant activities | [202] |
| Ganoderma | β-d-glucans | In vitro antioxidant activity | [259] |
| Entoloma lividoalbum | Water soluble β-glucan | High antioxidant activity | [260] |
| Tylopilus ballouii | Fucogalactomannan | Inhibiting superoxide and hydroxyl radicals | [261] |
| Ganoderma lucidum | α- and β-glucans | High antioxidant activity | [262] |
| Fistulina hepatica, Pleorotus squarrosulus, Polyporus grammocephalus, Phellinus linteus, Austreus hygrometricus, and Macrocybe gigantea | Polysaccharides | Significant antioxidant potential | [263] |
| Ganoderma tsugae | Polysaccharides | Best scavenging activity | [268] |
| Ganoderma lucidum, Hypsizygus marmoreus, Pleurotus ostreatus, Pleurotus nebrodensis, Lentinus edodes, Pleurotus eryngii, Flammulina velutipes, and Hericium erinaceus | Polysaccharide activity compared | Significant antioxidant potential | [249] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, S.; Gopal, J.; Muthu, M. Antioxidant Activity of Mushroom Extracts/Polysaccharides—Their Antiviral Properties and Plausible AntiCOVID-19 Properties. Antioxidants 2021, 10, 1899. https://doi.org/10.3390/antiox10121899
Chun S, Gopal J, Muthu M. Antioxidant Activity of Mushroom Extracts/Polysaccharides—Their Antiviral Properties and Plausible AntiCOVID-19 Properties. Antioxidants. 2021; 10(12):1899. https://doi.org/10.3390/antiox10121899
Chicago/Turabian StyleChun, Sechul, Judy Gopal, and Manikandan Muthu. 2021. "Antioxidant Activity of Mushroom Extracts/Polysaccharides—Their Antiviral Properties and Plausible AntiCOVID-19 Properties" Antioxidants 10, no. 12: 1899. https://doi.org/10.3390/antiox10121899
APA StyleChun, S., Gopal, J., & Muthu, M. (2021). Antioxidant Activity of Mushroom Extracts/Polysaccharides—Their Antiviral Properties and Plausible AntiCOVID-19 Properties. Antioxidants, 10(12), 1899. https://doi.org/10.3390/antiox10121899







