Genome-Wide RNAi Screening Identifies Novel Pathways/Genes Involved in Oxidative Stress and Repurposable Drugs to Preserve Cystic Fibrosis Airway Epithelial Cell Integrity
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
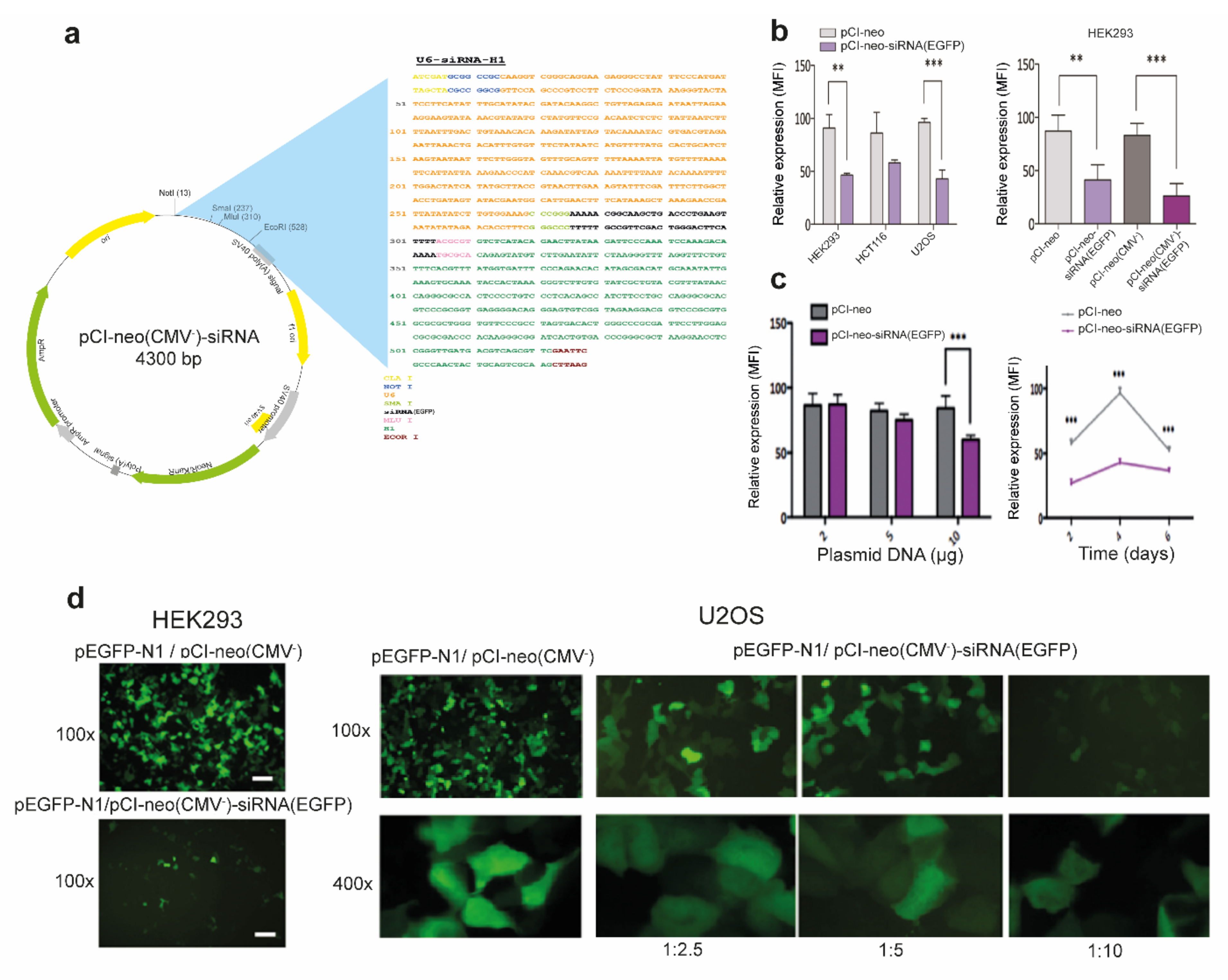
2.2. Construction of a Convergent siRNA Expression System
2.3. Plasmid Transfections
2.4. Cell Death Induction through Oxidative Stress
2.5. Randomized siRNA Library Generation
2.6. Randomized siRNA Library Transfection and Screening
2.7. Validation of siRNAs and Drugs Conferring Oxidative Stress Resistance
2.8. Differential Gene Expression Analysis
2.9. Identification of Putative siRNA Targets
2.10. Protein-Protein Interaction (PPI) Networks
2.11. Pathways Over-Representation Analysis (ORA)
2.12. Drug Database Mining
2.13. Statistical Analysis
3. Results
3.1. Efficient RNAi-Mediated Transcript Knockdown in Epithelial Cells through Convergent Transcription from RNA Polymerase III Promoters
3.2. Genome-Wide Screening Using a Randomized siRNA Library Uncovers siRNA Sequences Able to Confer Oxidative Stress Resistance in CF Airway Epithelial Cells
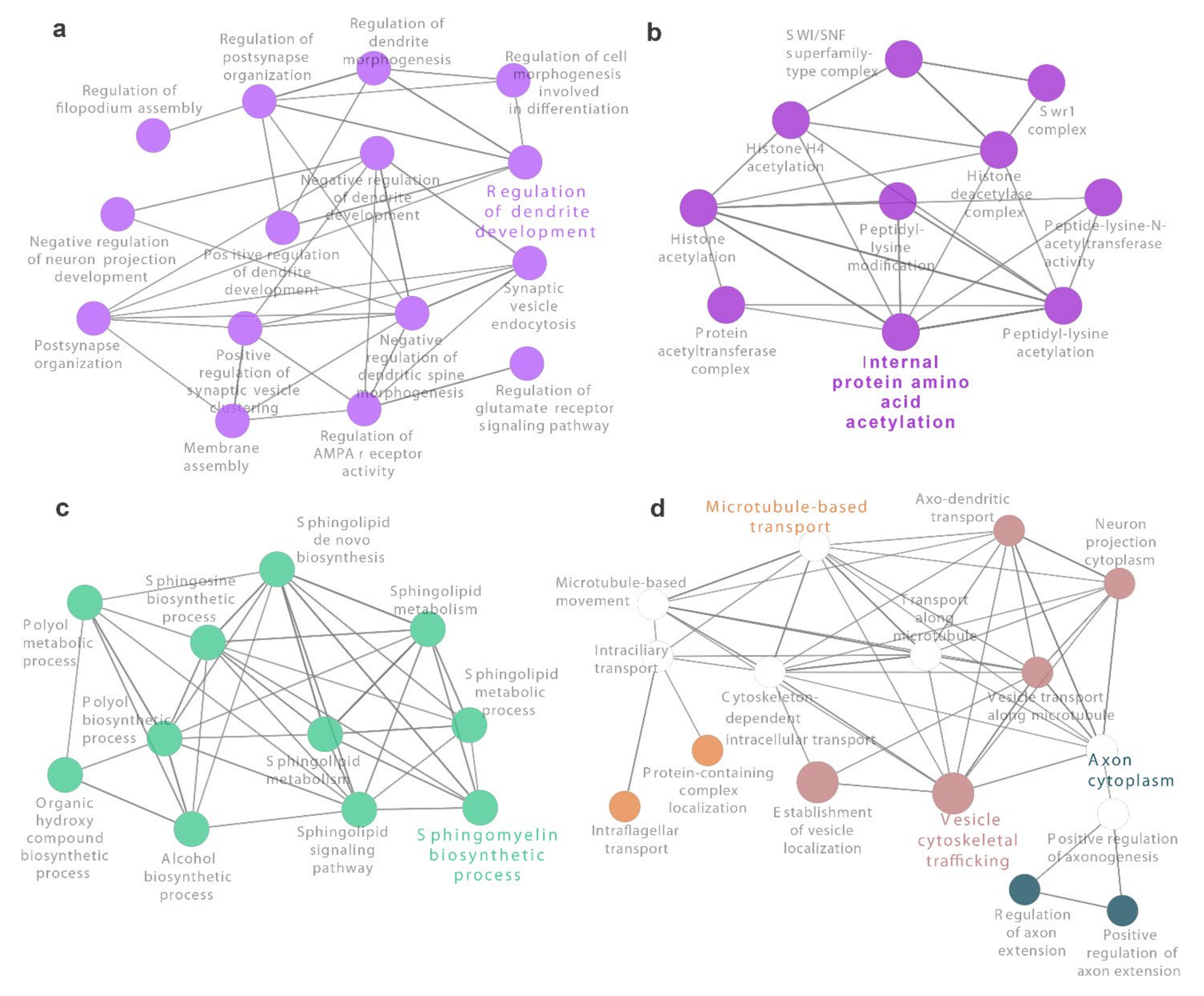
3.3. Identification of Novel Genes and Pathways Involved in Oxidative Stress in CF Airway Epithelial Cells
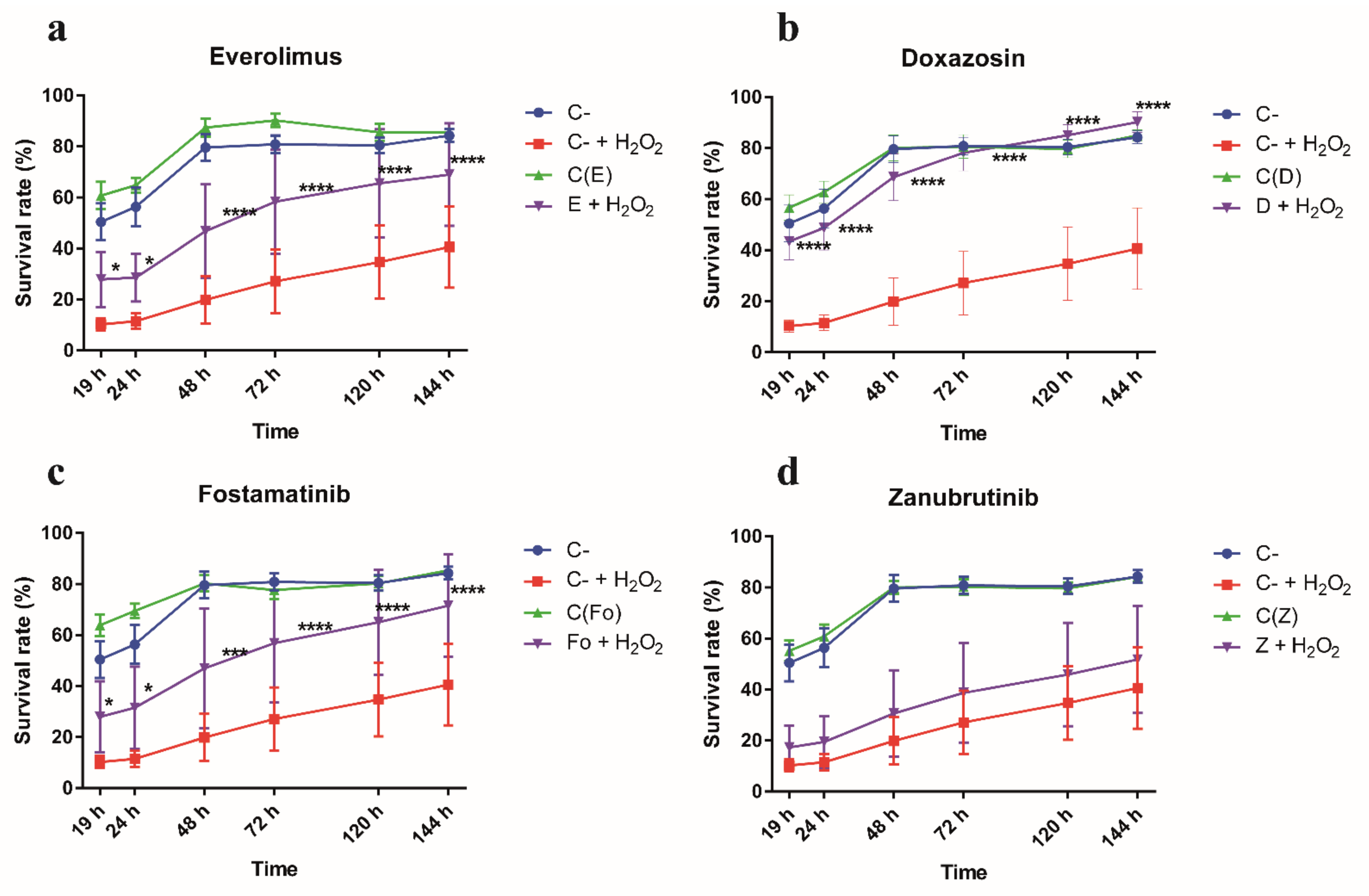
3.4. Gene Targets from RNAi-Based Genome-Wide Screening Disclose Repurposable Drugs Able to Protect CF Airway Epithelial Cells from Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, C.; Hoiby, N. Pathogenesis of cystic fibrosis. Lancet 1993, 341, 1065–1069. [Google Scholar] [CrossRef]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 2015, 14, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Parkins, M.D.; Somayaji, R.; Waters, V.J. Epidemiology, Biology, and Impact of Clonal Pseudomonas aeruginosa Infections in Cystic Fibrosis. Clin. Microbiol. Rev. 2018, 31, e00019-18. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; LiPuma, J.J. The Microbiome in Cystic Fibrosis. Clin. Chest. Med. 2016, 37, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Turkovic, L.; Caudri, D.; Rosenow, T.; Hall, G.; Stick, S. Presence of mucus plugging is predictive of long term lung function in children with cystic fibrosis. Eur. Respir. J. 2017, 50 (Suppl. 61), OA4401. [Google Scholar] [CrossRef]
- Rieber, N.; Hector, A.; Carevic, M.; Hartl, D. Current concepts of immune dysregulation in cystic fibrosis. Int. J. Biochem. Cell Biol. 2014, 52, 108–112. [Google Scholar] [CrossRef]
- Yuan, S.; Hollinger, M.; Lachowicz-Scroggins, M.E.; Kerr, S.C.; Dunican, E.M.; Daniel, B.M.; Ghosh, S.; Erzurum, S.C.; Willard, B.; Hazen, S.L.; et al. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci. Transl. Med. 2015, 7, 276ra27. [Google Scholar] [CrossRef]
- Erttmann, S.F.; Gekara, N.O. Hydrogen peroxide release by bacteria suppresses inflammasome-dependent innate immunity. Nat. Commun. 2019, 10, 3493. [Google Scholar] [CrossRef]
- Wood, L.G.; Fitzgerald, D.A.; Gibson, P.G.; Cooper, D.M.; Collins, C.E.; Garg, M.L. Oxidative stress in cystic fibrosis: Dietary and metabolic factors. J. Am. Coll. Nutr. 2001, 20, 157–165. [Google Scholar] [CrossRef]
- Ciofu, O.; Smith, S.; Lykkesfeldt, J. A systematic Cochrane Review of antioxidant supplementation lung disease for cystic fibrosis. Paediatr. Respir. Rev. 2020, 33, 28–29. [Google Scholar] [CrossRef]
- Rao Tata, P.; Rajagopal, J. Plasticity in the lung: Making and breaking cell identity. Development 2017, 144, 755–766. [Google Scholar] [CrossRef]
- Gruenert, D.C.; Willems, M.; Cassiman, J.J.; Frizzell, R.A. Established cell lines used in cystic fibrosis research. J. Cyst. Fibros. 2004, 3 (Suppl. 2), 191–196. [Google Scholar] [CrossRef] [PubMed]
- Cozens, A.L.; Yezzi, M.J.; Chin, L.; Simon, E.M.; Finkbeiner, W.E.; Wagner, J.A.; Gruenert, D.C. Characterization of immortal cystic fibrosis tracheobronchial gland epithelial cells. Proc. Natl. Acad. Sci. USA 1992, 89, 5171–5175. [Google Scholar] [CrossRef] [PubMed]
- Da Paula, A.C.; Ramalho, A.S.; Farinha, C.M.; Cheung, J.; Maurisse, R.; Gruenert, D.; Ousingsawat, J.; Kunzelmann, K.; Amaral, M. Characterization of novel airway submucosal gland cell models for cystic fibrosis studies. Cell. Physiol. Biochem. 2005, 15, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Cairns, M.J.; Dawes, I.W.; Arndt, G.M. Expressing functional siRNAs in mammalian cells using convergent transcription. BMC Biotechnol. 2003, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Kaykas, A.; Moon, R.T. A plasmid-based system for expressing small interfering RNA libraries in mammalian cells. BMC Cell Biol. 2004, 5, 16. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Tram, R.D.C. R: A Language and Environment for Statistical Computing; The R Project for Statistical Computing: Vienna, Austria, 2008; ISBN 3-900051-07-0. Available online: https://www.gbif.org/es/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 2 July 2021).
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. InterJ. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef]
- Carbon, S.; Douglass, E.; Dunn, N. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software Environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Li, F.; Zhou, Y.; Zhang, Y.; Wang, Z.; Zhang, R.; Zhu, J.; Ren, Y.; Tan, Y.; et al. Therapeutic target database 2020: Enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020, 48, D1031–D1041. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Airway Redox Homeostasis and Inflammation Gone Awry: From Molecular Pathogenesis to Emerging Therapeutics in Respiratory Pathology. Int. J. Mol. Sci. 2020, 21, 9317. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Berger, N.D.; Luijsterburg, M.S.; Piett, C.G.; Stanley, F.K.; Schräder, C.U.; Fang, S.; Chan, J.A.; Schriemer, D.C.; Nagel, Z.D.; et al. The CHD6 chromatin remodeler is an oxidative DNA damage response factor. Nat. Commun. 2019, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Pedrotti, S.; Busà, R.; Compagnucci, C.; Sette, C. The RNA recognition motif protein RBM11 is a novel tissue-specific splicing regulator. Nucleic Acids Res. 2012, 40, 1021–1032. [Google Scholar] [CrossRef]
- Chibaya, L.; Karim, B.; Zhang, H.; Jones, S.N. Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2003193118. [Google Scholar] [CrossRef]
- Wang, Z.; Su, D.; Sun, Z.; Liu, S.; Li, Q.; Guan, L.; Liu, Y.; Ma, X.; Hu, S. MDM2 phosphorylation mediates H2O2-induced lens epithelial cells apoptosis and age-related cataract. Biochem. Biophys. Res. Commun. 2020, 528, 112–119. [Google Scholar] [CrossRef]
- Kinnula, V.L.; Crapo, J.D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care Med. 2003, 167, 1600–1619. [Google Scholar] [CrossRef] [PubMed]
- Aguade-Gorgorio, J.; McComb, S.; Eckert, C.; Guinot, A.; Marovca, B.; Mezzatesta, C.; Jenni, S.; Abduli, L.; Schrappe, M.; Dobay, M.P.; et al. TNFR2 is required for RIP1-dependent cell death in human leukemia. Blood Adv. 2020, 4, 4823–4833. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Kanayama, A.; Miyamoto, Y. Apoptosis triggered by phagocytosis-related oxidative stress through FLIP S down-regulation and JNK activation. J. Leukoc. Biol. 2007, 82, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Redstone, S.C.J.; Fleming, A.M.; Burrows, C.J. Oxidative Modification of the Potential G-Quadruplex Sequence in the PCNA Gene Promoter Can Turn on Transcription. Chem. Res. Toxicol. 2019, 32, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fan, L.; Lu, C.; Yin, S.; Hu, H. Functional Role of p53 in the Regulation of Chemical-Induced Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 6039769. [Google Scholar] [CrossRef]
- Eriksson, S.E.; Ceder, S.; Bykov, V.J.N.; Wiman, K.G. P53 as a hub in cellular redox regulation and therapeutic target in cancer. J. Mol. Cell Biol. 2019, 11, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Kaur, R.; Akhter, S.; Legerski, R.J. Cdc5L interacts with ATR and is required for the S-phase cell-cycle checkpoint. EMBO Rep. 2009, 10, 1029–1035. [Google Scholar] [CrossRef]
- Mu, R.; Wang, Y.B.; Wu, M.; Yang, Y.; Song, W.; Li, T.; Zhang, W.N.; Tan, B.; Li, A.L.; Wang, N.; et al. Depletion of pre-mRNA splicing factor Cdc5L inhibits mitotic progression and triggers mitotic catastrophe. Cell Death Dis. 2014, 5, e1151. [Google Scholar] [CrossRef] [PubMed]
- Saran, U.; Foti, M.; Dufour, J.F. Cellular and molecular effects of the mTOR inhibitor everolimus. Clin. Sci. 2015, 129, 895–914. [Google Scholar] [CrossRef]
- Geahlen, R.L. Getting Syk: Spleen tyrosine kinase as a therapeutic target. Trends Pharmacol. Sci. 2014, 35, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Zanubrutinib: First Approval. Drugs 2020, 80, 91–97. [Google Scholar] [CrossRef]
- Roesch, E.A.; Nichols, D.P.; Chmiel, J.F. Inflammation in cystic fibrosis: An update. Pediatr. Pulmonol. 2018, 53, S30–S50. [Google Scholar] [CrossRef]
- Teramoto, S.; Tomita, T.; Matsui, H.; Ohga, E.; Matsuse, T.; Ouchi, Y. Hydrogen peroxide-induced apoptosis and necrosis in human lung fibroblasts: Protective roles of glutathione. Jpn. J. Pharmacol. 1999, 79, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wine, J.J.; Joo, N.S. Submucosal glands and airway defense. Proc. Am. Thorac. Soc. 2004, 1, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, J.F.; Schlossberg, H.; Yankaskas, J.R.; Dudus, L. Progenitor cells of the adult human airway involved in submucosal gland development. Development 1995, 121, 2031–2046. [Google Scholar] [CrossRef]
- Lynch, T.J.; Engelhardt, J.F. Progenitor cells in proximal airway epithelial development and regeneration. J. Cell. Biochem. 2014, 115, 1637–1645. [Google Scholar] [CrossRef]
- Liu, D.; Xu, Y. P53, oxidative stress, and aging. Antioxid. Redox Signal 2011, 15, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, M.; Sanchez, G.; Barbier, J.; Corcos, L.; Auboeuf, D. The emerging role of pre-messenger RNA splicing in stress responses: Sending alternative messages and silent messengers. RNA Biol. 2011, 8, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Disher, K.; Skandalis, A. Evidence of the modulation of mRNA splicing fidelity in humans by oxidative stress and p53. Genome 2007, 50, 946–953. [Google Scholar] [CrossRef]
- Merdzhanova, G.; Edmond, V.; De Seranno, S.; Van den Broeck, A.; Corcos, L.; Brambilla, C.; Brambilla, E.; Gazzeri, S.; Eymin, B. E2F1 controls alternative splicing pattern of genes involved in apoptosis through upregulation of the splicing factor SC35. Cell Death Differ. 2008, 15, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Shkreta, L.; Michelle, L.; Toutant, J.; Tremblay, M.L.; Chabot, B. The DNA damage response pathway regulates the alternative splicing of the apoptotic mediator Bcl-x. J. Biol. Chem. 2011, 286, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, M.; Sanchez, G.; De Cian, M.C.; Barbier, J.; Dardenne, E.; Gratadou, L.; Dujardin, G.; Le Jossic-Corcos, C.; Corcos, L.; Auboeuf, D. Cotranscriptional exon skipping in the genotoxic stress response. Nat. Struct. Mol. Biol. 2010, 17, 1358–1366. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Qin, X.; Owzar, K.; McCann, J.J.; Kastan, M.B. Dna-damage-induced alternative splicing of p53. Cancers 2021, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.J.; Santangelo, M.S.P.; Paronetto, M.P.; de la Mata, M.; Pelisch, F.; Boireau, S.; Glover-Cutter, K.; Ben-Dov, C.; Blaustein, M.; Lozano, J.J.; et al. DNA Damage Regulates Alternative Splicing through Inhibition of RNA Polymerase II Elongation. Cell 2009, 137, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.Z.; Jiang, J.; Duan, C.G. The Crosstalk Between Epigenetic Mechanisms and Alternative RNA Processing Regulation. Front. Genet. 2020, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.N.; Van Santen, M.A.; Schwienhorst, A.; Urlaub, H.; Lührmann, R. Stalling of spliceosome assembly at distinct stages by small-molecule inhibitors of protein acetylation and deacetylation. RNA 2009, 15, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Edmond, V.; Moysan, E.; Khochbin, S.; Matthias, P.; Brambilla, C.; Brambilla, E.; Gazzeri, S.; Eymin, B. Acetylation and phosphorylation of SRSF2 control cell fate decision in response to cisplatin. EMBO J. 2011, 30, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Kundu, S. A Molecular Interaction Map of Klebsiella pneumoniae and Its Human Host Reveals Potential Mechanisms of Host Cell Subversion. Front. Microbiol. 2021, 12, 613067. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.H.; Zhang, J.T.; Chen, C.; Xu, Z.H.; Lv, X.B.; Ye, L.; Yu, B.T. Identification of CDC5L as bridge gene between chronic obstructive pulmonary disease and lung adenocarcinoma. Epigenomics 2020, 12, 1515–1529. [Google Scholar] [CrossRef]
- Moore, M.J.; Wang, Q.; Kennedy, C.J.; Silver, P.A. An alternative splicing network links cell-cycle control to apoptosis. Cell 2010, 142, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Blanco, M.A. To Splice or Not to Splice, That Is the Treatment. Cell Chem. Biol. 2020, 27, 1453–1455. [Google Scholar] [CrossRef] [PubMed]
- Sidarovich, A.; Will, C.L.; Anokhina, M.M.; Ceballos, J.; Sievers, S.; Agafonov, D.E.; Samatov, T.; Bao, P.; Kastner, B.; Urlaub, H.; et al. Identification of a small molecule inhibitor that stalls splicing at an early step of spliceosome activation. eLife 2017, 6, e23533. [Google Scholar] [CrossRef] [PubMed]
- Ostedgaard, L.S.; Price, M.P.; Whitworth, K.M.; Abou Alaiwa, M.H.; Fischer, A.J.; Warrier, A.; Samuel, M.; Spate, L.D.; Allen, P.D.; Hilkin, B.M.; et al. Lack of airway submucosal glands impairs respiratory host defenses. eLife 2020, 9, e59653. [Google Scholar] [CrossRef]
- Wine, J.J. Parasympathetic control of airway submucosal glands: Central reflexes and the airway intrinsic nervous system. Auton. Neurosci. Basic. Clin. 2007, 133, 35–54. [Google Scholar] [CrossRef]
- Ballard, S.T.; Spadafora, D. Fluid secretion by submucosal glands of the tracheobronchial airways. Respir. Physiol. Neurobiol. 2007, 159, 271–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fischer, A.J.; Pino-Argumedo, M.I.; Hilkin, B.M.; Shanrock, C.R.; Gansemer, N.D.; Chaly, A.L.; Zarei, K.; Allen, P.D.; Ostedgaard, L.S.; Hoffman, E.A.; et al. Mucus strands from submucosal glands initiate mucociliary transport of large particles. JCI Insight. 2019, 4, e124863. [Google Scholar] [CrossRef] [PubMed]
- Saint-Criq, V.; Gray, M.A. Role of CFTR in epithelial physiology. Cell. Mol. Life Sci. 2017, 74, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Marshall, W.F. Intraflagellar transport and ciliary dynamics. Cold Spring Harb. Perspect. Biol. 2017, 9, a021998. [Google Scholar] [CrossRef]
- Wang, L.; Dynlacht, B.D. The regulation of cilium assembly and disassembly in development and disease. Development 2018, 145, dev151407. [Google Scholar] [CrossRef]
- Kantar, A.; Oggiano, N.; Giorgi, P.L.; Braga, P.C.; Fiorini, R. Polymorphonuclear leukocyte-generated oxygen metabolites decrease beat frequency of human respiratory cilia. Lung 1994, 172, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Siller, S.S.; Burke, M.C.; Li, F.Q.; Takemaru, K.I. Chibby functions to preserve normal ciliary morphology through the regulation of intraflagellar transport in airway ciliated cells. Cell Cycle 2015, 14, 3163–3172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhatt, J.M. Treatment of pulmonary exacerbations in cystic fibrosis. Eur. Respir. Rev. 2013, 22, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.I.; Kwon, T.; Tu, F.; Brooks, E.R.; Gupta, R.; Meyer, M.; Baker, J.C.; Marcotte, E.M.; Wallingford, J.B. Coordinated genomic control of ciliogenesis and cell movement by RFX2. eLife 2014, 2014, e01439. [Google Scholar] [CrossRef]
- Ito, T.; Kagoshima, M.; Sasaki, Y.; Li, C.; Udaka, N.; Kitsukawa, T.; Fujisawa, H.; Taniguchi, M.; Yagi, T.; Kitamura, H.; et al. Repulsive axon guidance molecule Sema3A inhibits branching morphogenesis of fetal mouse lung. Mech. Dev. 2000, 97, 35–45. [Google Scholar] [CrossRef]
- Volckaert, T.; Yuan, T.; Yuan, J.; Boateng, E.; Hopkins, S.; Zhang, J.S.; Thannickal, V.J.; Fässler, R.; De Langhe, S.P. Hippo signaling promotes lung epithelial lineage commitment by curbing Fgf10 and β-catenin signaling. Development 2019, 146, dev166454. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; He, Y.Y. PTEN in DNA damage repair. Cancer Lett. 2012, 319, 125–129. [Google Scholar] [CrossRef]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. MTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Rabanal-Ruiz, Y.; Otten, E.G.; Korolchuk, V.I. MTORC1 as the main gateway to autophagy. Essays Biochem. 2017, 61, 565–584. [Google Scholar] [CrossRef] [PubMed]
- O’grady, S.M. Oxidative stress, autophagy and airway ion transport. Am. J. Physiol. Cell Physiol. 2019, 316, C16–C32. [Google Scholar] [CrossRef] [PubMed]
- Sidos Santos Simon, M.I.; Dalle Molle, R.; Silva, F.M.; Rodrigues, T.W.; Feldmann, M.; Forte, G.C.; Marostica, P.J. Antioxidant Micronutrients and Essential Fatty Acids Supplementation on Cystic Fibrosis Outcomes: A Systematic Review. J. Acad. Nutr. Diet. 2020, 120, 1016–1033.e1. [Google Scholar] [CrossRef] [PubMed]
- Veltman, M.; De Sanctis, J.B.; Stolarczyk, M.; Klymiuk, N.; Bähr, A.; Brouwer, R.W.; Oole, E.; Shah, J.; Ozdian, T.; Liao, J.; et al. CFTR Correctors and Antioxidants Partially Normalize Lipid Imbalance but not Abnormal Basal Inflammatory Cytokine Profile in CF Bronchial Epithelial Cells. Front. Physiol. 2021, 12, 619442. [Google Scholar] [CrossRef] [PubMed]
- Schuler, W.; Sedrani, R.; Cottens, S.; Häberlin, B.; Schulz, M.; Schuurman, H.J.; Zenke, G.; Zerwes, H.G.; Schreier, M.H. SDZ RAD, a new rapamycin derivative: Pharmacological properties in vitro and in vivo. Transplantation 1997, 64, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Babamoto, K.; Hirokawa, W. Doxazosin: A new alpha 1-adrenergic antagonist. Clin. Pharm. 1992, 11, 415–427. [Google Scholar]
- Markham, A. Fostamatinib: First Global Approval. Drugs 2018, 78, 959–963. [Google Scholar] [CrossRef]
- Reilly, R.; Mroz, M.S.; Dempsey, E.; Wynne, K.; Keely, S.J.; McKone, E.F.; Hiebel, C.; Behl, C.; Coppinger, J.A. Targeting the PI3K/Akt/mTOR signalling pathway in Cystic Fibrosis. Sci. Rep. 2017, 7, 7642. [Google Scholar] [CrossRef]
- Summer, R.; Shaghaghi, H.; Schriner, D.L.; Roque, W.; Sales, D.; Cuevas-Mora, K.; Desai, V.; Bhushan, A.; Ramirez, M.I.; Romero, F. Activation of the mTORC1/PGC-1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 316, L1049–L1060. [Google Scholar] [CrossRef] [PubMed]
- Mercer, P.F.; Woodcock, H.V.; Eley, J.D.; Platé, M.; Sulikowski, M.G.; Durrenberger, P.F.; Franklin, L.; Nanthakumar, C.B.; Man, Y.; Genovese, F.; et al. Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax 2016, 71, 701–711. [Google Scholar] [CrossRef]
- Liedtke, C.M. α-Adrenergic regulation of Na-Cl cotransport in human airway epithelium. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1989, 257, L125–L129. [Google Scholar] [CrossRef]
- Li, T.; Yang, S.; She, X.; Yan, Q.; Zhang, P.; Zhu, H.; Wang, F.; Luo, X.; Sun, X. Modulation of α-adrenoceptor signalling protects photoreceptors after retinal detachment by inhibiting oxidative stress and inflammation. Br. J. Pharmacol. 2019, 176, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Tognolini, M.; Lodola, A. Targeting the Eph-ephrin System with Protein-Protein Interaction (PPI) Inhibitors. Curr. Drug Targets 2015, 16, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Mócsai, A.; Ruland, J.; Tybulewicz, V.L.J. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat. Rev. Immunol. 2010, 10, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.P.; Lau, C.W.; Schnapp, A.; Chow, C.W. Syk: A novel target for treatment of inflammation in lung disease. Inflamm. Allergy-Drug Targets 2009, 8, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, A. Spleen Tyrosine Kinase as a Target Therapy for Pseudomonas aeruginosa Infection. J. Innate Immun. 2018, 10, 255–263. [Google Scholar] [CrossRef]
- Ito, K.; Coates, M.S.; Alton, E.W.F.W.; Rapeport, G.W.; Davies, J.C. Pseudomonas aeruginosa induces p38MAP kinase-dependent IL-6 and CXCL8 release from bronchial epithelial cells via a Syk kinase pathway. PLoS ONE 2021, 16, e0246050. [Google Scholar] [CrossRef]
- Ramis, I.; Otal, R.; Carreño, C.; Eichhorn, P.; Orellana, A.; Maldonado, M.; De Alba, J.; Prats, N.; Fernández, J.C.; Vidal, B.; et al. A novel inhaled Syk inhibitor blocks mast cell degranulation and early asthmatic response. Pharmacol. Res. 2015, 99, 116–124. [Google Scholar] [CrossRef]
- Kost-Alimova, M.; Sidhom, E.H.; Satyam, A.; Chamberlain, B.T.; Dvela-Levitt, M.; Melanson, M.; Alper, S.L.; Santos, J.; Gutierrez, J.; Subramanian, A.; et al. A High-Content Screen for Mucin-1-Reducing Compounds Identifies Fostamatinib as a Candidate for Rapid Repurposing for Acute Lung Injury. Cell Rep. Med. 2020, 1, 100137. [Google Scholar] [CrossRef] [PubMed]




| Feature * | Adding Direct Interactors (1st Shell) | Adding Indirect Interactors (2nd Shell) | ||
|---|---|---|---|---|
| Only siRNA Targets | High Confidence Interactions | Very High Confidence Interactions | Very High Confidence Interactions | |
| Total genes | 14 | 603 | 125 | 650 |
| siRNA target genes | 14 (100%) | 115 (19.1%) | 26 (20.8%) | 26 (4.0%) |
| Total interactions | 8 | 2864 | 357 | 1497 |
| Oxidative stress genes | 0 (0%) | 20 (3.3%) | 4 (3.2%) | 20 (3.1%) |
| LCC size | 3 (21.4%) | 395 (65.5%) | 66 (52.8%) | 628 (96.6%) |
| Drug Name | DB Drug Code | TTD Drug Code |
|---|---|---|
| Pyridoxal phosphate | DB00114 | - |
| Amitriptyline | DB00321 | - |
| Epinephrine | DB00668 | - |
| Amrinone | DB01427 | - |
| Dalfampridine | DB06637 | - |
| Zanubrutinib | DB15035 | - |
| Tiludronic acid | DB01133 | - |
| Zinc | DB01593 | - |
| Zinc acetate | DB14487 | - |
| Zinc chloride | DB14533 | - |
| Zinc sulfate | DB14548 | - |
| Fostamatinib | DB12010 | - |
| Citric acid | DB04272 | - |
| Copper | DB09130 | - |
| Methoxamine | DB00723 | D09GYT |
| Phendimetrazine | DB01579 | D0T6SU |
| Imatinib | DB00619 | D0AZ3C |
| Sirolimus | DB00877 | D03LJR |
| Everolimus | DB01590 | D0K3QS |
| Temsirolimus | DB06287 | D0ES1Q |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Checa, J.; Martínez-González, I.; Maqueda, M.; Mosquera, J.L.; Aran, J.M. Genome-Wide RNAi Screening Identifies Novel Pathways/Genes Involved in Oxidative Stress and Repurposable Drugs to Preserve Cystic Fibrosis Airway Epithelial Cell Integrity. Antioxidants 2021, 10, 1936. https://doi.org/10.3390/antiox10121936
Checa J, Martínez-González I, Maqueda M, Mosquera JL, Aran JM. Genome-Wide RNAi Screening Identifies Novel Pathways/Genes Involved in Oxidative Stress and Repurposable Drugs to Preserve Cystic Fibrosis Airway Epithelial Cell Integrity. Antioxidants. 2021; 10(12):1936. https://doi.org/10.3390/antiox10121936
Chicago/Turabian StyleCheca, Javier, Itziar Martínez-González, Maria Maqueda, Jose Luis Mosquera, and Josep M. Aran. 2021. "Genome-Wide RNAi Screening Identifies Novel Pathways/Genes Involved in Oxidative Stress and Repurposable Drugs to Preserve Cystic Fibrosis Airway Epithelial Cell Integrity" Antioxidants 10, no. 12: 1936. https://doi.org/10.3390/antiox10121936
APA StyleCheca, J., Martínez-González, I., Maqueda, M., Mosquera, J. L., & Aran, J. M. (2021). Genome-Wide RNAi Screening Identifies Novel Pathways/Genes Involved in Oxidative Stress and Repurposable Drugs to Preserve Cystic Fibrosis Airway Epithelial Cell Integrity. Antioxidants, 10(12), 1936. https://doi.org/10.3390/antiox10121936






