Increased Kynurenine Indicates a Fatal Course of COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Analysis
2.3. Data Analysis
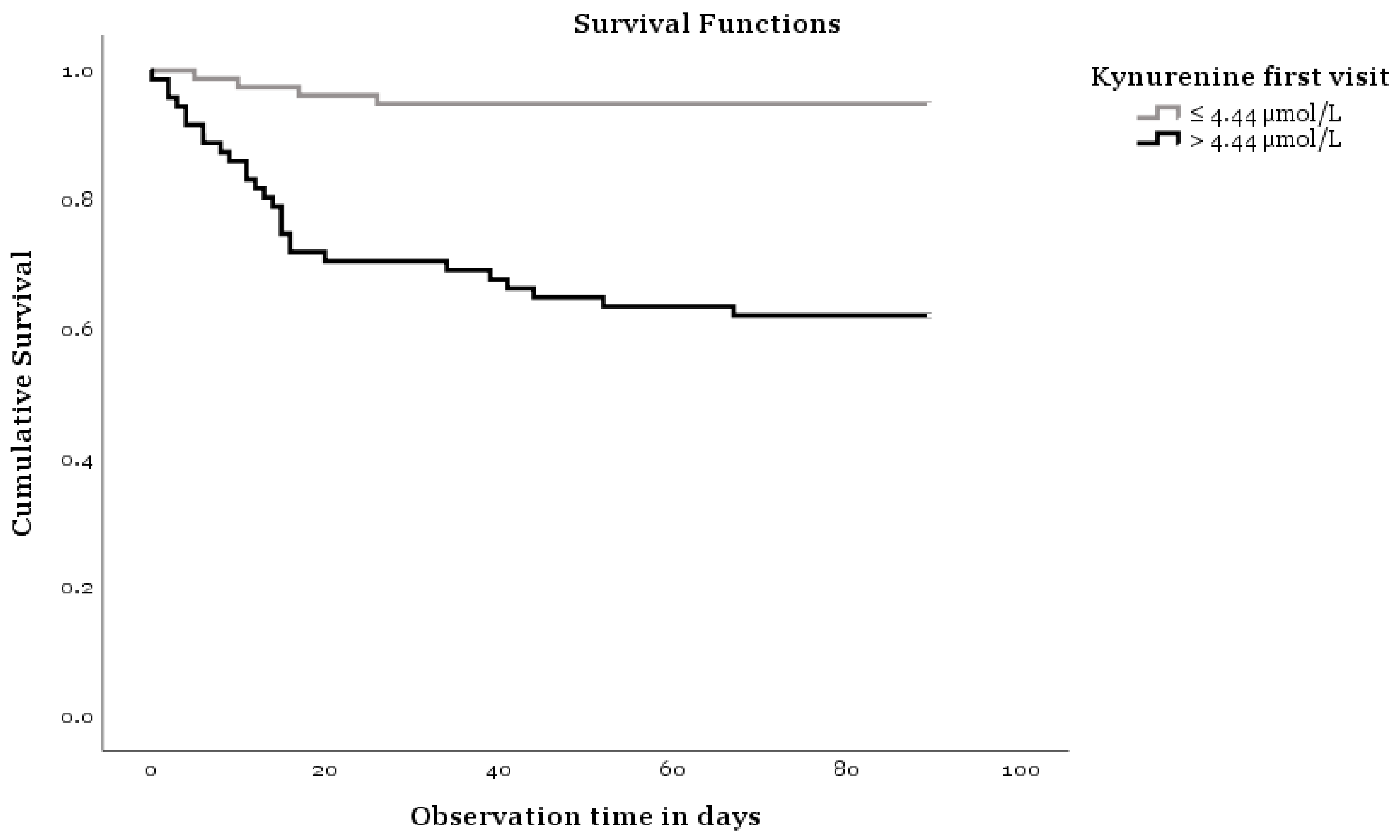
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyes Ocampo, J.; Lugo Huitron, R.; Gonzalez-Esquivel, D.; Ugalde-Muniz, P.; Jimenez-Anguiano, A.; Pineda, B.; Pedraza-Chaverri, J.; Rios, C.; Perez de la Cruz, V. Kynurenines with neuroactive and redox properties: Relevance to aging and brain diseases. Oxid. Med. Cell. Longev. 2014, 2014, 646909. [Google Scholar] [CrossRef]
- Niinisalo, P.; Raitakari, O.T.; Kahonen, M.; Hurme, M.; Lehtimaki, T.; Magnussen, C.; Viikari, J.; Juonala, M.; Kaaja, R. IDO activity forecasts obesity in males and premenopausal females in a 10-year follow-up study: The Cardiovascular Risk in Young Finns Study. Atherosclerosis 2021, 336, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Merlo, L.M.F.; DuHadaway, J.B.; Montgomery, J.D.; Peng, W.D.; Murray, P.J.; Prendergast, G.C.; Caton, A.J.; Muller, A.J.; Mandik-Nayak, L. Differential Roles of IDO1 and IDO2 in T and B Cell Inflammatory Immune Responses. Front. Immunol. 2020, 11, 1861. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Kimura, A.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 19961–19966. [Google Scholar] [CrossRef] [Green Version]
- Marfella, A.; Polese, C.; d’Alessio, P.; Beneduce, G.; Rossi, F.; Perna, M. An HPLC method for the simultaneous analysis of urinary neopterin and kynurenine. Pharmacol. Res. 1992, 26, 174–175. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of Kynurenine Pathway in Oxidative Stress during Neurodegenerative Disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capuron, L.; Neurauter, G.; Musselman, D.L.; Lawson, D.H.; Nemeroff, C.B.; Fuchs, D.; Miller, A.H. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol. Psychiatry 2003, 54, 906–914. [Google Scholar] [CrossRef]
- Wonodi, I.; Stine, O.C.; Sathyasaikumar, K.V.; Roberts, R.C.; Mitchell, B.D.; Hong, L.E.; Kajii, Y.; Thaker, G.K.; Schwarcz, R. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch. Gen. Psychiatry 2011, 68, 665–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartoli, F.; Misiak, B.; Callovini, T.; Cavaleri, D.; Cioni, R.M.; Crocamo, C.; Savitz, J.B.; Carra, G. The kynurenine pathway in bipolar disorder: A meta-analysis on the peripheral blood levels of tryptophan and related metabolites. Mol. Psychiatry 2020, 26, 3419–3429. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Brew, B.J.; Noonan, C.E.; Takikawa, O.; Cullen, K.M. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol. Appl. Neurobiol. 2005, 31, 395–404. [Google Scholar] [CrossRef]
- Wirleitner, B.; Rudzite, V.; Neurauter, G.; Murr, C.; Kalnins, U.; Erglis, A.; Trusinskis, K.; Fuchs, D. Immune activation and degradation of tryptophan in coronary heart disease. Eur. J. Clin. Investig. 2003, 33, 550–554. [Google Scholar] [CrossRef]
- Stone, T.W. Kynurenines in the CNS: From endogenous obscurity to therapeutic importance. Prog. Neurobiol. 2001, 64, 185–218. [Google Scholar] [CrossRef]
- Liu, D.; Ray, B.; Neavin, D.R.; Zhang, J.; Athreya, A.P.; Biernacka, J.M.; Bobo, W.V.; Hall-Flavin, D.K.; Skime, M.K.; Zhu, H.; et al. Beta-defensin 1, aryl hydrocarbon receptor and plasma kynurenine in major depressive disorder: Metabolomics-informed genomics. Transl. Psychiatry 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashi, A.A.; Davis, R.W.; Phair, R.D. The IDO Metabolic Trap Hypothesis for the Etiology of ME/CFS. Diagnostics 2019, 9, 82. [Google Scholar] [CrossRef] [Green Version]
- Ichiyama, A.; Nakamura, S.; Kawai, H.; Honjo, T.; Nishizuka, Y.; Hayaishi, O.; Senoh, S. Studies on the Metabolism of the Benzene Ring of Tryptophan in Mammalian Tissues. Ii. Enzymic Formation of Alpha-Aminomuconic Acid from 3-Hydroxyanthranilic Acid. J. Biol. Chem. 1965, 240, 740–749. [Google Scholar] [CrossRef]
- Lionetto, L.; Ulivieri, M.; Capi, M.; De Bernardini, D.; Fazio, F.; Petrucca, A.; Pomes, L.M.; De Luca, O.; Gentile, G.; Casolla, B.; et al. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: An observational cohort study. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166042. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Gostner, J.M.; Nilsson, S.; Andersson, L.M.; Fuchs, D.; Gisslen, M. Serum neopterin levels in relation to mild and severe COVID-19. BMC Infect. Dis. 2020, 20, 942. [Google Scholar] [CrossRef] [PubMed]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy-Bien, J.P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Goff, C.L.; Misset, B.; et al. Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. A Pilot Study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, I.; Eroglu, B.C.; Guven, G.S. Altered tryptophan absorption and metabolism could underlie long-term symptoms in survivors of coronavirus disease 2019 (COVID-19). Nutrition 2021, 90, 111308. [Google Scholar] [CrossRef]
- Lorkiewicz, P.; Waszkiewicz, N. Biomarkers of Post-COVID Depression. J. Clin. Med. 2021, 10, 4142. [Google Scholar] [CrossRef] [PubMed]
- Lawler, N.G.; Gray, N.; Kimhofer, T.; Boughton, B.; Gay, M.; Yang, R.; Morillon, A.C.; Chin, S.T.; Ryan, M.; Begum, S.; et al. Systemic Perturbations in Amine and Kynurenine Metabolism Associated with Acute SARS-CoV-2 Infection and Inflammatory Cytokine Responses. J. Proteome Res. 2021, 20, 2796–2811. [Google Scholar] [CrossRef] [PubMed]
- Danlos, F.X.; Grajeda-Iglesias, C.; Durand, S.; Sauvat, A.; Roumier, M.; Cantin, D.; Colomba, E.; Rohmer, J.; Pommeret, F.; Baciarello, G.; et al. Metabolomic analyses of COVID-19 patients unravel stage-dependent and prognostic biomarkers. Cell Death Dis. 2021, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Loeffelholz, M.J.; Alland, D.; Butler-Wu, S.M.; Pandey, U.; Perno, C.F.; Nava, A.; Carroll, K.C.; Mostafa, H.; Davies, E.; McEwan, A.; et al. Multicenter Evaluation of the Cepheid Xpert Xpress SARS-CoV-2 Test. J. Clin. Microbiol. 2020, 58, e00926-20. [Google Scholar] [CrossRef] [PubMed]
- Wolters, F.; Grunberg, M.; Huber, M.; Kessler, H.H.; Pruller, F.; Saleh, L.; Febreau, C.; Rahamat-Langendoen, J.; Thibault, V.; Melchers, W.J.G. European multicenter evaluation of Xpert(R) Xpress SARS-CoV-2/Flu/RSV test. J. Med. Virol. 2021, 93, 5798–5804. [Google Scholar] [CrossRef] [PubMed]
- Herve, C.; Beyne, P.; Jamault, H.; Delacoux, E. Determination of tryptophan and its kynurenine pathway metabolites in human serum by high-performance liquid chromatography with simultaneous ultraviolet and fluorimetric detection. J. Chromatogr. B Biomed. Sci. Appl. 1996, 675, 157–161. [Google Scholar] [CrossRef]
- Enko, D.; Zelzer, S.; Wenninger, J.; Holasek, S.; Schnedl, W.J.; Baranyi, A.; Herrmann, M.; Meinitzer, A. Interleukin-6 is associated with tryptophan metabolism and signs of depression in individuals with carbohydrate malabsorption. EXCLI J. 2020, 19, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Calzetta, L.; Aiello, M.; Frizzelli, A.; Rogliani, P.; Chetta, A. Dexamethasone in Patients Hospitalized with COVID-19: Whether, When and to Whom. J. Clin. Med. 2021, 10, 1607. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Moller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef]
- Ombrello, M.J.; Schulert, G.S. COVID-19 and cytokine storm syndrome: Are there lessons from macrophage activation syndrome? Transl. Res. 2021, 232, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Nie, M.; Pang, H.; Wang, B.; Hu, J.; Meng, X.; Li, K.; Ran, X.; Long, Q.; Deng, H.; et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021, 12, 1618. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.R.; Suleiman, M.; Perez-Lopez, A. Metabolomics in the Diagnosis and Prognosis of COVID-19. Front. Genet. 2021, 12, 721556. [Google Scholar] [CrossRef]
- Dogan, H.O.; Senol, O.; Bolat, S.; Yildiz, S.N.; Buyuktuna, S.A.; Sariismailoglu, R.; Dogan, K.; Hasbek, M.; Hekim, S.N. Understanding the pathophysiological changes via untargeted metabolomics in COVID-19 patients. J. Med. Virol. 2021, 93, 2340–2349. [Google Scholar] [CrossRef]
- Lopez-Hernandez, Y.; Monarrez-Espino, J.; Oostdam, A.H.; Delgado, J.E.C.; Zhang, L.; Zheng, J.; Valdez, J.J.O.; Mandal, R.; Gonzalez, F.L.O.; Moreno, J.C.B.; et al. Targeted metabolomics identifies high performing diagnostic and prognostic biomarkers for COVID-19. Sci. Rep. 2021, 11, 14732. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, F.; Kuroiwa, T.; Takikawa, O.; Kido, R. Human indolylamine 2,3-dioxygenase. Its tissue distribution, and characterization of the placental enzyme. Biochem. J. 1985, 230, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.C.; Metz, R.; Muller, A.J.; Merlo, L.M.; Mandik-Nayak, L. IDO2 in Immunomodulation and Autoimmune Disease. Front. Immunol. 2014, 5, 585. [Google Scholar] [CrossRef] [Green Version]
- Badawy, A.A.; Bano, S. Tryptophan Metabolism in Rat Liver After Administration of Tryptophan, Kynurenine Metabolites, and Kynureninase Inhibitors. Int. J. Tryptophan Res. 2016, 9, 51–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, Y.W.; Hajjar, J.; Hwu, P.; Naing, A. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J. Immunother. Cancer 2015, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Gong, J.; Liu, Y. Indoleamine 2,3-dioxygenase regulation of immune response (Review). Mol. Med. Rep. 2018, 17, 4867–4873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prendergast, G.C.; Smith, C.; Thomas, S.; Mandik-Nayak, L.; Laury-Kleintop, L.; Metz, R.; Muller, A.J. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol. Immunother. 2014, 63, 721–735. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Aldajani, W.A.; Salazar, F.; Sewell, H.F.; Knox, A.; Ghaemmaghami, A.M. Expression and regulation of immune-modulatory enzyme indoleamine 2,3-dioxygenase (IDO) by human airway epithelial cells and its effect on T cell activation. Oncotarget 2016, 7, 57606–57617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.; Park, H.Y.; Suh, Y.S.; Yoon, E.H.; Kim, J.; Jang, W.H.; Lee, W.S.; Park, S.G.; Choi, I.W.; Choi, I.; et al. Inhibition of acute lethal pulmonary inflammation by the IDO-AhR pathway. Proc. Natl. Acad. Sci. USA 2017, 114, E5881–E5890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, T.; Stefanoni, D.; Reisz, J.A.; Nemkov, T.; Bertolone, L.; Francis, R.O.; Hudson, K.E.; Zimring, J.C.; Hansen, K.C.; Hod, E.A.; et al. COVID-19 infection results in alterations of the kynurenine pathway and fatty acid metabolism that correlate with IL-6 levels and renal status. medRxiv 2020. [Google Scholar] [CrossRef]
- Giovannoni, F.; Li, Z.; Garcia, C.C.; Quintana, F.J. A potential role for AHR in SARS-CoV-2 pathology. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Christen, S.; Peterhans, E.; Stocker, R. Antioxidant activities of some tryptophan metabolites: Possible implication for inflammatory diseases. Proc. Natl. Acad. Sci. USA 1990, 87, 2506–2510. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.Y.; Zhang, M.; Yang, C.X.; Zhang, N.; Wang, X.C.; Yang, X.P.; Dong, X.Q.; Zheng, Y.T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 541–543. [Google Scholar] [CrossRef]
- Tan, M.; Liu, Y.; Zhou, R.; Deng, X.; Li, F.; Liang, K.; Shi, Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology 2020, 160, 261–268. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Ho, Y.C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Barhoumi, T.; Alghanem, B.; Shaibah, H.; Mansour, F.A.; Alamri, H.S.; Akiel, M.A.; Alroqi, F.; Boudjelal, M. SARS-CoV-2 Coronavirus Spike Protein-Induced Apoptosis, Inflammatory, and Oxidative Stress Responses in THP-1-Like-Macrophages: Potential Role of Angiotensin-Converting Enzyme Inhibitor (Perindopril). Front. Immunol. 2021, 12, 728896. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.D.; Shen, Q.; Cintron, S.A.; Hiebert, J.B. Post-COVID-19 Syndrome. Nurs. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chilosi, M.; Poletti, V.; Ravaglia, C.; Rossi, G.; Dubini, A.; Piciucchi, S.; Pedica, F.; Bronte, V.; Pizzolo, G.; Martignoni, G.; et al. The pathogenic role of epithelial and endothelial cells in early-phase COVID-19 pneumonia: Victims and partners in crime. Mod. Pathol. 2021, 34, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Giagulli, V.A.; Guastamacchia, E.; Magrone, T.; Jirillo, E.; Lisco, G.; De Pergola, G.; Triggiani, V. Worse progression of COVID-19 in men: Is testosterone a key factor? Andrology 2021, 9, 53–64. [Google Scholar] [CrossRef]


| Exitus (n = 31) | Recovery (n = 117) | |
|---|---|---|
| age (years) | 80.0 (52.0, 98.0) | 57.0 (18.0, 93.0) |
| Sex female | 14 (45.2%) | 55 (47.0%) |
| Sex male | 17 (54.8%) | 62 (53.0%) |
| ICU admission | 13 (41.9%) | 22 (18.8%) |
| normal ward | 18 (58.1%) | 95 (81.2%) |
| renal disease no | 15 (48.4%) | 98 (83.8%) |
| renal disease yes | 16 (51.6%) | 19 (16.2%) |
| CAD no | 15 (48.4%) | 93 (79.5%) |
| CAD yes | 16 (51.6%) | 24 (20.5%) |
| preexisting disease no | 3 (9.7%) | 51 (43.6%) |
| preexisting disease yes | 28 (90.3%) | 66 (56.4%) |
| cancer no | 24 (80.0%) | 106 (91.4%) |
| cancer yes | 6 (20.0%) | 10 (8.6%) |
| hypertension | 8 (25.8%) | 71 (60.7%) |
| oxygen therapy | 28 (90.3%) | 60 (51.7%) |
| pulmonary disease no | 25 (80.6%) | 101 (87.1%) |
| pulmonary disease yes | 6 (19.4%) | 15 (12.9%) |
| Plasma Value 1st Visit | N | Exitus Median (min, max) | N | Recovery Median (min, max) | p-Value |
|---|---|---|---|---|---|
| Kynurenine, µmol/L | 31 | 6.1 (3.1, 18.1) | 117 | 3.7 (1.1, 12.9) | <0.001 |
| CRP, mg/dL | 31 | 87.0 (3.8, 315.3) | 117 | 22.0 (0.6, 336.9) | <0.001 |
| Interleukin-6, pg/mL | 31 | 79.9 (7.5, 614.0) | 117 | 18.8 (1.5, 3086.0) | <0.001 |
| Ferritin, ng/mL | 31 | 619.0 (78.0, 23,706.0) | 117 | 336.0 (7.0, 14,553.0) | 0.008 |
| NTproBNP, pg/mL | 31 | 2110.0 (21.0, 70,000.0) | 117 | 125.0 (5.0, 70,000.0) | <0.001 |
| cTnT, pg/mL | 31 | 51.0 (3.0, 851.0) | 117 | 7.0 (3.0, 1020.0) | <0.001 |
| Creatinin, mg/dL | 31 | 1.4 (0.6, 13.4) | 117 | 1.0 (0.5, 8.4) | <0.001 |
| D-Dimer, mg/L | 21 | 1.8 (0.2, 33.0) | 91 | 0.7 (0.2, 33.0) | -*- |
| Parameter | Category | Hazard Ratio | Lower 95% Confidence Limit | Upper 95% Confidence Limit | p-Value |
|---|---|---|---|---|---|
| Sex | male versus female | 1.03 | 0.49 | 2.18 | 0.938 |
| Age, years | - | 1.04 | 1.01 | 1.07 | 0.008 |
| Kynurenine, µmol/L | - | 1.19 | 1.07 | 1.32 | 0.001 |
| Ferritin, ng/mL grouped | normal versus high | 1.71 | 0.51 | 5.73 | 0.389 |
| NTproBNP, pg/mL grouped | normal versus high | 6.78 | 0.82 | 55.90 | 0.075 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangge, H.; Herrmann, M.; Meinitzer, A.; Pailer, S.; Curcic, P.; Sloup, Z.; Holter, M.; Prüller, F. Increased Kynurenine Indicates a Fatal Course of COVID-19. Antioxidants 2021, 10, 1960. https://doi.org/10.3390/antiox10121960
Mangge H, Herrmann M, Meinitzer A, Pailer S, Curcic P, Sloup Z, Holter M, Prüller F. Increased Kynurenine Indicates a Fatal Course of COVID-19. Antioxidants. 2021; 10(12):1960. https://doi.org/10.3390/antiox10121960
Chicago/Turabian StyleMangge, Harald, Markus Herrmann, Andreas Meinitzer, Sabine Pailer, Pero Curcic, Zdenka Sloup, Magdalena Holter, and Florian Prüller. 2021. "Increased Kynurenine Indicates a Fatal Course of COVID-19" Antioxidants 10, no. 12: 1960. https://doi.org/10.3390/antiox10121960
APA StyleMangge, H., Herrmann, M., Meinitzer, A., Pailer, S., Curcic, P., Sloup, Z., Holter, M., & Prüller, F. (2021). Increased Kynurenine Indicates a Fatal Course of COVID-19. Antioxidants, 10(12), 1960. https://doi.org/10.3390/antiox10121960







