Intra-Articular Injection of (−)-Epigallocatechin 3-Gallate (EGCG) Ameliorates Cartilage Degeneration in Guinea Pigs with Spontaneous Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assessment
2.3. Senescence-Associated Beta-Galactosidase (SAβGal) Activity Analysis
2.4. Real-Time Quantitative PCR (RT-qPCR)
2.5. Animal Experiments
2.6. Endurance Test
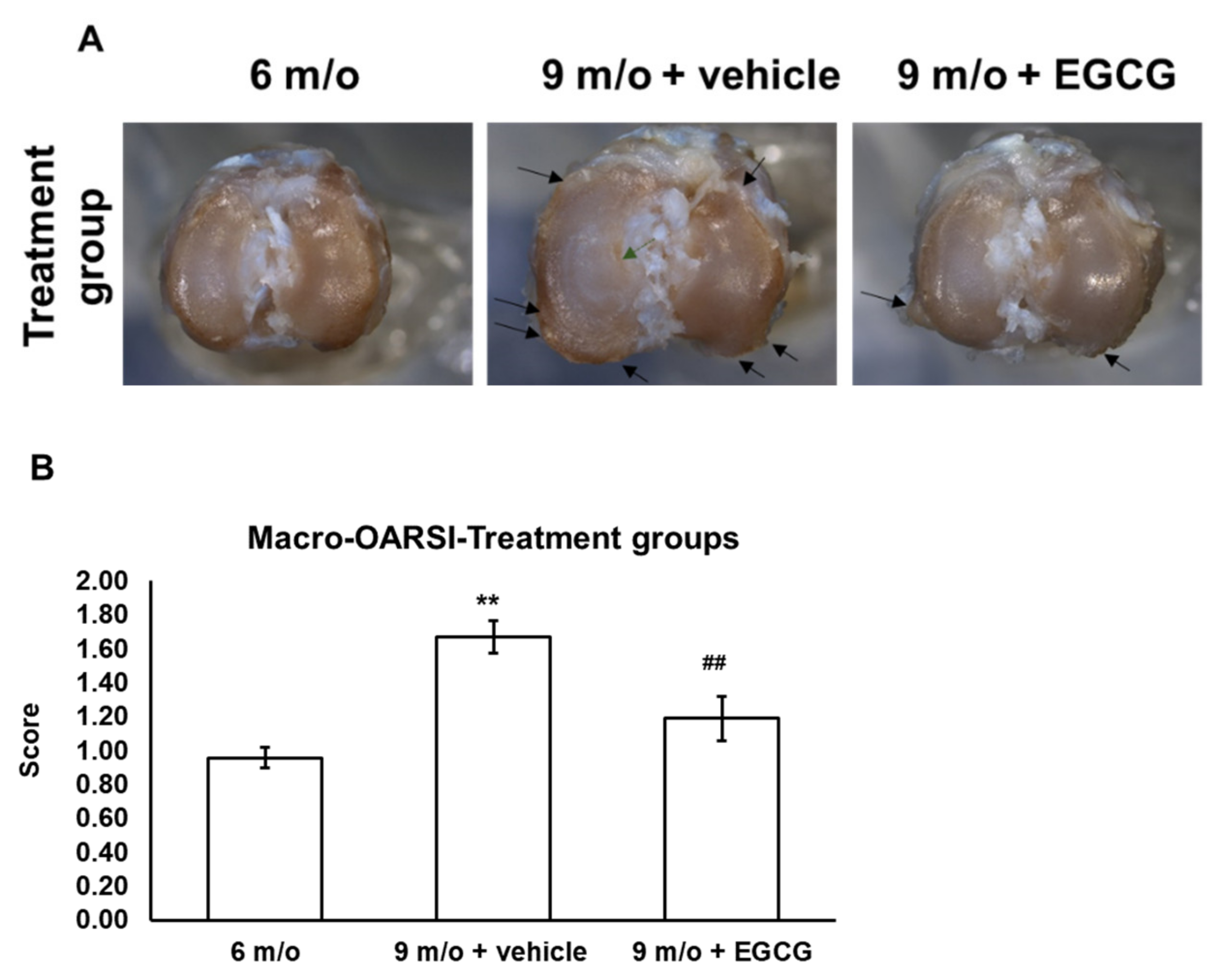
2.7. Gross Observations and Histopathological Analysis
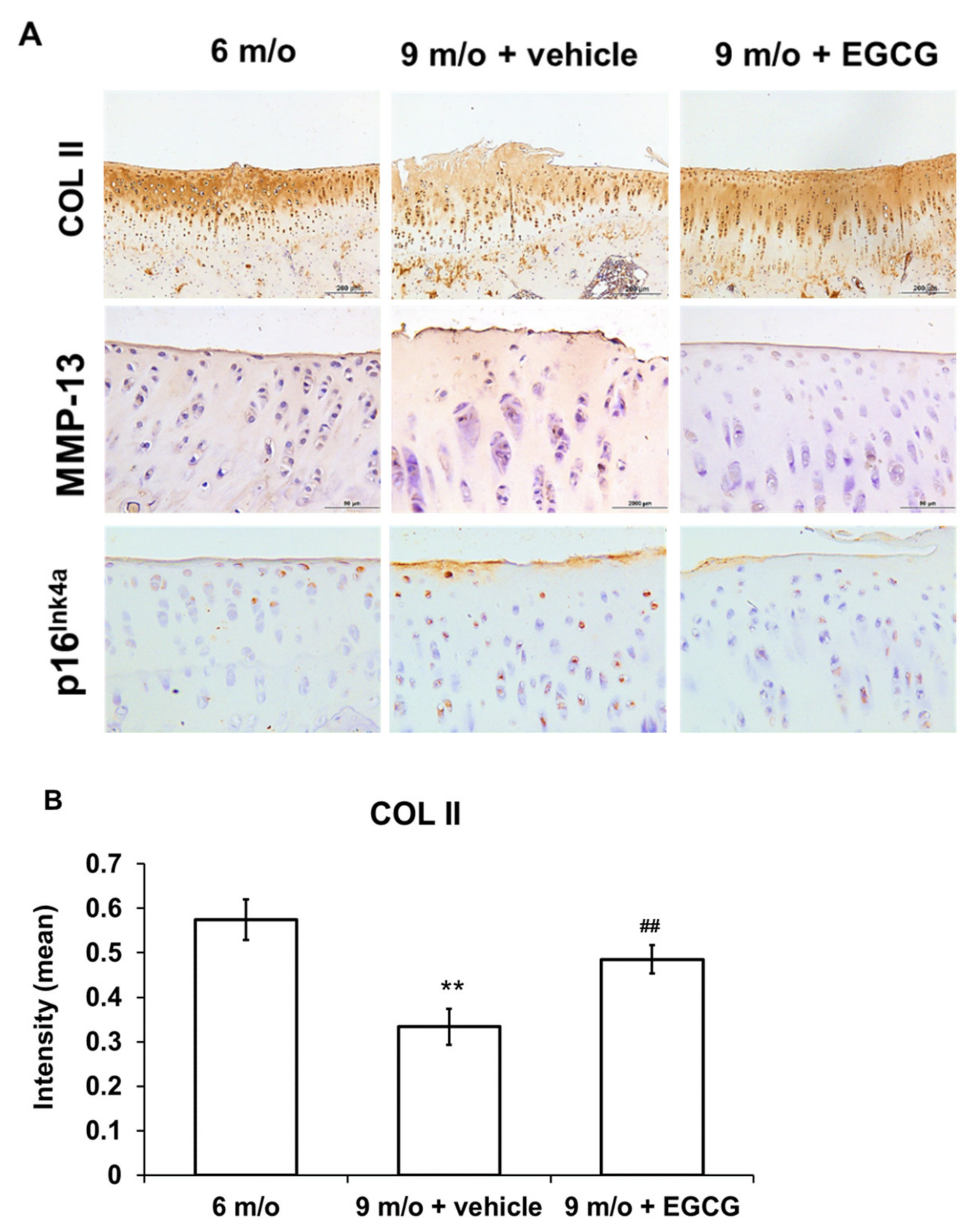
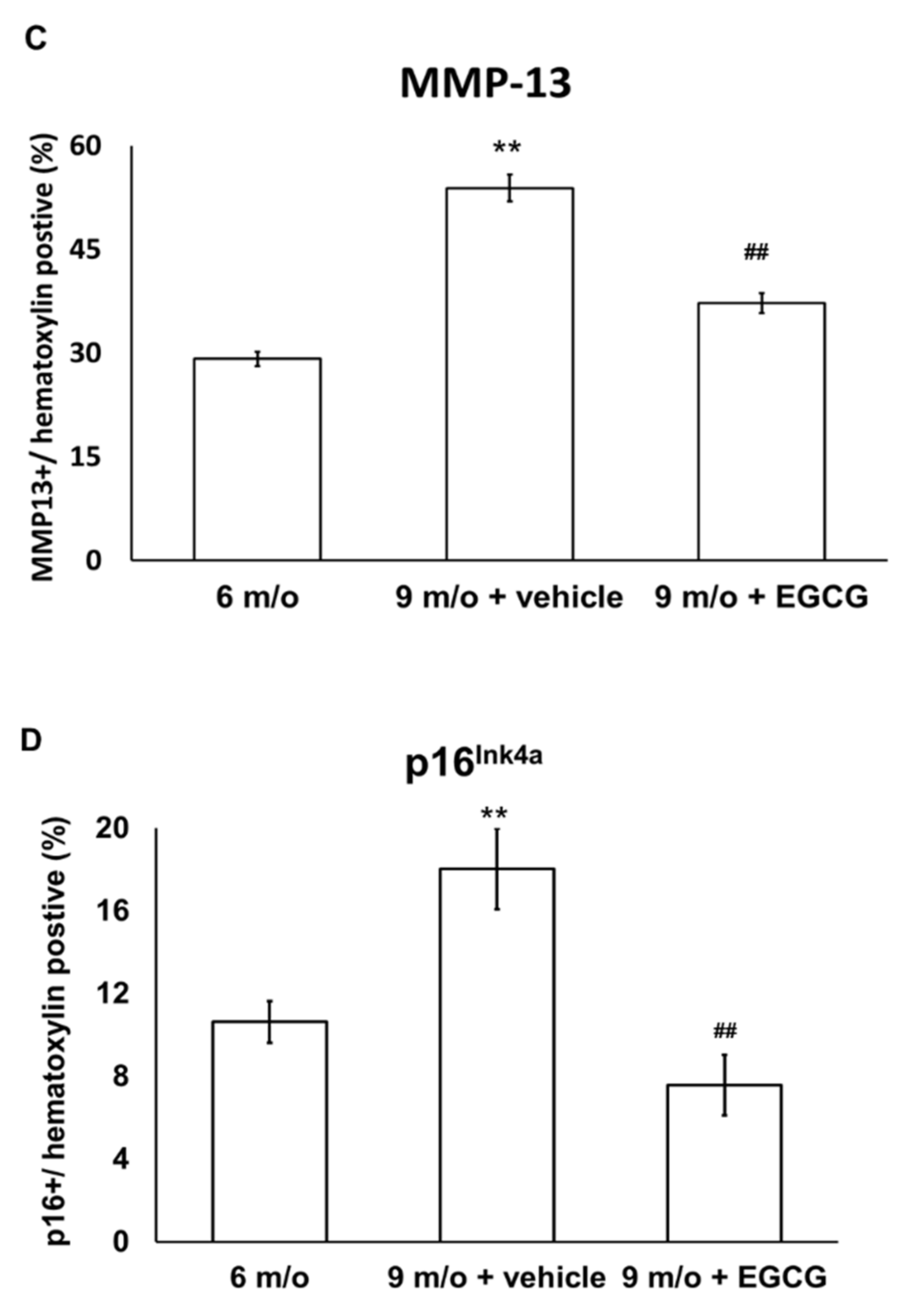
2.8. Immunohistochemistry (IHC) for Col II, MMP-13, and Marker of Cellular Senescence (p16 Ink4a)
2.9. Statistical Analysis
3. Results
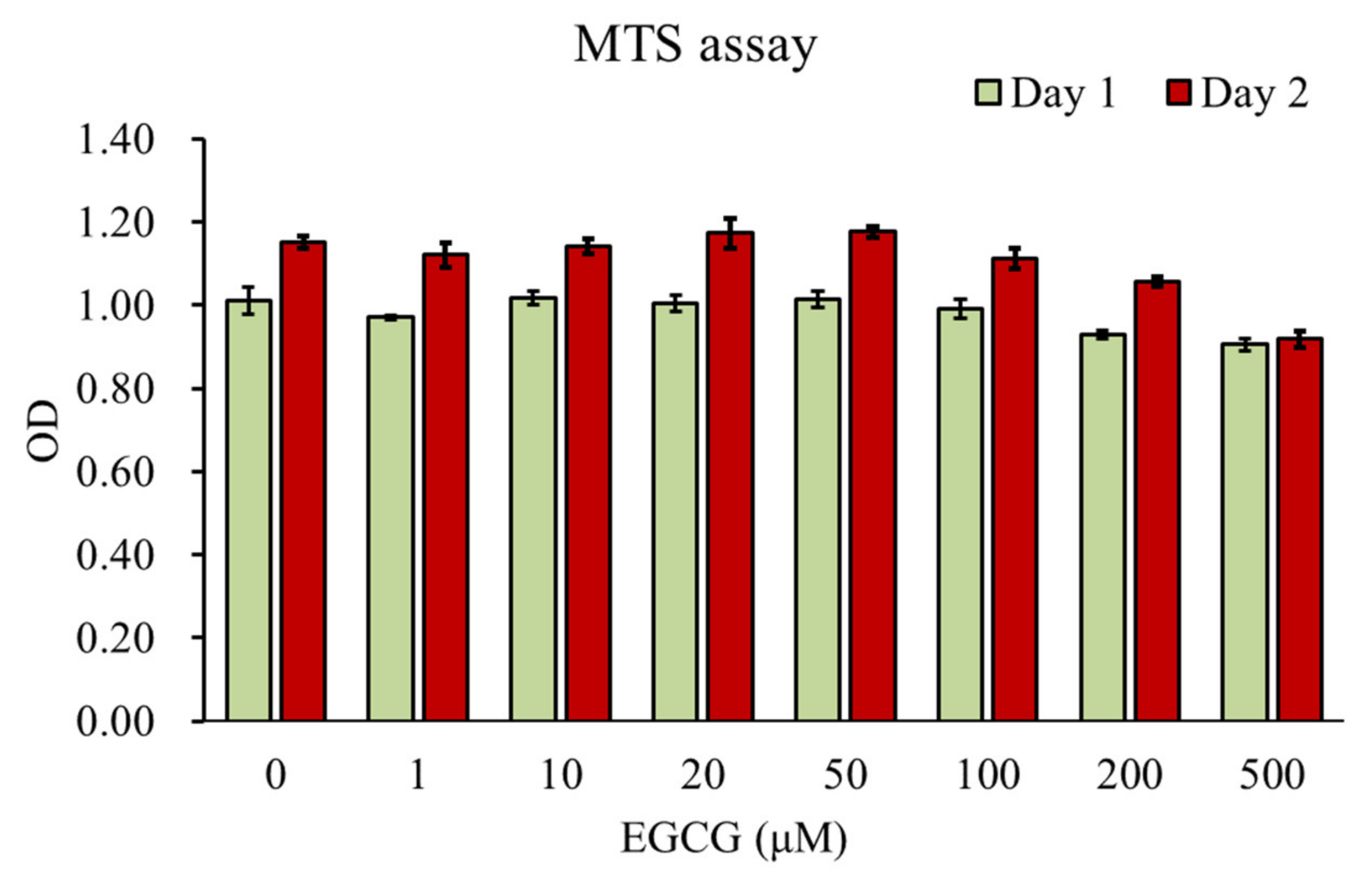
3.1. Effect of EGCG on Chondrocyte Viability
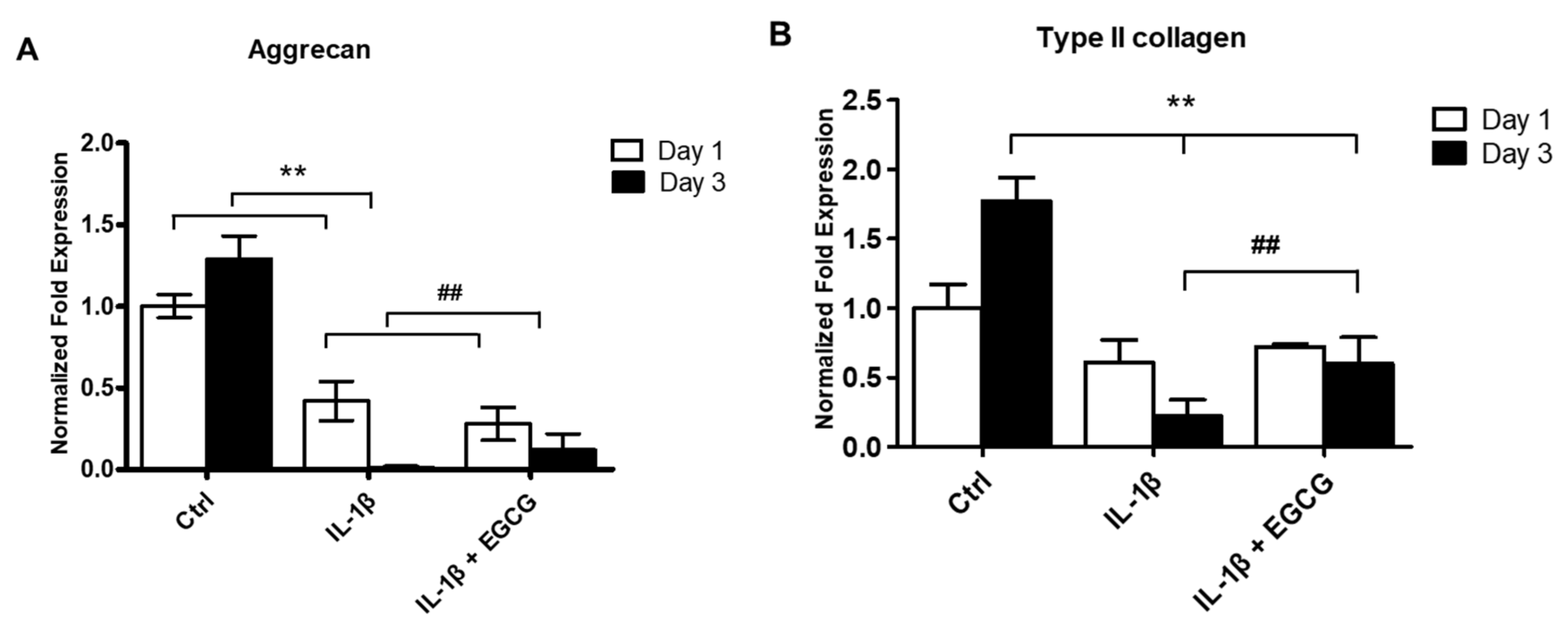
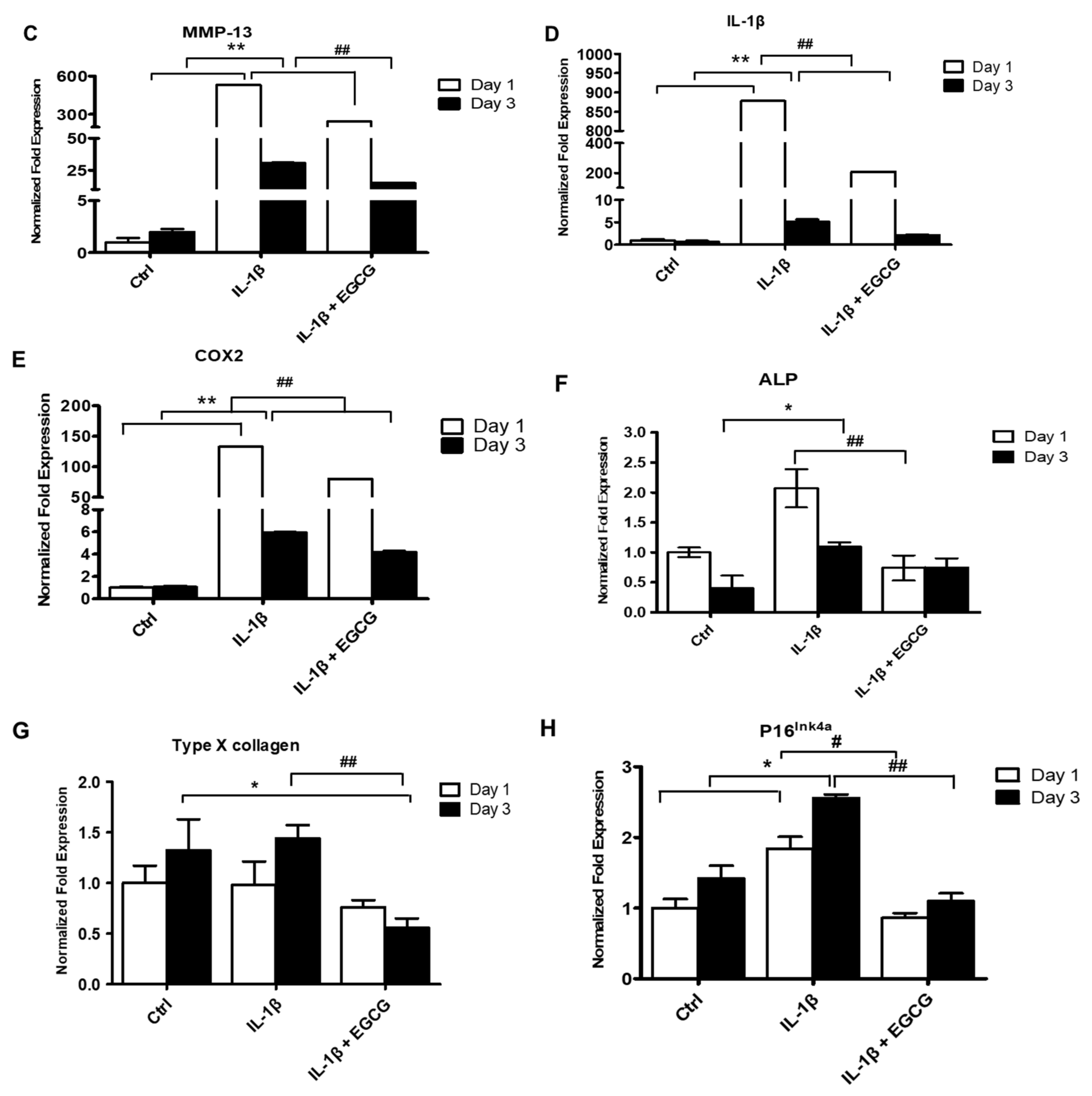
3.2. EGCG Suppresses ECM Degradation, Inflammatory Response, Chondrocyte Hypertrophic Differentiation, and Cell Senescence in IL-1β-Stimulated Human Chondrocytes
3.3. EGCG Attenuates SAβGal Activity in IL-1β-Stimulated Human Chondrocytes
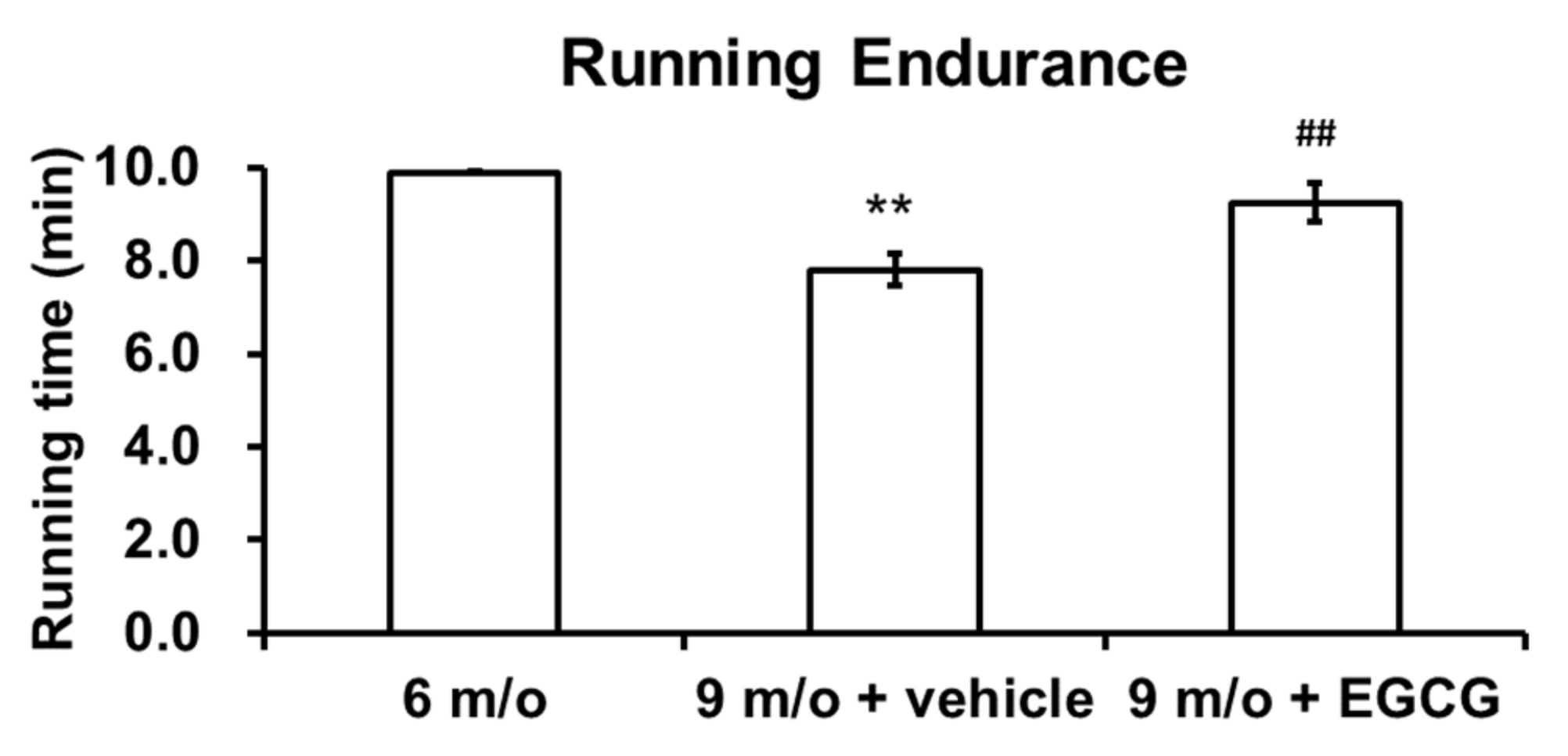
3.4. Intra-Articular Injection of EGCG Improves Running Endurance of OA Animals
3.5. EGCG Treatment Ameliorates Cartilage Degradation
3.6. EGCG Maintains the Content of Col II and Attenuates the Expression of MMP-13 and p16 Ink4a in OA Cartilage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | osteoarthritis |
| EGCG | (−)-Epigallocatechin 3-gallate |
| COX-2 | cyclooxygenase 2 |
| MMP | matrix metalloproteinase |
| TNF-α | tumor necrosis factor-α |
| ADAMTS | a disintegrin and metalloproteinase with thrombospondin motifs |
| Col II | collagen type II |
| Col X | collagen X |
| ECM | extracellular matrix |
| DH | Dunkin–Hartley |
| SAβGal | senescence-associated beta-galactosidase |
| IL-1β | interleukin-1β |
| ALP | alkaline phosphatase |
| GAG | glycosaminoglycan |
References
- Shane Anderson, A.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, A.; Ning, Y.; Wen, Y.; Cai, Y.; Xu, K.; Cai, Y.; Han, J.; Liu, L.; Du, Y.; Liang, X.U.; et al. Se of integrative epigenetic and mRNA expression analyses to identify significantly changed genes and functional pathways in osteoarthritic cartilage. Bone. Jt. Res. 2018, 7, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Van Meurs, J.B.; Boer, C.G.; Lopez-Delgado, L.; Riancho, J.A. Role of Epigenomics in Bone and Cartilage Disease. J. Bone. Miner. Res. 2019, 34, 215–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhou, L.; Zhang, Y.; Huang, L.; Shi, Q. Mesenchymal stem cells a promising strategy for treating knee osteoarthritis. Bone. Joint Res. 2020, 9, 719–728. [Google Scholar] [CrossRef]
- Liao, S.; Kao, Y.H.; Hiipakka, R.A. Green tea: Biochemical and biological basis for health benefits. Vitam. Horm. 2001, 62, 1–94. [Google Scholar] [CrossRef]
- Lee, S.; Yu, J.S.; Phung, H.M.; Lee, J.G.; Kim, K.H.; Kang, K.S. Potential Anti-Skin Aging Effect of (−)-Catechin Isolated from the Root Bark of Ulmus davidiana var. japonica in Tumor Necrosis Factor-α-Stimulated Normal Human Dermal Fibroblasts. Antioxidants (Basel) 2020, 9, 981. [Google Scholar] [CrossRef]
- Lin, S.Y.; Kang, L.; Chen, J.C.; Wang, C.Z.; Huang, H.H.; Lee, M.J.; Cheng, T.L.; Chang, C.F.; Lin, Y.S.; Chen, C.H. (−)-Epigallocatechin-3-gallate (EGCG) enhances healing of femoral bone defect. Phytomedicine 2019, 55, 165–171. [Google Scholar] [CrossRef]
- Kerasioti, E.; Apostolou, A.; Kafantaris, I.; Chronis, K.; Kokka, E.; Dimitriadou, C.; Tzanetou, E.N.; Priftis, A.; Koulocheri, S.D.; Haroutounian, S.A.; et al. Polyphenolic Composition of Rosa canina, Rosa sempervivens and Pyrocantha coccinea Extracts and Assessment of Their Antioxidant Activity in Human Endothelial Cells. Antioxidants (Basel) 2019, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Azain, M.; Crowe-White, K.; Mumaw, J.; Grimes, J.A.; Schmiedt, C.; Barletta, M.; Rayalam, S.; Park, H.J. Effect of Acute Ingestion of Green Tea Extract and Lemon Juice on Oxidative Stress and Lipid Profile in Pigs Fed a High-Fat Diet. Antioxidants (Basel) 2019, 8, 195. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.T.; Kang, L.; Wang, C.Z.; Huang, P.J.; Huang, H.T.; Lin, S.Y.; Chou, S.H.; Lu, C.C.; Shen, P.C.; Lin, Y.S.; et al. (−)-Epigallocatechin-3-Gallate Decreases Osteoclastogenesis via Modulation of RANKL and Osteoprotegrin. Molecules 2019, 24, 156. [Google Scholar] [CrossRef] [Green Version]
- Attanzio, A.; D’Anneo, A.; Pappalardo, F.; Bonina, F.P.; Livrea, M.A.; Allegra, M.; Tesoriere, L. Phenolic Composition of Hydrophilic Extract of Manna from Sicilian Fraxinus angustifolia Vahl and its Reducing, Antioxidant and Anti-Inflammatory Activity in Vitro. Antioxidants (Basel) 2019, 8, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.Y.; Kang, L.; Wang, C.Z.; Huang, H.H.; Cheng, T.L.; Huang, H.T.; Lee, M.J.; Lin, Y.S.; Ho, M.L.; Wang, G.J.; et al. (−)-Epigallocatechin-3-Gallate (EGCG) Enhances Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Molecules 2018, 23, 3221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.Y.; Kan, J.Y.; Lu, C.C.; Huang, H.H.; Cheng, T.L.; Huang, H.T.; Ho, C.J.; Lee, T.C.; Chuang, S.C.; Lin, Y.S.; et al. Green Tea Catechin (−)-Epigallocatechin-3-Gallate (EGCG) Facilitates Fracture Healing. Biomolecules 2020, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Ho, M.L.; Chang, J.K.; Hung, S.H.; Wang, G.J. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos. Int. 2005, 16, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.W.; Chen, C.H.; Wang, Y.H.; Ho, M.L.; Hung, S.H.; Chen, I.S.; Wang, G.J. (−)-Epigallocatechin gallate inhibition of osteoclastic differentiation via NF-kappaB. Biochem. Biophys. Res. Commun. 2009, 379, 1033–1037. [Google Scholar] [CrossRef]
- Chen, C.H.; Chang, J.K.; Hung, S.H.; Huang, H.T.; Wang, C.H.; Yeh, C.H.; Wang, G.J. Green tea catechins enhance the expression of osteoprotegerin (OPG) in pluripotent stem cells. J. Orthop. Surg. Taiwan 2003, 20, 178–183. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, H.; Jeong, M.J.; Kang, T.C. Epigallocatechin-3-Gallate and PEDF 335 Peptide, 67LR Activators, Attenuate Vasogenic Edema, and Astroglial Degeneration Following Status Epilepticus. Antioxidants (Basel) 2020, 9, 854. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, K.S.; Lim, D.; Yang, D.J.; Park, J.I.; Kim, K.W.; Jeong, J.H.; Choi, H.S.; Kim, D.K. Epigallocatechin-3-Gallate (EGCG)-Inducible SMILE Inhibits STAT3-Mediated Hepcidin Gene Expression. Antioxidants (Basel) 2020, 9, 514. [Google Scholar] [CrossRef]
- Kluknavsky, M.; Balis, P.; Skratek, M.; Manka, J.; Bernatova, I. (−)-Epicatechin Reduces the Blood Pressure of Young Borderline Hypertensive Rats During the Post-Treatment Period. Antioxidants (Basel) 2020, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Kuban-Jankowska, A.; Kostrzewa, T.; Musial, C.; Barone, G.; Lo Bosco, G.; Lo Celso, F.; Gorska-Ponikowska, M. Green Tea Catechins Induce Inhibition of PTP1B Phosphatase in Breast Cancer Cells with Potent Anti-Cancer Properties: In Vitro Assay, Molecular Docking, and Dynamics Studies. Antioxidants (Basel) 2020, 9, 1208. [Google Scholar] [CrossRef]
- Perdices, L.; Fuentes-Broto, L.; Segura, F.; Cuenca, N.; Orduna-Hospital, E.; Pinilla, I. Epigallocatechin Gallate Slows Retinal Degeneration, Reduces Oxidative Damage, and Modifies Circadian Rhythms in P23H Rats. Antioxidants (Basel) 2020, 9, 718. [Google Scholar] [CrossRef]
- Ahmed, S.; Rahman, A.; Hasnain, A.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic. Biol. Med. 2002, 33, 1097–1105. [Google Scholar] [CrossRef]
- Ahmed, S.; Wang, N.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J. Pharmacol. Exp. Ther. 2004, 308, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Haqqi, T.M. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res. Ther. 2011, 13, R93. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Liu, Q.; Liu, L.; Wu, H.; Zheng, L. Effect of epigallocatechin-3-gallate on proliferation and phenotype maintenance in rabbit articular chondrocytes in vitro. Exp. Ther. Med. 2015, 9, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Andriamanalijaona, R.; Kypriotou, M.; Baugé, C.; Renard, E.; Legendre, F.; Raoudi, M.; Boumediene, K.; Gatto, H.; Monginoux, P.; Pujol, J.P. Comparative effects of 2 antioxidants, selenomethionine and epigallocatechin-gallate, on catabolic and anabolic gene expression of articular chondrocytes. J. Rheumatol. 2005, 32, 1958–1967. [Google Scholar] [PubMed]
- Adcocks, C.; Collin, P.; Buttle, D.J. Catechins from green tea (Camellia sinensis) inhibit bovine and human cartilage proteoglycan and type II collagen degradation in vitro. J. Nutr. 2002, 132, 341–346. [Google Scholar] [CrossRef]
- Jin, Y.; Koh, R.H.; Kim, S.H.; Kim, K.M.; Park, G.K.; Hwang, N.S. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mater. Sci. Eng. C. Mater. Biol. Appl. 2020, 115, 111096. [Google Scholar] [CrossRef]
- Leong, D.J.; Choudhury, M.; Hanstein, R.; Hirsh, D.M.; Kim, S.J.; Majeska, R.J.; Schaffler, M.B.; Hardin, J.A.; Spray, D.C.; Goldring, M.B.; et al. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse post-traumatic osteoarthritis model. Arthritis Res. Ther. 2014, 16, 508. [Google Scholar] [CrossRef] [Green Version]
- Luk, H.Y.; Appell, C.; Chyu, M.C.; Chen, C.H.; Wang, C.Y.; Yang, R.S.; Shen, C.L. Impacts of Green Tea on Joint and Skeletal Muscle Health: Prospects of Translational Nutrition. Antioxidants (Basel) 2020, 9, 1050. [Google Scholar] [CrossRef]
- Heinecke, L.F.; Grzanna, M.W.; Au, A.Y.; Mochal, C.A.; Rashmir-Raven, A.; Frondoza, C.G. Inhibition of cyclooxygenase-2 expression and prostaglandin E2 production in chondrocytes by avocado soybean unsaponifiables and epigallocatechin gallate. Osteoarthr. Cartil. 2010, 18, 220–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.S.; Tseng, C.Y.; Lee, C.H.; Su, S.L.; Lee, H.S. Effects of (−)-epigallocatechin-3-gallate on cyclooxygenase 2, PGE(2), and IL-8 expression induced by IL-1beta in human synovial fibroblasts. Rheumatol. Int. 2010, 30, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Rasheed, N.; Al-Shaya, O. Epigallocatechin-3-O-gallate modulates global microRNA expression in interleukin-1β-stimulated human osteoarthritis chondrocytes: Potential role of EGCG on negative co-regulation of microRNA-140-3p and ADAMTS5. Eur. J. Nutr. 2018, 57, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahmed, S.; Islam, N.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: Suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis. Rheum. 2002, 46, 2079–2086. [Google Scholar] [CrossRef]
- Rasheed, Z.; Rasheed, N.; Al-Shobaili, H.A. Epigallocatechin-3-O-gallate up-regulates microRNA-199a-3p expression by down-regulating the expression of cyclooxygenase-2 in stimulated human osteoarthritis chondrocytes. J. Cell. Mol. Med. 2016, 20, 2241–2248. [Google Scholar] [CrossRef] [Green Version]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Campisi, J. Cellular senescence: Putting the paradoxes in perspective. Curr. Opin. Genet. Dev. 2011, 21, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Greene, M.A.; Loeser, R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, K.; Litherland, G.J.; Rai, T.S. Cellular senescence in osteoarthritis pathology. Aging. Cell 2017, 16, 210–218. [Google Scholar] [CrossRef]
- Jeon, O.H.; Kim, C.; Laberge, R.M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bapat, S.; Hubbard, D.; Munjal, A.; Hunter, M.; Fulzele, S. Pros and cons of mouse models for studying osteoarthritis. Clin. Transl. Med. 2018, 7, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.S.; Kuo, C.W.; Ko, J.Y.; Chen, Y.S.; Wang, S.Y.; Ke, H.J.; Kuo, P.C.; Lee, C.H.; Wu, J.C.; Lu, W.B.; et al. Irisin Mitigates Oxidative Stress, Chondrocyte Dysfunction and Osteoarthritis Development through Regulating Mitochondrial Integrity and Autophagy. Antioxidants (Basel) 2020, 9, 810. [Google Scholar] [CrossRef] [PubMed]
- Tessier, J.J.; Bowyer, J.; Brownrigg, N.J.; Peers, I.S.; Westwood, F.R.; Waterton, J.C.; Maciewicz, R.A. Characterisation of the guinea pig model of osteoarthritis by in vivo three-dimensional magnetic resonance imaging. Osteoarthr. Cartil. 2003, 11, 845–853. [Google Scholar] [CrossRef]
- Gregory, M.H.; Capito, N.; Kuroki, K.; Stoker, A.M.; Cook, J.L.; Sherman, S.L. A review of translational animal models for knee osteoarthritis. Arthritis 2012, 764621. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Huebner, J.L.; DeGroot, J.; Bendele, A. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthr. Cartil. 2010, 18, S35–S52. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.T.; Cheng, T.L.; Ho, C.J.; Huang, H.H.; Lu, C.C.; Chuang, S.C.; Li, J.Y.; Lee, T.C.; Chen, S.T.; Lin, Y.S.; et al. Intra-Articular Injection of (−)-Epigallocatechin 3-Gallate to Attenuate Articular Cartilage Degeneration by Enhancing Autophagy in a Post-Traumatic Osteoarthritis Rat Model. Antioxidants (Basel) 2021, 10, 8. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [Green Version]
- Hall, B.M.; Balan, V.; Gleiberman, A.S.; Strom, E.; Krasnov, P.; Virtuoso, L.P.; Rydkina, E.; Vujcic, S.; Balan, K.; Gitlin, I.I.; et al. P16(Ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging 2017, 9, 1867–1884. [Google Scholar] [CrossRef] [Green Version]
- Mohamad Kamal, N.S.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell. Biol. 2020, 99, 151108. [Google Scholar] [CrossRef]
- Chen, H.T.; Lee, M.J.; Chen, C.H.; Chuang, S.C.; Chang, L.F.; Ho, M.L.; Hung, S.H.; Fu, Y.C.; Wang, Y.H.; Wang, H.I.; et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J. Cell. Mol. Med. 2012, 16, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Ho, M.L.; Chang, L.H.; Kang, L.; Lin, Y.S.; Wu, S.C.; Chang, J.K. Parathyroid hormone-(1-34) ameliorated knee osteoarthritis in rats via autophagy. J. Appl. Physiol. 2018, 124, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.C.; Chen, C.H.; Chou, L.Y.; Cheng, T.L.; Kang, L.; Chuang, S.C.; Lin, Y.S.; Ho, M.L.; Wang, Y.H.; Lin, S.Y.; et al. Discoidin Domain Receptors 1 Inhibition Alleviates Osteoarthritis via Enhancing Autophagy. Int. J. Mol. Sci. 2020, 21, 6991. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Huang, T.H.; Cheng, T.L.; Chang, C.F.; Wang, C.Z.; Wu, M.H.; Kang, L. Exercise training ameliorates glucosamine-induced insulin resistance in ovariectomized rats. Menopause 2017, 24, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Kuo, S.M.; Tien, Y.C.; Shen, P.C.; Kuo, Y.W.; Huang, H.H. Steady Augmentation of Anti-Osteoarthritic Actions of Rapamycin by Liposome-Encapsulation in Collaboration with Low-Intensity Pulsed Ultrasound. Int. J. Nanomed. 2020, 15, 3771–3790. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.T.; Gou, Y.; Fang, J.K.; Hu, Y.P.; Lian, Q.Q.; Zhang, Y.Y.; Wang, Y.D.; Tian, F.M.; Zhang, L. Parathyroid hormone (1-34) ameliorates cartilage degeneration and subchondral bone deterioration in collagenase-induced osteoarthritis model in mice. Bone Joint Res. 2020, 9, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Chou, L.Y.; Chen, C.H.; Lin, Y.H.; Chuang, S.C.; Chou, H.C.; Lin, S.Y.; Fu, Y.C.; Chang, J.K.; Ho, M.L.; Wang, C.Z. Discoidin domain receptor 1 regulates endochondral ossification through terminal differentiation of chondrocytes. FASEB J. 2020, 34, 5767–5781. [Google Scholar] [CrossRef] [Green Version]
- Chou, L.Y.; Chen, C.H.; Chuang, S.C.; Cheng, T.L.; Lin, Y.H.; Chou, H.C.; Fu, Y.C.; Wang, Y.H.; Wang, C.Z. Discoidin Domain Receptor 1 Regulates Runx2 during Osteogenesis of Osteoblasts and Promotes Bone Ossification via Phosphorylation of p38. Int. J. Mol. Sci. 2020, 21, 7210. [Google Scholar] [CrossRef]
- He, Z.; Nie, P.; Lu, J.; Ling, Y.; Guo, J.; Zhang, B.; Hu, J.; Liao, J.; Gu, J.; Dai, B.; et al. Less mechanical loading attenuates osteoarthritis by reducing cartilage degeneration, subchondral bone remodelling, secondary inflammation, and activation of NLRP3 inflammasome. Bone Joint Res. 2020, 9, 731–741. [Google Scholar] [CrossRef]
- Chen, C.H.; Li, C.J.; Tai, I.C.; Lin, X.H.; Hsu, H.K.; Ho, M.L. The Fractionated Toona sinensis Leaf Extract Induces Apoptosis of Human Osteosarcoma Cells and Inhibits Tumor Growth in a Murine Xenograft Model. Integr. Cancer Ther. 2017, 16, 397–405. [Google Scholar] [CrossRef]
- St Hilaire, C.; Ziegler, S.G.; Markello, T.C.; Brusco, A.; Groden, C.; Gill, F.; Carlson-Donohoe, H.; Lederman, R.J.; Chen, M.Y.; Yang, D.; et al. NT5E mutations and arterial calcifications. N. Eng. J. Med. 2011, 364, 432–442. [Google Scholar] [CrossRef] [PubMed]
- March, L.; Cross, M.; Lo, C.; Arden, N.; Gates, L.; Leyland, K.; Hawker, G.; King, L.K. Osteoarthritis: A Serious Disease: Submitted to the U.S. Food and Drug Administration; OARSI: Mont Laurel, NJ, USA, 2016. [Google Scholar]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 3–6. [Google Scholar] [PubMed]
- Latourte, A.; Kloppenburg, M.; Richette, P. Emerging pharmaceutical therapies for osteoarthritis. Nat. Rev. Rheumatol. 2020, 16, 673–688. [Google Scholar] [CrossRef]
- Shlopov, B.V.; Gumanovskaya, M.L.; Hasty, K.A. Autocrine regulation of collagenase 3 (matrix metalloproteinase 13) during osteoarthritis. Arthritis Rheum. 2000, 43, 195–205. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Cole, A.; Murphy, G.; Bienias, J.L.; Im, H.J.; Loeser, R.F., Jr. Increased matrix metalloproteinase-13 production with aging by human articular chondrocytes in response to catabolic stimuli. J. Gerontol. Biol. Sci. Med. Sci. 2005, 60, 1118–1124. [Google Scholar] [CrossRef] [Green Version]
- Clancy, R.; Rediske, J.; Koehne, C.; Stoyanovsky, D.; Amin, A.; Attur, M.; Iyama, K.; Abramson, S.B. Activation of stress-activated protein kinase in osteoarthritic cartilage: Evidence for nitric oxide dependence. Osteoarthr. Cartil. 2001, 9, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Goldring, M.B.; Otero, M.; Plumb, D.A.; Dragomir, C.; Favero, M.; El Hachem, K.; Hashimoto, K.; Roach, H.I.; Olivotto, E.; Borzì, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cell Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef]
- Carlo, M.D., Jr.; Loeser, R.F. Increased oxidative stress with aging reduces chondrocyte survival: Correlation with intracellular glutathione levels. Arthritis Rheum. 2003, 48, 3419–3430. [Google Scholar] [CrossRef]
- Yudoh, K.; Nguyen v, T.; Nakamura, H.; Hongo-Masuko, K.; Kato, T.; Nishioka, K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: Oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res. Ther. 2005, 7, R380–R391. [Google Scholar] [CrossRef] [Green Version]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N.Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Spector, T.D.; Hart, D.J.; Nandra, D.; Doyle, D.V.; Mackillop, N.; Gallimore, J.R.; Pepys, M.B. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997, 40, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Zhai, G.; Hart, D.J.; Kato, B.S.; Wang, H.; Williams, F.M.; Spector, T.D. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis Rheum. 2009, 60, 2037–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.Y.; Tian, F.M.; Wang, W.Y.; Cheng, Y.; Song, H.P.; Zhang, Y.Z.; Zhang, L. Parathyroid hormone (1-34) prevents cartilage degradation and preserves subchondral bone micro-architecture in guinea pigs with spontaneous osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1869–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; Van de Sluis, B.; Kirkland, J.L.; Van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.-C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The senescence-associated secretory phenotype and its regulation. Cytokine 2019, 117, 15–22. [Google Scholar] [CrossRef]
- Haqqi, T.M.; Anthony, D.D.; Gupta, S.; Ahmad, N.; Lee, M.S.; Kumar, G.K.; Mukhtar, H. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc. Natl. Acad. Sci. USA 1999, 96, 4524–4529. [Google Scholar] [CrossRef] [Green Version]
- Meki, A.; Sobhi; Bahri, A.L.; Oraini, M.; Mehana, E.-S.; Deeb, E. The Protective Effect of Green Tea Extract Against the Oxidative Stress of The Experimental Arthritic Rats. Damascus Univ. J. Med. Sci. 2007, 32, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, K.; Lougiakis, N.; Rizou, S.V.; Kotsinas, A.; Kletsas, D.; Munoz-Espin, D.; Kastrinakis, N.G.; Pouli, N.; Marakos, P.; Townsend, P.; et al. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 2017, 16, 192–197. [Google Scholar] [CrossRef]


| Human Gene | PCR Primers Sequence (Forward and Reverse) | Annealing Temp. (°C) |
|---|---|---|
| Collagen type IIA1 (81bp product) | Forward: 5′-CAA CAC TGC CAA CGT CCA GAT-3′ | 61 |
| Reverse: 5′-TCT TGC AGT GGT AGG TGA TGT TCT-3′ | ||
| Aggrecan (189bp product) | Forward: 5′-ACA GCT GGG GAC ATT AGT GG -3′ | 61 |
| Reverse: 5′-GTG GAA TGC AGA GGT GGT TT-3′ | ||
| MMP-13 (161bp product) | Forward:5′-CTT CCC AAC CGT ATT GAT GCT-3′ | 61 |
| Reverse: 5′-CTG GTT TCC TGA GAA CAG GAG-3′ | ||
| IL-1β (219 bp product) | Forward:5′-GCA ATG AGG ATG ACT TGT TCT-3′ | 61 |
| Reverse: 5′-GGT CAT TCT CCT GGA AGG TCT-3′ | ||
| PTGS2(COX-2) (157 bp product) | Forward:5′-TGA GCA TCT ACG GTT TGC TG-3′ | 61 |
| Reverse: 5′-TGC TTG TCT GGA ACA ACT GC-3′ | ||
| Collagen type XA1 (85bp product) | Forward: 5′-AGC CAG GGT TGC CAG GAC CA-3′ | 61 |
| Reverse: 5′-TTT TCC CAC TCC AGG AGG GC-3′ | ||
| ALP (64bp product) | Forward: 5′-AAC TTC CAG ACC ATT GGC TTG A-3′ | 64 |
| Reverse: 5′-TTG CCG CGT GTC GTG TT-3′ | ||
| P16 Ink4a (134bp product) | Forward: 5′-CCAGAGGCAGTAACCATGCC-3′ | 61 |
| Reverse: 5′- TTGTGGCCCTGTAGGACCTTC -3′ | ||
| GAPDH | Forward: 5′-TCT CCT CTG ACT TCA ACA GCG AC-3′ | 61 |
| Reverse: 5′-CCC TGT TGC TGT AGC CAA ATT C-3′ | ||
| Cycling conditions | Denature:95 °C for 30 s, 95 °C for 4 min, followed by 35 cycles of 95 °C for 10 s, 58.4–61.5 °C (shown in column of Annealing Temp.) for 15 s and 72 °C for 15 s. | |
| OARSI Scoring | 6 m/o | 9 m/o + Vehicle | 9m/o + EGCG |
|---|---|---|---|
| Articular cartilage structure | 0.90 ± 0.10 | 1.64 ± 0.13 * | 0.92 ± 0.10 # |
| Proteoglycan content | 0.95 ± 0.11 | 1.4 ± 0.18 * | 0.94 ± 0.11 # |
| cellularity | 1.39 ± 0.06 | 1.65 ± 0.09 | 1.91 ± 0.10 |
| Tidemark integrity | 0.29 ± 0.10 | 0.32 ± 0.12 | 0.23 ± 0.09 |
| Additional features osteophyte | 0.07 ± 0.01 | 0.06 ± 0.02 | 0.16 ± 0.09 |
| Total | 3.6 ± 0.39 | 5.11 ± 0.53 ** | 4.16 ± 0.49 ## |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-T.; Cheng, T.-L.; Yang, C.-D.; Chang, C.-F.; Ho, C.-J.; Chuang, S.-C.; Li, J.-Y.; Huang, S.-H.; Lin, Y.-S.; Shen, H.-Y.; et al. Intra-Articular Injection of (−)-Epigallocatechin 3-Gallate (EGCG) Ameliorates Cartilage Degeneration in Guinea Pigs with Spontaneous Osteoarthritis. Antioxidants 2021, 10, 178. https://doi.org/10.3390/antiox10020178
Huang H-T, Cheng T-L, Yang C-D, Chang C-F, Ho C-J, Chuang S-C, Li J-Y, Huang S-H, Lin Y-S, Shen H-Y, et al. Intra-Articular Injection of (−)-Epigallocatechin 3-Gallate (EGCG) Ameliorates Cartilage Degeneration in Guinea Pigs with Spontaneous Osteoarthritis. Antioxidants. 2021; 10(2):178. https://doi.org/10.3390/antiox10020178
Chicago/Turabian StyleHuang, Hsuan-Ti, Tsung-Lin Cheng, Chung-Da Yang, Chi-Fen Chang, Cheng-Jung Ho, Shu-Chun Chuang, Jhong-You Li, Shih-Hao Huang, Yi-Shan Lin, Hsin-Yi Shen, and et al. 2021. "Intra-Articular Injection of (−)-Epigallocatechin 3-Gallate (EGCG) Ameliorates Cartilage Degeneration in Guinea Pigs with Spontaneous Osteoarthritis" Antioxidants 10, no. 2: 178. https://doi.org/10.3390/antiox10020178







