Coenzyme Q10 Analogues: Benefits and Challenges for Therapeutics
Abstract
:1. Introduction
2. The Relevance of Coenzyme Q10
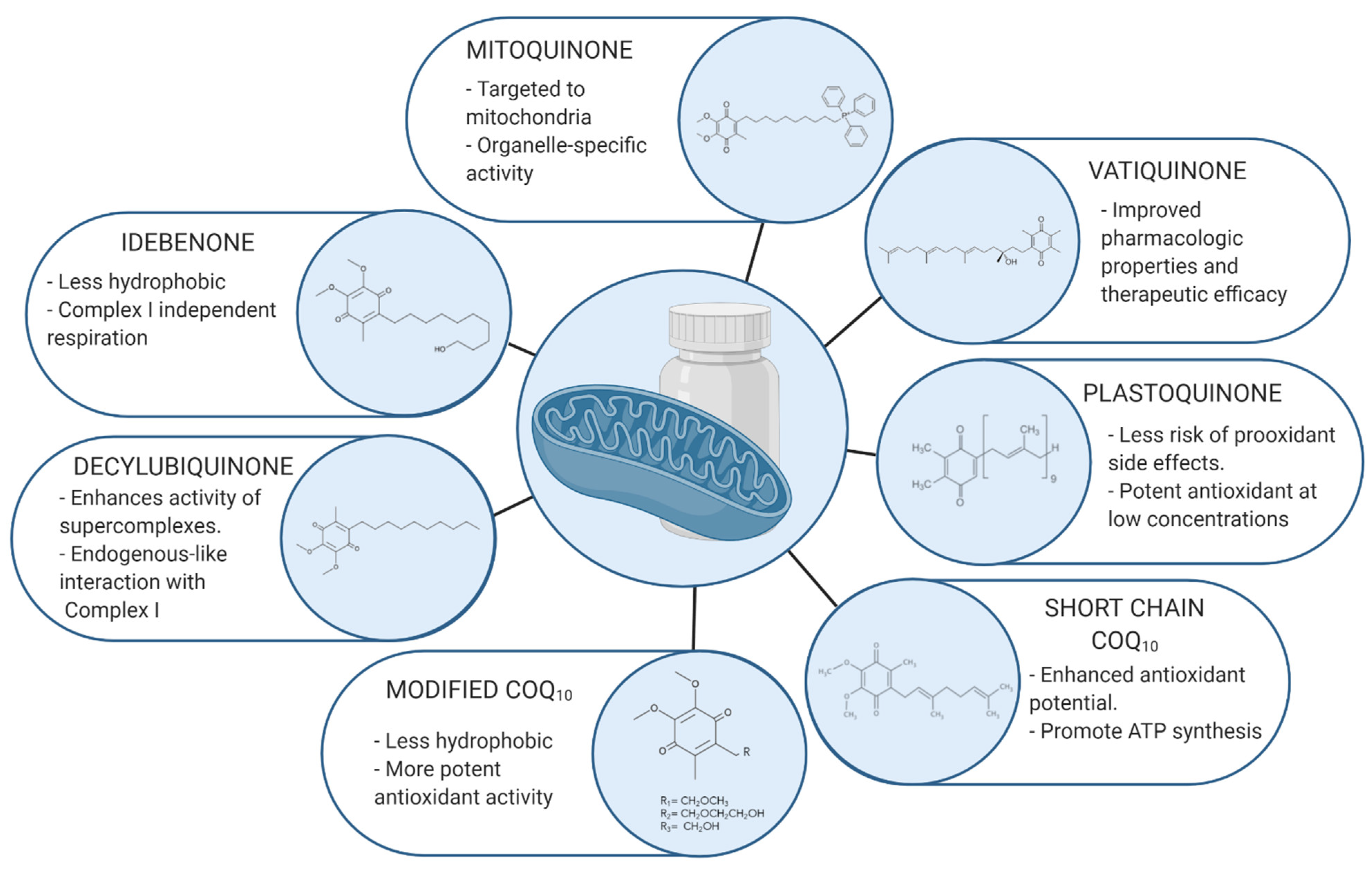
3. Most Relevant Coenzyme Q10 Analogues
3.1. Idebenone
3.2. Mitoquinone
3.3. Decylubiquinone
3.4. Plastoquinone and SKQ1
3.5. C6 Position
3.6. Short Chain Coenzyme Q10
3.7. EPI-743/Vatiquinone
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ROS | Reactive Oxygen Species |
| CoQ10 | Coenzyme Q10 |
| MRC | Mitochondrial respiratory chain |
| MSA | Multiple system atrophy |
| NQO1 | Cytoplasmic enzyme NADH-quinone oxidoreductase 1 |
| G3PDH | Glycerophosphate |
| MELAS | Mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes |
| LHON | Leber’s hereditary optic neuropathy |
| mtDNA | Mitochondrial DNA |
| FRDA | Friedreich Ataxia |
| dTPP | Decyltriphenylphosphonium |
| ETC | Electron transport chain |
| IV | Intravenous |
| CVD | Cardiovascular disease |
| DKD | Diabetic Kidney Disease |
| RNS | Reactive Nitrogen Species |
| HIF1α | Hypoxia-inducible factor 1-alpha |
| ALT | Alanine transaminase |
| AST | Aspartate aminotransferase |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| TH | Tyrosine hydroxylase |
| PD | Parkinson Disease |
| AD | Alzheimer Disease |
| Aβ | β-amyloid |
References
- Santos, A.L.; Sinha, S.; Lindner, A.B. The Good, the Bad, and the Ugly of ROS: New Insights on Aging and Aging-Related Diseases from Eukaryotic and Prokaryotic Model Organisms. Oxid. Med. Cell. Longev. 2018, 2018, 1941285. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Harmful and Beneficial Role of ROS. Oxid. Med. Cell. Longev. 2016, 2016, 7909186. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.M.; Pestronk, A.; David, W.; Rothstein, J.; Simpson, E.; Appel, S.H.; Andres, P.L.; Mahoney, K.; Allred, P.; Alexander, K.; et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: A phase 1, randomised, first-in-man study. Lancet Neurol. 2013, 12, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Matsui, A.; Ikeda, T.; Enomoto, K.; Hosoda, K.; Nakashima, H.; Omae, K.; Watanabe, M.; Hibi, T.; Kitajima, M. Increased formation of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, in human breast cancer tissue and its relationship to GSTP1 and COMT genotypes. Cancer Lett. 2000, 151, 87–95. [Google Scholar] [CrossRef]
- Chattopadhyay, M.; Khemka, V.K.; Chatterjee, G.; Ganguly, A.; Mukhopadhyay, S.; Chakrabarti, S. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Mol. Cell. Biochem. 2015, 399, 95–103. [Google Scholar] [CrossRef]
- Chong, W.C.; Shastri, M.D.; Eri, R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. Int. J. Mol. Sci. 2017, 18, 771. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant Therapy: Current Status and Future Prospects. Curr. Med. Chem. 2011, 18, 3871–3888. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Ávila, M.; Vega, A.F.; de la Mata, M.; Pavón, A.D.; de Miguel, M.; Calero, C.P.; Paz, M.V.; Cotán, D.; et al. Coenzyme q10 therapy. Mol. Syndr. 2014, 5, 187–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor-Maldonado, C.J.; Suárez-Rivero, J.M.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q(10): Novel Formulations and Medical Trends. Int. J. Mol. Sci. 2020, 21, 8432. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, H.N.; Chopra, R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 2007, 7, S78–S88. [Google Scholar] [CrossRef]
- Bergamini, C.; Moruzzi, N.; Sblendido, A.; Lenaz, G.; Fato, R. A Water Soluble CoQ10 Formulation Improves Intracellular Distribution and Promotes Mitochondrial Respiration in Cultured Cells. PLoS ONE 2012, 7, e33712. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Nakamura, K.; Abe, J.; Hyodo, M.; Haga, S.; Ozaki, M.; Harashima, H. Mitochondrial delivery of Coenzyme Q10 via systemic administration using a MITO-Porter prevents ischemia/reperfusion injury in the mouse liver. J. Control. Release 2015, 213, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef]
- Desbats, M.A.; Lunardi, G.; Doimo, M.; Trevisson, E.; Salviati, L. Genetic bases and clinical manifestations of coenzyme Q10 (CoQ 10) deficiency. J. Inherit. Metab. Dis. 2015, 38, 145–156. [Google Scholar] [CrossRef]
- Siemieniuk, E.; Skrzydlewska, E. Coenzyme Q10: Its biosynthesis and biological significance in animal organisms and in humans. Postepy Hig. Med. Dosw. 2005, 59, 150–159. [Google Scholar]
- Papucci, L.; Schiavone, N.; Witort, E.; Donnini, M.; Lapucci, A.; Tempestini, A.; Formigli, L.; Zecchi-Orlandini, S.; Orlandini, G.; Carella, G.; et al. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J. Biol. Chem. 2003, 278, 28220–28228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echtay, K.S.; Winkler, E.; Klingenberg, M. Coenzyme Q is an obligatory cofactor for uncoupling protein function. Nature 2000, 408, 609–613. [Google Scholar] [CrossRef]
- James, A.M.; Smith, R.A.; Murphy, M.P. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch. Biochem. Biophys. 2004, 423, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Alcocer-Gomez, E.; Culic, O.; Carrion, A.M.; de Miguel, M.; Diaz-Parrado, E.; Perez-Villegas, E.M.; Bullon, P.; Battino, M.; Sanchez-Alcazar, J.A. NLRP3 inflammasome is activated in fibromyalgia: The effect of coenzyme Q10. Antioxid. Redox Signal. 2014, 20, 1169–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chokchaiwong, S.; Kuo, Y.T.; Lin, S.H.; Hsu, Y.C.; Hsu, S.P.; Liu, Y.T.; Chou, A.J.; Kao, S.H. Coenzyme Q10 serves to couple mitochondrial oxidative phosphorylation and fatty acid beta-oxidation, and attenuates NLRP3 inflammasome activation. Free Radic. Res. 2018, 52, 1445–1455. [Google Scholar] [CrossRef]
- Liang, S.; Ping, Z.; Ge, J. Coenzyme Q10 Regulates Antioxidative Stress and Autophagy in Acute Myocardial Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2017, 2017, 9863181. [Google Scholar] [CrossRef] [PubMed]
- Turunen, M.; Wehlin, L.; Sjoberg, M.; Lundahl, J.; Dallner, G.; Brismar, K.; Sindelar, P.J. beta2-Integrin and lipid modifications indicate a non-antioxidant mechanism for the anti-atherogenic effect of dietary coenzyme Q10. Biochem. Biophys. Res. Commun. 2002, 296, 255–260. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Guaras, A.; Perales-Clemente, E.; Calvo, E.; Acin-Perez, R.; Loureiro-Lopez, M.; Pujol, C.; Martinez-Carrascoso, I.; Nunez, E.; Garcia-Marques, F.; Rodriguez-Hernandez, M.A.; et al. The CoQH2/CoQ Ratio Serves as a Sensor of Respiratory Chain Efficiency. Cell Rep. 2016, 15, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Carbone, C.; Pignatello, R.; Musumeci, T.; Puglisi, G. Chemical and technological delivery systems for idebenone: A review of literature production. Expert Opin. Drug Deliv. 2012, 9, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Meier, T.; Buyse, G. Idebenone: An emerging therapy for Friedreich ataxia. J. Neurol. 2009, 256, 25. [Google Scholar] [CrossRef] [PubMed]
- Di Prospero, N.A.; Sumner, C.J.; Penzak, S.R.; Ravina, B.; Fischbeck, K.H.; Taylor, J.P. Safety, Tolerability, and Pharmacokinetics of High-Dose Idebenone in Patients With Friedreich Ataxia. Arch. Neurol. 2007, 64, 803–808. [Google Scholar] [CrossRef]
- Bodmer, M.; Vankan, P.; Dreier, M.; Kutz, K.W.; Drewe, J. Pharmacokinetics and metabolism of idebenone in healthy male subjects. Eur. J. Clin. Pharmacol. 2009, 65, 493. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, V.; Schiavone, M.; Galber, C.; Carini, M.; Da Ros, T.; Petronilli, V.; Argenton, F.; Carelli, V.; Lopez, M.J.A.; Salviati, L.; et al. The idebenone metabolite QS10 restores electron transfer in complex I and coenzyme Q defects. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 901–908. [Google Scholar] [CrossRef]
- Esposti, M.D.; Ngo, A.; Ghelli, A.; Benelli, B.; Carelli, V.; McLennan, H.; Linnane, A.W. The Interaction of Q Analogs, Particularly Hydroxydecyl Benzoquinone (Idebenone), with the Respiratory Complexes of Heart Mitochondria. Arch. Biochem. Biophys. 1996, 330, 395–400. [Google Scholar] [CrossRef]
- Fato, R.; Bergamini, C.; Leoni, S.; Lenaz, G. Mitochondrial production of reactive oxygen species: Role of Complex I and quinone analogues. BioFactors 2008, 32, 31–39. [Google Scholar] [CrossRef]
- Rauchová, H.; Drahota, Z.; Bergamini, C.; Fato, R.; Lenaz, G. Modification of respiratory-chain enzyme activities in brown adipose tissue mitochondria by idebenone (hydroxydecyl-ubiquinone). J. Bioenerg. Biomembr. 2008, 40, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Hoffmann-Enger, B.; Deppe, H.; Soeberdt, M.; Haefeli, R.H.; Rummey, C.; Feurer, A.; Gueven, N. Features of idebenone and related short-chain quinones that rescue ATP levels under conditions of impaired mitochondrial complex I. PLoS ONE 2012, 7, e36153. [Google Scholar] [CrossRef] [Green Version]
- Giorgio, V.; Petronilli, V.; Ghelli, A.; Carelli, V.; Rugolo, M.; Lenaz, G.; Bernardi, P. The effects of idebenone on mitochondrial bioenergetics. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 363–369. [Google Scholar] [CrossRef]
- Haefeli, R.H.; Erb, M.; Gemperli, A.C.; Robay, D.; Fruh, I.C.; Anklin, C.; Dallmann, R.; Gueven, N. NQO1-dependent redox cycling of idebenone: Effects on cellular redox potential and energy levels. PLoS ONE 2011, 6, e17963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, A.M.; Cochemé, H.M.; Smith, R.A.J.; Murphy, M.P. Interactions of Mitochondria-targeted and Untargeted Ubiquinones with the Mitochondrial Respiratory Chain and Reactive Oxygen Species: Implications for the use of exogenous ubiquinones as therapies and experimental tools. J. Biol. Chem. 2005, 280, 21295–21312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauchova, H.; Vokurkova, M.; Drahota, Z. Idebenone-induced recovery of glycerol-3-phosphate and succinate oxidation inhibited by digitonin. Physiol. Res. 2012, 61, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Rodenburg, R.J. Mitochondrial complex I-linked disease. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 938–945. [Google Scholar] [CrossRef]
- Lee, S.; Sheck, L.; Crowston, J.G.; Van Bergen, N.J.; O’Neill, E.C.; O’Hare, F.; Kong, Y.X.G.; Chrysostomou, V.; Vincent, A.L.; Trounce, I.A. Impaired Complex-I-Linked Respiration and ATP Synthesis in Primary Open-Angle Glaucoma Patient Lymphoblasts. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2431–2437. [Google Scholar] [CrossRef] [Green Version]
- Kernt, M.; Arend, N.; Buerger, A.; Mann, T.; Haritoglou, C.; Ulbig, M.W.; Kampik, A.; Hirneiss, C. Idebenone Prevents Human Optic Nerve Head Astrocytes From Oxidative Stress, Apoptosis, and Senescence by Stabilizing BAX/Bcl-2 Ratio. J. Glaucoma 2013, 22, 404–412. [Google Scholar] [CrossRef]
- Grieb, P.; Ryba, M.S.; Debicki, G.S.; Gordon-Krajcer, W.; Januszewski, S.; Chrapusta, S.J. Changes in oxidative stress in the rat brain during post-cardiac arrest reperfusion, and the effect of treatment with the free radical scavenger idebenone. Resuscitation 1998, 39, 107–113. [Google Scholar] [CrossRef]
- Mordente, A.; Martorana, G.E.; Minotti, G.; Giardina, B. Antioxidant Properties of 2,3-Dimethoxy-5-methyl- 6-(10-hydroxydecyl)-1,4-benzoquinone (Idebenone). Chem. Res. Toxicol. 1998, 11, 54–63. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, C.; Oliveira, C.R. Mitochondrial function is differentially affected upon oxidative stress. Free Radic. Biol. Med. 1999, 26, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Rustin, P.; von Kleist-Retzow, J.-C.; Chantrel-Groussard, K.; Sidi, D.; Munnich, A.; Rötig, A. Effect of idebenone on cardiomyopathy in Friedreich’s ataxia: A preliminary study. Lancet 1999, 354, 477–479. [Google Scholar] [CrossRef]
- Theodorou-Kanakari, A.; Karampitianis, S.; Karageorgou, V.; Kampourelli, E.; Kapasakis, E.; Theodossiadis, P.; Chatziralli, I. Current and Emerging Treatment Modalities for Leber’s Hereditary Optic Neuropathy: A Review of the Literature. Adv. Ther. 2018, 35, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Klopstock, T.; Yu-Wai-Man, P.; Dimitriadis, K.; Rouleau, J.; Heck, S.; Bailie, M.; Atawan, A.; Chattopadhyay, S.; Schubert, M.; Garip, A.; et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain 2011, 134, 2677–2686. [Google Scholar] [CrossRef]
- Klopstock, T.; Metz, G.; Yu-Wai-Man, P.; Büchner, B.; Gallenmüller, C.; Bailie, M.; Nwali, N.; Griffiths, P.G.; von Livonius, B.; Reznicek, L.; et al. Persistence of the treatment effect of idebenone in Leber’s hereditary optic neuropathy. Brain 2013, 136, e230. [Google Scholar] [CrossRef] [Green Version]
- Hausse, A.O.; Aggoun, Y.; Bonnet, D.; Sidi, D.; Munnich, A.; Rötig, A.; Rustin, P. Idebenone and reduced cardiac hypertrophy in Friedreich’s ataxia. Heart 2002, 87, 346–349. [Google Scholar] [CrossRef]
- Mariotti, C.; Solari, A.; Torta, D.; Marano, L.; Fiorentini, C.; Di Donato, S. Idebenone treatment in Friedreich patients: One-year-long randomized placebo-controlled trial. Neurology 2003, 60, 1676. [Google Scholar] [CrossRef] [PubMed]
- Giovanni, D.S.; Valeria, P.; Bahaa, F.; Majid, A.F. Monitoring cardiac function during idebenone therapy in Friedreich’s ataxia. Curr. Pharm. Des. 2015, 21, 479–483. [Google Scholar] [CrossRef]
- Long-Term Safety and Tolerability of Idebenone in Friedreich’s Ataxia Patients (MICONOS Extension). Available online: https://ClinicalTrials.gov/show/NCT00993967 (accessed on 20 January 2021).
- Study to Assess the Safety and Tolerability of Idebenone in the Treatment of Friedreich’s Ataxia Patients. Available online: https://ClinicalTrials.gov/show/NCT00697073 (accessed on 20 January 2021).
- A Study of Efficacy, Safety and Tolerability of Idebenone in the Treatment of Friedreich’s Ataxia (FRDA) Patients. Available online: https://ClinicalTrials.gov/show/NCT00905268 (accessed on 20 January 2021).
- Idebenone to Treat Friedreich’s Ataxia. Available online: https://ClinicalTrials.gov/show/NCT00229632 (accessed on 20 January 2021).
- Phase III Study of Idebenone in Duchenne Muscular Dystrophy (DMD). Available online: https://ClinicalTrials.gov/show/NCT01027884 (accessed on 20 January 2021).
- Long-Term Safety, Tolerability and Efficacy of Idebenone in Duchenne Muscular Dystrophy (DELPHI Extension). Available online: https://ClinicalTrials.gov/show/NCT00758225 (accessed on 20 January 2021).
- A Phase III Double-Blind Study with Idebenone in Patients with Duchenne Muscular Dystrophy (DMD) Taking Glucocorticoid Steroids. Available online: https://ClinicalTrials.gov/show/NCT02814019 (accessed on 20 January 2021).
- Idebenone for Primary Progressive Multiple Sclerosis. Available online: https://ClinicalTrials.gov/show/NCT01854359 (accessed on 20 January 2021).
- Clinical Trial of Idebenone in Primary Progressive Multiple Sclerosis (IPPoMS). Available online: https://ClinicalTrials.gov/show/NCT00950248 (accessed on 20 January 2021).
- Idebenone Treatment of Early Parkinson’s Diseasesymptoms. Available online: https://ClinicalTrials.gov/show/NCT03727295 (accessed on 20 January 2021).
- Study to Assess Efficacy, Safety and Tolerability of Idebenone in the Treatment of Leber’s Hereditary Optic Neuropathy. Available online: https://ClinicalTrials.gov/show/NCT00747487 (accessed on 20 January 2021).
- Study of Idebenone in the Treatment of Mitochondrial Encephalopathy Lactic Acidosis & Stroke-Like Episodes. Available online: https://ClinicalTrials.gov/show/NCT00887562 (accessed on 20 January 2021).
- Sugizaki, T.; Tanaka, K.-I.; Asano, T.; Kobayashi, D.; Hino, Y.; Takafuji, A.; Shimoda, M.; Mogushi, K.; Kawahara, M.; Mizushima, T. Idebenone has preventative and therapeutic effects on pulmonary fibrosis via preferential suppression of fibroblast activity. Cell Death Discov. 2019, 5, 146. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.E. Chapter 31-Medical Foods and Dietary Approaches in Cognitive Decline, Mild Cognitive Impairment, and Dementia. In Diet and Nutrition in Dementia and Cognitive Decline; Martin, C.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 343–356. [Google Scholar] [CrossRef]
- Ikejiri, Y.; Fau-Ishii, K.M.E.; Fau-Nishimoto, K.I.K.; Fau-Yasuda, M.N.K.; Fau-Sasaki, M.Y.M.; Sasaki, M. Idebenone improves cerebral mitochondrial oxidative metabolism in a patient with MELAS. Neurology 1996, 47, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Petrov, S.; Shmirova, V.; Kozlova, I.; Antonov, A.A. Application of a idebenone in therapy of glaucoma optic neuropathy. Glaucoma 2007, 6, 29–34. [Google Scholar]
- Orsucci, D.; Fau-Ienco, E.C.M.M.; Fau-LoGerfo, A.I.E.; Fau-Siciliano, G.L.A.; Siciliano, G. Targeting mitochondrial dysfunction and neurodegeneration by means of coenzyme Q10 and its analogues. Curr. Med. Chem. 2011, 18, 4053–4064. [Google Scholar] [CrossRef]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, R.A.J.; Murphy, M.P. Selective Targeting of a Redox-active Ubiquinone to Mitochondria within Cells: Antioxidant and antiapoptotic properties. J. Biol. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.A.J.; Porteous, C.M.; Coulter, C.V.; Murphy, M.P. Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem. 1999, 263, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. Selective targeting of bioactive compounds to mitochondria. Trends Biotechnol. 1997, 15, 326–330. [Google Scholar] [CrossRef]
- Magwere, T.; West, M.; Riyahi, K.; Murphy, M.P.; Smith, R.A.J.; Partridge, L. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech. Ageing Dev. 2006, 127, 356–370. [Google Scholar] [CrossRef]
- Smith, R.A.J.; Hartley, R.C.; Cochemé, H.M.; Murphy, M.P. Mitochondrial pharmacology. Trends Pharmacol. Sci. 2012, 33, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Koopman, W.J.H.; Verkaart, S.; Visch, H.-J.; van der Westhuizen, F.H.; Murphy, M.P.; van den Heuvel, L.W.P.J.; Smeitink, J.A.M.; Willems, P.H.G.M. Inhibition of complex I of the electron transport chain causes O2−·-mediated mitochondrial outgrowth. Am. J. Physiol. Cell Physiol. 2005, 288, C1440–C1450. [Google Scholar] [CrossRef] [PubMed]
- Porteous, C.M.; Logan, A.; Evans, C.; Ledgerwood, E.C.; Menon, D.K.; Aigbirhio, F.; Smith, R.A.J.; Murphy, M.P. Rapid uptake of lipophilic triphenylphosphonium cations by mitochondria in vivo following intravenous injection: Implications for mitochondria-specific therapies and probes. Biochim. Biophys. Acta Gen. Subj. 2010, 1800, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Junior, R.F.R.; Dabkowski, E.R.; Shekar, K.C.; Connell, K.A.O.; Hecker, P.A.; Murphy, M.P. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic. Biol. Med. 2018, 117, 18–29. [Google Scholar] [CrossRef]
- Matthew, J.R.; Jessica, R.S.-P.; Chelsea, A.C.S.; Nina, Z.B.; Lauren, M.C.; Hannah, L.R.; Kayla, A.; Chonchol, M.W.; Rachel, A.G.-R.; Michael, P.M.; et al. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef]
- Chacko, B.K.; Srivastava, A.; Johnson, M.S.; Benavides, G.A.; Chang, M.J.; Ye, Y.; Jhala, N.; Murphy, M.P.; Kalyanaraman, B.; Darley-Usmar, V.M. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology 2011, 54, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gane, E.J.; Weilert, F.; Orr, D.W.; Keogh, G.F.; Gibson, M.; Lockhart, M.M.; Frampton, C.M.; Taylor, K.M.; Smith, R.A.J.; Murphy, M.P. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010, 30, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Chandran, K.; Kalivendi, S.V.; Joseph, J.; Antholine, W.E.; Hillard, C.J.; Kanthasamy, A.; Kanthasamy, A.; Kalyanaraman, B. Neuroprotection by a mitochondria-targeted drug in a Parkinson’s disease model. Free Radic. Biol. Med. 2010, 49, 1674–1684. [Google Scholar] [CrossRef] [Green Version]
- Ünal, İ.; Çalışkan-Ak, E.; Üstündağ, Ü.V.; Ateş, P.S.; Alturfan, A.A.; Altinoz, M.A.; Elmaci, I.; Emekli-Alturfan, E. Neuroprotective effects of mitoquinone and oleandrin on Parkinson’s disease model in zebrafish. Int. J. Neurosci. 2020, 130, 574–582. [Google Scholar] [CrossRef]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The Mitochondria-Targeted Antioxidant MitoQ Prevents Loss of Spatial Memory Retention and Early Neuropathology in a Transgenic Mouse Model of Alzheimer’s Disease. J. Neurosci. 2011, 31, 15703. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.F.; Gruber, J.; Cheah, I.K.; Goo, C.K.; Cheong, W.F.; Shui, G.; Sit, K.P.; Wenk, M.R.; Halliwell, B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic. Biol. Med. 2014, 71, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Pinho, B.R.; Duarte, A.I.; Canas, P.M.; Moreira, P.I.; Murphy, M.P.; Oliveira, J.M.A. The interplay between redox signalling and proteostasis in neurodegeneration: In vivo effects of a mitochondria-targeted antioxidant in Huntington’s disease mice. Free Radic. Biol. Med. 2020, 146, 372–382. [Google Scholar] [CrossRef]
- Miquel, E.; Cassina, A.; Martínez-Palma, L.; Souza, J.M.; Bolatto, C.; Rodríguez-Bottero, S.; Logan, A.; Smith, R.A.J.; Murphy, M.P.; Barbeito, L.; et al. Neuroprotective effects of the mitochondria-targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2014, 70, 204–213. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Shen, R.; Fang, J.; Yang, Y.; Dai, W.; Zhu, Y.; Zhou, M. Mitochondrial-targeted antioxidant MitoQ provides neuroprotection and reduces neuronal apoptosis in experimental traumatic brain injury possibly via the Nrf2-ARE pathway. Am. J. Transl. Res. 2018, 10, 1887–1899. [Google Scholar]
- A Trial of MitoQ for the Treatment of People with Parkinson’s Disease. Available online: https://ClinicalTrials.gov/show/NCT00329056 (accessed on 20 January 2021).
- MitoQ for Fatigue in Multiple Sclerosis (MS). Available online: https://ClinicalTrials.gov/show/NCT04267926 (accessed on 20 January 2021).
- MitoQ for Fatigue in Multiple Sclerosis. Available online: https://ClinicalTrials.gov/show/NCT03166800 (accessed on 20 January 2021).
- MitoQ for the Treatment of Metabolic Dysfunction in Asthma. Available online: https://ClinicalTrials.gov/show/NCT04026711 (accessed on 20 January 2021).
- The Efficacy of Oral Mitoquinone (MitoQ) Supplementation for Improving Physiological in Middle-Aged and Older Adults. Available online: https://ClinicalTrials.gov/show/NCT02597023 (accessed on 20 January 2021).
- Trial of MitoQ for Raised Liver Enzymes Due to Hepatitis C. Available online: https://ClinicalTrials.gov/show/NCT00433108 (accessed on 20 January 2021).
- A Study to Compare MitoQ and Placebo to Treat Non-Alcoholic Fatty Liver Disease (NAFLD). Available online: https://ClinicalTrials.gov/show/NCT01167088 (accessed on 20 January 2021).
- Lenaz, G.; Bovina, C.; Castelluccio, C.; Fato, R.; Formiggini, G.; Genova, M.L.; Marchetti, M.; Pich, M.M.; Pallotti, F.; Castelli, G.P.; et al. Mitochondrial complex I defects in aging. Mol. Cell Biochem. 1997, 174, 329–333. [Google Scholar] [CrossRef]
- Armstrong, J.S.; Whiteman, M.; Rose, P.; Jones, D.P. The Coenzyme Q10 analog decylubiquinone inhibits the redox-activated mitochondrial permeability transition: Role of mitcohondrial [correction mitochondrial] complex III. J. Biol. Chem. 2003, 278, 49079–49084. [Google Scholar] [CrossRef] [Green Version]
- Hano, N.; Nakashima, Y.; Shinzawa-Itoh, K.; Yoshikawa, S. Effect of the side chain structure of coenzyme Q on the steady state kinetics of bovine heart NADH: Coenzyme Q oxidoreductase. J. Bioenerg. Biomembr. 2003, 35, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Esposti, M.D.; Ngo, A.; McMullen, G.L.; Ghelli, A.; Sparla, F.; Benelli, B.; Ratta, M.; Linnane, A.W. The specificity of mitochondrial complex I for ubiquinones. Biochem. J. 1996, 313 Pt 1, 327–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telford, J.E.; Kilbride, S.M.; Davey, G.P. Decylubiquinone increases mitochondrial function in synaptosomes. J. Biol. Chem. 2010, 285, 8639–8645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schagger, H.; Pfeiffer, K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000, 19, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Acin-Perez, R.; Fernandez-Silva, P.; Peleato, M.L.; Perez-Martos, A.; Enriquez, J.A. Respiratory active mitochondrial supercomplexes. Mol. Cell 2008, 32, 529–539. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Soiferman, D.; Moore, D.G.; Burte, F.; Saada, A. Evaluating the therapeutic potential of idebenone and related quinone analogues in Leber hereditary optic neuropathy. Mitochondrion 2017, 36, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Hosni-Ahmed, A.; Sims, M.; Jones, T.S.; Patil, R.; Patil, S.; Abdelsamed, H.; Yates, C.R.; Miller, D.D.; Pfeffer, L.M. EDL-360: A Potential Novel Antiglioma Agent. J. Cancer Sci. Ther. 2014, 6, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Jun, D.Y.; Rue, S.W.; Han, K.H.; Taub, D.; Lee, Y.S.; Bae, Y.S.; Kim, Y.H. Mechanism underlying cytotoxicity of thialysine, lysine analog, toward human acute leukemia Jurkat T cells. Biochem. Pharmacol. 2003, 66, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, X.; Yang, Y.; Wei, B.; Li, Q.; Mao, G.; He, Y.; Li, Y.; Zheng, L.; Zhang, Q.; et al. Decylubiquinone suppresses breast cancer growth and metastasis by inhibiting angiogenesis via the ROS/p53/ BAI1 signaling pathway. Angiogenesis 2020, 23, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Murad, L.B.; Guimaraes, M.R.; Vianna, L.M. Effects of decylubiquinone (coenzyme Q10 analog) supplementation on SHRSP. Biofactors 2007, 30, 13–18. [Google Scholar] [CrossRef]
- Chakraborthy, A.; Ramani, P.; Sherlin, H.J.; Premkumar, P.; Natesan, A. Antioxidant and pro-oxidant activity of Vitamin C in oral environment. Indian J. Dent. Res. 2014, 25, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Vlachantoni, D.; Bramall, A.N.; Murphy, M.P.; Taylor, R.W.; Shu, X.; Tulloch, B.; Van Veen, T.; Turnbull, D.M.; McInnes, R.R.; Wright, A.F. Evidence of severe mitochondrial oxidative stress and a protective effect of low oxygen in mouse models of inherited photoreceptor degeneration. Hum. Mol. Genet. 2011, 20, 322–335. [Google Scholar] [CrossRef] [Green Version]
- Antonenko, Y.N.; Avetisyan, A.V.; Bakeeva, L.E.; Chernyak, B.V.; Chertkov, V.A.; Domnina, L.V.; Ivanova, O.Y.; Izyumov, D.S.; Khailova, L.S.; Klishin, S.S.; et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: Synthesis and in vitro studies. Biochemistry (Mosc.) 2008, 73, 1273–1287. [Google Scholar] [CrossRef]
- Borisova-Mubarakshina, M.M.; Vetoshkina, D.V.; Ivanov, B.N. Antioxidant and signaling functions of the plastoquinone pool in higher plants. Physiol. Plant 2019, 166, 181–198. [Google Scholar] [CrossRef]
- Mubarakshina, M.M.; Ivanov, B.N. The production and scavenging of reactive oxygen species in the plastoquinone pool of chloroplast thylakoid membranes. Physiol. Plant 2010, 140, 103–110. [Google Scholar] [CrossRef]
- Szechynska-Hebda, M.; Karpinski, S. Light intensity-dependent retrograde signalling in higher plants. J. Plant Physiol. 2013, 170, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Pinnola, A. The rise and fall of Light-Harvesting Complex Stress-Related proteins as photoprotection agents during evolution. J. Exp. Bot. 2019, 70, 5527–5535. [Google Scholar] [CrossRef] [PubMed]
- Green, D.E. The electromechanochemical model for energy coupling in mitochondria. Biochim. Biophys. Acta 1974, 346, 27–78. [Google Scholar] [CrossRef]
- Skulachev, V.P.; Anisimov, V.N.; Antonenko, Y.N.; Bakeeva, L.E.; Chernyak, B.V.; Erichev, V.P.; Filenko, O.F.; Kalinina, N.I.; Kapelko, V.I.; Kolosova, N.G.; et al. An attempt to prevent senescence: A mitochondrial approach. Biochim. Biophys. Acta 2009, 1787, 437–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skulachev, M.V.; Antonenko, Y.N.; Anisimov, V.N.; Chernyak, B.V.; Cherepanov, D.A.; Chistyakov, V.A.; Egorov, M.V.; Kolosova, N.G.; Korshunova, G.A.; Lyamzaev, K.G.; et al. Mitochondrial-targeted plastoquinone derivatives. Effect on senescence and acute age-related pathologies. Curr. Drug Targets 2011, 12, 800–826. [Google Scholar] [CrossRef]
- Skulachev, V.P.; Antonenko, Y.N.; Cherepanov, D.A.; Chernyak, B.V.; Izyumov, D.S.; Khailova, L.S.; Klishin, S.S.; Korshunova, G.A.; Lyamzaev, K.G.; Pletjushkina, O.Y.; et al. Prevention of cardiolipin oxidation and fatty acid cycling as two antioxidant mechanisms of cationic derivatives of plastoquinone (SkQs). Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 878–889. [Google Scholar] [CrossRef] [Green Version]
- Plotnikov, E.Y.; Fau-Jankauskas, S.S.S.D.; Fau-Rokitskaya, T.I.J.S.; Fau-Chupyrkina, A.A.R.T.; Fau-Pevzner, I.B.C.A.; Fau-Zorova, L.D.P.I.; Fau-Isaev, N.K.Z.L.; Fau-Antonenko, Y.N.I.N.; Fau-Skulachev, V.P.A.Y.; Fau-Zorov, D.B.S.V.; et al. Mild uncoupling of respiration and phosphorylation as a mechanism providing nephro- and neuroprotective effects of penetrating cations of the SkQ family. Biochemistry 2012, 77, 1029–1037. [Google Scholar] [CrossRef]
- Garlid, K.F.; Nakashima, R.A.; Nakashima, R.A. Studies on the mechanism of uncoupling by amine local anesthetics. Evidence for mitochondrial proton transport mediated by lipophilic ion pairs. J. Biol. Chem. 1983, 258, 7974–7980. [Google Scholar] [CrossRef]
- Feniouk, B.A.; Skulachev, V.P. Cellular and Molecular Mechanisms of Action of Mitochondria-Targeted Antioxidants. Curr. Aging Sci. 2017, 10, 41–48. [Google Scholar] [CrossRef]
- Skulachev, V.P. A biochemical approach to the problem of aging: “megaproject” on membrane-penetrating ions. The first results and prospects. Biochemistry (Mosc.) 2007, 72, 1385–1396. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Troger, A.; Spahiu, V.; Perekhvatova, N.; Skulachev, M.; Petrov, A.; Chernyak, B.; Asbell, P. The Role of SKQ1 (Visomitin) in Inflammation and Wound Healing of the Ocular Surface. Ophthalmol. Ther. 2019, 8, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weniger, M.; Reinelt, L.; Neumann, J.; Holdt, L.; Ilmer, M.; Renz, B.; Hartwig, W.; Werner, J.; Bazhin, A.V.; D’Haese, J.G. The Analgesic Effect of the Mitochondria-Targeted Antioxidant SkQ1 in Pancreatic Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 4650489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demyanenko, I.A.; Zakharova, V.V.; Ilyinskaya, O.P.; Vasilieva, T.V.; Fedorov, A.V.; Manskikh, V.N.; Zinovkin, R.A.; Pletjushkina, O.Y.; Chernyak, B.V.; Skulachev, V.P.; et al. Mitochondria-Targeted Antioxidant SkQ1 Improves Dermal Wound Healing in Genetically Diabetic Mice. Oxid. Med. Cell. Longev. 2017, 2017, 6408278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titova, E.; Shagieva, G.; Ivanova, O.; Domnina, L.; Domninskaya, M.; Strelkova, O.; Khromova, N.; Kopnin, P.; Chernyak, B.; Skulachev, V.; et al. Mitochondria-targeted antioxidant SkQ1 suppresses fibrosarcoma and rhabdomyosarcoma tumour cell growth. Cell Cycle 2018, 17, 1797–1811. [Google Scholar] [CrossRef] [Green Version]
- Kolosova, N.G.; Tyumentsev, M.A.; Muraleva, N.A.; Kiseleva, E.; Vitovtov, A.O.; Stefanova, N.A. Antioxidant SkQ1 Alleviates Signs of Alzheimer’s Disease-like Pathology in Old OXYS Rats by Reversing Mitochondrial Deterioration. Curr. Alzheimer Res. 2017, 14, 1283–1292. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, C.; Lei, B.; Xu, X.; Lu, B. Mitochondria-targeted antioxidant SkQ1 improves spermatogenesis in Immp2l mutant mice. Andrologia 2018, 50, e12848. [Google Scholar] [CrossRef]
- Bakeeva, L.E. Age-Related Changes in Ultrastructure of Mitochondria. Effect of SkQ1. Biochemistry (Mosc.) 2015, 80, 1582–1588. [Google Scholar] [CrossRef]
- Tsybul’ko, E.; Krementsova, A.; Symonenko, A.; Rybina, O.; Roshina, N.; Pasyukova, E. The Mitochondria-Targeted Plastoquinone-Derivative SkQ1 Promotes Health and Increases Drosophila melanogaster Longevity in Various Environments. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Karakhanova, S.; Soltek, S.; Werner, J.; Philippov, P.P.; Bazhin, A.V. In vivo immunoregulatory properties of the novel mitochondria-targeted antioxidant SkQ1. Mol. Immunol. 2012, 52, 19–29. [Google Scholar] [CrossRef]
- Ahmed, E.; Donovan, T.; Yujiao, L.; Zhang, Q. Mitochondrial Targeted Antioxidant in Cerebral Ischemia. J. Neurol. Neurosci. 2015, 6, 2–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loshchenova, P.S.; Sinitsyna, O.I.; Fedoseeva, L.A.; Stefanova, N.A.; Kolosova, N.G. Influence of Antioxidant SkQ1 on Accumulation of Mitochondrial DNA Deletions in the Hippocampus of Senescence-Accelerated OXYS Rats. Biochemistry (Mosc.) 2015, 80, 596–603. [Google Scholar] [CrossRef]
- Gueven, N.; Nadikudi, M.; Daniel, A.; Chhetri, J. Targeting mitochondrial function to treat optic neuropathy. Mitochondrion 2017, 36, 7–14. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Egorov, M.V.; Krasilshchikova, M.S.; Lyamzaev, K.G.; Manskikh, V.N.; Moshkin, M.P.; Novikov, E.A.; Popovich, I.G.; Rogovin, K.A.; Shabalina, I.G.; et al. Effects of the mitochondria-targeted antioxidant SkQ1 on lifespan of rodents. Aging 2011, 3, 1110–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anisimov, V.N.; Bakeeva, L.E.; Egormin, P.A.; Filenko, O.F.; Isakova, E.F.; Manskikh, V.N.; Mikhelson, V.M.; Panteleeva, A.A.; Pasyukova, E.G.; Pilipenko, D.I.; et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 5. SkQ1 prolongs lifespan and prevents development of traits of senescence. Biochemistry (Mosc.) 2008, 73, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Neroev, V.V.; Archipova, M.M.; Bakeeva, L.E.; Fursova, A.; Grigorian, E.N.; Grishanova, A.Y.; Iomdina, E.N.; Ivashchenko, Z.N.; Katargina, L.A.; Khoroshilova-Maslova, I.P.; et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 4. Age-related eye disease. SkQ1 returns vision to blind animals. Biochemistry (Mosc.) 2008, 73, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Study of SkQ1 as Treatment for Dry-Eye Syndrome. Available online: https://ClinicalTrials.gov/show/NCT03764735 (accessed on 20 January 2021).
- Vehicle-Controlled Study of SkQ1 as Treatment for Dry-Eye Syndrome. Available online: https://ClinicalTrials.gov/show/NCT04206020 (accessed on 20 January 2021).
- A Clinical Study to Assess the Safety and Efficacy of an Ophthalmic Solution (SkQ1) in the Treatment of Dry Eye Syndrome (DES). Available online: https://ClinicalTrials.gov/show/NCT02121301 (accessed on 20 January 2021).
- Rouen, P.A.; White, M.L. Dry Eye Disease: Prevalence, Assessment, and Management. Home Healthc. Now 2018, 36, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.; Perekhvatova, N.; Skulachev, M.; Stein, L.; Ousler, G. SkQ1 Ophthalmic Solution for Dry Eye Treatment: Results of a Phase 2 Safety and Efficacy Clinical Study in the Environment and During Challenge in the Controlled Adverse Environment Model. Adv. Ther. 2016, 33, 96–115. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, S.; Yang, T.; Yang, J. Synthesis and antioxidant activities of Coenzyme Q analogues. Eur. J. Med. Chem. 2014, 86, 710–713. [Google Scholar] [CrossRef]
- Kawamukai, M. Biosynthesis of coenzyme Q in eukaryotes. Biosci. Biotechnol. Biochem. 2016, 80, 23–33. [Google Scholar] [CrossRef]
- Aberg, F.; Appelkvist, E.L.; Dallner, G.; Ernster, L. Distribution and redox state of ubiquinones in rat and human tissues. Arch. Biochem. Biophys. 1992, 295, 230–234. [Google Scholar] [CrossRef]
- Chan, T.S.; Teng, S.; Wilson, J.X.; Galati, G.; Khan, S.; O’Brien, P.J. Coenzyme Q cytoprotective mechanisms for mitochondrial complex I cytopathies involves NAD(P)H: Quinone oxidoreductase 1(NQO1). Free Radic. Res. 2002, 36, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Mine, Y.; Okamoto, T. Intracellular reduction of coenzyme Q homologues with a short isoprenoid side chain induces apoptosis of HeLa cells. J. Biochem. 2018, 163, 329–339. [Google Scholar] [CrossRef]
- Kagan, V.E.; Serbinova, E.A.; Koynova, G.M.; Kitanova, S.A.; Tyurin, V.A.; Stoytchev, T.S.; Quinn, P.J.; Packer, L. Antioxidant action of ubiquinol homologues with different isoprenoid chain length in biomembranes. Free Radic. Biol. Med. 1990, 9, 117–126. [Google Scholar] [CrossRef]
- Kishi, T.; Okamoto, T.; Takahashi, T.; Goshima, K.; Yamagami, T. Cardiostimulatory action of coenzyme Q homologues on cultured myocardial cells and their biochemical mechanisms. Clin. Investig. 1993, 71, S71–S75. [Google Scholar] [CrossRef] [PubMed]
- Esaka, Y.; Nagahara, Y.; Hasome, Y.; Nishio, R.; Ikekita, M. Coenzyme Q2 induced p53-dependent apoptosis. Biochim. Biophys. Acta 2005, 1724, 49–58. [Google Scholar] [CrossRef]
- Lenaz, G. Quinone specificity of complex I. Biochim. Biophys. Acta 1998, 1364, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Cadenas, E.; Boveris, A.; Ragan, C.I.; Stoppani, A.O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch. Biochem. Biophys. 1977, 180, 248–257. [Google Scholar] [CrossRef]
- Lenaz, G.; Bovina, C.; D’Aurelio, M.; Fato, R.; Formiggini, G.; Genova, M.L.; Giuliano, G.; Pich, M.M.; Paolucci, U.; Castelli, G.P.; et al. Role of mitochondria in oxidative stress and aging. Ann. N. Y. Acad. Sci. 2002, 959, 199–213. [Google Scholar] [CrossRef]
- O’Brien, P.J. Molecular mechanisms of quinone cytotoxicity. Chem. Biol. Interact. 1991, 80, 1–41. [Google Scholar] [CrossRef]
- Monks, T.J.; Hanzlik, R.P.; Cohen, G.M.; Ross, D.; Graham, D.G. Quinone chemistry and toxicity. Toxicol. Appl. Pharmacol. 1992, 112, 2–16. [Google Scholar] [CrossRef]
- Long, D.J., II; Jaiswal, A.K. NRH:quinone oxidoreductase2 (NQO2). Chem. Biol. Interact. 2000, 129, 99–112. [Google Scholar] [CrossRef]
- Colucci, M.A.; Moody, C.J.; Couch, G.D. Natural and synthetic quinones and their reduction by the quinone reductase enzyme NQO1: From synthetic organic chemistry to compounds with anticancer potential. Org. Biomol. Chem. 2008, 6, 637–656. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A.; Chatelain-Egger, F.; Vella, F.; Delagrange, P.; Ferry, G. Quinone reductase 2 substrate specificity and inhibition pharmacology. Chem. Biol. Interact. 2005, 151, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Cerqua, C.; Casarin, A.; Pierrel, F.; Fonseca, L.V.; Viola, G.; Salviati, L.; Trevisson, E. Vitamin K2 cannot substitute Coenzyme Q10 as electron carrier in the mitochondrial respiratory chain of mammalian cells. Sci. Rep. 2019, 9, 6553. [Google Scholar] [CrossRef] [Green Version]
- Grant, J.; Saldanha, J.W.; Gould, A.P. A Drosophila model for primary coenzyme Q deficiency and dietary rescue in the developing nervous system. Dis. Model. Mech. 2010, 3, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Shrader, W.D.; Amagata, A.; Barnes, A.; Enns, G.M.; Hinman, A.; Jankowski, O.; Kheifets, V.; Komatsuzaki, R.; Lee, E.; Mollard, P.; et al. alpha-Tocotrienol quinone modulates oxidative stress response and the biochemistry of aging. Bioorg. Med. Chem. Lett. 2011, 21, 3693–3698. [Google Scholar] [CrossRef]
- Enns, G.M.; Kinsman, S.L.; Perlman, S.L.; Spicer, K.M.; Abdenur, J.E.; Cohen, B.H.; Amagata, A.; Barnes, A.; Kheifets, V.; Shrader, W.D.; et al. Initial experience in the treatment of inherited mitochondrial disease with EPI-743. Mol. Genet. Metab. 2012, 105, 91–102. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oestreicher, J.; Morgan, B. Glutathione: Subcellular distribution and membrane transport (1). Biochem. Cell Biol. 2019, 97, 270–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn-Kirby, A.H.; Amagata, A.; Maeder, C.I.; Mei, J.J.; Sideris, S.; Kosaka, Y.; Hinman, A.; Malone, S.A.; Bruegger, J.J.; Wang, L.; et al. Targeting ferroptosis: A novel therapeutic strategy for the treatment of mitochondrial disease-related epilepsy. PLoS ONE 2019, 14, e0214250. [Google Scholar] [CrossRef] [Green Version]
- Hirschhorn, T.; Stockwell, B.R. The development of the concept of ferroptosis. Free Radic. Biol. Med. 2019, 133, 130–143. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Zhang, Y.; Kuang, X.; Liu, Y.; Guo, M.; Ma, L.; Zhang, D.; Li, Q. Iron Metabolism and Ferroptosis in Epilepsy. Front. Neurosci. 2020, 14, 601193. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, C.; Zhao, Y.; Gao, G. Mitochondria regulation in ferroptosis. Eur. J. Cell Biol. 2020, 99, 151058. [Google Scholar] [CrossRef] [PubMed]
- Abdalkader, M.; Lampinen, R.; Kanninen, K.M.; Malm, T.M.; Liddell, J.R. Targeting Nrf2 to Suppress Ferroptosis and Mitochondrial Dysfunction in Neurodegeneration. Front. Neurosci. 2018, 12, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bebber, C.M.; Muller, F.; Clemente, L.P.; Weber, J.; von Karstedt, S. Ferroptosis in Cancer Cell Biology. Cancers (Basel) 2020, 12, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaldan, S.; Bush, A.I.; Devos, D.; Rolland, A.S.; Moreau, C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic. Biol. Med. 2019, 133, 221–233. [Google Scholar] [CrossRef]
- Enns, G.M.; Cowan, T.M. Glutathione as a Redox Biomarker in Mitochondrial Disease-Implications for Therapy. J. Clin. Med. 2017, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Sadun, A.A.; Chicani, C.F.; Ross-Cisneros, F.N.; Barboni, P.; Thoolen, M.; Shrader, W.D.; Kubis, K.; Carelli, V.; Miller, G. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary optic neuropathy. Arch. Neurol. 2012, 69, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, D.; Catteruccia, M.; Piemonte, F.; Pastore, A.; Tozzi, G.; Dionisi-Vici, C.; Pontrelli, G.; Corsetti, T.; Livadiotti, S.; Kheifets, V.; et al. EPI-743 reverses the progression of the pediatric mitochondrial disease—Genetically defined Leigh Syndrome. Mol. Genet. Metab. 2012, 107, 383–388. [Google Scholar] [CrossRef]
- Pastore, A.; Petrillo, S.; Tozzi, G.; Carrozzo, R.; Martinelli, D.; Dionisi-Vici, C.; Di Giovamberardino, G.; Ceravolo, F.; Klein, M.B.; Miller, G.; et al. Glutathione: A redox signature in monitoring EPI-743 therapy in children with mitochondrial encephalomyopathies. Mol. Genet. Metab. 2013, 109, 208–214. [Google Scholar] [CrossRef] [PubMed]
- An Exploratory Open Label Study of EPI-743 (Vincerinone TM) in Children with Autism Spectrum Disorder. Available online: https://ClinicalTrials.gov/show/NCT02226458 (accessed on 20 January 2021).
- Safety and Efficacy of EPI-743 in Patients with Friedreich’s Ataxia. Available online: https://ClinicalTrials.gov/show/NCT01728064 (accessed on 20 January 2021).
- Kouga, T.; Takagi, M.; Miyauchi, A.; Shimbo, H.; Iai, M.; Yamashita, S.; Murayama, K.; Klein, M.B.; Miller, G.; Goto, T.; et al. Japanese Leigh syndrome case treated with EPI-743. Brain Dev. 2018, 40, 145–149. [Google Scholar] [CrossRef] [PubMed]
- A Study to Evaluate Efficacy and Safety of Vatiquinone for Treating Mitochondrial Disease in Participants with Refractory Epilepsy. Available online: https://ClinicalTrials.gov/show/NCT04378075 (accessed on 20 January 2021).
- Phase 2 Study of EPI-743 in Children with Pearson Syndrome. Available online: https://ClinicalTrials.gov/show/NCT02104336 (accessed on 20 January 2021).
- EPI-743 in Friedreich’s Ataxia Point Mutations. Available online: https://ClinicalTrials.gov/show/NCT01962363 (accessed on 20 January 2021).
- EPI-743 for Mitochondrial Respiratory Chain Diseases. Available online: https://ClinicalTrials.gov/show/NCT01370447 (accessed on 20 January 2021).
- EPI-743 in Cobalamin C Defect: Effects on Visual and Neurological Impairment. Available online: https://ClinicalTrials.gov/show/NCT01793090 (accessed on 20 January 2021).
- Finsterer, J.; Zarrouk-Mahjoub, S. Is vatiquinone truly beneficial for Leigh syndrome? Brain Dev. 2018, 40, 443. [Google Scholar] [CrossRef] [PubMed]
- Safety and Efficacy Study of EPI-743 in Children with Leigh Syndrome. Available online: https://ClinicalTrials.gov/show/NCT01721733 (accessed on 20 January 2021).
- Phase 2 Study of EPI-743 for Treatment of Rett Syndrome. Available online: https://ClinicalTrials.gov/show/NCT01822249 (accessed on 20 January 2021).
| Coenzyme Q10 Analogue | Medical Applications | Level of Study | References |
|---|---|---|---|
| Idebenone | LHON | Approved for patients’ treatment | [46,47,48] |
| Friedreich ataxia | Patients’ treatment | [27,49,50,51] | |
| Pulmonary fibrosis | Tested in vivo | [64] | |
| Dementia | Patients’ treatment | [65] | |
| MELAS | Patients’ treatment | [66] | |
| Glaucoma | Patients’ treatment | [67] | |
| Mitoquinone | Heart failure | Tested in vivo | [76] |
| Hypertension | Tested in vivo | [77] | |
| Diabetic kidney disease | Tested in vivo | [78] | |
| Alcoholic fatty liver disease | Tested in vivo | [79] | |
| Hepatitis C | Patients’ treatment | [80] | |
| Parkinson’s disease | Patients’ treatment | [81,82] | |
| Alzheimer’s disease | Tested in vivo | [83,84] | |
| Huntington’s disease | Tested in vivo | [85] | |
| Amyotrophic lateral sclerosis | Tested in vivo | [86] | |
| Traumatic brain injury | Tested in vivo | [87] | |
| Decylubiquinone | LHON | In vitro studies | [102] |
| Cancer | Tested in vivo | [103,104,105] | |
| Hypertension | Tested in vivo | [106] | |
| SkQ1 | Inflammation | Tested in vivo | [122,123] |
| Wound healing | Tested in vivo | [122,124] | |
| Tumor growth suppression | In vitro studies | [125] | |
| Alzheimer’s disease | Tested in vivo | [126] | |
| Fertility | Tested in vivo | [127] | |
| Aging | Tested in vivo | [128,129] | |
| Immunoregulation | Tested in vivo | [130] | |
| Ischemia | Tested in vivo | [131] | |
| Dry eye treatment | Patients’ treatment | [140,141] | |
| CoQ10 with modifications on C6 | ROS related diseases | - | - |
| Short-Chain CoQ10 | CoenzymeQ10 deficiency-related syndromes | Tested in vivo | [145] |
| Apoptosis modulation | In vitro studies | [146] | |
| EPI-743 | LHON | Patients’ treatment | [172] |
| Leigh syndrome | Patients’ treatment | [175,177,184] | |
| Parkinson’s disease | Patients’ treatment | [178] | |
| Rett syndrome | Patients’ treatment | [185] | |
| Pearson’s syndrome | Patients’ treatment | [180] | |
| Epilepsy | In vitro studies | [164] | |
| Friedreich’s ataxia | Patients’ treatment | [167,181,182] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q10 Analogues: Benefits and Challenges for Therapeutics. Antioxidants 2021, 10, 236. https://doi.org/10.3390/antiox10020236
Suárez-Rivero JM, Pastor-Maldonado CJ, Povea-Cabello S, Álvarez-Córdoba M, Villalón-García I, Munuera-Cabeza M, Suárez-Carrillo A, Talaverón-Rey M, Sánchez-Alcázar JA. Coenzyme Q10 Analogues: Benefits and Challenges for Therapeutics. Antioxidants. 2021; 10(2):236. https://doi.org/10.3390/antiox10020236
Chicago/Turabian StyleSuárez-Rivero, Juan M., Carmen J. Pastor-Maldonado, Suleva Povea-Cabello, Mónica Álvarez-Córdoba, Irene Villalón-García, Manuel Munuera-Cabeza, Alejandra Suárez-Carrillo, Marta Talaverón-Rey, and José A. Sánchez-Alcázar. 2021. "Coenzyme Q10 Analogues: Benefits and Challenges for Therapeutics" Antioxidants 10, no. 2: 236. https://doi.org/10.3390/antiox10020236
APA StyleSuárez-Rivero, J. M., Pastor-Maldonado, C. J., Povea-Cabello, S., Álvarez-Córdoba, M., Villalón-García, I., Munuera-Cabeza, M., Suárez-Carrillo, A., Talaverón-Rey, M., & Sánchez-Alcázar, J. A. (2021). Coenzyme Q10 Analogues: Benefits and Challenges for Therapeutics. Antioxidants, 10(2), 236. https://doi.org/10.3390/antiox10020236








