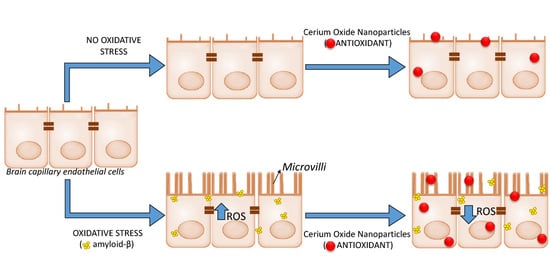
Oxidative Stress Boosts the Uptake of Cerium Oxide Nanoparticles by Changing Brain Endothelium Microvilli Pattern
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
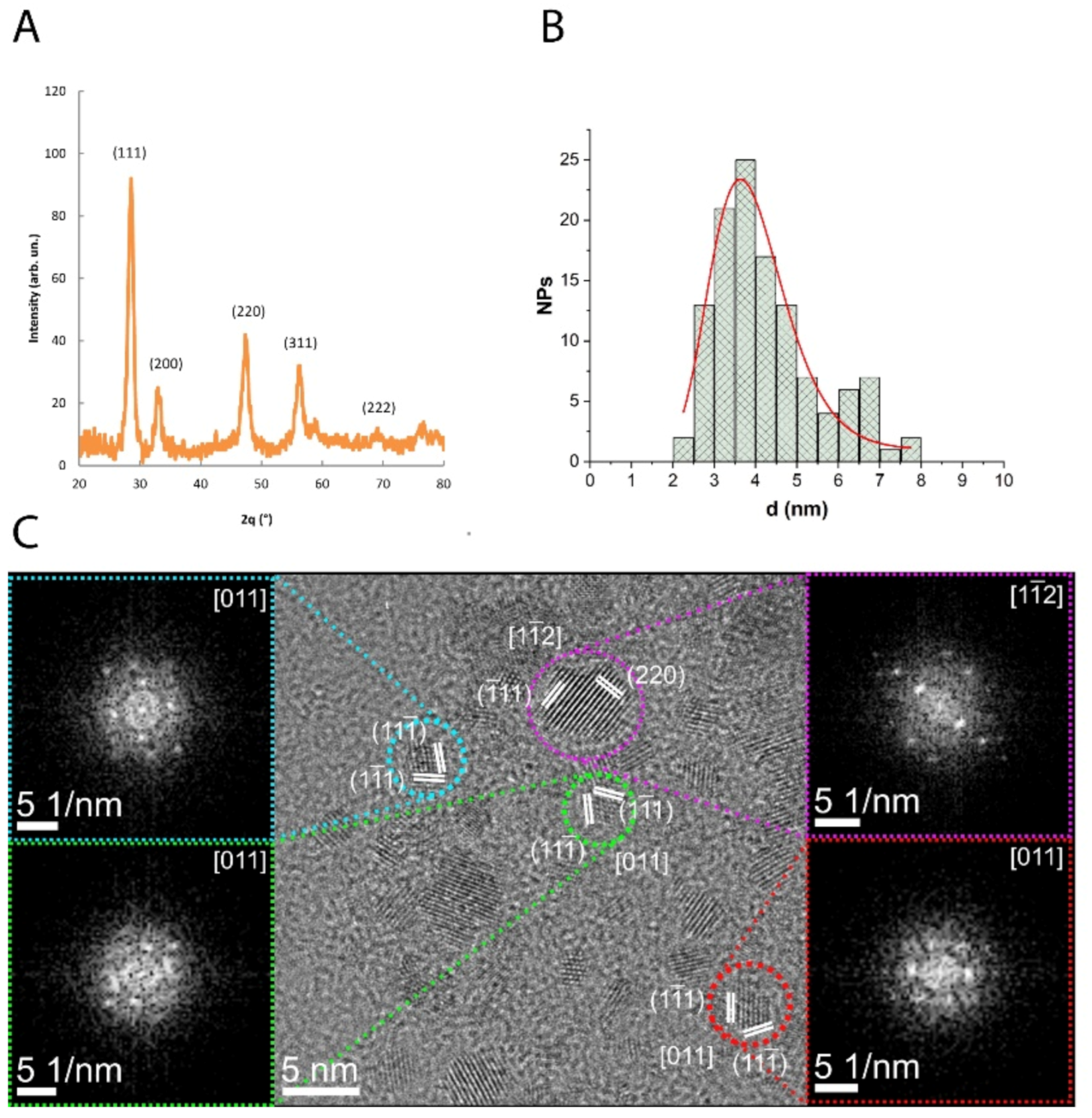
2.2. CNP Synthesis and Characterization
2.3. Preparation and Characterization of Aβ Oligomers
2.4. Cells and Culture Conditions
2.5. Cell Viability in the Presence of CNP or Aβ Oligomers
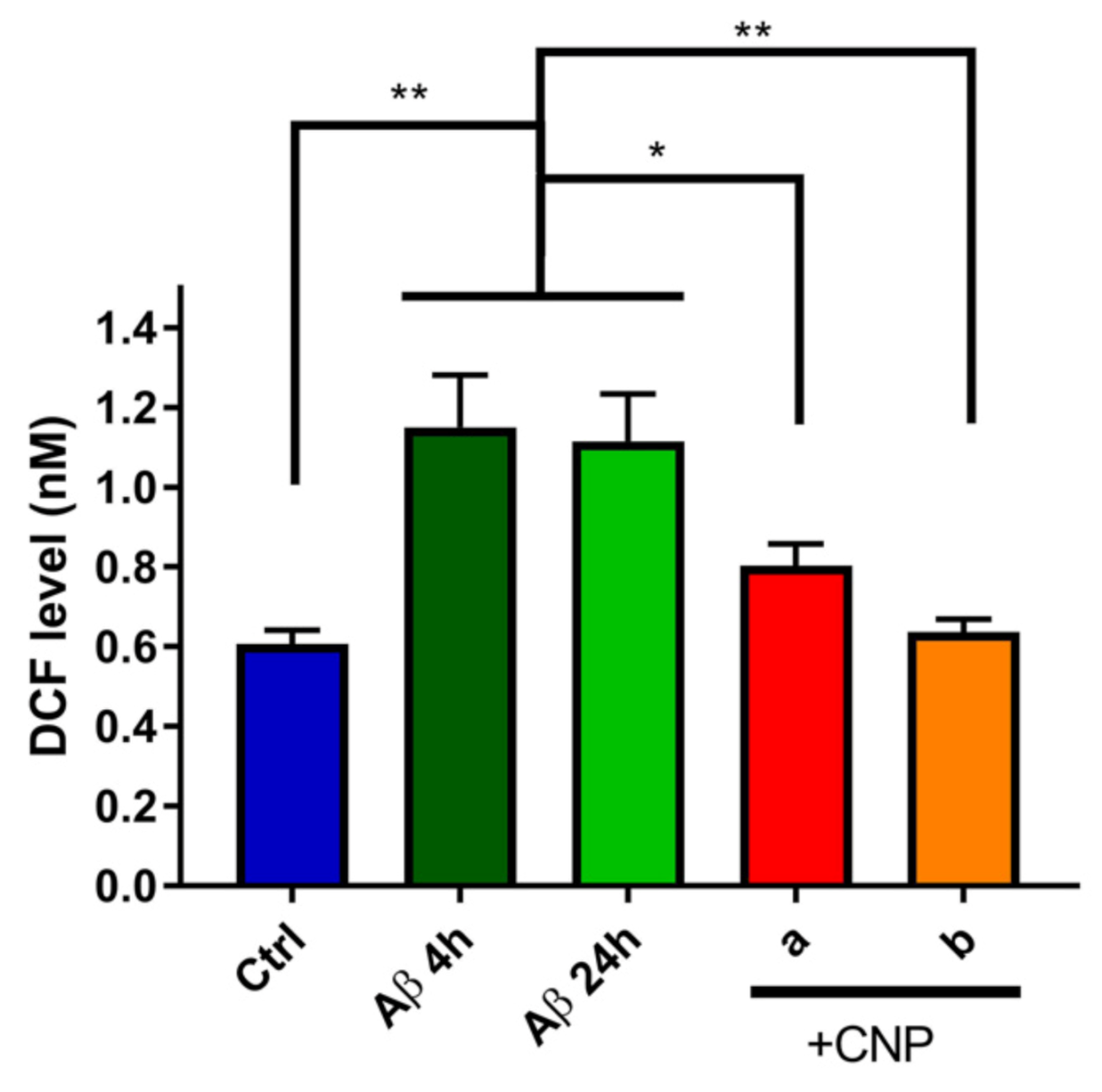
2.6. Free Radical Scavenging Activity of CNP after hCMEC/D3 Exposure to Aβ Oligomers
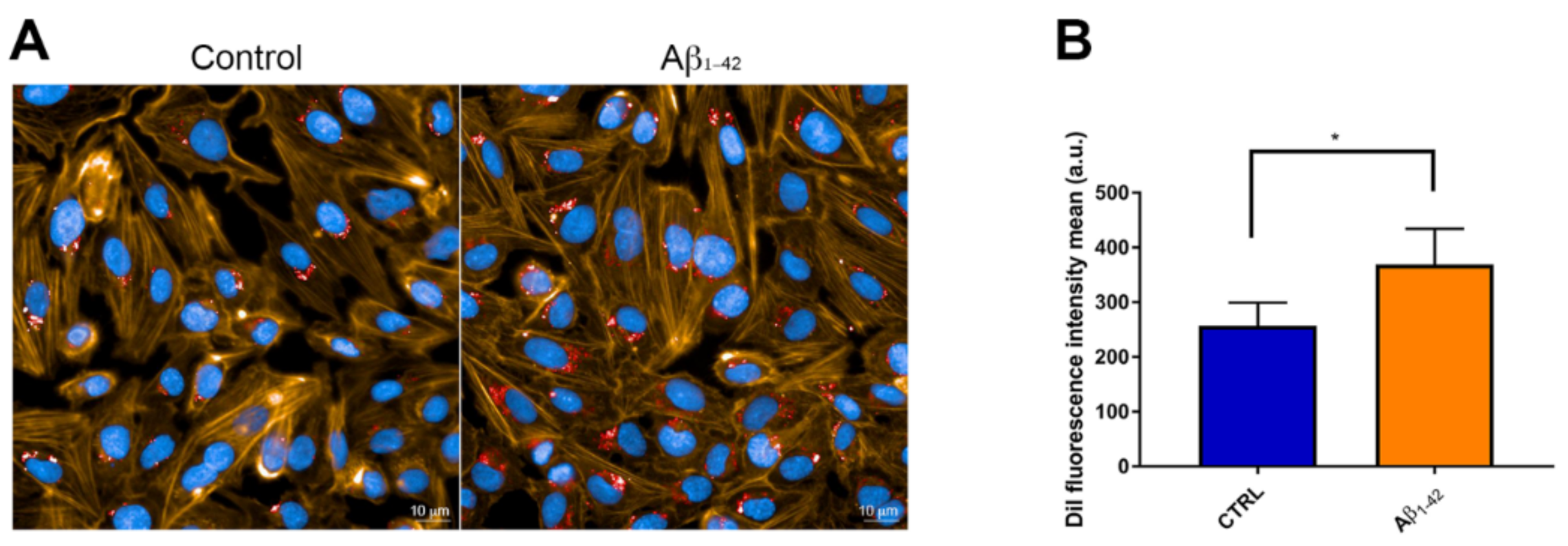
2.7. Uptake of CNP by hCMEC/D3 Cells
2.8. Binding of CNP to Aβ
2.9. Visualization of Microvilli-Like Protrusions by SEM
2.10. In Vitro BBB Model
2.11. Immunoblotting for pERM/ERM Levels
2.12. Statistical Analysis
3. Results
3.1. Characterization of CNP
3.2. Cell Viability in the Presence of CNP or Aβ Oligomers
3.3. Evaluation of the Antioxidant Activity of CNP in Cerebral Endothelial Cells Exposed to Aβ
3.4. CNP Cellular Uptake after Exposure to Aβ
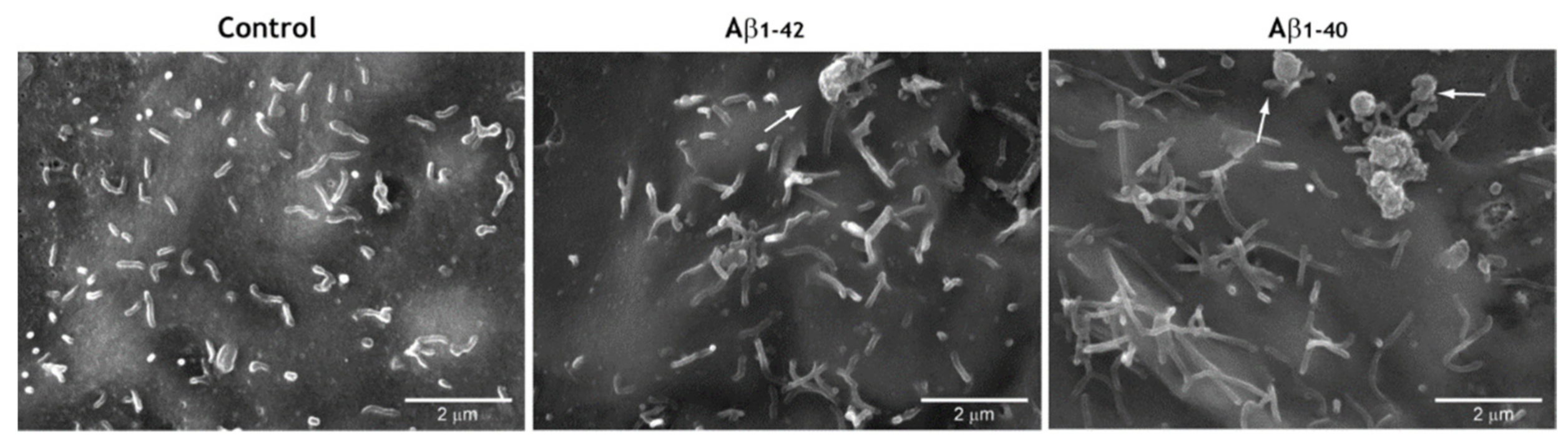
3.5. Pro-Oxidant Stimuli Affect the Architecture of Endothelial Microvilli
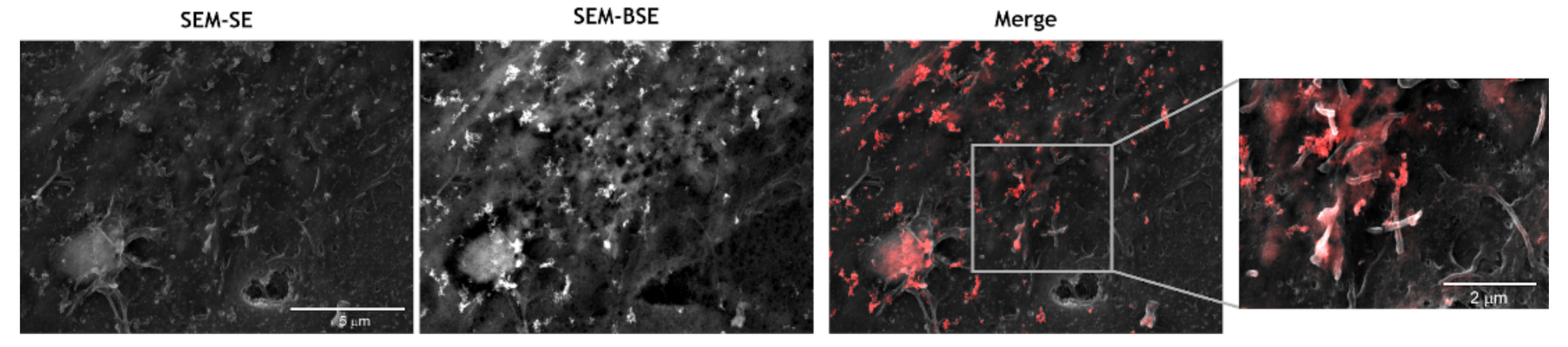
3.6. CNP Tropism for Endothelial Microvilli
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Disease International. Available online: www.alzint.org/ (accessed on 8 February 2021).
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease At 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The Role of Brain Vasculature in Neurodegenerative Disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Van de Haar, H.J.; Jansen, J.F.A.; van Osch, M.J.P.; van Buchem, M.A.; Muller, M.; Wong, S.M.; Hofman, P.A.M.; Burgmans, S.; Verhey, F.R.J.; Backes, W.H. Neurovascular Unit Impairment in Early Alzheimer’s Disease Measured with Magnetic Resonance Imaging. Neurobiol. Aging 2016, 45, 190–196. [Google Scholar] [CrossRef]
- Park, L.; Wang, G.; Moore, J.; Girouard, H.; Zhou, P.; Anrather, J.; Iadecola, C. The Key Role of Transient Receptor Potential Melastatin-2 Channels in Amyloid-Β-Induced Neurovascular Dysfunction. Nat. Commun. 2014, 5, 5318. [Google Scholar] [CrossRef] [Green Version]
- Park, L.; Wang, G.; Zhou, P.; Zhou, J.; Pitstick, R.; Previti, M.L.; Younkin, L.; Younkin, S.G.; Van Nostrand, W.E.; Cho, S.; et al. Scavenger Receptor Cd36 is Essential for the Cerebrovascular Oxidative Stress and Neurovascular Dysfunction Induced by Amyloid-Beta. Proc. Natl. Acad. Sci. USA 2011, 108, 5063–5068. [Google Scholar] [CrossRef] [Green Version]
- Askarova, S.; Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C. Role of Aβ-Receptor for Advanced Glycation End Products Interaction in Oxidative Stress and Cytosolic Phospholipase A2 Activation in Astrocytes and Cerebral Endothelial Cells. Neuroscience 2011, 199, 375–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marco, L.Y.; Venneri, A.; Farkas, E.; Evans, P.C.; Marzo, A.; Frangi, A.F. Vascular Dysfunction in the Pathogenesis of Alzheimer’s Disease—A Review of Endothelium-Mediated Mechanisms and Ensuing Vicious Circles. Neurobiol. Dis. 2015, 82, 593–606. [Google Scholar] [CrossRef] [Green Version]
- Alliev, G.; Priyadarshini, M.; Reddy, V.P.; Grieg, N.H.; Kaminsky, Y.; Cacabelos, R.; Ashraf, G.M.; Jabir, N.R.; Kamal, M.A.; Nikolenko, V.N.; et al. Oxidative Stress Mediated Mitochondrial and Vascular Lesions as Markers in the Pathogenesis of Alzheimer Disease. Curr. Med. Chem. 2014, 21, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Enciu, A.M.; Gherghiceanu, M.; Popescu, O.B. Triggers and Effectors of Oxidative Stress at Blood-Brain Barrier Level: Relevance for Brain Ageing and Neurodegeneration. Oxid. Med. Cell. Longev. 2013, 2013, 297512. [Google Scholar] [CrossRef] [PubMed]
- Bulbarelli, A.; Lonati, E.; Brambilla, A.; Orlando, A.; Cazzaniga, E.; Piazza, F.; Ferrarese, C.; Masserini, M.; Sancini, G. Aβ42 Production in Brain Capillary Endothelial Cells after Oxygen and Glucose Deprivation. Mol. Cell. Neurosci. 2012, 49, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.; Premkumar, D.; Pax, A.; Cohen, D.; Lieberburg, I. Production and Increased Detection of Amyloid Beta Protein and Amyloidogenic Fragments in Brain Microvessels, Meningeal Vessels and Choroid Plexus in Alzheimer’s Disease. Mol. Brain Res. 1996, 35, 58–68. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Battaglini, M.; Marino, A.; Ciofani, G. Antioxidants and Nanotechnology: Promises and Limits of Potentially Disruptive Approaches in the Treatment of Central Nervous System Diseases. Adv. Healthc. Mater. 2020, 9, e1901589. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative Stress Induced-Neurodegenerative Diseases: The Need for Antioxidants That Penetrate the Blood Brain Barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin- and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef] [Green Version]
- Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Angelova, A. Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells. Molecules 2019, 24, 3058. [Google Scholar] [CrossRef] [Green Version]
- Santonocito, D.; Sarpietro, M.G.; Carbone, C.; Panico, A.; Campisi, A.; Siciliano, E.A.; Sposito, G.; Castelli, F.; Puglia, C. Curcumin Containing PEGylated Solid Lipid Nanoparticles for Systemic Administration: A Preliminary Study. Molecules 2020, 25, 2991. [Google Scholar] [CrossRef] [PubMed]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological Potential of Cerium Oxide Nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.; Tredici, I.G.; Ghigna, P.; Castillo-Michel, H.; Falqui, A.; Di Benedetto, C.; Alberti, G.; Ricci, V.; Anselmi-Tamburini, U.; Sommi, P. Dependence of the Ce(iii)/Ce(iv) Ratio on Intracellular Localization in Ceria Nanoparticles Internalized by Human Cells. Nanoscale 2017, 9, 1527–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Angelo, B.; Santucci, S.; Benedetti, E.; Di Loreto, S.; Phani, R.A.; Falone, S.; Amicarelli, F.; Ceru, M.P.; Cimini, A. Cerium Oxide Nanoparticles Trigger Neuronal Survival in A Human Alzheimer Disease Model by Modulating Bdnf Pathway. Curr. Nanosci. 2009, 5, 167–176. [Google Scholar] [CrossRef]
- Kwon, H.J.; Moon-Yong, C.; Dokyoon, K.; Dong, K.K.; Min, S.; Kwangsoo, S.; Taeghwan, H.; Inhee, M.J. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s Disease. ACS Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef]
- Dowding, J.M.; Song, W.; Bossy, K.; Karakoti, A.; Kumar, A.; Kim, A.; Bossy, B.; Seal, S.; Ellisman, M.H.; Perkins, G.; et al. Cerium Oxide Nanoparticles Protect Against Aβ-Induced Mitochondrial Fragmentation and Neuronal Cell Death. Cell Death Differ. 2014, 21, 1622–1632. [Google Scholar] [CrossRef]
- Li, M.; Peng, S.; Can, X.; Jinsong, R.; Xiaogang, Q. Cerium Oxide Caged Metal Chelator: Anti-Aggregation and Anti-Oxidation Integrated H2o2-Responsive Controlled Drug Release for Potential Alzheimer’s Disease Treatment. Chem. Sci. 2013, 4, 2536–2542. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-Charge-Dependent Cell Localization and Cytotoxicity of Cerium Oxide Nanoparticles. ACS Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef] [Green Version]
- Santra, S.; Kaittanis, C.; Grimm, J.; Perez, J.M. Drug/Dye-Loaded, Multifunctional Iron Oxide Nanoparticles for Combined Targeted Cancer Therapy and Dual Optical/Magnetic Resonance Imaging. Small 2009, 5, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Minniti, S.; Gregori, M.; Sancini, G.; Cagnotto, A.; Couraud, P.O.; Ordóñez-Gutiérrez, L.; Wandosell, F.; Salmona, M.; Re, F. The Hunt for Brain Aβ Oligomers by Peripherally Circulating Multi-Functional Nanoparticles: Potential Therapeutic Approach for Alzheimer Disease. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dal Magro, R.; Simonelli, S.; Cox, A.; Formicola, B.; Corti, R.; Cassina, V.; Nardo, L.; Mantegazza, F.; Salerno, D.; Grasso, G.; et al. The Extent of Human Apolipoprotein A-I Lipidation Strongly Affects the β-Amyloid Efflux Across the Blood-Brain Barrier in Vitro. Front. Neurosci. 2019, 13, 419. [Google Scholar] [CrossRef] [PubMed]
- Nardo, L.; Re, F.; Brioschi, S.; Cazzaniga, E.; Orlando, A.; Minniti, S.; Lamperti, M.; Gregori, M.; Cassina, V.; Brogioli, D.; et al. Fluorimetric Detection of the Earliest Events in Amyloid β Oligomerization and Its Inhibition by Pharmacologically Active Liposomes. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 746–756. [Google Scholar] [CrossRef]
- Weksler, B.M.; Romero, I.A.; Couraud, P.O. The hCMEC/D3 Cell Line as A Model of the Human Blood Brain Barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregori, M.; Orlando, A.; Re, F.; Sesana, S.; Nardo, L.; Salerno, D.; Mantegazza, F.; Salvati, E.; Zito, A.; Malavasi, F.; et al. Novel Antitransferrin Receptor Antibodies Improve the Blood-Brain Barrier Crossing Efficacy of Immunoliposomes. J. Pharm. Sci. 2016, 150, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.M.; Kokjohn, T.A.; Kalback, W.; Luehrs, D.; Galasko, D.R.; Chevallier, N.; Koo, E.H.; Emmerling, M.R.; Roher, A.E. Amyloid-Beta Peptides Interact with Plasma Proteins and Erythrocytes: Implications for Their Quantitation in Plasma. Biochem. Biophys. Res. Commun. 2000, 268, 750–756. [Google Scholar] [CrossRef]
- Cox, A.; Andreozzi, P.; Dal Magro, R.; Fiordaliso, F.; Corbelli, A.; Talamini, L.; Chinello, C.; Raimondo, F.; Magni, F.; Tringali, M.; et al. Evolution of Nanoparticle Protein Corona across the Blood-Brain Barrier. ACS Nano 2018, 12, 7292–7300. [Google Scholar] [CrossRef]
- Formicola, B.; Dal Magro, R.; Montefusco-Pereira, C.V.; Lehr, C.M.; Koch, M.; Russo, L.; Grasso, G.; Deriu, M.A.; Danani, A.; Bourdoulous, S.; et al. The Synergistic Effect of Chlorotoxin-mApoE in Boosting Drug-Loaded Liposomes Across The BBB. J. Nanobiotechnol. 2019, 17, 115. [Google Scholar] [CrossRef]
- Pezzini, I.; Marino, A.; Del Turco, S.; Nesti, C.; Doccini, S.; Cappello, V.; Gemmi, M.; Parlanti, P.; Santorelli, F.M.; Mattoli, V.; et al. Cerium Oxide Nanoparticles: The Regenerative Redox Machine in Bioenergetic Imbalance. Nanomedicine 2017, 12, 403–416. [Google Scholar] [CrossRef]
- Hanafy, B.I.; Cave, G.W.V.; Barnett, Y.; Pierscionek, B. Treatment of Human Lens Epithelium with High Levels of Nanoceria Leads to Reactive Oxygen Species Mediated Apoptosis. Molecules 2020, 25, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomic, J.L.; Pensalfini, A.; Head, E.; Glabe, C.G. Soluble Fibrillar Oligomer Levels Are Elevated in Alzheimer’s Disease Brain and Correlate with Cognitive Dysfunction. Neurobiol. Dis. 2009, 35, 352–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an Understanding of Amyloid-Β Oligomers: Characterization, Toxicity Mechanisms, and Inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef]
- Xu, J.; Chen, S.; Ku, G.; Ahmed, S.H.; Xu, J.; Chen, H.; Hsu, C.Y. Amyloid Beta Peptide-Induced Cerebral Endothelial Cell Death Involves Mitochondrial Dysfunction and Caspase Activation. J. Cereb. Blood Flow Metab. 2001, 21, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Thal, D.R.; Griffin, W.S.; de Vos, R.A.; Ghebremedhin, E. Cerebral Amyloid Angiopathy and Its Relationship to Alzheimer’s Disease. Acta Neuropathol. 2008, 115, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Kirkitadze, M.D.; Bitan, G.; Teplow, D.B. Paradigm Shifts in Alzheimer’s Disease and Other Neurodegenerative Disorders: The Emerging Role of Oligomeric Assemblies. J. Neurosci. Res. 2002, 69, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Durán-Prado, M.; Frontiñán, J.; Santiago-Mora, R.; Peinado, J.R.; Parrado-Fernández, C.; Gómez-Almagro, M.V.; Moreno, M.; López-Domínguez, J.A.; Villalba, J.M.; Alcaín, F.J. Coenzyme Q10 Protects Human Endothelial Cells from Β-Amyloid Uptake and Oxidative Stress-Induced Injury. PLoS ONE 2014, 9, e109223. [Google Scholar] [CrossRef]
- Tscheka, C.; Hittinger, M.; Lehr, C.M.; Schneider-Daum, N.; Schneider, M. Macrophage Uptake of Cylindrical Microparticles Investigated with Correlative Microscopy. Eur. J. Pharm. Biopharm. 2015, 95, 151–155. [Google Scholar] [CrossRef]
- Dal Magro, R.; Ornaghi, F.; Cambianica, I.; Beretta, S.; Re, F.; Musicanti, C.; Rigolio, R.; Donzelli, E.; Canta, A.; Ballarini, E.; et al. ApoE-Modified Solid Lipid Nanoparticles: A Feasible Strategy to Cross the Blood-Brain Barrier. J. Control. Release 2017, 249, 103–110. [Google Scholar] [CrossRef]
- Cox, A.; Vinciguerra, D.; Re, F.; Magro, R.D.; Mura, S.; Masserini, M.; Couvreur, P.; Nicolas, J. Protein-Functionalized Nanoparticles Derived from End-Functional Polymers and Polymer Prodrugs for Crossing the Blood-Brain Barrier. Eur. J. Pharm. Biopharm. 2019, 142, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Bana, L.; Minniti, S.; Salvati, E.; Sesana, S.; Zambelli, V.; Cagnotto, A.; Orlando, A.; Cazzaniga, E.; Zwart, R.; Scheper, W.; et al. Liposomes Bi-Functionalized with Phosphatidic Acid and an Apoe-Derived Peptide Affect Aβ Aggregation Features and Cross The Blood-Brain-Barrier: Implications for Therapy of Alzheimer Disease. Nanomedicine 2014, 10, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Songjiang, Z.; Lixiang, W. Amyloid-Beta Associated with Chitosan Nano-Carrier Has Favorable Immunogenicity and Permeates The Bbb. Aaps Pharmscitech 2009, 10, 900–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabbiani, G.; Majno, G. Endothelial Microvilli in the Vessels of The Rat Gasserian Ganglion and Testis. Z. Zellforsch. Mikrosk. Anat. 1969, 97, 111–117. [Google Scholar] [CrossRef]
- Makarov, V.; Zueva, L.; Sanabria, P.; Wessinger, W.D.; Golubeva, T.; Khmelinskii, I.; Inyushin, M. On the Role of the Blood Vessel Endothelial Microvilli in the Blood Flow in Small Capillaries. J. Biophys. 2015, 2015, 529746. [Google Scholar] [CrossRef] [Green Version]
- Carman, C.V.; Jun, C.D.; Salas, A.; Springer, T.A. Endothelial Cells Proactively form Microvilli-Like Membrane Projections Upon Intercellular Adhesion Molecule 1 Engagement of Leukocyte LFA-1. J. Immunol. 2003, 171, 6135–6144. [Google Scholar] [CrossRef] [Green Version]
- Arita-Okubo, S.; Kim-Kaneyama, J.R.; Lei, X.F.; Fu, W.G.; Ohnishi, K.; Takeya, M.; Miyauchi, A.; Honda, H.; Itabe, H.; Miyazaki, T.; et al. Role of Hic-5 in the Formation of Microvilli-Like Structures and the Monocyte-Endothelial Interaction that Accelerates Atherosclerosis. Cardiovasc. Res. 2015, 150, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Eugène, E.; Hoffmann, I.; Pujol, C.; Couraud, P.O.; Bourdoulous, S.; Nassif, X. Microvilli-Like Structures Are Associated with the Internalization of Virulent Capsulated Neisseria Meningitidis into Vascular Endothelial Cells. J. Cell Sci. 2002, 115, 1231–1241. [Google Scholar]
- Dietrich, W.D.; Busto, R.; Ginsberg, M.D. Cerebral Endothelial Microvilli: Formation Following Global Forebrain Ischemia. J. Neuropathol. Exp. Neurol. 1984, 43, 72–83. [Google Scholar] [CrossRef]
- Lossinsky, A.S.; Vorbrodt, A.W.; Wisniewski, H.M. Scanning and Transmission Electron Microscopic Studies of Microvascular Pathology in the Osmotically Impaired Blood-Brain Barrier. J. Neurocytol. 1995, 24, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Gautreau, A.; Louvard, D.; Arpin, M. Morphogenic Effects of Ezrin Require a Phosphorylation-Induced Transition from Oligomers to Monomers at the Plasma Membrane. J. Cell Biol. 2000, 150, 193–203. [Google Scholar] [CrossRef]
- Yamane, J.; Ohnishi, H.; Sasaki, H.; Narimatsu, H.; Ohgushi, H.; Tachibana, K. Formation of Microvilli and Phosphorylation of Erm Family Proteins by Cd43, A Potent Inhibitor for Cell Adhesion: Cell Detachment Is a Potential Cue for Erm Phosphorylation and Organization of Cell Morphology. Cell Adhes. Migr. 2011, 5, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Yonemura, S.; Hirao, M.; Doi, Y.; Takahashi, N.; Kondo, T.; Tsukita, S. Ezrin/Radixin/Moesin (Erm) Proteins Bind to a Positively Charged Amino Acid Cluster in The Juxta-Membrane Cytoplasmic Domain of CD44, CD43, and ICAM-2. J. Cell Biol. 1998, 140, 885–895. [Google Scholar] [CrossRef] [Green Version]
- Varland, S.; Vandekerckhove, J.; Drazic, A. Actin Post-Translational Modifications: The Cinderella of Cytoskeletal Control. Trends Biochem. Sci. 2019, 44, 502–516. [Google Scholar] [CrossRef] [Green Version]
- Hung, R.J.; Pak, C.W.; Terman, J.R. Direct Redox Regulation of F-Actin Assembly and Disassembly by Mical. Science 2011, 334, 1710–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kommaddi, R.P.; Tomar, D.S.; Karunakaran, S.; Bapat, D.; Nanguneri, S.; Ray, A.; Schneider, B.L.; Nair, D.; Ravindranath, V. Glutaredoxin1 Diminishes Amyloid Beta-Mediated Oxidation of F-Actin and Reverses Cognitive Deficits in an Alzheimer’s Disease Mouse Model. Antioxid. Redox Signal. 2019, 31, 1321–1338. [Google Scholar] [CrossRef]
- Wong, S.W.; Sun, S.; Cho, M.; Lee, K.K.; Mak, A.F. H2O2 Exposure Affects Myotube Stiffness and Actin Filament Polymerization. Ann. Biomed. Eng. 2015, 43, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Usatyuk, P.V.; Enika, D.; Natarajan, V.; Rifkind, J.M. Vascular Endothelial Barrier Dysfunction Mediated by Amyloid-Beta Proteins. J. Alzheimers Dis. 2009, 17, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, G.; Panther, D.J.; Phillips, J.L.; Tarasevich, B.J.; Dohnalkova, A.; Hu, D.; Teeguarden, J.G.; Pounds, J.G. Submicrometer and Nanoscale Inorganic Particles Exploit the Actin Machinery to Be Propelled along Microvilli-Like Structures into Alveolar Cells. ACS Nano 2007, 1, 463–475. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Magro, R.; Vitali, A.; Fagioli, S.; Casu, A.; Falqui, A.; Formicola, B.; Taiarol, L.; Cassina, V.; Marrano, C.A.; Mantegazza, F.; et al. Oxidative Stress Boosts the Uptake of Cerium Oxide Nanoparticles by Changing Brain Endothelium Microvilli Pattern. Antioxidants 2021, 10, 266. https://doi.org/10.3390/antiox10020266
Dal Magro R, Vitali A, Fagioli S, Casu A, Falqui A, Formicola B, Taiarol L, Cassina V, Marrano CA, Mantegazza F, et al. Oxidative Stress Boosts the Uptake of Cerium Oxide Nanoparticles by Changing Brain Endothelium Microvilli Pattern. Antioxidants. 2021; 10(2):266. https://doi.org/10.3390/antiox10020266
Chicago/Turabian StyleDal Magro, Roberta, Agostina Vitali, Stefano Fagioli, Alberto Casu, Andrea Falqui, Beatrice Formicola, Lorenzo Taiarol, Valeria Cassina, Claudia Adriana Marrano, Francesco Mantegazza, and et al. 2021. "Oxidative Stress Boosts the Uptake of Cerium Oxide Nanoparticles by Changing Brain Endothelium Microvilli Pattern" Antioxidants 10, no. 2: 266. https://doi.org/10.3390/antiox10020266
APA StyleDal Magro, R., Vitali, A., Fagioli, S., Casu, A., Falqui, A., Formicola, B., Taiarol, L., Cassina, V., Marrano, C. A., Mantegazza, F., Anselmi-Tamburini, U., Sommi, P., & Re, F. (2021). Oxidative Stress Boosts the Uptake of Cerium Oxide Nanoparticles by Changing Brain Endothelium Microvilli Pattern. Antioxidants, 10(2), 266. https://doi.org/10.3390/antiox10020266