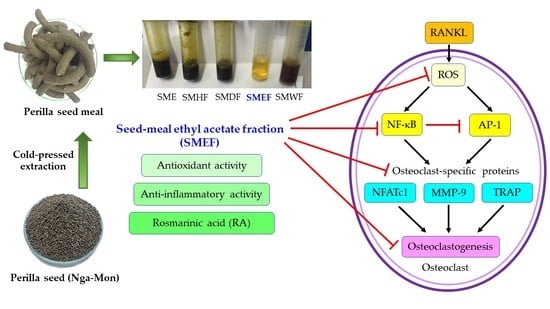
Inhibitory Effect of a Rosmarinic Acid-Enriched Fraction Prepared from Nga-Mon (Perilla frutescens) Seed Meal on Osteoclastogenesis through the RANK Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Extract and Fraction Collection and Preparation
2.3. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical-Scavenging Assay
2.4. 2,2-Azino-bis-3-ethylbenzthiazoline-6-sulfonic Acid (ABTS) Radical-Scavenging Assay
2.5. Superoxide Anion (O2•−)Radical-Scavenging Assay
2.6. Nitric Oxide (NO) Radical-Scavenging Assay
2.7. Ferric Reducing/Antioxidant Power (FRAP) Assay
2.8. Total Phenolic Content (TPC)
2.9. Total Flavonoid Content (TFC)
2.10. RA Identification
2.11. Cell Culture and Cytotoxicity Assay
2.12. Proinflammatory Protein (NO, iNOS, and COX-2) Production
2.13. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
2.14. TRAP Activity Assay
2.15. Western Blot Analysis
2.16. Gelatin Zymography
2.17. Measurement of Intracellular ROS
2.18. Statistical Analysis
3. Results
3.1. Yield and Antioxidant Activity of PSM Fractions
3.2. Anti-Inflammatory Activity of PSM Fractions
3.3. TPC, TFC, and RA content of the PSM fractions
3.4. Quantitative HPLC Analysis of RA Compound from PSM Fractions
3.5. Cytotoxicity of SMEF in RAW 264.7 Cells
3.6. Effect of SMEF on RANKL-Induced Osteoclast Differentiation
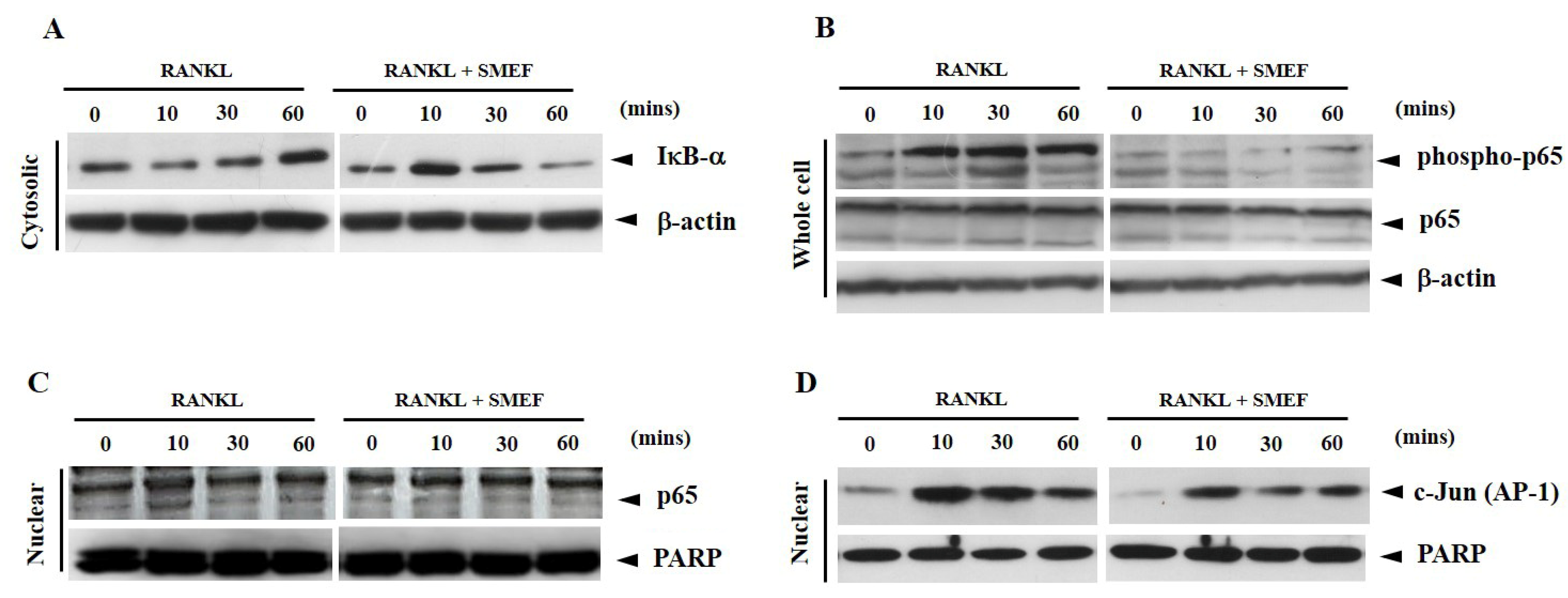
3.7. Effect of SMEF on RANKL-Induced NF-κB and AP-1 Activation
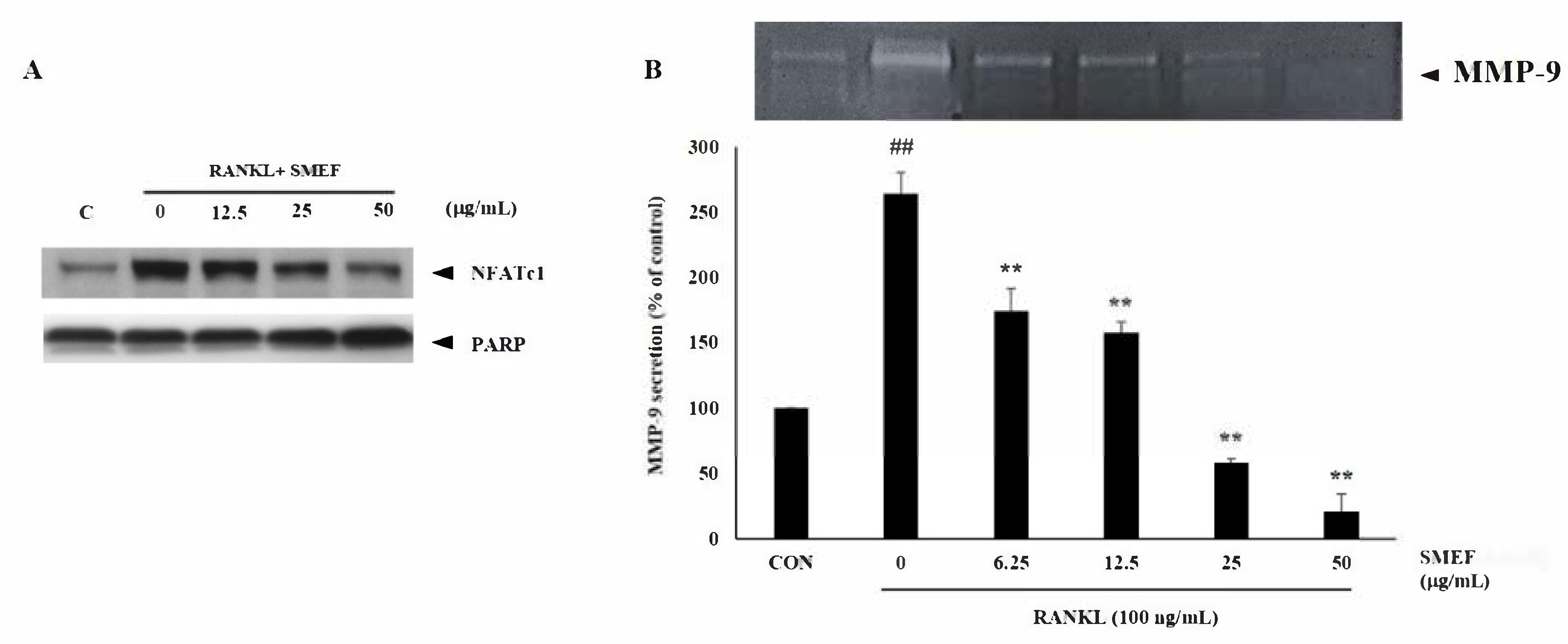
3.8. Effect of SMEF on Osteoclastic-Specific Protein Expression
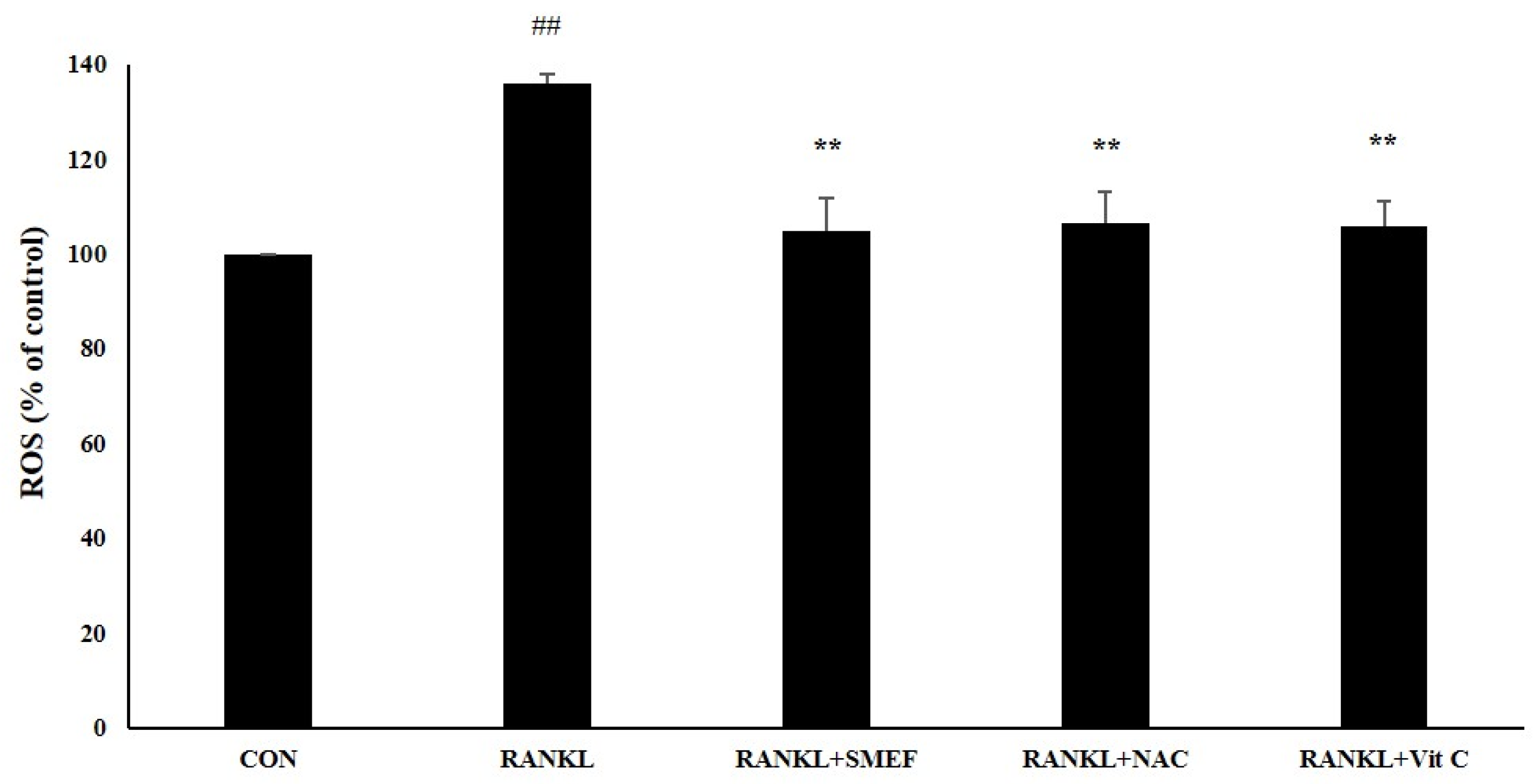
3.9. Effect of SMEF on RANKL-Induced ROS Generation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. Mech. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases. Miner. Bone. Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.Y.; Kim, H.Y.; Min, J.Y.; Kim, H.M.; Jeong, H.J. An osteoclastogenesis system, the RANKL/RANK signalling pathway, contributes to aggravated allergic inflammation. Br. J. Pharmacol. 2019, 176, 1664–1679. [Google Scholar] [CrossRef] [PubMed]
- Agidigbi, T.S.; Kim, C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases. Int. J. Mol. Sci. 2019, 20, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, T.; Yao, Z.Q.; Li, F.; Zhang, Q.; Badell, I.R.; Schwarz, E.M.; Takeshita, S.; Wagner, E.F.; Noda, M.; Matsuo, K.; et al. NF-kappa B p50 and p52 regulate receptor activator of NF-kappa B ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J. Biol. Chem. 2007, 282, 18245–18253. [Google Scholar] [CrossRef] [Green Version]
- Jules, J.; Ashley, J.W.; Feng, X. Selective targeting of RANK signaling pathways as new therapeutic strategies for osteoporosis. Expert. Opin. Ther. Tar. 2010, 14, 923–934. [Google Scholar] [CrossRef]
- Cheng, C.; Wentworth, K.; Shoback, D.M. New frontiers in osteoporosis therapy. Annu. Rev. Med. 2020, 71, 277–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, M.Z. Antiresorptive drugs (bisphosphonates), atypical fractures and rebound effect: New evidence of similitude. Homeopathy. 2012, 101, 231–242. [Google Scholar] [CrossRef]
- Phromnoi, K.; Suttajit, M.; Saenjum, C. Polyphenols and rosmarinic acid contents, antioxidant and antiinflammatory activities of different solvent fractions from NgaMon (Perilla frutescens) leaf. J. Pharm. Nutr. Sci. 2019, 9, 239–246. [Google Scholar]
- Pintha, K.; Tantipaiboonwong, P.; Yodkeeree, S.; Chaiwangyen, W.; Chumphukam, O.; Khantamat, O.; Khanaree, C.; Kangwan, N.; Thongchuai, B.; Suttajit, M. Thai perilla (Perilla frutescens) leaf extract inhibits human breast cancer invasion and migration. Maejo Int. J. Sci. Technol. 2018, 12, 112–123. [Google Scholar]
- Khanaree, C.; Pintha, K.; Tantipaiboonwong, P.; Suttajit, M.; Chewonarin, T. The effect of Perilla frutescens leaf on 1, 2-dimethylhydrazine-induced initiation of colon carcinogenesis in rats. J. Food. Biochem. 2018, 42. [Google Scholar] [CrossRef]
- Kangwan, N.; Pintha, K.; Lekawanvijit, S.; Suttajit, M. Rosmarinic Acid enriched fraction from Perilla frutescens leaves strongly protects indomethacin-induced gastric ulcer in rats. Biomed Res. Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Han, T.; Yang, G.; Cao, X.L.; Li, H.Z.; Pei, H.R.; Zhang, Z.J. Preparative and scaled-up separation of high-purity alpha-linolenic acid from perilla seed oil by conventional and pH-zone refining counter current chromatography. J. Sep. Sci. 2019, 42, 2360–2370. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Rahman, M.S.; Kim, A.N.; Son, Y.; Gu, S.; Lee, M.H.; Kim, J.I.; Ha, T.J.; Kwak, D.; Kim, H.J.; et al. Effect of freeze-thaw pretreatment on yield and quality of perilla seed oil. LWT. 2020, 122. [Google Scholar] [CrossRef]
- Siriamornpun, S.; Li, D.; Yang, L.; Suttajit, S.; Suttajit, M. Variation of lipid and fatty acid compositions in Thai Perilla seeds grown at different locations. Songklanakarin J. Sci. Technol. 2006, 28, 17–21. [Google Scholar]
- Suttajit, M.; Khanaree, C.; Tantipaiboonwong, P.; Pintha, K. Omega-3, omega-6 fatty acids and nutrients of Nga-mon seeds in Northern Thailand. Naresuan Phayao J. 2015, 80–86. [Google Scholar]
- Adimcilar, V.; Kalaycioglu, Z.; Aydogdu, N.; Dirmenci, T.; Kahraman, A.; Erim, F.B. Rosmarinic and carnosic acid contents and correlated antioxidant and antidiabetic activities of 14 Salvia species from Anatolia. J. Pharm. Biomed. Anal. 2019, 175, 5. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, B.R.; Kim, J.E.; Bae, S.J.; Choi, Y.J.; Lee, S.J.; Gong, J.E.; Lee, H.S.; Lee, C.Y.; Kim, B.H.; et al. Therapeutic effects of cold-pressed perilla oil mainly consisting of linolenic acid, oleic acid and linoleic acid on UV-induced photoaging in NHDF cells and SKH-1 hairless mice. Molecules. 2020, 25, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chumphukam, O.; Tipsuwan, W.; Khanaree, C.; Pintha, K.; Tantipaiboonwong, P.; Chaiwangyen, W.; Roytrakul, S.; Suttajit, M.; Topanurak, S. Alpha-linolenic acid content and expression of KASII and FAD3 in perilla seed associated with altitude of cultivation areas. Scienceasia. 2019, 45, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Rakariyatham, K.; Wu, X.; Tang, Z.H.; Han, Y.H.; Wang, Q.; Xiao, H. Synergism between luteolin and sulforaphane in anti-inflammation. Food. Funct. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yuan, T.; Yin, N.N.; Ma, X.F.; Zhang, Z.B.; Zhu, Z.; Shaukat, A.; Deng, G.Z. Luteoloside protects the uterus from Staphylococcus aureus-induced inflammation, apoptosis, and injury. Inflammation. 2018, 41, 1702–1716. [Google Scholar] [CrossRef]
- Inoue, K.I.; Takano, H.; Shiga, A.; Fujita, Y.; Makino, H.; Yanagisawa, R.; Ichinose, T.; Kato, Y.; Yamada, T.; Yoshikawa, T. Effects of volatile constituents of a rosemary extract on allergic airway inflammation related to house dust mite allergen in mice. Int. J. Mol. Med. 2005, 16, 315–319. [Google Scholar] [CrossRef]
- Ko, C.H.; Lau, K.M.; Choy, W.Y.; Leung, P.C. Effects of tea catechins, epigallocatechin, gallocatechin, and gallocatechin gallate, on bone metabolism. J. Agric. Food Chem. 2009, 57, 7293–7297. [Google Scholar] [CrossRef]
- Omori, A.; Yoshimura, Y.; Deyama, Y.; Suzuki, K. Rosmarinic acid and arbutin suppress osteoclast differentiation by inhibiting superoxide and NFATc1 downregulation in RAW 264.7 cells. Biomed. Rep. 2015, 3, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Sanbongi, C.; Takano, H.; Osakabe, N.; Sasa, N.; Natsume, M.; Yanagisawa, R.; Inoue, K.; Sadakane, K.; Ichinose, T.; Yoshikawa, T. Rosmarinic acid in perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin. Exp. Allergy 2004, 34, 971–977. [Google Scholar] [CrossRef]
- Takano, H.; Osakabe, N.; Sanbongi, C.; Yanagisawa, R.; Inoue, K.; Yasuda, A.; Natsume, M.; Baba, S.; Ichiishi, E.; Yoshikawa, T. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp. Biol. Med. 2004, 229, 247–254. [Google Scholar] [CrossRef]
- Phromnoi, K.; Sinchaiyakij, P.; Khanaree, C.; Nuntaboon, P.; Chanwikrai, Y.; Chaiwangsri, T.; Suttajit, M. Anti-inflammatory and antioxidant activities of medicinal plants used by traditional healers for antiulcer treatment. Sci. Pharm. 2019, 87. [Google Scholar] [CrossRef] [Green Version]
- Limtrakul, P.; Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Srisomboon, J. Anti-aging and tyrosinase inhibition effects of Cassia fistula flower butanolic extract. BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef] [Green Version]
- Saenjum, C.; Chaiyasut, C.; Kadchumsang, S.; Chansakaow, S.; Suttajit, M. Antioxidant activity and protective effects on DNA damage of Caesalpinia sappan L. extract. J. Med. Plant Res. 2010, 4, 1594–1600. [Google Scholar]
- Ghosh, M.; Kim, I.S.; Lee, Y.M.; Hong, S.M.; Lee, T.H.; Lim, J.H.; Debnath, T.; Lim, B.O. The effects of Aronia melanocarpa ‘viking’ extracts in attenuating RANKL-induced osteoclastic differentiation by inhibiting ROS generation and c-FOS/NFATc1 signaling. Molecules. 2018, 23, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooppachai, C.; Limtrakul, P.; Yodkeeree, S. Dicentrine potentiates TNF-alpha-induced apoptosis and suppresses invasion of A549 lung adenocarcinoma cells via modulation of NF-kappa B and AP-1 activation. Molecules 2019, 24, 4100. [Google Scholar] [CrossRef] [Green Version]
- Banjerdpongchai, R.; Wudtiwai, B.; Khaw-on, P.; Rachakhom, W.; Duangnil, N.; Kongtawelert, P. Hesperidin from Citrus seed induces human hepatocellular carcinoma HepG2 cell apoptosis via both mitochondrial and death receptor pathways. Tumor. Biol. 2016, 37, 227–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koerdt, S.; Siebers, J.; Bloch, W.; Ristow, O.; Kuebler, A.C.; Reuther, T. Role of oxidative and nitrosative stress in autogenous bone grafts to the mandible using guided bone regeneration and a deproteinized bovine bone material. J. Cranio. maxill. Surg. 2014, 42, 560–567. [Google Scholar] [CrossRef]
- Li, H.T.; Huang, C.H.; Zhu, J.; Gao, K.H.; Fang, J.; Li, H.Z. Lutein suppresses oxidative stress and inflammation by Nrf2 activation in an osteoporosis rat model. Med. Sci. Monitor. 2018, 24, 5071–5075. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.K.; Chen, C.; Zhu, X.D.; Li, Y.F.; Yu, R.H.; Xu, W. Glycyrrhizin suppresses RANKL-induced osteoclastogenesis and oxidative stress through inhibiting NF-kappa B and MAPK and activating AMPK/Nrf2. Calcif. Tissue Int. 2018, 103, 324–337. [Google Scholar] [CrossRef]
- Shen, J.; Yao, R.; Jing, M.; Zhou, Z.Y. Sinomenine regulates inflammatory response and oxidative stress via nuclear factor kappa B (NF-kappa B) and NF-E2-related factor 2 (Nrf2) signaling pathways in ankle fractures in children. Med. Sci. Monitor. 2018, 24, 6649–6655. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R.; Kaser, A.; Pines, A.; Dotan, I. Gut, inflammation and osteoporosis: Basic and clinical concepts. Gut. 2008, 57, 684–694. [Google Scholar] [CrossRef]
- Egger, F.; Jakab, M.; Fuchs, J.; Oberascher, K.; Brachtl, G.; Ritter, M.; Kerschbaum, H.H.; Gaisberger, M. Effect of glycine on BV-2 microglial cells treated with interferon-gamma and lipopolysaccharide. Int. J. Mol. Sci. 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.P.; Schaper, F.; Teubner, A.; Lammert, F.; Heinrich, P.C.; Matern, S.; Siewert, E. Interleukin-6 plays a crucial role in the hepatic expression of SOCS3 during acute inflammatory processes in vivo. J. Hepatol. 2005, 43, 704–710. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ethnomedicinal, phytochemical and pharmacological investigations of Perilla frutescens (L.) britt. Molecules 2019, 24, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, T.T.; Zhang, L.R.; Yang, X.H. Ferulic acid, a natural polyphenol, protects against osteoporosis by activating SIRT1 and NF-kappa B in neonatal rats with glucocorticoid-induced osteoporosis. Biomed. Pharmacother. 2019, 120, 15. [Google Scholar] [CrossRef] [PubMed]
- Phumthum, M.; Srithi, K.; Inta, A.; Junsongduang, A.; Tangjitman, K.; Pongamornkul, W.; Trisonthi, C.; Balslev, H. Ethnomedicinal plant diversity in Thailand. J. Ethnopharmacol. 2018, 214, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Rahim, R.A.; Jayusman, P.A.; Muhammad, N.; Ahmad, F.; Mokhtar, N.; Mohamed, I.N.; Mohamed, N.; Shuid, A.N. Recent Advances in nanoencapsulation systems using PLGA of bioactive phenolics for protection against chronic diseases. Int. J. Environ. Res. Public Health. 2019, 16, 17. [Google Scholar]
- Sarmadi, B.; Ismail, A.; Yusof, L.; Yunoh, M.F.M. Mechanism of action of cocoa on bone metabolism in calcium- and estrogen-deficient rat model of osteoporosis: Evidence for site and dose-related responses and involvement of IGF-I. J. Funct. Foods. 2020, 66, 12. [Google Scholar] [CrossRef]
- Luo, C.X.; Zou, L.; Sun, H.J.; Peng, J.Y.; Gao, C.; Bao, L.C.; Ji, R.P.; Jin, Y.; Sun, S.Y. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Tenore, G.C.; Daglia, M.; Tundis, R.; Loizzo, M.R.; Nabavi, S.M. The cellular protective effects of rosmarinic acid: From bench to bedside. Curr. Neurovasc. Res. 2015, 12, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Veras, K.S.; Fachel, F.N.S.; Pittol, V.; Garcia, K.R.; Bassani, V.L.; dos Santos, V.; Henriques, A.T.; Teixeira, H.F.; Koester, L.S. Compatibility study of rosmarinic acid with excipients used in pharmaceutical solid dosage forms using thermal and non-thermal techniques. Saudi. Pharm. J. 2019, 27, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Cheng, C.P.; Chang, D.M. Plectranthus amboinicus attenuates inflammatory bone erosion in mice with collagen-induced arthritis by downregulation of RANKL-induced NFATc1 expression. J. Rheumatol. 2011, 38, 1844–1857. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, H.; Sun, K.; Liu, J.; Mou, Y.; Qi, D.; Zhou, C.; Abudunaibi, M.; Tasiken, B.; Li, J.; et al. Vinpocetine inhibits RANKL-induced osteoclastogenesis and attenuates ovariectomy-induced bone loss. Biomed. Pharmacother. 2020, 123. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.K.; Chang, T.S.; Kang, K.S.; Hwang, G.S. Inhibitory effect of brazilin on osteoclast differentiation and its mechanism of action. Int. Immunopharmacol. 2015, 29, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.Z.; He, L.G.; Wang, S.; Wang, K.; Zhang, Y.Y.; Tao, L.; Li, X.J.; Liu, S.W. Aconine inhibits RANKL-induced osteoclast differentiation in RAW264.7 cells by suppressing NF-kappa B and NFATc1 activation and DC-STAMP expression. Acta Pharmacologica Sinica. 2016, 37, 255–263. [Google Scholar]
- Osawa, K.; Fukushima, H.; Jimi, E. The role of nuclear factor-KB signaling in bone formation: One bite provides dual tastes. J. Oral Biosci. 2015, 57, 14–17. [Google Scholar] [CrossRef]
- Zhu, X.L.; Zeng, Z.Y.; Qiu, D.B.; Chen, J.M. Vγ9Vδ2 T cells inhibit immature dendritic cell transdifferentiation into osteoclasts through downregulation of RANK, c-Fos and ATP6V0D2. Int. J. Mol. Med. 2018, 42, 2071–2079. [Google Scholar] [CrossRef]
- Torre, E. Molecular signaling mechanisms behind polyphenol-induced bone anabolism. Phytochem. Rev. 2017, 16, 1183–1226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wen, H.; Yang, X.B.; Zhang, K.; He, B.R.; Zhang, X.L.; Kong, L.B. Stimuli and relevant signaling cascades for NFATc1 in bone cell homeostasis: Friend or foe? Curr. Stem. Cell. Res. Ther. 2019, 14, 239–243. [Google Scholar] [CrossRef]
- Paiva, K.B.S.; Granjeiro, J.M. Bone tissue remodeling and development: Focus on matrix metalloproteinase functions. Arch. Biochem. Biophys. 2014, 561, 74–87. [Google Scholar] [CrossRef]
- Franco, G.C.N.; Kajiya, M.; Nakanishi, T.; Ohta, K.; Rosalen, P.L.; Groppo, F.C.; Ernst, C.W.O.; Boyesen, J.L.; Bartlett, J.D.; Stashenko, P.; et al. Inhibition of matrix metalloproteinase-9 activity by doxycycline ameliorates RANK ligand-induced osteoclast differentiation in vitro and in vivo. Exp. Cell Res. 2011, 317, 1454–1464. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.H.; Liu, T.T.; Li, J.; Xu, J.; Chen, F.; Hu, L.H.; Zhang, B.L.; Zi, C.T.; Wang, X.J.; Sheng, J. Oxidation derivative of (-)-epigallocatechin-3-gallate (EGCG) inhibits RANKL-induced osteoclastogenesis by suppressing RANK signaling pathways in RAW 264.7 cells. Biomed. Pharmacother. 2019, 118, 9. [Google Scholar] [CrossRef]
- Zhou, L.; Song, H.Y.; Zhang, Y.Q.; Ren, Z.Z.; Li, M.H.; Fu, Q. Polyphyllin VII attenuated RANKL-induced osteoclast differentiation via inhibiting of TRAF6/c-Src/PI3K pathway and ROS production. BMC Musculoskelet. Disord. 2020, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.W.; Jiang, J.W.; Lu, X.Y.; Zhang, T.; Zhao, K.X.; Han, W.Q.; Yang, W.L.; Qian, Y. Garcinol suppresses RANKL-induced osteoclastogenesis and its underlying mechanism. J. Cell. Physiol. 2019, 234, 7498–7509. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, J.K.; Jang, H.D. Genistein inhibits osteoclastic differentiation of RAW 264.7 cells via regulation of ROS production and scavenging. Int. J. Mol. Sci. 2014, 15, 10605–10621. [Google Scholar] [CrossRef] [Green Version]
- Satue, M.; Arriero, M.D.; Monjo, M.; Ramis, J.M. Quercitrin and Taxifolin stimulate osteoblast differentiation in MC3T3-E1 cells and inhibit osteoclastogenesis in RAW 264.7 cells. Biochem. Pharmacol. 2013, 86, 1476–1486. [Google Scholar] [CrossRef]
- Yan, L.L.; Lu, L.L.; Hu, F.B.; Shetti, D.; Wei, K. Piceatannol attenuates RANKL-induced osteoclast differentiation and bone resorption by suppressing MAPK, NE-kappa B and AKT signalling pathways and promotes Caspase3-mediated apoptosis of mature osteoclasts. R. Soc. Open Sci. 2019, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Brondani, J.E.; Comim, F.V.; Flores, L.M.; Martini, L.A.; Premaor, M.O. Fruit and vegetable intake and bones: A systematic review and meta-analysis. PLoS One. 2019, 14, 16. [Google Scholar] [CrossRef]
- Kim, J.M.; Liceaga, A.M.; Yoon, K.Y. Purification and identification of an antioxidant peptide from perilla seed (Perilla frutescens) meal protein hydrolysate. Food. Sci. Nutr. 2019, 7, 1645–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.Y.; Yoon, K.Y. Functional properties of enzymatic hydrolysate and peptide fractions from perilla seed meal protein. Pol. J. Food. Nutr. Sci. 2019, 69, 119–127. [Google Scholar] [CrossRef]
- Chumphukam, O.; Pintha, K.; Khanaree, C.; Chewonarin, T.; Chaiwangyen, W.; Tantipaiboonwong, P.; Suttajit, M.; Khantamat, O. Potential anti-mutagenicity, antioxidant, and anti-inflammatory capacities of the extract from perilla seed meal. J. Food Biochem. 2018, 42. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Gondal, T.A.; Imran, A.; Shahbaz, M.; Amir, R.M.; Sajid, M.W.; Qaisrani, T.B.; Atif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci-Basel. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Chkhikvishvili, I.; Sanikidze, T.; Gogia, N.; McHedlishvili, T.; Enukidze, M.; Machavariani, M.; Vinokur, Y.; Rodov, V. Rosmarinic acid-rich extracts of summer savory (Satureja hortensis L.) protect jurkat T Cells against oxidative stress. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fachel, F.N.S.; Dal Pra, M.; Azambuja, J.H.; Endres, M.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Barschak, A.G.; Teixeira, H.F.; Braganhol, E. Glioprotective effect of chitosan-coated rosmarinic acid nanoemulsions against lipopolysaccharide-induced inflammation and oxidative stress in rat astrocyte primary cultures. Cell. Mol. Neurobiol. 2020, 40, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Khouya, T.; Ramchoun, M.; Amrani, S.; Harnafi, H.; Rouis, M.; Couchie, D.; Simmet, T.; Alem, C. Anti-inflammatory and anticoagulant effects of polyphenol-rich extracts from Thymus atlanticus: An in vitro and in vivo study. J. Ethnopharmacol. 2020, 252, 8. [Google Scholar] [CrossRef]
- Zhu, F.X.; Asada, T.; Sato, A.; Koi, Y.; Nishiwaki, H.; Tamura, H. Rosmarinic acid extract for antioxidant, antiallergic, and alpha-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J. Agric. Food Chem. 2014, 62, 885–892. [Google Scholar] [CrossRef]
- Guo, S.F.; Zhu, W.; Yin, Z.Q.; Xiao, D.; Zhang, Q.; Liu, T.; Ni, J.D.; Ouyang, Z.X.; Xie, H.M. Proanthocyanidins attenuate breast cancer-induced bone metastasis by inhibiting Irf-3/c-jun activation. Anti-Cancer Drugs. 2019, 30, 998–1005. [Google Scholar]
- Kwak, H.B.; Lee, B.K.; Oh, J.; Yeon, J.T.; Choi, S.W.; Cho, H.J.; Lee, M.S.; Kim, J.J.; Bae, J.M.; Kim, S.H.; et al. Inhibition of osteoclast differentiation and bone resorption by rotenone, through down-regulation of RANKL-induced c-Fos and NFATc1 expression. Bone. 2010, 46, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.H.; Rodriguez-Gonzalez, M.; Hernandez, M.; Recinos, C.C.; Seldeen, K.L.; Troen, B.R. AP-1 and Mitf interact with NFATc1 to stimulate cathepsin K promoter activity in osteoclast precursors. J. Cell. Biochem. 2019, 120, 12382–12392. [Google Scholar] [CrossRef] [PubMed]
- Goettsch, C.; Babelova, A.; Trummer, O.; Erben, R.G.; Rauner, M.; Rammelt, S.; Weissmann, N.; Weinberger, V.; Benkhoff, S.; Kampschulte, M.; et al. NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J. Clin. Investig. 2013, 123, 4731–4738. [Google Scholar] [CrossRef] [Green Version]



| Fractions and Standards | Radical Scavenging Assay IC50 (µg/mL) | Reducing Power Assay mg Fe/g Fraction | |||
|---|---|---|---|---|---|
| DPPH• | ABTS•+ | O2•− | NO | FRAP | |
| SME | 76.6 ± 5.1 b | 20.7 ± 0.4 c | 28.7 ± 1.39 c | 40.6 ± 1.32 c | 1058.2 ± 110.3 d |
| SMHF | >200 d | 86.1 ± 5.9 e | 81.9 ± 3.25 d | 95.9 ± 3.04 e | 240.6 ± 15.0 e |
| SMDF | 162.9 ± 11.7 c | 16.2 ± 0.2 b | 92.8 ± 3.37 e | 100.1 ± 3.20 f | 460.4 ± 17.1 e |
| SMEF | 9.2 ± 0.7 a | 3.6 ± 0.1 a | 19.5 ± 1.13 b | 26.6 ± 0.87 b | 8627.3 ± 351.7 b |
| SMWF | 25.6 ± 1.4 a | 30.6 ± 0.5 d | 32.0 ± 1.79 c | 43.9 ± 1.24 d | 792.1 ± 31.7 d |
| L-ascorbic acid | 13.56 ± 1.29 b | 2.06 ± 0.03 a | 13.1 ± 0.47 b | - | 7948.6 ± 217.0 c |
| RA | - | - | 6.9 ± 0.26 a | 17.7 ± 1.02 a | 20,572.7 ± 181.9 a |
| Fractions and Standards | IC50 (µg/mL) | ||
|---|---|---|---|
| NO | iNOS | COX-2 | |
| SME | 28.1 ± 1.6 d | 34.4 ± 1.5 d | 39.4 ± 1.7 d |
| SMHF | 43.0 ± 1.6 e | 38.3 ± 0.6 e | 42.6 ± 0.1 d |
| SMDF | >50 | >50 | >50 |
| SMEF | 21.2 ± 1.4 c | 24.2 ± 2.7 c | 26.9 ± 2.4 c |
| SMWF | 29.7 ± 2.0 d | 42.2 ± 7.6 f | 49.6 ± 7.8 e |
| RA | 13.2 ± 0.6 b | 17.5 ± 2.3 b | 21.3 ± 2.8 b |
| Curcumin | 7.5 ± 0.7 a | 8.9 ± 5.6 a | 9.4 ± 5.7 a |
| Fractions | TPC | TFC | RA |
|---|---|---|---|
| mg GAE/g Fraction | mg CAE/g Fraction | mg/g Fraction | |
| SME | 64.6 ± 1.3 b | 68.3 ± 5.8 b | 23.7 ± 1.1 c |
| SMHF | 18.3 ± 3.7 a | 7.3 ± 0.7 a | 2.9 ± 0.5 a |
| SMDF | 67.4 ± 8.9 b | 18.4 ± 7.1a | 1.4 ± 0.3 a |
| SMEF | 291.4 ± 6.0 c | 493.4 ± 15.9 c | 243.8 ± 3.2 d |
| SMWF | 57.4 ± 2.9 b | 57.3 ± 4.6 b | 11.4 ± 0.8 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phromnoi, K.; Suttajit, M.; Saenjum, C.; Limtrakul, P. Inhibitory Effect of a Rosmarinic Acid-Enriched Fraction Prepared from Nga-Mon (Perilla frutescens) Seed Meal on Osteoclastogenesis through the RANK Signaling Pathway. Antioxidants 2021, 10, 307. https://doi.org/10.3390/antiox10020307
Phromnoi K, Suttajit M, Saenjum C, Limtrakul P. Inhibitory Effect of a Rosmarinic Acid-Enriched Fraction Prepared from Nga-Mon (Perilla frutescens) Seed Meal on Osteoclastogenesis through the RANK Signaling Pathway. Antioxidants. 2021; 10(2):307. https://doi.org/10.3390/antiox10020307
Chicago/Turabian StylePhromnoi, Kanokkarn, Maitree Suttajit, Chalermpong Saenjum, and Pornngarm Limtrakul (Dejkriengkraikul). 2021. "Inhibitory Effect of a Rosmarinic Acid-Enriched Fraction Prepared from Nga-Mon (Perilla frutescens) Seed Meal on Osteoclastogenesis through the RANK Signaling Pathway" Antioxidants 10, no. 2: 307. https://doi.org/10.3390/antiox10020307
APA StylePhromnoi, K., Suttajit, M., Saenjum, C., & Limtrakul, P. (2021). Inhibitory Effect of a Rosmarinic Acid-Enriched Fraction Prepared from Nga-Mon (Perilla frutescens) Seed Meal on Osteoclastogenesis through the RANK Signaling Pathway. Antioxidants, 10(2), 307. https://doi.org/10.3390/antiox10020307