Exacerbation of AMD Phenotype in Lasered CNV Murine Model by Dysbiotic Oral Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Biofilm Culture
2.2. Animals
2.3. Laser-Induced Choroidal Neovascularization (Li-CNV) AMD Model
2.4. Ligature-Enhanced Periodontitis (PD) in Pre-Existing CNV (AMD) Model
2.5. Fundus-Imaging Analysis
2.6. Optical Coherence Tomography (OCT)Analysis
2.7. Fundus Fluorescein Angiography (FFA) Analysis
2.8. Analysis of Retinal Thickness
2.9. Immunofluorescence and Confocal-Imaging Analysis
2.10. Quantitative PCR Assays
2.11. Statistical Analysis
3. Results
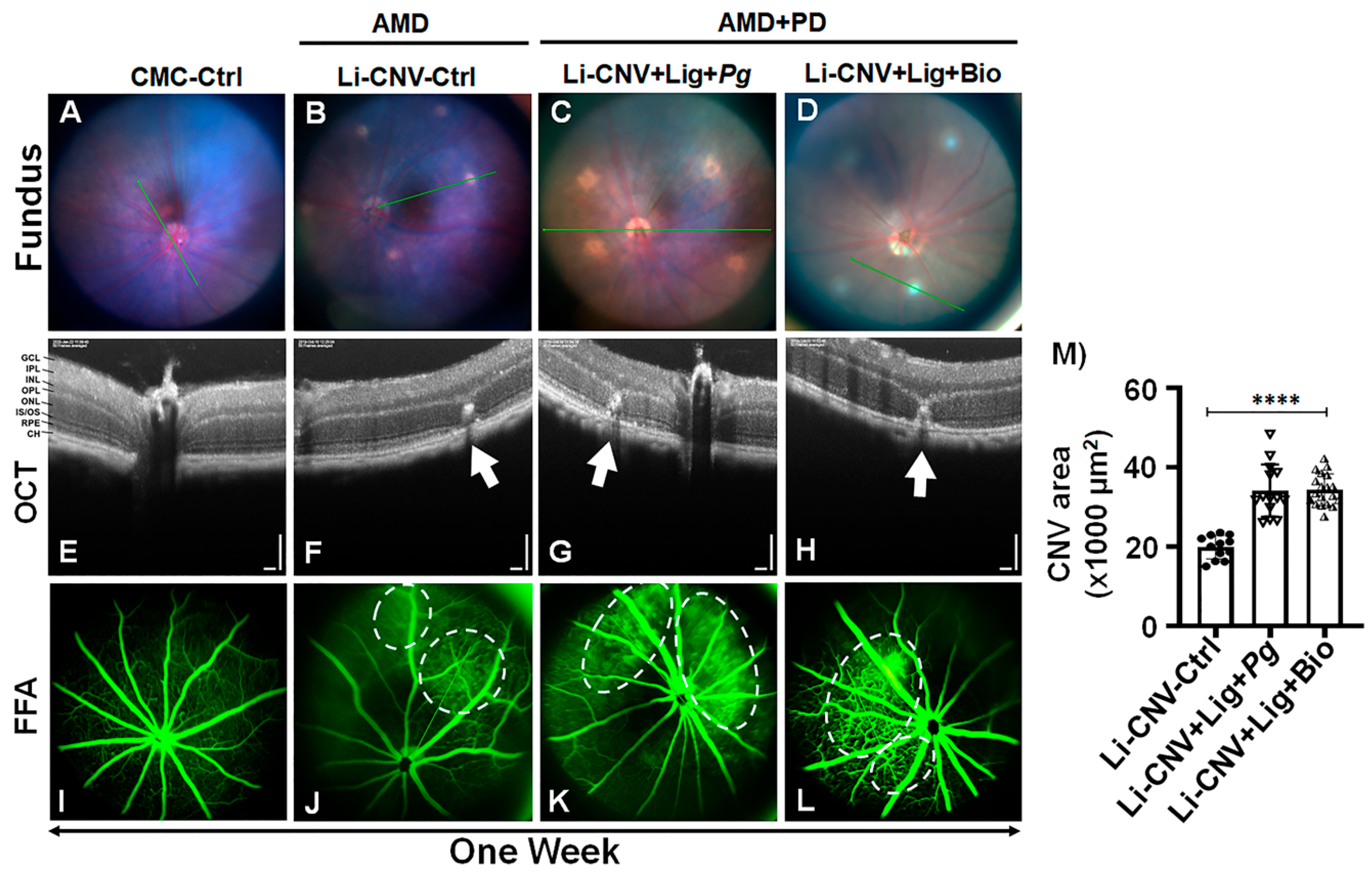
3.1. Establishment of Experimentally Induced AMD+PD Murine Model
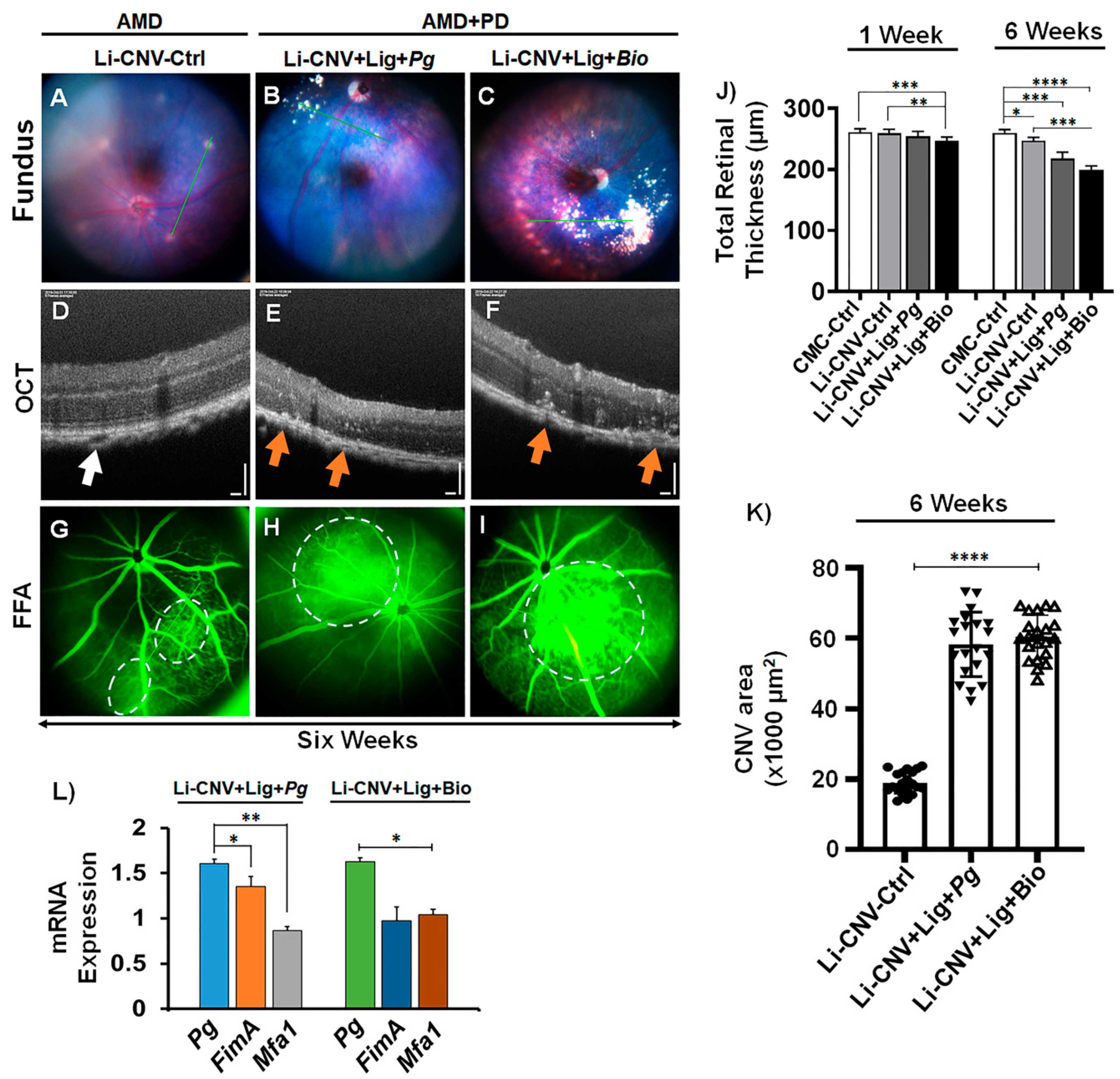
3.2. Increased Vascular Leakage and CNV Area in AMD+PD Mice Retinae.
3.3. Exacerbation of Pathological Angiogenesis and Inflammation in AMD+PD Mice Retinae
3.4. Pg 16S-rRNA Gene Expression in the Retinae of AMD+PD Murine Model
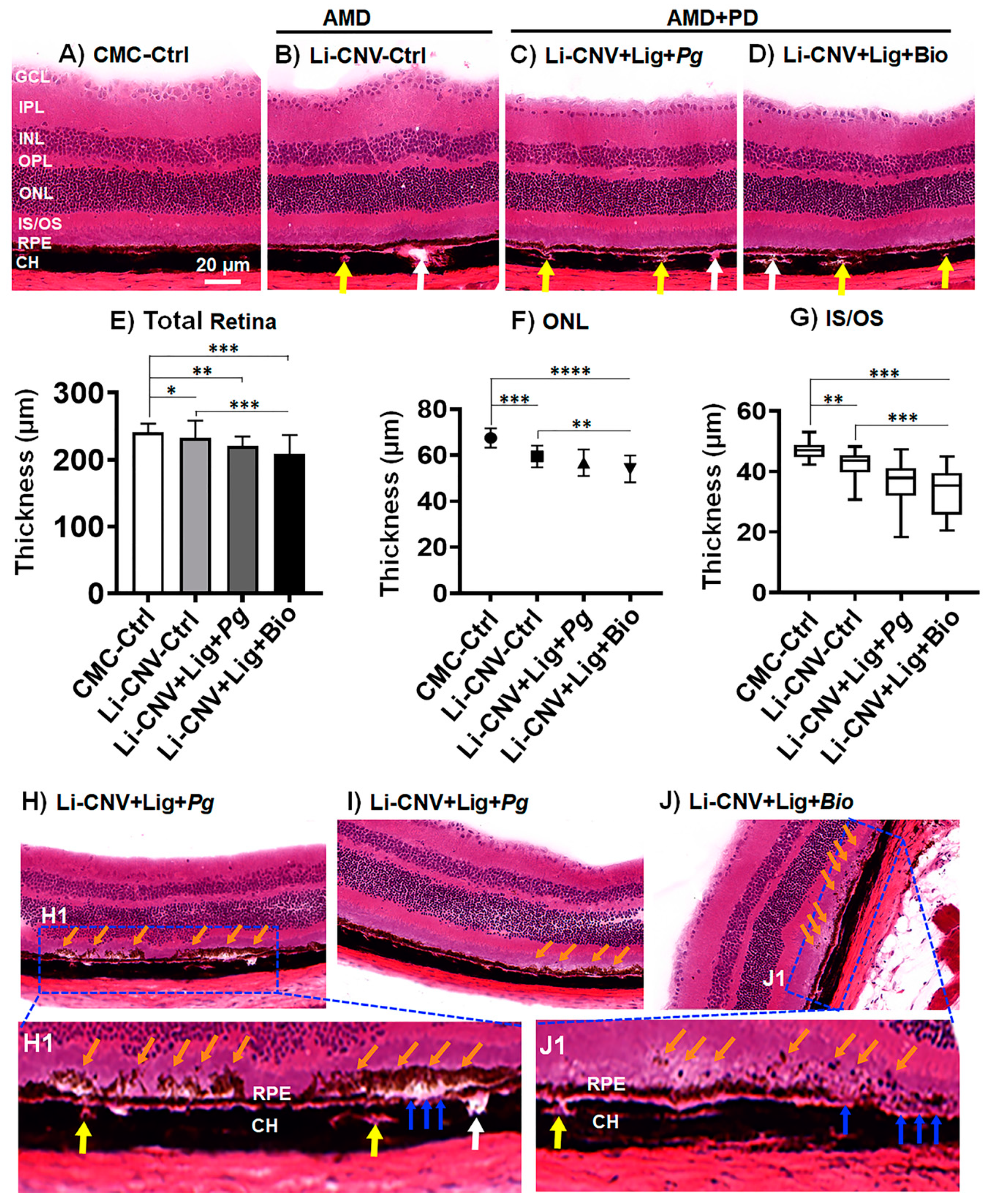
3.5. Reduced Retinal Thickness in AMD+PD Mice
3.6. Subretinal Drusen-like Deposits in AMD+PD Mice
3.7. Increased Choroidal/Retinal Neovasculogenesis in the Retinae of AMD+PD Mice
3.8. Increased Angiogenesis, Pro-Inflammatory and Decreased Anti-Inflammatory Mediators in AMD+PD Mice Retinae
3.9. Up-Regulated Expression of Oxidative-Stress Genes and Down-Regulated Antioxidant Genes in AMD+PD Mice Retinae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, D.S.; O’Colmain, B.J.; Muñoz, B.; Tomany, S.C.; Mccarty, C.; De Jong, P.T.V.M.; Nemesure, B.; Mitchell, P.; Kempen, J. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Pennington, K.L.; DeAngelis, M.M. Epidemiology of age-related macular degeneration (AMD): Associations with cardiovascular disease phenotypes and lipid factors. Eye Vis. 2016, 3, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, W.L.; Su, X.; Li, B.X.; Cheung, C.M.G.; Klein, B.E.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Javed, F.; Sculean, A.; Romanos, G.E. Association between age-related macular degeneration and periodontal and peri-implant diseases: A systematic review. Acta Ophthalmol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the epidemiology of age-related macular degeneration. Asia-Pac. J. Ophthalmol. 2017, 6, 493–497. [Google Scholar] [CrossRef]
- NEI. Age-Related Macular Degeneration (AMD) Data and Statistics. Available online: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/eye-health-data-and-statistics/age-related-macular-degeneration-amd-data-and-statistics (accessed on 19 December 2020).
- Brown, M.M.; Brown, G.C.; Lieske, H.B.; Tran, I.; Turpcu, A.; Colman, S. Societal costs associated with neovascular age-related macular degeneration in the United States. Retina 2016, 36, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Zimbrón, L.F.; Zamora-Alvarado, R.; La Paz, L.O.-D.; Velez-Montoya, R.; Zenteno, E.; Gulias-Cañizo, R.; Quiroz-Mercado, H.; Gonzalez-Salinas, R. Age-related macular degeneration: New paradigms for treatment and management of AMD. Oxidative Med. Cell. Longev. 2018, 8374647. [Google Scholar] [CrossRef]
- Lane, J.; Rohan, E.M.F.; Sabeti, F.; Essex, R.W.; Maddess, T.; Dawel, A.; Robbins, R.A.; Barnes, N.; He, X.; McKone, E. Impacts of impaired face perception on social interactions and quality of life in age-related macular degeneration: A qualitative study and new community resources. PLoS ONE 2018, 13, e0209218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; Hollander, A.I.D. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.R. Risk factors for age-related macular degeneration. Prog. Retin. Eye Res. 2001, 20, 227–253. [Google Scholar] [CrossRef]
- Cascella, R.; Ragazzo, M.; Strafella, C.; Missiroli, F.; Borgiani, P.; Angelucci, F.; Marsella, L.T.; Cusumano, A.; Novelli, G.; Ricci, F.; et al. Age-related macular degeneration: Insights into inflammatory genes. J. Ophthalmol. 2014, 582842. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Forrester, J.V.; Xu, H. Dysregulation in retinal para-inflammation and age-related retinal degeneration in CCL2 or CCR2 deficient mice. PLoS ONE 2011, 6, e22818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef]
- Hageman, G.S. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001, 20, 705–732. [Google Scholar] [CrossRef]
- Hoare, A.; Soto, C.; Rojas-Celis, V.; Bravo, D. Chronic inflammation as a link between periodontitis and carcinogenesis. Mediat. Inflamm. 2019, 2019, 1029857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjunan, P.; Swaminathan, R.; Yuan, J.; Al-Shabrawey, M.; Espinosa-Heidmann, D.G.; Nussbaum, J.; Martin, P.M.; Cutler, C.W. Invasion of human retinal pigment epithelial cells by Porphyromonas gingivalis leading to vacuolar/cytosolic localization and autophagy dysfunction in-vitro. Sci. Rep. 2020, 10, 7468. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.X.P.; Cheung, C.M.G.; Sim, S.; Chu, C.W.; Wilm, A.; Lin, C.B.; Mathur, R.; Wong, D.; Chan, C.M.; Bhagarva, M.; et al. Human pharyngeal microbiota in age-related macular degeneration. PLoS ONE 2018, 13, e0201768. [Google Scholar] [CrossRef] [Green Version]
- Zinkernagel, M.S.; Zysset-Burri, D.C.; Keller, I.; Berger, L.E.; Leichtle, A.B.; Largiadèr, C.R.; Fiedler, G.M.; Wolf, S. Association of the intestinal microbiome with the development of neovascular age-related macular degeneration. Sci. Rep. 2017, 7, 40826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowan, S.; Taylor, A. Gut microbiota modify risk for dietary glycemia-induced age-related macular degeneration. Gut Microbes 2018, 9, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Hu, X.; Miao, L.; Ge, X.; Deng, Y.; Bible, P.W.; Wei, L. Epigenetics, microbiota, and intraocular inflammation: New paradigms of immune regulation in the eye. Prog. Retin. Eye Res. 2018, 64, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Nakata, I.; Nagahama Study Group; Yamashiro, K.; Kawaguchi, T.; Nakanishi, H.; Akagi-Kurashige, Y.; Miyake, M.; Tsujikawa, A.; Yamada, R.; Matsuda, F.; et al. Calcium, ARMS2 genotype and Chlamydia pneumoniae infection in early age-related macular degeneration: A multivariate analysis from the Nagahama Study. Sci. Rep. 2015, 5, 9345. [Google Scholar] [CrossRef]
- Miller, D.M.; Legra, J.M.; Dubovy, S.R.; Suner, I.J.; Dix, R.D.; Sedmak, D.D.; Cousins, S.W. The association of exudative age-related macular degeneration with prior infection. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1747. [Google Scholar]
- Karesvuo, P.; Gursoy, U.K.; Pussinen, P.J.; Suominen, A.L.; Huumonen, S.; Vesti, E.; Könönen, E. Alveolar bone loss associated with age-related macular degeneration in males. J. Periodontol. 2013, 84, 58–67. [Google Scholar] [CrossRef]
- Arjunan, P. Eye on the enigmatic link: Dysbiotic oral pathogens in ocular diseases: The flip side. Int. Rev. Immunol. 2020, 1–24. [Google Scholar] [CrossRef]
- Wagley, S.; Marra, K.V.; Salhi, R.A.; Gautam, S.; Campo, R.; Veale, P.; Veale, J.; Arroyo, J.G. Periodontal disease and age-related macular degeneratioN. Retina 2015, 35, 982–988. [Google Scholar] [CrossRef]
- Shin, Y.U.; Lim, H.W.; Hong, E.H.; Kang, M.H.; Seong, M.; Nam, E.; Cho, H. The association between periodontal disease and age-related macular degeneration in the Korea National health and nutrition examination survey. Medicine 2017, 96, e6418. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.-T.; Hsia, N.-Y.; Chen, S.-C.; Lin, C.-L.; Chen, I.-A.; Wu, I.-T.; Palanisamy, K.; Shen, T.-C.; Li, C.-Y. Risk of age-related macular degeneration in patients with periodontitis. Retina 2020, 40, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, A.; Puchalska-Niedbal, L. Stan jamy ustnej pacjentow chorych na zwyrodnienie plamki zwiazane z wiekiem (AMD)—Doniesienie wstepne [Oral status as a potential source of infection in AMD patients—Introduction]. Klin. Oczna 2012, 114, 29–32. [Google Scholar]
- Pockpa, Z.; Struillou, X.; Coulibaly, N.; Weber, M.; Soueidan, A.; Badran, Z. Potential relationship between periodontal diseases and eye diseases. Med. Hypotheses 2017, 99, 63–66. [Google Scholar] [CrossRef]
- Pockpa, Z.A.D.; Struillou, X.; Kone, D.; Mobio, G.S.; Soueidan, A.; Badran, Z. Periodontal diseases and age-related macular degeneration: Is there a link? A review. Perm. J. 2019, 23. [Google Scholar] [CrossRef]
- Eke, P.I.; Borgnakke, W.S.; Genco, R.J. Recent epidemiologic trends in periodontitis in the USA. Periodontology 2000 2020, 82, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Winning, L.; Linden, G.J. Periodontitis and systemic disease: Association or causality? Curr. Oral Health Rep. 2017, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, C.-J.; Chang, M.-L.; Taylor, A. Associations between periodontal microbiota and death rates. Sci. Rep. 2016, 6, 35428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjunan, P.; El-Awady, A.; Dannebaum, R.; Kunde-Ramamoorthy, G.; Cutler, C. High-throughput sequencing reveals key genes and immune homeostatic pathways activated in myeloid dendritic cells by Porphyromonas gingivalis 381 and its fimbrial mutants. Mol. Oral Microbiol. 2015, 31, 78–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjunan, P.; Meghil, M.M.; Pi, W.; Xu, J.; Lang, L.; El-Awady, A.; Sullivan, W.; Rajendran, M.; Rabelo, M.S.; Wang, T.; et al. Oral pathobiont activates anti-apoptotic pathway, promoting both immune suppression and oncogenic cell proliferation. Sci. Rep. 2018, 8, 16607. [Google Scholar] [CrossRef] [Green Version]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Eke, P.I.; Wei, L.; Borgnakke, W.S.; Thornton-Evans, G.; Zhang, X.; Lu, H.; McGuire, L.C.; Genco, R.J. Periodontitis prevalence in adults ≥ 65 years of age, in the USA. Periodontology 2000 2016, 72, 76–95. [Google Scholar] [CrossRef]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on prevalence of periodontitis in adults in the united states: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Du, Z.; Wu, X.; Song, M.; Li, P.; Wang, L. Oxidative damage induces MCP-1 secretion and macrophage aggregation in age-related macular degeneration (AMD). Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 2469–2476. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000 2007, 43, 160–232. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, Y.; Wang, J.; Sternberg, P.; Freeman, M.L.; Grossniklaus, H.E.; Cai, J. Age-related retinopathy in NRF2-deficient mice. PLoS ONE 2011, 6, e19456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjunan, P.; Lin, X.; Tang, Z.; Du, Y.; Kumar, A.; Liu, L.; Yin, X.; Huang, L.; Chen, W.; Chen, Q.; et al. VEGF-B is a potent antioxidant. Proc. Natl. Acad. Sci. USA 2018, 115, 10351–10356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, S.; Lal, N. Antioxidant enzymes in periodontitis. J. Oral Biol. Craniofacial Res. 2017, 7, 54–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapple, I.L.C.; Brock, G.; Eftimiadi, C.; Matthews, J.B. Glutathione in gingival crevicular fluid and its relation to local antioxidant capacity in periodontal health and disease. Mol. Pathol. 2002, 55, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontology 2000 2013, 62, 59–94. [Google Scholar] [CrossRef]
- Lamont, R.J.; Hajishengallis, G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 2015, 21, 172–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajishengallis, G. Porphyromonas gingivalis-host interactions: Open war or intelligent guerilla tactics? Microbes Infect. 2009, 11, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, M.; Ohara, N. Molecular mechanisms of Porphyromonas gingivalis-host cell interaction on periodontal diseases. Jpn. Dent. Sci. Rev. 2017, 53, 134–140. [Google Scholar] [CrossRef]
- Kimura, S.; Nagai, A.; Onitsuka, T.; Koga, T.; Fujiwara, T.; Kaya, H.; Hamada, S. Induction of experimental periodontitis in mice with Porphyromonas gingivalis—Adhered ligatures. J. Periodontol. 2000, 71, 1167–1173. [Google Scholar] [CrossRef]
- Tikoo, P.; Bali, D.; Changela, R.; Gugnani, S.; Pandit, N. Porphyromonas gingivalis: Its virulence and vaccine. J. Int. Clin. Dent. Res. Organ. 2015, 7, 51. [Google Scholar] [CrossRef]
- Takii, R.; Kadowaki, T.; Baba, A.; Tsukuba, T.; Yamamoto, K. A functional virulence complex composed of gingipains, adhesins, and lipopolysaccharide shows high affinity to host cells and matrix proteins and escapes recognition by host immune systems. Infect. Immun. 2005, 73, 883–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Tribble, G.D.; Lamont, R.J. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontology 2000 2009, 52, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, H.; Lorand, L.; Duncan, M.J. Transglutaminase 2 is essential for adherence of Porphyromonas gingivalis to host cells. Proc. Natl. Acad. Sci. USA 2014, 111, 5355–5360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, M.J.; Nakao, S.; Skobe, Z.; Xie, H. Interactions of Porphyromonas gingivalis with epithelial cells. Infect. Immun. 1993, 61, 2260–2265. [Google Scholar] [CrossRef] [Green Version]
- Ezzo, P.J.; Cutler, C.W. Microorganisms as risk indicators for periodontal disease. Periodontology 2000 2003, 32, 24–35. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Wang, M.; Liang, S.; Triantafilou, M.; Triantafilou, K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc. Natl. Acad. Sci. USA 2008, 105, 13532–13537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Wu, J.; Lin, L.; Huang, Y.; Chen, Q.; Ji, Y. Porphyromonas gingivalis stimulates the release of nitric oxide by inducing expression of inducible nitric oxide synthases and inhibiting endothelial nitric oxide synthases. J. Periodontal Res. 2010, 45, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Moser, B.; Roth-Walter, F.; Giacona, M.B.; Harja, E.; Papapanou, P.N.; Schmidt, A.M.; Lalla, E. Infection with a periodontal pathogen increases mononuclear cell adhesion to human aortic endothelial cells. Atherosclerosis 2007, 190, 271–281. [Google Scholar] [CrossRef]
- Xie, M.; Tang, Q.; Yu, S.; Sun, J.; Mei, F.; Zhao, J.; Chen, L. Porphyromonas gingivalis disrupts vascular endothelial homeostasis in a TLR-NF-κB axis dependent manner. Int. J. Oral Sci. 2020, 12, 28. [Google Scholar] [CrossRef]
- Farrugia, C.; Stafford, G.; Murdoch, C. Porphyromonas gingivalis outer membrane vesicles increase vascular permeability. J. Dent. Res. 2020, 99, 1494–1501. [Google Scholar] [CrossRef]
- Hajishengallis, G. Oral bacteria and leaky endothelial junctions in remote extraoral sites. FEBS J. 2020. [Google Scholar] [CrossRef]
- Mougeot, J.-L.C.; Stevens, C.B.; Paster, B.J.; Brennan, M.T.; Lockhart, P.B.; Mougeot, F.K.B. Porphyromonas gingivalis is the most abundant species detected in coronary and femoral arteries. J. Oral Microbiol. 2017, 9, 1281562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronert, M.A.; Hofheinz, H.; Manassa, E.; Asgarouladi, H.; Olbrisch, R.R. The beginning of a new era in tissue expansion: Self-filling osmotic tissue expander—Four-year clinical experience. Plast. Reconstr. Surg. 2004, 114, 1025–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutler, C.W.; Kalmar, J.R.; Arnold, R.R. Phagocytosis of virulent Porphyromonas gingivalis by human polymorphonuclear leukocytes requires specific immunoglobulin G. Infect. Immun. 1991, 59, 2097–2104. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Tang, Z.; Hou, X.; Lennartsson, J.; Li, Y.; Koch, A.W.; Scotney, P.; Lee, C.; Arjunan, P.; Dong, L.; et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 6152–6157. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 2013, 394, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Elashiry, M.; Elashiry, M.M.; Elsayed, R.; Rajendran, M.; Auersvald, C.; Zeitoun, R.; Rashid, M.H.; Ara, R.; Meghil, M.M.; Liu, Y.; et al. Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo. J. Extracell. Vesicles 2020, 9, 1795362. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Mander, S.; Hussein, K.A.; Elsherbiny, N.M.; Smith, S.B.; Al-Shabrawey, M.; Tawfik, A. Hyperhomocysteinemia disrupts retinal pigment epithelial structure and function with features of age-related macular degeneration. Oncotarget 2016, 7, 8532–8545. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Puleo, D.A. Animal models for periodontal disease. J. Biomed. Biotechnol. 2011, 754857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal models of age related macular degeneration. Mol. Asp. Med. 2012, 33, 487–509. [Google Scholar] [CrossRef] [Green Version]
- Ibbett, P.; Goverdhan, S.V.; Pipi, E.; Chouhan, J.K.; Keeling, E.; Angus, E.M.; Scott, J.A.; Gatherer, M.; Page, A.; Teeling, J.L.; et al. A lasered mouse model of retinal degeneration displays progressive outer retinal pathology providing insights into early geographic atrophy. Sci. Rep. 2019, 9, 7475. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Kumar, A.; Lee, C.; Wang, B.; Arjunan, P.; Dong, L.; Maminishkis, A.; Tang, Z.; Li, Y.; Zhang, F.; et al. PDGF-CC blockade inhibits pathological angiogenesis by acting on multiple cellular and molecular targets. Proc. Natl. Acad. Sci. USA 2010, 107, 12216–12221. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Hou, X.; Lee, C.; Li, Y.; Maminishkis, A.; Tang, Z.; Zhang, F.; Langer, H.F.; Arjunan, P.; Dong, L.; et al. Platelet-derived growth factor-DD targeting arrests pathological angiogenesis by modulating glycogen synthase kinase-3β phosphorylation. J. Biol. Chem. 2010, 285, 15500–15510. [Google Scholar] [CrossRef] [Green Version]
- Nahavandipour, A.; Nielsen, M.K.; Sørensen, T.L.; Subhi, Y. Systemic levels of interleukin-6 in patients with age-related macular degeneration: A systematic review and meta-analysis. Acta Ophthalmol. 2020, 98, 434–444. [Google Scholar] [CrossRef]
- Tawfig, N. Proinflammatory cytokines and periodontal disease. J. Dent. Probl. Solut. 2016, 3, 012–017. [Google Scholar] [CrossRef] [Green Version]
- Jing, G.; Wang, J.J.; Zhang, S.X. ER stress and apoptosis: A new mechanism for retinal cell death. Exp. Diabetes Res. 2012, 589589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Wei, Y.; Zhang, T.; Zhang, Z.; Qiu, S.; Zhou, X.; Zhang, S. Effects of GSK2606414 on cell proliferation and endoplasmic reticulum stress-associated gene expression in retinal pigment epithelial cells. Mol. Med. Rep. 2017, 15, 3105–3110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, T.M.; Shinde, V.M.; Starr, C.R.; Kruglov, A.A.; Boitet, E.; Kotla, P.; Zolotukhin, S.; Gross, A.K.; Gorbatyuk, M.S. An activated unfolded protein response promotes retinal degeneration and triggers an inflammatory response in the mouse retina. Cell Death Dis. 2014, 5, e1578. [Google Scholar] [CrossRef] [Green Version]
- Gorbatyuk, M.; Gorbatyuk, O. Review: Retinal degeneration: Focus on the unfolded protein response. Mol. Vis. 2013, 19, 1985. [Google Scholar]
- Bellezza, I. Oxidative stress in age-related macular degeneration: Nrf2 as therapeutic target. Front. Pharmacol. 2018, 9, 1280. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.; Al Saigh, M.; McCulloch, C.; Glogauer, M. The role of NrF2 in the regulation of periodontal health and disease. J. Dent. Res. 2017, 96, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Hyttinen, J.M.T.; Toropainen, E.; Kaarniranta, K. Endoplasmic reticulum stress in age-related macular degeneration: Trigger for neovascularization. Mol. Med. 2010, 16, 535–542. [Google Scholar] [CrossRef]
- Samanta, A.; Aziz, A.A.; Jhingan, M.; Singh, S.R.; Khanani, A.M.; Chhablani, J. Emerging therapies in neovascular age-related macular degeneration in 2020. Asia-Pac. J. Ophthalmol. 2020, 9, 250–259. [Google Scholar] [CrossRef]
- Ryan, S.J. The development of an experimental model of subretinal neovascularization in disciform macular degeneration. Trans. Am. Ophthalmol. Soc. 1979, 77, 707–745. [Google Scholar]
- Dobi, E.T.; Puliafito, C.A.; Destro, M. A new model of experimental choroidal neovascularization in the rat. Arch. Ophthalmol. 1989, 107, 264–269. [Google Scholar] [CrossRef]
- Tobe, T.; Ortega, S.; Luna, J.D.; Ozaki, H.; Okamoto, N.; Derevjanik, N.L.; Vinores, S.A.; Basilico, C.; Campochiaro, P.A. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am. J. Pathol. 1998, 153, 1641–1646. [Google Scholar] [CrossRef] [Green Version]
- Miller, H.; Miller, B.; Ishibashi, T.; Ryan, S.J. Pathogenesis of laser-induced choroidal subretinal neovascularization. Investig. Ophthalmol. Vis. Sci. 1990, 31, 899–908. [Google Scholar]
- Miller, J.W.; Adamis, A.P.; Shima, D.T.; D’Amore, P.A.; Moulton, R.S.; O’Reilly, M.S.; Folkman, J.; Dvorak, H.F.; Brown, L.F.; Berse, B.; et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am. J. Pathol. 1994, 145, 574–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graves, D.T.; Fine, D.; Teng, Y.-T.A.; Van Dyke, T.E.; Hajishengallis, G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J. Clin. Periodontol. 2008, 35, 89–105. [Google Scholar] [CrossRef] [Green Version]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [Green Version]
- Dioguardi, M.; Crincoli, V.; Laino, L.; Alovisi, M.; Sovereto, D.; Mastrangelo, F.; Russo, L.L.; Muzio, L.L. The role of periodontitis and periodontal bacteria in the onset and progression of Alzheimer’s disease: A systematic review. J. Clin. Med. 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, I.; Singhrao, S.K. Is there a link between genetic defects in the complement cascade and Porphyromonas gingivalis in Alzheimer’s disease? J. Oral Microbiol. 2020, 12, 1676486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhrao, S.K.; Olsen, I. Are Porphyromonas gingivalis outer membrane vesicles microbullets for sporadic Alzheimer’s disease manifestation? J. Alzheimer’s Dis. Rep. 2018, 2, 219–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhrao, S.K.; Olsen, I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J. Oral Microbiol. 2019, 11, 1563405. [Google Scholar] [CrossRef] [Green Version]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimer’s Dis. 2013, 36, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Han, X.; Wittchen, E.S.; Hartnett, M.E. TNF-α mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent β-catenin activation. Mol. Vis. 2016, 22, 116–128. [Google Scholar]
- Shi, X.; Semkova, I.; Müther, P.S.; Dell, S.; Kociok, N.; Joussen, A.M. Inhibition of TNF-α reduces laser-induced choroidal neovascularization. Exp. Eye Res. 2006, 83, 1325–1334. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, D.; He, S.; Spee, C.; Ryan, S.J.; Hinton, D.R. Transcriptional regulation of bone morphogenetic protein 4 by tumor necrosis factor and its relationship with age-related macular degeneration. FASEB J. 2011, 25, 2221–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ma, W.; Han, S.; Meng, Z.; Zhaoyang, M.; Yin, Y.; Wang, Y.; Yanling, W. TGF-β participates choroid neovascularization through Smad2/3-VEGF/TNF-α signaling in mice with Laser-induced wet age-related macular degeneration. Sci. Rep. 2017, 7, 9672. [Google Scholar] [CrossRef] [Green Version]
- Kohno, T.; Mizukami, H.; Suzuki, M.; Saga, Y.; Takei, Y.; Shimpo, M.; Matsushita, T.; Okada, T.; Hanazono, Y.; Kume, A.; et al. Interleukin-10-mediated inhibition of angiogenesis and tumor growth in mice bearing VEGF-producing ovarian cancer. Cancer Res. 2003, 63, 5091–5094. [Google Scholar]
- Lopez, P.F.; Sippy, B.D.; Lambert, H.M.; Thach, A.B.; Hinton, D.R. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1996, 37, 855–868. [Google Scholar]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The role of inflammation in age-related macular degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef]
- Izumi-Nagai, K.; Nagai, N.; Ozawa, Y.; Mihara, M.; Ohsugi, Y.; Kurihara, T.; Koto, T.; Satofuka, S.; Inoue, M.; Tsubota, K.; et al. Interleukin-6 Receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am. J. Pathol. 2007, 170, 2149–2158. [Google Scholar] [CrossRef] [Green Version]
- Andriessen, E.M.; Wilson, A.M.; Mawambo, G.; Dejda, A.; Miloudi, K.; Sennlaub, F.; Sapieha, P. Gut microbiota influences pathological angiogenesis in obesity-driven choroidal neovascularization. EMBO Mol. Med. 2016, 8, 1366–1379. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Huang, W.-C.; Pang, J.-H.S.; Wu, Y.-H.; Cheng, C.-Y. Quercetin inhibits the production of IL-1β-induced inflammatory cytokines and chemokines in ARPE-19 cells via the MAPK and NF-κB signaling pathways. Int. J. Mol. Sci. 2019, 20, 2957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vladau, M.; Cimpean, A.M.; Balica, R.A.; Jitariu, A.A.; Popovici, R.A.; Raica, M. VEGF/VEGFR2 axis in periodontal disease progression and angiogenesis: Basic approach for a new therapeutic strategy. In Vivo 2015, 30, 53–60. [Google Scholar]
- Chauhan, S.K.; Saban, D.R.; Dohlman, T.H.; Dana, R. CCL-21 conditioned regulatory T cells induce allotolerance through enhanced homing to lymphoid tissue. J. Immunol. 2014, 192, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Shapira, L.; Borinski, R.; Sela, M.N.; Soskolne, A. Superoxide formation and chemiluminescence of peripheral polymorphonuclear leukocytes in rapidly progressive periodontitis patients. J. Clin. Periodontol. 1991, 18, 44–48. [Google Scholar] [CrossRef]
- Liu, C.; Mo, L.; Niu, Y.; Li, X.; Zhou, X.; Xu, X. The role of reactive oxygen species and autophagy in periodontitis and their potential linkage. Front. Physiol. 2017, 8, 439. [Google Scholar] [CrossRef]
- Vo, T.T.T.; Chu, P.-M.; Tuan, V.P.; Te, J.S.-L.; Lee, I.-T. The promising role of antioxidant phytochemicals in the prevention and treatment of periodontal disease via the inhibition of oxidative stress pathways: Updated insights. Antioxidants 2020, 9, 1211. [Google Scholar] [CrossRef]
- Henry, L.G.; McKenzie, R.M.E.; Robles, A.; Fletcher, H.M. Oxidative stress resistance in Porphyromonas gingivalis. Future Microbiol. 2012, 7, 497–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strand, K.R.; Sun, C.; Li, T.; Jenney, F.E.; Schut, G.J.; Adams, M.W.W. Oxidative stress protection and the repair response to hydrogen peroxide in the hyperthermophilic archaeon Pyrococcus furiosus and in related species. Arch. Microbiol. 2010, 192, 447–459. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noailles, A.; Maneu, V.; Campello, L.; Lax, P.; Cuenca, N. Systemic inflammation induced by lipopolysaccharide aggravates inherited retinal dystrophy. Cell Death Dis. 2018, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Suuronen, T.; Kaarniranta, K.; Ojala, J. ER stress in Alzheimer’s disease: A novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflamm. 2009, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Dong, D.; Ni, M.; Ligaya, P.; Xiong, S.; Ye, W.; Virrey, J.J.; Mao, C.; Ye, R.; Wang, M.; Pen, L.; et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008, 68, 498–505. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Aberami, S.; Nikhalashree, S.; Bharathselvi, M.; Biswas, J.; Sulochana, K.N.; Coral, K. Elemental concentrations in Choroid-RPE and retina of human eyes with age-related macular degeneration. Exp. Eye Res. 2019, 186, 107718. [Google Scholar] [CrossRef]
- Dias, I.H.K.; Chapple, I.L.C.; Milward, M.; Grant, M.M.; Hill, E.; Brown, J.; Griffiths, H.R. Sulforaphane restores cellular glutathione levels and reduces chronic periodontitis neutrophil hyperactivity in vitro. PLoS ONE 2013, 8, e66407. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Sachdeva, M.M.; Cano, M.; Handa, J.T. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp. Eye Res. 2014, 119, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Abokyi, S.; To, C.-H.; Lam, T.T.; Tse, D.Y. Central role of oxidative stress in age-related macular degeneration: Evidence from a review of the molecular mechanisms and animal models. Oxid. Med. Cell. Longev. 2020, 2020, 7901270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambros, M.L.; Plafker, S.M. Oxidative stress and the Nrf2 anti-oxidant transcription factor in age-related macular degeneration. Adv. Exp. Med. Biol. 2016, 854, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano, M.; Thimmalappula, R.; Fujihara, M.; Nagai, N.; Sporn, M.; Wang, A.L.; Neufeld, A.H.; Biswal, S.; Handa, J.T. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and age-related macular degeneration. Vis. Res. 2010, 50, 652–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velmurugan, G.V.; Sundaresan, N.R.; Gupta, M.P.; White, C. Defective Nrf2-dependent redox signalling contributes to microvascular dysfunction in type 2 diabetes. Cardiovasc. Res. 2013, 100, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Sima, C.; Aboodi, G.M.; Lakschevitz, F.S.; Sun, C.; Goldberg, M.B.; Glogauer, M. Nuclear factor erythroid 2-related factor 2 down-regulation in oral neutrophils is associated with periodontal oxidative damage and severe chronic periodontitis. Am. J. Pathol. 2016, 186, 1417–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.M.; Stefanovic, N.; Tan, G.; Wilkinson-Berka, J.L.; De Haan, J.B. Lack of the antioxidant glutathione peroxidase-1 (GPx1) exacerbates retinopathy of prematurity in mice. Investig. Ophthalmol. Vis. Sci. 2013, 54, 555–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almerich-Silla, J.M.; Montiel-Company, J.M.; Pastor, S.; Serrano, F.; Puig-Silla, M.; Dasí, F. Oxidative stress parameters in saliva and its association with periodontal disease and types of bacteria. Dis. Markers 2015, 2015, 653537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Oveson, B.C.; Jo, Y.; Lauer, T.W.; Usui, S.; Komeima, K.; Xie, B.; Campochiaro, P.A. Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage. Antioxid. Redox Signal. 2009, 11, 715–724. [Google Scholar] [CrossRef]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arjunan, P.; Swaminathan, R.; Yuan, J.; Elashiry, M.; Tawfik, A.; Al-Shabrawey, M.; Martin, P.M.; Muthusamy, T.; Cutler, C.W. Exacerbation of AMD Phenotype in Lasered CNV Murine Model by Dysbiotic Oral Pathogens. Antioxidants 2021, 10, 309. https://doi.org/10.3390/antiox10020309
Arjunan P, Swaminathan R, Yuan J, Elashiry M, Tawfik A, Al-Shabrawey M, Martin PM, Muthusamy T, Cutler CW. Exacerbation of AMD Phenotype in Lasered CNV Murine Model by Dysbiotic Oral Pathogens. Antioxidants. 2021; 10(2):309. https://doi.org/10.3390/antiox10020309
Chicago/Turabian StyleArjunan, Pachiappan, Radhika Swaminathan, Jessie Yuan, Mohamed Elashiry, Amany Tawfik, Mohamed Al-Shabrawey, Pamela M. Martin, Thangaraju Muthusamy, and Christopher W. Cutler. 2021. "Exacerbation of AMD Phenotype in Lasered CNV Murine Model by Dysbiotic Oral Pathogens" Antioxidants 10, no. 2: 309. https://doi.org/10.3390/antiox10020309
APA StyleArjunan, P., Swaminathan, R., Yuan, J., Elashiry, M., Tawfik, A., Al-Shabrawey, M., Martin, P. M., Muthusamy, T., & Cutler, C. W. (2021). Exacerbation of AMD Phenotype in Lasered CNV Murine Model by Dysbiotic Oral Pathogens. Antioxidants, 10(2), 309. https://doi.org/10.3390/antiox10020309









