Abstract
Fuchs endothelial corneal dystrophy (FECD) is characterized by the gradual deterioration of corneal endothelial cells (CECs) and is the most common cause of corneal transplantation worldwide. CECs apoptosis caused by oxidative stress plays a pivotal role in the pathogenesis of FECD. Antioxidant compounds have been of considerable significance as a candidate treatment in the management of corneal diseases. Based on these findings, the objective of this study was to evaluate the effects of an aloe extract with antioxidant properties, in an “in vitro” model of FECD. Human corneal epithelial (HCE) cells were preincubated with aloe extract 100 μg/mL, two hours before hydrogen peroxide (H2O2) stimulus. H2O2 challenge significantly reduced the cell viability, increased the generation of Reactive Oxygen Species (ROS) and malondialdehyde levels. Moreover, m-RNA expression and activity of Nrf-2, Catalase and Superoxide dismutase (SOD) were reduced together with an enhanced expression of IL-1β, tumor necrosis factor-α (TNF-α), IL-6, and cyclooxygenase 2 (COX-2). Furthermore, Bcl-2, Caspase-3 and Caspase-8 expression were down-regulated while Bax was up-regulated by H2O2 stimulus. Aloe extract blunted the oxidative stress-induced inflammatory cascade triggered by H2O2 and modulated apoptosis. Aloe extract defends HCE cells from H2O2-induced injury possibly due its antioxidant and anti-inflammatory activity, indicating that eye drops containing aloe extract may be used as an adjunctive treatment for FECD.
Keywords:
ROS; oxidative stress; inflammation; apoptosis; corneal epithelial cells; Aloe vera; antioxidant 1. Introduction
Fuchs endothelial corneal dystrophy (FECD) is a bilateral hereditary and slowly progressive disease which affects the endothelial layer of the cornea of both eyes. The prevalence of FECD is approximately four percent of the adult population. Early manifestation can be observed in patient from 30 to 40 years old but Fuchs usually become symptomatic over 40 years of age or later. Women are more commonly affected than men are. In addition, family history increases the chance of developing the disease. Symptoms are usually represented by blurred and cloudy vision, especially in the morning that improves during the day, photophobia and feeling of foreign body inside the eye. Fuchs dystrophy can be classified in four stages, which vary from early signs of guttae formation to end-stage subepithelial scarring. Diagnosis is confirmed through ophthalmological examination, corneal pachymetry ad in-vivo confocal. It is characterized by the gradual deterioration of corneal endothelial cells (CECs) developing a characteristic feature (cornea guttate) and is the most common cause of corneal transplantation worldwide [1,2]. The deterioration of corneal endothelial cells and the consequent guttae formation usually start in the centre of the cornea and slowly involves the periphery as well. The longer the guttae spreads, the more CECs are destroyed and the density of endothelial cells decreases, as these two parameters are inversely proportional.
In this stage, the changes in Fuchs endothelial cells include a modification of the cell size called “polymegethism” and a modification of the cell outline called “pleomorphism”. The corneal endothelium (CE) is a monolayer formed by the CECs situated on the corneal surface that has different functions including: maintaining corneal deturgescence thanks to his barrier function and regulating corneal hydration, nutrition and transparency. As the CE does not divide in vivo, loss of endothelial cells seen in FECD is permanent. CECs apoptosis caused by oxidative stress may play a pivotal role in the pathogenesis of FECD [3,4]. Previous studies have already demonstrated a higher level of reactive oxygen species (ROS) in the cornea of FECD patients when compared with the healthy one [5]. The imbalance between oxidant and antioxidant factors in CECs is responsible for the development of endothelial oxidative DNA damage. It is caused by a decreased expression of Nrf2 transcription factor and its antioxidant targets like superoxide dismutases, glutathione S-transferases and peroxiredoxin, which are involved in the scavenging of ROS. The aberrant Nrf2 expression influences the antioxidant system in FECD corneal endothelium and induces free radicals and other reactive species to accumulate, leading to an alteration of tissue homeostasis and activating the p53-dependent apoptotic pathway [6,7].
Reactive oxygen species could be produced after photochemical reactions caused by the exposure to UV light or ionizing radiation. During physiological conditions, there is a cellular equilibrium between ROS production and degradation and low levels of ROS can be found [8]. The imbalance between ROS production and the antioxidant scavenging systems (AOX) causes Oxidative stress (OS). OS is related to several disorders, including Parkinson’s and Alzheimer’s diseases, cancer, atherosclerosis, diabetes and rheumatoid arthritis [9,10,11]. Furthermore, OS is also responsible for different ocular pathologies, such as ocular surface disorders, different syndromes of the eye anterior and posterior segment and retinal diseases. The cornea is a transparent and avascular tissue in the anterior segment of the eye and is one of the most densely innervated tissues in the body with refractive and barrier functions [12]. Due to its external localization, it is directly exposed to different factors, such as air pollution, cigarette smoke and UV radiations, which can induce oxidative damage, and different ocular pathologies such as FECD [13].
Corneal tissue has developed physiological antioxidant systems, which contain free radical scavengers, including superoxide dismutase, glutathione peroxidase and catalase [14]. The imbalance between prooxidant and antioxidant is primarily attributed to the down-regulation of the antioxidant enzymes principally: Lactate dehydrogenase, catalase and glutathione peroxidase. This imbalance leads to structural and functional changes in the corneal tissue. As a consequence of the increased oxidative stress there is a reduction of the number of corneal fibroblasts and corneal endothelial cells in corneas, due to the triggering of the apoptotic process [15,16,17]. Both elevated ROS levels and oxidative stress play a key role in the development of many corneal diseases, including Fuchs endothelial corneal dystrophy, keratoconus, granular corneal dystrophy type 2 and bullous keratopathy [18,19].
Previous papers have investigated the possibility of using phytotherapic treatments as new strategies for ROS related diseases [20,21]. Health plants are currently used in a number of consumer products due to their medicinal or aesthetic properties. Among them, the Aloe vera extracts are known for their curative properties, and are used both internally and externally on humans in the form of alternative medications, as well as in the home for first aid. Aloe vera has demonstrated wound-healing properties and showed immunomodulatory, antioxidant and anti-inflammatory effects [22]. Indeed, different studies showed that Aloe vera treatment induced inhibition of the inflammatory reactions, reducing the expression of proinflammatory cytokines, like interleukine-6 (IL-6), IL-1β and tumor necrosis factor-α (TNF-α) [23]. Moreover, Aloe vera extracts revealed a modulating effect on the inflammatory cytokine network and antioxidant function including ROS scavenging in eye tissue [24]. Furthermore, previous work established that the use of Nrf2 agonists with antioxidant activity caused cytoprotective effects, a significant decrease in ROS production and ameliorated oxidative stress-levels in FECD corneas, confirming the idea that the implementation of antioxidant system may play a role in the treatment of corneal ROS related diseases [25].
Finally, Jurkunas et al. demonstrated that immortalized normal corneal endothelial cells stimulated with H2O2 may display morphological changes and cellular apoptosis similar to those observed in FECD [5]. For all these reasons, the aim of the present study is to investigate the effects of an Aloe vera extract in an “in vitro” model of FECD induced by H2O2 stimulation in human corneal cells.
2. Materials and Methods
2.1. Cell Culture
Epithelial Adenovirus 12-SV40 hybrid transformed HCE cells (ATCC® CRL-11135™) were obtained from LGC Standards S.r.l Milan, Italy. Cells were cultured in a medium prepared with DMEM-F12 basic media supplemented with 10% heat-inactivated FBS, recombinant human epidermal growth factor (5 ng/mL), 5 μg/mL insulin plus 1% antibiotics (penicillin/streptomycin), and incubated at 37 °C with 5% of CO2. The culture’s medium was replaced with a time interval of 2 days and the cells were re-plated.
2.2. Treatments of Cells
HCE cells were put in culture using six well plates and a density of 3 × 105 cells/well. To establish an oxidative stress model 500 μM H2O2 (Sigma Aldrich, Milan, Italy) was added to the culture medium for 24 h. HCE cells were preincubated with aloe extract 100 μg/mL 2 h before H2O2 stimulus. HCE cells treated with 1% DMSO were used as control. The oxidative stress model, the doses and the incubation time of Aloe vera extract were chosen according to previously published papers [5,24,26,27].
2.3. MTT Assay
Cell viability was assessed using the MTT assay. HCE cells were treated with H2O2 (500 μM), H2O2 + aloe extract (100 μg/mL), when reaching confluence. In particular, CTRL, H2O2, H2O2 + aloe extract, were examined in a 96-well plate with a density of 8 × 104 cells/well for 24 h in order to evaluate the cytotoxic effect as previously described [28,29,30].
2.4. Ros Measurament
To evaluate the effect of aloe extract on OS, the production of Total Reactive Oxygen Species (ROS) in HCE cells was measured using an assay kit (Thermo Fisher, Carlsbad, CA, USA). Briefly, after the treatments the HCE cells were cleaned with PBS once, and then were incubated at 37 °C for 30 min with 2′,7′-dichlorofluorescein diacetate. DCFH-DA fluorescence distribution of 1 × 104 cells was detected using a flow cytometry at two different wavelength 488 nm for excitation and 525 nm for emission [13].
2.5. Malondialdehyde Assay
The effects of aloe extract as antioxidants against lipid peroxidation in HCE cells were examined using as marker the levels of malondialdehyde (MDA) as previously described in details [31,32,33].
2.6. Real Time Quantitative PCR Amplification (RT-qPCR)
The m-RNA expression of SOD2, Catalase, Nrf2, Il-1β, TNF-α IL-6, COX-2, Bcl-2, Bax, Caspase-3 and Caspase-8 was evaluated as previously described [34,35,36]. Primers used to identify both targets and reference genes are catalogued in Table 1.

Table 1.
Primer list.
2.7. Measurements of Cytokines
Nrf2, IL-1β, TNF-α, IL-6, PGE2 and Caspase-8 levels were measured in the cell culture supernatants, Bax, Bcl-2 and Caspase-3 levels were evaluated in the cell culture extracts using an Enzyme-Linked Immunosorbent Assay (ELISA) kits (Abcam, Cambridge, UK or Thermo Fisher, Waltham, MA, USA), in agreement with the instructions reported by the manufacturer [37,38,39,40].
2.8. Catalase and SOD Activity Measurement
The Catalase and SOD activity were evaluated in agreement with the manufacturer’s protocol of commercial kits (Thermo Fisher, Waltham, MA, USA). All the samples were measured in duplicate and the results were interpolated with the Catalase or SOD standard curve and the results were expresses in units/mL.
2.9. Collection of Plant Samples and Extract Preparation
Aloe vera plants (Aloe barbadensis Miller) were subjected to vegetative propagation as previously described [41]. Briefly, young shoots were cut from the mother plant and subsequently were planted in 0.5 kg plastic pots with incorporated commercial ground. Plants received tap water once every two weeks and grown under ambient irradiance of (400–1400 μmol m−2 s−1) and a temperature of (25 ± 1 °C). After 12 months of growth, the earliest fully developed leaves were used for the extract preparation. Fresh leaves were cleaned with distillated water and cut into fragments of around 20 g each. The extraction was performed by grinding a sample of 20 g with 100 mL of 100% methanol using a high performance grinder, followed by agitation for 4 h at 4 °C. After, the extract was evaporated in a bath at 60 °C and then lyophilized for 24 h The obtained powder was weighed and stored until the use.
2.10. Phytochemical Analysis of Aloe vera Extract
In Aloe vera plants, several phytochemical constituents are present such as: anthraquinones, fatty acids, alkaloids, carbohydrates, enzymes, vitamins, mineral and other miscellaneous compounds [20]. A phytochemical screening was performed the Aloe vera methanolic extract was subjected to for the presence or absence of various phytochemical according to standard protocols [42,43].
2.11. Statistical Analysis
Data are shown as the mean ± SD and the values reported are the result of at least five experiments performed in duplicate (two wells for each treatment). To ensure reproducibility, all assays were replicated three times. The various groups were compared and evaluated using one-way ANOVA with Tukey post-test for comparison between the different groups. A p value < 0.05 was considered significant. Graphs were prepared using GraphPad Prism (version 8.0 for macOS, San Diego, CA, USA).
3. Results
3.1. Phytochemical Screening of Aloe vera Extract
A wide range of various phytochemicals; alkaloids, flavonoids, glycosides, phenolic compounds, tannins and saponin, steroids and terpenoids, glycosides were tested with their appropriate protocols and reagents. The Aloe vera extract showed presence of most of the phytochemicals tested, the characterization is depicted in Table 2.

Table 2.
Qualitative analyses of the phytochemical components of Aloe vera extract.
3.2. Effects of Aloe Extract on Cell Vitality and Oxidative Stress
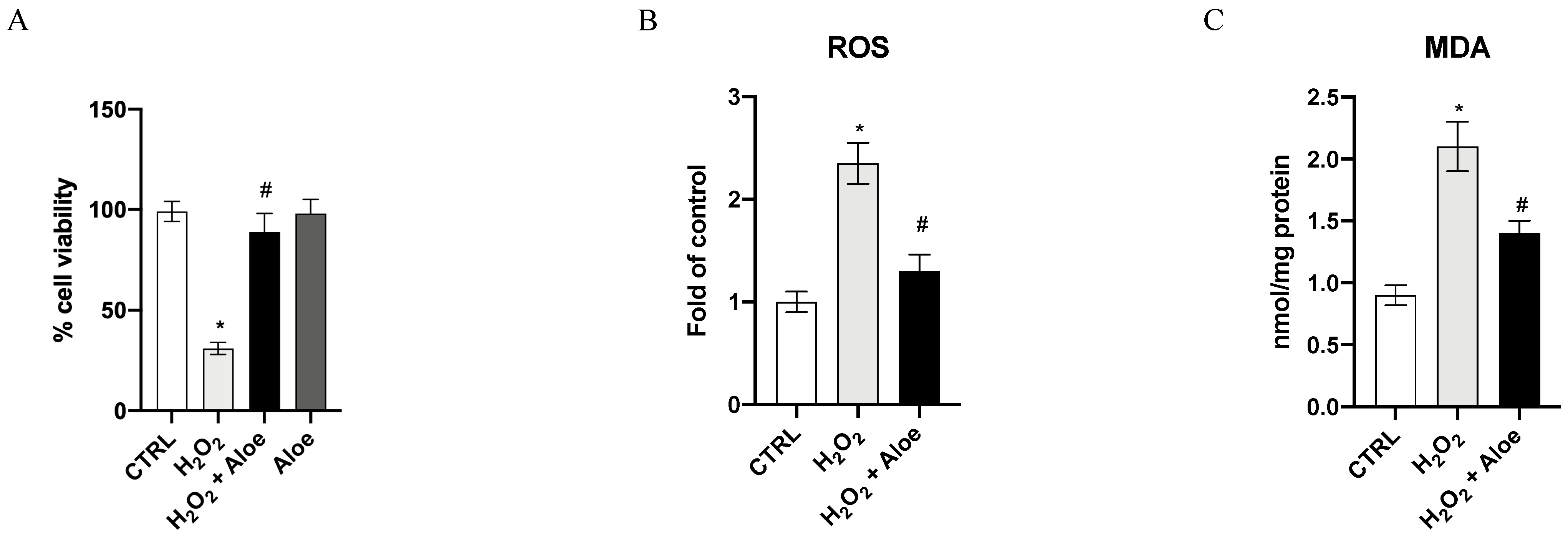
The viability of HCE cells was drastically reduced after exposure to 500 μM H2O2 for 24 h, as compared with the control group (p < 0.0001 versus control group; Figure 1A). Pre-treatment for 2h with aloe extract at 100 μg/mL significantly increased the cell viability of HCE cells incubated with H2O2 (p < 0.0001 vs. H2O2 group; Figure 1A). Furthermore, the incubation of aloe extract did not affect cell viability, thereby showing that this natural extract does not have a cytotoxic effect. To examine the ROS level in this oxidative stress setting and the effects of aloe extract, we measured the ROS production. An exposure to H2O2 at a concentration of 500 μM for 24 h resulted in high levels of ROS compared to the control group (p < 0.0001 vs. control group; Figure 1B). Whereas, aloe extract at a concentration of 100 μg/mL significantly suppressed the production of ROS (p < 0.0001 vs. H2O2 group; Figure 1B), indicating that aloe extract suppressed ROS production under the oxidative stress induced by H2O2. An important aspect of damage caused by ROS, especially by H2O2, is the oxidation of lipids, such as MDA. We observed a significant increase in MDA generation by H2O2 stimulus (p < 0.0001 vs. control group; Figure 1C). Moreover, aloe extract at 100 μg/mL significantly inhibited MDA levels compared to the H2O2 group (p < 0.0001 vs. H2O2 group; Figure 1C).
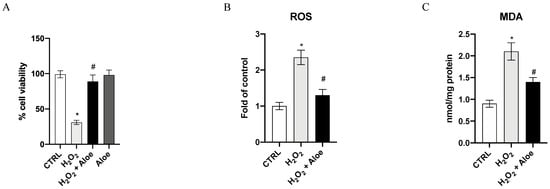
Figure 1.
Effects of pre-treatment with aloe extract on cell viability (A), ROS production (B), MDA generation (C). Values are expressed as the means ± SD. * p < 0.0001 vs. CTRL; # p < 0.0001 vs. H2O2.
3.3. Effects of Aloe Extract on mRNA Expression and Activity of Antioxidant Markers
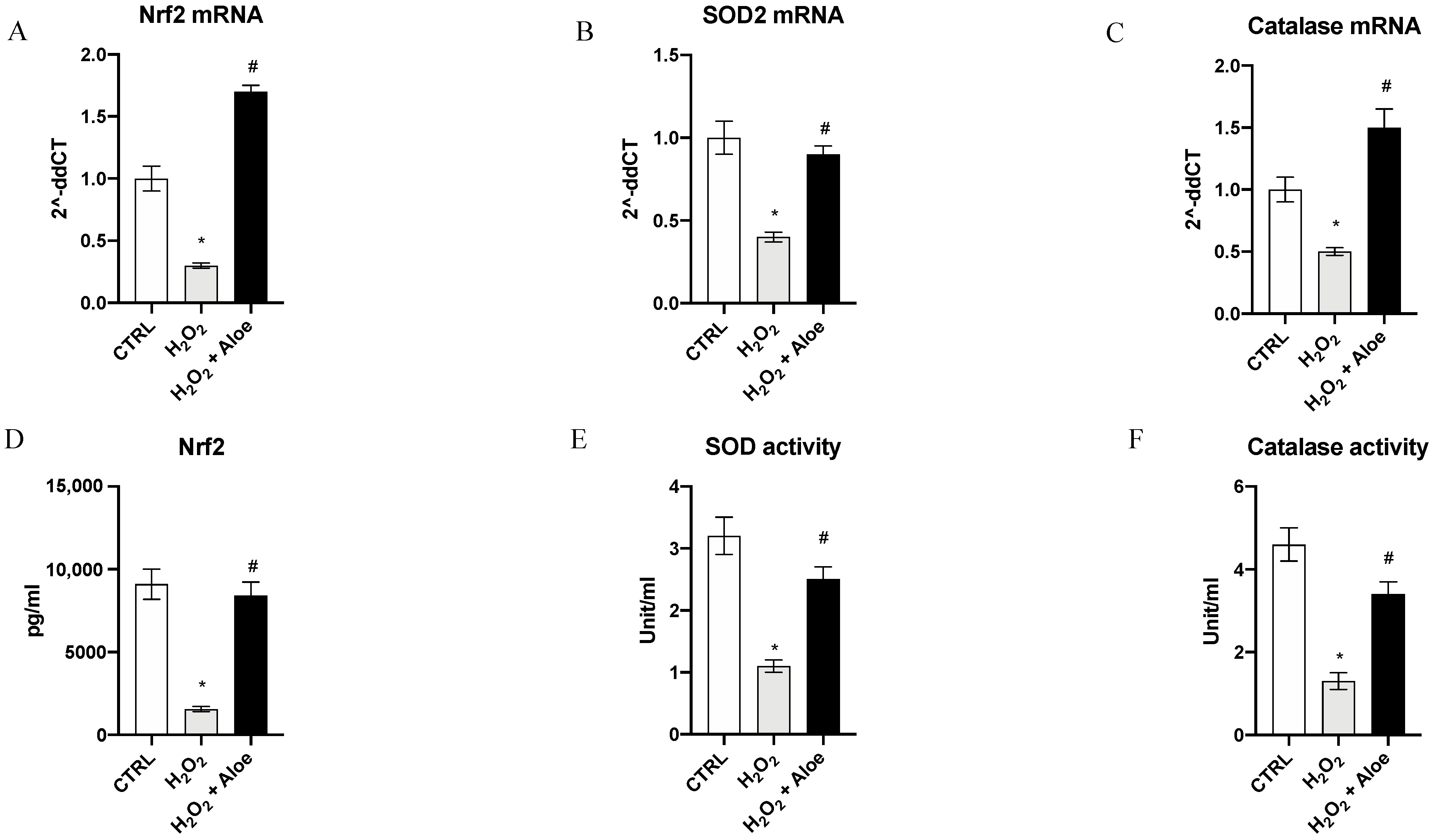
To analyze the effects of aloe extract during oxidative stress, we measured the gene expression and mature protein levels of Nrf2, chief regulator of the antioxidant system, and the enzyme activities and gene expression of SOD2 (one of the major antioxidant defense systems against free radicals) and catalase (one of the most important antioxidant enzymes) involved in antioxidant defenses. Exposure of HCE cells to H2O2 for 24 h significantly decreased mRNA expression of Nrf2, SOD2 and Catalase compared to the control group (p < 0.0001 vs. control group; Figure 2). While, 2h of pre-treatment with aloe extract significantly upregulated the gene expression of Nrf2, SOD2 and Catalase when compared to cell cultures challenged with H2O2 alone (p < 0.0001 vs. H2O2 group; Figure 2). Moreover, Nrf2 mature protein levels were significantly reduced in the H2O2 group as compared to the control group (p < 0.0001 vs. control group; Figure 2). Meanwhile, 2h pre-treatment with aloe extract significantly increased Nrf2 levels in the cells after exposure to H2O2 stimulus (p < 0.0001 vs. H2O2 group; Figure 2). In addition, it was revealed that catalase and SOD activity was decreased in HCE cells after exposure to H2O2 at a concentration of 500 μM for 24 h (p < 0.0001 versus control group) while 2h pre-treatment with aloe extract at 100 μg/mL significantly upregulated the catalase and SOD activity in HCE cells after exposure to H2O2 (p < 0.0001 vs. H2O2 group; Figure 2).
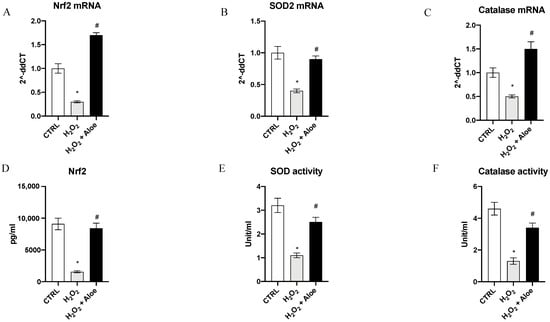
Figure 2.
Effects of pre-treatment with aloe extract on Nrf2 (A), SOD2 (B), Catalase (C) mRNA expression. Effects of pre-treatment with aloe extract on Nrf2 levels (D), SOD activity (E), Catalase activity (F). Values are expressed as the means ± SD. * p < 0.0001 vs. CTRL; # p < 0.0001 vs. H2O2.
3.4. Effects of Aloe Extracts on Inflammatory Markers
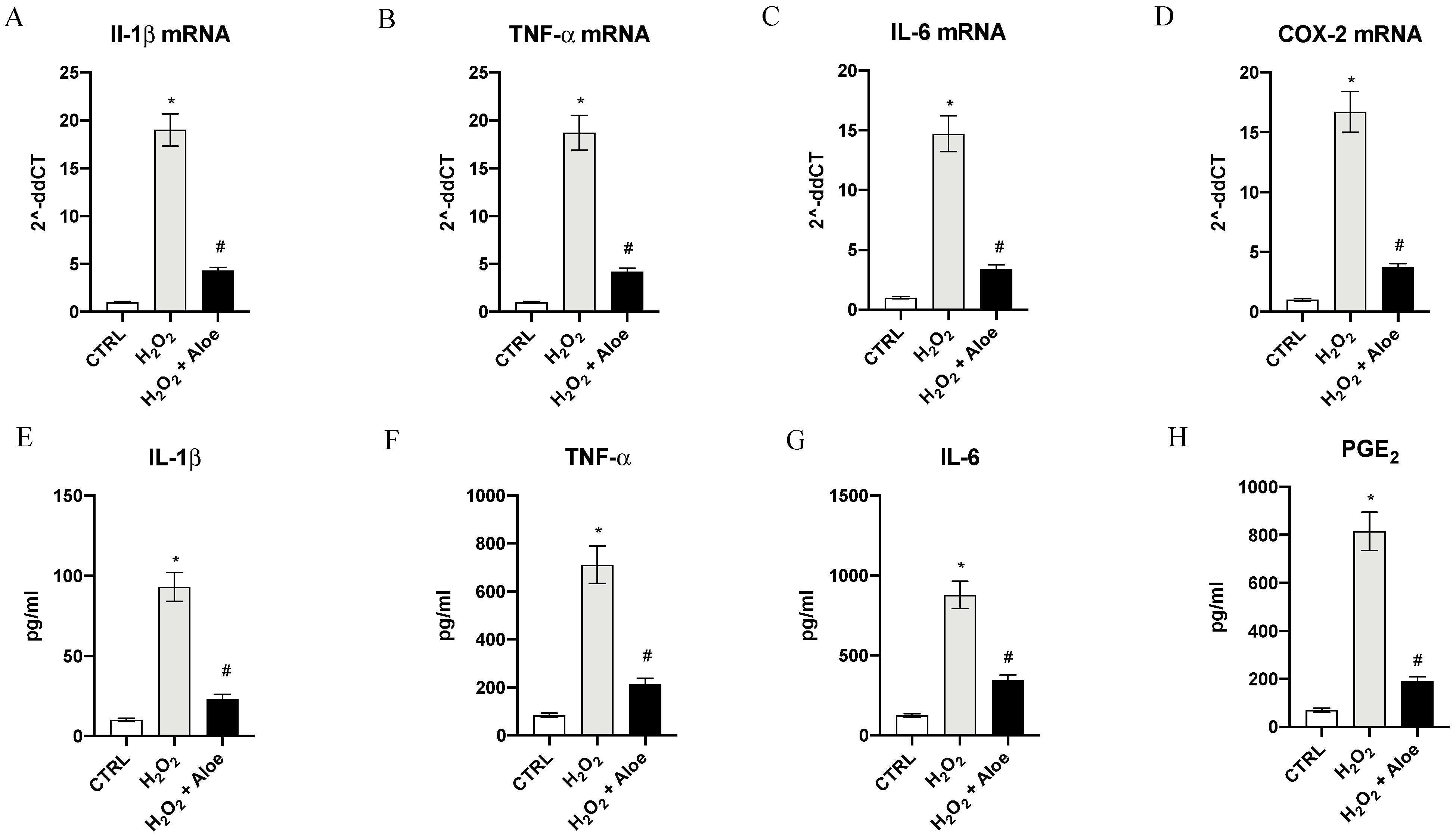
As estimated, H2O2 induced a significant upregulation of mRNA expression of proinflammatory enzyme COX-2, a common feature of inflammation caused by oxidative stress, and a marked expression of proinflammatory cytokines IL-1β, IL-6 and TNF-α compared to control group (p < 0.0001 vs. control group; Figure 3). Pre-treatment with aloe extract in H2O2 stimulated HCE cells suppressed the increased mRNA for the inflammatory enzyme COX-2 and caused a marked reduction in the expression of the message of the inflammatory cytokine TNF-α, IL-6 and IL-1β (p < 0.0001 vs. H2O2 group; Figure 3). To confirm the anti-inflammatory effect of the aloe extract we measured the mature protein levels in the supernatants of HCE cells stimulated with H2O2. TNF-α, IL-6 and IL-1β levels were markedly increased (p < 0.0001 vs. control group; Figure 3). By contrast aloe extract pre-treatment blunted the increase of TNF-α, IL-6 and IL-1β in the HCE cells stimulated with H2O2 (p < 0.0001 vs. H2O2 group; Figure 3). PGE2 levels metabolite of COX-2 was also markedly released in the supernatants of HCE cells upon H2O2 stimulation (p < 0.0001 vs. control group; Figure 3). Pre-treatment with aloe extract resulted in a powerful reduction of PGE2 levels (p < 0.0001 vs. H2O2 group; Figure 3).
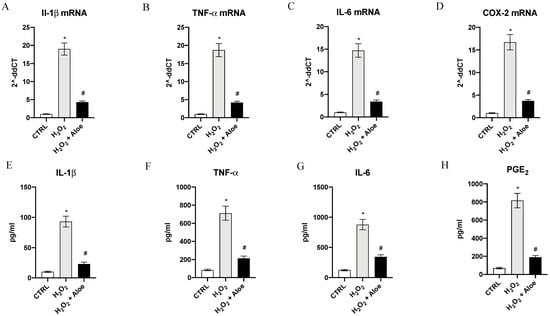
Figure 3.
Effects of pre-treatment with aloe extract on IL-1β (A), TNF-α (B), IL-6 (C), COX-2 (D) mRNA expression. Effects of pre-treatment with aloe extract on IL-1β (E), TNF-α (F), IL-6 (G), PGE2 (H) levels. Values are expressed as the means ± SD. * p < 0.0001 vs. CTRL; # p < 0.0001 vs. H2O2.
3.5. Effects of Aloe Extract on Apoptosis
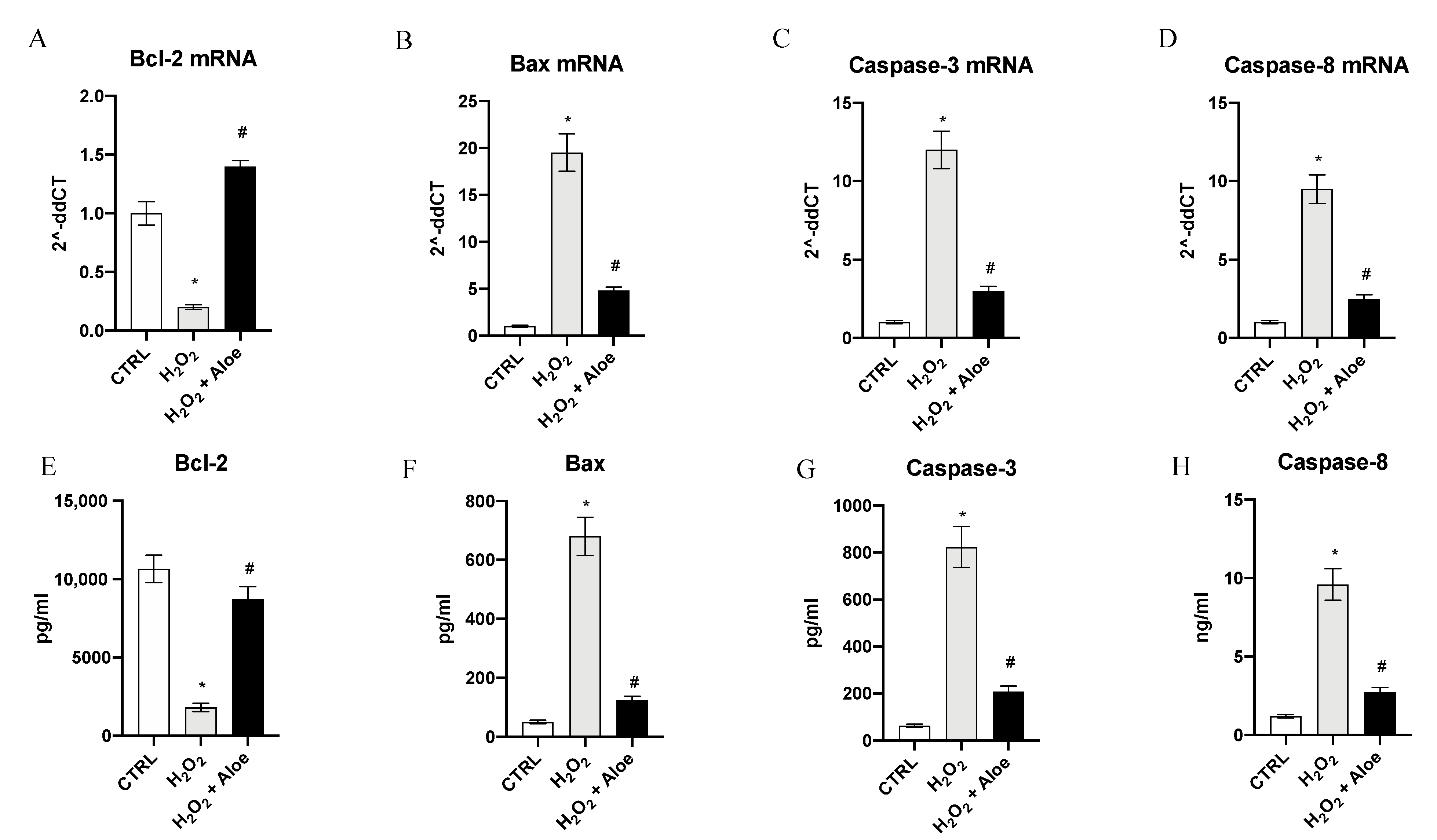
To examine the role of aloe extract in regulating apoptosis in HCE cells stimulated with H2O2, the mRNA expression of Bcl-2, Bax, Caspase-3 and Caspase-8 was evaluated. HCE cells challenged with H2O2 for 24 h showed a marked reduction of Bcl-2 expression with a concomitant up-regulation in the mRNA expression of Bax, Caspase-3 and Caspase-8 compared to control cells (p < 0.0001 vs. control group; Figure 4). Conversely, when cells were pretreated with Aloe extract for 2 h, mRNA expression of Bcl-2, Bax, Caspase-3 and Caspase-8 were reversed (p < 0.0001 vs. H2O2 group; Figure 4). To better evaluate the antiapoptotic effect of aloe extract we measured the protein levels of Bcl-2, Bax, Caspase-3 and Caspase-8 in HCE cells challenged with H2O2. After HCE cells were challenged with H2O2 for 24 h Bcl-2 protein expression levels were significantly decreased with a contemporaneous increase in protein expression of Bax, Caspase-3 and Caspase-8 compared to control cells (p < 0.0001 vs. control group; Figure 4). By contrast, when cells were pretreated with aloe extract for 2 h, a significant growth in Bcl-2 protein expression with a simultaneous reduction of Bax, Caspase-3 and Caspase-8 protein levels were observed (p < 0.0001 vs. H2O2 group; Figure 4).
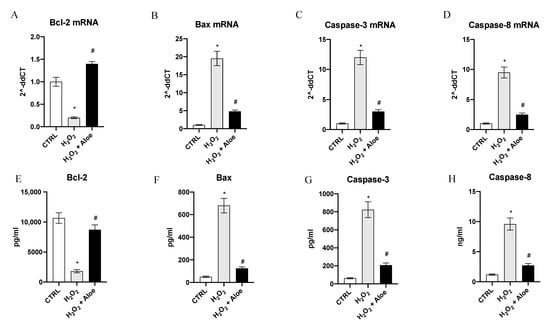
Figure 4.
Effects of pre-treatment with aloe extract on Bcl-2 (A), Bax (B), Caspase-3 (C), Caspase-8 (D) mRNA expression. Effects of pre-treatment with aloe extract on Bcl-2 (E), Bax (F), Caspase-3 (G), Caspase-8 (H) levels. Values are expressed as the means ± SD. * p < 0.0001 vs. CTRL; # p < 0.0001 vs. H2O2.
4. Discussion
Fuchs is a corneal endothelium degenerative condition characterized by the accumulation of focal guttae, contributing to oedema of the cornea and vision loss. The corneal endothelium is a single layer that serves as a barrier, maintains a particular level of corneal hydration and preserves corneal stromal clarity through precise spatial collagen fiber arrangement. End-stage FECD corneal endothelial cells are reduced in number and appear attenuated, inducing progressive stromal swelling and resulting in blurred vision. These pathological conditions are related to CECs apoptosis caused by an exaggerated ROS production that lead to oxidative stress. ROS can be divided into two forms: radical and non-radical species. Hydrogen peroxide (H2O2), superoxide anion (O2−), ozone (O3), and nitric oxide (NO) belong to the first category. Oxidative stress may have distinct effects, and the cellular response depends on its behaviour. The signalling role of ROS has also been shown to be important for the integrity of living organisms and their aging process. In addition, ROS may be involved in various damage mechanisms, such as membrane lipid peroxidation, protein structure damage and also in different ocular pathologies such as ocular surface disorders.
In this scenario, evaluating the efficacy of natural extracts could be of interest. Indeed, medicinal plants and their derivatives have shown several beneficial effects, including the reduction of reactive oxygen species (ROS) (antioxidant activity), the prevention of cell apoptosis, and the modulation of pro-inflammatory factors. In particular, among medicinal plants, Aloe vera (A. barbadensis Miller) has a number of pharmacological properties, such as antioxidant, immunomodulatory, bactericidal, antiviral, antifungal and anti-inflammatory due to the presence of a variety of chemicals like flavonoids, anthraquinones, enzymes, vitamins, and phenolic acids. In addition, in previous studies, Aloe vera extract showed the ability to speed up re-epithelialization and minimize fibrosis in superficial corneal lesions [24,41].
In light of these preceding observations, in the present study, we evaluated the efficacy of an extract from Aloe vera in an “in vitro” model of FECD, induced by H2O2 stimulus. Previous papers have investigated the possibility that hydrogen peroxide, its products, and/or other oxidant species may be in part responsible for the functional and structural alterations of corneal endothelial cells during ocular inflammatory disease processes [44]. Moreover, preceding works have demonstrated that the corneal endothelium is susceptible to oxidative stress, which leads to inflammation and apoptosis [5,45,46,47]. Our results have shown that hydrogen peroxide causes the alteration and apoptosis of corneal endothelial cells according to previous published papers [48,49]. Pre-incubation with aloe extract significantly reduced oxidative stress markers and upregulated the expression of Nrf2, SOD2 and Catalase when compared to cells cultures challenged with H2O2 alone. These effects may be attributed to Aloin, a substance with many biological activities present in Aloe vera. Indeed, Aloin has been demonstrated to have anti-oxidant effects in two different models of oxidative stress damage in skin fibroblasts and macrophages [27,50]. These results are in accordance with other studies that demonstrated the protective effects of Nrf2 agonists on oxidative stress in corneal endothelium due to the antioxidant activity of these compounds, corroborating the idea that the implementation of antioxidant systems may play a role in the treatment of corneal ROS related diseases [25].
Moreover, pre-treatment with aloe extract in H2O2 stimulated HCE cells, suppressed the increased mRNA for the COX-2 enzyme and caused a marked reduction in the expression of the message and protein levels of the inflammatory cytokine TNF-α, IL-6 and IL-1 β when compared to cell cultures challenged with H2O2 alone. These findings may be related to several Aloe compounds such as Aloin and Aloe-emodin that exhibited a great suppression of pro-inflammatory cytokine expression in murine macrophages and in human gingival fibroblast under oxidative stress stimulus [20,27]. Furthermore, our results agree with the findings of other previous studies showing that Aloe vera’s anti-inflammatory activity is linked to the downregulations of the arachidonic acid pathway via cyclooxygenase [51]. Moreover, it has been elucidated that the presence of sterols in the Aloe vera extract may reduce the production of the phospholipase A2 enzyme, which is accountable for the release of arachidonic acid precursors in the synthesis of prostaglandins. As a consequence, Aloe vera extract’s anti-inflammatory activity is the result of the inhibition of both prostaglandin and leukotriene synthesis [51]. Furthermore, it has been demonstrated that the herb’s extracts may inhibit the inflammatory process in different ways by reducing the number of circulating cytokines and also inhibiting the adhesion ability of leukocytes in the injury site. [52]. In addition, previous papers have demonstrated that medical herbal plants have antioxidant effects in human corneal cells and their administration to the eye influences local homeostasis among other effects on the cytokine network [53]. In particular, it has been well-demonstrated that Aloe vera extract is full of various biologically active constituents with different therapeutic properties, such as: Wound-healing properties and immunomodulatory, anti-inflammatory and antioxidant effects [20,54] Additionally, our results demonstrated that pre-treatment with Aloe vera extract for two hours increased Bcl-2 levels and reduced Bax, Caspase-3 and Caspase-8 mRNA expression and protein levels compared to cell cultures challenged with H2O2 alone. These results agree with the findings of previous studies showing that nature-derived antioxidant application had anti-inflammatory and anti-apoptotic activities, and may avoid the aggravation of different illnesses caused by oxidative stress in eye models [55,56,57].
Moreover, previous papers have already established that the activity of Aloe vera extracts did not have toxic effects even at high concentrations and can be helpful as a complimentary treatment for eye disorders. Aloe vera has well-established pharmacological proprieties that allow the activation and inhibition of different enzymes, modulating the metabolism of cells, the expression of anti-inflammatory, antifungal and antibacterial proprieties [58]. Additionally, in a previous study the healing of corneal epithelial lesions mechanically induced in rabbits after Aloe vera application was observed, without toxic effect [54,59]. Furthermore, Aloe vera extracts demonstrated a concentration-dependent ROS scavenging action that can be attributed to the phenolic compounds present in the extracts and this is considered its principal beneficial activity [60].
Nevertheless, the present study is subject to some limitations, for instance Aloe vera’s antioxidant properties are limited by simultaneous insufficient prevention of lipid peroxidation [61]. In addition, Aloe vera extracts have an inhibitory activity on NO production (which is also an effector radical in biological processes) because of constitutive and inducible NO synthase inhibition [62]. This effect could be a limitation because a proper level of NO allows the maintainence of homeostasis of the ocular surface and may work as a health preventive factor [63]. Furthermore, we evaluated only the effects of Aloe vera extract in this “in vitro” model of FECD, additional studies should be performed to compare the efficacy of this extract to other extracts from medical plants with antioxidant activities. Also, we did not asses in detail the potential ophthalmological side effects arising from human Aloe vera use, and such aspects should be carefully evaluated in future studies when considering a longer disease course and human lifespan.
To date there are no non-surgical treatments for FECD [64], but the present experiment demonstrated the efficacy of this extract from Aloe vera as treatment for FECD due to its modulating effect on the inflammatory cytokine network and antioxidant function, including ROS scavenging in eye tissue. Since, Aloe vera extracts are currently on the market for the treatment of other pathologies and severe side effects were not reported, the extract could be readily available for clinical trials in FECD patients. Therefore, these results exalt the potential future application of Aloe extract as a complimentary treatment option for patients with FECD, in a possible formulation of eye drops.
5. Conclusions
In conclusion, our findings suggest that the application of Aloe vera extract may protect the cornea from oxidative stress and may provide a scientific basis for the use of this extract in the treatment of corneal inflammation. This effect, in light of its high translational potential, merits confirmation in a clinical setting.
Author Contributions
Conceptualization, I.C.; formal analysis, D.A. and G.P.; Funding acquisition, F.S.; methodology, N.I.; supervision, F.S. and G.P.; validation, D.A. and G.C.; writing—original draft, I.C. and F.M.; writing—review and editing, G.P. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by departmental funding assigned to Francesco Squadrito.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are grateful to Ahsan Bhatti MD, Singleton Hospital, Swansea, Wales, UK for the language support during the revision‘s process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matthaei, M.; Hribek, A.; Clahsen, T.; Bachmann, B.; Cursiefen, C.; Jun, A.S. Fuchs Endothelial Corneal Dystrophy: Clinical, Genetic, Pathophysiologic, and Therapeutic Aspects. Annu. Rev. Vis. Sci. 2019, 5, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Soh, Y.Q.; Peh, G.S.; Mehta, J.S. Evolving therapies for Fuchs’ endothelial dystrophy. Regen. Med. 2018, 13, 97–115. [Google Scholar] [CrossRef]
- Kim, E.C.; Meng, H.; Jun, A.S. N-Acetylcysteine increases corneal endothelial cell survival in a mouse model of Fuchs endothelial corneal dystrophy. Exp. Eye Res. 2014, 127, 20–25. [Google Scholar] [CrossRef]
- Engler, C.; Kelliher, C.; Spitze, A.R.; Speck, C.L.; Eberhart, C.G.; Jun, A.S. Unfolded protein response in fuchs endothelial corneal dystrophy: A unifying pathogenic pathway? Am. J. Ophthalmol. 2010, 149, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Jurkunas, U.V.; Bitar, M.S.; Funaki, T.; Azizi, B. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am. J. Pathol. 2010, 177, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef] [PubMed]
- Jurkunas, U.V.; Rawe, I.; Bitar, M.S.; Zhu, C.; Harris, D.L.; Colby, K.; Joyce, N.C. Decreased expression of peroxiredoxins in Fuchs’ endothelial dystrophy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2956–2963. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Agil, A.; Durán, R.; Barrero, F.; Morales, B.; Araúzo, M.; Alba, F.; Miranda, M.T.; Prieto, I.; Ramírez, M.; Vives, F. Plasma lipid peroxidation in sporadic Parkinson’s. Role of the L-dopa. J. Neurol. Sci. 2006, 240, 31–36. [Google Scholar] [CrossRef]
- Conner, E.M.; Grisham, M.B. Inflammation, free radicals, and antioxidants. Nutrition 1996, 12, 274–277. [Google Scholar] [CrossRef]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar] [PubMed]
- Yin, Y.; Zong, R.; Bao, X.; Zheng, X.; Cui, H.; Liu, Z.; Zhou, Y. Oxidative Stress Suppresses Cellular Autophagy in Corneal Epithelium. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3286–3293. [Google Scholar] [CrossRef] [PubMed]
- Atalla, L.R.; Sevanian, A.; Rao, N.A. Immunohistochemical localization of glutathione peroxidase in ocular tissue. Curr. Eye Res. 1988, 7, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Alio, J.L.; Ayala, M.J.; Mulet, M.E.; Artola, A.; Ruiz, J.M.; Bellot, J. Antioxidant therapy in the treatment of experimental acute corneal inflammation. Ophthalmic Res. 1995, 27, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Augustin, A.J.; Spitznas, M.; Kaviani, N.; Meller, D.; Koch, F.H.; Grus, F.; Göbbels, M.J. Oxidative reactions in the tear fluid of patients suffering from dry eyes. Graefes Arch. Clin. Exp. Ophthalmol. 1995, 233, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Cejková, J.; Ardan, T.; Simonová, Z.; Cejka, C.; Malec, J.; Jirsová, K.; Filipec, M.; Dotrelová, D.; Brůnová, B. Nitric oxide synthase induction and cytotoxic nitrogen-related oxidant formation in conjunctival epithelium of dry eye (Sjögren’s syndrome). Nitric Oxide 2007, 17, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Arnal, E.; Peris-Martínez, C.; Menezo, J.L.; Johnsen-Soriano, S.; Romero, F.J. Oxidative stress in keratoconus? Investig. Ophthalmol. Vis. Sci. 2011, 52, 8592–8597. [Google Scholar] [CrossRef] [PubMed]
- Buddi, R.; Lin, B.; Atilano, S.R.; Zorapapel, N.C.; Kenney, M.C.; Brown, D.J. Evidence of oxidative stress in human corneal diseases. J. Histochem. Cytochem. 2002, 50, 341–351. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Weintraub, S.T.; Yu, B.P. Isolation and identification of a phenolic antioxidant from Aloe barbadensis. Free Radic. Biol. Med. 2000, 28, 261–265. [Google Scholar] [CrossRef]
- Choi, S.; Chung, M.H. A review on the relationship between Aloe vera components and their biologic effects. Semin. Integr. Med. 2003, 1, 53–62. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.K.; Gupta, A.; Bishayee, A.; Pandey, A.K. Therapeutic potential of Aloe vera-A miracle gift of nature. Phytomedicine 2019, 60, 152996. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Paduch, R. Aloe vera extract activity on human corneal cells. Pharm. Biol. 2012, 50, 147–154. [Google Scholar] [CrossRef]
- Ziaei, A.; Schmedt, T.; Chen, Y.; Jurkunas, U.V. Sulforaphane decreases endothelial cell apoptosis in fuchs endothelial corneal dystrophy: A novel treatment. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6724–6734. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Miao, Q.; Jin, K.; Lou, L.; Ye, X.; Xi, Y.; Ye, J. Protective Role of Hinokitiol Against H2O2-Induced Injury in Human Corneal Epithelium. Curr. Eye Res. 2017, 4, 47–53. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, T.; Sheng, L.; Wang, Z.; Tao, H.; Zhang, Q.; Zhang, Y.; Qi, Z. Aloin suppresses lipopolysaccharide‑induced inflammation by inhibiting JAK1‑STAT1/3 activation and ROS production in RAW264.7 cells. Int. J. Mol. Med. 2018, 42, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. β-Caryophyllene Inhibits Cell Proliferation through a Direct Modulation of CB2 Receptors in Glioblastoma Cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef] [PubMed]
- Picciolo, G.; Pallio, G.; Altavilla, D.; Vaccaro, M.; Oteri, G.; Irrera, N.; Squadrito, F. β-Caryophyllene Reduces the Inflammatory Phenotype of Periodontal Cells by Targeting CB2 Receptors. Biomedicines 2020, 8, 164. [Google Scholar] [CrossRef]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mazzon, E.; Mannino, F.; Squadrito, V.; Arcoraci, V.; Minutoli, L.; Campo, G.M.; et al. β-Caryophyllene Mitigates Collagen Antibody Induced Arthritis (CAIA) in Mice Through a Cross-Talk between CB2 and PPAR-γ Receptors. Biomolecules 2019, 9, 326. [Google Scholar] [CrossRef]
- Syed Najmuddin, S.U.; Romli, M.F.; Hamid, M.; Alitheen, N.B.; Nik Abd Rahman, N.M. Anti-cancer effect of Annona Muricata Linn Leaves Crude Extract (AMCE) on breast cancer cell line. BMC Complement. Altern. Med. 2016, 16, 311. [Google Scholar] [CrossRef] [PubMed]
- Pallio, G.; Bitto, A.; Pizzino, G.; Galfo, F.; Irrera, N.; Squadrito, F.; Squadrito, G.; Pallio, S.; Anastasi, G.P.; Cutroneo, G.; et al. Adenosine Receptor Stimulation by Polydeoxyribonucleotide Improves Tissue Repair and Symptomology in Experimental Colitis. Front. Pharmacol. 2016, 7, 273. [Google Scholar] [CrossRef]
- Minutoli, L.; Marini, H.; Rinaldi, M.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Calò, M.; Adamo, E.B.; Trichilo, V.; et al. A dual inhibitor of cyclooxygenase and 5-lipoxygenase protects against kainic acid-induced brain injury. Neuromol. Med. 2015, 17, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Bitto, A.; Pallio, G.; Mannino, F.; Arcoraci, V.; Aliquò, F.; Minutoli, L.; De Ponte, C.; D’andrea, P.; et al. Cadmium-Induced Oxidative Stress Impairs Glycemic Control in Adolescents. Oxid. Med. Cell Longev. 2017, 2017, 6341671. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; Arcoraci, V.; Mannino, F.; Vermiglio, G.; Pallio, G.; Minutoli, L.; Bagnato, G.; Anastasi, G.P.; Mazzon, E.; Bramanti, P.; et al. Activation of A2A Receptor by PDRN Reduces Neuronal Damage and Stimulates WNT/β-CATENIN Driven Neurogenesis in Spinal Cord Injury. Front. Pharmacol. 2018, 9, 506. [Google Scholar] [CrossRef] [PubMed]
- Pallio, G.; Micali, A.; Benvenga, S.; Antonelli, A.; Marini, H.R.; Puzzolo, D.; Macaione, V.; Trichilo, V.; Santoro, G.; Irrera, N.; et al. Myo-inositol in the protection from cadmium-induced toxicity in mice kidney: An emerging nutraceutical challenge. Food Chem. Toxicol. 2019, 132, 110675. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Micali, A.; Rinaldi, M.; Irrera, N.; Marini, H.; Puzzolo, D.; Pisani, A.; Lorenzini, C.; Valenti, A.; Laurà, R.; et al. Polydeoxyribonucleotide, an Adenosine-A2(A) Receptor Agonist, Preserves Blood Testis Barrier from Cadmium-Induced Injury. Front. Pharmacol. 2017, 7, 537. [Google Scholar] [CrossRef]
- Pizzino, G.; Bitto, A.; Pallio, G.; Irrera, N.; Galfo, F.; Interdonato, M.; Mecchio, A.; De Luca, F.; Minutoli, L.; Squadrito, F.; et al. Blockade of the JNK signalling as a rational therapeutic approach to modulate the early and late steps of the inflammatory cascade in polymicrobial sepsis. Mediators Inflamm. 2015, 2015, 591572. [Google Scholar] [CrossRef] [PubMed]
- Interdonato, M.; Bitto, A.; Pizzino, G.; Irrera, N.; Pallio, G.; Mecchio, A.; Cuspilici, A.; Minutoli, L.; Altavilla, D.; Squadrito, F. Levels of heavy metals in adolescents living in the industrialised area of Milazzo-Valle del Mela (Northern Sicily). J. Environ. Public Health 2014, 2014, 326845. [Google Scholar] [CrossRef]
- Bitto, A.; Giuliani, D.; Pallio, G.; Irrera, N.; Vandini, E.; Canalini, F.; Zaffe, D.; Ottani, A.; Minutoli, L.; Rinaldi, M.; et al. Effects of COX1-2/5-LOX blockade in Alzheimer transgenic 3xTg-AD mice. Inflamm. Res. 2017, 66, 389–398. [Google Scholar] [CrossRef]
- Cesar, V.; Jozić, I.; Begović, L.; Vuković, T.; Mlinarić, S.; Lepeduš, H.; Borović Šunjić, S.; Žarković, N. Cell-Type-Specific Modulation of Hydrogen Peroxide Cytotoxicity and 4-Hydroxynonenal Binding to Human Cellular Proteins In Vitro by Antioxidant Aloe vera Extract. Antioxidants 2018, 7, 125. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Chapman and Hall Limited: London, UK, 1998. [Google Scholar]
- Karunyadevi, S.; Arun, N.; Surekha, V. Screening of phytochemical compounds, antioxidant and antimicrobial activity of Aloe vera and Arkaa. Adv. Biotec. 2009, 9, 38–43. [Google Scholar]
- Hull, D.S.; Csukas, S.; Green, K.; Livingston, V. Hydrogen peroxide and corneal endothelium. Acta Ophthalmol. 1981, 59, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Crupi, R.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Petrosino, S.; Evangelista, M.; Di Paola, R.; et al. N-Palmitoylethanolamine-oxazoline (PEA-OXA): A new therapeutic strategy to reduce neuroinflammation, oxidative stress associated to vascular dementia in an experimental model of repeated bilateral common carotid arteries occlusion. Neurobiol Dis. 2019, 125, 77–91. [Google Scholar] [CrossRef]
- Cho, K.S.; Lee, E.H.; Choi, J.S.; Joo, C.K. Reactive oxygen species-induced apoptosis and necrosis in bovine corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 1999, 40, 911–919. [Google Scholar]
- Joyce, N.C.; Zhu, C.C.; Harris, D.L. Relationship among oxidative stress DNA damage and proliferative capacity in human corneal endothelium. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Del Maestro, R.F.; Thaw, H.H.; Bjork, J.; Planker, M.; Arfors, K.E. Free radicals as mediators of tissue injury. Acta Physiol. Scand. Suppl. 1980, 492, 43–57. [Google Scholar] [PubMed]
- Fridovich, I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J. Biol. Chem. 1970, 245, 4053–4057. [Google Scholar] [CrossRef]
- Liu, F.W.; Liu, F.C.; Wang, Y.R.; Tsai, H.I.; Yu, H.P. Aloin Protects Skin Fibroblasts from Heat Stress-Induced Oxidative Stress Damage by Regulating the Oxidative Defense System. PLoS ONE 2015, 10, 0143528. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, B.; Avila, G.; Segura, D.; Escalante, B. Antiinflammatory activity of extracts from Aloe vera gel. J. Ethnopharmacol. 1996, 55, 69–75. [Google Scholar] [CrossRef]
- Duansak, D.; Somboonwong, J.; Patumraj, S. Effects of Aloe vera on leukocyte adhesion and TNF-alpha and IL-6 levels in burn wounded rats. Clin. Hemorheol. Microcirc. 2003, 29, 239–246. [Google Scholar] [PubMed]
- Chen, M.; Hu, D.N.; Pan, Z.; Lu, C.W.; Xue, C.Y.; Aass, I. Curcumin protects against hyperosmoticity-induced IL-1beta elevation in human corneal epithelial cell via MAPK pathways. Exp. Eye Res. 2010, 90, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Green, K.; Tsai, J.; Luxenberg, M.N. Effect of Aloe vera on corneal epithelial wound healing. J. Toxicol. Cutan. Ocul. Toxicol. 1996, 15, 301–304. [Google Scholar] [CrossRef]
- Choi, W.; Lee, J.B.; Cui, L.; Li, Y.; Li, Z.; Choi, J.S.; Lee, H.S.; Yoon, K.C. Therapeutic Efficacy of Topically Applied Antioxidant Medicinal Plant Extracts in a Mouse Model of Experimental Dry Eye. Oxid. Med. Cell Longev. 2016, 2016, 4727415. [Google Scholar] [CrossRef]
- Sandhu, P.S.; Singh, B.; Gupta, V.; Parveen, B.; Dharmendra, K. Potential Herbs Used in Ocular Diseases. J. Pharm. Sci. Res. 2011, 3, 1127–1140. [Google Scholar]
- Bjørklund, G.; Dadar, M.; Martins, N.; Chirumbolo, S.; Goh, B.H.; Smetanina, K.; Lysiuk, R. Brief Challenges on Medicinal Plants: An Eye-Opening Look at Ageing-Related Disorders. Basic Clin. Pharmacol. Toxicol. 2018, 22, 539–558. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.S.; Burk, M.; Loprinzi, C.L.; Hill, M.; Schomberg, P.J.; Nearhood, K.; O’Fallon, J.R.; Laurie, J.A.; Shanahan, T.G.; Moore, R.L.; et al. Phase III doubleblind evaluation of an aloe vera gel as a prophylactic agent for radiation-induced skin toxicity. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 345–349. [Google Scholar] [CrossRef]
- Esposito, E.; Campolo, M.; Casili, G.; Lanza, M.; Franco, D.; Filippone, A.; Peritore, A.F.; Cuzzocrea, S. Protective Effects of Xyloglucan in Association with the Polysaccharide Gelose in an Experimental Model of Gastroenteritis and Urinary Tract Infections. Int. J. Mol. Sci. 2018, 19, 1844. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, N.; Candoken, E.; Akev, N. Implications for degenerative disorders: Antioxidative activity, total phenols, flavonoids, ascorbic acid, beta-carotene and beta-tocopherol in Aloe vera. Oxid. Med. Cell Longev. 2009, 2, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, K.L.; Viljoen, A.M.; Jäger, A.K. Screening of Aloe species for antioxidant activity. S. Afr. J. Bot. 2003, 69, 599–602. [Google Scholar] [CrossRef]
- Chen, X.D.; Huang, L.Y.; Wu, B.Y.; Jiang, Q.; Wang, Z.C.; Lin, X.H. Effect of Aloe vera polysaccharide on the release of cytokines and nitric oxide in cultured human keratinocytes. Chin. Crit. Care Med. 2005, 17, 296–298. [Google Scholar]
- Park, J.H.; Kim, J.Y.; Kim, D.J.; Kim, M.; Chang, M.; Chuck, R.S.; Park, C.Y. Effect of Nitric Oxide on Human Corneal Epithelial Cell Viability and Corneal Wound Healing. Sci. Rep. 2017, 7, 8093. [Google Scholar] [CrossRef] [PubMed]
- Rose, L.; Kelliher, C.; Jun, A.S. Endothelial keratoplasty: Historical perspectives, current techniques, future directions. Can. J. Ophthalmol. 2009, 44, 401–405. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).