Antioxidant Effect of a Probiotic Product on a Model of Oxidative Stress Induced by High-Intensity and Duration Physical Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Eligibility Criteria and Randomization
2.3. Intervention
2.4. Physical Exercise Oxidative Stress Model
2.5. Study Procedures
2.6. Study Variables
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Lipid, Protein, and DNA-Related Oxidative Stress Biomarkers and Antioxidative Enzymes
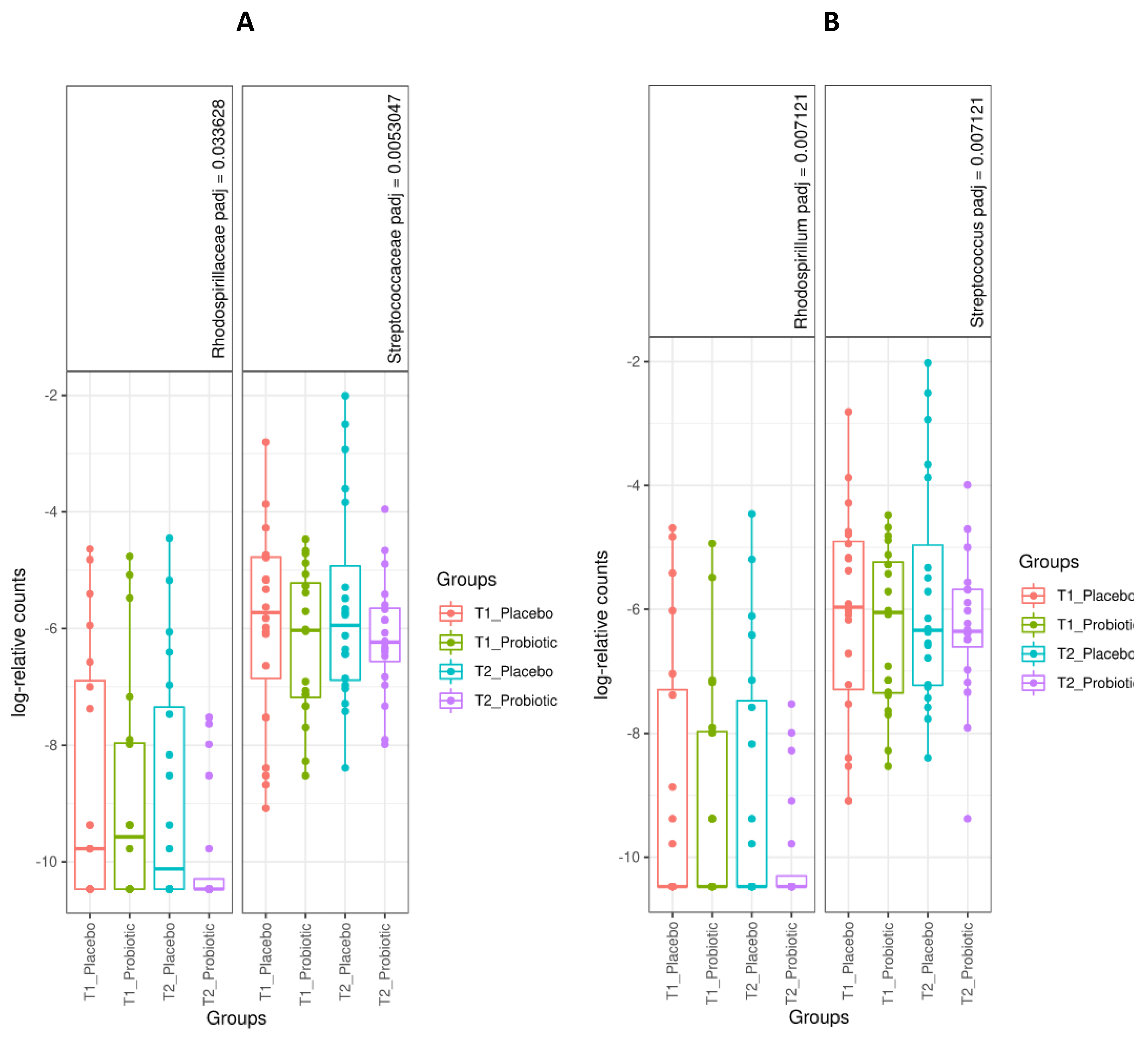
3.3. Microbiome Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox. Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Simpson, D.S.A.; Oliver, P.L. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative stress in cardiovascular diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Available online: https://www.hindawi.com/journals/omcl/2020/8609213/ (accessed on 27 January 2021).
- Romano, A.D.; Serviddio, G.; de Matthaeis, A.; Bellanti, F.; Vendemiale, G. Oxidative stress and aging. J. Nephrol. 2010, 23 (Suppl. S15), S29–S36. [Google Scholar]
- Vasquez, E.C.; Pereira, T.M.C.; Campos-Toimil, M.; Baldo, M.P.; Peotta, V.A. Gut microbiota, diet, and chronic diseases: The role played by oxidative stress. Oxid. Med. Cell Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Jones, R.M.; Mercante, J.W.; Neish, A.S. Reactive oxygen production induced by the gut microbiota: Pharmacotherapeutic implications. Curr. Med. Chem. 2012, 19, 1519–1529. [Google Scholar] [CrossRef]
- Neish, A.S. Microbes in gastrointestinal health and disease. Gastroenterology 2009, 136, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.; Kasper, D.L. Microbiota-stimulated immune mechanisms to maintain gut homeostasis. Curr. Opin. Immunol. 2010, 22, 455–460. [Google Scholar] [CrossRef]
- Ismail, A.S.; Hooper, L.V. Epithelial cells and their neighbors. IV. bacterial contributions to intestinal epithelial barrier integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G779–G784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, C.; Michels, M.; Ávila, P.; Burger, H.; Milioli, M.V.M.; Dal-Pizzol, F. The protective effects of fecal microbiota transplantation in an experimental model of necrotizing enterocolitis. J. Pediatr. Surg. 2019, 54, 1578–1583. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox mechanism of reactive oxygen species in exercise. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox. Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, T.; Muraoka, I. Exercise-induced oxidative stress and the effects of antioxidant intake from a physiological viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pingitore, A.; Lima, G.P.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef]
- Powers, S.K.; DeRuisseau, K.C.; Quindry, J.; Hamilton, K.L. Dietary antioxidants and exercise. J. Sports Sci. 2004, 22, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Genomic Sequence and Pre-Clinical Safety Assessment of Bifidobacterium Longum CECT 7347, a Probiotic Able to Reduce the Toxicity and Inflammatory Potential of Gliadin-Derived Peptides|Abstract. Available online: https://www.longdom.org/abstract/genomic-sequence-and-preclinical-safety-assessment-of-embifidobacterium-longumem-cect-7347-a-probiotic-able-to-reduce-th-33128.html (accessed on 27 January 2021).
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Krotkiewski, M.; Brzezinska, Z. Lipid peroxides production after strenuous exercise and in relation to muscle morphology and capillarization. Muscle Nerve 1996, 19, 1530. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [Green Version]
- Criswell, D.; Powers, S.; Dodd, S.; Lawler, J.; Edwards, W.; Renshler, K.; Grinton, S. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Med. Sci. Sports Exerc. 1993, 25, 1135–1140. [Google Scholar] [CrossRef]
- Hammeren, J.; Powers, S.; Criswell, D.; Martin, A.; Lowenthal, D.; Pollock, M. Exercise training-induced alterations in skeletal muscle oxidative and antioxidant enzyme activity in senescent rats. Int. J. Sports Med. 1992, 13, 412–416. [Google Scholar] [CrossRef]
- Theofilidis, G.; Bogdanis, G.C.; Koutedakis, Y.; Karatzaferi, C. Monitoring exercise-induced muscle fatigue and adaptations: Making sense of popular or emerging indices and biomarkers. Sports 2018, 6, 153. [Google Scholar] [CrossRef] [Green Version]
- De Salazar, L.; Torregrosa-García, A.; Luque-Rubia, A.J.; Ávila-Gandía, V.; Domingo, J.C.; López-Román, F.J. Oxidative stress in endurance cycling is reduced dose-dependently after one month of re-esterified DHA supplementation. Antioxidants 2020, 9, 1145. [Google Scholar] [CrossRef]
- Torregrosa-García, A.; Ávila-Gandía, V.; Luque-Rubia, A.J.; Abellán-Ruiz, M.S.; Querol-Calderón, M.; López-Román, F.J. Pomegranate extract improves maximal performance of trained cyclists after an exhausting endurance trial: A randomised controlled trial. Nutrients 2019, 11, 721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Sánchez, A.; Alacid, F.; Rubio-Arias, J.A.; Fernández-Lobato, B.; Ramos-Campo, D.J.; Aguayo, E. Consumption of watermelon juice enriched in L-citrulline and pomegranate ellagitannins enhanced metabolism during physical exercise. J. Agric. Food Chem. 2017, 65, 4395–4404. [Google Scholar] [CrossRef]
- López-Román, F.J.; Ávila-Gandía, V.; Contreras-Fernández, C.J.; Luque-Rubia, A.J.; Villegas-García, J.A. Effect of docosahexaenoic acid supplementation on differences of endurance exercise performance in competitive and non-competitive male cyclists. Gazz. Med. Ital. Arch. Sci. Med. 2019, 178, 411–416. [Google Scholar] [CrossRef]
- Ramos-Campo, D.J.; Ávila-Gandía, V.; López-Román, F.J.; Miñarro, J.; Contreras, C.; Soto-Méndez, F.; Domingo Pedrol, J.C.; Luque-Rubia, A.J. Supplementation of re-esterified docosahexaenoic and eicosapentaenoic acids reduce inflammatory and muscle damage markers after exercise in endurance athletes: A randomized, controlled crossover trial. Nutrients 2020, 12, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Michalickova, D.; Kotur-Stevuljevic, J.; Miljkovic, M.; Dikic, N.; Kostic-Vucicevic, M.; Andjelkovic, M.; Koricanac, V.; Djordjevic, B. Effects of probiotic supplementation on selected parameters of blood prooxidant-antioxidant balance in elite athletes: A double-blind randomized placebo-controlled study. J. Hum. Kinet. 2018, 64, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martarelli, D.; Verdenelli, M.C.; Scuri, S.; Cocchioni, M.; Silvi, S.; Cecchini, C.; Pompei, P. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr. Microbiol. 2011, 62, 1689–1696. [Google Scholar] [CrossRef]
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Välimäki, I.A.; Vuorimaa, T.; Ahotupa, M.; Kekkonen, R.; Korpela, R.; Vasankari, T. Decreased training volume and increased carbohydrate intake increases oxidized LDL levels. Int. J. Sports Med. 2012, 33, 291–296. [Google Scholar] [CrossRef]
- Ardeshirlarijani, E.; Tabatabaei-Malazy, O.; Mohseni, S.; Qorbani, M.; Larijani, B.; Baradar Jalili, R. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: A meta-analysis of randomized trials. Daru 2019, 27, 827–837. [Google Scholar] [CrossRef]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef]
- Escamilla, J.; Lane, M.A.; Maitin, V. Cell-free supernatants from probiotic lactobacillus casei and lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr. Cancer 2012, 64, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C.; et al. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef]
- Jacouton, E.; Mach, N.; Cadiou, J.; Lapaque, N.; Clément, K.; Doré, J.; van Hylckama Vlieg, J.E.T.; Smokvina, T.; Blottière, H.M. Lactobacillus rhamnosus CNCMI-4317 modulates fiaf/angptl4 in intestinal epithelial cells and circulating level in mice. PLoS ONE 2015, 10, e0138880. [Google Scholar] [CrossRef] [Green Version]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W.J.H. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.R.; Cadet, J.; Möller, L.; Poulsen, H.E.; Viña, J. Are we sure we know how to measure 8-Oxo-7,8-dihydroguanine in DNA from human cells? Arch. Biochem. Biophys. 2004, 423, 57–65. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free radical-induced damage to DNA: Mechanisms and measurement. Free Radic. Biol. Med. 2002, 32, 1102–1115. [Google Scholar] [CrossRef]
- Mikhed, Y.; Görlach, A.; Knaus, U.G.; Daiber, A. Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair. Redox. Biol. 2015, 5, 275–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Ni, X. ROS-mediated DNA methylation pattern alterations in carcinogenesis. Curr. Drug Targets 2015, 16, 13–19. [Google Scholar] [CrossRef]
- Samuel, B.S.; Gordon, J.I. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc. Natl. Acad. Sci. USA 2006, 103, 10011–10016. [Google Scholar] [CrossRef] [Green Version]
- Samuel, B.S.; Hansen, E.E.; Manchester, J.K.; Coutinho, P.M.; Henrissat, B.; Fulton, R.; Latreille, P.; Kim, K.; Wilson, R.K.; Gordon, J.I. Genomic and metabolic adaptations of methanobrevibacter smithii to the human gut. Proc. Natl. Acad. Sci. USA 2007, 104, 10643–10648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, F.; Larsen, N.; de Mello Tieghi, T.; Adorno, M.A.T.; Kot, W.; Saad, S.M.I.; Jespersen, L.; Sivieri, K. Modulation of gut microbiota from obese individuals by in vitro fermentation of citrus pectin in combination with bifidobacterium longum BB-46. Appl. Microbiol. Biotechnol. 2018, 102, 8827–8840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Deng, Q.; Xu, J.; Wang, X.; Hu, C.; Tang, H.; Huang, F. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019, 116, 1202–1211. [Google Scholar] [CrossRef]
- Eren, A.M.; Sogin, M.L.; Morrison, H.G.; Vineis, J.H.; Fisher, J.C.; Newton, R.J.; McLellan, S.L. A single genus in the gut microbiome reflects host preference and specificity. ISME J. 2015, 9, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise alters gut microbiota composition and function in lean and obese humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Lebas, M.; Garault, P.; Carrillo, D.; Codoñer, F.M.; Derrien, M. Metabolic response of Faecalibacterium prausnitzii to cell-free supernatants from lactic acid bacteria. Microorganisms 2020, 8, 1528. [Google Scholar] [CrossRef]
- Edamatsu, T.; Fujieda, A.; Itoh, Y. Phenyl sulfate, indoxyl sulfate and p-cresyl sulfate decrease glutathione level to render cells vulnerable to oxidative stress in renal tubular cells. PLoS ONE 2018, 13, e0193342. [Google Scholar] [CrossRef] [Green Version]
- Asai, H.; Hirata, J.; Hirano, A.; Hirai, K.; Seki, S.; Watanabe-Akanuma, M. Activation of aryl hydrocarbon receptor mediates suppression of hypoxia-inducible factor-dependent erythropoietin expression by indoxyl sulfate. Am. J. Physiol. Cell Physiol. 2016, 310, C142–C150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleftheriadis, T.; Pissas, G.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Kynurenine, by activating aryl hydrocarbon receptor, decreases erythropoietin and increases hepcidin production in HepG2 cells: A new mechanism for anemia of inflammation. Exp. Hematol. 2016, 44, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Kuwabara, R.; de Haan, B.J.; Smink, A.M.; de Vos, P. Acetate and butyrate improve β-cell metabolism and mitochondrial respiration under oxidative stress. Int. J. Mol. Sci. 2020, 21, 1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yardeni, T.; Tanes, C.E.; Bittinger, K.; Mattei, L.M.; Schaefer, P.M.; Singh, L.N.; Wu, G.D.; Murdock, D.G.; Wallace, D.C. Host mitochondria influence gut microbiome diversity: A role for ROS. Sci. Signal. 2019, 12, 588. [Google Scholar] [CrossRef]



| Variables | Test #1 | Test #2 (6-Week Probiotic/Placebo Intake) | Test #1 vs. Test #2 | Between-Group Difference p Value F Snedecor | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline Mean (SE) | After Exercise Mean (SE) | Mean Difference (95% CI), p Value | Baseline Mean (SE) | After Exercise Mean (SE) | Mean Difference (95% CI), p Value | Δ Mean Difference (95% CI) p Value | ||
| Urinary isoprostane, pg/day | ||||||||
| Placebo group | 1.3 (0.5) | 2.5 (0.7) | 1.2 (0.5 to 1.9) p = 0.05 | 1.2 (0.5) | 2.1 (0.7) | 0.9 (0.3 to 1.5) p < 0.05 | –0.3 (−0.8 to 0.2) p = 0.292 | p = 0.213 F = 1.601 |
| Probiotic group | 2.1 (0.5) | 3.3 (0.7) | 1.3 (0.6 to 2.0) p < 0.05 | 2.2 (0.5) | 3.6 (0.7) | 1.4 (0.9 to 2.0) p < 0.001 | 0.1 (−0.3 to 0.7) p = 0.476 | |
| Serum MDA, ng/mL | ||||||||
| Placebo group | 347.4 (84.8) | 491.1 (145.3) | 143.7 (−25.8 to 313.2) p = 0.094 | 312.9 (64.3) | 454.4 (113.3) | 141.5 (−52.8 to 335.8) p = 0.149 | −2.2 (−147 to 142.6) p = 0.975 | p < 0.05 F = 4.195 |
| Probiotic group | 433.2 (82.9) | 687.4 (142.0) | 254 (88 to 419.8) p < 0.05 | 358 (62.9) | 404.6 (110.7) | 46.6 (−143 to 236.4) p = 0.623 | –207.6 (−0.341 to −66.1) p < 0.05 | |
| Serum Ox-LDL, pg/mL | ||||||||
| Placebo group | 740.3 (82.9) | 899.6 (64.1) | 159.3 (81.9 to 236.7) p < 0.001 | 779.9 (64.2) | 977.4 (78.4) | 196.6 (83.0 to 310.2) p < 0.05 | 37.3 (−83.5 to 158.0) p = 0.536 | p < 0.063 F = 3.653 |
| Probiotic group | 646.2 (60.1) | 809.0 (62.6) | 162.9 (87.2 to 238.5) p < 0.001 | 772.9 (67.6) | 813.3 (77.1) | 40.4 (−70.6 to 151.4) p = 0.467 | −122.5 (−240 to −4.5) p < 0.05 | |
| Urinary 8-OHdG, pg/day | ||||||||
| Placebo group | 10.7 (0.2) | 23.1 (3.8) | 12.4 (8.3 to 16.6) p < 0.001 | 11.8 (2.4) | 23.4 (3.3) | 11.5 (8.1 to 15.0) p < 0.001 | −0.9 (−4.6 to 2.8) p = 0.620 | p < 0.001 F = 15.144 |
| Probiotic group | 13.3 (2.0) | 29.0 (3.7) | 15.7 (11.6 to 19.7) p < 0.001 | 13.6 (2.4) | 18.4 (3.2) | 4.8 (1.4 to 8.1) p < 0.001 | −10.9 (−14.5 to −7.3) p < 0.01 | |
| Serum protein carbonyl, pmol/mg protein | ||||||||
| Placebo group | 124.0 (16.3) | 160.0 (18.0) | 36.0 (18.4 to 53.6) p < 0.001 | 112.4 (19.9) | 162.0 (20.6) | 49.6 (32.6 to 66.2) p < 0.001 | 13.6 (−4.4 to 31.6) p = 0.135 | p = 0.434 F = 0.625 |
| Probiotic group | 166.8 (15.9) | 204.2 (17.6) | 37.4 (20.1 to 54.6) p < 0.001 | 162.9 (17.5) | 204 (20.1) | 41.1 (24.8 to 57.3) p < 0.001 | 3.7 (−13.9 to 21.3) p = 0.671 | |
| Serum GPx, pg/mL | ||||||||
| Placebo group | 526.9 (84.9) | 788.0 (92.1) | 261.1 (162.2 to 360.0) p < 0.001 | 633.8 (80.3) | 1111.7 (214.5) | 477.8 (112.5 to 843.2) p < 0.05 | 216.7 (−156.4 to 598.9) p = 0.248 | p = 0.253 F = 1.598 |
| Probiotic group | 473.4 (83.0) | 594.8 (90.0) | 121.4 (24.7 to 218.0) p < 0.05 | 598.5 (78.4) | 610.0 (209.6) | 11.6 (−345.4 to 368.5) p = 0.948 | −109.9 (−474.4 to 254.7) p = 0.546 | |
| Serum SOD, ng/mL | ||||||||
| Placebo group | 24.1 (2.6) | 34.5 (3.3) | 10.5 (5.9 to 15.1) p < 0.001 | 22.1 (2.2) | 29.9 (2.7) | 7.8 (3.7 to 11.9) p < 0.001 | −2.9 (−8.6 to 3.2) p = 0.358 | p = 0.267 F = 1.274 |
| Probiotic group | 29.2 (2.6) | 33.1 (3.3) | 3.9 (−0.7 to 8.5) p = 0.094 | 24.9 (2.2) | 30.7 (2.7) | 5.8 (1.7 to 10) p < 0.05 | 2 (−4 to 7.8) p = 0.511 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez Macarro, M.; Ávila-Gandía, V.; Pérez-Piñero, S.; Cánovas, F.; García-Muñoz, A.M.; Abellán-Ruiz, M.S.; Victoria-Montesinos, D.; Luque-Rubia, A.J.; Climent, E.; Genovés, S.; et al. Antioxidant Effect of a Probiotic Product on a Model of Oxidative Stress Induced by High-Intensity and Duration Physical Exercise. Antioxidants 2021, 10, 323. https://doi.org/10.3390/antiox10020323
Sánchez Macarro M, Ávila-Gandía V, Pérez-Piñero S, Cánovas F, García-Muñoz AM, Abellán-Ruiz MS, Victoria-Montesinos D, Luque-Rubia AJ, Climent E, Genovés S, et al. Antioxidant Effect of a Probiotic Product on a Model of Oxidative Stress Induced by High-Intensity and Duration Physical Exercise. Antioxidants. 2021; 10(2):323. https://doi.org/10.3390/antiox10020323
Chicago/Turabian StyleSánchez Macarro, Maravillas, Vicente Ávila-Gandía, Silvia Pérez-Piñero, Fernando Cánovas, Ana María García-Muñoz, María Salud Abellán-Ruiz, Desirée Victoria-Montesinos, Antonio J. Luque-Rubia, Eric Climent, Salvador Genovés, and et al. 2021. "Antioxidant Effect of a Probiotic Product on a Model of Oxidative Stress Induced by High-Intensity and Duration Physical Exercise" Antioxidants 10, no. 2: 323. https://doi.org/10.3390/antiox10020323
APA StyleSánchez Macarro, M., Ávila-Gandía, V., Pérez-Piñero, S., Cánovas, F., García-Muñoz, A. M., Abellán-Ruiz, M. S., Victoria-Montesinos, D., Luque-Rubia, A. J., Climent, E., Genovés, S., Ramon, D., Chenoll, E., & López-Román, F. J. (2021). Antioxidant Effect of a Probiotic Product on a Model of Oxidative Stress Induced by High-Intensity and Duration Physical Exercise. Antioxidants, 10(2), 323. https://doi.org/10.3390/antiox10020323








