Abstract
Honeybee products have positive effects on the reproductive performance of mammals. Many honeybee product constituents are biologically active, with antioxidant, antimicrobial, antiviral, anti-inflammatory, immunomodulatory, antifungal, wound-healing, and cardio-protective properties. Honeybee products also improve male and female fertility rates by enhancing gamete cryopreservation, in vitro maturation and fertilization, and embryo development. Previously published studies confirmed their efficacy for alleviating reproductive toxicity caused by contaminants and lifestyle habits that impair overall health and well-being. However, high-dose oral administration of honeybee products may adversely affect the reproductive system, and unfavorable effects were alleviated by treatment cessation. For this reason, this review proposes that bioactive components from bee products can be used as a strategy for improving the reproductive performance and health of mammals.
1. Introduction
Procreation is a pivotal innate physiological event for all creatures. For humankind, reproductive health and fertility are of particular importance for maintaining social, mental, and physical health status. Supporting adequate reproductive performance in food-producing animals is also important to humans, enabling mass production to maintain food security. However, reproductive events and fertility of individuals/organisms are greatly impacted by modern lifestyle circumstances such as increased exposure to environmental and behavioral stresses. According to a World Health Organization report, 60–80 million couples, representing 8–12% of couples worldwide, are currently experiencing infertility [1,2,3]. Similarly, poor reproductive efficiency is evoked in production animals owing to management practices for producing animal products in massive quantities [4]. Therefore, research endeavors to improve and maintain adequate reproductive health are essential for humankind and animals.
The use of natural products as alternatives to synthetic prophylactic and therapeutic drugs is increasingly recommended to improve many aspects of human and animal health [5]. Honeybee products, including honey, propolis, royal jelly, bee pollen, beeswax, drone brood, bee venom, and bee bread, contain several natural bioactive components with various pharmaceutical and nutritional properties. Given the chemical constitutes of these products, honeybee products may have beneficial prophylactic and therapeutic effects on reproductive health in mammals. For example, honey is an energy-rich product that contains substantial concentrations of polyphenolic compounds with antioxidant activity [6,7,8,9], which can improve reproductive events by improving energy status [10,11] and/or redox status [12]. Additionally, propolis has many pharmaceutical properties thanks to its enrichment with flavonoids, which are polyphenolic compounds required for maintaining reproductive health, particularly under stressful conditions such as heat stress [13,14]. Furthermore, products such as royal jelly and drone brood contain sex hormones, and thus can be used to modulate endocrine system functions [15]. Many studies have shown the positive effects of honeybee products on reproductive health in humans [16,17] and animals [14,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. However, the potential hazards of honeybee products have also been discussed [37,38]. This review describes honeybee products, emphasizing the unique chemical constituents of each product and their effects on reproductive performance in humans and animals. This will help in exploring the treasure of the natural active compounds in these products, allowing opportunities to detect novel pharmaceutical molecules for safe reproductive health manipulation.
2. Honeybee Species and Bioactive Components of Honeybee Products
There are approximately 20,000 known species of bees. Honeybees represent a small portion of all bees, with eight recognized species (Apis mellifera, A. mellifera; Apis cerana, A. cerana; Apis dorsata, A. dorsata; Apis florea, A. florea; Apis andreniformis, A. andreniformis; Apis laboriosa, A. laboriosa; Apis koschevnikovi, A. koschevnikovi; and Apis nigrocincta, A. nigrocincta) and 43 subspecies (Table 1). Among these species, A. mellifera and A. cerana are domesticated by humans, while the other species are wild. In Europe and America, A. mellifera (Western honeybee) is the species universally managed by beekeepers. This species has several subspecies, including A. mellifera ligustica (Italian bee), A. mellifera mellifera (European dark bee), and A. mellifera carnica (Carniolan honeybee). A. cerana (Asiatic honeybee) is the common species bred for honey production in the tropics [1,2,39,40].

Table 1.
The major species of honeybees and their geographical distribution.
Apitherapy, a branch of alternative medicine that uses honeybee products, has been applied to protect from and treat diseases for many centuries [10,12]. Nevertheless, the physical properties and chemical compositions of honeybee products are highly varied and depend on factors such as plant type, climatic conditions, and geographical region. Moreover, the metabolites, physiology, endogenous enzymes, and flora of the collecting insects can affect the physical properties and chemical composition of honeybee products [39,40]. In the following section, different honeybee products and their major and minor chemical constitutes will be shown.
2.1. Honey
Honey is a sweet substance produced by honeybees from collected flower nectar and plant secretions, which are then combined with specific bee substances, deposited, dehydrated, and stored in honeycombs to ripen [13]. Although many honeybee species, wasps, and ants produce different types of honey [4], the legal definition of honey according to the European Union Council [41] is honey that is produced by A. mellifera honeybees [4,13].
Chemically, honey is a natural food substance mainly composed of simple sugars, along with minor constituents such as minerals, vitamins, amino acids, organic acids, flavonoids and other phenolic compounds, and aromatic substances [13]. Generally, sugars comprise 95–99% of honey dry matter, mainly in the form of fructose (32–38% of total sugars). In addition, several other monosaccharides (glucose), disaccharides (sucrose and maltose), and oligosaccharides (maltotriose and panose) are found in honey. Low concentrations of proteins (0.5%) in the form of enzymes (amylase, sucrase or α-glucosidase, and glucose oxidase) and more than 20 individual amino acids (proline is the most important) have also been identified [8]. Organic acids (0.57%; gluconic, acetic, butyric, citric, formic, lactic, malic, pyroglutamic, and succinic acids) as well as major and minor minerals (calcium, magnesium, sodium, potassium, phosphorus, sulfur, zinc, iron, copper, and manganese) are also components of honey. In addition, vitamins such as ascorbic acid (vitamin C), thiamine (vitamin B1), riboflavin (vitamin B2), nicotinic acid (vitamin B3), pantothenic acid (vitamin B5), pyridoxine (vitamin B6), biotin (vitamin B8), folic acid (vitamin B9), and cyanocobalamin (vitamin B12) are present in measurable concentrations [40,42]. Honey also contains significant amounts of bioactive polyphenols, including phenolic acids (vanillic, caffeic, syringic, p-coumaric, ferulic, ellagic, 3-hydroxybenzoic, chlorogenic, 4-hydroxybenzoic, rosmarinic, gallic, and benzoic acids) and flavonoids (quercetin, kaempferol, myricetin, pinobanksin, pinocembrin, chrysin, galangin, hesperetin, and others) [8,43]. The unique chemical composition of honey confers several nutritional and medicinal properties [42]. Honey has a long history of use in traditional medicine owing to its antioxidant, hepato-protective, cardio-protective, anti-inflammatory, antidiabetic, hypolipidemic, anticancer, gastrointestinal-protective, and wound-healing properties. In addition, honey produces antibacterial effects against several microorganisms, including Escherichia coli, Shigella spp., Helicobacter pylori, and Salmonella spp. [13].
2.2. Royal Jelly
Royal jelly is a thick, viscous, and milky natural substance that is formed as a result of incomplete digestion of honeydew in the stomach of a bee worker [44]. In the hive, royal jelly is fed to larvae developing into female workers and male drones until they are three days old, while individuals developing into future queens are fed royal jelly throughout the larval period. Moreover, royal jelly is provided as a special nutrient for adult queens throughout their lives [13,45,46,47]. Nutritionally, royal jelly is a high-value nutrient, consisting of proteins (27%–41%), carbohydrates (30%), and lipids (3%–19%). It also contains a unique group of nine soluble proteins called major royal jelly proteins (MRJPs), of which MRJPs 1–5 account for approximately 82%. Several antioxidant peptides have been isolated from royal jelly hydrolysate, some with robust hydroxyl radical scavenging activity. Moreover, royal jelly peptides such as jelleines, royalisin, and apisimin exhibit antimicrobial activity [15]. Fatty acids are the major lipid constituents (mainly hydroxydecanoic acid), followed by phenolic lipids, waxes, steroids, and finally phospholipids [48]. Royal jelly also provides a valuable energy source thanks to its enrichment with carbohydrates (3.4%–7.7% glucose, 2.3%–7.8% fructose) and energy-rich molecules such as adenosine monophosphate [15]. In addition, royal jelly contains phytosterols, flavonoids, vitamins (B9, B1, niacin, B3, B5, B2, B6, and beta-carotene), minerals (iron, potassium, zinc, magnesium, and copper), and hormones (prolactin, testosterone, estradiol, and progesterone) [15]. The diverse royal jelly chemical composition confers several pharmaceutical and nutraceutical properties, such as antibacterial, antioxidant, anti-inflammatory, antidiabetic, hypotensive, hepato-protective, antitumor, and hypoglycemic properties [46,47,48,49]. Moreover, immunomodulatory activities and estrogen-like effects have been reported. Accordingly, royal jelly has been widely used in commercial medical products, health foods, and cosmetics for more than 35 years [49].
2.3. Propolis
Propolis (bee glue) is a dark-colored resinous substance that honeybees produce by mixing collected plant parts (buds, floral buds, leaves, branches, and barks) and salivary gland secretions. In ancient Greek, propolis means defense of the city; the antiseptic and antimicrobial substance is used in the hive to maintain colony health. Thus, propolis has been identified as a natural antibiotic candidate [50]. Raw propolis is typically composed of 50% plant resin, 30% wax, 10% essential and aromatic oil, 5% pollen, and 5% other organic substances. The color of propolis varies from green to brown and reddish, depending on its botanical source [1]. Propolis cannot be commercialized as a raw material; it must be purified and dewaxed via solvent extraction to remove inert materials and preserve the phenolic fractions [13]. Propolis has been classified into seven main types according to the plant source: (1) poplar propolis: the most widespread type of propolis (Europe, North America, New Zealand, and non-tropical regions of Asia), (2) green propolis or Baccharis propolis, (3) red propolis or Clusia (Brazil, Cuba, Venezuela, and Mexico), (4) eucalyptus propolis, (5) Taiwanese green propolis or Macaranga propolis (Okinawa prefecture in Japan, Taiwan, and Indonesia), (6) birch propolis (Russia), and (7) Mediterranean propolis (Greece, Sicily, and Malta) [13]. Among the types of propolis, poplar and Brazil green propolis are the most commercially available and widely studied [50,51]. The biological activities of propolis, including antioxidant, antimicrobial, antiviral, anti-inflammatory, antifungal, wound-healing, and cardio-protective properties, have been ascribed to the action of phenolic compounds and terpenoids [11,14].
2.4. Bee Venom
Bee venom, also known as “apitoxin”, is a colorless, acidic liquid that is synthesized in the honeybee venom gland. Honeybee defense mechanisms against threatening attacks involve the injection of bee venom into the body of the attacker, evoking several neurological, immunological, and inflammatory responses [52,53]. The chemical constituents that distinguish bee venom are a group of amphipathic polycationic peptides, predominantly melittin and apamin. Bee venom also contains peptides such as mast-cell degranulating peptide, adolapin, tertiapin, secapin, melittin F, and cardiopep [54], as well as amines such as histamine and catecholamines [55]. Some of these peptides trigger cell lysis, while others act as neurotoxins [56]. Melittin acts as a detergent, binding and breaking down the cell membrane lipid bilayers [57]. Apamin exerts a highly specific toxicity mechanism, blocking the small conductance Ca2+-dependent K+ channels (SK channels) expressed in the central nervous system, cardiovascular system, and smooth muscle [7]. Despite the toxicity, bee venom has been used as an analgesic, antimutagenic, antinociceptive, and radio-protective immunomodulatory agent and was identified as a potential Parkinson’s disease therapy [7]. Furthermore, some substances extracted from bee venom can be included in domestic animal diets as antimicrobial agents to enhance productive performance and health status [36].
2.5. Bee Pollen
Bee pollen is a mixture of flower pollen (male germ element), nectar, enzymes, honey, beeswax, and honeybee salivary secretions gathered and produced by worker honeybees [13]. Bee pollen is a rich source of nutrients such as protein (25%), essential amino acids, oil (6%), polyunsaturated fatty acids (13% linoleic acid and 39% linolenic acid), 28 minerals, 12 vitamins, 11 enzymes or coenzymes, carbohydrates (35%–61%; sucrose, glucose, and fructose), carotenoids, flavonoids, and phytosterols [58,59]. Bee pollen is an energy-boosting food that is used by humans as a diet supplement. The high content of protein, fat, and minerals (particularly Ca, Mg, Fe, and P) in bee pollen provides a nutritional value similar to that of dried legumes. B5 and B3, vitamin C, and B2 levels are comparable to those of beef, vegetables (lettuce and tomatoes), and skimmed milk powder, respectively [60]. Bee pollen is used in complementary and alternative medicine to cure prostatitis, stomach ulcers, and infectious diseases, as well as to prevent and treat high-altitude-sickness syndrome. A wide range of therapeutic properties have been reported, including antimicrobial, antioxidant, hepato-protective, chemo-preventive and anti-carcinogenic, anti-atherosclerotic, anti-inflammatory, antiallergenic, and immunomodulatory activities [60,61,62].
2.6. Drone Brood, Beeswax, and Bee Bread
Drone brood (apilarnil) is a lesser-known honeybee product. Drone brood is a milky, yellowish-gray color that consists of the dried powder from 3–7-day-old drone larvae collected from drone cells [63]. Drone brood contains approximately 25%–35% dry matter, 9%–12% protein, 6%–10% carbohydrate, 5%–8% lipid, 2% ash, vitamins (A, B1, B6, and choline), and minerals (Ca, P, Na, Zn, Mn, Fe, Cu, and K) [64].
Beeswax is a substance produced by honeybee wax gland complexes to make combs; the greatest quantity is produced during the colony growth phase in late spring [65]. Chemically, beeswax contains more than 300 identified components. Hydrocarbons such as heptacosane, nonacosane, hentriacontane, pentacosane, and tricosane are the main components, along with free fatty acids and fatty alcohols, linear wax monoesters, hydroxymonoesters, complex wax esters, and more than 50 other aromatic components [66]. Beeswax is used as an additive in a variety of industrial, pharmaceutical, and cosmetic products; however, to the best of our knowledge, no specific studies on the relationship between beeswax and reproduction have been published [13].
Bee bread contains abundant high-quality proteins; carbohydrates; fatty acids; vitamins (B-group, C, K, E, and D), especially vitamin P (rutin) and provitamin A (carotene); various minerals; oligo-elements (especially K and Fe); essential oils; enzymes; pigments; and other biologically active natural substances [67]. No other natural product contains such high concentrations of vitamin P (rutin; 13 mg/100 g of bee bread), which can improve the condition of blood vessels. Moreover, bee bread boosts the immune system and has been used to tackle chronic inflammation of the prostate, male sterility and impotence, endocrine glands disorders, and decreased libido, as well as to improve intra-uterine nutrition. Bee bread also produces a strong antiseptic effect on a wide range of pathogenic microorganisms [67]. A summary of the chemical composition of honeybee products is shown in Table 2.

Table 2.
Chemical composition of different honeybee products.
3. Honeybee Products and Reproductive Health
3.1. Biological Effects of Honeybee Products on Reproduction
Owing to the diversity of chemical constituents in honeybee products, a wide range of biological effects on reproductive functions could be achieved via several biological mechanisms/pathways. For example, most honeybee products contain phenolic compounds, widely known to affect reproduction in mammals. Phenolic compounds can regulate gonadal steroidogenesis and the functionality and metabolism of sex steroid hormones [70,71]. In addition, phenolic compounds can affect the expression of genes and activity of enzymes (aromatase, topoisomerases I and II, and extracellular signal-regulated kinases) involved in the regulation of reproductive events. Phenolic compounds can also contribute to cellular apoptotic/proliferation, epigenetic, antioxidant, and inflammatory pathways [72]. Metabolic status may also be affected by phenols owing to their ability to regulate metabolic hormone signals such as growth hormone, insulin-like growth factors, and triiodothyronine, as well as lipid, protein, and carbohydrate metabolism. Furthermore, sex hormones have been detected in some bee products, such as royal jelly and drone brood, which can thus modulate endocrine system functions [15]. Drone brood is rich in male sex hormones, especially testosterone, producing an androgenic effect that enhances male sex features. Drone brood can also be classified as a natural anabolic agent that can increase body muscle weight in male individuals [63,73]. Additionally, nutrients, vitamins, and minerals can directly affect reproductive tissues, reinforcing their functions and improving gametogenesis and/or the quality of gametes [74]. Therefore, honeybee products can provide effective tools for improving reproductive functions in vivo and assisted reproductive technique (ART) outputs in vitro. The potential effects of honeybee products on mammalian reproductive functions and ARTs are shown in Figure 1.
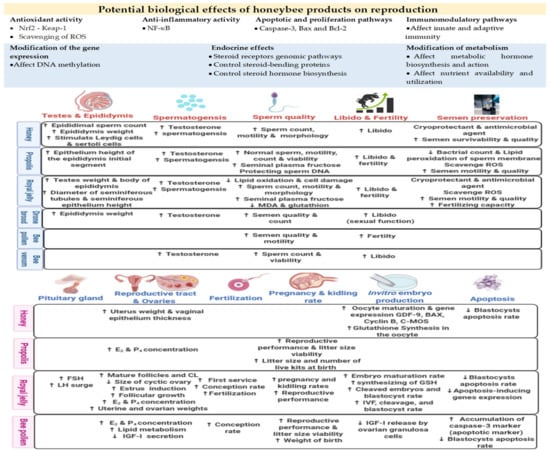
Figure 1.
Potential effects of honeybee products on reproductive functions and assisted reproductive techniques (ARTs) in mammals. BAX: bcl-2-like protein 4, Bcl-2: B-cell lymphoma 2, C-MOS: complementary metal–oxide–semiconductor, E2: estradiol-17β, FSH: follicle stimulating hormone, GDF-9: growth differentiation factor 9, IGF-I: insulin-like growth factor I, LH: luteinizing hormone, Nrf2-Keap-1: nuclear factor erythroid 2-related factor 2-Keap-1, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells, P4: progesterone, ROS: reactive oxygen species.
3.2. Applications for Improving Male Fertility
Recent studies on the effects of honeybee products on male reproductive performance in mammalian species are shown in Table 3. Several studies have supported the traditional use of honey as a natural product to enhance reproductive efficiency and fertility in males [31,32,33]. Honey has been demonstrated to improve libido, erectile function, spermatogenesis, epididymal sperm count, and normal sperm percentage, and reduce the percentage of sperm head and tail abnormalities and chromatin damage in mammalian species, including humans [16,17] and rats [31,32,33]. The positive effects of honey on male reproductive performance are attributed to several different mechanisms. Honey can increase the activity of enzymes that affect sperm quality, such as sorbitol dehydrogenase [31], which converts sorbitol into fructose, an important nutrient for sperm metabolism and motility. Additionally, honey administration improves spermatogenesis by enhancing male sex hormones, specifically testosterone. This enhancement was mediated by luteinizing hormone synthesis, Leydig cell viability, upregulation of steroidogenic acute regulatory protein expression, and aromatase activity in the testes [75]. In addition, bioactive compounds with antioxidant activity (e.g., antioxidant polyphenols such as flavonoids and phenolic acids) can provide reproductive organs with a robust defense system against oxidative stress induced by elevated levels of reactive oxygen species (ROS) [33]. Furthermore, honey can treat dysfunctional erection or impotence by modulating the biosynthesis of nitric oxide, a chemical substance involved in vasodilatation that affects erectile function [16,17].

Table 3.
Summary of some recent studies on the effects of different honeybee products on reproductive performance of males in different mammalian species.
Propolis is another honeybee product shown to improve the reproductive performance of mammalian males. Several studies confirmed that administration of propolis improved sperm count and testis, seminal vesicle, and epididymis weights, and reduced the percentage of sperm head and tail abnormalities [14,19,20,21,22,23]. Moreover, the administration of propolis extract increased serum testosterone levels, coupled with increased activity and expression of testicular steroidogenesis enzymes including 3β-hydroxysteroid dehydrogenase (3β-HSD) and 17β-hydroxysteroid dehydrogenase (17β-HSD) [24], improving testosterone levels [25]. Propolis also shows strong antioxidant and modulatory activity on cellular mitochondrial energy production [19].
Royal jelly also produced many beneficial effects on male reproductive performance in mammals. As indicated by studies conducted on adult male rats [23,26,27,28] and rabbits [22,29,30], royal jelly enhanced spermatogenesis, steroidogenesis, sperm quality traits, and libido of treated males. Interestingly, royal jelly contains acetylcholine (1 mg/g dry weight), a peripheral and central neurotransmitter. Acetylcholine can stimulate gonadotropin secretion at both hypothalamic and hypophyseal levels, consequently increasing plasma testosterone levels. Most studies have focused on the major products of honeybees (honey, propolis, and royal jelly); however, beneficial effects of other honeybee products, such as drone brood [34,35], bee venom [36], and bee pollen [18], on male reproductive health have also been reported. These products contain active constituents that can affect reproductive functions, such as apistimul in drone brood [34].
3.3. Applications for Improving Female Fertility
Table 4 summarizes recent studies on the effects of honeybee products on female reproductive performance in mammalian species. Honey [76], royal jelly [46,77,78,79,80,81], propolis [82,83], and bee pollen [82,83,84,85] were demonstrated to improve female reproductive performance in several mammals. Treatment with royal jelly improved estrus response and pregnancy rate in ewes by enhancing ovarian follicle growth and development and estradiol secretion [80]. Interestingly, Gimenez-Diaz [78] and Kridli [86] reported that royal jelly can mimic the actions of gonadotropins to improve estrus response and conception rate in ewes, providing a promising natural alternative to equine chorionic gonadotropin (eCG). The positive effects of royal jelly on reproductive performance may be partially ascribed to its estrogenic effects [81].

Table 4.
Effect of bee products on reproductive performance of females of different mammalian species.
The positive effects of bee pollen on female reproductive performance are well-documented. Bee pollen can improve rabbit doe reproductive performance and milk production and enhance the immune status and growth performance of their offspring [84,85,86]. These results were attributed to the high micronutrient content (e.g., polyunsaturated fatty acids, minerals, vitamins, amino acids) and biological actions of flavonoids, carotenoids, and phenolic compounds in bee pollen [81]. Furthermore, bee pollen can regulate sex steroidogenesis, specifically progesterone and estradiol [87,88]. Hormonal balance is important for adjusting the mechanisms underlying the fate of ovarian follicles, involving cross-dialog between pro-apoptotic (caspase-3, Bax) and pro-survival anti-apoptotic (Bcl-2) molecules [87,88]. The dose and time of administration of bee pollen can both affect these pathways; positive and negative effects on ovarian follicle development and growth could be obtained using different administration methods [87].
In models designed to study menopausal symptoms, Zaid et al. [76] noticed that daily consumption of Tualang honey for two weeks by ovariectomized female rats suppressed menopause-related reproductive disorders, including uterine atrophy, vaginal epithelium atrophy, and osteoporosis. The positive effects of Tualang honey are likely due to flavonoids, particularly kaempferol and quercetin, which have weak estrogenic activity. Further, flavonoids are strong free radical scavengers, protecting organisms from the destructive actions of ROS [72,76]. Interestingly, local vaginal application of honey and royal jelly before sexual intercourse improved fertility in couples having trouble conceiving [88].
4. Honeybee Products and Assisted Reproductive Techniques
4.1. Male-Associated Assisted Reproductive Techniques
The efficiency of ARTs depends on the appropriate manipulation of gametes (sperm cells) during each stage of handling, starting from semen collection, followed by dilution, cooling, cryopreservation, and finally transfer to the female reproductive tract. During these processes, sperm cells might be exposed to thermal and physicochemical stresses, which can cause serious damage to the sperm cell membrane and DNA, thereby decreasing semen quality [89,90].
One of the important factors that can affect sperm cell quality is the medium in which sperm cells are placed for ART. Ideal media should minimize and protect sperm cells against physicochemical stresses; thus, the composition should prevent alterations to the structure and function of sperm cells [90]. Several studies have described the benefits of honey as a natural cryoprotectant agent for maintaining semen quality kinetic parameters, sperm cell membrane and DNA integrity, and sperm cell morphology when included in semen cryopreservation media (human [91], cattle bull semen [92,93], buffalo bull semen [94], and rabbits [95,96]) and liquid storage media [97].
Honey contains a mixture of sugars, proteins, enzymes, amino acids and organic acids, vitamins, phenolic acids, and flavonoids that confer high antioxidant activity. An appropriate mixture of nutrients and bioactive components is necessary for maintaining adequate sperm quality. For example, simple sugars (glucose, fructose, and sucrose) in honey provide sperm cells with a preferable energy source [94,98,99,100]. Additionally, the presence of components, such as organic acids (gluconic acid), phenolic acids, and flavonoids, with antioxidant and antibacterial activities can play a crucial protective role against the harmful effects of ROS and microbial attack [32]. Thus far, inhibitory effects of honey on 60 species of gram-positive and gram-negative bacteria (Staphylococcus aureus, Escherichia coli, Staphylococcus epidermidis, and Bacillus cereus) have been reported; these effects are comparable in strength to antibiotics penicillin, streptomycin, and kanamycin [101].
Other studies have reported the beneficial effects of including royal jelly in sperm cell-processing media on sperm cell quality and, later, fertility in some mammals (rams [102,103], goats [104], and buffalo bull [105,106]). The protective role of royal jelly was mainly ascribed to its unique amino acid profile [107]. Royal jelly contains many vital amino acids, including valine, aspartic acid, isoleucine, tyrosine, glycine, lysine, proline, cysteine, and leucine. Proline has been demonstrated to maintain sperm cell membrane integrity under stressful conditions [108] and both cysteine and proline act as robust antioxidant agents to eliminate ROS and stimulate glutathione enzyme synthesis and activity during semen cooling–freezing processes [108]. Moreover, royal jelly vitamins (C and E) and 10-hydroxy-2-decenoic acid provide protective effects to the sperm cell membrane [109,110]. However, high concentrations of royal jelly produced negative impacts on sperm cell quality [103], thus careful consideration of the concentration in media is essential.
Propolis has also been included in semen preservation media, providing antioxidant and antimicrobial activity [111,112,113] and maintaining semen characteristics such as motility, viability, and DNA integrity [114]. The effects of honeybee products on male ARTs are shown in Table 5.

Table 5.
Effect of honeybee products on assisted reproductive techniques (ARTs) in males.
4.2. Female-Associated Assisted Reproductive Techniques
For females (Table 6), in vitro maturation (IVM) and in vitro fertilization (IVF) are the most common ARTs, the outcomes of which relate to oocyte quality. Thus, improving the oocyte microenvironment during IVM and IVF has the potential to improve ART outcomes, with corresponding improvements in reproductive efficiency in humans and animals. Under normal circumstances, oocytes receive essential elements, proteins, growth factors, hormones, antioxidants, and other compounds directly from the female reproductive fluids. Consequently, artificially formulated media for oocyte IVM or IVF should contain elements that correspond to the natural sources in the fluids of the female reproductive tract [115,116]. Given the chemical constituents of honeybee products, the inclusion of the honeybee product(s) in IVM or IVF media may have positive effects on oocyte maturation, oocyte fertilizability, and division and embryo development. The addition of honey (black seed bee honey) to sheep IVM medium has been shown to improve oocyte maturation rate, glutathione levels, and gene expression of GDF-9, BAX, cyclin B, C-MOS, and IGF1 genes. Similarly, the addition of royal jelly to goat oocyte [117] medium or ovine IVM medium [118] improved oocyte and nuclear maturation rate, fertilization rate, and blastocyst formation. These improvements seem to be a result of decreased expression of apoptosis-inducing genes, increased glutathione-S-transferase enzymes content, and enhanced mitochondrial activity, which lead to a reduction in blastocyst apoptosis rates [87,117,118,119]. The positive effect of royal jelly on oocyte maturation is related to the presence of antioxidant amino acids (cysteine, lysine, and arginine) and polyphenols [117].

Table 6.
Effect of honeybee products on assisted reproductive techniques (ARTs) in females.
5. Honeybee Products and Reproductive Disorder Mitigation
5.1. Reproductive Toxicity of Pollutants and Heavy Metals
Recently, synthetic chemicals used for different industrial and agricultural purposes have been increasing environmental pollution. Exposure to these chemicals via direct contact during manufacturing and handling, consumption of polluted foods/water, or inhalation of polluted air can cause reproductive toxicity and increase the risk of subfertility/infertility.
Heavy metals such as cadmium chloride (CdCl2) [120], copper (Cu) [121], and aluminum chloride (Al Cl3) [122] have been confirmed to induce subfertility/infertility in male and female mammals. These heavy metals can exert reproductive toxicity through several mechanisms. Heavy metals can accumulate inside vital organs and gonads and can evoke oxidative and inflammatory stress, leading to negative impacts on reproductive function, hormone balance, gamete quality, and fertilizability of gametes, as well as producing teratogenic effects in fetuses [120,121,122,123,124]. The efficacy of honeybee products for alleviating heavy metal reproductive toxicity has been demonstrated. Royal jelly was shown to fix hormonal alterations, oxidative status, inflammatory response, and apoptotic cascades induced following Cd-exposure, presumably due to its potent antioxidant activity [120]. Similarly, propolis protected against the toxic effects of excess Cu [121] or Al Cl3 [122] on testicular tissue and semen quality traits in rats, as well as by scavenging ROS and improving testicular and blood plasma redox status.
Synthetic pesticides, such as chlorpyrifos (organophosphorus insecticide), cypermethrin, and triphenyltin, are used to control a variety of agricultural, animal farming, and indoor pests [123,124,125,126,127,128]. Such chemicals can induce direct reproductive toxicity and may also act as endocrine disruptors, producing estrogen or androgen-like effects [125] and leading to various health hazards. Several studies have confirmed the protective role of propolis against pesticide-induced reproductive toxicity in males [25,122] and females [126]. The protective role of propolis is ascribed to the antioxidant and anti-inflammatory actions of polyphenols baicalin, lucenin 2, and quercetin against neuroprotective toxicity. Moreover, the anti-inflammatory activity of alkaloids and hepato-protective role of the organosilicon compounds in propolis were proposed as protective mechanisms [125]. Interestingly, honeybee products could also be used to reduce the toxicity of naturally occurring toxins. El-Nekeety et al. [126] reported that royal jelly resulted in a significant reduction in the toxic hazards of fumonisins (mycotoxins), likely due to increased glutathione peroxidase synthesis and suppression of lipid peroxidation and free radical generation by antioxidant enzymes. The protective effects of honeybee products against chemical stress induced reproductive toxicity in mammals are summarized in Table 7.

Table 7.
Protective effects of honeybee products against chemical stresses-induced reproductive toxicity in mammals.
5.2. Unhealthy Lifestyle and Psychological Stresses
Lifestyle factors related to individual habits and ways of life can substantially impact overall health and well-being, including reproductive health. Nutrition, weight, exercise, psychological stress, and other factors can have substantial effects on fertility (Table 8). Lifestyle factors such as cigarette smoking and drug addiction can negatively influence fertility [127]. For instance, smoke from cigarettes, household wood and coal fires, barbecue grills, and automobile exhaust can elevate levels of harmful toxins, such as nicotine [128] and benzo[a]-pyrene [129], in the bloodstream. These toxic compounds are associated with decreased sperm count and motility and an increased percentage of morphologically abnormal sperm, sperm chromatin damage, erectile dysfunction, and early pregnancy loss [130]. Studies using animal models found that the negative impacts of toxins could be mitigated by the consumption of honey [131], royal jelly [128], and propolis [129]. The positive impacts of honeybee products are related to their antioxidant and endocrine-modulating activity, as some bee products, specifically royal jelly, include sex hormones among their constituents [85]. Human exposure to harmful synthetic chemicals may also occur because of the use of chemicals in food and healthcare products, which are considered safe for humans. For example, monosodium glutamate (MSG; flavor enhancer) is used as an ingredient in various food products; however, negative impacts of MSG on male infertility (testicular hemorrhage, degeneration and alteration of sperm cell population and morphology) have been documented [132]. Similarly, sodium fluoride, a main component of toothpaste, can induce reproductive disorders [133]. Bee propolis has been identified as a suitable supplement for alleviating such negative effects [133,134].

Table 8.
Impact of honeybee products on animal reproductive disorders induced by unhealthy lifestyle and psychological stresses.
Furthermore, psychological stress contributes to many reproductive disorders and dysfunctions. Haron et al. [135] found that administering Tualang honey (1.2 g/kg daily) to restraint-stressed pregnant rats conferred beneficial effects on reproductive parameters, such as corticosterone level and pregnancy outcome. Another study on rats exposed to prenatal restraint stress reported that impaired reproductive function in male rat offspring could be improved by feeding dams honey (1.2 g/kg, three times per day) from day 11 of pregnancy until delivery [136]. Moreover, Rajabzadeh et al. [137] reported that dissolving 0.2 mL of 5% honey in the water of rats exposed to auditory stress significantly improved fertility rates and fetus health.
6. Precautions and Hazards
As shown through the review, honeybee products have several beneficial effects on the reproductive health of mammals. However, adverse effects of honeybee products on reproduction in mammals have been also reported, specifically when they are consumed during critical periods of the reproductive cycle, such as puberty and pregnancy. The effects of royal jelly on the reproductive system of puberty male rats were investigated in vivo after daily administration of royal jelly at doses of 200, 400, and 800 mg/kg for four weeks. The high-dose royal jelly oral administration adversely affected the reproductive system by decreasing testis weight, changing testis microstructure, and increasing sperm deformity rate of the assayed rats, but the unfavorable effects were alleviated by treatment cessation [37]. The adverse effects of royal jelly were attributed to the estrogenic activity of a high dose of royal jelly. In males, the improper intake of substances with estrogenic activity can adversely affect the reproductive performance of males at different reproductive windows [138,139]. In another study, a low dose of Indonesian propolis (380 mg/kg) and a high-dose of propolis (1400 mg/kg) were provided to mice for 18 days of gestation to confirm the safety of consuming propolis during pregnancy [38]. The low dose of propolis increased weight, crown-rump length, and ossification center thickness compared with the control group. Conversely, the high-dose of propolis reduced weight, crown-rump length, and ossification center thickness and caused hypertrophy of the placenta, inhibiting fetal development. These results may be attributed to the immunomodulatory properties of propolis, as fetal resorption may occur due to rejection via the immune system pathway. Increased macrophage activity in the endometrium during pregnancy leads to increased production of NO and TNF-α, which are toxic to embryo development. Further, it was found that colony-stimulating factor-1 (CSF-1) increased resorption in pregnant mice; CSF-1 plays an important role in the differentiation of macrophages. The anticancer activity of propolis was also proposed as an underlying mechanism of fetal growth retardation in high-dose groups. It is known that most anticancer agents are teratogens, and vice versa. Thus, some natural products that have anticancer effects may also be teratogenic. However, teratogenic effects were not observed after the administration of low doses [38]. Therefore, honeybee product dose should be carefully considered, as high concentrations of royal jelly and propolis can negatively impact sperm cell quality and fetal development [104].
7. Conclusions
The study of the unique chemical composition of honeybee products and their effects on the reproductive performance of mammals provides opportunities to detect pharmaceutical molecules for safe reproductive health manipulation. The biological activities (e.g., antioxidant, antimicrobial, antiviral, anti-inflammatory, immunomodulatory, antifungal, wound-healing, and cardio-protective) of honeybee products were ascribed to the phenolic compound and terpenoid constituents. Honeybee products have been demonstrated to improve libido, erectile function, spermatogenesis, epididymal sperm count, and normal sperm percentage, as well as to reduce sperm head and tail abnormalities and chromatin damage in many mammalian species. The benefits of using bee honey as a natural cryoprotectant agent in semen cryopreservation and liquid storage media were also reported. Moreover, honeybee products can improve female reproductive performance and milk production and fetal immune status and growth performance. Given the chemical constituents of honeybee products, inclusion in in vitro maturation (IVM) or in vitro fertilization (IVF) media may produce positive effects on oocyte maturation, fertilizability, and division and embryo development. Many studies have also confirmed the efficacy of honeybee products for alleviating the reproductive toxicity of chemical contaminants and pollutants. However, high-dose oral administration of honeybee products may adversely affect the reproductive system; thus, doses should be carefully considered when administering such products. Overall, the bioactive components of honeybee products, when wisely used, can provide a natural approach for improving the reproductive performance and health of mammals.
Author Contributions
Conceptualization, N.M.H.; resources, N.M.H., E.M.H., and J.S.-G.; writing—review and editing, N.M.H., E.M.H., and J.S.-G.; visualization, N.M.H., E.M.H., and J.S.-G. All authors have read and agreed to the published version of the manuscript.
Funding
No fund was received for this review article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Zulkhairi Amin, F.A.; Sabri, S.; Mohammad, S.M.; Ismail, M.; Chan, K.W.; Ismail, N.; Norhaizan, M.E.; Zawawi, N. Therapeutic properties of stingless bee honey in comparison with european bee honey. Adv. Pharmacol. Sci. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, A.; Lensky, Y. Bee Products: Properties, Applications, and Apitherapy; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 1475793715. [Google Scholar]
- El-Desoky, N.I.; Hashem, N.M.; Elkomy, A.; Abo-Elezz, Z.R. Physiological response and semen quality of rabbit bucks supplemented with Moringa leaves ethanolic extract during summer season. Animal 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G.; Antunes, M.D.; Faleiro, M.L. Honey as a complementary medicine. Integr. Med. Insights 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Szczêsna, T. Protein content and amino acid composition of bee-collected pollen from selected botanical origins. J. Apic. Sci. 2006, 50, 81–90. [Google Scholar]
- Silici, S. Bal Arısı Ürünleri ve Apiterapi. Turk. J. Agric. Food Sci. Technol. 2019, 7, 1249. [Google Scholar] [CrossRef]
- Hashem, N.M.; Abd El-Hady, A.M.; Hassan, O.A. Inclusion of phytogenic feed additives comparable to vitamin E in diet of growing rabbits: Effects on metabolism and growth. Ann. Agric. Sci. 2017, 62. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Bee venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M. Bee Products—Chemical and Biological Properties; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319596891. [Google Scholar]
- Hashem, N.M.; El-Hady, A.A.; Hassan, O. Effect of vitamin E or propolis supplementation on semen quality, oxidative status and hemato-biochemical changes of rabbit bucks during hot season. Livest. Sci. 2013, 157, 520–526. [Google Scholar] [CrossRef]
- Maghsoudlou, A.; Sadeghi Mahoonak, A.; Mohebodini, H.; Toldra, F. Royal jelly: Chemistry, storage and bioactivities. J. Apic. Sci. 2019, 63, 17–40. [Google Scholar] [CrossRef]
- Igbokwe, V.U.; Samuel, O. Pure Honey Potent Fertility Booster: Activities of Honey on Sperm. IOSR J. Dent. Med. Sci. 2013, 9, 43–47. [Google Scholar]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Hanoun, A.; Bovera, F. Effect of different levels of bee pollen on performance and blood profile of New Zealand White bucks and growth performance of their offspring during summer and winter months. J. Anim. Physiol. Anim. Nutr. 2011, 95, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Handayani, N.; Gofur, A. Does propolis extract alleviate male reproductive performance through gonadotropic hormone levels and sperm quality? IOP Conf. Ser. Earth Environ. Sci. 2019, 276. [Google Scholar] [CrossRef]
- De Moraes, G.V.; Mataveli, M.; de Moura, L.P.P.; Scapinello, C.; Mora, F.; Osmari, M.P. Inclusion of propolis in rabbit diets and semen characteristics. Arq. Ciências Veterinárias Zool. UNIPAR 2015, 17, 227–231. [Google Scholar] [CrossRef]
- Gabr, S. Effect of oral administration of rabbit bucks with egyptian propolis during summer, in Egypt. Egypt. J. Rabbit Sci. 2013, 23, 161–178. [Google Scholar] [CrossRef]
- El-Sherbiny, A. Effect of some bee products on reproductive phenomena of male New Zealand white rabbits. Egypt. J. Rabbit Sci. 2015, 25, 119–136. [Google Scholar] [CrossRef]
- Capucho, C.; Sette, R.; de Souza Predes, F.; de Castro Monteiro, J.; Pigoso, A.A.; Barbieri, R.; Dolder, M.A.H.; Severi-Aguiar, G.D.C. Green Brazilian propolis effects on sperm count and epididymis morphology and oxidative stress. Food Chem. Toxicol. 2012, 50, 3956–3962. [Google Scholar] [CrossRef] [PubMed]
- Rizk, S.M.; Zaki, H.F.; Mina, M.A.M. Propolis attenuates doxorubicin-induced testicular toxicity in rats. Food Chem. Toxicol. 2014, 67, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; Kamel, K.I.; Hassan, M.S.; El-Morsy, A.M.A. Protective role of propolis against reproductive toxicity of triphenyltin in male rabbits. Food Chem. Toxicol. 2010, 48, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ekusa, A.; Iwai, K.; Yonekura, M.; Takahata, Y.; Morimatsu, F. Royal jelly peptides inhibit lipid peroxidation in vitro and in vivo. J. Nutr. Sci. Vitaminol. (Tokyo) 2008, 54, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A. Effect of royal jelly on sexual efficiency in adult male rats. Iraqi J. Vet. Sci. 2009, 23, 155–160. [Google Scholar]
- Shi, Z.; Enayatullah, H.; Lv, Z.; Dai, H.; Wei, Q.; Shen, L.; Karwand, B.; Shi, F. Freeze-dried royal jelly proteins enhanced the testicular development and spermatogenesis in pubescent male mice. Animals 2019, 9. [Google Scholar] [CrossRef]
- El-Hanoun, A.M.; Elkomy, A.E.; Fares, W.A.; Shahien, E.H. Impact of royal jelly to improve reproductive performance of male rabits under hot sumer conditions. World Rabbit Sci. 2014, 22, 241–248. [Google Scholar] [CrossRef]
- Khadr, A.; Abdou, A.; El-Sherbiny, A. Age of puberty and fertility of male new zealand white rabbits orally administered with royal jelly or/ and bee honey. J. Anim. Poult. Prod. 2015, 6, 201–217. [Google Scholar] [CrossRef]
- Abdul-Ghani, A.S.; Dabdoub, N.; Muhammad, R.; Abdul-Ghani, R.; Qazzaz, M. Effect of Palestinian honey on spermatogenesis in rats. J. Med. Food 2008, 11, 799–802. [Google Scholar] [CrossRef]
- Mohamed, M.; Sulaiman, S.A.; Jaafar, H.; Sirajudeen, K.N.S. Effect of different doses of Malaysian honey on reproductive parameters in adult male rats. Andrologia 2012, 44, 182–186. [Google Scholar] [CrossRef]
- Syazana, N.S.; Hashida, N.H.; Majid, A.M.; Durriyyah Sharifah, H.A.; Kamaruddin, M.Y. Effects of Gelam honey on sperm quality and testis of rat. Sains Malaysiana 2011, 40, 1243–1246. [Google Scholar]
- Bolatovna, K.S.; Rustenov, A.; Eleuqalieva, N.; Omirzak, T.; Akhanov, U.K. Improving reproductive qualities of pigs using the drone brood homogenate. Biol. Med. 2015, 7, 2. [Google Scholar]
- Shoinbayeva, K.B.; Omirzak, T.; Bigara, T.; Abubakirova, A.; Dauylbay, A. Biologically active preparation and reproductive function of stud rams. Asian J. Pharm. 2017, 11, 184–191. [Google Scholar]
- El-Hanoun, A.; El-Komy, A.; El-Sabrout, K.; Abdella, M. Effect of bee venom on reproductive performance and immune response of male rabbits. Physiol. Behav. 2020, 223, 112987. [Google Scholar] [CrossRef]
- Yang, A.; Zhou, M.; Zhang, L.; Xie, G.; Chen, H.; Liu, Z.; Ma, W. Influence of royal jelly on the reproductive function of puberty male rats. Food Chem. Toxicol. 2012, 50, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Fikri, A.M.; Sulaeman, A.; Handharyani, E.; Marliyati, S.A.; Fahrudin, M. The effect of propolis administration on fetal development. Heliyon 2019, 5, e02672. [Google Scholar] [CrossRef]
- Uddin, V.; Zuccato, V.; Maza, F.; Schievano, E. Entomological origin of honey discriminated by NMR chloroform extracts in ecuadorian honey. Int. J. Nutr. Food Eng. 2015, 9, 494–497. [Google Scholar]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Braz. J. Pharmacogn. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- Council, E.U. Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Communities L 2002, 10, 47–52. [Google Scholar]
- Patricia, V.; Oliverio, V.; Triny, L.; Favián, M. Meliponini biodiversity and medicinal uses of pot-honey from El Oro province in Ecuador. Emirates J. Food Agric. 2015, 27, 502–506. [Google Scholar] [CrossRef]
- Piszcz, P.; Głód, B.K. Antioxidative properties of selected polish honeys. J. Apic. Sci. 2019, 63, 81–91. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, I. Chemistry and bioactivity of royal jelly from Greece. J. Agric. Food Chem. 2005, 53, 8987–8992. [Google Scholar] [CrossRef]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. More than royal food-Major royal jelly protein genes in sexuals and workers of the honeybee Apis mellifera. Front. Zool. 2013, 10, 1–10. [Google Scholar] [CrossRef]
- Hamid, N.A.; Bakar, A.B.A.; Zain, A.A.M.; Hussain, N.H.N.; Othman, Z.A.; Zakaria, Z.; Mohamed, M. Composition of Royal Jelly (RJ) and its anti-androgenic effect on reproductive parameters in a polycystic ovarian syndrome (PCOS) animal model. Antioxidants 2020, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Balkanska, R.; Marghitas, L.-A.; Pavel, C.I. Antioxidant activity and total polyphenol content of royal jelly from Bulgaria. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 578–585. [Google Scholar] [CrossRef]
- Nozaki, R.; Tamura, S.; Ito, A.; Moriyama, T.; Yamaguchi, K.; Kono, T. A rapid method to isolate soluble royal jelly proteins. Food Chem. 2012, 134, 2332–2337. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Šturm, L.; Ulrih, N.P. Advances in the Propolis chemical composition between 2013 and 2018: A review. eFood 2019, 1, 24. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Hellner, M.; Winter, D.; Von Georgi, R.; Münstedt, K. Apitherapy: Usage and experience in German beekeepers. Evid.-Based Complement. Altern. Med. 2008, 5, 475–479. [Google Scholar] [CrossRef]
- El-Seedi, H.; El-Wahed, A.A.; Yosri, N.; Musharraf, S.G.; Chen, L.; Moustafa, M.; Zou, X.; Al-Mousawi, S.; Guo, Z.; Khatib, A.; et al. Antimicrobial properties of apis mellifera’s bee venom. Toxins 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee Venom: An updating review of its bioactive molecules and its health applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Bae, H. Anti-inflammatory applications of melittin, a major component of bee venom: Detailed mechanism of action and adverse effects. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Sobral, F.; Sampaio, A.; Falcão, S.; Queiroz, M.J.R.P.; Calhelha, R.C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal. Food Chem. Toxicol. 2016, 94, 172–177. [Google Scholar] [CrossRef]
- Pucca, M.B.; Cerni, F.A.; Oliveira, I.S.; Jenkins, T.P.; Argemí, L.; Sørensen, C.V.; Ahmadi, S.; Barbosa, J.E.; Laustsen, A.H. Bee Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Front. Immunol. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Dong, J.; Zhang, H. Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide. Innov. Food Sci. Emerg. Technol. 2009, 10, 42–46. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Alagawany, M.; Farag, M.R.; Elnesr, S.S. Beneficial impacts of bee pollen in animal production, reproduction and health. J. Anim. Physiol. Anim. Nutr. 2019, 103, 477–484. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki Michałand Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E.; Iriti, M. Polyphenols from bee pollen: Structure, absorption, metabolism and biological activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Antioxidant and tyrosinase inhibitory properties of aqueous ethanol extracts from monofloral bee pollen. J. Apic. Sci. 2015, 59, 119–128. [Google Scholar] [CrossRef]
- Sawczuk, R.; Karpinska, J.; Miltyk, W. What do we need to know about drone brood homogenate and what is known. J. Ethnopharmacol. 2019, 245. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. Royal jelly, bee brood: Composition, health, medicine: A review. Lipids 2011, 3, 8–19. [Google Scholar]
- Kacániová, M.; Vuković, N.; Chlebo, R.; Haščík, P.; Rovná, K.; Cubon, J.; Dzugan, M.; Pasternakiewicz, A. The antimicrobial activity of honey, bee pollen loads and beeswax from Slovakia. Arch. Biol. Sci. 2012, 64, 927–934. [Google Scholar] [CrossRef]
- Aguilar, F.; Autrup, H.; Barlow, S.; Castle, L.; Crebelli, R.; Engel, K.; Gontard, N.; Gott, D.; Grilli, S.; Gürtler, R.; et al. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food Adopted on 7 March 2008. EFSA J. 2008, 1–29. [Google Scholar]
- Milojkovic, V. Bee Bread (Perga)—The Source of Health, Vitality and Longevity. In Proceedings of the Apiquality & Apimedica 2018 the XI-th Congress of the XI-th Romanian Society of Apitherapy, Sibiu, Romania, 11–16 October 2018. [Google Scholar]
- Fuenmayor, B.C.; Zuluaga, D.C.; Díaz, M.C.; de Quicazán, C.M.; Cosio, M.; Mannino, S. Evaluation of the physicochemical and functional properties of Colombian bee pollen. Rev. MVZ Córdoba 2014, 4003–4014. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Bakier, S.; Stocki, M. GC-MS investigation of the chemical composition of honeybee drone and queen larva homogenate. J. Apic. Sci. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Hashem, N.; Gonzalez-Bulnes, A.; Simal-Gandara, J. Polyphenols in Farm Animals: Source of Reproductive Gain or Waste? Antioxidants 2020. [Google Scholar] [CrossRef]
- Hashem, N.M.; El-Azrak, K.M.; Nour El-Din, A.N.M.; Sallam, S.M.; Taha, T.A.; Salem, M.H. Effects of Trifolium alexandrinum phytoestrogens on oestrous behaviour, ovarian activity and reproductive performance of ewes during the non-breeding season. Anim. Reprod. Sci. 2018. [Google Scholar] [CrossRef]
- Hashem, N.M.; El-Azrak, K.M.; Sallam, S.M.A. Hormonal concentrations and reproductive performance of Holstein heifers fed Trifolium alexandrinum as a phytoestrogenic roughage. Anim. Reprod. Sci. 2016, 170. [Google Scholar] [CrossRef]
- Sİlİcİ, S. Chemical Content and Bioactive Properties of Drone Larvae (Apilarnil). Mellifera 2019, 19, 14–22. [Google Scholar]
- Hosny, N.S.; Hashem, N.M.; Morsy, A.S.; Abo-Elezz, Z.R. Effects of organic selenium on the physiological response, blood metabolites, redox status, semen quality, and fertility of rabbit bucks kept under natural heat stress conditions. Front. Vet. Sci. 2020, 7, 290. [Google Scholar] [CrossRef]
- Banihani, S.A. Mechanisms of honey on testosterone levels. Heliyon 2019, 5, e02029. [Google Scholar] [CrossRef]
- Zaid, S.S.M.; Sulaiman, S.A.; Sirajudeen, K.N.M.; Othman, N.H. The effects of tualang honey on female reproductive organs, tibia bone and hormonal profile in ovariectomised rats—Animal model for menopause. BMC Complement. Altern. Med. 2010, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Husein, M.Q.; Haddad, S.G. A new approach to enhance reproductive performance in sheep using royal jelly in comparison with equine chorionic gonadotropin. Anim. Reprod. Sci. 2006, 93, 24–33. [Google Scholar] [CrossRef]
- Gimenez-Diaz, C. Improved reproductive response of sheep in intrauterine insemination program with the use of royal jelly. Afr. J. Biotechnol. 2012, 11, 12518–12521. [Google Scholar] [CrossRef]
- Husein, M.Q.; Kridli, R.T. Reproductive responses following royal jelly treatment administered orally or intramuscularly into progesterone-treated Awassi ewes. Anim. Reprod. Sci. 2002, 74, 45–53. [Google Scholar] [CrossRef]
- Kridli, R.T.; Husein, M.Q.; Humphrey, W.D. Effect of royal jelly and GnRH on the estrus synchronization and pregnancy rate in ewes using intravaginal sponges. Small Rumin. Res. 2003, 49, 25–30. [Google Scholar] [CrossRef]
- Ghanbari, E.; Khazaei, M.R.; Khazaei, M.; Nejati, V. Royal jelly promotes ovarian follicles growth and increases steroid hormones in immature rats. Int. J. Fertil. Steril. 2018, 11, 263–269. [Google Scholar] [CrossRef]
- Attia, Y.A.; Bovera, F.; Abd Elhamid, A.E.H.; Nagadi, S.A.; Mandour, M.A.; Hassan, S.S. Bee pollen and propolis as dietary supplements for rabbit: Effect on reproductive performance of does and on immunological response of does and their offspring. J. Anim. Physiol. Anim. Nutr. 2019, 103, 959–968. [Google Scholar] [CrossRef]
- Attia, Y.; Bovera, F.; El-Tahawy, W.; El-Hanoun, A.; Al-Harthi, M.; Habiba, H.I. Productive and reproductive performance of rabbits does as affected by bee pollen and/or propolis, inulin and/or mannan-oligosaccharides. World Rabbit Sci. 2015, 23, 273–282. [Google Scholar] [CrossRef]
- Kolesarova, A.; Bakova, Z.; Capcarova, M.; Galik, B.; Juracek, M.; Simko, M.; Toman, R.; Sirotkin, A.V. Consumption of bee pollen affects rat ovarian functions. J. Anim. Physiol. Anim. Nutr. 2013, 97, 1059–1065. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Hanoun, A.; Tag El-Din, A.E.; Bovera, F.; Shewika, Y.E. Effect of bee pollen levels on productive, reproductive and blood traits of NZW rabbits. J. Anim. Physiol. Anim. Nutr. 2011, 95, 294–303. [Google Scholar] [CrossRef]
- Kridli, R.T.; Al-Khetib, S.S. Reproductive responses in ewes treated with eCG or increasing doses of royal jelly. Anim. Reprod. Sci. 2006, 92, 75–85. [Google Scholar] [CrossRef]
- Adriana, K.; Capcarova, M.; Bakova, Z.; Branislav, G.; Miroslav, J.; Milan, S.; Sirotkin, A.V. The effect of bee pollen on secretion activity, markers of proliferation and apoptosis of porcine ovarian granulosa cells in vitro. J. Environ. Sci. Heal. Part B Pestic. Food Contam. Agric. Wastes 2011, 46, 207–212. [Google Scholar] [CrossRef]
- Abdelhafiz, A.T.; Muhamad, J.A. Midcycle pericoital intravaginal bee honey and royal jelly for male factor infertility. Int. J. Gynecol. Obstet. 2008, 101, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Makarevich, A.V.; Spalekova, E.; Olexikova, L.; Lukac, N.; Kubovicova, E.; Hegedusova, Z. Functional characteristics of ram cooling-stored spermatozoa under the influence of epidermal growth factor. Gen. Physiol. Biophys. 2011, 30, S36–S43. [Google Scholar] [CrossRef]
- Hashem, N.; Gonzalez-Bulnes, A. State-of-the-Art and Prospective of Nanotechnologies for Smart Reproductive Management of Farm Animals. Animals 2020. [Google Scholar] [CrossRef]
- Fakhrildin, M.-B.M.-R.; Alsaadi, R.A.-R. Honey Supplementation to Semen-Freezing Medium ImprovesHuman Sperm Parameters Post-Thawing. J. Fam. Reprod. Heal. 2014, 8, 27–31. [Google Scholar]
- Chung, E.L.T.; Nayan, N.; Nasir, N.S.M.; Hing, P.S.A.; Ramli, S.; Rahman, M.H.A.; Kamalludin, M.H. Effect of honey as an additive for cryopreservation on bull semen quality from different cattle breeds under tropical condition. J. Anim. Heal. Prod 2019, 7, 171–178. [Google Scholar] [CrossRef]
- Yimer, N.; Sarsaifi, K.; Haron, A.W. Effect of honey supplementation into Tris Extender on Cryopreservation of Bull Spermatozoa Application of assisted reproductive biotechnology in Rusa deer View project Enhancement of the quality of semen cryopreservation View project. Malays. J. Anim. Sci. 2016, 18, 47–54. [Google Scholar]
- El-Nattat, W.S.; El-Sheshtawy, R.I.; El-Batawy, K.A.; Shahba, M.I.; El-Seadawy, I.E. Research article Preservability of buffalo bull semen in tris-citrate extender enriched with bee’s honey. J. Innov. Pharm. Biol. Sci. 2016, 3, 180–185. [Google Scholar]
- Mu, A. Effect of bee honey and royal jelly addition to extender on rabbit semen fertilizing capacity at room temperaturE. J. Chem. Inf. Model. 2019, 53, 1689–1699. [Google Scholar] [CrossRef]
- El-Speiy, M.; El-Sawy, M.; Badri, F.; Sadaka, T. Effect of Honey Bees Supplementation for Semen Extender on Cryopreservation, Bacterial Activity and Fertility Traits of Rabbits. Egypt. J. Rabbit Sci. 2017, 27, 1–22. [Google Scholar] [CrossRef]
- Machebe, N.S.; Ugwu, S.O.; Akandi, A. Survivability of boar sperm stored under room temperature in extenders containing some natural products. Open Access Anim. Physiol. 2015, 57. [Google Scholar] [CrossRef]
- Chua, L.S.; Rahaman, N.L.A.; Adnan, N.A.; Eddie Tan, T.T. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Gautam, S.; Sharma, A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010, 118, 391–397. [Google Scholar] [CrossRef]
- Khan, F.R.; Abadin, Z.U.; Rauf, N. Honey: Nutritional and medicinal value. Int. J. Clin. Pract. 2007, 61, 1705–1707. [Google Scholar] [CrossRef]
- Mullai, V.; Menon, T. Bactericidal activity of different types of honey against clinical and environmental isolates of Pseudomonas aeruginosa. J. Altern. Complement. Med. 2007, 13, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Masoumi, R.; Rostami, B.; Shahir, M.H.; Taghilou, P.; Arslan, H.O. Effects of supplementation of Tris-egg yolk extender with royal jelly on chilled and frozen-thawed ram semen characteristics. Cryobiology 2019, 88, 75–80. [Google Scholar] [CrossRef]
- Moradi, A.R.; Malekinejad, H.; Farrokhi-Ardabili, F.; Bernousi, I. Royal Jelly improves the sperm parameters of ram semen during liquid storage and serves as an antioxidant source. Small Rumin. Res. 2013, 113, 346–352. [Google Scholar] [CrossRef]
- Alcay, S.; Toker, M.B.; Onder, N.T.; Gokce, E. Royal jelly supplemented soybean lecithin-based extenders improve post-thaw quality and incubation resilience of goat spermatozoa. Cryobiology 2017, 74, 81–85. [Google Scholar] [CrossRef]
- Shahzad, Q.; Mehmood, M.U.; Khan, H.; ul Husna, A.; Qadeer, S.; Azam, A.; Naseer, Z.; Ahmad, E.; Safdar, M.; Ahmad, M. Royal jelly supplementation in semen extender enhances post-thaw quality and fertility of Nili-Ravi buffalo bull sperm. Anim. Reprod. Sci. 2016, 167, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, S.M. Effect of royal jelly on the fertilizing ability of buffalo spermatozoa in vitro. J. Buffalo Sci. 2012, 1, 1–4. [Google Scholar] [CrossRef]
- Kodai, T.; Umebayashi, K.; Nakatani, T.; Ishiyama, K.; Noda, N. Compositions of royal jelly II. Organic acid glycosides and sterols of the royal jelly of honeybees (Apis mellifera). Chem. Pharm. Bull. 2007, 55, 1528–1531. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Al-Gabri, N.A.; Hashem, N.M.; Gonzalez-Bulnes, A. Supplementation with Proline Improves Haemato-Biochemical and Reproductive Indicators in Male Rabbits Affected by Environmental Heat-Stress. Animals 2021, 11. [Google Scholar] [CrossRef]
- Rahnama, G.; Deldar, H.; Ansari Pirsaraei, Z.; Kazemifard, M. Oral administration of royal jelly may improve the preservation of rooster spermatozoa. J. Anim. Physiol. Anim. Nutr. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Toker, M.B.; Alcay, S.; Gokce, E.; Ustuner, B. Cryopreservation of ram semen with antioxidant supplemented soybean lecithin-based extenders and impacts on incubation resilience. Cryobiology 2016, 72, 205–209. [Google Scholar] [CrossRef]
- El-Seadawy, I.E.S.; El-Nattat, W.S.; El-Tohamy, M.M.; Aziza, S.A.H.; El-Senosy, Y.A.; Hussein, A.S. Preservability of rabbit semen after chilled storage in tris based extender enriched with different concentrations of Propolis ethanolic extract (PEE). Asian Pac. J. Reprod. 2017, 6, 68–76. [Google Scholar] [CrossRef]
- Virgili, F.; Marino, M. Regulation of cellular signals from nutritional molecules: A specific role for phytochemicals, beyond antioxidant activity. Free Radic. Biol. Med. 2008, 45, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M. Antimicrobial Effects of Propolis on Preservation of Ram’s Semen Extender and Its Fertility Rate. J. Anim. Poult. Prod. 2017, 8, 203–213. [Google Scholar] [CrossRef]
- Khalifa, E.I.; Mohamed, M.Y. Possibility of using propolis as natural antibiotic instead of synthetic antibiotics in ram semen extenders. Egypt. J. Sheep Goat Sci. 2016, 11, 1–14. [Google Scholar] [CrossRef]
- Barakat, I.A.H.; Alajmi, R.A.; Zoheir, K.M.A.; Salem, L.M.; Al-Hemidiy, A.R. Gene expression and maturation evaluation of sheep oocytes cultured in medium supplemented with natural antioxidant source. S. Afr. J. Anim. Sci. 2018, 48, 261–270. [Google Scholar] [CrossRef]
- Zhu, J.; Moawad, A.R.; Wang, C.Y.; Li, H.F.; Ren, J.Y.; Dai, Y.F. Advances in in vitro production of sheep embryos. Int. J. Vet. Sci. Med. 2018, 6, S15–S26. [Google Scholar] [CrossRef] [PubMed]
- Veshkini, A.; Mohammadi-Sangcheshmeh, A.; Ghanem, N.; Abazari-kia, A.H.; Mottaghi, E.; Kamaledini, R.; Deldar, H.; Ozturk, I.; Gastal, E.L. Oocyte maturation with royal jelly increases embryo development and reduces apoptosis in goats. Anim. Reprod. 2018, 15, 124–134. [Google Scholar] [CrossRef]
- Eshtiyaghi, M.; Deldar, H.; Pirsaraei, Z.A.; Shohreh, B. Royal jelly may improve the metabolism of glucose and redox state of ovine oocytes matured in vitro and embryonic development following in vitro fertilization. Theriogenology 2016, 86, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Kaabi, A.M.; Barakat, I.A.H.; Alajmi, R.A.; Abdel-Daim, M.M. Use of black seed (Nigella sativa) honey bee to improve sheep oocyte maturation medium. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Hassan, A.A.; Hammodi, A.S.; Kasem, Y.Y. Effect of royal jelly on reproductive performance in cadmium-treated male rats. Iraqi J. Vet. Sci. 2012, 26, 225–231. [Google Scholar]
- Seven, I.; Tatli Seven, P.; Gul Baykalir, B.; Parlak Ak, T.; Ozer Kaya, S.; Yaman, M. Bee glue (propolis) improves reproductive organs, sperm quality and histological changes and antioxidant parameters of testis tissues in rats exposed to excess copper. Andrologia 2020, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mahran, A.; AlRashidy, A.; ElMawla, A. Role of propolis in improving male rat fertility affected with aluminum chloride cytotoxicity. Hematology 2011, 1, 189. [Google Scholar] [CrossRef]
- Al-Sanafi, A.; Mohssin, S.; Abdulla, S. Effect of Royal Jelly on male Infertility. Iraqi J. Vet. Sci. 2012, 1, 1–12. [Google Scholar]
- ElMazoudy, R.H.; Attia, A.A.; El-Shenawy, N.S. Protective role of propolis against reproductive toxicity of chlorpyrifos in male rats. Pestic. Biochem. Physiol. 2011, 101, 175–181. [Google Scholar] [CrossRef]
- Khatab, A.E.; Hashem, N.M.; El-Kodary, L.M.; Lotfy, F.M.; Hassan, G.A. Evaluation of the Effects of Cypermethrin on Female Reproductive Function by Using Rabbit Model and of the Protective Role of Chinese Propolis. Biomed. Environ. Sci. 2016, 29. [Google Scholar] [CrossRef]
- El-Nekeety, A.A.; El-Kholy, W.; Abbas, N.F.; Ebaid, A.; Amra, H.A.; Abdel-Wahhab, M.A. Efficacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon 2007, 50, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Biedenharn, K.R.; Fedor, J.M.; Agarwal, A. Lifestyle factors and reproductive health: Taking control of your fertility. Reprod. Biol. Endocrinol. 2013, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Azad, F.; Nejati, V.; Shalizar-Jalali, A.; Najafi, G.; Rahmani, F. Royal jelly protects male mice against nicotine-induced reproductive failure. Vet. Res. Forum 2018, 9, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Troncoso, N.; Sanchez, F.; Garbarino, J.A.; Vanella, A. Propolis protects human spermatozoa from DNA damage caused by benzo [a] pyrene and exogenous reactive oxygen species. Life Sci. 2006, 78, 1401–1406. [Google Scholar] [CrossRef]
- Mohamed, M. Honey and male reproductive health. Honey Curr. Res. Clin. Appl. 2012, 131–142. [Google Scholar]
- Mohamed, M.; Sulaiman, S.A.; Sirajudeen, K.N.S. Protective effect of honey against cigarette smoke induced-impaired sexual behavior and fertility of male rats. Toxicol. Ind. Health 2013, 29, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Eweka, A.O.; OmIniabohs, F.A.E. Histological studies of the effects of monosodium glutamate on the kidney of adult Wistar rats. Internet J. Heal. 2007, 6, 2. [Google Scholar]
- ElMetwally, A. Immunohistopathologic Study On The Ameliorative Effect Of Propolis Against Fluoride Cytotoxicity On Rabbit Buck Fertility. Alexandria J. Vet. Sci. 2017, 53, 1. [Google Scholar] [CrossRef]
- Khaled, F.A.; Yousef, M.I.; Kamel, K.I. The protective role of propolis against the reproductive toxicity of mono-sodium glutamine in male rabbits. IJCS 2016, 4, 4–9. [Google Scholar]
- Haron, M.N.; Rahman, W.F.W.A.; Sulaiman, S.A.; Mohamed, M. Tualang honey ameliorates restraint stress-induced impaired pregnancy outcomes in rats. Eur. J. Integr. Med. 2014, 6, 657–663. [Google Scholar] [CrossRef]
- Haron, M.N.; Mohamed, M. Effect of honey on the reproductive system of male rat offspring exposed to prenatal restraint stress. Andrologia 2016, 48, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Rajabzadeh, A.; Sagha, M.; Gholami, M.R.; Hemmati, R. Honey and vitamin E restore the plasma level of gonadal hormones and improve the fertilization capacity in noise-stressed rats. Crescent J. Med. Biol. Sci. 2015, 2, 64–68. [Google Scholar]
- Hashem, N.M.; Abo-elsoud, M.A.; Nour El-Din, A.N.M.; Kamel, K.I.; Hassan, G.A. Prolonged exposure of dietary phytoestrogens on semen characteristics and reproductive performance of rabbit bucks. Domest. Anim. Endocrinol. 2018, 64. [Google Scholar] [CrossRef]
- Abo-elsoud, M.A.; Hashem, N.M.; Nour El-Din, A.N.M.; Kamel, K.I.; Hassan, G.A. Soybean isoflavone affects in rabbits: Effects on metabolism, antioxidant capacity, hormonal balance and reproductive performance. Anim. Reprod. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).