Oxidative Stress in Drug-Induced Liver Injury (DILI): From Mechanisms to Biomarkers for Use in Clinical Practice
Abstract
1. Introduction
1.1. Epidemiology
1.2. Diagnosis
2. Potential Mechanisms Involved in DILI Pathogenesis
2.1. Drug Factors
2.2. Metabolic Mechanisms
2.2.1. Oxidative Stress
2.2.2. Mitochondrial Dysfunction
2.2.3. Endoplasmic Reticulum (ER) Stress
2.3. BSEP Inhibition
2.4. Activation of the Immune Response
2.4.1. The Hapten Hypothesis
2.4.2. The Danger Hypothesis
2.4.3. The Pharmacological Interaction (p-i) Hypothesis
2.4.4. The Altered Peptide Repertoire Hypothesis
2.4.5. The Multiple Determinant Hypothesis
2.4.6. The Inflammatory Stress Hypothesis
3. Risk Factors
3.1. Age
3.2. Gender
3.3. Alcohol Consumption
3.4. Drug Metabolism Genetic Polymorphisms
3.4.1. Cytochrome P450 Family
3.4.2. UDP-Glucuronosyltransferases
3.4.3. N-Acetyl Transferases
3.4.4. Glutathione-S-Transferases
3.4.5. Transporters
3.5. Antioxidant Defense System Genetic Polymorphisms
3.6. HLA Haplotypes
3.7. Other Genetic Polymorphisms Associated with DILI Susceptibility
4. Biomarkers
4.1. Diagnosis
4.2. Prediction
4.3. Prognosis
4.4. Extracellular Vesicles
5. Role of Oxidative Stress in DILI: Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Roth, R.A.; Ganey, P.E. Intrinsic versus idiosyncratic drug-induced hepatotoxicity—Two villains or one? J. Pharmacol. Exp. Ther. 2010, 332, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, J.H.; Bjornsson, E.S. Drug-Induced Liver Injury—Types and Phenotypes. N. Engl. J. Med. 2019, 381, 264–273. [Google Scholar] [CrossRef]
- Andrade, R.J.; Chalasani, N.; Bjornsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, M.I.; Kaplowitz, N.; et al. Drug-induced liver injury. Nat. Rev. Dis. Prim. 2019, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Bernal, W.; Hyyrylainen, A.; Gera, A.; Audimoolam, V.K.; McPhail, M.J.; Auzinger, G.; Rela, M.; Heaton, N.; O’Grady, J.G.; Wendon, J.; et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J. Hepatol. 2013, 59, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Gyamlani, G.G.; Parikh, C.R. Acetaminophen toxicity: Suicidal vs. accidental. Crit. Care 2002, 6, 155–159. [Google Scholar] [CrossRef]
- Larson, A.M.; Polson, J.; Fontana, R.J.; Davern, T.J.; Lalani, E.; Hynan, L.S.; Reisch, J.S.; Schiodt, F.V.; Ostapowicz, G.; Shakil, A.O.; et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005, 42, 1364–1372. [Google Scholar] [CrossRef]
- Reuben, A.; Tillman, H.; Fontana, R.J.; Davern, T.; McGuire, B.; Stravitz, R.T.; Durkalski, V.; Larson, A.M.; Liou, I.; Fix, O.; et al. Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann. Intern. Med. 2016, 164, 724–732. [Google Scholar] [CrossRef]
- Chen, M.; Suzuki, A.; Borlak, J.; Andrade, R.J.; Lucena, M.I. Drug-induced liver injury: Interactions between drug properties and host factors. J. Hepatol. 2015, 63, 503–514. [Google Scholar] [CrossRef]
- Carrascosa, M.F.; Salcines-Caviedes, J.R.; Lucena, M.I.; Andrade, R.J. Acute liver failure following atorvastatin dose escalation: Is there a threshold dose for idiosyncratic hepatotoxicity? J. Hepatol. 2015, 62, 751–752. [Google Scholar] [CrossRef]
- Lammert, C.; Einarsson, S.; Saha, C.; Niklasson, A.; Bjornsson, E.; Chalasani, N. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: Search for signals. Hepatology 2008, 47, 2003–2009. [Google Scholar] [CrossRef]
- Bell, L.N.; Chalasani, N. Epidemiology of idiosyncratic drug-induced liver injury. Semin. Liver Dis. 2009, 29, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-Cabrera, J.; Sanjuan-Jimenez, R.; Clavijo, E.; Medina-Caliz, I.; Gonzalez-Jimenez, A.; Garcia-Cortes, M.; Ortega-Alonso, A.; Jimenez-Perez, M.; Gonzalez-Grande, R.; Stephens, C.; et al. Incidence and prevalence of acute hepatitis E virus infection in patients with suspected Drug-Induced Liver Injury in the Spanish DILI Registry. Liver Int. 2020. [Google Scholar] [CrossRef]
- Garcia-Cortes, M.; Robles-Diaz, M.; Stephens, C.; Ortega-Alonso, A.; Lucena, M.I.; Andrade, R.J. Drug induced liver injury: An update. Arch. Toxicol. 2020. [Google Scholar] [CrossRef]
- De Valle, M.B.; Av Klinteberg, V.; Alem, N.; Olsson, R.; Bjornsson, E. Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment. Pharmacol. Ther. 2006, 24, 1187–1195. [Google Scholar] [CrossRef]
- De Abajo, F.J.; Montero, D.; Madurga, M.; Garcia Rodriguez, L.A. Acute and clinically relevant drug-induced liver injury: A population based case-control study. Br. J. Clin. Pharmacol. 2004, 58, 71–80. [Google Scholar] [CrossRef]
- Sgro, C.; Clinard, F.; Ouazir, K.; Chanay, H.; Allard, C.; Guilleminet, C.; Lenoir, C.; Lemoine, A.; Hillon, P. Incidence of drug-induced hepatic injuries: A French population-based study. Hepatology 2002, 36, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, E.S.; Bergmann, O.M.; Bjornsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013, 144, 1419–1425, 1425.e1–1425.e3, quiz e1419–e1420. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Medina-Caliz, I.; Gonzalez-Jimenez, A.; Garcia-Cortes, M.; Lucena, M.I. Hepatic Damage by Natural Remedies. Semin. Liver Dis. 2018, 38, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Eickhoff, A. Herbal hepatotoxicity in traditional and modern medicine: Actual key issues and new encouraging steps. Front. Pharmacol. 2015, 6, 72. [Google Scholar] [CrossRef]
- Navarro, V.J.; Lucena, M.I. Hepatotoxicity induced by herbal and dietary supplements. Semin. Liver Dis. 2014, 34, 172–193. [Google Scholar] [CrossRef]
- Kullak-Ublick, G.A.; Andrade, R.J.; Merz, M.; End, P.; Benesic, A.; Gerbes, A.L.; Aithal, G.P. Drug-induced liver injury: Recent advances in diagnosis and risk assessment. Gut 2017, 66, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.S.; Hoofnagle, J.H. Categorization of drugs implicated in causing liver injury: Critical assessment based on published case reports. Hepatology 2016, 63, 590–603. [Google Scholar] [CrossRef]
- Andrade, R.J.; Aithal, G.P.; Björnsson, E.S.; Kaplowitz, N.; Kullak-Ublick, G.A.; Larrey, D.; Karlsen, T.H. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef]
- Benichou, C.; Solal Celigny, P. Standardization of definitions and criteria for causality assessment of adverse drug reactions. Drug-induced blood cytopenias: Report of an international consensus meeting. Nouv. Rev. Fr. Hematol. 1991, 33, 257–262. [Google Scholar] [PubMed]
- Aithal, G.P.; Watkins, P.B.; Andrade, R.J.; Larrey, D.; Molokhia, M.; Takikawa, H.; Hunt, C.M.; Wilke, R.A.; Avigan, M.; Kaplowitz, N.; et al. Case definition and phenotype standardization in drug-induced liver injury. Clin. Pharmacol. Ther. 2011, 89, 806–815. [Google Scholar] [CrossRef]
- Navarro, V.J.; Senior, J.R. Drug-Related Hepatotoxicity. N. Engl. J. Med. 2006, 354, 731–739. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Raj, S. Drug-induced liver injury with skin reactions: Drugs and host risk factors, clinical phenotypes and prognosis. Liver Int. 2019, 39, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Devarbhavi, H.; Raj, S.; Aradya, V.H.; Rangegowda, V.T.; Veeranna, G.P.; Singh, R.; Reddy, V.; Patil, M. Drug-induced liver injury associated with Stevens-Johnson syndrome/toxic epidermal necrolysis: Patient characteristics, causes, and outcome in 36 cases. Hepatology 2016, 63, 993–999. [Google Scholar] [CrossRef]
- Sanabria-Cabrera, J.; Medina-Caliz, I.; Stankeviciute, S.; Rodriguez-Nicolas, A.; Almarza-Torres, M.; Lucena, M.I.; Andrade, R.J. Drug-Induced liver Injury Associated with Severe Cutaneous Hypersensitivity Reactions: A Complex Entity in Need of a Multidisciplinary Approach. Curr. Pharm. Des. 2019, 25, 3855–3871. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Raj, S.; Joseph, T.; Singh, R.; Patil, M. Features and Treatment of Dapsone-Induced Hepatitis, Based on Analysis of 44 Cases and Literature Review. Clin. Gastroenterol. Hepatol. 2017, 15, 1805–1807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Devarbhavi, H.; Karanth, D.; Prasanna, K.S.; Adarsh, C.K.; Patil, M. Drug-Induced liver injury with hypersensitivity features has a better outcome: A single-center experience of 39 children and adolescents. Hepatology 2011, 54, 1344–1350. [Google Scholar] [CrossRef]
- Drug-Induced Liver Injury (DILI): Current Status and Future Directions for Drug Development and the Post-Market Setting; A Consensus by a CIOMS Working Group; Council for International Organizations of Medical Sciences (CIOMS): Geneva, Switzerland, 2020.
- Danan, G.; Benichou, C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J. Clin. Epidemiol. 1993, 46, 1323–1330. [Google Scholar] [CrossRef]
- Benesic, A.; Rotter, I.; Dragoi, D.; Weber, S.; Buchholtz, M.L.; Gerbes, A.L. Development and Validation of a Test to Identify Drugs That Cause Idiosyncratic Drug-Induced Liver Injury. Clin. Gastroenterol. Hepatol. 2018, 16, 1488–1494.e5. [Google Scholar] [CrossRef] [PubMed]
- Benesic, A.; Rahm, N.L.; Ernst, S.; Gerbes, A.L. Human monocyte-derived cells with individual hepatocyte characteristics: A novel tool for personalized in vitro studies. Lab Investig. 2012, 92, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug Metabolism in the Liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Andrade, R.J.; Robles, M.; Ulzurrun, E.; Lucena, M.I. Drug-induced liver injury: Insights from genetic studies. Pharmacogenomics 2009, 10, 1467–1487. [Google Scholar] [CrossRef]
- Uetrecht, J. Mechanistic Studies of Idiosyncratic DILI: Clinical Implications. Front. Pharmacol. 2019, 10, 837. [Google Scholar] [CrossRef]
- Hughes, J.D.; Blagg, J.; Price, D.A.; Bailey, S.; Decrescenzo, G.A.; Devraj, R.V.; Ellsworth, E.; Fobian, Y.M.; Gibbs, M.E.; Gilles, R.W.; et al. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg. Med. Chem. Lett. 2008, 18, 4872–4875. [Google Scholar] [CrossRef]
- Chen, M.; Borlak, J.; Tong, W. High lipophilicity and high daily dose of oral medications are associated with significant risk for drug-induced liver injury. Hepatology 2013, 58, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.R.; Uetrecht, J.; Shear, N.H. Idiosyncratic drug reactions: The reactive metabolite syndromes. Lancet 2000, 356, 1587–1591. [Google Scholar] [CrossRef]
- Uetrecht, J. Idiosyncratic drug reactions: Current understanding. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 513–539. [Google Scholar] [CrossRef]
- Stepan, A.F.; Walker, D.P.; Bauman, J.; Price, D.A.; Baillie, T.A.; Kalgutkar, A.S.; Aleo, M.D. Structural alert/reactive metabolite concept as applied in medicinal chemistry to mitigate the risk of idiosyncratic drug toxicity: A perspective based on the critical examination of trends in the top 200 drugs marketed in the United States. Chem. Res. Toxicol. 2011, 24, 1345–1410. [Google Scholar] [CrossRef] [PubMed]
- Cubero, F.J.; Zoubek, M.E.; Hu, W.; Peng, J.; Zhao, G.; Nevzorova, Y.A.; Al Masaoudi, M.; Bechmann, L.P.; Boekschoten, M.V.; Muller, M.; et al. Combined Activities of JNK1 and JNK2 in Hepatocytes Protect Against Toxic Liver Injury. Gastroenterology 2016, 150, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Kaplowitz, N. Mechanisms of drug-induced liver injury. Clin. Liver Dis. 2013, 17, 507–518. [Google Scholar] [CrossRef]
- Iorga, A.; Dara, L.; Kaplowitz, N. Drug-Induced Liver Injury: Cascade of Events Leading to Cell Death, Apoptosis or Necrosis. Int. J. Mol. Sci. 2017, 18, 1018. [Google Scholar] [CrossRef]
- Walgren, J.L.; Mitchell, M.D.; Thompson, D.C. Role of metabolism in drug-induced idiosyncratic hepatotoxicity. Crit. Rev. Toxicol. 2005, 35, 325–361. [Google Scholar] [CrossRef]
- Torres, S.; Baulies, A.; Insausti-Urkia, N.; Alarcon-Vila, C.; Fucho, R.; Solsona-Vilarrasa, E.; Nunez, S.; Robles, D.; Ribas, V.; Wakefield, L.; et al. Endoplasmic Reticulum Stress-Induced Upregulation of STARD1 Promotes Acetaminophen-Induced Acute Liver Failure. Gastroenterology 2019, 157, 552–568. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Glutathione and mitochondria. Front. Pharmacol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Ye, H.; Nelson, L.J.; Gomez Del Moral, M.; Martinez-Naves, E.; Cubero, F.J. Dissecting the molecular pathophysiology of drug-induced liver injury. World J. Gastroenterol. 2018, 24, 1373–1385. [Google Scholar] [CrossRef]
- Wendel, A.; Feuerstein, S.; Konz, K.H. Acute paracetamol intoxication of starved mice leads to lipid peroxidation in vivo. BioChem Pharmacol. 1979, 28, 2051–2055. [Google Scholar] [CrossRef]
- Knight, T.R.; Fariss, M.W.; Farhood, A.; Jaeschke, H. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol. Sci. 2003, 76, 229–236. [Google Scholar] [CrossRef]
- Jaeschke, H.; Ramachandran, A. Oxidant Stress and Lipid Peroxidation in Acetaminophen Hepatotoxicity. React. Oxyg. Species (Apex) 2018, 5, 145–158. [Google Scholar] [CrossRef]
- Felser, A.; Blum, K.; Lindinger, P.W.; Bouitbir, J.; Krähenbühl, S. Mechanisms of hepatocellular toxicity associated with dronedarone—A comparison to amiodarone. Toxicol. Sci. 2013, 131, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Hong, I.; Kim, M.; Lee, B.H.; Kim, J.H.; Kang, K.S.; Kim, H.L.; Yoon, B.I.; Chung, H.; Kong, G.; et al. Gene expression profiles of murine fatty liver induced by the administration of methotrexate. Toxicology 2008, 249, 75–84. [Google Scholar] [CrossRef]
- Rabinowich, L.; Shibolet, O. Drug Induced Steatohepatitis: An Uncommon Culprit of a Common Disease. BioMed Res. Int. 2015, 2015, 168905. [Google Scholar] [CrossRef]
- Zimmerman, H.J.; Ishak, K.G. Valproate-induced hepatic injury: Analyses of 23 fatal cases. Hepatology 1982, 2, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Cover, C.; Mansouri, A.; Knight, T.R.; Bajt, M.L.; Lemasters, J.J.; Pessayre, D.; Jaeschke, H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp Ther. 2005, 315, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
- Jaeschke, H.; McGill, M.R.; Ramachandran, A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012, 44, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Henstock, P.V.; Dunn, M.C.; Smith, A.R.; Chabot, J.R.; de Graaf, D. Cellular imaging predictions of clinical drug-induced liver injury. Toxicol. Sci. 2008, 105, 97–105. [Google Scholar] [CrossRef]
- Niu, H.; Sanabria-Cabrera, J.; Alvarez-Alvarez, I.; Robles-Diaz, M.; Stankeviciute, S.; Aithal, G.P.; Bjornsson, E.S.; Andrade, R.J.; Lucena, M.I. Prevention and management of idiosyncratic drug-induced liver injury: Systematic review and meta-analysis of randomised clinical trials. Pharmacol. Res. 2021, 164, 105404. [Google Scholar] [CrossRef] [PubMed]
- Keays, R.; Harrison, P.M.; Wendon, J.A.; Forbes, A.; Gove, C.; Alexander, G.J.; Williams, R. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: A prospective controlled trial. BMJ 1991, 303, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; Hynan, L.S.; Rossaro, L.; Fontana, R.J.; Stravitz, R.T.; Larson, A.M.; Davern, T.J., 2nd; Murray, N.G.; McCashland, T.; Reisch, J.S.; et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009, 137, 856–864.e1. [Google Scholar] [CrossRef]
- Baniasadi, S.; Eftekhari, P.; Tabarsi, P.; Fahimi, F.; Raoufy, M.R.; Masjedi, M.R.; Velayati, A.A. Protective effect of N-acetylcysteine on antituberculosis drug-induced hepatotoxicity. Eur. J. Gastroenterol. Hepatol. 2010, 22, 1235–1238. [Google Scholar] [CrossRef]
- Gu, J.; Tang, S.J.; Tan, S.Y.; Wu, Q.; Zhang, X.; Liu, C.X.; Gao, X.S.; Yuan, B.D.; Han, L.J.; Gao, A.P.; et al. An open-label, randomized and multi-center clinical trial to evaluate the efficacy of Silibinin in preventing drug-induced liver injury. Int. J. Clin. Exp. Med. 2015, 8, 4320–4327. [Google Scholar] [PubMed]
- Luangchosiri, C.; Thakkinstian, A.; Chitphuk, S.; Stitchantrakul, W.; Petraksa, S.; Sobhonslidsuk, A. A double-blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug-induced liver injury. BMC Complement. Altern. Med. 2015, 15, 334. [Google Scholar] [CrossRef]
- Marjani, M.; Baghaei, P.; Kazempour Dizaji, M.; Gorji Bayani, P.; Fahimi, F.; Tabarsi, P.; Velayati, A.A. Evaluation of Hepatoprotective Effect of Silymarin Among Under Treatment Tuberculosis Patients: A Randomized Clinical Trial. Iran J. Pharm. Res. 2016, 15, 247–252. [Google Scholar]
- Zhang, S.; Pan, H.; Peng, X.; Lu, H.; Fan, H.; Zheng, X.; Xu, G.; Wang, M.; Wang, J. Preventive use of a hepatoprotectant against anti-tuberculosis drug-induced liver injury: A randomized controlled trial. J. Gastroenterol. Hepatol. 2016, 31, 409–416. [Google Scholar] [CrossRef]
- Heo, E.; Kim, D.K.; Oh, S.H.; Lee, J.K.; Park, J.H.; Chung, H.S. Effect of Prophylactic Use of Silymarin on Anti-tuberculosis Drugs Induced Hepatotoxicity. Tuberc. Respir. Dis. (Seoul) 2017, 80, 265–269. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; An, Q.; Zhang, S.; Shen, L. Efficacy of silibinin capsules in the prevention of liver injury induced by anti-tuberculosis drugs. Chin. J. Antitubere 2017, 39, 757–760. [Google Scholar]
- Asgarshirazi, M.; Shariat, M.; Sheikh, M. Comparison of efficacy of folic acid and silymarin in the management of antiepileptic drug induced liver injury: A randomized clinical trial. Hepatobiliary Pancreat Dis. Int. 2017, 16, 296–302. [Google Scholar] [CrossRef]
- Marjani, M.; Fahim, F.; Sadr, M.; Kazempour Dizaji, M.; Moniri, A.; Khabiri, S.; Tabarsi, P.; Velayati, A.A. Evaluation of Silymarin for management of anti-tuberculosis drug induced liver injury: A randomized clinical trial. Gastroenterol. Hepatol. Bed Bench 2019, 12, 138–142. [Google Scholar] [PubMed]
- Li, X.; Zhou, J.; Chen, S.; Guan, M.; Wang, Y.; Zhao, L.; Ying, H.; Zhou, Y. Role of bicyclol in preventing chemotherapeutic agent-induced liver injury in patients over 60 years of age with cancer. J. Int. Med. Res. 2014, 42, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.H.; Li, L.; Zhang, X.; Gu, J.; Du, Y.D.; Cai, C.; Xiao, H.P. Role of bicyclol in preventing drug-induced liver injury in tuberculosis patients with liver disease. Int. J. Tuberc. Lung Dis. 2015, 19, 475–480. [Google Scholar] [CrossRef]
- Tang, R. Analysis of the efficacy of bicyclol tablets in the treatment of liver injury caused by anti-tuberculosis drug. Xinxueguanbing Fangzhi Zhishi 2013, 10, 83–85. [Google Scholar]
- Naiqiong, W.; Liansheng, W.; Zhanying, H.; Yuanlin, G.; Chenggang, Z.; Ying, G.; Qian, D.; Dongchen, L.; Yanjun, Z.; Jianjun, L. A Multicenter and Randomized Controlled Trial of Bicyclol in the Treatment of Statin-Induced Liver Injury. Med. Sci. Monit. 2017, 23, 5760–5766. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Mo, Y.; Zhang, D. Magnesium isoglycyrrhizinate prevention of chemotherapy-induced liver damage during initial treatment of patients with gastrointestinal tumors. Zhonghua Gan Zang Bing Za Zhi 2015, 23, 204–208. [Google Scholar] [CrossRef]
- Li-na, T.; Feng, L.; Zan, S.; Yuanjue, S.; Yang, Y. Magnesium isoglycyrrhizinate used in the treatment of chemotherapeutic drugs-induced acute liver dysfunction: A phase III clinical trial. Tumori 2012, 32, 738–743. [Google Scholar]
- Wang, Y.; Wang, Z.; Gao, M.; Zhong, H.; Chen, C.; Yao, Y.; Zhang, Z.; Zhang, X.; Li, F.; Zhang, J.; et al. Efficacy and safety of magnesium isoglycyrrhizinate injection in patients with acute drug-induced liver injury: A phase II trial. Liver Int. 2019, 39, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Wen, F.; Yang, W.; Deng, Y.B.; Li, M.; Zhang, P.F.; Tang, R.L.; Li, Q.; Wei, Y.Q. The role of tiopronin for the prevention of chemotherapy-related liver toxicity in advanced colorectal cancer patients treated with mFOLFOX7: A prospective analysis. Tumori 2014, 100, 446–451. [Google Scholar] [CrossRef]
- Hatamkhani, S.; Khalili, H.; Karimzadeh, I.; Dashti-Khavidaki, S.; Abdollahi, A.; Jafari, S. Carnitine for prevention of antituberculosis drug-induced hepatotoxicity: A randomized, clinical trial. J. Gastroenterol. Hepatol. 2014, 29, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Koido, M.; Kawakami, E.; Fukumura, J.; Noguchi, Y.; Ohori, M.; Nio, Y.; Nicoletti, P.; Aithal, G.P.; Daly, A.K.; Watkins, P.B.; et al. Polygenic architecture informs potential vulnerability to drug-induced liver injury. Nat. Med. 2020, 26, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Dara, L.; Win, S.; Than, T.A.; Yuan, L.; Abbasi, S.Q.; Liu, Z.X.; Kaplowitz, N. Regulation of drug-induced liver injury by signal transduction pathways: Critical role of mitochondria. Trends Pharmacol. Sci. 2013, 34, 243–253. [Google Scholar] [CrossRef]
- Haasio, K.; Koponen, A.; Penttila, K.E.; Nissinen, E. Effects of entacapone and tolcapone on mitochondrial membrane potential. Eur. J. Pharmacol. 2002, 453, 21–26. [Google Scholar] [CrossRef]
- Bova, M.P.; Tam, D.; McMahon, G.; Mattson, M.N. Troglitazone induces a rapid drop of mitochondrial membrane potential in liver HepG2 cells. Toxicol. Lett. 2005, 155, 41–50. [Google Scholar] [CrossRef]
- Ong, M.M.K.; Latchoumycandane, C.; Boelsterli, U.A. Troglitazone-Induced Hepatic Necrosis in an Animal Model of Silent Genetic Mitochondrial Abnormalities. Toxicol. Sci. 2006, 97, 205–213. [Google Scholar] [CrossRef]
- Dykens, J.A.; Jamieson, J.D.; Marroquin, L.D.; Nadanaciva, S.; Xu, J.J.; Dunn, M.C.; Smith, A.R.; Will, Y. In vitro assessment of mitochondrial dysfunction and cytotoxicity of nefazodone, trazodone, and buspirone. Toxicol. Sci. 2008, 103, 335–345. [Google Scholar] [CrossRef]
- Boelsterli, U.A. Mechanisms of NSAID-induced hepatotoxicity: Focus on nimesulide. Drug Saf. 2002, 25, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Török, M.; Zahno, A.; Waldhauser, K.M.; Brecht, K.; Krähenbühl, S. Toxicity of statins on rat skeletal muscle mitochondria. Cell. Mol. Life Sci. CMLS 2006, 63, 2415–2425. [Google Scholar] [CrossRef]
- Tolosa, L.; Carmona, A.; Castell, J.V.; Gómez-Lechón, M.J.; Donato, M.T. High-content screening of drug-induced mitochondrial impairment in hepatic cells: Effects of statins. Arch. Toxicol. 2015, 89, 1847–1860. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.M.; Yang, Y.; Watkins, P.B.; Howell, B.A.; Siler, S.Q. Elucidating Differences in the Hepatotoxic Potential of Tolcapone and Entacapone With DILIsym((R)), a Mechanistic Model of Drug-Induced Liver Injury. CPT Pharmacometr. Syst. Pharmacol. 2016, 5, 31–39. [Google Scholar] [CrossRef]
- Berson, A.; Cazanave, S.; Descatoire, V.; Tinel, M.; Grodet, A.; Wolf, C.; Feldmann, G.; Pessayre, D. The Anti-Inflammatory Drug, Nimesulide (4-Nitro-2-phenoxymethane-sulfoanilide), Uncouples Mitochondria and Induces Mitochondrial Permeability Transition in Human Hepatoma Cells: Protection by Albumin. J. Pharm. Exp. Ther. 2006, 318, 444. [Google Scholar] [CrossRef]
- Lim, P.L.; Liu, J.; Go, M.L.; Boelsterli, U.A. The mitochondrial superoxide/thioredoxin-2/Ask1 signaling pathway is critically involved in troglitazone-induced cell injury to human hepatocytes. Toxicol. Sci. 2008, 101, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Masubuchi, Y.; Kano, S.; Horie, T. Mitochondrial permeability transition as a potential determinant of hepatotoxicity of antidiabetic thiazolidinediones. Toxicology 2006, 222, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Tay, V.K.; Wang, A.S.; Leow, K.Y.; Ong, M.M.; Wong, K.P.; Boelsterli, U.A. Mitochondrial permeability transition as a source of superoxide anion induced by the nitroaromatic drug nimesulide in vitro. Free Radic. Biol. Med. 2005, 39, 949–959. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Nieminen, A.L.; Qian, T.; Trost, L.C.; Elmore, S.P.; Nishimura, Y.; Crowe, R.A.; Cascio, W.E.; Bradham, C.A.; Brenner, D.A.; et al. The mitochondrial permeability transition in cell death: A common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1998, 1366, 177–196. [Google Scholar] [CrossRef]
- Cho, T.; Wang, X.; Uetrecht, J. Rotenone Increases Isoniazid Toxicity but Does Not Cause Significant Liver Injury: Implications for the Hypothesis that Inhibition of the Mitochondrial Electron Transport Chain Is a Common Mechanism of Idiosyncratic Drug-Induced Liver Injury. Chem. Res. Toxicol. 2019, 32, 1423–1431. [Google Scholar] [CrossRef]
- Lee, K.K.; Fujimoto, K.; Zhang, C.; Schwall, C.T.; Alder, N.N.; Pinkert, C.A.; Krueger, W.; Rasmussen, T.; Boelsterli, U.A. Isoniazid-induced cell death is precipitated by underlying mitochondrial complex I dysfunction in mouse hepatocytes. Free Radic Biol. Med. 2013, 65, 584–594. [Google Scholar] [CrossRef]
- Stewart, J.D.; Horvath, R.; Baruffini, E.; Ferrero, I.; Bulst, S.; Watkins, P.B.; Fontana, R.J.; Day, C.P.; Chinnery, P.F. Polymerase gamma gene POLG determines the risk of sodium valproate-induced liver toxicity. Hepatology 2010, 52, 1791–1796. [Google Scholar] [CrossRef]
- Fromenty, B. Alteration of mitochondrial DNA homeostasis in drug-induced liver injury. Food Chem. Toxicol. 2020, 135, 110916. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Haouzi, D.; Descatoire, V.; Demeilliers, C.; Sutton, A.; Vadrot, N.; Fromenty, B.; Feldmann, G.; Pessayre, D.; Berson, A. Tacrine inhibits topoisomerases and DNA synthesis to cause mitochondrial DNA depletion and apoptosis in mouse liver. Hepatology 2003, 38, 715–725. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Nakayama, S.; Horie, T. Role of mitochondrial permeability transition in diclofenac-induced hepatocyte injury in rats. Hepatology 2002, 35, 544–551. [Google Scholar] [CrossRef]
- Grazioli, S.; Pugin, J. Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.E.; Amaral, S.S.; Pires, D.A.; Nogueira, L.L.; Soriani, F.M.; Lima, B.H.F.; Lopes, G.A.O.; Russo, R.C.; Ávila, T.V.; Melgaço, J.G.; et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 2012, 56, 1971–1982. [Google Scholar] [CrossRef]
- McGill, M.R.; Staggs, V.S.; Sharpe, M.R.; Lee, W.M.; Jaeschke, H.; Acute Liver Failure Study, G. Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology 2014, 60, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.M.; Young, A.L.; Patankar, Y.R.; Berwin, B.L.; Wang, L.; von Herrmann, K.M.; Weier, J.M.; Havrda, M.C. Editor’s Highlight: Nlrp3 Is Required for Inflammatory Changes and Nigral Cell Loss Resulting From Chronic Intragastric Rotenone Exposure in Mice. Toxicol. Sci. 2017, 159, 64–75. [Google Scholar] [CrossRef]
- Stephens, C.; Andrade, R.J.; Lucena, M.I. Mechanisms of drug-induced liver injury. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Baulies, A.; Ribas, V.; Nunez, S.; Torres, S.; Alarcon-Vila, C.; Martinez, L.; Suda, J.; Ybanez, M.D.; Kaplowitz, N.; Garcia-Ruiz, C.; et al. Lysosomal Cholesterol Accumulation Sensitizes To Acetaminophen Hepatotoxicity by Impairing Mitophagy. Sci. Rep. 2015, 5, 18017. [Google Scholar] [CrossRef] [PubMed]
- Uzi, D.; Barda, L.; Scaiewicz, V.; Mills, M.; Mueller, T.; Gonzalez-Rodriguez, A.; Valverde, A.M.; Iwawaki, T.; Nahmias, Y.; Xavier, R.; et al. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J. Hepatol. 2013, 59, 495–503. [Google Scholar] [CrossRef]
- Schroder, M.; Kaufman, R.J. ER stress and the unfolded protein response. Mutat. Res. 2005, 569, 29–63. [Google Scholar] [CrossRef]
- Nagy, G.; Kardon, T.; Wunderlich, L.; Szarka, A.; Kiss, A.; Schaff, Z.; Bánhegyi, G.; Mandl, J. Acetaminophen induces ER dependent signaling in mouse liver. Arch. BioChem Biophys. 2007, 459, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Szarka, A.; Lotz, G.; Dóczi, J.; Wunderlich, L.; Kiss, A.; Jemnitz, K.; Veres, Z.; Bánhegyi, G.; Schaff, Z.; et al. BGP-15 inhibits caspase-independent programmed cell death in acetaminophen-induced liver injury. Toxicol. Appl. Pharmacol. 2010, 243, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Hur, K.Y.; So, J.S.; Ruda, V.; Frank-Kamenetsky, M.; Fitzgerald, K.; Koteliansky, V.; Iwawaki, T.; Glimcher, L.H.; Lee, A.H. IRE1α activation protects mice against acetaminophen-induced hepatotoxicity. J. Exp. Med. 2012, 209, 307–318. [Google Scholar] [CrossRef]
- Maiuri, A.R.; Breier, A.B.; Turkus, J.D.; Ganey, P.E.; Roth, R.A. Calcium Contributes to the Cytotoxic Interaction Between Diclofenac and Cytokines. Toxicol. Sci. 2016, 149, 372–384. [Google Scholar] [CrossRef]
- Morgan, R.E.; Trauner, M.; van Staden, C.J.; Lee, P.H.; Ramachandran, B.; Eschenberg, M.; Afshari, C.A.; Qualls, C.W., Jr.; Lightfoot-Dunn, R.; Hamadeh, H.K. Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol. Sci. 2010, 118, 485–500. [Google Scholar] [CrossRef]
- Stieger, B. Role of the bile salt export pump, BSEP, in acquired forms of cholestasis. Drug Metab. Rev. 2010, 42, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Whitington, P.F.; Freese, D.K.; Alonso, E.M.; Schwarzenberg, S.J.; Sharp, H.L. Clinical and biochemical findings in progressive familial intrahepatic cholestasis. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 134–141. [Google Scholar] [CrossRef]
- Fattinger, K.; Funk, C.; Pantze, M.; Weber, C.; Reichen, J.; Stieger, B.; Meier, P.J. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: A potential mechanism for hepatic adverse reactions. Clin. Pharmacol. Ther. 2001, 69, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.; Ponelle, C.; Scheuermann, G.; Pantze, M. Cholestatic potential of troglitazone as a possible factor contributing to troglitazone-induced hepatotoxicity: In vivo and in vitro interaction at the canalicular bile salt export pump (Bsep) in the rat. Mol. Pharmacol. 2001, 59, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Aleo, M.D.; Luo, Y.; Swiss, R.; Bonin, P.D.; Potter, D.M.; Will, Y. Human drug-induced liver injury severity is highly associated with dual inhibition of liver mitochondrial function and bile salt export pump. Hepatology 2014, 60, 1015–1022. [Google Scholar] [CrossRef]
- Köck, K.; Ferslew, B.C.; Netterberg, I.; Yang, K.; Urban, T.J.; Swaan, P.W.; Stewart, P.W.; Brouwer, K.L. Risk factors for development of cholestatic drug-induced liver injury: Inhibition of hepatic basolateral bile acid transporters multidrug resistance-associated proteins 3 and 4. Drug Metab. Dispos. 2014, 42, 665–674. [Google Scholar] [CrossRef]
- Wuillemin, N.; Adam, J.; Fontana, S.; Krahenbuhl, S.; Pichler, W.J.; Yerly, D. HLA haplotype determines hapten or p-i T cell reactivity to flucloxacillin. J. Immunol. 2013, 190, 4956–4964. [Google Scholar] [CrossRef]
- Pradeu, T.; Cooper, E.L. The danger theory: 20 years later. Front. Immunol. 2012, 3, 287. [Google Scholar] [CrossRef]
- Pirmohamed, M.; Naisbitt, D.J.; Gordon, F.; Park, B.K. The danger hypothesis—Potential role in idiosyncratic drug reactions. Toxicology 2002, 181–182, 55–63. [Google Scholar] [CrossRef]
- Burgdorf, S.; Kurts, C. Endocytosis mechanisms and the cell biology of antigen presentation. Curr. Opin. Immunol. 2008, 20, 89–95. [Google Scholar] [CrossRef]
- Mak, A.; Uetrecht, J. Immune mechanisms of idiosyncratic drug-induced liver injury. J. Clin. Transl. Res. 2017, 3, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Luyendyk, J.P.; Ganey, P.E.; Roth, R.A. Inflammatory stress and idiosyncratic hepatotoxicity: Hints from animal models. Pharmacol. Rev. 2009, 61, 262–282. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Uetrecht, J.P. The danger hypothesis applied to idiosyncratic drug reactions. Handb. Exp. Pharmacol. 2010, 493–509. [Google Scholar] [CrossRef]
- Mariathasan, S.; Newton, K.; Monack, D.M.; Vucic, D.; French, D.M.; Lee, W.P.; Roose-Girma, M.; Erickson, S.; Dixit, V.M. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 2004, 430, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J. Pharmacological interaction of drugs with antigen-specific immune receptors: The p-i concept. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 301–305. [Google Scholar] [CrossRef]
- Pichler, W.J.; Beeler, A.; Keller, M.; Lerch, M.; Posadas, S.; Schmid, D.; Spanou, Z.; Zawodniak, A.; Gerber, B. Pharmacological interaction of drugs with immune receptors: The p-i concept. Allergol. Int. 2006, 55, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J. The p-i Concept: Pharmacological Interaction of Drugs With Immune Receptors. World Allergy Organ. J. 2008, 1, 96–102. [Google Scholar] [CrossRef]
- Adam, J.; Pichler, W.J.; Yerly, D. Delayed drug hypersensitivity: Models of T-cell stimulation. Br. J. Clin. Pharmacol. 2011, 71, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, B.; Mauri-Hellweg, D.; Zanni, M.; Bettens, F.; Pichler, W.J. Direct, MHC-dependent presentation of the drug sulfamethoxazole to human alphabeta T cell clones. J. Clin. Investig. 1997, 100, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Kindmark, A.; Jawaid, A.; Harbron, C.G.; Barratt, B.J.; Bengtsson, O.F.; Andersson, T.B.; Carlsson, S.; Cederbrant, K.E.; Gibson, N.J.; Armstrong, M.; et al. Genome-wide pharmacogenetic investigation of a hepatic adverse event without clinical signs of immunopathology suggests an underlying immune pathogenesis. Pharmacogenom. J. 2008, 8, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Y.; Chung, W.H.; Huang, H.W.; Chen, Y.T.; Hung, S.I. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J. Allergy Clin. Immunol. 2012, 129, 1562–1569.e1565. [Google Scholar] [CrossRef]
- Ostrov, D.A.; Grant, B.J.; Pompeu, Y.A.; Sidney, J.; Harndahl, M.; Southwood, S.; Oseroff, C.; Lu, S.; Jakoncic, J.; de Oliveira, C.A.; et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc. Natl. Acad. Sci. USA 2012, 109, 9959–9964. [Google Scholar] [CrossRef]
- Yun, J.; Cai, F.; Lee, F.J.; Pichler, W.J. T-cell-mediated drug hypersensitivity: Immune mechanisms and their clinical relevance. Asia Pac. Allergy 2016, 6, 77–89. [Google Scholar] [CrossRef]
- Li, A.P. A review of the common properties of drugs with idiosyncratic hepatotoxicity and the “multiple determinant hypothesis” for the manifestation of idiosyncratic drug toxicity. Chem. Biol. Interact. 2002, 142, 7–23. [Google Scholar] [CrossRef]
- Ulrich, R.G. Idiosyncratic toxicity: A convergence of risk factors. Annu. Rev. Med. 2007, 58, 17–34. [Google Scholar] [CrossRef]
- Roth, R.A.; Maiuri, A.R.; Ganey, P.E. Idiosyncratic Drug-Induced Liver Injury: Is Drug-Cytokine Interaction the Linchpin? J. Pharmacol. Exp. Ther. 2017, 360, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.J.; Ganey, P.E.; Roth, R.A. Idiosyncratic drug-induced liver injury and the role of inflammatory stress with an emphasis on an animal model of trovafloxacin hepatotoxicity. Toxicol. Sci. 2010, 118, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef]
- Kato, R.; Uetrecht, J. Supernatant from Hepatocyte Cultures with Drugs That Cause Idiosyncratic Liver Injury Activates Macrophage Inflammasomes. Chem. Res. Toxicol. 2017, 30, 1327–1332. [Google Scholar] [CrossRef]
- Mak, A.; Kato, R.; Weston, K.; Hayes, A.; Uetrecht, J. Editor’s Highlight: An Impaired Immune Tolerance Animal Model Distinguishes the Potential of Troglitazone/Pioglitazone and Tolcapone/Entacapone to Cause IDILI. Toxicol. Sci. 2018, 161, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Lucena, M.I.; Sanabria, J.; García-Cortes, M.; Stephens, C.; Andrade, R.J. Drug-induced liver injury in older people. Lancet Gastroenterol. Hepatol. 2020, 5, 862–874. [Google Scholar] [CrossRef]
- Chalasani, N.; Bonkovsky, H.L.; Fontana, R.; Lee, W.; Stolz, A.; Talwalkar, J.; Reddy, K.R.; Watkins, P.B.; Navarro, V.; Barnhart, H.; et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology 2015, 148, 1340–1352.e7. [Google Scholar] [CrossRef]
- Lucena, M.I.; Andrade, R.J.; Kaplowitz, N.; Garcia-Cortes, M.; Fernandez, M.C.; Romero-Gomez, M.; Bruguera, M.; Hallal, H.; Robles-Diaz, M.; Rodriguez-Gonzalez, J.F.; et al. Phenotypic characterization of idiosyncratic drug-induced liver injury: The influence of age and sex. Hepatology 2009, 49, 2001–2009. [Google Scholar] [CrossRef]
- Hunt, C.M.; Yuen, N.A.; Stirnadel-Farrant, H.A.; Suzuki, A. Age-related differences in reporting of drug-associated liver injury: Data-mining of WHO Safety Report Database. Regul. Toxicol. Pharmacol. 2014, 70, 519–526. [Google Scholar] [CrossRef]
- Weersink, R.A.; Alvarez-Alvarez, I.; Medina-Caliz, I.; Sanabria-Cabrera, J.; Robles-Diaz, M.; Ortega-Alonso, A.; Garcia-Cortes, M.; Bonilla, E.; Niu, H.; Soriano, G.; et al. Clinical Characteristics and Outcome of Drug-Induced Liver Injury in the Older Patients: From the Young-Old to the Oldest-Old. Clin. Pharmacol. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.; Robles-Diaz, M.; Medina-Caliz, I.; Garcia-Cortes, M.; Ortega-Alonso, A.; Sanabria-Cabrera, J.; Gonzalez-Jimenez, A.; Alvarez-Alvarez, I.; Slim, M.; Jimenez-Perez, M.; et al. Comprehensive analysis and insights gained from long-term experience of the Spanish DILI registry. J. Hepatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Fountain, F.F.; Tolley, E.; Chrisman, C.R.; Self, T.H. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: A 7-year evaluation from a public health tuberculosis clinic. Chest 2005, 128, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.M.; Goldberg, S.V.; Buskin, S.E. Hepatotoxicity associated with isoniazid preventive therapy: A 7-year survey from a public health tuberculosis clinic. JAMA 1999, 281, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Bhaskaran, K.; Pealing, L.; Root, A.; Smeeth, L.; van Staa, T.P.; Klungel, O.H.; Reynolds, R.F.; Douglas, I. Quantification of the risk of liver injury associated with flucloxacillin: A UK population-based cohort study. J. Antimicrob. ChemoTher. 2017, 72, 2636–2646. [Google Scholar] [CrossRef]
- Bryant, A.E., 3rd; Dreifuss, F.E. Valproic acid hepatic fatalities. III. U.S. experience since 1986. Neurology 1996, 46, 465–469. [Google Scholar] [CrossRef]
- Bjornsson, E.; Talwalkar, J.; Treeprasertsuk, S.; Kamath, P.S.; Takahashi, N.; Sanderson, S.; Neuhauser, M.; Lindor, K. Drug-induced autoimmune hepatitis: Clinical characteristics and prognosis. Hepatology 2010, 51, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- DeLemos, A.S.; Foureau, D.M.; Jacobs, C.; Ahrens, W.; Russo, M.W.; Bonkovsky, H.L. Drug-induced liver injury with autoimmune features. Semin. Liver Dis. 2014, 34, 194–204. [Google Scholar] [CrossRef]
- Lawrenson, R.A.; Seaman, H.E.; Sundstrom, A.; Williams, T.J.; Farmer, R.D. Liver damage associated with minocycline use in acne: A systematic review of the published literature and pharmacovigilance data. Drug Saf. 2000, 23, 333–349. [Google Scholar] [CrossRef]
- Robles-Diaz, M.; Lucena, M.I.; Kaplowitz, N.; Stephens, C.; Medina-Caliz, I.; Gonzalez-Jimenez, A.; Ulzurrun, E.; Gonzalez, A.F.; Fernandez, M.C.; Romero-Gomez, M.; et al. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology 2014, 147, 109–118.e5. [Google Scholar] [CrossRef]
- Dakhoul, L.; Ghabril, M.; Gu, J.; Navarro, V.; Chalasani, N.; Serrano, J.; United States Drug Induced Liver Injury, N. Heavy Consumption of Alcohol is Not Associated With Worse Outcomes in Patients With Idiosyncratic Drug-induced Liver Injury Compared to Non-Drinkers. Clin. Gastroenterol. Hepatol. 2018, 16, 722–729.e2. [Google Scholar] [CrossRef]
- Garcia-Cortes, M.; Stephens, C.; Lucena, M.I.; Fernandez-Castaner, A.; Andrade, R.J. Causality assessment methods in drug induced liver injury: Strengths and weaknesses. J. Hepatol. 2011, 55, 683–691. [Google Scholar] [CrossRef]
- Zimmerman, H.J. Effects of alcohol on other hepatotoxins. Alcohol. Clin. Exp. Res. 1986, 10, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K.; Aithal, G.P.; Leathart, J.B.; Swainsbury, R.A.; Dang, T.S.; Day, C.P. Genetic susceptibility to diclofenac-induced hepatotoxicity: Contribution of UGT2B7, CYP2C8, and ABCC2 genotypes. Gastroenterology 2007, 132, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Urban, T.J.; Daly, A.K.; Aithal, G.P. Genetic basis of drug-induced liver injury: Present and future. Semin. Liver Dis. 2014, 34, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Tailor, A.; Faulkner, L.; Naisbitt, D.J.; Park, B.K. The chemical, genetic and immunological basis of idiosyncratic drug-induced liver injury. Hum. Exp. Toxicol. 2015, 34, 1310–1317. [Google Scholar] [CrossRef]
- McGill, M.R.; Jaeschke, H. Biomarkers of drug-induced liver injury: Progress and utility in research, medicine, and regulation. Expert Rev. Mol. Diagn. 2018, 18, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.; Lucena, M.I.; Andrade, R.J. Genetic variations in drug-induced liver injury (DILI): Resolving the puzzle. Front. Genet. 2012, 3, 253. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.W.; Bartlett, J.A.; Andersen, J.W.; Sanne, I.; Wilkinson, G.R.; Hinkle, J.; Rousseau, F.; Ingram, C.D.; Shaw, A.; Lederman, M.M.; et al. Pharmacogenetics of nevirapine-associated hepatotoxicity: An Adult AIDS Clinical Trials Group collaboration. Clin. Infect. Dis. 2006, 43, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.D.; Haas, D.W.; Motsinger, A.A.; Donahue, J.P.; Erdem, H.; Raffanti, S.; Rebeiro, P.; George, A.L.; Kim, R.B.; Haines, J.L.; et al. Drug transporter and metabolizing enzyme gene variants and nonnucleoside reverse-transcriptase inhibitor hepatotoxicity. Clin. Infect. Dis. 2006, 43, 779–782. [Google Scholar] [CrossRef]
- Lang, C.; Meier, Y.; Stieger, B.; Beuers, U.; Lang, T.; Kerb, R.; Kullak-Ublick, G.A.; Meier, P.J.; Pauli-Magnus, C. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet. Genom. 2007, 17, 47–60. [Google Scholar] [CrossRef]
- Ulzurrun, E.; Stephens, C.; Crespo, E.; Ruiz-Cabello, F.; Ruiz-Nunez, J.; Saenz-Lopez, P.; Moreno-Herrera, I.; Robles-Diaz, M.; Hallal, H.; Moreno-Planas, J.M.; et al. Role of chemical structures and the 1331T>C bile salt export pump polymorphism in idiosyncratic drug-induced liver injury. Liver Int. 2013, 33, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Ahn, B.M.; Yi, J.; Lee, J.H.; Lee, J.H.; Nam, S.W.; Chon, C.Y.; Han, K.H.; Ahn, S.H.; Jang, I.J.; et al. MRP2 haplotypes confer differential susceptibility to toxic liver injury. Pharmacogenet. Genom. 2007, 17, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Yimer, G.; Amogne, W.; Habtewold, A.; Makonnen, E.; Ueda, N.; Suda, A.; Worku, A.; Haefeli, W.E.; Burhenne, J.; Aderaye, G.; et al. High plasma efavirenz level and CYP2B6*6 are associated with efavirenz-based HAART-induced liver injury in the treatment of naive HIV patients from Ethiopia: A prospective cohort study. Pharmacogenom. J. 2012, 12, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Rotger, M.; Colombo, S.; Furrer, H.; Bleiber, G.; Buclin, T.; Lee, B.L.; Keiser, O.; Biollaz, J.; Decosterd, L.; Telenti, A.; et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet. Genom. 2005, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, X.; Wu, S.Q.; Chen, G.; Zhang, M.M.; Wang, M.G.; Wang, F.J.; Sandford, A.J.; He, J.Q. Association of CYP2B6 gene polymorphisms and anti-tuberculosis drug-induced hepatotoxicity in a Chinese population. Infect. Genet. Evol. 2017, 51, 198–202. [Google Scholar] [CrossRef]
- Ariyoshi, N.; Iga, Y.; Hirata, K.; Sato, Y.; Miura, G.; Ishii, I.; Nagamori, S.; Kitada, M. Enhanced susceptibility of HLA-mediated ticlopidine-induced idiosyncratic hepatotoxicity by CYP2B6 polymorphism in Japanese. Drug Metab. Pharmacokinet. 2010, 25, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Chern, H.D.; Su, W.J.; Wu, J.C.; Chang, S.C.; Chiang, C.H.; Chang, F.Y.; Lee, S.D. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology 2003, 37, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Becquemont, L.; Mary-Krause, M.; de Waziers, I.; Beaune, P.; Funck-Brentano, C.; Jaillon, P. Combined glutathione-S-transferase M1 and T1 genetic polymorphism and tacrine hepatotoxicity. Clin. Pharmacol. Ther. 2000, 67, 432–437. [Google Scholar] [CrossRef]
- Roy, B.; Chowdhury, A.; Kundu, S.; Santra, A.; Dey, B.; Chakraborty, M.; Majumder, P.P. Increased risk of antituberculosis drug-induced hepatotoxicity in individuals with glutathione S-transferase M1 ’null’ mutation. J. Gastroenterol. Hepatol. 2001, 16, 1033–1037. [Google Scholar] [CrossRef]
- Watanabe, I.; Tomita, A.; Shimizu, M.; Sugawara, M.; Yasumo, H.; Koishi, R.; Takahashi, T.; Miyoshi, K.; Nakamura, K.; Izumi, T.; et al. A study to survey susceptible genetic factors responsible for troglitazone-associated hepatotoxicity in Japanese patients with type 2 diabetes mellitus. Clin. Pharmacol. Ther. 2003, 73, 435–455. [Google Scholar] [CrossRef]
- Huang, Y.S.; Su, W.J.; Huang, Y.H.; Chen, C.Y.; Chang, F.Y.; Lin, H.C.; Lee, S.D. Genetic polymorphisms of manganese superoxide dismutase, NAD(P)H:quinone oxidoreductase, glutathione S-transferase M1 and T1, and the susceptibility to drug-induced liver injury. J. Hepatol. 2007, 47, 128–134. [Google Scholar] [CrossRef]
- Lucena, M.I.; Andrade, R.J.; Martinez, C.; Ulzurrun, E.; Garcia-Martin, E.; Borraz, Y.; Fernandez, M.C.; Romero-Gomez, M.; Castiella, A.; Planas, R.; et al. Glutathione S-transferase m1 and t1 null genotypes increase susceptibility to idiosyncratic drug-induced liver injury. Hepatology 2008, 48, 588–596. [Google Scholar] [CrossRef]
- Lucena, M.I.; Garcia-Martin, E.; Andrade, R.J.; Martinez, C.; Stephens, C.; Ruiz, J.D.; Ulzurrun, E.; Fernandez, M.C.; Romero-Gomez, M.; Castiella, A.; et al. Mitochondrial superoxide dismutase and glutathione peroxidase in idiosyncratic drug-induced liver injury. Hepatology 2010, 52, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Acuna, G.; Foernzler, D.; Leong, D.; Rabbia, M.; Smit, R.; Dorflinger, E.; Gasser, R.; Hoh, J.; Ott, J.; Borroni, E.; et al. Pharmacogenetic analysis of adverse drug effect reveals genetic variant for susceptibility to liver toxicity. Pharmacogenom. J. 2002, 2, 327–334. [Google Scholar] [CrossRef]
- Martignoni, E.; Cosentino, M.; Ferrari, M.; Porta, G.; Mattarucchi, E.; Marino, F.; Lecchini, S.; Nappi, G. Two patients with COMT inhibitor–induced hepatic dysfunction and UGT1A9 genetic polymorphism. Neurology 2005, 65, 1820. [Google Scholar] [CrossRef] [PubMed]
- Cirulli, E.T.; Nicoletti, P.; Abramson, K.; Andrade, R.J.; Bjornsson, E.S.; Chalasani, N.; Fontana, R.J.; Hallberg, P.; Li, Y.J.; Lucena, M.I.; et al. A Missense Variant in PTPN22 is a Risk Factor for Drug-induced Liver Injury. Gastroenterology 2019, 156, 1707–1716.e2. [Google Scholar] [CrossRef]
- Yu, K.; Geng, X.; Chen, M.; Zhang, J.; Wang, B.; Ilic, K.; Tong, W. High daily dose and being a substrate of cytochrome P450 enzymes are two important predictors of drug-induced liver injury. Drug Metab. Dispos. 2014, 42, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.T.; Chen, T. Hepatotoxicity of Herbal Supplements Mediated by Modulation of Cytochrome P450. Int. J. Mol. Sci. 2017, 18, 2353. [Google Scholar] [CrossRef] [PubMed]
- Lazarska, K.E.; Dekker, S.J.; Vermeulen, N.P.E.; Commandeur, J.N.M. Effect of UGT2B7*2 and CYP2C8*4 polymorphisms on diclofenac metabolism. Toxicol. Lett. 2018, 284, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K. Drug-induced liver injury: Past, present and future. Pharmacogenomics 2010, 11, 607–611. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Thorgeirsson, U.P.; Black, M.; Timbrell, J.A.; Snodgrass, W.R.; Potter, W.Z.; Jollow, H.R.; Keiser, H.R. Increased incidence of isoniazid hepatitis in rapid acetylators: Possible relation to hydranize metabolites. Clin. Pharmacol. Ther. 1975, 18, 70–79. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Zimmerman, H.J.; Ishak, K.G.; Thorgeirsson, U.P.; Timbrell, J.A.; Snodgrass, W.R.; Nelson, S.D. Isoniazid liver injury: Clinical spectrum, pathology, and probable pathogenesis. Ann. Intern. Med. 1976, 84, 181–192. [Google Scholar] [CrossRef]
- Yamamoto, T.; Suou, T.; Hirayama, C. Elevated serum aminotransferase induced by isoniazid in relation to isoniazid acetylator phenotype. Hepatology 1986, 6, 295–298. [Google Scholar] [CrossRef]
- Gronhagen-Riska, C.; Hellstrom, P.E.; Froseth, B. Predisposing factors in hepatitis induced by isoniazid-rifampin treatment of tuberculosis. Am. Rev. Respir. Dis. 1978, 118, 461–466. [Google Scholar] [CrossRef]
- Dickinson, D.S.; Bailey, W.C.; Hirschowitz, B.I.; Soong, S.J.; Eidus, L.; Hodgkin, M.M. Risk factors for isoniazid (NIH)-induced liver dysfunction. J. Clin. Gastroenterol. 1981, 3, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Gurumurthy, P.; Krishnamurthy, M.S.; Nazareth, O.; Parthasarathy, R.; Sarma, G.R.; Somasundaram, P.R.; Tripathy, S.P.; Ellard, G.A. Lack of relationship between hepatic toxicity and acetylator phenotype in three thousand South Indian patients during treatment with isoniazid for tuberculosis. Am. Rev. Respir. Dis. 1984, 129, 58–61. [Google Scholar] [CrossRef]
- Sarma, G.R.; Immanuel, C.; Kailasam, S.; Narayana, A.S.; Venkatesan, P. Rifampin-induced release of hydrazine from isoniazid. A possible cause of hepatitis during treatment of tuberculosis with regimens containing isoniazid and rifampin. Am. Rev. Respir. Dis. 1986, 133, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Sarma, G.R.; Janardhanam, B.; Ramachandran, P.; Santha, T.; Sivasubramanian, S.; Somasundaram, P.R.; Tripathy, S.P. Hepatic toxicity in South Indian patients during treatment of tuberculosis with short-course regimens containing isoniazid, rifampicin and pyrazinamide. Tubercle 1986, 67, 99–108. [Google Scholar] [CrossRef]
- Pande, J.N.; Singh, S.P.; Khilnani, G.C.; Khilnani, S.; Tandon, R.K. Risk factors for hepatotoxicity from antituberculosis drugs: A case-control study. Thorax 1996, 51, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Wu, J.C.; Lee, C.N.; Yen, F.S.; Lu, C.L.; Lin, T.P.; Lee, S.D. A prospective clinical study of isoniazid-rifampicin-pyrazinamide-induced liver injury in an area endemic for hepatitis B. J. Gastroenterol. Hepatol. 1997, 12, 87–91. [Google Scholar] [CrossRef]
- Ohno, M.; Yamaguchi, I.; Yamamoto, I.; Fukuda, T.; Yokota, S.; Maekura, R.; Ito, M.; Yamamoto, Y.; Ogura, T.; Maeda, K.; et al. Slow N-acetyltransferase 2 genotype affects the incidence of isoniazid and rifampicin-induced hepatotoxicity. Int. J. Tuberc. Lung Dis. 2000, 4, 256–261. [Google Scholar]
- Huang, Y.S.; Chern, H.D.; Su, W.J.; Wu, J.C.; Lai, S.L.; Yang, S.Y.; Chang, F.Y.; Lee, S.D. Polymorphism of the N-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatitis. Hepatology 2002, 35, 883–889. [Google Scholar] [CrossRef]
- Cho, H.J.; Koh, W.J.; Ryu, Y.J.; Ki, C.S.; Nam, M.H.; Kim, J.W.; Lee, S.Y. Genetic polymorphisms of NAT2 and CYP2E1 associated with antituberculosis drug-induced hepatotoxicity in Korean patients with pulmonary tuberculosis. Tuberculosis (Edinb) 2007, 87, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Possuelo, L.G.; Castelan, J.A.; de Brito, T.C.; Ribeiro, A.W.; Cafrune, P.I.; Picon, P.D.; Santos, A.R.; Teixeira, R.L.; Gregianini, T.S.; Hutz, M.H.; et al. Association of slow N-acetyltransferase 2 profile and anti-TB drug-induced hepatotoxicity in patients from Southern Brazil. Eur. J. Clin. Pharmacol. 2008, 64, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yi, J.; Zhou, C.; Shen, X. Pharmacogenetic study of drug-metabolising enzyme polymorphisms on the risk of anti-tuberculosis drug-induced liver injury: A meta-analysis. PLoS ONE 2012, 7, e47769. [Google Scholar] [CrossRef]
- Chan, S.L.; Chua, A.P.G.; Aminkeng, F.; Chee, C.B.E.; Jin, S.; Loh, M.; Gan, S.H.; Wang, Y.T.; Brunham, L.R. Association and clinical utility of NAT2 in the prediction of isoniazid-induced liver injury in Singaporean patients. PLoS ONE 2017, 12, e0186200. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Wilffert, B.; Tong, R.; van Soolingen, D.; van den Hof, S.; Alffenaar, J.W. The association between the NAT2 genetic polymorphisms and risk of DILI during anti-TB treatment: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2018, 84, 2747–2760. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Mandal, R.K.; Elasbali, A.M.; Dar, S.A.; Jawed, A.; Wahid, M.; Mahto, H.; Lohani, M.; Mishra, B.N.; Akhter, N.; et al. Pharmacogenetic association between NAT2 gene polymorphisms and isoniazid induced hepatotoxicity: Trial sequence meta-analysis as evidence. BioSci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.; Kirkham, J.; Dwan, K.; Sloan, D.J.; Davies, G.; Jorgensen, A.L. NAT2 variants and toxicity related to anti-tuberculosis agents: A systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2019, 23, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Suvichapanich, S.; Wattanapokayakit, S.; Mushiroda, T.; Yanai, H.; Chuchottawon, C.; Kantima, T.; Nedsuwan, S.; Suwankesawong, W.; Sonsupap, C.; Pannarunothai, R.; et al. Genomewide Association Study Confirming the Association of NAT2 with Susceptibility to Antituberculosis Drug-Induced Liver Injury in Thai Patients. Antimicrob. Agents ChemoTher. 2019, 63. [Google Scholar] [CrossRef]
- Nicoletti, P.; Devarbhavi, H.; Goel, A.; Venkatesan, R.; Eapen, C.E.; Grove, J.I.; Zafer, S.; Bjornsson, E.; Lucena, M.I.; Andrade, R.J.; et al. Genetic Risk Factors in Drug-Induced Liver Injury Due to Isoniazid-Containing Antituberculosis Drug Regimens. Clin. Pharmacol. Ther. 2020. [Google Scholar] [CrossRef]
- Nasseri, G.; Zahedi, T.; Mousavi-Kazerooni, F.; Saadat, M. Prevalence of Null Genotypes of Glutathione S-Transferase T1 (GSTT1) and M1 (GSTM1) in Seven Iranian Populations. Iran J. Public Health 2015, 44, 1655–1661. [Google Scholar]
- Sakatis, M.Z.; Reese, M.J.; Harrell, A.W.; Taylor, M.A.; Baines, I.A.; Chen, L.; Bloomer, J.C.; Yang, E.Y.; Ellens, H.M.; Ambroso, J.L.; et al. Preclinical strategy to reduce clinical hepatotoxicity using in vitro bioactivation data for >200 compounds. Chem. Res. Toxicol. 2012, 25, 2067–2082. [Google Scholar] [CrossRef] [PubMed]
- Zollner, G.; Thueringer, A.; Lackner, C.; Fickert, P.; Trauner, M. Alterations of canalicular ATP-binding cassette transporter expression in drug-induced liver injury. Digestion 2014, 90, 81–88. [Google Scholar] [CrossRef]
- Yimer, G.; Ueda, N.; Habtewold, A.; Amogne, W.; Suda, A.; Riedel, K.D.; Burhenne, J.; Aderaye, G.; Lindquist, L.; Makonnen, E.; et al. Pharmacogenetic & pharmacokinetic biomarker for efavirenz based ARV and rifampicin based anti-TB drug induced liver injury in TB-HIV infected patients. PLoS ONE 2011, 6, e27810. [Google Scholar] [CrossRef]
- Miura, Y.; Imamura, C.K.; Fukunaga, K.; Katsuyama, Y.; Suyama, K.; Okaneya, T.; Mushiroda, T.; Ando, Y.; Takano, T.; Tanigawara, Y. Sunitinib-induced severe toxicities in a Japanese patient with the ABCG2 421 AA genotype. BMC Cancer 2014, 14, 964. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.; Khoury, H.; Prip-Buus, C.; Cepanec, C.; Pessayre, D.; Degoul, F. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics 2003, 13, 145–157. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, Y.H.; Noh, J.R.; Choi, D.H.; Kim, K.S.; Lee, C.H. Enhanced Production of Adenosine Triphosphate by Pharmacological Activation of Adenosine Monophosphate-Activated Protein Kinase Ameliorates Acetaminophen-Induced Liver Injury. Mol. Cells 2015, 38, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, M.; Ando, F.; Iguchi, A.; Shimokata, H. Glutathione peroxidase 1 Pro198Leu variant contributes to the metabolic syndrome in men in a large Japanese cohort. Am. J. Clin. Nutr. 2008, 87, 1939–1944. [Google Scholar] [CrossRef]
- Hamanishi, T.; Furuta, H.; Kato, H.; Doi, A.; Tamai, M.; Shimomura, H.; Sakagashira, S.; Nishi, M.; Sasaki, H.; Sanke, T.; et al. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in japanese type 2 diabetic patients. Diabetes 2004, 53, 2455–2460. [Google Scholar] [CrossRef]
- Kaliyaperumal, K.; Grove, J.I.; Delahay, R.M.; Griffiths, W.J.H.; Duckworth, A.; Aithal, G.P. Pharmacogenomics of drug-induced liver injury (DILI): Molecular biology to clinical applications. J. Hepatol. 2018, 69, 948–957. [Google Scholar] [CrossRef]
- Lucena, M.I.; Molokhia, M.; Shen, Y.; Urban, T.J.; Aithal, G.P.; Andrade, R.J.; Day, C.P.; Ruiz-Cabello, F.; Donaldson, P.T.; Stephens, C.; et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology 2011, 141, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.; Lopez-Nevot, M.A.; Ruiz-Cabello, F.; Ulzurrun, E.; Soriano, G.; Romero-Gomez, M.; Moreno-Casares, A.; Lucena, M.I.; Andrade, R.J. HLA alleles influence the clinical signature of amoxicillin-clavulanate hepatotoxicity. PLoS ONE 2013, 8, e68111. [Google Scholar] [CrossRef]
- Donaldson, P.T.; Daly, A.K.; Henderson, J.; Graham, J.; Pirmohamed, M.; Bernal, W.; Day, C.P.; Aithal, G.P. Human leucocyte antigen class II genotype in susceptibility and resistance to co-amoxiclav-induced liver injury. J. Hepatol. 2010, 53, 1049–1053. [Google Scholar] [CrossRef]
- Hautekeete, M.L.; Horsmans, Y.; Van Waeyenberge, C.; Demanet, C.; Henrion, J.; Verbist, L.; Brenard, R.; Sempoux, C.; Michielsen, P.P.; Yap, P.S.; et al. HLA association of amoxicillin-clavulanate--induced hepatitis. Gastroenterology 1999, 117, 1181–1186. [Google Scholar] [CrossRef]
- O’Donohue, J.; Oien, K.A.; Donaldson, P.; Underhill, J.; Clare, M.; MacSween, R.N.; Mills, P.R. Co-amoxiclav jaundice: Clinical and histological features and HLA class II association. Gut 2000, 47, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Pariente, E.A.; Hamoud, A.; Goldfain, D.; Latrive, J.P.; Gislon, J.; Cassan, P.; Morin, T.; Staub, J.L.; Ramain, J.P.; Bertrand, J.L.; et al. Hepatitis caused by clometacin (Dupéran). Retrospective study of 30 cases. A model of autoimmune drug-induced hepatitis? Gastroenterol. Clin. Biol. 1989, 13, 769–774. [Google Scholar]
- Aithal, G.P. Hepatotoxicity related to antirheumatic drugs. Nat. Rev. Rheumatol 2011, 7, 139–150. [Google Scholar] [CrossRef]
- Petros, Z.; Kishikawa, J.; Makonnen, E.; Yimer, G.; Habtewold, A.; Aklillu, E. HLA-B(*)57 Allele Is Associated with Concomitant Anti-tuberculosis and Antiretroviral Drugs Induced Liver Toxicity in Ethiopians. Front. Pharmacol. 2017, 8, 90. [Google Scholar] [CrossRef]
- Nicoletti, P.; Aithal, G.P.; Bjornsson, E.S.; Andrade, R.J.; Sawle, A.; Arrese, M.; Barnhart, H.X.; Bondon-Guitton, E.; Hayashi, P.H.; Bessone, F.; et al. Association of Liver Injury From Specific Drugs, or Groups of Drugs, With Polymorphisms in HLA and Other Genes in a Genome-Wide Association Study. Gastroenterology 2017, 152, 1078–1089. [Google Scholar] [CrossRef]
- Daly, A.K.; Donaldson, P.T.; Bhatnagar, P.; Shen, Y.; Pe’er, I.; Floratos, A.; Daly, M.J.; Goldstein, D.B.; John, S.; Nelson, M.R.; et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat. Genet. 2009, 41, 816–819. [Google Scholar] [CrossRef]
- Mosedale, M.; Watkins, P.B. Understanding Idiosyncratic Toxicity: Lessons Learned from Drug-Induced Liver Injury. J. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, P.; Werk, A.N.; Sawle, A.; Shen, Y.; Urban, T.J.; Coulthard, S.A.; Bjornsson, E.S.; Cascorbi, I.; Floratos, A.; Stammschulte, T.; et al. HLA-DRB1*16: 01-DQB1*05: 02 is a novel genetic risk factor for flupirtine-induced liver injury. PharmacoGenet. Genom. 2016, 26, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Spraggs, C.F.; Budde, L.R.; Briley, L.P.; Bing, N.; Cox, C.J.; King, K.S.; Whittaker, J.C.; Mooser, V.E.; Preston, A.J.; Stein, S.H.; et al. HLA-DQA1*02:01 is a major risk factor for lapatinib-induced hepatotoxicity in women with advanced breast cancer. J. Clin. Oncol. 2011, 29, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Schaid, D.J.; Spraggs, C.F.; McDonnell, S.K.; Parham, L.R.; Cox, C.J.; Ejlertsen, B.; Finkelstein, D.M.; Rappold, E.; Curran, J.; Cardon, L.R.; et al. Prospective validation of HLA-DRB1*07:01 allele carriage as a predictive risk factor for lapatinib-induced liver injury. J. Clin. Oncol. 2014, 32, 2296–2303. [Google Scholar] [CrossRef] [PubMed]
- Parham, L.R.; Briley, L.P.; Li, L.; Shen, J.; Newcombe, P.J.; King, K.S.; Slater, A.J.; Dilthey, A.; Iqbal, Z.; McVean, G.; et al. Comprehensive genome-wide evaluation of lapatinib-induced liver injury yields a single genetic signal centered on known risk allele HLA-DRB1*07:01. Pharmacogenom. J. 2016, 16, 180–185. [Google Scholar] [CrossRef]
- Singer, J.B.; Lewitzky, S.; Leroy, E.; Yang, F.; Zhao, X.; Klickstein, L.; Wright, T.M.; Meyer, J.; Paulding, C.A. A genome-wide study identifies HLA alleles associated with lumiracoxib-related liver injury. Nat. Genet. 2010, 42, 711–714. [Google Scholar] [CrossRef]
- Urban, T.J.; Nicoletti, P.; Chalasani, N.; Serrano, J.; Stolz, A.; Daly, A.K.; Aithal, G.P.; Dillon, J.; Navarro, V.; Odin, J.; et al. Minocycline hepatotoxicity: Clinical characterization and identification of HLA-B *35:02 as a risk factor. J. Hepatol. 2017, 67, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Bartlett, J.A.; Sanne, I.; Lederman, M.M.; Hinkle, J.; Rousseau, F.; Dunn, D.; Pavlos, R.; James, I.; Mallal, S.A.; et al. Associations between HLA-DRB1*0102, HLA-B*5801, and hepatotoxicity during initiation of nevirapine-containing regimens in South Africa. J. Acquir. Immune Defic. Syndr. 2013, 62, e55–e57. [Google Scholar] [CrossRef]
- Martin, A.M.; Nolan, D.; James, I.; Cameron, P.; Keller, J.; Moore, C.; Phillips, E.; Christiansen, F.T.; Mallal, S. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS 2005, 19, 97–99. [Google Scholar] [CrossRef]
- Yuan, J.; Guo, S.; Hall, D.; Cammett, A.M.; Jayadev, S.; Distel, M.; Storfer, S.; Huang, Z.; Mootsikapun, P.; Ruxrungtham, K.; et al. Toxicogenomics of nevirapine-associated cutaneous and hepatic adverse events among populations of African, Asian, and European descent. AIDS 2011, 25, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.F.; Johnson, T.; Wang, X.; Carpenter, C.; Graves, A.P.; Warren, L.; Xue, Z.; King, K.S.; Fraser, D.J.; Stinnett, S.; et al. HLA-B*57:01 Confers Susceptibility to Pazopanib-Associated Liver Injury in Patients with Cancer. Clin. Cancer Res. 2016, 22, 1371–1377. [Google Scholar] [CrossRef]
- Hirata, K.; Takagi, H.; Yamamoto, M.; Matsumoto, T.; Nishiya, T.; Mori, K.; Shimizu, S.; Masumoto, H.; Okutani, Y. Ticlopidine-induced hepatotoxicity is associated with specific human leukocyte antigen genomic subtypes in Japanese patients: A preliminary case-control study. Pharmacogenom. J. 2008, 8, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, M.; Takagi, H.; Mori, M. HLA-A33/B44/DR6 Is Highly Related to Intrahepatic Cholestasis Inducedby Tiopronin. Dig. Dis. Sci. 2000, 45, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Phillips, E.; Dellinger, A.; Nicoletti, P.; Schutte, R.; Li, D.; Ostrov, D.A.; Fontana, R.J.; Watkins, P.B.; Stolz, A.; et al. HLA-B*14:01 and HLA-B*35:01 are associated with trimethoprim-sulfamethoxazole induced liver injury. Hepatology 2020. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Balamurugan, A.; Saha, P.K.; Pandey, R.M.; Mehra, N.K. Evaluation of clinical and immunogenetic risk factors for the development of hepatotoxicity during antituberculosis treatment. Am. J. Respir. Crit. Care Med. 2002, 166, 916–919. [Google Scholar] [CrossRef]
- Monshi, M.M.; Faulkner, L.; Gibson, A.; Jenkins, R.E.; Farrell, J.; Earnshaw, C.J.; Alfirevic, A.; Cederbrant, K.; Daly, A.K.; French, N.; et al. Human leukocyte antigen (HLA)-B*57:01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology 2013, 57, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, P.; Aithal, G.P.; Chamberlain, T.C.; Coulthard, S.; Alshabeeb, M.; Grove, J.I.; Andrade, R.J.; Bjornsson, E.; Dillon, J.F.; Hallberg, P.; et al. Drug-Induced Liver Injury due to Flucloxacillin: Relevance of Multiple Human Leukocyte Antigen Alleles. Clin. Pharmacol. Ther. 2019, 106, 245–253. [Google Scholar] [CrossRef]
- Mallal, S.; Phillips, E.; Carosi, G.; Molina, J.M.; Workman, C.; Tomazic, J.; Jagel-Guedes, E.; Rugina, S.; Kozyrev, O.; Cid, J.F.; et al. HLA-B*5701 screening for hypersensitivity to abacavir. N. Engl. J. Med. 2008, 358, 568–579. [Google Scholar] [CrossRef]
- Lucena, M.I.; Kaplowitz, N.; Hallal, H.; Castiella, A.; Garcia-Bengoechea, M.; Otazua, P.; Berenguer, M.; Fernandez, M.C.; Planas, R.; Andrade, R.J. Recurrent drug-induced liver injury (DILI) with different drugs in the Spanish Registry: The dilemma of the relationship to autoimmune hepatitis. J. Hepatol. 2011, 55, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Strettell, M.D.; Donaldson, P.T.; Thomson, L.J.; Santrach, P.J.; Moore, S.B.; Czaja, A.J.; Williams, R. Allelic basis for HLA-encoded susceptibility to type 1 autoimmune hepatitis. Gastroenterology 1997, 112, 2028–2035. [Google Scholar] [CrossRef]
- De Boer, Y.S.; van Gerven, N.M.; Zwiers, A.; Verwer, B.J.; van Hoek, B.; van Erpecum, K.J.; Beuers, U.; van Buuren, H.R.; Drenth, J.P.; den Ouden, J.W.; et al. Genome-wide association study identifies variants associated with autoimmune hepatitis type 1. Gastroenterology 2014, 147, 443–452.e445. [Google Scholar] [CrossRef]
- De Boer, Y.S.; Kosinski, A.S.; Urban, T.J.; Zhao, Z.; Long, N.; Chalasani, N.; Kleiner, D.E.; Hoofnagle, J.H. Features of Autoimmune Hepatitis in Patients With Drug-induced Liver Injury. Clin. Gastroenterol. Hepatol. 2017, 15, 103–112.e102. [Google Scholar] [CrossRef]
- Clarke, F.; Purvis, H.A.; Sanchez-Blanco, C.; Gutierrez-Martinez, E.; Cornish, G.H.; Zamoyska, R.; Guermonprez, P.; Cope, A.P. The protein tyrosine phosphatase PTPN22 negatively regulates presentation of immune complex derived antigens. Sci. Rep. 2018, 8, 12692. [Google Scholar] [CrossRef]
- Vang, T.; Nielsen, J.; Burn, G.L. A switch-variant model integrates the functions of an autoimmune variant of the phosphatase PTPN22. Sci. Signal 2018, 11. [Google Scholar] [CrossRef]
- Yilmaz, B.; Spalinger, M.R.; Biedermann, L.; Franc, Y.; Fournier, N.; Rossel, J.-B.; Juillerat, P.; Rogler, G.; Macpherson, A.J.; Scharl, M. The presence of genetic risk variants within PTPN2 and PTPN22 is associated with intestinal microbiota alterations in Swiss IBD cohort patients. PLoS ONE 2018, 13, e0199664. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.; Andrade, R.J. Genetic Predisposition to Drug-Induced Liver Injury. Clin. Liver Dis. 2020, 24, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Ingelman-Sundberg, M.; Sim, S.C.; Gomez, A.; Rodriguez-Antona, C. Influence of cytochrome P450 polymorphisms on drug therapies: Pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol. Ther. 2007, 116, 496–526. [Google Scholar] [CrossRef] [PubMed]
- Aithal, G.P. Pharmacogenetic testing in idiosyncratic drug-induced liver injury: Current role in clinical practice. Liver Int. 2015, 35, 1801–1808. [Google Scholar] [CrossRef]
- Stephens, C.; Lucena, M.I.; Andrade, R.J. Drug-Induced Liver Disease: Mechanism and Diagnosis. Evid. Based Gastroenterol. Hepatol. 2019, 4e, 715–728. [Google Scholar]
- Xiao, L.L.; Zhang, F.; Zhao, Y.L.; Zhang, L.J.; Xie, Z.Y.; Huang, K.Z.; Ouyang, X.X.; Wu, X.X.; Xu, X.W.; Li, L.J. Using advanced oxidation protein products and ischaemia-modified albumin to monitor oxidative stress levels in patients with drug-induced liver injury. Sci. Rep. 2020, 10, 18128. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Jaeschke, H. Biomarkers of drug-induced liver injury. Adv. Pharmacol. 2019, 85, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wu, D.; Jiang, W.; Li, J.; Long, J.; Jia, C.; Zhou, T. Molecular Biomarkers in Drug-Induced Liver Injury: Challenges and Future Perspectives. Front. Pharmacol. 2019, 10, 1667. [Google Scholar] [CrossRef]
- Garcia-Cortes, M.; Ortega-Alonso, A.; Lucena, M.I.; Andrade, R.J.; Spanish Group for the Study of Drug-Induced Liver Disease, l. Drug-induced liver injury: A safety review. Expert Opin. Drug Saf. 2018, 17, 795–804. [Google Scholar] [CrossRef]
- Antoine, D.J.; Williams, D.P.; Kipar, A.; Jenkins, R.E.; Regan, S.L.; Sathish, J.G.; Kitteringham, N.R.; Park, B.K. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol. Sci. 2009, 112, 521–531. [Google Scholar] [CrossRef]
- Church, R.J.; Kullak-Ublick, G.A.; Aubrecht, J.; Bonkovsky, H.L.; Chalasani, N.; Fontana, R.J.; Goepfert, J.C.; Hackman, F.; King, N.M.P.; Kirby, S.; et al. Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: An international collaborative effort. Hepatology 2019, 69, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Howell, L.S.; Ireland, L.; Park, B.K.; Goldring, C.E. MiR-122 and other microRNAs as potential circulating biomarkers of drug-induced liver injury. Expert Rev. Mol. Diagn. 2018, 18, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Bandiera, S.; Pfeffer, S.; Baumert, T.F.; Zeisel, M.B. miR-122--a key factor and therapeutic target in liver disease. J. Hepatol. 2015, 62, 448–457. [Google Scholar] [CrossRef]
- McGill, M.R.; Sharpe, M.R.; Williams, C.D.; Taha, M.; Curry, S.C.; Jaeschke, H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Investig. 2012, 122, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Devarbhavi, H. An Update on Drug-induced Liver Injury. J. Clin. Exp. Hepatol. 2012, 2, 247–259. [Google Scholar] [CrossRef]
- Andrade, R.J.; Robles-Diaz, M. Diagnostic and prognostic assessment of suspected drug-induced liver injury in clinical practice. Liver Int. 2020, 40, 6–17. [Google Scholar] [CrossRef]
- Nelson, R.L.; Povey, M.S.; Hopkinson, D.A.; Harris, H. Electrophoresis of human l-glutamate dehydrogenase: Tissue distribution and preliminary population survey. BioChem. Genet. 1977, 15, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Hou, F.F.; Nie, J. AOPPs and the progression of kidney disease. Kidney Int. Suppl. 2014, 4, 102–106. [Google Scholar] [CrossRef]
- Roy, D.; Quiles, J.; Gaze, D.C.; Collinson, P.; Kaski, J.C.; Baxter, G.F. Role of reactive oxygen species on the formation of the novel diagnostic marker ischaemia modified albumin. Heart 2006, 92, 113–114. [Google Scholar] [CrossRef]
- Gaskell, H.; Ge, X.; Nieto, N. High-Mobility Group Box-1 and Liver Disease. Hepatol. Commun. 2018, 2, 1005–1020. [Google Scholar] [CrossRef]
- Andersson, U.; Lindberg, J.; Wang, S.; Balasubramanian, R.; Marcusson-Stahl, M.; Hannula, M.; Zeng, C.; Juhasz, P.J.; Kolmert, J.; Backstrom, J.; et al. A systems biology approach to understanding elevated serum alanine transaminase levels in a clinical trial with ximelagatran. Biomarkers 2009, 14, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.A.; Giachelli, C.M.; Scatena, M. The role of osteopontin in inflammatory processes. J. Cell Commun. Signal. 2009, 3, 311–322. [Google Scholar] [CrossRef]
- Srungaram, P.; Rule, J.A.; Yuan, H.J.; Reimold, A.; Dahl, B.; Sanders, C.; Lee, W.M.; Acute Liver Failure Study Group. Plasma osteopontin in acute liver failure. Cytokine 2015, 73, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Yokosuka, O.; Kanda, T.; Fukai, K.; Imazeki, F.; Muramatsu, M.; Seki, N.; Miyazaki, M.; Ochiai, T.; Hirasawa, H.; et al. Serum osteopontin levels in patients with acute liver dysfunction. Scand. J. Gastroenterol. 2006, 41, 102–110. [Google Scholar] [CrossRef]
- Matsui, A.; Mochida, S.; Ohno, A.; Nagoshi, S.; Hirose, T.; Fujiwara, K. Plasma osteopontin levels in patients with fulminant hepatitis. Hepatol. Res. 2004, 29, 202–206. [Google Scholar] [CrossRef]
- Devhare, P.B.; Ray, R.B. Extracellular vesicles: Novel mediator for cell to cell communications in liver pathogenesis. Mol. Aspects Med. 2018, 60, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Moran, L.; Cubero, F.J. Extracellular vesicles in liver disease and beyond. World J. Gastroenterol. 2018, 24, 4519–4526. [Google Scholar] [CrossRef] [PubMed]
- Umbaugh, D.S.; Jaeschke, H. Extracellular vesicles: Roles and applications in drug-induced liver injury. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Blaya, D.; Aguilar-Bravo, B.; Hao, F.; Casacuberta-Serra, S.; Coll, M.; Perea, L.; Vallverdu, J.; Graupera, I.; Pose, E.; Llovet, L.; et al. Expression of microRNA-155 in inflammatory cells modulates liver injury. Hepatology 2018, 68, 691–706. [Google Scholar] [CrossRef]
- Erhard, F.; Haas, J.; Lieber, D.; Malterer, G.; Jaskiewicz, L.; Zavolan, M.; Dolken, L.; Zimmer, R. Widespread context dependency of microRNA-mediated regulation. Genome Res. 2014, 24, 906–919. [Google Scholar] [CrossRef]
- Koberle, V.; Pleli, T.; Schmithals, C.; Augusto Alonso, E.; Haupenthal, J.; Bonig, H.; Peveling-Oberhag, J.; Biondi, R.M.; Zeuzem, S.; Kronenberger, B.; et al. Differential stability of cell-free circulating microRNAs: Implications for their utilization as biomarkers. PLoS ONE 2013, 8, e75184. [Google Scholar] [CrossRef]
- Van der Pol, E.; Boing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.; Shirai, Y.; Oda, S.; Yokoi, T. Identification of Specific MicroRNA Biomarkers in Early Stages of Hepatocellular Injury, Cholestasis, and Steatosis in Rats. Toxicol. Sci. 2018, 166, 228–239. [Google Scholar] [CrossRef]
- Hu, J.; Xu, Y.; Hao, J.; Wang, S.; Li, C.; Meng, S. MiR-122 in hepatic function and liver diseases. Protein Cell 2012, 3, 364–371. [Google Scholar] [CrossRef]
- Russo, M.W.; Steuerwald, N.; Norton, H.J.; Anderson, W.E.; Foureau, D.; Chalasani, N.; Fontana, R.J.; Watkins, P.B.; Serrano, J.; Bonkovsky, H.L. Profiles of miRNAs in serum in severe acute drug induced liver injury and their prognostic significance. Liver Int. 2017, 37, 757–764. [Google Scholar] [CrossRef]
- Starkey Lewis, P.J.; Dear, J.; Platt, V.; Simpson, K.J.; Craig, D.G.; Antoine, D.J.; French, N.S.; Dhaun, N.; Webb, D.J.; Costello, E.M.; et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology 2011, 54, 1767–1776. [Google Scholar] [CrossRef]
- Lopez-Riera, M.; Conde, I.; Tolosa, L.; Zaragoza, A.; Castell, J.V.; Gomez-Lechon, M.J.; Jover, R. New microRNA Biomarkers for Drug-Induced Steatosis and Their Potential to Predict the Contribution of Drugs to Non-alcoholic Fatty Liver Disease. Front. Pharmacol. 2017, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Harrill, A.H.; Roach, J.; Fier, I.; Eaddy, J.S.; Kurtz, C.L.; Antoine, D.J.; Spencer, D.M.; Kishimoto, T.K.; Pisetsky, D.S.; Park, B.K.; et al. The effects of heparins on the liver: Application of mechanistic serum biomarkers in a randomized study in healthy volunteers. Clin. Pharmacol. Ther. 2012, 92, 214–220. [Google Scholar] [CrossRef]
- Mosedale, M.; Eaddy, J.S.; Trask, O.J., Jr.; Holman, N.S.; Wolf, K.K.; LeCluyse, E.; Ware, B.R.; Khetani, S.R.; Lu, J.; Brock, W.J.; et al. miR-122 Release in Exosomes Precedes Overt Tolvaptan-Induced Necrosis in a Primary Human Hepatocyte Micropatterned Coculture Model. Toxicol. Sci. 2018, 161, 149–158. [Google Scholar] [CrossRef]
- Holman, N.S.; Church, R.J.; Nautiyal, M.; Rose, K.A.; Thacker, S.E.; Otieno, M.A.; Wolf, K.K.; LeCluyse, E.; Watkins, P.B.; Mosedale, M. Hepatocyte-Derived Exosomes Promote Liver Immune Tolerance: Possible Implications for Idiosyncratic Drug-Induced Liver Injury. Toxicol. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.; Tolosa, L. High-Content Screening for the Detection of Drug-Induced Oxidative Stress in Liver Cells. Antioxidants 2021, 10, 106. [Google Scholar] [CrossRef]
- Cipriano, M.; Pinheiro, P.F.; Sequeira, C.O.; Rodrigues, J.S.; Oliveira, N.G.; Antunes, A.M.M.; Castro, M.; Marques, M.M.; Pereira, S.A.; Miranda, J.P. Nevirapine Biotransformation Insights: An Integrated In Vitro Approach Unveils the Biocompetence and Glutathiolomic Profile of a Human Hepatocyte-Like Cell 3D Model. Int. J. Mol. Sci. 2020, 21, 3998. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Oda, S.; Yokoi, T. Cell-based assay using glutathione-depleted HepaRG and HepG2 human liver cells for predicting drug-induced liver injury. Toxicol In Vitro 2018, 48, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Z.; Qing, T.; Ren, Z.; Yu, D.; Couch, L.; Ning, B.; Mei, N.; Shi, L.; Tolleson, W.H.; et al. Activation of the Nrf2 signaling pathway in usnic acid-induced toxicity in HepG2 cells. Arch. Toxicol. 2017, 91, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Doshi, U.; Suzuki, A.; Chang, C.W.; Borlak, J.; Li, A.P.; Tong, W. Evaluation of multiple mechanism-based toxicity endpoints in primary cultured human hepatocytes for the identification of drugs with clinical hepatotoxicity: Results from 152 marketed drugs with known liver injury profiles. Chem. Biol. Interact. 2016, 255, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Garside, H.; Marcoe, K.F.; Chesnut-Speelman, J.; Foster, A.J.; Muthas, D.; Kenna, J.G.; Warrior, U.; Bowes, J.; Baumgartner, J. Evaluation of the use of imaging parameters for the detection of compound-induced hepatotoxicity in 384-well cultures of HepG2 cells and cryopreserved primary human hepatocytes. Toxicol In Vitro 2014, 28, 171–181. [Google Scholar] [CrossRef]
- Wink, S.; Hiemstra, S.W.; Huppelschoten, S.; Klip, J.E.; van de Water, B. Dynamic imaging of adaptive stress response pathway activation for prediction of drug induced liver injury. Arch. Toxicol. 2018, 92, 1797–1814. [Google Scholar] [CrossRef] [PubMed]
- Ware, B.R.; Berger, D.R.; Khetani, S.R. Prediction of Drug-Induced Liver Injury in Micropatterned Co-cultures Containing iPSC-Derived Human Hepatocytes. Toxicol. Sci. 2015, 145, 252–262. [Google Scholar] [CrossRef]
- Ayuso, P.; García-Martín, E.; Agúndez, J.A.G. Variability of the Genes Involved in the Cellular Redox Status and Their Implication in Drug Hypersensitivity Reactions. Antioxidants 2021, 10, 294. [Google Scholar] [CrossRef]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef]
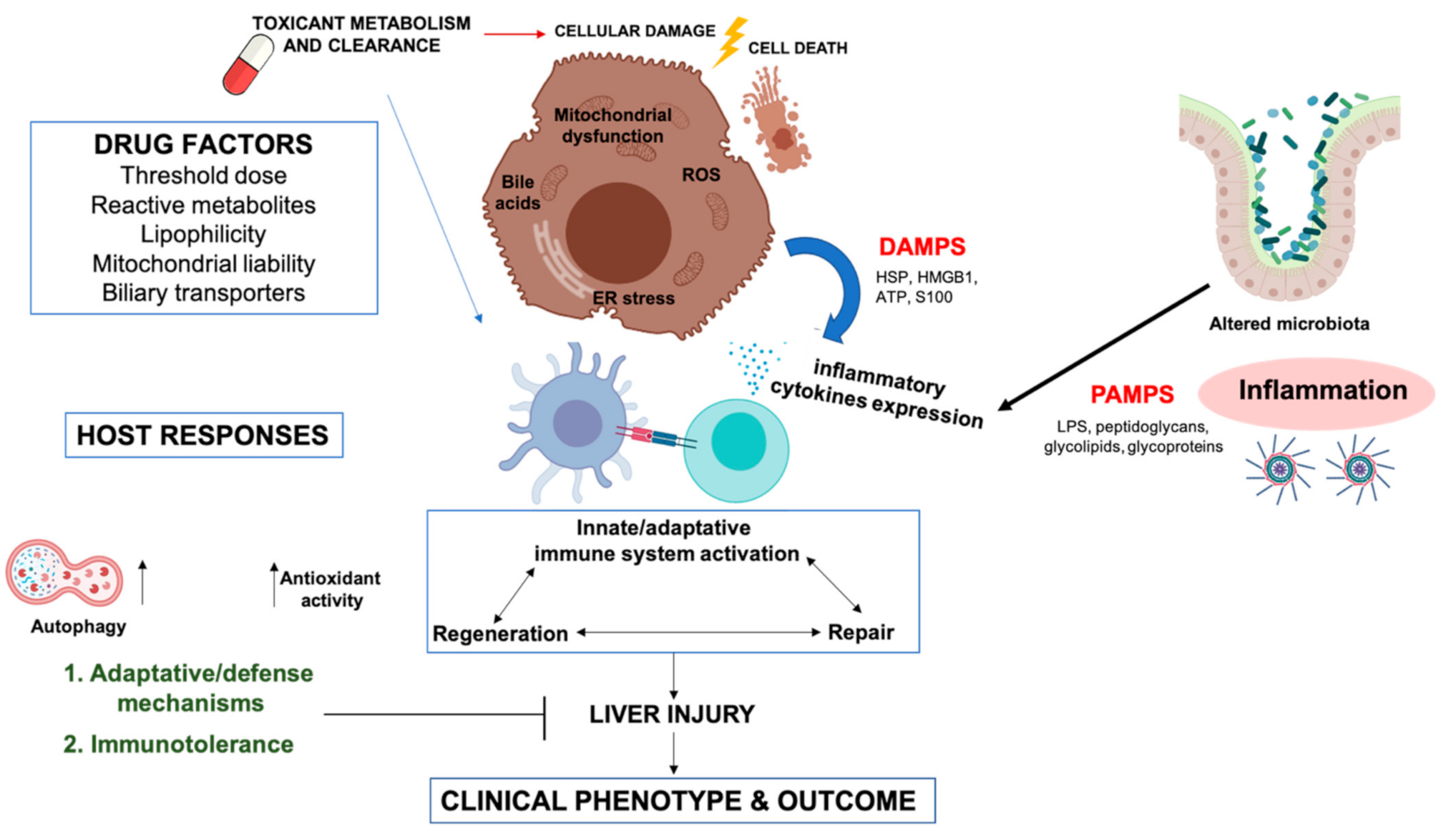
| Drug | Indication | Type | Drug | Indication | Type |
|---|---|---|---|---|---|
| Abacavir | Antiretroviral | H/C | Allopurinol | Gout prophylaxis | H |
| Amiodarone | Anti-arrhythmic | H | Amodiaquine | Malaria treatment | H |
| Amoxicillin–clavulanic acid | Antibiotic | C/M | Angiotensin -converting enzyme inhibitors | Hypertension | C |
| Atorvastatin | Hypercholesterolemia | H/C | Azathioprine | Immunosuppressor | C |
| Bosentan | Hypertension | H/M | Carbamazepine | Anticonvulsant | H/C/M |
| Chlorpromazine | Antipsychotic | C/M | Clozapine | Antipsychotic | H/M |
| Cyclosporine A | Immunosuppressor | C | Dantrolene | Muscle relaxant | H |
| Diclofenac | Analgesic | H | Disulfiram | Alcoholism | H |
| Erythromycin | Antibiotic | C/M | Felbamate | Anticonvulsant | H |
| Fenofibrate | Hypertriglyceridemia and dyslipidemia | H | Floxuridine | Antineoplastic | H/C |
| Flucloxacillin | Antibiotic | C | Flupirtine | Analgesic | H |
| Flutamide | Nonsteroidal antiandrogen | H/C/M | Gabapentin | Anticonvulsant | C |
| Halothane | Anesthetic | H | Hydralazine | Antihypertensive | H |
| Ibuprofen | NSAID | H | Infliximab | Monoclonal antibody (Crohn’s disease, rheumatoid arthritis) | H |
| Isoniazid | Anti-tuberculotic | H | Ketoconazole | Fungicidal | H/C |
| Lamotrigine | Anticonvulsant | H | Lapatinib | Breast cancer | H |
| Leflunomide | Immunomodulatory agent | H/C | Lisinopril | Antihypertensive | H |
| Methotrexate | Antineoplastic | H | Methyldopa | Antihypertensive | H |
| Minocycline | Antibiotic | H | Nefazodone | Antidepressant | H |
| Nevirapine | Nonnucleoside reverse transcriptase inhibitor | C | Nitrofurantoin | Antibiotic | H/M |
| Pazopanib | Antitumor activity | M | Phenytoin | Anticonvulsant | H/M |
| Propylthiouracil | Antithyroid | H | Pyrazinamide | Anti-tuberculotic | H |
| Quinidine | Antiarrhythmic | C/M | Rifampicin | Anti-tuberculotic | H |
| Statins | Hypolipidemic | H/C | Sulfasalazine | Antirheumatic | M |
| Sulfonamides | Antibiotic | H/C | Sulindac | NSAID | H/C/M |
| Tamoxifen | Nonsteroidal antiestrogen | H/C/M | Terbinafine | Fungicidal | H/C |
| Thioguanine | Antitumor activity | M | Ticlopidine | Anti-platelet | C |
| TMP-SMX | Antibiotic | H | Tolcapone | Parkinson’s disease therapy | H |
| Tolvaptan | Hyponatremia treatment | H/M | Valproic acid | Anticonvulsant | H |
| Genetic Variation | Association | Drug Studied | DILI Association | References |
|---|---|---|---|---|
| Drug transporters genes | ||||
| ABCB1 3435T | Transporter | Nevirapine | ↓ Risk | [169,170] |
| ABCB11 1331C | Transporter | Various | ↑ Risk | [171,172] |
| ABCB4 haplotypes | Transporter | Various | ↑ Risk | [171] |
| ABCC2 haplotypes | Transporter | Various | ↑ Risk | [164,173] |
| Cytochrome P450 genes | ||||
| CYP2B6*6 | Phase I | Efavirenz | ↑ Risk | [174] |
| Nevirapine | ↑ Risk | [175] | ||
| Anti TBC | ↓ Risk | [176] | ||
| CYP2B6 rs7254579 | Phase I | Ticlopidine | ↑ Risk | [177] |
| CYP2C8 haplotypes | Phase I | Diclofenac | ↑ Risk | [164] |
| Repaglinide | ↑ Risk | [164] | ||
| CYP2E1 c1/c1 | Phase I | Isoniazid | ↑ Risk | [178] |
| Phase II enzymes genes | ||||
| GST T1/M1 null | Phase II | Various | ↑ Risk | [179,180,181,182,183] |
| GPX1 198T | Phase II | Various | ↑ Risk | [184] |
| NAT2 slow acetylators | Phase II | Anti-TBC | ↑ Risk | [177,180,181] |
| SOD2 47C | Phase II | Various | ↑ Risk | [182,184] |
| UGT2B7*2 | Phase II | Diclofenac | ↑ Risk | [164] |
| UGT1A6/1A9 | Phase II | Tolcapone | ↑ Risk | [185,186] |
| Others | ||||
| PTPN2 | Tyrosine phosphatase | Various | ↑ Risk | [187] |
| POLG | mtDNA polymerase γ | Valproic acid | ↑ Risk | [100] |
| Drug | Genetic Variation | Odds Ratio | Population | References |
|---|---|---|---|---|
| Amoxicillin-clavulanate | A*02:01 | 2.2 | Caucasian | [223] |
| A*30:02 | 6.7 | Caucasian | [224] | |
| B*18:01 | 2.9 | Caucasian | [224] | |
| DRB1*07:01 | 0.18 ^ | Caucasian | [225] | |
| DRB1*15:01-DQB1*06:02 | 3 | Caucasian | [223,224,225,226,227] | |
| Clometacin | B*08 | - | Caucasian | [228] |
| Diclofenac | DRB1*13 | ^ | Caucasian | [229] |
| Efavirenz + Anti-TB | B*57:02 | 8.1 | African | [230] |
| B*57:03 | 26.8 | African | [230] | |
| Fenofibrate | A*33:01 | 58.7 | Caucasian | [231] |
| Flucloxacillin | B*57:01 | 80.6 | Caucasian | [232] |
| B*57:03 | 79.2 | Caucasian | [233] | |
| DRB1*0701-DQB1*0303 | 9.7 | Caucasian | [232] | |
| Flupirtine | DRB1*16:01-DQB1*05:02 | 18.7 | Caucasian | [234] |
| Lapatinib | DQA1*02:01 | 9–14.1 | Caucasian | [235,236] |
| DQB1*02:02 | 6.9–8.6 | Caucasian | [235,236] | |
| DQB1*07:01 | 6.9–14.1 | Caucasian | [235,236,237] | |
| Lumiracoxib | DRB1*15:01-DQB1*06:02-DRB5*01:01-DQA1*01:02 | 5 | Caucasian | [238] |
| Minocycline | B*35:02 | 29.6 | Caucasian | [239] |
| Nevirapine | B*58:01 | - | African | [240] |
| DRB1*01:01 | 3–4.8 | Caucasian | [241,242] | |
| DRB1*01:02 | - | African | [240] | |
| Pazopanib | B*57:01 | 2 | - | [243] |
| Terbinafine | A*33:01 | 40.5 | Caucasian | [231] |
| Ticlopidine | A*33:01 | 163.1 | Caucasian | [231] |
| A*33:03 | 13 | Japanese | [244] | |
| B*44:03 | 6.6 | Japanese | [244] | |
| Cw*1403 | 7.3 | Japanese | [244] | |
| DQB1*06:04 | 10.1 | Japanese | [244] | |
| DRB1*13:02 | 9 | Japanese | [244] | |
| Tiopronin | A*33 | - | Japanese | [245] |
| Trimethoprim-Sulfamethoxazole | B*14:01 | 9.2 | Caucasian | [246] |
| B*35:01 | - | Africans | [246] | |
| Ximelagatran | DRB1*07:01-DQA1*02 | 4.4 | Caucasian | [136] |
| DQB1*02:01 | - | Indian | [247] |
| Serum Biomarkers | Advantage | Limitations | Comments | References |
|---|---|---|---|---|
| AOPPs, IMA |
|
|
| [262] |
| APAP-CYS |
|
|
HPLC-EC HPLC-mass spectrometry | [263] |
| Bilirubin |
|
|
| [261,264] |
| GLDH |
|
|
| [161,255,256] |
| HMGB1 |
|
|
| [23,265] |
| K18 |
|
|
| [266,267] |
| MCSFR |
|
|
| [267] |
| miR-122 |
|
|
| [268,269] |
| mtDNA |
|
|
| [167,270] |
| OPN |
|
|
| [264,267] |
| Transaminases |
|
|
| [23,271] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanueva-Paz, M.; Morán, L.; López-Alcántara, N.; Freixo, C.; Andrade, R.J.; Lucena, M.I.; Cubero, F.J. Oxidative Stress in Drug-Induced Liver Injury (DILI): From Mechanisms to Biomarkers for Use in Clinical Practice. Antioxidants 2021, 10, 390. https://doi.org/10.3390/antiox10030390
Villanueva-Paz M, Morán L, López-Alcántara N, Freixo C, Andrade RJ, Lucena MI, Cubero FJ. Oxidative Stress in Drug-Induced Liver Injury (DILI): From Mechanisms to Biomarkers for Use in Clinical Practice. Antioxidants. 2021; 10(3):390. https://doi.org/10.3390/antiox10030390
Chicago/Turabian StyleVillanueva-Paz, Marina, Laura Morán, Nuria López-Alcántara, Cristiana Freixo, Raúl J. Andrade, M Isabel Lucena, and Francisco Javier Cubero. 2021. "Oxidative Stress in Drug-Induced Liver Injury (DILI): From Mechanisms to Biomarkers for Use in Clinical Practice" Antioxidants 10, no. 3: 390. https://doi.org/10.3390/antiox10030390
APA StyleVillanueva-Paz, M., Morán, L., López-Alcántara, N., Freixo, C., Andrade, R. J., Lucena, M. I., & Cubero, F. J. (2021). Oxidative Stress in Drug-Induced Liver Injury (DILI): From Mechanisms to Biomarkers for Use in Clinical Practice. Antioxidants, 10(3), 390. https://doi.org/10.3390/antiox10030390











