Protective Activity and Underlying Mechanism of Ginseng Seeds against UVB-Induced Damage in Human Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Ginseng Seeds Extract
2.3. Antioxidant Activities
2.4. Phytosterols, Tocopherols, and Tocotrienols
2.5. Ginsenosides
2.6. Fatty Acids
2.7. Cell Viability and UVB Irradiation
2.8. Measurement of ROS
2.9. Measurement of MMPs and Total Collagen
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activities and Phytochemical Content
3.2. Protective Effect against UVB-Induced Damage
3.3. Measurement of ROS, MMPs, and Collagen Production
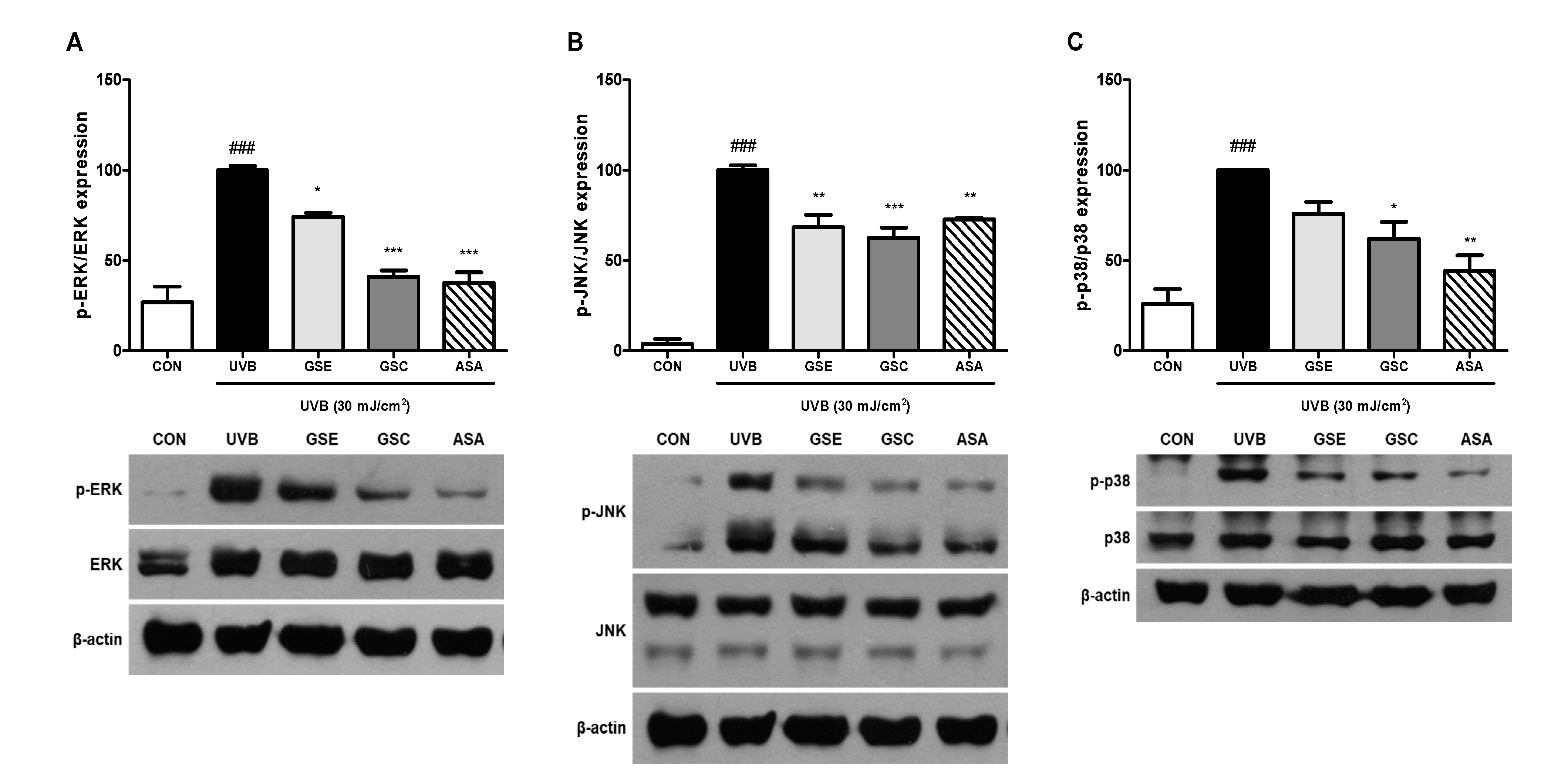
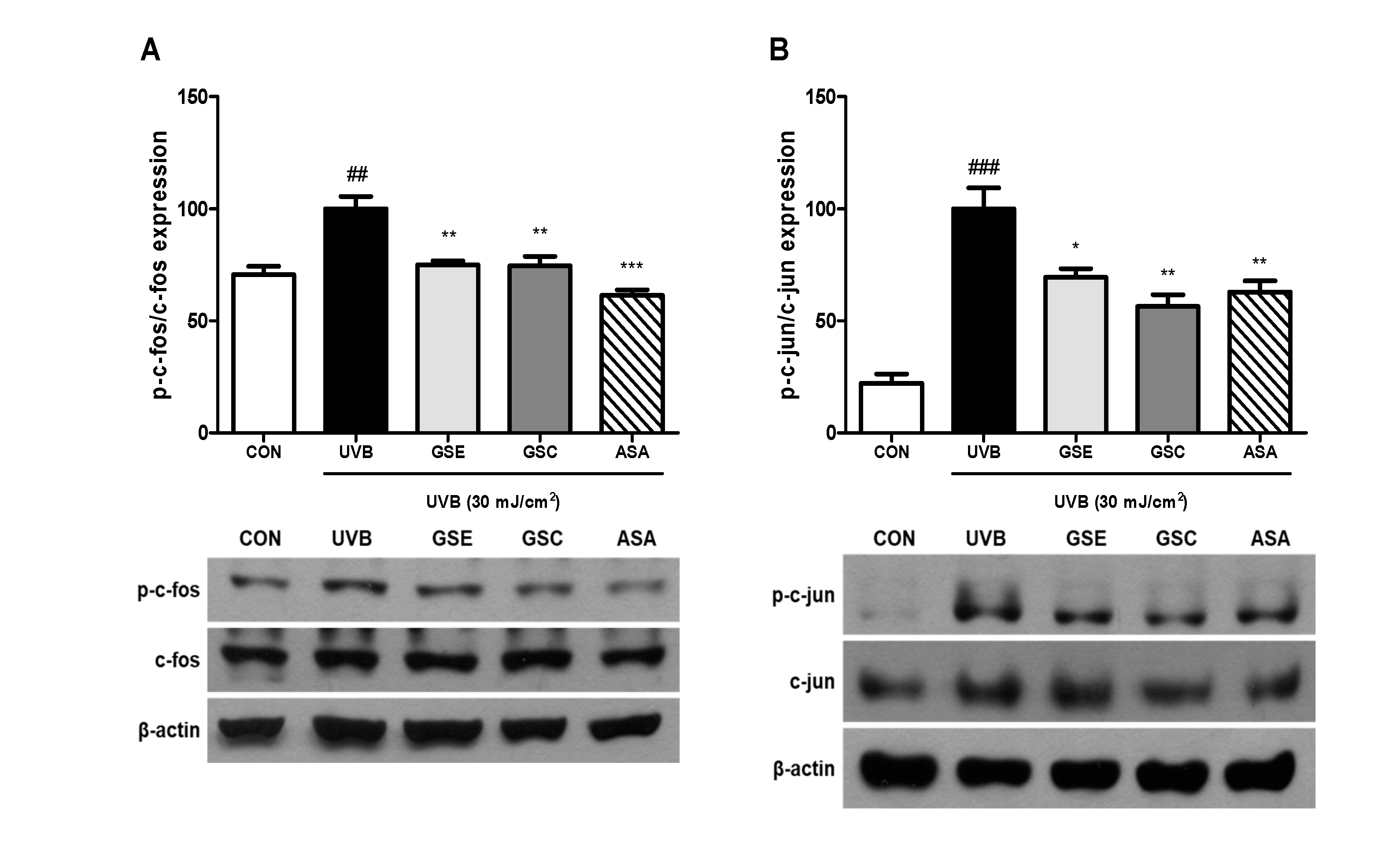
3.4. Phosphorylation of MAPK and AP-1
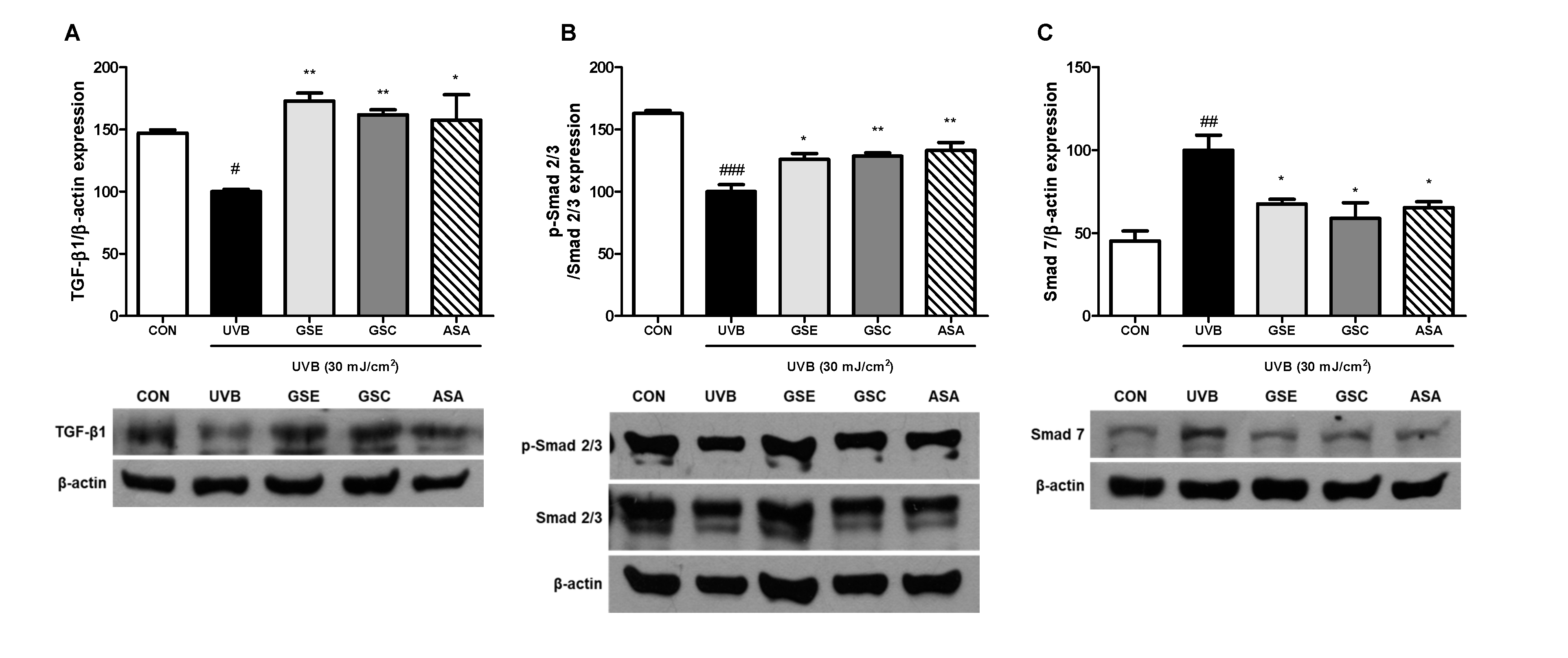
3.5. Regulation of TGF-β and Smad Signaling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2008, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Wondrak, G.T.; Jacobson, M.K.; Jacobson, E.L. Endogenous UVA-photosensitizers: Mediators of skin photodamage and novel targets for skin photoprotection. Photochem. Photobiol. Sci. 2006, 5, 215–237. [Google Scholar] [CrossRef]
- Yamaba, H.; Habba, M.; Kunita, M.; Sakaida, T.; Tanaka, H.; Yashiro, Y.; Nakata, S. Morphological change of skin fibroblasts induced by UV irradiations is involved in photoaging. Exp. Dermatol. 2016, 3, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, K.C.; Fan, P.C.; Tsai, S.Y.; Shih, I.C.; Chiang, H.M. Ixora parviflora protects against UVB-induced photoaging by inhibiting the expression of MMPs, MAP kinases, and COX-2 and by promoting type Ⅰ procollagen synthesis. Evid-Based Complement. Altern. Med. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sardy, M. Role of matrix metalloproteinases in skin ageing. Connect. Tissue Res. 2009, 50, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Fisher, G.J. Ultraviolet (UV) light irradiation induced signal transduction in skin photoaging. J. Dermatol. Sci. Suppl. 2005, 1, S1–S8. [Google Scholar] [CrossRef]
- Chen, S.J.; Yuan, W.; Mori, Y.; Levenson, A.; Varga, J.; Trojanowska, M. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-β: Involvement of Smad3. J. Investig. Dermatol. 1999, 112, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Crowe, D.L.; Castillo, C.; Wuenschell, C.; Chai, Y.; Warburton, D. Smad7 is a TGF-β-inducible attenuator of Smad2/3-mediated inhibition of embryonic lung morphogenesis. Mech. Dev. 2000, 93, 71–81. [Google Scholar] [CrossRef]
- Tanaka, H.; Yamaba, H.; Kosugi, N.; Mizutani, H.; Nakata, S. Fermentable metabolite of Zymomonas mobilis controls collagen reduction in photoaging skin by improving TGF-β/Smad signaling suppression. Arch. Dermatol. Res. 2008, 300, 57–64. [Google Scholar] [CrossRef]
- Vogler, B.K.; Pittler, M.H.; Ernst, E. The efficacy of ginseng. A systematic review of randomized clinical trials. Eur. J. Clin. Pharmacol. 1999, 55, 567–575. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, W.Y. Purification and characterization of polyphenol oxidase from fresh ginseng. J. Ginseng. Res. 2013, 37, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Jung, N.P. The effect of ginseng saponin on the mouse immune system. Korean J. Ginseng. Sci. 1987, 11, 130–135. [Google Scholar]
- Ko, S.K.; Cho, O.S.; Bae, H.M.; Im, B.O.; Lee, O.H.; Lee, B.Y. Quantitative analysis of ginsenosides composition in flower buds of various ginseng plants. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 154–157. [Google Scholar] [CrossRef]
- Seo, E.; Kim, S.; Lee, S.J.; Oh, B.C.; Jun, H.S. Ginseng Berry Extract Supplementation Improves Age-Related Decline of Insulin Signaling in Mice. Nutrients 2015, 7, 3038–3053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.K.; Song, S.Y.; Oh, W.K.; Kaewsuwan, S.; Tran, T.L.; Kim, W.S.; Sung, J.H. Wound-healing effect of ginsenoside Rd from leaves of Panax ginseng via cyclic AMP-dependent protein kinase pathway. Eur. J. Pharmacol. 2013, 702, 285–293. [Google Scholar] [CrossRef]
- Choi, J.E.; Li, X.; Han, Y.H.; Li, K.T. Changes of saponin contents of leaves, stems and flower-buds of Panax ginseng C. A. Meyer by harvesting days. Korean J. Med. Crop. Sci. 2009, 17, 251–256. [Google Scholar]
- Hu, J.N.; Lee, J.H.; Shin, J.A.; Choi, J.E.; Lee, K.T. Determination of ginsenosides content in Korean ginseng seeds and roots by high performance liquid chromatography. Food Sci. Biotechnol. 2008, 17, 430–433. [Google Scholar]
- Svetashev, V.I.; Burundukova, O.L.; Muzarok, T.I.; Zhuravlev, Y.N. Fatty acid composition of seeds from wild and cultivated ginseng (Panax ginseng Meyer): Occurrence of a high level of petroselinic aicd. J. Am. Oil Chem. Soc. 2016, 93, 1319–1323. [Google Scholar] [CrossRef]
- Beveridge, T.H.J.; Li, T.S.C.; Drover, J.C.G. Phytosterol content in American ginseng seed oil. J. Agric. Food Chem. 2002, 50, 744–750. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Watson, R.E.B.; Nicolaou, A.; Rhodes, L.E. Omega-3 polyunsaturated fatty acids: Photoprotective macronutrients. Exp. Dermatol. 2011, 20, 537–543. [Google Scholar] [CrossRef]
- Kim, E.; Kim, D.; Yoo, S.; Hong, Y.H.; Han, S.Y.; Jeong, S.; Jeong, D.; Kim, J.H.; Cho, J.Y.; Park, J. The skin protective effects of compound K, a metabolite of ginsenoside Rb1 from Panax ginseng. J. Ginseng. Res. 2018, 42, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Mora-Ranjeva, M.P.; Charveron, M.; Fabre, B.; Milon, A.; Muller, I. Incorporation of phytosterols in human keratinocytes. Consequences on UVA-induced lipid peroxidation and calcium ionophore-induced prostaglandin release. Chem. Phys. Lipids 2006, 141, 216–224. [Google Scholar] [CrossRef]
- Cheung, L.M.; Cheung, P.C.K.; Ooi, V.E.C. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Choi, Y.; Jeong, H.S.; Lee, J. Antioxidant activity of methanolic extracts from some grains consumed in Korea. Food Chem. 2007, 103, 130–138. [Google Scholar] [CrossRef]
- Sung, J.; Lee, J. Antioxidant and antiproliferative activities of grape seeds from different cultivars. Food Sci. Biotechnol. 2010, 19, 321–326. [Google Scholar] [CrossRef]
- Choi, N.; Kim, H.; Kim, B.H.; Lee, J.; Kim, I.H. Production of phytosteryl ester from echium oil in a recirculating packed bed reactor using an immobilized lipase. J. Oleo Sci. 2017, 66, 1329–1335. [Google Scholar] [CrossRef] [Green Version]
- Sim, U.; Sung, J.; Lee, H.; Heo, H.; Jeong, H.S.; Lee, J. Effect of calcium chloride and sucrose on the composition of bioactive compounds and antioxidant activities in buckwheat sprouts. Food Chem. 2020, 312, 126075. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.W.; Kim, M.Y.; Lee, Y.J.; Li, M.; Shin, Y.S.; Lee, J.; Jeong, H.S. Influence of organic acids and heat treatment on ginsenoside conversion. J. Ginseng. Res. 2018, 42, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Shin, J.A.; Qin, Y.; Kwon, J.I.; Lee, K.T. A Study on the Relationship of Fat Content in Human Milk on Carotenoids Content and Fatty Acid Compositions in Korea. Nutrients 2019, 11, 2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochemical. 2012, 114, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Afanas’ev, I. Signaling by reactive oxygen and nitrogen species in skin diseases. Curr. Drug Metab. 2010, 11, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef] [Green Version]
- Park, H.W.; In, G.; Kim, J.H.; Cho, B.G.; Han, G.H.; Chang, I.M. Metabolomic approach for discrimination of processed ginseng genus (Panax ginseng and Panax quinquefolius) using UPLC-QTOF MS. J. Ginseng. Res. 2014, 38, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Arcan, I.; Yemenicioğlu, A. Antioxidant activity and phenolic content of fresh and dry nuts with or without seed coat. J. Food Comp. Anal. 2009, 22, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Viswanath, V.; Urooj, A.; Malleshi, N.G. Evaluation of antioxidant and antimicrobial properties of finger millet polyphenols (Eleusine coracana). Food Chem. 2009, 114, 340–346. [Google Scholar] [CrossRef]
- Yamada, Y.; Obayashi, M.; Ishikawa, T.; Kiso, Y.; Ono, Y.; Yamashita, K. Dietary tocotrienol reduces UVB-induced skin damage and sesamin enhances tocotrienol effects in hairless mice. J. Nutr. Sci. Vitaminol. 2008, 54, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berneburg, M.; Plettenberg, H.; Krutmann, J. Photoaging of human skin. Photodermatol. Photoimmunol. Photomed. 2000, 16, 239–244. [Google Scholar] [CrossRef]
- Ogata, Y.; Enghild, J.J.; Nagase, H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J. Biol. Chem. 1992, 267, 3581–3584. [Google Scholar] [CrossRef]
- Sugaru, E.; Sakai, M.; Horigome, K.; Tokunaga, T.; Kitoh, M.; Hume, W.E.; Nagata, R.; Nakagawa, T.; Taiji, M. SMP-534 inhibits TGF-beta-induced ECM production in fibroblast cells and reduces mesangial matrix accumulation in experimental glomerulonephritis. Am. J. Physiol. Renal Physiol. 2005, 289, F998–F1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisdom, R.; Huynh, L.; Hsia, D.; Kim, S. RAS and TGF-β exert antagonistic effects on extracellular matrix gene expression and fibroblast transformation. Oncogene 2005, 24, 7043–7054. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| GSE | GSC | ||
|---|---|---|---|
| Antioxidant activities and compounds | |||
| DPPH radical scavenging (TE (1) mg/g residue) | 6.96 ± 0.43 | 16.02 ± 2.81 | |
| ABTS radical scavenging (TE mg/g residue) | 17.35 ± 0.33 | 49.34 ± 0.89 | |
| Total polyphenolic contents (GAE (2) mg/g residue) | 4.37 ± 0.04 | 5.87 ± 0.07 | |
| Total flavonoid contents (CE (3) mg/g residue) | 1.62 ± 0.05 | 2.70 ± 0.04 | |
| Phytosterols (mg/g residue) | |||
| Campesterol | 6.35 ± 0.10 | 6.11 ± 0.41 | |
| Stigmasterol | 15.62 ± 0.60 | 2.96 ± 0.55 | |
| β-sitosterol | 20.51 ± 0.57 | 4.01 ± 0.35 | |
| Ginsenosides (mg/g residue) | |||
| Rg1 | 0.04 ± 0.01 | 0.69 ± 0.00 | |
| Re | 0.81 ± 0.10 | 1.22 ± 0.09 | |
| Rk3 | N.D. (4) | 0.45 ± 0.12 | |
| Rh2 | 0.26 ± 0.05 | N.D. | |
| Rh4 | 0.80 ± 0.04 | 10.45 ± 0.16 | |
| Tocopherols and tocotrienol (μg/g residue) | |||
| α-Tocopherol | 30.19 ± 0.22 | N.D. | |
| α-Tocotrienol | 202.97 ± 3.66 | 12.14 ± 0.56 | |
| γ-Tocopherol | 50.74 ± 0.05 | 15.88 ± 1.08 | |
| Fatty acids (mg/g residue) | |||
| Lauric acid | 12:0 | N.D. | 0.14 ± 0.00 |
| Myristic acid | 14:0 | N.D. | 0.19 ± 0.00 |
| Palmitic acid | 16:0 | 5.28 ± 0.24 | 3.15 ± 0.04 |
| Hexadecenoic acid | 16:1 | 0.14 ± 0.00 | 0.66 ± 0.03 |
| Stearic acid | 18:0 | 0.35 ± 0.01 | 0.64 ± 0.01 |
| Oleic acid | 18:1(n-9) | 6.74 ± 0.36 | 9.88 ± 0.48 |
| Vaccenic acid | 18:1(n-7) | 0.98 ± 0.06 | 1.64 ± 0.05 |
| Linolelaidic acid | 18:2t | 0.25 ± 0.03 | 0.41 ± 0.02 |
| Linoleic acid | 18:2(n-6) | 19.33 ± 0.96 | 11.50 ± 0.55 |
| Linolenic acid | 18:3t | N.D. | 0.42 ± 0.02 |
| Linolenic acid | 18:3(n-3) | 0.33 ± 0.01 | 0.15 ± 0.01 |
| Eicosanoic acid | 20:1 | 0.11 ± 0.01 | N.D. |
| Total | 33.50 ± 1.62 | 28.88 ± 1.19 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, H.; Lee, H.; Yang, J.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Protective Activity and Underlying Mechanism of Ginseng Seeds against UVB-Induced Damage in Human Fibroblasts. Antioxidants 2021, 10, 403. https://doi.org/10.3390/antiox10030403
Heo H, Lee H, Yang J, Sung J, Kim Y, Jeong HS, Lee J. Protective Activity and Underlying Mechanism of Ginseng Seeds against UVB-Induced Damage in Human Fibroblasts. Antioxidants. 2021; 10(3):403. https://doi.org/10.3390/antiox10030403
Chicago/Turabian StyleHeo, Huijin, Hana Lee, Jinwoo Yang, Jeehye Sung, Younghwa Kim, Heon Sang Jeong, and Junsoo Lee. 2021. "Protective Activity and Underlying Mechanism of Ginseng Seeds against UVB-Induced Damage in Human Fibroblasts" Antioxidants 10, no. 3: 403. https://doi.org/10.3390/antiox10030403






