Abstract
Hypoxia is a common and severe stress to an organism’s homeostatic mechanisms, and hypoxia during gestation is associated with significantly increased incidence of maternal complications of preeclampsia, adversely impacting on the fetal development and subsequent risk for cardiovascular and metabolic disease. Human and animal studies have revealed a causative role of increased uterine vascular resistance and placental hypoxia in preeclampsia and fetal/intrauterine growth restriction (FGR/IUGR) associated with gestational hypoxia. Gestational hypoxia has a major effect on mitochondria of uteroplacental cells to overproduce reactive oxygen species (ROS), leading to oxidative stress. Excess mitochondrial ROS in turn cause uteroplacental dysfunction by damaging cellular macromolecules, which underlies the pathogenesis of preeclampsia and FGR. In this article, we review the current understanding of hypoxia-induced mitochondrial ROS and their role in placental dysfunction and the pathogenesis of pregnancy complications. In addition, therapeutic approaches selectively targeting mitochondrial ROS in the placental cells are discussed.
1. Introduction
Preeclampsia and fetal growth restriction (FGR) are two most common pregnancy complications worldwide, affecting 5–10% of pregnancy [1,2,3]. According to the International Society for the Study of Hypertension in Pregnancy (ISSHP) [4], preeclampsia is defined as gestational hypertension with systolic blood pressure (BP) ≥ 140 and/or diastolic BP ≥ 90 mm Hg at or after 20 weeks’ gestation, accompanied by one or more of the following conditions: (1) proteinuria, (2) other maternal organ dysfunction such as acute kidney injury, liver dysfunction, neurological complications and hematological complications; and (3) uteroplacental dysfunction such as FGR, abnormal umbilical artery Doppler wave form analysis, or stillbirth. FGR refers the failure of a fetus to reach its genetic/biological growth potential and is clinically defined as an ultrasonographic estimated fetal weight or abdominal circumference below the 10th percentile for gestational age by the American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine [2,5]. Preeclampsia is heterogeneous, exhibiting in the early onset (premature delivery prior to 34 weeks’ gestation) and late-onset forms (delivery at or after 34 weeks’ gestation). Early onset preeclampsia is frequently associated with FGR [6]. Preeclampsia and FGR are major causes of maternal and perinatal morbidity and mortality [7,8]. Moreover, they also predispose for various diseases in later life of the mother and offspring, including cardiovascular disease, metabolic syndrome, and neurologic impairment [8,9,10,11].
It is now recognized that both preeclampsia and FGR originate from the placenta due to uteroplacental dysfunction conferred by gestational hypoxia [1,12,13,14,15]. Gestational hypoxia is associated with overproduction in reactive oxygen species (ROS) in the placenta, leading to oxidative stress [12,16,17,18]. ROS can be beneficial or deleterious, depending on their levels in cells. At low levels, ROS function as signaling molecules in regulating a variety of cellular processes [19]. However, due to their highly reactivity, the overwhelming accumulation of ROS may indiscriminately damage cellular macromolecules such as lipids, proteins and DNAs and consequently impairs cellular functions. Oxidative stress has been implicated in many human diseases including hypertension [20,21]. Not surprisingly, uteroplacental tissues/cells of preeclampsia and FGR display a heightened level of oxidative stress [22], which plays a central role in the pathogenesis of both complications [18,23]. Mitochondria consume most cellular oxygen (O2) to produce ATP to sustain cellular functions and a significant portion of ROS is also generated during the oxidation-phosphorylation coupling. Mitochondria are severely impacted by reduced O2 availability as hypoxia alters mitochondrial structure/dynamics and electron transfer complexes and enhances production of ROS [24,25]. It appears that mitochondria are the predominant sources to generate ROS in the placenta under both physiological and pathophysiological conditions [22,26]. Here, we review current knowledge of placental mitochondrial ROS in the pathogenesis of preeclampsia and FGR. In addition, we also discuss the potential therapeutic strategy by targeting mitochondrial ROS in the management of these two pregnancy complications.
2. Hypoxia, Hypoxia Inducible Factors (HIFs) and Preeclampsia/FGR
2.1. Maladaptation of Uteroplacental Circulation in Preeclampsia/FGR
Uterine vessels undergo substantially structural and functional adaptation during pregnancy. Structural changes of uterine vessels involve an increase arterial lumen with the remodeling of spiral arteries [27], whereas functional changes of uterine arteries implicate modulating ion channel expression/activity to lowering intrinsic myogenic tone [14]. The uterine vascular adaptation along with the formation of the placenta establishes the low-resistance and high-flow uteroplacental circulation, resulting in a dramatic increase in uteroplacental perfusion to meet the requirement of placental and fetal growth/development. However, uteroplacental hemodynamic is altered in preeclampsia and FGR, displaying increased uteroplacental vascular resistance and reduced uteroplacental perfusion [27,28,29,30,31,32]. Impaired trophoblast invasion and subsequent deficient spiral arteries are prominent features of preeclampsia and FGR [33]. As expected, inhibition of trophoblast invasion in rat pregnancy results in uteroplacental hypoperfusion [34].
2.2. Hypoxia and Hypoxia Inducible Factors (HIFs)
Oxygen is essential to the survival of the most eukaryotic organisms. In general, hypoxia constitutes a severe stress to cells. Mounting evidence suggests that hypoxia is a key player in the pathogenesis of preeclampsia and FGR [14,15,35]. Hypoxia-inducible factors (HIFs) are key molecules that regulate cellular responses to hypoxia and play important roles in both physiological and pathophysiological contexts. HIFs are transcription factors consisting of HIF-α subunit (HIF-1α or HIF-2α) and HIF-1β. Both α and β subunits are constitutively expressed and only the α subunit is regulated by O2 levels. Under normoxia, the α subunit is hydroxylated by prolyl hydroxylase domain proteins (PHDs), which use O2 and α-ketoglutarate as substrates. The hydroxylated α subunit is then recognized and polyubiquitylated by the von Hippel-Lindau (VHL) protein that serves as an E3 ubiquitin ligase, followed by proteasomal degradation. However, under hypoxic conditions, PHDs are enzymatically inactive and the α subunit are no longer hydroxylated. Thus, the α subunit is stabilized and translocated to the nucleus, where the α subunit dimers with HIF-1β and activates expression of target genes, playing critical roles in mediating cellular responses to hypoxia.
2.3. Hypoxia, Hypoxia to Normoxia Transition and Normal Pregnancy
Intriguingly, hypoxia is essential for development including embryonic and placental development [36,37,38]. Measured at weeks 7–10 in the first trimester, the partial O2 pressure is ~20 mm Hg in the placenta and 60 mm Hg in the decidua [39]. The embryonic and placental development thus occurs under a hypoxic environment. Hypoxia in this period is not considered to be pathological as low O2 promotes trophoblast proliferation and angiogenesis/vasculogenesis in the placenta [40,41]. At weeks 11–16, placental O2 tension reaches ~60 mm Hg, approaching the O2 level in the decidua (~70 mm Hg) [39]. The rise in O2 is believed to trigger trophoblast to differentiate [40], promoting the transition of trophoblasts from the proliferative to an invasive phenotype to complete spiral artery remodeling. The establishment of the uteroplacental circulation allows to adequately supply O2 and nutrients to the placenta to meet the metabolic and biosynthetic demands for the rapid growth/development of the placenta and the fetus in the second and third trimesters.
2.4. Hypoxia and Preeclampsia/FGR
The expression of HIF-1α in the placenta occurs concurrently with placental O2 tension, displaying high expression in the first trimester and declining starting from week 9 of gestation [42,43]. Inactivation of HIF-1α in maternal tissues at E8.5 leads to reduced trophoblast proliferation and increased apoptosis in mouse placentas [44]. Most importantly, the temporal expression pattern of HIF-1α in trophoblasts is key to the shift from proliferation to invasiveness. Antisense inhibition of HIF-1α expression triggers a switch of trophoblasts from proliferative to invasive phenotype [42]. Failure to complete this transition would compromise spiral artery modeling. Placental hypoxia apparently persists beyond the first trimester in preeclampsia and FGR as HIF-1α and HIF-2α expression in the placenta keeps elevated throughout pregnancy [45,46,47,48,49]. Further evidence to support the pivotal role of hypoxia in the pathogenesis of preeclampsia and FGR comes from studies in which humans and animals experience hypobaric and/or normobaric hypoxia and from in or ex vivo studies using trophoblasts and placental explants. Pregnancy at high altitude is associated with ~3-fold increases in the incidence of preeclampsia and FGR [50,51,52] and overexpression of placental HIF-1α [53]. Pregnant ewes exposed to hypobaric hypoxia at high altitude have elevated blood pressure and uterine vascular resistance and reduced birthweight of newborn lambs [54,55]. Additionally, numerous animal studies demonstrated that chronic normobaric hypoxia during pregnancy increases placental HIF expression, inhibits trophoblast invasion, and promotes preeclampsia-like symptoms and FGR [56,57,58,59,60,61,62,63]. In vitro studies also demonstrated that hypoxia boosts HIFs expression in trophoblast cell lines [64,65]. Furthermore, the global gene expression displayed a strikingly similar pattern in placentas from preeclampsia and high-altitude pregnancy and ex vivo hypoxia-treated placental explants [66]. Pregnant mice with global overexpression of HIF-1α display phenotypes of preeclampsia and FGR, showing elevated blood pressure, proteinuria and reduced fetal weight [67]. Similar findings and impaired spiral artery remodeling are also observed with prolonged expression of trophoblast-specific HIF-1α [68]. In response to hypoxia, the induction of HIF-1α and HIF-2α also stimulates the production soluble Fms-like tyrosine kinase-1 (sFlt-1) in trophoblasts [64,69]. sFlt-1, an anti-angiogenic factor, is increased in circulation in preeclampsia and FGR [70,71,72]. It is believed that sFlt-1 contributes to the pathogenesis of preeclampsia by prompting endothelial dysfunction, disrupting angiogenesis, and impairing trophoblast invasion [70,73,74,75,76]. Low O2 tension is an inducer of angiogenesis. HIF-1α is a key regulator of placental vascular development. Genetic deletion of HIF-1α or HIF-2α results in defective placental vascularization [77]. In contrast, high altitude pregnancy increases HIF-1α expression in the placenta, which is associated with greater vascularity [78].
The functional adaptation of uterine arteries also contributes to the marked increase in uterine blood flow in pregnancy. Large-conductance Ca2+-activated K+ (BKCa) channels and ryanodine receptors in uterine arteries appear to be major determinants in initiating the functional adaptation in pregnancy [79,80,81,82,83]. The coupling of ryanodine receptor-mediated Ca2+ sparks to BKCa channel-mediated spontaneous transient outward currents (STOCs) is an important mechanism to regulate arterial myogenic tone, the primary constituent in setting basal vascular tone/resistance [84,85]. K+ efflux carried by STOCs causes membrane hyperpolarization and subsequent CaV1.2 closure, resulting in vascular smooth muscle cell relaxation. Estrogen increases the expression of the BKCa channel β1 subunit and ryanodine receptors in uterine arteries in pregnancy, leading to reduced vascular tone and increased uterine blood flow by promoting Ca2+ spark-STOC coupling [80,81,82,83,86]. This adaptation is disrupted by high-altitude hypoxia, resulting in increased uterine vascular resistance [87,88,89]. HIF-1α is a major contributor to the gestational hypoxia-induced uterine vascular maladaptation and HIF-1α-responsive microRNA-210 (miR-210) appears to be a key mediator [55,90,91].
3. Mitochondrial Reactive Oxygen Species (ROS): The GOOD, the Bad and the Ugly
3.1. Overview of Mitochondrial (ROS)
ROS are reactive molecules derived from molecular O2. The major forms of ROS include free radicals superoxide (O2•−), hydroxyl radicals (OH•) and nonradical hydrogen peroxide (H2O2). ROS are produced from various sources in cells, including NADPH oxidases (NOXs), xanthine oxidase, cytochrome P450 enzymes, endoplasmic reticulum and mitochondria [92,93,94,95]. Among them, the mitochondrion appears to be a major source of ROS under physiological and pathophysiological conditions (see discussion in following sections). ROS in cells are normally maintained at physiological levels, balanced by enzymatic (i.e., superoxide dismutase (SOD), catalase, glutathione peroxidases (GPXs) and peroxiredoxins (PRXs)) and non-enzymatic (i.e., glutathione and vitamins C and E) antioxidant systems. An imbalance between ROS generation and elimination usually cause ROS accumulation, leading to oxidative stress. Oxidative stress contributes to a host of human diseases. As expected, excess ROS appear to be causative factors of uteroplacental dysfunction, contributing to the development of pregnancy complications.
Mitochondria are multifunctional organelles critical to cellular metabolism, Ca2+ homeostasis, redox homeostasis, and cell fate. They are the powerhouses of cells and generate ATP via oxidative phosphorylation. Besides ATP, mitochondria also produce precursors for synthesizing marcomolecules such as DNA/RNA, proteins and lipids [96]. In addition, mitochondria are involved in maintaining cellular Ca2+ homeostasis [97,98]. Moreover, mitochondria regulate cell death via releasing apoptotic factors such as cytochrome c and caspase activation [99]. ROS are produced as the byproducts of oxidative phosphorylation, participating in regulating cellular redox homeostasis [100].
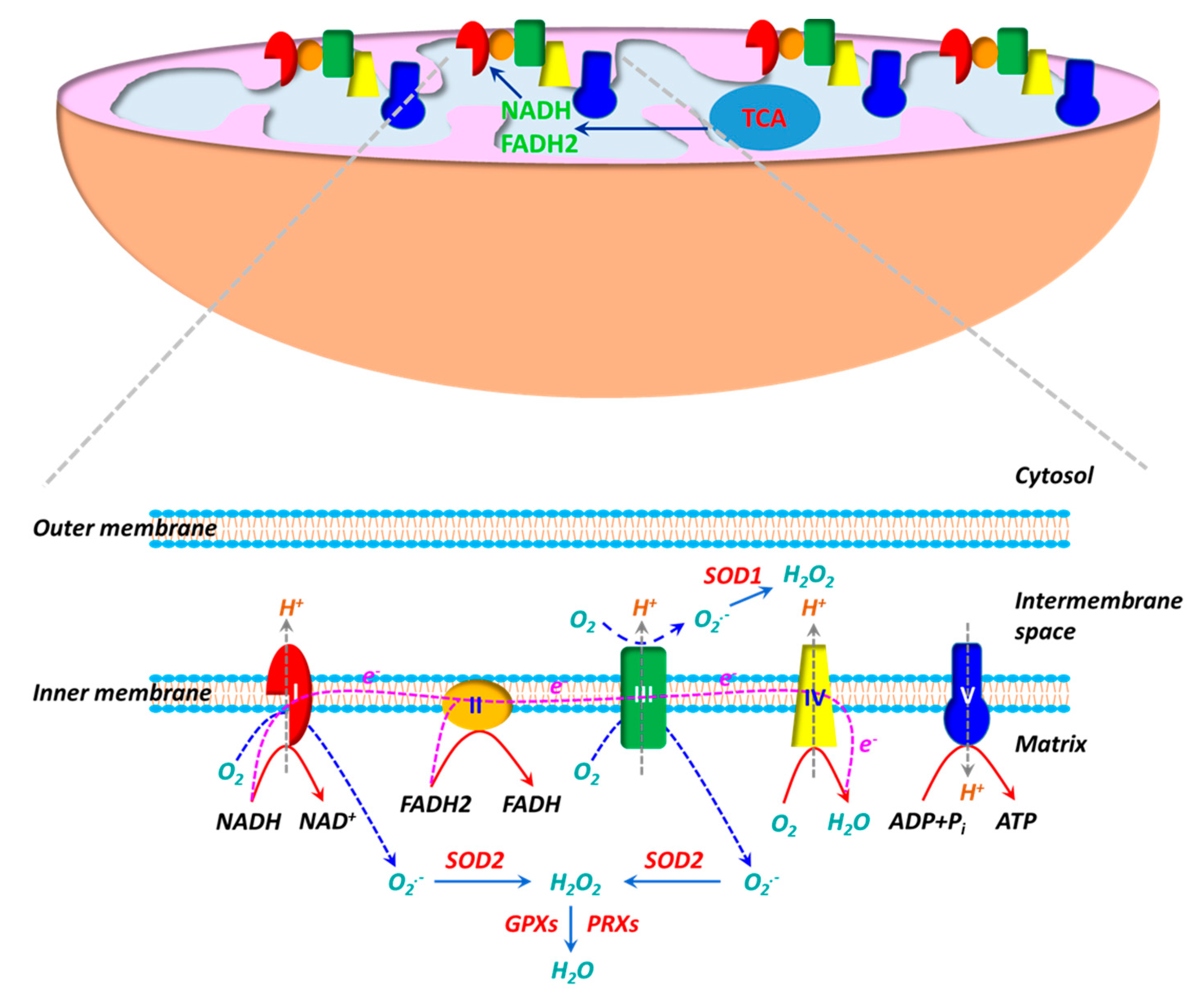
The electron transport chain (ETC), consisting of five multi-subunit protein complexes (Complexes I-V), is located in the inner mitochondrial membrane. Enzymatic reactions in the tricarboxylic acid (TCA) cycle generate reducing equivalents NADH and FADH2, which deposit electrons to the ETC at Complexes I and II, respectively. During the electron flow from NADH or FADH2 to O2 through the ETC, protons (H+) are pumped from the mitochondrial matrix into the intermembrane space by Complexes I, III and IV. O2 acts as an electron acceptor and is reduced to H2O at Complex IV. H+ ions flow down their gradient and back into the matrix through Complex V (ATP synthase) and the energy stored in the H+ gradient is coupled to the synthesis of ATP from ADP and Pi. ROS production is inherent to the oxidative metabolism in mitochondria (Figure 1). Approximate 1–2% of O2 reacts with electrons leaked from Complexes I and III during oxidative phosphorylation, leading to partial reduction of O2 and generation of superoxide (O2•−) [101,102,103]. Due to the high rate of O2 consumption in mitochondria, a significant fraction of O2•− is generated during oxidative phosphorylation. It is believed that mitochondria are the main sources of ROS in cells. O2•− generated in Complex I is exclusively released into mitochondrial matrix, whereas O2•− produced in Complex III is deposited into both mitochondrial matrix and intermembrane space. O2•− is dismutated to hydrogen peroxide (H2O2) by SOD2 (Mn-SOD) in the matrix and SOD1 (Cu, Zn-SOD) in the intermembrane space [102,104]. H2O2 can react with Fe2+/Cu+ via the Fenton reaction to yield hydroxy radical (OH•) [105], whereas O2•− may react with nitro oxide (NO) to produce peroxynitrite (ONOO−)[106]). Both OH• and ONOO− are strong oxidants. Mitochondria have their own antioxidant mechanisms to quench ROS. H2O2 is primarily decomposed into H2O by GPX1/4 and PRX3/5 [104,107].
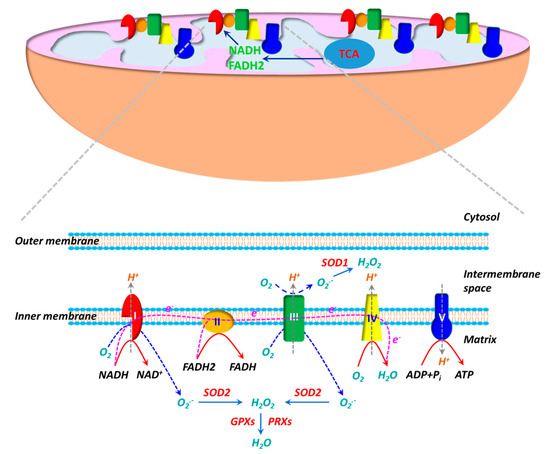
Figure 1.
The mitochondrion is the powerhouse in living cells. ATP, the “energy currency”, is synthesized during oxidative phosphorylation. Oxidative phosphorylation is carried out by a series of multi-heteromeric protein complexes (Complexes I-V) of the electron transport chain (ETC) located in the inner mitochondrial membrane. Electrons from NADH and FADH2 produced in tricarboxylic acid (TCA) cycle flow through Complexes I/II to Complex IV, where O2 serves as the final electron acceptor to generate H2O. During the electron transfer, protons (H+) are pumped out of the mitochondrial matrix into the intermembrane space at Complexes I, III and IV, resulting in a proton gradient and a transmembrane electrical potential. The energy stored in proton gradient is used to synthesize ATP from ADP when protons flow back through Complex V (ATP synthase). The mitochondrion is also an important source of reactive oxygen species (ROS). The electron flow in ETC is not perfect and a fraction of electrons are leaked at Complexes I/III, leading to partial reduction of O2 to generate superoxide (O2•−). O2•− generated at Complex I is released into the matrix, whereas O2•− produced at Complex III is delivered into both the matrix and the intermembrane space. O2•− is then dismutated to H2O2 by superoxide dismutase 1 (SOD1) in the intermembrane space and by SOD2 in the matrix. H2O2 is subsequently decomposed to H2O by glutathione peroxidases (GPXs) and peroxiredoxins (PRXs).
3.2. Hypoxia and Mitochondrial ROS
The other important function of mitochondria is O2 sensing. O2 is used as the final electron acceptor in the ETC during oxidative phosphorylation. Mitochondria consume more than 90% cellular O2 to generate ATP. Mitochondria are thus extremely sensitive to O2 supply and undergo metabolic adaptation when O2 deficiency (i.e., hypoxia) occurs. The ETC is proposed to function as an O2 sensor [108]. The primary site of ROS production during hypoxia appears to be Complex III [109,110]. In response to hypoxia, ROS are released from Complex III, which in turn stabilize HIFα (Figure 2). This notion has been confirmed with genetic knockout and pharmacological intervention [109,111,112]. Additionally, hypoxia also stimulates ROS release from Complex I, which also contributes to HIF stabilization [113,114]. HIFα is then translocated into the nucleus and dimerises with HIF1β subunit and regulates the expression of genes involved in a diversity of biological processes through binding to the hypoxia response element (HRE). Consequently, HIFs may reduce mitochondrial activity by altering the expression of key enzymes in the TCA cycle and subunits in the ETC. This HIF-mediated adaptation to hypoxia is important for cell’s survival.
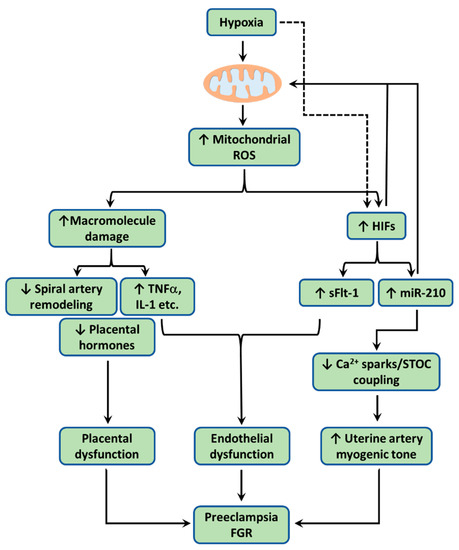
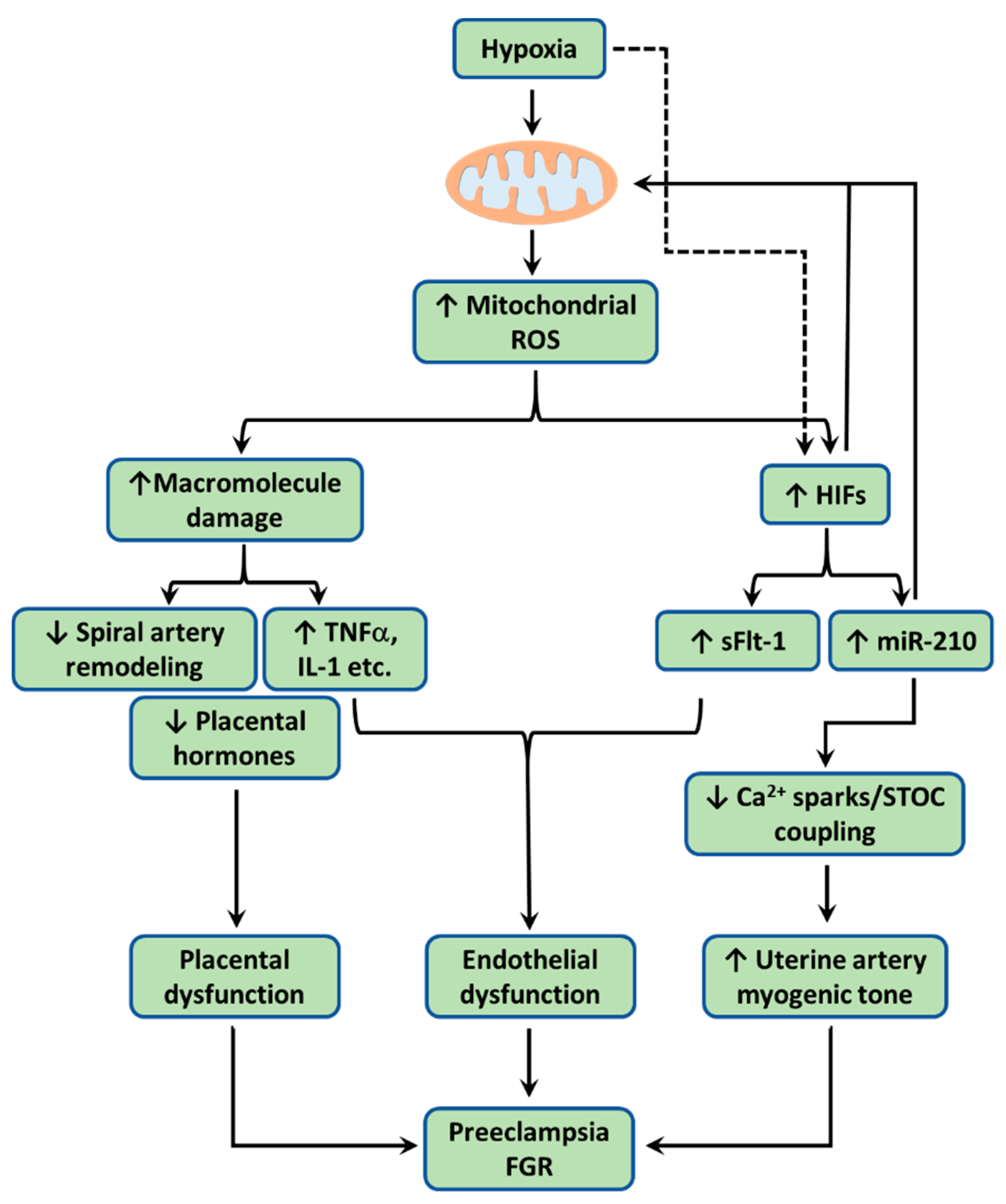
Figure 2.
When the uteroplacental cells encounter hypoxia, mitochondria overproduce reactive oxygen species (ROS). Excess ROS cause lipid peroxidation, protein carbonyl, and DNA damage, resulting mitochondrial dysfunction. Dysfunctional mitochondria engender placental dysfunction by (1) promoting trophoblast apoptosis, reducing trophoblast invasion, and consequently impairing spiral artery remodeling, (2) increasing production/release of pro-inflammatory cytokines including TNFα, IL-1β, and IL-6, and (3) suppressing production/release of placental hormones including estrogen, chorionic gonadotropin (CG), and placental lactogen (PL). Hypoxia also via mitochondrial ROS stabilizes hypoxia inducible factors (HIFs), which induces the expression of microRNA-210 (miR-210) and soluble Fms-like tyrosine kinase-1 (sFlt-1). HIF-1 could also in turn reduces mitochondrial metabolism. Once released into the maternal circulation, both pro-inflammatory cytokines and sFlt-1 cause endothelial dysfunction and/or impaired angiogenesis. Pro-inflammatory cytokines and sFlt-1 also act via autocrine/paracrine signaling to disrupt placental function. HIF-responsive miR-210 acts on mitochondria to downregulate iron-sulfur cluster scaffold (ISCU) in the electron transport chain (ETC) to increase mitochondrial ROS. miR-210 suppresses the Ca2+ spark/spontaneous transient outward current (STOC) coupling in uterine arteries directly or indirectly via miR-210-induced mitochondrial ROS, leading to increased arterial myogenic tone. Together, these changes conferred by hypoxia-induced mitochondrial ROS contribute to the pathogenesis of preeclampsia and FGR.
3.3. Dual Roles of Mitochondrial ROS: Signaling Molecules and Detrimental Effects
Evidently, ROS production is a normal part of cell functions and their level inside the cell is delicately balanced by antioxidants. ROS generated in mitochondria appear to exert their actions in a compartmentalized manner. O2•− in the mitochondrial matrix is largely retained to impact mitochondrial function due to its negative charge. However, O2•− in the intermembrane space may be transported to the cytosol via voltage-dependent anion channels [115]. On the other hand, nonpolar H2O2 is able to diffuse from mitochondria into the cytosol to alter other cellular processes. ROS can be either beneficial at low levels or potentially toxic if unchecked. ROS at low levels are signaling molecules to regulate a variety of cellular processes to sustain cellular proliferation, differentiation, angiogenesis, inflammation, and activate stress-responsive survival pathways [116,117,118,119,120]. ROS signaling is predominantly conferred by H2O2 that exerts the regulatory role through oxidative modification of cysteine residues on target proteins such as enzymes and transcription factors [117]. The oxidation of the cysteine thiol (Cys-SH) to the sulfenic form (Cys-SOH) results in allosteric changes in the protein leading to alternation of its function. In addition, ROS also possess antimicrobial action and participate in regulating autophagy [118,121,122].
However, ROS are highly reactive and their overproduction can cause oxidative damage to macromolecules such as lipids, proteins and nucleic acids and even elicits cell apoptotic cell death. Lipid peroxidation often occurs as the oxidative damage of polyunsaturated fatty acids due to the attack of carbon-carbon double bond(s) by ROS, leading to impairment of membrane function [123]. Malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) are two main lipid peroxidation products. Protein oxidation often leads to irreversibly forming a reactive carbonyl moiety in a protein, such as an aldehyde and ketone. Protein carbonylation causes protein misfolding and/or altering protein conformation, leading to protein inactivation [124]. ROS also cause DNA damage by causing base lesions and double strand breaks, which are potentially mutagenic [125]. 8-Hydroxydeoxyguanosine (8-oxodG) is one of the common products of ROS-induced DNA damage [126]. Overall, these ROS modifications would disrupt membrane integrity, alter chromatin structure/gene expression, and inactivate protein function, leading to cellular dysfunction and even cell death. For example, excess O2•− has been shown to inactivate mitochondrial aconitase by disrupting the iron–sulfur cluster [127], whereas H2O2 could induce mitochondrial lipid peroxidation [128]. Mitochondria DNAs are susceptible to oxidative damage and the resultant mutations in turn alter ETC activity and escalate ROS production [129]. Therefore, mitochondrial structure and function could be impaired by ROS overproduction in mitochondria.
4. Mitochondrial ROS and Preeclampsia/FGR
4.1. Mitochondrial ROS Production in Preeclamptic/FGR Uteroplacental Tissues
Normal pregnancy is characterized by mild oxidative stress, which is exaggerated in preeclampsia and FGR [130]. Numerous studies reveal that uteroplacental ROS production is increased, inferenced directly from the measurement of O2•−/H2O2 or indirectly from the measurement of markers of oxidative stress (i.e., MDA, 4-hydroxynonenal, 8-isoprostane) [90,131,132,133,134,135,136,137,138,139,140,141]. On the other hand, the enzymatic antioxidant system in uteroplacental tissues/cells appears to be impaired in preeclampsia and FGR. The expression and/or activity of SODs, GPXs, thioredoxin reductases (TRXRs), and catalase are reduced in preeclamptic and FGR placentas and trophoblasts [132,133,134,135,136,142,143,144,145,146,147]. Evidently, these changes engender overwhelming ROS accumulation in uteroplacental cells, leading to heightened oxidative stress. Abundance of SOD1 and catalase in preeclamptic myometrium is similarly decreased [148].
The association of mitochondrial dysfunction with preeclampsia is first reported in 1989 [149]. In this study, a high incidence of pre-eclampsia in a family with mitochondrial dysfunction is observed. Since then, emerging evidence has implicated mitochondrial dysfunction in the etiology of pregnancy complications [150,151]. Placental oxidative phosphorylation capacity is reduced in preeclampsia [152]. Comparative proteomic analysis reveal that various proteins involved in oxidative phosphorylation/ETC are altered in preeclamptic placentas [153,154]. Various ETC complexes as well as their subunits such as Complexes I-IV, cytochrome c oxidase (COX), are found to be downregulated in both preeclamptic and FGR placentas [155,156,157,158]. Accordingly, activity of one or more of ETC complexes in the placenta is suppressed in preeclampsia and FGR [157,158,159,160,161]. However, the work from Cetin’s laboratory demonstrates that cytotrophoblast ETC activity is increased in placentas of FGR [162]. Animal models of preeclampsia in rats and FGR in pigs also show downregulation of placental ETC components and activities [163,164,165]. The reduced expression and activities of placental ETC complexes are associated with increased mitochondrial H2O2 production [163]. Placental insufficiency is common feature shared by preeclampsia and preterm birth [166]. Preeclampsia is also a major contributor to preterm birth [167]. Placenta from preterm birth displays reduced mitochondrial calcium uptake 1 (MICU1) [168], a component of mitochondrial calcium uniporter (MCU) that participates in regulating mitochondrial Ca2+ homeostasis. MICU1 functions as a gatekeeper to prevent mitochondrial Ca2+ overload and its deficiency leads to Ca2+ overload and subsequent ROS overproduction and apoptosis [169].
Detection of oxidative modifications of intracellular molecules is frequently used as a marker of ROS formation. The increase in placental mitochondrial ROS in preeclampsia and FGR is often inferred from the observations of escalated lipid peroxidation in mitochondria in early studies. For example, Wang and Walsh observed that levels of MDA are elevated in mitochondria isolated from preeclamptic placentas [131]. Shibata and colleagues detected accumulation of 4-HNE-modified proteins in both cytosol and mitochondria with a dominance in mitochondria [170]. The development of fluorescent probes has enabled monitoring ROS generation in cells and even in mitochondria. For instance, the increase in mitochondrial O2•− in the preeclamptic placenta is confirmed using MitoSOX Red [171]. A similar increase in mitochondrial H2O2 is also detected using Amplex red in the placentas of reduced uterine perfusion pressure (RUPP) rat model of preeclampsia [163]. Intriguingly, Holland et al. reported that H2O2 (detected with Amplex Ultra Red) production and antioxidant activity were increased in term preeclamptic placentas but decreased in pre-term preeclamptic placentas [141]. 2′,7′-Dichlorodihydrofluorescein diacetate (H2DCFDA) is commonly used for measuring cellular H2O2 [172]. Increased ROS in preeclamptic placentas are detected with H2DCFDA [157]. Using this probe in the absence or presence of the mitochondrial targeted antioxidant mitoQ, it is determined that the increased cellular H2O2 is predominantly from mitochondria in both labyrinth and junctional zones of the placenta from a rat model of gestational hypoxia [173]. The increase in mitochondrial O2•− in primary trophoblasts in response to chronic hypoxia is also detected using MitoSOX Red [171].
4.2. Uteroplacental Tissues Exhibit Oxidative Stress due to Hypoxia-Induced Mitochondrial Dysfunction in Preeclampsia/FGR
Hypoxia is a common feature of preeclampsia and FGR [17,174]. Placental hypoxia is long believed to induce oxidative stress by stimulating ROS generation in mitochondria and other compartments of uteroplacental cells, leading to placental dysfunction and subsequent development of preeclampsia and FGR [17,18,174]. The placenta is a metabolically active organ and has a high nutrient and energy demand. Oxidative phosphorylation in the placenta increases progressively throughout normal pregnancy [175]. Unsurprisingly, the placenta at term consumed ~60–70% glucose and ~50% of O2 supplied by the uteroplacental circulation [176,177]. ATP generated by mitochondria fuels placental functions, including placental growth, protein and hormone synthesis and active transport, which are essential for placental/fetal growth and development. The placental function is disrupted by hypoxia [178]. The high-rate consumption of O2 makes mitochondria to be the major source of ROS in placental cells.
The link between hypoxia and oxidative stress in uteroplacental tissues/cells has been established based on both in vitro and in vivo studies. Human placentas from high-altitude pregnancy exhibit decreased activities of SODs, GPXs, and TXRs along with increased levels of lipid peroxidation, protein carbonyl, and nitrotyrosine [179]. In first trimester placental explants, the expression of SOD1 and SOD2 is reduced by hypoxia [180]. Uterine arteries from pregnant sheep at high altitude also have elevated ROS [90]. In a rat model of hypoxic pregnancy, an increase in 4-HNE is observed in the placenta [181]. Gestational hypoxia also increases peroxynitrite formation in placentas of guineapigs [182] and catechol-O-methyltransferase-deficient (COMT−/−) in mice [183]. Exposure of BeWo trophoblastic cells to hypoxia elevates intracellular ROS levels and mitoQ largely prevents hypoxia-induced ROS increase [184]. This finding is corroborated by Aljunaidy and colleagues who demonstrate that gestational hypoxia-induced increase in placental ROS in rat is blocked by mitoQ [173]. These observations suggest a predominant mitochondrial contribution to the accumulation of cytosolic ROS. Unsurprisingly, mitochondrial-derived superoxide production in human primary trophoblasts is increased after hypoxia exposure [171]. Similarly, in vitro hypoxia/reoxygenation also increases mitochondrial H2O2 production in healthy term placenta [141].
Mitochondrial proteins and enzyme activities are often used as markers of mitochondrial content. Hypoxia is also found to impact mitochondrial content in the uteroplacental unit. Compared to sea-level pregnancy, high-altitude pregnancy reduces Complex I level in human placenta [178]. Mitochondrial content is reduced in both placental explants and cultured BeWo cells following hypoxia exposure [184]. Activities of Complexes I and/or IV of the ETC are reduced in the hypoxic guinea pig placentas [182] and in hypoxic rat placentas [185]. Correspondingly, Complex I- and Complex-IV-mediated respiration is repressed by hypoxia in cultured trophoblast-like JEG3 cells [178]. The HIF-responsive miR-210 appears to be a major mediator of hypoxia-induced changes in the ETC. The expression of uteroplacental miR-210 is upregulated in preeclampsia, FGR and high-altitude pregnancy [55,178,186,187,188,189]. MiR-210 transfection suppresses the expression of iron-sulfur cluster scaffold (ISCU), succinate dehydrogenase (SDHD), NDUFA4, and cytochrome c oxidase assembly protein (COX10) in trophoblasts [178,190]. Any alternation of ETC complexes could potentially influence ETC flow and mitochondria-mediated ROS production. Trophoblasts transfected with miR-210 have reduced mitochondrial respiration [157,190]. MiR-210-induced downregulation of ISCU disrupts the electron flow within Complex I and reduces its activity, leading to increased ROS production [191,192].
4.3. Mitochondrial ROS Overproduction and Uteroplacental Dysfunction in Preeclampsia/FGR
4.3.1. Mitochondrial ROS Leads to Mitochondrial Dysfunction
The excess ROS in preeclamptic/FGR placentas cause damage to macromolecules as evidenced by: (1) increased indices of lipid peroxidation such as MDA and 4-HNE [131,135,143,193,194], (2) elevated levels of protein carbonyl [143,195], and (3) raised levels of DNA damage markers such as 8-OHdG and γH2AX and increased DNA fragmentation [196,197,198,199] (Figure 2). The DNA damage could be imitated by exposing decidual stromal cells to H2O2 or hypoxia/reperfusion [199] and in animal models of preeclampsia and FGR [164,200,201]. Mitochondria are susceptible to damage by ROS, resulting in altered structure and function. Mitochondrial dysfunction promotes oxidative stress and the overproduction of ROS in turn could cause further mitochondrial damage, form a vicious cycle [202,203]. Lipid peroxidation appears to primarily occur in mitochondria of preeclamptic placentas [170]. Mitochondrial swelling, a hallmark of mitochondrial dysfunction, is frequently detected in preeclamptic/FGR placentas/trophoblasts [147,153,154,157]. Similar findings are also observed in animal models of preeclampsia and FGR [147,164]. Swollen mitochondria are usually associated with irregular cristae (i.e., folds in the inner membrane of a mitochondrion) [154,157]. The complexes of ETC are embedded in the inner membranes of mitochondria. The disruption of cristae would perturb the structure/function of the ETC. Indeed, mitochondria overproduce ROS once they become swollen [204]. There are also increased mutations in the trophoblast mitochondrial genome observed in African American women with preeclampsia [205], probably due to ROS-induced DNA damage. Vishnyakova and colleagues detected both increased mitochondrial DNA copy number and mitochondrial Complex I activity in preeclamptic placentas [206]. The authors speculate that the high respiration rate in Complex I may contributes to the increased mitochondrial ROS production in preeclamptic placentas. Intriguingly, mitochondrial DNA copy number could be regulated by mitochondrial ROS. H2O2 exposure increases yeast mitochondrial DNA copy number [207]. Moreover, mitochondrial DNA copy number is positively associated with increased oxidative stress in placental tissues from gestational diabetes [208].
4.3.2. Mitochondrial ROS Impair Trophoblast Invasion/Spiral Artery Remodeling
As aforementioned, the failure of trophoblast invasion/spiral artery remodeling results in placental dysfunction and is the major contributor to the development of preeclampsia and FGR [33,209]. Mounting evidence from the human and animal models of preeclampsia and FGR implicates hypoxia in the impaired trophoblast invasion and defective spiral artery remodeling [57,61,67,68,210,211] (Figure 2). The inhibition of invasion by hypoxia has been imitated in vitro using primary trophoblasts and trophoblast cell lines [212,213]. In the cultured human extravillous trophoblast cell line HTR-8/SVneo, hypoxic treatment increased mitochondrial ROS and decreased invasive ability [214]. Importantly, H2O2 exposure decreased the invasion of human trophoblast cell-lines HTR-8/SVneo and TCL-1 [215,216]. Trophoblast apoptosis is commonly observed in preeclampsia and FGR [217,218,219]. The invasion of trophoblast is greatly impacted by trophoblast apoptosis [220]. Increased trophoblast apoptosis could limit trophoblast invasion. Hypoxia enhances trophoblastic apoptosis in cultured villous tissues and primary trophoblasts [221,222]. Hypoxia/reoxygenation-induced mitochondrial cytochrome c release and apoptosis in trophoblasts of the cultured villous tissue could be diminished by the free radical scavenger, desferrioxamine [223]. Similarly, hypoxia also stimulates H2O2 production in HTR-8/SVneo cells and promotes apoptosis [224]. The hypoxia-induced apoptosis is reduced by catalase. These observations suggest a potential role of ROS in trophoblast apoptosis induced by hypoxia. This notion is corroborated by the observations that H2O2 directly induces apoptosis or reduces viability in trophoblast cell lines [216,225,226]. Moreover, mitochondrial ROS stimulated by rotenone is found to promote trophoblast apoptosis [227]. Mitochondria-derived ROS stabilizes HIF-1α. HIF-1α appears to be the principal mediator of comprised spiral artery remodeling as discussed in previous sections. Specific overexpression of HIF-1α in trophoblasts disrupts trophoblast invasion and results in failure to remodel spiral arteries [68]. On the other hand, knockdown of HIF-1α reduces hypoxia-induced apoptosis of HTR8/SVneo cells [65]. As discussed previously, HIF-1-responsive miR-210 in the placenta is elevated in preeclampsia, FGR and high-altitude pregnancy and inhibits both trophoblast invasion and uterine vascular adaptation [55,178,186,187,188,189,213]. MiR-210 targets and downregulates ISCU, NDUFA4, and SDHD of the ETC, leading to reduced mitochondrial respiration and increased ROS production [188,190,191,192] (Figure 2). As expected, miR-210-induced mitochondrial dysfunction contributes to the impaired trophoblast invasion [190].
4.3.3. Mitochondrial ROS Impair Placental Hormone Production/Secretion
The placenta secrets a variety of hormones to regulate placental growth and transport as well as maternal physiology, which are essential for fetal growth/development [228,229,230]. Accumulating evidence reveals that the production/secretion of various placental hormones is impaired by hypoxia via ROS (Figure 2). Estrogen is primarily synthesized and secreted by the placenta during pregnancy and is essential for the adaptive changes of maternal cardiovascular function during pregnancy including hemodynamic adaptation of the uteroplacental circulation, placental cell differentiation, proliferation and angiogenesis [231,232]. Compared to normal pregnancy, circulating estrogen level is reduced in preeclamptic and FGR pregnancy [233,234,235]. High altitude pregnancy also has less circulating estrogen than sea-level pregnancy [236,237]. As expected, estrogen production in preeclamptic placental explants is suppressed compared to normotensive term counterparts [238]. Intriguingly, the reduction of estrogen synthesis in preeclamptic placentas is replicated by treating first trimester placental explants with H2O2 [238]. The reduced estrogen production is probably due to H2O2-mediated inhibition of aromatase activity [239]. Human chorionic gonadotropin (hCG) produced by the trophoblasts participates in regulating placental development, trophoblast invasion, and angiogenesis/vasculogenesis [240]. Altered hCG is a marker of preeclampsia and FGR [241,242]. Low hCG in the late first trimester is associated with FGR [242]. Hypoxia downregulates hCG expression/production in JEG-3, BeWo, and JAr cells [243]. The reduction of hCG by hypoxia could be simulated by exposing JEG-3 cells to H2O2 [244], suggesting that ROS probably mediate the hypoxia-induced downregulation of hCG in the placenta. Human placental lactogen (hPL) is also produced by trophoblasts and promotes placental/fetal growth primarily through insulin-like growth factors (IGFs) [245,246]. Deletion of the P0 transcript of IGF2 gene results in reduced placental growth and FGR [247]. Interestingly, increasing mitochondrial ROS by inhibiting Complex I activity with rotenone reduced hPL and IGF2 expression [248], implicating a pivotal role of mitochondrial ROS in the etiology of FGR.
4.3.4. Mitochondrial ROS Promote Placental Release of sFlt-1
The trophoblastic production and release of the antiangiogenic factor sFlt-1 are increased in preeclampsia and FGR [71,201,249,250]. The release of sFlt-1 into the maternal circulation is responsible for inducing proteinuria and hypertension by causing endothelial dysfunction [70,251,252,253]. Apparently, sFlt-1 release is mainly induced by hypoxia as sFlt-1 levels in placentas from high-altitude pregnancy are higher than placentas from sea-level pregnancy [69]. This is confirmed in a rat model of chronic hypoxia [254] and in vitro studies using placenta explants and trophoblasts [69,201,255,256,257]. Concurrently, exposure to hypoxia also increases oxidative stress in placental tissues and trophoblasts [255,256,257] (Figure 2). Significantly, H2O2 promotes sFlt-1expression in placental villous explants and BeWo cells [201]. Furthermore, the concomitant increases in mitochondrial ROS and sFlt-1 production are blocked by the mitochondria-targeted hydrogen sulfide donor AP39, thus establishing mitochondrial ROS as the link between hypoxia and sFlt-1 release [171]. Intriguingly, sFlt-1 also exerts its autocrine/paracrine effects on the placenta. The administration of sFlt-1 into pregnant mice increased oxidative stress in placentas, causing mitochondrial swelling in trophoblasts and apoptosis [258,259]. Similarly, induction of human sFlt-1 in pregnant mice impairs placental vascularization and nutrient exchange function, leading to FGR [260].
4.3.5. Mitochondrial ROS Promote Placental Release of Cytokines
Trophoblasts and immune cells in the placenta produce cytokines. Preeclampsia/FGR is commonly associated with increased expression/production of pro-inflammatory cytokines such as TNF-α, IL-1α/β, IL-6 and IL-8 and decreased expression/production of anti-inflammatory cytokine IL-10 in the placenta [261,262,263,264,265,266,267]. The altered expression/production of placental cytokines is believed to be the consequence of hypoxia [268]. Indeed, hypoxia or hypoxia/reoxygenation increases the expression/production of TNF-α, IL-1α, IL-1α, IL-6, and IL-8 in cultured placental explants and BeWo cells [184,269,270] (Figure 2). Expectedly, H2O2 stimulates TNF-α and IL-1β production and reduces IL-10 generation in placental explants [271]. In addition, hypoxia-induced increase in pro-inflammatory cytokine expression is associated with elevated mitochondrial ROS in BeWo cells [184]. The inhibition of mitochondrial ROS by diphenyleneiodonium diminishes lipopolysaccharide-induced cytokine production in human immune cells harboring TNF receptor 1 mutations [272]. In a way similar to sFlT-1, pro-inflammatory cytokines cause endothelial dysfunction once released into the maternal circulation from the placenta [273,274]. Locally, TNF-α inhibits trophoblast fusion and hCG-β expression [275]. An in vitro study reveals that TNF-α and IL-6 also increase trophoblast apoptosis, resulting in increased trophoblasts shed from the placenta [276].
4.3.6. Mitochondrial ROS Impair Uterine Vascular Adaptation
Pregnancy is associated with dramatically reduced uterine vascular resistance and increased uterine blood flow in both human and experimental animals [277,278,279,280,281,282,283]. In addition to structural remodeling of uterine arteries, functional changes in the uterine arteries also contributes to the uterine vascular adaptation [14,232,284]. However, gestational hypoxia thwarts the adaptation of the uteroplacental circulation. Uterine blood flow is reduced and uterine vascular resistance is increased in preeclamptic, FGR, and high-altitude pregnancy and in animal models of chronic hypoxia [28,31,32,55,60,62,285,286,287]. Mechanistically, the maladaptation of the uteroplacental circulation is in part due to the impaired Ca2+ spark/STOC coupling conferred by hypoxia-induced upregulation of miR-210 and ROS overproduction in uterine arteries [55,89,90,91,288,289] (Figure 2). Given the concomitant increase of both miR-210 and oxidative stress in uterine arteries in response to gestational hypoxia and the role of miR-210 in promoting mitochondrial ROS, it is expected that mitochondrial ROS in uterine arteries probably contributes to the dysfunction of uterine arteries in pregnancy complications associated with hypoxia, which merits further investigation.
5. Potential Interventions to Prevent/Manage Preeclampsia/FGR
As aforementioned, oxidative stress is a leading contributor to the pathogenesis of preeclampsia/FGR. It is expected that restoring redox homeostasis with the therapeutic approach of antioxidants would be effective for the treatment of both complications. Despite of success in preclinical studies, the antioxidant therapy for preeclampsia/FGR has proved largely unsuccessful in clinical trials. For example, the supplement with vitamins C and E does not reduce the incidence of both disorders and sometimes even results in deleterious outcomes [290,291,292]. Many factors could contribute to the failure of antioxidant therapy for the treatment of preeclampsia/FGR in clinical trials. Among them are poor bioavailability of antioxidants in tissues/cells undergoing oxidative stress and sites of ROS production in the cells and indiscriminate abolishment of ROS including those are essential for physiological signaling. These findings require to develop optimal antioxidant therapies “selectively inhibiting only the disease-relevant ROS sources and leaving all others intact” [293]. Placental mitochondria-targeted antioxidants may alleviate oxidative stress without suppressing ROS from non-mitochondrial sources that are indispensable to physiological signaling and ROS-mediated cellular functions in other maternal and fetal tissues.
5.1. Interventions Selectively Target Placental Cells
Preeclampsia and FGR are most common pregnancy complications with high morbidity and mortality for both the mother and fetus. Although our understanding of the pathophysiology of both disorders has advanced significantly, there are still no effective cures available. The only applicable treatment for both preeclampsia and FGR is either delaying labor or inducing labor. Therefore, there is a great need to develop effective therapy for preeclampsia and FGR. Given that both preeclampsia and FGR are originated from the uteroplacental dysfunction, it is anticipated that improving uteroplacental function is a promising therapeutic approach. To this end, it is important to specifically deliver drugs to the placenta to minimize both side effects in other maternal tissues and fetal exposure. Recent studies reveal that targeting ligands can be selectively delivered to the placenta in mice by conjugating with various peptides which bind to surface receptors in placental cells. Tumor-homing peptide sequences CGKRK and iRGD are found to bind to human and mouse placental tissues [294]. These peptides facilitate targeted delivery of peptide-decorated liposomes containing IGF2 to the mouse placenta, improving fetal weight in the P0 mouse model of FGR [294]. Moreover, the synthetic peptide CNKGLRNK is found to selectively bind to the endothelium of the uterine spiral arteries and placental labyrinth in mice and administration of CNKGLRNK-decorated liposomes containing nitric oxide donor 2-[[4-[(nitrooxy)methyl]benzoyl]thio]-benzoic acid methyl ester (SE175) reduces placental 4-HNE, increases mean spiral artery diameter and fetal weight [295]. Similarly, an alternative placental-specific drug delivery system is developed by Zhang and colleagues using synthetic placental chondroitin sulfate A-binding peptide (CSA-BP) based on the finding that Plasmodium falciparum-infected erythrocytes bind to CSA on the surfaces of trophoblasts [296]. Following intravenous administration, CSA-BP-conjugated nanoparticles accumulate in the mouse placenta without detection in fetal tissues [296]. Encouragingly, placental CSA-BP conjugated to lipid-polymer nanoparticles containing sFlt-1 siRNAs effectively reduces placental and serum sFlt-1 levels in mice [297].
5.2. Interventions Selecetively Target Mitochondrial ROS
As discussed in previous sections, studies over last decades have implicated that hypoxia-induced mitochondrial ROS production is a major contributor to placental dysfunction and subsequent development of preeclampsia and FGR. Thus, relieving mitochondria-associated oxidative stress to restore placental function presents a promising strategy for treating these disorders. This therapeutic potential is auspiciously supported by findings from numerous in vitro and in vivo studies as well as animal models. A number of antioxidants have been found to effectively improve mitochondrial ROS-induced placental dysfunction. In this section, we focus the discussion on potential antioxidant therapies of selenium, melatonin, and mitochondria-targeted MitoQ in preeclampsia and FGR.
5.2.1. Selenium
Selenium, an essential trace element, participates in regulating cell functions in the form of selenocysteine which is incorporated into in selenoproteins including antioxidant enzymes GPXs and TXRs, two major antioxidant enzymes in mitochondria. Disrupting selenocysteine incorporation into selenoproteins by knock-downing selenocysteine insertion binding protein 2 (SECISBP2) in trophoblast cell lines JAR and JEG-3 cells leads to elevated oxidative stress, impaired trophoblast invasion, and reduced the expression of hCGβ [298]. Selenium deficiency is associated with preeclampsia and FGR [299]. In rat models, selenium deprivation reproduces preeclampsia-like symptoms and FGR, accompanied with reduced activities of placental GPXs and TXRs and increased lipid peroxides/protein carbonyls in the placenta [300,301]. In BeWo, JEG-3 and Swan-71, selenium supplement increases the expression/activities of GPXs and TXRs and effectively diminishes rotenone- and antimycin-induced mitochondrial oxidative stress [227,302]. In small-scale clinical trials, selenium supplement is found to decrease the incidence of preeclampsia [303,304] and decreases the circulating level of sFlt-1 [305]. A recent large-scale trial conducted in Norway demonstrates that higher maternal dietary selenium intake is significantly associated with a lower risk of FGR [306].
5.2.2. Melatonin
It is well-known that melatonin secreted by the pineal gland participates in regulating circadian rhythm. Interestingly, melatonin possesses antioxidant property by directly scavenging ROS and indirectly promote the expression/activity of antioxidant enzymes [307,308]. Melatonin is also synthesized and its receptors are expressed in human placentas [309,310,311], implicating its autocrine/paracrine effects in regulating placental function. The expression of aralkylamine N-acetyltransferase, a key enzyme in melatonin synthesis, and melatonin receptors 1 and 2 in the placenta is reduced in preeclampsia and FGR [312,313]. Melatonin is believed to enter mitochondria through peptide transporters PEPT1 and PEPT2 [314], which enables melatonin to function as an antioxidant to counter overproduction of mitochondrial ROS-induced placental dysfunction in preeclampsia and FGR. Melatonin prevented hypoxia/reoxygenation-induced ROS, apoptosis, autophagy, and inflammation in cultured human primary villous trophoblasts [315,316,317]. Melatonin also increases antioxidant defense by upregulating the expression of glutamate cysteine ligase (the first and rate-limiting enzymes in the production of the cellular antioxidant glutathione (GSH)), NADPH: quinone acceptor oxidoreductase 1 and thioredoxin in placental tissues [318]. Moreover, melatonin suppresses sFlt-1 secretion in primary trophoblasts [318]. In the RUPP rat model, melatonin lowers mean arterial pressure and placental expression of sFlt-1 [319]. Both human and animal studies prove beneficial effects of melatonin in treating preeclampsia and FGR. Maternal administration of melatonin improves ischemia/reperfusion-induced oxidative DNA and impaired mitochondrial respiration in the placenta, leading to improved fetal growth [200,320]. Promisingly, melatonin treatment significantly prolonged the interval from diagnosis to delivery in women with preeclampsia [321]. However, antenatal treatment with melatonin during the last one-third of gestation is found to decrease birth weight in high-altitude pregnant sheep [322], suggesting existence of species-dependent melatonin effects.
5.2.3. mitoQ
A number of mitochondria-target antioxidants have been developed by conjugating the lipophilic triphenylphosphonium (TPP+) cation to antioxidant moieties [323,324]. Due to their positive charges, these compounds can easily pass biological membranes and accumulate several-hundred-fold within mitochondria. Therefore, they are able to protect against mitochondrial oxidative damage. Among them, mitoQ, consisting of a ubiquinone moiety attached to TPP+, has shown promise in animal models of cardiovascular diseases and in a clinical trial [325,326,327]. The potential therapeutics for preeclampsia and FGR with mitoQ has also been intensively investigated. Following administration, mitoQ is found to accumulate in rat and sheep placentas [328,329]. In a rat model of prenatal hypoxia, treatment with mitoQ prevents hypoxia-induced decrease in mitochondrial activity and increase in placental ROS [173,185,328,330,331]. In both rat and sheep, impaired fetal growth is improved by mitoQ in hypoxic pregnancy [328,329]. Similarly, mitoQ also improves ETC activities, reduces mean arterial pressure, and mends fetal growth in a rat RUPP model [163]. Interestingly, in a mouse RUPP model, mitoQ administration during late pregnancy (GD 13.5–17.5) reduces placental oxidative stress, lowers blood pressure, and improves fetal birth weight, whereas mitoQ application during early pregnancy (GD 7.5–11.5) exacerbates RUPP-induced preeclampsia-like symptoms [147]. Therefore, timing is an important factor to be considered when administrating antioxidants during pregnancy as mitochondrial ROS could participate in regulating placental development in early pregnancy [147,332]. In a way similar to mitoQ, AP39, consisting of a H2S-donating moiety (dithiolethione) coupled to TPP+, also prevents ROS production, lowers HIF-1α, and decreases sFlt-1 production in cultured human primary trophoblast culture [171].
6. Conclusions
Placental homeostasis is essential for the well-beings of the mother and normal growth of the fetus. Mitochondria play a central role in maintaining placental homeostasis. Mitochondrial function is sensitive to changes in O2. Although a low O2 environment is indispensable for embryonic development and placentation, prolonged hypoxia is detrimental to the placenta as it promotes mitochondrial dysfunction and ROS overproduction. Numerous studies demonstrate that excessive ROS cause damages to lipids, proteins and DNAs, resulting in impaired trophoblast invasion/spiral artery remodeling, reduced hormone production/secretion, and increased release of bioactive factors into the maternal circulation. Together, these changes contribute to placental dysfunction and subsequent development of preeclampsia and FGR. Ultimately, targeting mitochondrial ROS may offer avenues for the development of therapeutics. Indeed, promising preclinical results have been obtained in animal models with mitochondria-targeted antioxidants. However, more efforts will be needed for the successful translation of these scientific findings into clinical applications for preeclampsia and FGR. It should also bear in mind that ROS possess both beneficial and detrimental roles in human body. It will be another great challenge to preserve physiological signaling of ROS while alleviating pathological ROS damage.
Author Contributions
Conceptualization and writing, X.-Q.H. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by National Institutes of Health Grants HD083132 (L.Z.), HL128209 (L.Z.), HL137649 (L.Z.) and HL149608 (L.Z.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huppertz, B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 2008, 51, 970–975. [Google Scholar] [CrossRef]
- Devaskar, S.U.; Chu, A. Intrauterine Growth Restriction: Hungry for an Answer. Physiology (Bethesda) 2016, 31, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine; Martins, J.G.; Biggio, J.R.; Abuhamad, A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and management of fetal growth restriction: (Replaces Clinical Guideline Number 3, April 2012). Am. J. Obstet. Gynecol. 2020, 223, B2–B17. [Google Scholar] [CrossRef]
- Mateus, J.; Newman, R.B.; Zhang, C.; Pugh, S.J.; Grewal, J.; Kim, S.; Grobman, W.A.; Owen, J.; Sciscione, A.C.; Wapner, R.J.; et al. Fetal growth patterns in pregnancy-associated hypertensive disorders: NICHD Fetal Growth Studies. Am. J. Obstet. Gynecol. 2019, 221, 635.e1–635.e6. [Google Scholar] [CrossRef] [PubMed]
- Hutcheon, J.A.; Lisonkova, S.; Joseph, K.S. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 391–403. [Google Scholar] [CrossRef]
- Unterscheider, J.; O’Donoghue, K.; Daly, S.; Geary, M.P.; Kennelly, M.M.; McAuliffe, F.M.; Hunter, A.; Morrison, J.J.; Burke, G.; Dicker, P.; et al. Fetal growth restriction and the risk of perinatal mortality-case studies from the multicentre PORTO study. BMC Pregnancy Childbirth 2014, 14, 63. [Google Scholar] [CrossRef]
- von Beckerath, A.K.; Kollmann, M.; Rotky-Fast, C.; Karpf, E.; Lang, U.; Klaritsch, P. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am. J. Obstet. Gynecol. 2013, 208, 130.e1–136.e6. [Google Scholar] [CrossRef] [PubMed]
- Tooher, J.; Thornton, C.; Makris, A.; Ogle, R.; Korda, A.; Hennessy, A. All Hypertensive Disorders of Pregnancy Increase the Risk of Future Cardiovascular Disease. Hypertension 2017, 70, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Castro, C.; Rascher, W.; Hartner, A. Intrauterine growth restriction-impact on cardiovascular diseases later in life. Mol. Cell. Pediatr. 2018, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J. Oxygen, the Janus gas; its effects on human placental development and function. J. Anat. 2009, 215, 27–35. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef]
- Ducsay, C.A.; Goyal, R.; Pearce, W.J.; Wilson, S.; Hu, X.Q.; Zhang, L. Gestational Hypoxia and Developmental Plasticity. Physiol. Rev. 2018, 98, 1241–1334. [Google Scholar] [CrossRef]
- Tong, W.; Giussani, D.A. Preeclampsia link to gestational hypoxia. J. Dev. Orig. Health Dis. 2019, 10, 322–333. [Google Scholar] [CrossRef]
- Aljunaidy, M.M.; Morton, J.S.; Cooke, C.M.; Davidge, S.T. Prenatal hypoxia and placental oxidative stress: Linkages to developmental origins of cardiovascular disease. Am. J. Physiol. Regul. Integr. Comp. Physiol 2017, 313, R395–R399. [Google Scholar] [CrossRef]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.L. Oxidative stress in placental pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Silvestro, S.; Calcaterra, V.; Pelizzo, G.; Bramanti, P.; Mazzon, E. Prenatal Hypoxia and Placental Oxidative Stress: Insights from Animal Models to Clinical Evidences. Antioxidants 2020, 9, 414. [Google Scholar] [CrossRef]
- D’Autreaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Briones, A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. 2011, 34, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef] [PubMed]
- Solaini, G.; Baracca, A.; Lenaz, G.; Sgarbi, G. Hypoxia and mitochondrial oxidative metabolism. Biochim. Biophys. Acta 2010, 1797, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, D.C.; Brune, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.J.; Bartho, L.A.; Perkins, A.V.; Holland, O.J. Placental mitochondria and reactive oxygen species in the physiology and pathophysiology of pregnancy. Clin. Exp. Pharmacol. Physiol. 2020, 47, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Lunell, N.O.; Sarby, B.; Lewander, R.; Nylund, L. Comparison of uteroplacental blood flow in normal and in intrauterine growth-retarded pregnancy. Measurements with Indium-113m and a computer-linked gammacamera. Gynecol. Obstet. Investig. 1979, 10, 106–118. [Google Scholar] [CrossRef]
- Lunell, N.O.; Nylund, L.E.; Lewander, R.; Sarby, B. Uteroplacental blood flow in pre-eclampsia measurements with indium-113m and a computer-linked gamma camera. Clin. Exp. Hypertens. B 1982, 1, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.; Goldfrad, C.; Carpenter, R.G.; Campbell, S. Transvaginal uterine and umbilical artery Doppler examination of 12-16 weeks and the subsequent development of pre-eclampsia and intrauterine growth retardation. Ultrasound Obstet. Gynecol. 1997, 9, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Leiberman, J.R.; Meizner, I.; Mazor, M.; Insler, V. Blood supply to the uterus in preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1988, 28, 23–27. [Google Scholar] [CrossRef]
- Konje, J.C.; Howarth, E.S.; Kaufmann, P.; Taylor, D.J. Longitudinal quantification of uterine artery blood volume flow changes during gestation in pregnancies complicated by intrauterine growth restriction. BJOG 2003, 110, 301–305. [Google Scholar] [CrossRef]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: Relationship to clinical outcome. Hypertension 2013, 62, 1046–1054. [Google Scholar] [CrossRef]
- Verlohren, S.; Geusens, N.; Morton, J.; Verhaegen, I.; Hering, L.; Herse, F.; Dudenhausen, J.W.; Muller, D.N.; Luft, F.C.; Cartwright, J.E.; et al. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension 2010, 56, 304–310. [Google Scholar] [CrossRef]
- Murray, A.J. Oxygen delivery and fetal-placental growth: Beyond a question of supply and demand? Placenta 2012, 33 (Suppl. 2), e16–e22. [Google Scholar] [CrossRef]
- Webster, W.S.; Abela, D. The effect of hypoxia in development. Birth Defects Res. C Embryo Today 2007, 81, 215–228. [Google Scholar] [CrossRef]
- Dunwoodie, S.L. The role of hypoxia in development of the Mammalian embryo. Dev. Cell 2009, 17, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Iqbal, K.; Kozai, K. Hypoxia and Placental Development. Birth Defects Res. 2017, 109, 1309–1329. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Watson, A.; Burton, G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am. J. Obstet. Gynecol. 2001, 184, 998–1003. [Google Scholar] [CrossRef]
- Genbacev, O.; Zhou, Y.; Ludlow, J.W.; Fisher, S.J. Regulation of human placental development by oxygen tension. Science 1997, 277, 1669–1672. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Charnock-Jones, D.S.; Jauniaux, E. Regulation of vascular growth and function in the human placenta. Reproduction 2009, 138, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Caniggia, I.; Mostachfi, H.; Winter, J.; Gassmann, M.; Lye, S.J.; Kuliszewski, M.; Post, M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J. Clin. Investig. 2000, 105, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Conrad, K.P. Expression, ontogeny, and regulation of hypoxia-inducible transcription factors in the human placenta. Biol. Reprod. 2000, 63, 559–569. [Google Scholar] [CrossRef]
- Kenchegowda, D.; Natale, B.; Lemus, M.A.; Natale, D.R.; Fisher, S.A. Inactivation of maternal Hif-1alpha at mid-pregnancy causes placental defects and deficits in oxygen delivery to the fetal organs under hypoxic stress. Dev. Biol. 2017, 422, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Whitelock, K.A.; Weissfeld, L.A.; Daftary, A.R.; Markovic, N.; Conrad, K.P. Selective overexpression of the hypoxia-inducible transcription factor, HIF-2alpha, in placentas from women with preeclampsia. Biol. Reprod. 2001, 64, 499–506. [Google Scholar] [CrossRef]
- Nishi, H.; Nakada, T.; Hokamura, M.; Osakabe, Y.; Itokazu, O.; Huang, L.E.; Isaka, K. Hypoxia-inducible factor-1 transactivates transforming growth factor-beta3 in trophoblast. Endocrinology 2004, 145, 4113–4118. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Brandon, H.M.; Daftary, A.; Ness, R.; Conrad, K.P. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 2004, 25, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Stubert, J.; Schattenberg, F.; Richter, D.U.; Dieterich, M.; Briese, V. Trophoblastic progranulin expression is upregulated in cases of fetal growth restriction and preeclampsia. J. Perinat. Med. 2012, 40, 475–481. [Google Scholar] [CrossRef]
- Korkes, H.A.; De Oliveira, L.; Sass, N.; Salahuddin, S.; Karumanchi, S.A.; Rajakumar, A. Relationship between hypoxia and downstream pathogenic pathways in preeclampsia. Hypertens. Pregnancy 2017, 36, 145–150. [Google Scholar] [CrossRef]
- Palmer, S.K.; Moore, L.G.; Young, D.; Cregger, B.; Berman, J.C.; Zamudio, S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 m) in Colorado. Am. J. Obstet. Gynecol. 1999, 180, 1161–1168. [Google Scholar] [CrossRef]
- Keyes, L.E.; Armaza, J.F.; Niermeyer, S.; Vargas, E.; Young, D.A.; Moore, L.G. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatri. Res. 2003, 54, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.G.; Charles, S.M.; Julian, C.G. Humans at high altitude: Hypoxia and fetal growth. Respir. Physiol. Neurobiol. 2011, 178, 181–190. [Google Scholar] [CrossRef]
- Zamudio, S.; Wu, Y.; Ietta, F.; Rolfo, A.; Cross, A.; Wheeler, T.; Post, M.; Illsley, N.P.; Caniggia, I. Human placental hypoxia-inducible factor-1alpha expression correlates with clinical outcomes in chronic hypoxia in vivo. Am. J. Pathol. 2007, 170, 2171–2179. [Google Scholar] [CrossRef]
- Parraguez, V.C.; Atlagich, M.; Diaz, R.; Bruzzone, M.E.; Behn, C.; Raggi, L.A. Effect of hypobaric hypoxia on lamb intrauterine growth: Comparison between high- and low-altitude native ewes. Reprod. Fertil. Dev. 2005, 17, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Dasgupta, C.; Xiao, D.; Huang, X.; Yang, S.; Zhang, L. MicroRNA-210 Targets Ten-Eleven Translocation Methylcytosine Dioxygenase 1 and Suppresses Pregnancy-Mediated Adaptation of Large Conductance Ca(2+)-Activated K(+) Channel Expression and Function in Ovine Uterine Arteries. Hypertension 2017. [Google Scholar] [CrossRef] [PubMed]
- de Grauw, T.J.; Myers, R.E.; Scott, W.J. Fetal growth retardation in rats from different levels of hypoxia. Biol. Neonatol. 1986, 49, 85–89. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, D.; Hu, Y.; Wang, Z.; Paradis, A.; Mata-Greenwood, E.; Zhang, L. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension 2013, 62, 599–607. [Google Scholar] [CrossRef]
- Matheson, H.; Veerbeek, J.H.; Charnock-Jones, D.S.; Burton, G.J.; Yung, H.W. Morphological and molecular changes in the murine placenta exposed to normobaric hypoxia throughout pregnancy. J. Physiol. 2016, 594, 1371–1388. [Google Scholar] [CrossRef]
- Kimball, R.; Wayment, M.; Merrill, D.; Wahlquist, T.; Reynolds, P.R.; Arroyo, J.A. Hypoxia reduces placental mTOR activation in a hypoxia-induced model of intrauterine growth restriction (IUGR). Physiol. Rep. 2015, 3, e12651. [Google Scholar] [CrossRef]
- Aljunaidy, M.M.; Morton, J.S.; Cooke, C.L.; Davidge, S.T. Maternal vascular responses to hypoxia in a rat model of intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R1068–R1075. [Google Scholar] [CrossRef]
- Thompson, L.P.; Pence, L.; Pinkas, G.; Song, H.; Telugu, B.P. Placental Hypoxia During Early Pregnancy Causes Maternal Hypertension and Placental Insufficiency in the Hypoxic Guinea Pig Model. Biol. Reprod. 2016, 95, 128. [Google Scholar] [CrossRef]
- Turan, S.; Aberdeen, G.W.; Thompson, L.P. Chronic hypoxia alters maternal uterine and fetal hemodynamics in the full-term pregnant guinea pig. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R330–R339. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L.S.; Rennie, M.Y.; Hoggarth, J.; Yu, L.X.; Rahman, A.; Kingdom, J.C.; Seed, M.; Macgowan, C.K.; Sled, J.G. Feto- and utero-placental vascular adaptations to chronic maternal hypoxia in the mouse. J. Physiol. 2018, 596, 3285–3297. [Google Scholar] [CrossRef]
- Sasagawa, T.; Nagamatsu, T.; Morita, K.; Mimura, N.; Iriyama, T.; Fujii, T.; Shibuya, M. HIF-2alpha, but not HIF-1alpha, mediates hypoxia-induced up-regulation of Flt-1 gene expression in placental trophoblasts. Sci. Rep. 2018, 8, 17375. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, C.; Wang, P.; Gao, J.; Liu, X.; Li, Y.; Yan, S.; Shi, Y. HIF1alpha affects trophoblastic apoptosis involved in the onset of preeclampsia by regulating FOXO3a under hypoxic conditions. Mol. Med. Rep. 2020, 21, 2484–2492. [Google Scholar] [CrossRef]
- Soleymanlou, N.; Jurisica, I.; Nevo, O.; Ietta, F.; Zhang, X.; Zamudio, S.; Post, M.; Caniggia, I. Molecular evidence of placental hypoxia in preeclampsia. J. Clin. Endocrinol. Metab. 2005, 90, 4299–4308. [Google Scholar] [CrossRef]
- Tal, R.; Shaish, A.; Barshack, I.; Polak-Charcon, S.; Afek, A.; Volkov, A.; Feldman, B.; Avivi, C.; Harats, D. Effects of hypoxia-inducible factor-1alpha overexpression in pregnant mice: Possible implications for preeclampsia and intrauterine growth restriction. Am. J. Pathol. 2010, 177, 2950–2962. [Google Scholar] [CrossRef]
- Albers, R.E.; Kaufman, M.R.; Natale, B.V.; Keoni, C.; Kulkarni-Datar, K.; Min, S.; Williams, C.R.; Natale, D.R.C.; Brown, T.L. Trophoblast-Specific Expression of Hif-1alpha Results in Preeclampsia-Like Symptoms and Fetal Growth Restriction. Sci. Rep. 2019, 9, 2742. [Google Scholar] [CrossRef] [PubMed]
- Nevo, O.; Soleymanlou, N.; Wu, Y.; Xu, J.; Kingdom, J.; Many, A.; Zamudio, S.; Caniggia, I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1085–R1093. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Nevo, O.; Many, A.; Xu, J.; Kingdom, J.; Piccoli, E.; Zamudio, S.; Post, M.; Bocking, A.; Todros, T.; Caniggia, I. Placental expression of soluble fms-like tyrosine kinase 1 is increased in singletons and twin pregnancies with intrauterine growth restriction. J. Clin. Endocrinol. Metab. 2008, 93, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Holme, A.M.; Roland, M.C.; Henriksen, T.; Michelsen, T.M. In vivo uteroplacental release of placental growth factor and soluble Fms-like tyrosine kinase-1 in normal and preeclamptic pregnancies. Am. J. Obstet. Gynecol. 2016, 215, 782.e1–782.e9. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmed, A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ. Res. 2004, 95, 884–891. [Google Scholar] [CrossRef]
- Nevo, O.; Lee, D.K.; Caniggia, I. Attenuation of VEGFR-2 expression by sFlt-1 and low oxygen in human placenta. PLoS ONE 2013, 8, e81176. [Google Scholar] [CrossRef]
- Sanchez-Aranguren, L.C.; Espinosa-Gonzalez, C.T.; Gonzalez-Ortiz, L.M.; Sanabria-Barrera, S.M.; Riano-Medina, C.E.; Nunez, A.F.; Ahmed, A.; Vasquez-Vivar, J.; Lopez, M. Soluble Fms-Like Tyrosine Kinase-1 Alters Cellular Metabolism and Mitochondrial Bioenergetics in Preeclampsia. Front. Physiol. 2018, 9, 83. [Google Scholar] [CrossRef]
- Su, M.T.; Wang, C.Y.; Tsai, P.Y.; Chen, T.Y.; Tsai, H.L.; Kuo, P.L. Aspirin enhances trophoblast invasion and represses soluble fms-like tyrosine kinase 1 production: A putative mechanism for preventing preeclampsia. J. Hypertens. 2019, 37, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Cowden Dahl, K.D.; Fryer, B.H.; Mack, F.A.; Compernolle, V.; Maltepe, E.; Adelman, D.M.; Carmeliet, P.; Simon, M.C. Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Mol. Cell. Biol. 2005, 25, 10479–10491. [Google Scholar] [CrossRef] [PubMed]
- Tissot van Patot, M.C.; Bendrick-Peart, J.; Beckey, V.E.; Serkova, N.; Zwerdlinger, L. Greater vascularity, lowered HIF-1/DNA binding, and elevated GSH as markers of adaptation to in vivo chronic hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L525–L532. [Google Scholar] [CrossRef]
- Rosenfeld, C.R.; Cornfield, D.N.; Roy, T. Ca(2+)-activated K(+) channels modulate basal and E(2)beta-induced rises in uterine blood flow in ovine pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H422–H431. [Google Scholar] [CrossRef]
- Rosenfeld, C.R.; Roy, T.; DeSpain, K.; Cox, B.E. Large-conductance Ca2+-dependent K+ channels regulate basal uteroplacental blood flow in ovine pregnancy. J. Soc. Gynecol. Investig. 2005, 12, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.R.; Liu, X.T.; DeSpain, K. Pregnancy modifies the large conductance Ca2+-activated K+ channel and cGMP-dependent signaling pathway in uterine vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1878–H1887. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, X.Q.; Xiao, D.; Zhu, R.; Huang, X.; Yang, S.; Wilson, S.; Zhang, L. Pregnancy upregulates large-conductance Ca(2+)-activated K(+) channel activity and attenuates myogenic tone in uterine arteries. Hypertension 2011, 58, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Song, R.; Romero, M.; Dasgupta, C.; Huang, X.; Holguin, M.A.; Williams, V.; Xiao, D.; Wilson, S.M.; Zhang, L. Pregnancy Increases Ca(2+) Sparks/Spontaneous Transient Outward Currents and Reduces Uterine Arterial Myogenic Tone. Hypertension 2019, 73, 691–702. [Google Scholar] [CrossRef]
- Nelson, M.T.; Cheng, H.; Rubart, M.; Santana, L.F.; Bonev, A.D.; Knot, H.J.; Lederer, W.J. Relaxation of arterial smooth muscle by calcium sparks. Science 1995, 270, 633–637. [Google Scholar] [CrossRef]
- Knot, H.J.; Standen, N.B.; Nelson, M.T. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J. Physiol. 1998, 508 Pt 1, 211–221. [Google Scholar] [CrossRef]
- Nagar, D.; Liu, X.T.; Rosenfeld, C.R. Estrogen regulates β1-subunit expression in Ca(2+)-activated K(+) channels in arteries from reproductive tissues. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1417–H1427. [Google Scholar] [CrossRef]
- Chang, K.; Xiao, D.; Huang, X.; Xue, Z.; Yang, S.; Longo, L.D.; Zhang, L. Chronic hypoxia inhibits sex steroid hormone-mediated attenuation of ovine uterine arterial myogenic tone in pregnancy. Hypertension 2010, 56, 750–757. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, X.Q.; Xiao, D.; Zhu, R.; Huang, X.; Yang, S.; Wilson, S.M.; Zhang, L. Chronic hypoxia suppresses pregnancy-induced upregulation of large-conductance Ca2+-activated K+ channel activity in uterine arteries. Hypertension 2012, 60, 214–222. [Google Scholar] [CrossRef]
- Hu, X.Q.; Song, R.; Romero, M.; Dasgupta, C.; Min, J.; Hatcher, D.; Xiao, D.; Blood, A.; Wilson, S.M.; Zhang, L. Gestational Hypoxia Inhibits Pregnancy-Induced Upregulation of Ca(2+) Sparks and Spontaneous Transient Outward Currents in Uterine Arteries Via Heightened Endoplasmic Reticulum/Oxidative Stress. Hypertension 2020, 76, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Hu, X.Q.; Huang, X.; Zhou, J.; Wilson, S.M.; Yang, S.; Zhang, L. Chronic hypoxia during gestation enhances uterine arterial myogenic tone via heightened oxidative stress. PLoS ONE 2013, 8, e73731. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Dasgupta, C.; Xiao, J.; Yang, S.; Zhang, L. Long-term high altitude hypoxia during gestation suppresses large conductance Ca(2+) -activated K(+) channel function in uterine arteries: A causal role for microRNA-210. J. Physiol. 2018, 596, 5891–5906. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine Oxidoreductase-Derived Reactive Species: Physiological and Pathological Effects. Oxid. Med. Cell. Longev. 2016, 2016, 3527579. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Veith, A.; Moorthy, B. Role of Cytochrome P450s in the Generation and Metabolism of Reactive Oxygen Species. Curr. Opin. Toxicol. 2018, 7, 44–51. [Google Scholar] [CrossRef]
- Ahn, C.S.; Metallo, C.M. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 2015, 3, 1. [Google Scholar] [CrossRef]
- Contreras, L.; Drago, I.; Zampese, E.; Pozzan, T. Mitochondria: The calcium connection. Biochim. Biophys. Acta 2010, 1797, 607–618. [Google Scholar] [CrossRef]
- Luongo, T.S.; Lambert, J.P.; Gross, P.; Nwokedi, M.; Lombardi, A.A.; Shanmughapriya, S.; Carpenter, A.C.; Kolmetzky, D.; Gao, E.; van Berlo, J.H.; et al. The mitochondrial Na(+)/Ca(2+) exchanger is essential for Ca(2+) homeostasis and viability. Nature 2017, 545, 93–97. [Google Scholar] [CrossRef]
- Tait, S.W.; Green, D.R. Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Collins, Y.; Chouchani, E.T.; James, A.M.; Menger, K.E.; Cocheme, H.M.; Murphy, M.P. Mitochondrial redox signalling at a glance. J. Cell Sci. 2012, 125, 801–806. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Perevoschikova, I.V.; Goncalves, R.L.; Hey-Mogensen, M.; Brand, M.D. The determination and analysis of site-specific rates of mitochondrial reactive oxygen species production. Methods Enzymol. 2013, 526, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fischbacher, A.; von Sonntag, C.; Schmidt, T.C. Hydroxyl radical yields in the Fenton process under various pH, ligand concentrations and hydrogen peroxide/Fe(II) ratios. Chemosphere 2017, 182, 738–744. [Google Scholar] [CrossRef]
- Szabo, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J. Mitochondrial Antioxidants and the Maintenance of Cellular Hydrogen Peroxide Levels. Oxid. Med. Cell. Longev. 2018, 2018, 7857251. [Google Scholar] [CrossRef]
- Guzy, R.D.; Schumacker, P.T. Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 2006, 91, 807–819. [Google Scholar] [CrossRef]
- Guzy, R.D.; Hoyos, B.; Robin, E.; Chen, H.; Liu, L.; Mansfield, K.D.; Simon, M.C.; Hammerling, U.; Schumacker, P.T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005, 1, 401–408. [Google Scholar] [CrossRef]
- Bell, E.L.; Klimova, T.A.; Eisenbart, J.; Moraes, C.T.; Murphy, M.P.; Budinger, G.R.; Chandel, N.S. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J. Cell Biol. 2007, 177, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Maltepe, E.; Goldwasser, E.; Mathieu, C.E.; Simon, M.C.; Schumacker, P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 1998, 95, 11715–11720. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; McClintock, D.S.; Feliciano, C.E.; Wood, T.M.; Melendez, J.A.; Rodriguez, A.M.; Schumacker, P.T. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: A mechanism of O2 sensing. J. Biol. Chem. 2000, 275, 25130–25138. [Google Scholar] [CrossRef] [PubMed]
- Paddenberg, R.; Ishaq, B.; Goldenberg, A.; Faulhammer, P.; Rose, F.; Weissmann, N.; Braun-Dullaeus, R.C.; Kummer, W. Essential role of complex II of the respiratory chain in hypoxia-induced ROS generation in the pulmonary vasculature. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L710–L719. [Google Scholar] [CrossRef] [PubMed]
- Bastian, A.; Matsuzaki, S.; Humphries, K.M.; Pharaoh, G.A.; Doshi, A.; Zaware, N.; Gangjee, A.; Ihnat, M.A. AG311, a small molecule inhibitor of complex I and hypoxia-induced HIF-1alpha stabilization. Cancer Lett. 2017, 388, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Antunes, F.; Canali, R.; Rettori, D.; Cadenas, E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J. Biol. Chem. 2003, 278, 5557–5563. [Google Scholar] [CrossRef]
- Janssen-Heininger, Y.M.; Mossman, B.T.; Heintz, N.H.; Forman, H.J.; Kalyanaraman, B.; Finkel, T.; Stamler, J.S.; Rhee, S.G.; van der Vliet, A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic. Biol. Med. 2008, 45, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox. Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Pereira, R.D.; De Long, N.E.; Wang, R.C.; Yazdi, F.T.; Holloway, A.C.; Raha, S. Angiogenesis in the placenta: The role of reactive oxygen species signaling. Biomed. Res. Int. 2015, 2015, 814543. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M. Reactive oxygen species: A novel antimicrobial. Int. J. Antimicrob. Agents 2018, 51, 299–303. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005, 24, 1311–1317. [Google Scholar] [CrossRef]
- Poetsch, A.R. The genomics of oxidative DNA damage, repair, and resulting mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef]
- Gardner, P.R.; Raineri, I.; Epstein, L.B.; White, C.W. Superoxide radical and iron modulate aconitase activity in mammalian cells. J. Biol. Chem. 1995, 270, 13399–13405. [Google Scholar] [CrossRef]
- Radi, R.; Sims, S.; Cassina, A.; Turrens, J.F. Roles of catalase and cytochrome c in hydroperoxide-dependent lipid peroxidation and chemiluminescence in rat heart and kidney mitochondria. Free Radic. Biol. Med. 1993, 15, 653–659. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Y.; Zhang, Y. Mitochondrial reactive oxygen species cause major oxidative mitochondrial DNA damages and repair pathways. J. Biosci. 2020, 45, 84. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vazquez, C.M.; Mate, A.; Sobrevia, L.; Marin, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S.W. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 1998, 19, 581–586. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S.W. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in pre-eclampsia. Placenta 2001, 22, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, J.M.; van Rijn, B.B.; Franx, A.; Bruinse, H.W.; de Roos, R.; Stroes, E.S.; van Faassen, E.E. Placental superoxide is increased in pre-eclampsia. Placenta 2001, 22, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Madazli, R.; Benian, A.; Aydin, S.; Uzun, H.; Tolun, N. The plasma and placental levels of malondialdehyde, glutathione and superoxide dismutase in pre-eclampsia. J. Obstet. Gynaecol. 2002, 22, 477–480. [Google Scholar] [CrossRef]
- Atamer, Y.; Kocyigit, Y.; Yokus, B.; Atamer, A.; Erden, A.C. Lipid peroxidation, antioxidant defense, status of trace metals and leptin levels in preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 119, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, O.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, M.; Gilbert, J.S.; LaMarca, B.B.; Sholook, M.; Chandler, D.L.; Wang, Y.; Granger, J.P. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am. J. Hypertens. 2008, 21, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Dhingra, R.; Arya, D.S.; Kalaivani, M.; Bhatla, N.; Kumar, R. Role of oxidative stress markers and antioxidants in the placenta of preeclamptic patients. J. Obstet. Gynaecol. Res. 2010, 36, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Kweider, N.; Huppertz, B.; Wruck, C.J.; Beckmann, R.; Rath, W.; Pufe, T.; Kadyrov, M. A role for Nrf2 in redox signalling of the invasive extravillous trophoblast in severe early onset IUGR associated with preeclampsia. PLoS ONE 2012, 7, e47055. [Google Scholar] [CrossRef]
- Shaker, O.G.; Sadik, N.A. Pathogenesis of preeclampsia: Implications of apoptotic markers and oxidative stress. Hum. Exp. Toxicol. 2013, 32, 1170–1178. [Google Scholar] [CrossRef]
- Holland, O.J.; Cuffe, J.S.M.; Dekker Nitert, M.; Callaway, L.; Kwan Cheung, K.A.; Radenkovic, F.; Perkins, A.V. Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. Cell Death Dis. 2018, 9, 1150. [Google Scholar] [CrossRef]
- Many, A.; Hubel, C.A.; Fisher, S.J.; Roberts, J.M.; Zhou, Y. Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am. J. Pathol. 2000, 156, 321–331. [Google Scholar] [CrossRef]
- Vanderlelie, J.; Venardos, K.; Clifton, V.L.; Gude, N.M.; Clarke, F.M.; Perkins, A.V. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta 2005, 26, 53–58. [Google Scholar] [CrossRef]
- Roland, L.; Beauchemin, D.; Acteau, G.; Fradette, C.; St-Pierre, I.; Bilodeau, J.F. Effects of labor on placental expression of superoxide dismutases in preeclampsia. Placenta 2010, 31, 392–400. [Google Scholar] [CrossRef]
- Roland-Zejly, L.; Moisan, V.; St-Pierre, I.; Bilodeau, J.F. Altered placental glutathione peroxidase mRNA expression in preeclampsia according to the presence or absence of labor. Placenta 2011, 32, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Wang, L.; Zhang, G.; Zhang, Z.; Guo, W. Oxidative stress activated by Keap-1/Nrf2 signaling pathway in pathogenesis of preeclampsia. Int. J. Clin. Exp. Pathol. 2020, 13, 382–392. [Google Scholar]
- Yang, Y.; Xu, P.; Zhu, F.; Liao, J.; Wu, Y.; Hu, M.; Fu, H.; Qiao, J.; Lin, L.; Huang, B.; et al. The Potent Antioxidant MitoQ Protects Against Preeclampsia During Late Gestation but Increases the Risk of Preeclampsia When Administered in Early Pregnancy. Antioxid. Redox Signal. 2021, 34, 118–136. [Google Scholar] [CrossRef]
- Vishnyakova, P.A.; Volodina, M.A.; Tarasova, N.V.; Marey, M.V.; Kan, N.E.; Khodzhaeva, Z.S.; Vysokikh, M.Y.; Sukhikh, G.T. Alterations in antioxidant system, mitochondrial biogenesis and autophagy in preeclamptic myometrium. BBA Clin. 2017, 8, 35–42. [Google Scholar] [CrossRef]
- Torbergsen, T.; Oian, P.; Mathiesen, E.; Borud, O. Pre-eclampsia-a mitochondrial disease? Acta Obstet. Gynecol. Scand. 1989, 68, 145–148. [Google Scholar] [CrossRef]
- Holland, O.; Dekker Nitert, M.; Gallo, L.A.; Vejzovic, M.; Fisher, J.J.; Perkins, A.V. Review: Placental mitochondrial function and structure in gestational disorders. Placenta 2017, 54, 2–9. [Google Scholar] [CrossRef]
- Karaa, A.; Elsharkawi, I.; Clapp, M.A.; Balcells, C. Effects of mitochondrial disease/dysfunction on pregnancy: A retrospective study. Mitochondrion 2019, 46, 214–220. [Google Scholar] [CrossRef]
- Yung, H.W.; Colleoni, F.; Dommett, E.; Cindrova-Davies, T.; Kingdom, J.; Murray, A.J.; Burton, G.J. Noncanonical mitochondrial unfolded protein response impairs placental oxidative phosphorylation in early-onset preeclampsia. Proc. Natl. Acad. Sci. USA 2019, 116, 18109–18118. [Google Scholar] [CrossRef]
- Shi, Z.; Long, W.; Zhao, C.; Guo, X.; Shen, R.; Ding, H. Comparative proteomics analysis suggests that placental mitochondria are involved in the development of pre-eclampsia. PLoS ONE 2013, 8, e64351. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, X.; Cai, W.; Zhou, M.; Shao, P.; Yang, Z.; Fu, R.; Cao, J.; Liu, Y.; Yu, F.; et al. Proteomics Analysis Reveals Abnormal Electron Transport and Excessive Oxidative Stress Cause Mitochondrial Dysfunction in Placental Tissues of Early-Onset Preeclampsia. Proteom. Clin. Appl. 2018, 12, e1700165. [Google Scholar] [CrossRef] [PubMed]
- Furui, T.; Kurauchi, O.; Tanaka, M.; Mizutani, S.; Ozawa, T.; Tomoda, Y. Decrease in cytochrome c oxidase and cytochrome oxidase subunit I messenger RNA levels in preeclamptic pregnancies. Obstet. Gynecol. 1994, 84, 283–288. [Google Scholar] [PubMed]
- Matsubara, S.; Minakami, H.; Sato, I.; Saito, T. Decrease in cytochrome c oxidase activity detected cytochemically in the placental trophoblast of patients with pre-eclampsia. Placenta 1997, 18, 255–259. [Google Scholar] [CrossRef]
- Muralimanoharan, S.; Maloyan, A.; Mele, J.; Guo, C.; Myatt, L.G.; Myatt, L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta 2012, 33, 816–823. [Google Scholar] [CrossRef]
- Zsengeller, Z.K.; Rajakumar, A.; Hunter, J.T.; Salahuddin, S.; Rana, S.; Stillman, I.E.; Ananth Karumanchi, S. Trophoblast mitochondrial function is impaired in preeclampsia and correlates negatively with the expression of soluble fms-like tyrosine kinase 1. Pregnancy Hypertens. 2016, 6, 313–319. [Google Scholar] [CrossRef]
- Beyramzadeh, M.; Dikmen, Z.G.; Erturk, N.K.; Tuncer, Z.S.; Akbiyik, F. Placental respiratory chain complex activities in high risk pregnancies. J. Matern. Fetal Neonatal Med. 2017, 30, 2911–2917. [Google Scholar] [CrossRef]
- Guitart-Mampel, M.; Gonzalez-Tendero, A.; Ninerola, S.; Moren, C.; Catalan-Garcia, M.; Gonzalez-Casacuberta, I.; Juarez-Flores, D.L.; Ugarteburu, O.; Matalonga, L.; Cascajo, M.V.; et al. Cardiac and placental mitochondrial characterization in a rabbit model of intrauterine growth restriction. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1157–1167. [Google Scholar] [CrossRef]
- Guitart-Mampel, M.; Juarez-Flores, D.L.; Youssef, L.; Moren, C.; Garcia-Otero, L.; Roca-Agujetas, V.; Catalan-Garcia, M.; Gonzalez-Casacuberta, I.; Tobias, E.; Milisenda, J.C.; et al. Mitochondrial implications in human pregnancies with intrauterine growth restriction and associated cardiac remodelling. J. Cell. Mol. Med. 2019, 23, 3962–3973. [Google Scholar] [CrossRef]
- Mando, C.; De Palma, C.; Stampalija, T.; Anelli, G.M.; Figus, M.; Novielli, C.; Parisi, F.; Clementi, E.; Ferrazzi, E.; Cetin, I. Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E404–E413. [Google Scholar] [CrossRef]
- Vaka, V.R.; McMaster, K.M.; Cunningham, M.W., Jr.; Ibrahim, T.; Hazlewood, R.; Usry, N.; Cornelius, D.C.; Amaral, L.M.; LaMarca, B. Role of Mitochondrial Dysfunction and Reactive Oxygen Species in Mediating Hypertension in the Reduced Uterine Perfusion Pressure Rat Model of Preeclampsia. Hypertension 2018, 72, 703–711. [Google Scholar] [CrossRef]
- Luo, Z.; Luo, W.; Li, S.; Zhao, S.; Sho, T.; Xu, X.; Zhang, J.; Xu, W.; Xu, J. Reactive oxygen species mediated placental oxidative stress, mitochondrial content, and cell cycle progression through mitogen-activated protein kinases in intrauterine growth restricted pigs. Reprod. Biol. 2018, 18, 422–431. [Google Scholar] [CrossRef]
- Vaka, V.R.; McMaster, K.M.; Cornelius, D.C.; Ibrahim, T.; Jayaram, A.; Usry, N.; Cunningham, M.W., Jr.; Amaral, L.M.; LaMarca, B. Natural killer cells contribute to mitochondrial dysfunction in response to placental ischemia in reduced uterine perfusion pressure rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R441–R447. [Google Scholar] [CrossRef]
- Morgan, T.K. Role of the Placenta in Preterm Birth: A Review. Am. J. Perinatol. 2016, 33, 258–266. [Google Scholar] [CrossRef]
- Davies, E.L.; Bell, J.S.; Bhattacharya, S. Preeclampsia and preterm delivery: A population-based case-control study. Hypertens. Pregnancy 2016, 35, 510–519. [Google Scholar] [CrossRef]
- Vishnyakova, P.A.; Tarasova, N.V.; Volodina, M.A.; Tsvirkun, D.V.; Sukhanova, I.A.; Kurchakova, T.A.; Kan, N.E.; Medzidova, M.K.; Sukhikh, G.T.; Vysokikh, M.Y. Gestation age-associated dynamics of mitochondrial calcium uniporter subunits expression in feto-maternal complex at term and preterm delivery. Sci. Rep. 2019, 9, 5501. [Google Scholar] [CrossRef]
- Mallilankaraman, K.; Doonan, P.; Cardenas, C.; Chandramoorthy, H.C.; Muller, M.; Miller, R.; Hoffman, N.E.; Gandhirajan, R.K.; Molgo, J.; Birnbaum, M.J.; et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell 2012, 151, 630–644. [Google Scholar] [CrossRef]
- Shibata, E.; Nanri, H.; Ejima, K.; Araki, M.; Fukuda, J.; Yoshimura, K.; Toki, N.; Ikeda, M.; Kashimura, M. Enhancement of mitochondrial oxidative stress and up-regulation of antioxidant protein peroxiredoxin III/SP-22 in the mitochondria of human pre-eclamptic placentae. Placenta 2003, 24, 698–705. [Google Scholar] [CrossRef]
- Covarrubias, A.E.; Lecarpentier, E.; Lo, A.; Salahuddin, S.; Gray, K.J.; Karumanchi, S.A.; Zsengeller, Z.K. AP39, a Modulator of Mitochondrial Bioenergetics, Reduces Antiangiogenic Response and Oxidative Stress in Hypoxia-Exposed Trophoblasts: Relevance for Preeclampsia Pathogenesis. Am. J. Pathol. 2019, 189, 104–114. [Google Scholar] [CrossRef]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef]
- Aljunaidy, M.M.; Morton, J.S.; Kirschenman, R.; Phillips, T.; Case, C.P.; Cooke, C.M.; Davidge, S.T. Maternal treatment with a placental-targeted antioxidant (MitoQ) impacts offspring cardiovascular function in a rat model of prenatal hypoxia. Pharmacol. Res. 2018, 134, 332–342. [Google Scholar] [CrossRef]
- Hung, T.H.; Burton, G.J. Hypoxia and reoxygenation: A possible mechanism for placental oxidative stress in preeclampsia. Taiwan J. Obstet. Gynecol. 2006, 45, 189–200. [Google Scholar] [CrossRef]
- Holland, O.J.; Hickey, A.J.R.; Alvsaker, A.; Moran, S.; Hedges, C.; Chamley, L.W.; Perkins, A.V. Changes in mitochondrial respiration in the human placenta over gestation. Placenta 2017, 57, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W.; Kennaugh, J.M.; Battaglia, F.C.; Makowski, E.L.; Meschia, G. Metabolic and circulatory studies of fetal lamb at midgestation. Am. J. Physiol. 1986, 250, E538–E544. [Google Scholar] [CrossRef]
- Carter, A.M. Placental oxygen consumption. Part I: In vivo studies—A review. Placenta 2000, 21 (Suppl A), S31–S37. [Google Scholar] [CrossRef]
- Colleoni, F.; Padmanabhan, N.; Yung, H.W.; Watson, E.D.; Cetin, I.; Tissot van Patot, M.C.; Burton, G.J.; Murray, A.J. Suppression of mitochondrial electron transport chain function in the hypoxic human placenta: A role for miRNA-210 and protein synthesis inhibition. PLoS ONE 2013, 8, e55194. [Google Scholar] [CrossRef]
- Zamudio, S.; Kovalenko, O.; Vanderlelie, J.; Illsley, N.P.; Heller, D.; Belliappa, S.; Perkins, A.V. Chronic hypoxia in vivo reduces placental oxidative stress. Placenta 2007, 28, 846–853. [Google Scholar] [CrossRef][Green Version]
- Kohan-Ghadr, H.R.; Kilburn, B.A.; Kadam, L.; Johnson, E.; Kolb, B.L.; Rodriguez-Kovacs, J.; Hertz, M.; Armant, D.R.; Drewlo, S. Rosiglitazone augments antioxidant response in the human trophoblast and prevents apoptosisdagger. Biol. Reprod. 2019, 100, 479–494. [Google Scholar] [CrossRef]
- Richter, H.G.; Camm, E.J.; Modi, B.N.; Naeem, F.; Cross, C.M.; Cindrova-Davies, T.; Spasic-Boskovic, O.; Dunster, C.; Mudway, I.S.; Kelly, F.J.; et al. Ascorbate prevents placental oxidative stress and enhances birth weight in hypoxic pregnancy in rats. J. Physiol. 2012, 590, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Telugu, B.P.; Thompson, L.P. Sexual dimorphism of mitochondrial function in the hypoxic guinea pig placenta. Biol. Reprod. 2019, 100, 208–216. [Google Scholar] [CrossRef]
- Rueda-Clausen, C.F.; Stanley, J.L.; Thambiraj, D.F.; Poudel, R.; Davidge, S.T.; Baker, P.N. Effect of prenatal hypoxia in transgenic mouse models of preeclampsia and fetal growth restriction. Reprod. Sci. 2014, 21, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Vangrieken, P.; Al-Nasiry, S.; Bast, A.; Leermakers, P.A.; Tulen, C.B.M.; Janssen, G.M.J.; Kaminski, I.; Geomini, I.; Lemmens, T.; Schiffers, P.M.H.; et al. Hypoxia-induced mitochondrial abnormalities in cells of the placenta. PLoS ONE 2021, 16, e0245155. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, E.; Kirschenman, R.; Spaans, F.; Holody, C.D.; Phillips, T.E.J.; Case, C.P.; Cooke, C.M.; Murphy, M.P.; Lemieux, H.; Davidge, S.T. Nanoparticle-encapsulated antioxidant improves placental mitochondrial function in a sexually dimorphic manner in a rat model of prenatal hypoxia. FASEB J. 2021, 35, e21338. [Google Scholar] [CrossRef]
- Pineles, B.L.; Romero, R.; Montenegro, D.; Tarca, A.L.; Han, Y.M.; Kim, Y.M.; Draghici, S.; Espinoza, J.; Kusanovic, J.P.; Mittal, P.; et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am. J. Obstet. Gynecol. 2007, 196, 261.e1–261.e6. [Google Scholar] [CrossRef]
- Zhu, X.M.; Han, T.; Sargent, I.L.; Yin, G.W.; Yao, Y.Q. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am. J. Obstet. Gynecol. 2009, 200, 661.e1–661.e7. [Google Scholar] [CrossRef]
- Lee, D.C.; Romero, R.; Kim, J.S.; Tarca, A.L.; Montenegro, D.; Pineles, B.L.; Kim, E.; Lee, J.; Kim, S.Y.; Draghici, S.; et al. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: Siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am. J. Pathol. 2011, 179, 590–602. [Google Scholar] [CrossRef]
- Ishibashi, O.; Ohkuchi, A.; Ali, M.M.; Kurashina, R.; Luo, S.S.; Ishikawa, T.; Takizawa, T.; Hirashima, C.; Takahashi, K.; Migita, M.; et al. Hydroxysteroid (17-beta) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: A novel marker for predicting preeclampsia. Hypertension 2012, 59, 265–273. [Google Scholar] [CrossRef]
- Anton, L.; DeVine, A.; Polyak, E.; Olarerin-George, A.; Brown, A.G.; Falk, M.J.; Elovitz, M.A. HIF-1alpha Stabilization Increases miR-210 Eliciting First Trimester Extravillous Trophoblast Mitochondrial Dysfunction. Front. Physiol. 2019, 10, 699. [Google Scholar] [CrossRef]
- Favaro, E.; Ramachandran, A.; McCormick, R.; Gee, H.; Blancher, C.; Crosby, M.; Devlin, C.; Blick, C.; Buffa, F.; Li, J.L.; et al. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS ONE 2010, 5, e10345. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y.; Zhang, H.; Huang, P.; Luthra, R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 2010, 29, 4362–4368. [Google Scholar] [CrossRef]
- Morikawa, S.; Kurauchi, O.; Tanaka, M.; Yoneda, M.; Uchida, K.; Itakura, A.; Furugori, K.; Mizutani, S.; Tomoda, Y. Increased mitochondrial damage by lipid peroxidation in trophoblast cells of preeclamptic placentas. Biochem. Mol. Biol. Int. 1997, 41, 767–775. [Google Scholar] [CrossRef]
- Serdar, Z.; Gur, E.; Colakoethullary, M.; Develioethlu, O.; Sarandol, E. Lipid and protein oxidation and antioxidant function in women with mild and severe preeclampsia. Arch. Gynecol. Obstet. 2003, 268, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zusterzeel, P.L.; Rutten, H.; Roelofs, H.M.; Peters, W.H.; Steegers, E.A. Protein carbonyls in decidua and placenta of pre-eclamptic women as markers for oxidative stress. Placenta 2001, 22, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Nikaido, T.; Toki, T.; Kita, N.; Kanai, M.; Ashida, T.; Ohira, S.; Konishi, I. Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch. 2004, 444, 49–55. [Google Scholar] [CrossRef]
- Fujimaki, A.; Watanabe, K.; Mori, T.; Kimura, C.; Shinohara, K.; Wakatsuki, A. Placental oxidative DNA damage and its repair in preeclamptic women with fetal growth restriction. Placenta 2011, 32, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Kimura, C.; Watanabe, K.; Iwasaki, A.; Mori, T.; Matsushita, H.; Shinohara, K.; Wakatsuki, A. The severity of hypoxic changes and oxidative DNA damage in the placenta of early-onset preeclamptic women and fetal growth restriction. J. Matern. Fetal Neonatal Med. 2013, 26, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, S.; Kidane, D.; Guller, S.; Luo, T.; Norwitz, N.G.; Arcuri, F.; Toti, P.; Norwitz, E.R. In vivo and in vitro evidence for placental DNA damage in preeclampsia. PLoS ONE 2014, 9, e86791. [Google Scholar] [CrossRef]
- Nagai, R.; Watanabe, K.; Wakatsuki, A.; Hamada, F.; Shinohara, K.; Hayashi, Y.; Imamura, R.; Fukaya, T. Melatonin preserves fetal growth in rats by protecting against ischemia/reperfusion-induced oxidative/nitrosative mitochondrial damage in the placenta. J. Pineal Res. 2008, 45, 271–276. [Google Scholar] [CrossRef]
- Ushida, T.; Kotani, T.; Tsuda, H.; Imai, K.; Nakano, T.; Hirako, S.; Ito, Y.; Li, H.; Mano, Y.; Wang, J.; et al. Molecular hydrogen ameliorates several characteristics of preeclampsia in the Reduced Uterine Perfusion Pressure (RUPP) rat model. Free Radic. Biol. Med. 2016, 101, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal. Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Wu, S.B.; Wu, Y.T.; Wei, Y.H. Oxidative stress response elicited by mitochondrial dysfunction: Implication in the pathophysiology of aging. Exp. Biol. Med. 2013, 238, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.I.; Jou, M.J. Mitochondrial swelling and generation of reactive oxygen species induced by photoirradiation are heterogeneously distributed. Ann. N. Y. Acad. Sci. 2004, 1011, 112–122. [Google Scholar] [CrossRef]
- Ding, D.; Scott, N.M.; Thompson, E.E.; Chaiworapongsa, T.; Torres, R.; Billstrand, C.; Murray, K.; Dexheimer, P.J.; Ismail, M.; Kay, H.; et al. Increased protein-coding mutations in the mitochondrial genome of African American women with preeclampsia. Reprod. Sci. 2012, 19, 1343–1351. [Google Scholar] [CrossRef]
- Vishnyakova, P.A.; Volodina, M.A.; Tarasova, N.V.; Marey, M.V.; Tsvirkun, D.V.; Vavina, O.V.; Khodzhaeva, Z.S.; Kan, N.E.; Menon, R.; Vysokikh, M.Y.; et al. Mitochondrial role in adaptive response to stress conditions in preeclampsia. Sci. Rep. 2016, 6, 32410. [Google Scholar] [CrossRef]
- Hori, A.; Yoshida, M.; Shibata, T.; Ling, F. Reactive oxygen species regulate DNA copy number in isolated yeast mitochondria by triggering recombination-mediated replication. Nucleic Acids Res. 2009, 37, 749–761. [Google Scholar] [CrossRef]
- Qiu, C.; Hevner, K.; Abetew, D.; Sedensky, M.; Morgan, P.; Enquobahrie, D.A.; Williams, M.A. Mitochondrial DNA copy number and oxidative DNA damage in placental tissues from gestational diabetes and control pregnancies: A pilot study. Clin. Lab. 2013, 59, 655–660. [Google Scholar] [CrossRef]
- Staff, A.C.; Fjeldstad, H.E.; Fosheim, I.K.; Moe, K.; Turowski, G.; Johnsen, G.M.; Alnaes-Katjavivi, P.; Sugulle, M. Failure of physiological transformation and spiral artery atherosis: Their roles in preeclampsia. Am. J. Obstet. Gynecol. 2020. [Google Scholar] [CrossRef]
- Tissot van Patot, M.; Grilli, A.; Chapman, P.; Broad, E.; Tyson, W.; Heller, D.S.; Zwerdlinger, L.; Zamudio, S. Remodelling of uteroplacental arteries is decreased in high altitude placentae. Placenta 2003, 24, 326–335. [Google Scholar] [CrossRef]
- Cartwright, J.E.; Keogh, R.J.; Tissot van Patot, M.C. Hypoxia and placental remodelling. Adv. Exp. Med. Biol. 2007, 618, 113–126. [Google Scholar] [CrossRef]
- Onogi, A.; Naruse, K.; Sado, T.; Tsunemi, T.; Shigetomi, H.; Noguchi, T.; Yamada, Y.; Akasaki, M.; Oi, H.; Kobayashi, H. Hypoxia inhibits invasion of extravillous trophoblast cells through reduction of matrix metalloproteinase (MMP)-2 activation in the early first trimester of human pregnancy. Placenta 2011, 32, 665–670. [Google Scholar] [CrossRef]
- Li, L.; Huang, X.; He, Z.; Xiong, Y.; Fang, Q. miRNA-210-3p regulates trophoblast proliferation and invasiveness through fibroblast growth factor 1 in selective intrauterine growth restriction. J. Cell. Mol. Med. 2019, 23, 4422–4433. [Google Scholar] [CrossRef]
- Na, J.Y.; Seok, J.; Park, S.; Kim, J.S.; Kim, G.J. Effects of selenium on the survival and invasion of trophoblasts. Clin. Exp. Reprod. Med. 2018, 45, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, G.Y.; Wang, J.; Lu, S.L.; Cao, J.; Sun, L.Z. A novel bridge between oxidative stress and immunity: The interaction between hydrogen peroxide and human leukocyte antigen G in placental trophoblasts during preeclampsia. Am. J. Obstet. Gynecol. 2012, 206, 447.e7–447.e16. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Fukushima, K.; Takao, T.; Seki, H.; Takeda, S.; Wake, N. Oxidative stress produced by xanthine oxidase induces apoptosis in human extravillous trophoblast cells. J. Reprod. Dev. 2013, 59, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Smith, S.D.; Yusuf, K.; Huettner, P.C.; Kraus, F.T.; Sadovsky, Y.; Nelson, D.M. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am. J. Obstet. Gynecol. 2002, 186, 1056–1061. [Google Scholar] [CrossRef]
- Kadyrov, M.; Kingdom, J.C.; Huppertz, B. Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am. J. Obstet. Gynecol. 2006, 194, 557–563. [Google Scholar] [CrossRef]
- Tomas, S.Z.; Prusac, I.K.; Roje, D.; Tadin, I. Trophoblast apoptosis in placentas from pregnancies complicated by preeclampsia. Gynecol. Obstet. Investig. 2011, 71, 250–255. [Google Scholar] [CrossRef]
- Huppertz, B.; Kadyrov, M.; Kingdom, J.C. Apoptosis and its role in the trophoblast. Am. J. Obstet. Gynecol. 2006, 195, 29–39. [Google Scholar] [CrossRef]
- Levy, R.; Smith, S.D.; Chandler, K.; Sadovsky, Y.; Nelson, D.M. Apoptosis in human cultured trophoblasts is enhanced by hypoxia and diminished by epidermal growth factor. Am. J. Physiol. Cell Physiol. 2000, 278, C982–C988. [Google Scholar] [CrossRef]
- Heazell, A.E.; Lacey, H.A.; Jones, C.J.; Huppertz, B.; Baker, P.N.; Crocker, I.P. Effects of oxygen on cell turnover and expression of regulators of apoptosis in human placental trophoblast. Placenta 2008, 29, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Skepper, J.N.; Charnock-Jones, D.S.; Burton, G.J. Hypoxia-reoxygenation: A potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ. Res. 2002, 90, 1274–1281. [Google Scholar] [CrossRef]
- Li, J.; Tong, C.; Xu, P.; Wang, L.; Han, T.L.; Wen, L.; Luo, X.; Tan, B.; Zhu, F.; Gui, S.; et al. QSOX1 regulates trophoblastic apoptosis in preeclampsia through hydrogen peroxide production. J. Matern. Fetal Neonatal Med. 2019, 32, 3708–3715. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Liang, J.; Qian, J.; Jin, L.; Du, M.; Li, M.; Li, D. Opposing role of JNK-p38 kinase and ERK1/2 in hydrogen peroxide-induced oxidative damage of human trophoblast-like JEG-3 cells. Int. J. Clin. Exp. Pathol. 2014, 7, 959–968. [Google Scholar]
- Tang, C.; Pan, J.; Li, H.; He, B.; Hong, L.; Teng, X.; Li, D. Cyclosporin A protects trophoblasts from H2O2-induced oxidative injury via FAK-Src pathway. Biochem. Biophys. Res. Commun. 2019, 518, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.; Vanderlelie, J.J.; Holland, O.; Perkins, A.V. Overexpression of Endogenous Anti-Oxidants with Selenium Supplementation Protects Trophoblast Cells from Reactive Oxygen Species-Induced Apoptosis in a Bcl-2-Dependent Manner. Biol. Trace Elem. Res. 2017, 177, 394–403. [Google Scholar] [CrossRef]
- Murphy, V.E.; Smith, R.; Giles, W.B.; Clifton, V.L. Endocrine regulation of human fetal growth: The role of the mother, placenta, and fetus. Endocr. Rev. 2006, 27, 141–169. [Google Scholar] [CrossRef]
- Newbern, D.; Freemark, M. Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 409–416. [Google Scholar] [CrossRef]
- Napso, T.; Yong, H.E.J.; Lopez-Tello, J.; Sferruzzi-Perri, A.N. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol. 2018, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Berkane, N.; Liere, P.; Oudinet, J.P.; Hertig, A.; Lefevre, G.; Pluchino, N.; Schumacher, M.; Chabbert-Buffet, N. From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocr. Rev. 2017, 38, 123–144. [Google Scholar] [CrossRef]
- Hu, X.Q.; Song, R.; Zhang, L. Effect of Oxidative Stress on the Estrogen-NOS-NO-KCa Channel Pathway in Uteroplacental Dysfunction: Its Implication in Pregnancy Complications. Oxid. Med. Cell. Longev. 2019, 2019, 9194269. [Google Scholar] [CrossRef]
- Jobe, S.O.; Tyler, C.T.; Magness, R.R. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension 2013, 61, 480–487. [Google Scholar] [CrossRef]
- Perez-Sepulveda, A.; Monteiro, L.J.; Dobierzewska, A.; Espana-Perrot, P.P.; Venegas-Araneda, P.; Guzman-Rojas, A.M.; Gonzalez, M.I.; Palominos-Rivera, M.; Irarrazabal, C.E.; Figueroa-Diesel, H.; et al. Placental Aromatase Is Deficient in Placental Ischemia and Preeclampsia. PLoS ONE 2015, 10, e0139682. [Google Scholar] [CrossRef] [PubMed]
- Pecks, U.; Rath, W.; Kleine-Eggebrecht, N.; Maass, N.; Voigt, F.; Goecke, T.W.; Mohaupt, M.G.; Escher, G. Maternal Serum Lipid, Estradiol, and Progesterone Levels in Pregnancy, and the Impact of Placental and Hepatic Pathologies. Geburtshilfe Frauenheilkd. 2016, 76, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Sobrevilla, L.A.; Romero, I.; Kruger, F.; Whittembury, J. Low estrogen excretion during pregnancy at high altitude. Am. J. Obstet. Gynecol. 1968, 102, 828–833. [Google Scholar] [CrossRef]
- Zamudio, S.; Leslie, K.K.; White, M.; Hagerman, D.D.; Moore, L.G. Low serum estradiol and high serum progesterone concentrations characterize hypertensive pregnancies at high altitude. J. Soc. Gynecol. Investig. 1994, 1, 197–205. [Google Scholar] [CrossRef]
- Wan, J.; Hu, Z.; Zeng, K.; Yin, Y.; Zhao, M.; Chen, M.; Chen, Q. The reduction in circulating levels of estrogen and progesterone in women with preeclampsia. Pregnancy Hypertens. 2018, 11, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Morioka, N.; Wakatsuki, A.; Nakano, Y.; Sagara, Y. Role of the free radical-scavenger system in aromatase activity of the human ovary. Horm. Res. 1993, 39 (Suppl. 1), 22–27. [Google Scholar] [CrossRef]
- Fournier, T.; Guibourdenche, J.; Evain-Brion, D. Review: hCGs: Different sources of production, different glycoforms and functions. Placenta 2015, 36 (Suppl. 1), S60–S65. [Google Scholar] [CrossRef]
- Barjaktarovic, M.; Korevaar, T.I.M.; Jaddoe, V.W.V.; de Rijke, Y.B.; Peeters, R.P.; Steegers, E.A.P. Human chorionic gonadotropin and risk of pre-eclampsia: Prospective population-based cohort study. Ultrasound Obstet. Gynecol. 2019, 54, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Barjaktarovic, M.; Korevaar, T.I.; Jaddoe, V.W.; de Rijke, Y.B.; Visser, T.J.; Peeters, R.P.; Steegers, E.A. Human chorionic gonadotropin (hCG) concentrations during the late first trimester are associated with fetal growth in a fetal sex-specific manner. Eur. J. Epidemiol. 2017, 32, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Strohmer, H.; Kiss, H.; Mosl, B.; Egarter, C.; Husslein, P.; Knofler, M. Hypoxia downregulates continuous and interleukin-1-induced expression of human chorionic gonadotropin in choriocarcinoma cells. Placenta 1997, 18, 597–604. [Google Scholar] [CrossRef]
- McAleer, M.F.; Tuan, R.S. Metallothionein protects against severe oxidative stress-induced apoptosis of human trophoblastic cells. In Vitro Mol. Toxicol. 2001, 14, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Handwerger, S.; Freemark, M. The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J. Pediatr. Endocrinol. Metab. 2000, 13, 343–356. [Google Scholar] [CrossRef]
- Karabulut, A.K.; Layfield, R.; Pratten, M.K. Growth promoting effects of human placental lactogen during early organogenesis: A link to insulin-like growth factors. J. Anat. 2001, 198, 651–662. [Google Scholar] [CrossRef]
- Constancia, M.; Hemberger, M.; Hughes, J.; Dean, W.; Ferguson-Smith, A.; Fundele, R.; Stewart, F.; Kelsey, G.; Fowden, A.; Sibley, C.; et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 2002, 417, 945–948. [Google Scholar] [CrossRef]
- Walker, O.S.; Ragos, R.; Wong, M.K.; Adam, M.; Cheung, A.; Raha, S. Reactive oxygen species from mitochondria impacts trophoblast fusion and the production of endocrine hormones by syncytiotrophoblasts. PLoS ONE 2020, 15, e0229332. [Google Scholar] [CrossRef]
- Gu, Y.; Lewis, D.F.; Wang, Y. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J. Clin. Endocrinol. Metab. 2008, 93, 260–266. [Google Scholar] [CrossRef]
- Xu, B.; Thornton, C.; Tooher, J.; Ogle, R.; Lim, S.; Makris, A.; Hennessy, A. Effects of anti-hypertensive drugs on production of soluble fms-like tyrosine kinase 1 and soluble endoglin from human normal and pre-eclamptic placentas in vitro. Clin. Exp. Pharmacol. Physiol. 2009, 36, 839–842. [Google Scholar] [CrossRef]
- Wang, A.; Rana, S.; Karumanchi, S.A. Preeclampsia: The role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 2009, 24, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Karumanchi, S.A. Angiogenic Factors in Preeclampsia: From Diagnosis to Therapy. Hypertension 2016, 67, 1072–1079. [Google Scholar] [CrossRef]
- Possomato-Vieira, J.S.; Khalil, R.A. Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia. Adv. Pharmacol. 2016, 77, 361–431. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Kalkunte, S.; Sharma, S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension 2011, 57, 505–514. [Google Scholar] [CrossRef]
- Li, H.; Gu, B.; Zhang, Y.; Lewis, D.F.; Wang, Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta 2005, 26, 210–217. [Google Scholar] [CrossRef]
- George, E.M.; Colson, D.; Dixon, J.; Palei, A.C.; Granger, J.P. Heme Oxygenase-1 Attenuates Hypoxia-Induced sFlt-1 and Oxidative Stress in Placental Villi through Its Metabolic Products CO and Bilirubin. Int. J. Hypertens. 2012, 2012, 486053. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Deng, Q.; Luo, X.; Chen, Y.; Shan, N.; Qi, H. Oxidative stress-induced Gadd45alpha inhibits trophoblast invasion and increases sFlt1/sEng secretions via p38 MAPK involving in the pathology of pre-eclampsia. J. Matern. Fetal Neonatal Med. 2016, 29, 3776–3785. [Google Scholar] [CrossRef]
- Tam Tam, K.B.; Lamarca, B.; Arany, M.; Cockrell, K.; Fournier, L.; Murphy, S.; Martin, J.N., Jr.; Granger, J.P. Role of reactive oxygen species during hypertension in response to chronic antiangiogenic factor (sFlt-1) excess in pregnant rats. Am. J. Hypertens. 2011, 24, 110–113. [Google Scholar] [CrossRef][Green Version]
- Jiang, Z.; Zou, Y.; Ge, Z.; Zuo, Q.; Huang, S.Y.; Sun, L. A Role of sFlt-1 in Oxidative Stress and Apoptosis in Human and Mouse Pre-Eclamptic Trophoblasts. Biol. Reprod. 2015, 93, 73. [Google Scholar] [CrossRef] [PubMed]
- Vogtmann, R.; Kuhnel, E.; Dicke, N.; Verkaik-Schakel, R.N.; Plosch, T.; Schorle, H.; Stojanovska, V.; Herse, F.; Koninger, A.; Kimmig, R.; et al. Human sFLT1 Leads to Severe Changes in Placental Differentiation and Vascularization in a Transgenic hsFLT1/rtTA FGR Mouse Model. Front. Endocrinol. (Lausanne) 2019, 10, 165. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S.W. TNF alpha concentrations and mRNA expression are increased in preeclamptic placentas. J. Reprod. Immunol. 1996, 32, 157–169. [Google Scholar] [CrossRef]
- Benyo, D.F.; Smarason, A.; Redman, C.W.; Sims, C.; Conrad, K.P. Expression of inflammatory cytokines in placentas from women with preeclampsia. J. Clin. Endocrinol. Metab. 2001, 86, 2505–2512. [Google Scholar] [CrossRef]
- Hahn-Zoric, M.; Hagberg, H.; Kjellmer, I.; Ellis, J.; Wennergren, M.; Hanson, L.A. Aberrations in placental cytokine mRNA related to intrauterine growth retardation. Pediatr. Res. 2002, 51, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.T.; Breidenbach, M.; Honscheid, B.; Friebe-Hoffmann, U.; Engel, H.; Gohring, U.J.; Uekermann, L.; Kurbacher, C.M.; Schondorf, T. Preeclamptic women are deficient of interleukin-10 as assessed by cytokine release of trophoblast cells in vitro. Cytokine 2003, 23, 119–125. [Google Scholar] [CrossRef]
- Makris, A.; Xu, B.; Yu, B.; Thornton, C.; Hennessy, A. Placental deficiency of interleukin-10 (IL-10) in preeclampsia and its relationship to an IL10 promoter polymorphism. Placenta 2006, 27, 445–451. [Google Scholar] [CrossRef]
- Weel, I.C.; Romão-Veiga, M.; Matias, M.L.; Fioratti, E.G.; Peraçoli, J.C.; Borges, V.T.; Araujo, J.P., Jr.; Peraçoli, M.T. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. J. Reprod. Immunol. 2017, 123, 40–47. [Google Scholar] [CrossRef]
- Aggarwal, R.; Jain, A.K.; Mittal, P.; Kohli, M.; Jawanjal, P.; Rath, G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal. 2019, 33, e22834. [Google Scholar] [CrossRef]
- Keelan, J.A.; Mitchell, M.D. Placental cytokines and preeclampsia. Front. Biosci. 2007, 12, 2706–2727. [Google Scholar] [CrossRef]
- Benyo, D.F.; Miles, T.M.; Conrad, K.P. Hypoxia stimulates cytokine production by villous explants from the human placenta. J. Clin. Endocrinol. Metab. 1997, 82, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Charnock-Jones, D.S.; Skepper, J.N.; Burton, G.J. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: A potential mediator of the inflammatory response in preeclampsia. Am. J. Pathol. 2004, 164, 1049–1061. [Google Scholar] [CrossRef]
- Nunes, P.R.; Peracoli, M.T.S.; Romao-Veiga, M.; Matias, M.L.; Ribeiro, V.R.; Da Costa Fernandes, C.J.; Peracoli, J.C.; Rodrigues, J.R.; De Oliveira, L. Hydrogen peroxide-mediated oxidative stress induces inflammasome activation in term human placental explants. Pregnancy Hypertens. 2018, 14, 29–36. [Google Scholar] [CrossRef]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef]
- Raghupathy, R. Cytokines as key players in the pathophysiology of preeclampsia. Med. Princ. Pract. 2013, 22 (Suppl. 1), 8–19. [Google Scholar] [CrossRef]
- Harmon, A.C.; Cornelius, D.C.; Amaral, L.M.; Faulkner, J.L.; Cunningham, M.W., Jr.; Wallace, K.; LaMarca, B. The role of inflammation in the pathology of preeclampsia. Clin. Sci. (Lond.) 2016, 130, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Leisser, C.; Saleh, L.; Haider, S.; Husslein, H.; Sonderegger, S.; Knofler, M. Tumour necrosis factor-alpha impairs chorionic gonadotrophin beta-subunit expression and cell fusion of human villous cytotrophoblast. Mol. Hum. Reprod. 2006, 12, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Liu, B.; Zhao, H.B.; Stone, P.; Chen, Q.; Chamley, L. IL-6, TNFalpha and TGFbeta promote nonapoptotic trophoblast deportation and subsequently causes endothelial cell activation. Placenta 2010, 31, 75–80. [Google Scholar] [CrossRef]
- Rosenfeld, C.R.; Morriss, F.H., Jr.; Makowski, E.L.; Meschia, G.; Battaglia, F.C. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecol. Investig. 1974, 5, 252–268. [Google Scholar] [CrossRef]
- Ford, S.P.; Reynolds, L.P.; Ferrell, C.L. Blood flow, steroid secretion and nutrient uptake of the gravid uterus during the periparturient period in sows. J. Anim. Sci. 1984, 59, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Keyes, L.E.; Majack, R.; Dempsey, E.C.; Moore, L.G. Pregnancy stimulation of DNA synthesis and uterine blood flow in the guinea pig. Pediatr. Res. 1997, 41, 708–715. [Google Scholar] [CrossRef]
- Dowell, R.T.; Kauer, C.D. Maternal hemodynamics and uteroplacental blood flow throughout gestation in conscious rats. Methods Find Exp. Clin. Pharmacol. 1997, 19, 613–625. [Google Scholar]
- Konje, J.C.; Kaufmann, P.; Bell, S.C.; Taylor, D.J. A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am. J. Obstet. Gynecol. 2001, 185, 608–613. [Google Scholar] [CrossRef]
- Palmer, S.K.; Zamudio, S.; Coffin, C.; Parker, S.; Stamm, E.; Moore, L.G. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet. Gynecol. 1992, 80, 1000–1006. [Google Scholar]
- Magness, R.R.; Phernetton, T.M.; Gibson, T.C.; Chen, D.B. Uterine blood flow responses to ICI 182 780 in ovariectomized oestradiol-17beta-treated, intact follicular and pregnant sheep. J. Physiol. 2005, 565, 71–83. [Google Scholar] [CrossRef]
- Osol, G.; Mandala, M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 2009, 24, 58–71. [Google Scholar] [CrossRef]
- Zamudio, S.; Palmer, S.K.; Droma, T.; Stamm, E.; Coffin, C.; Moore, L.G. Effect of altitude on uterine artery blood flow during normal pregnancy. J. Appl. Physiol. (1985) 1995, 79, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Takata, M.; Nakatsuka, M.; Kudo, T. Differential blood flow in uterine, ophthalmic, and brachial arteries of preeclamptic women. Obstet. Gynecol. 2002, 100, 931–939. [Google Scholar] [CrossRef]
- Browne, V.A.; Toledo-Jaldin, L.; Davila, R.D.; Lopez, L.P.; Yamashiro, H.; Cioffi-Ragan, D.; Julian, C.G.; Wilson, M.J.; Bigham, A.W.; Shriver, M.D.; et al. High-end arteriolar resistance limits uterine artery blood flow and restricts fetal growth in preeclampsia and gestational hypertension at high altitude. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1221–R1229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Huang, X.; Hu, X.Q.; Xiao, D.; Zhang, L. Gestational hypoxia increases reactive oxygen species and inhibits steroid hormone-mediated upregulation of Ca(2+)-activated K(+) channel function in uterine arteries. Hypertension 2014, 64, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Huang, X.; Xiao, D.; Zhang, L. Direct effect of chronic hypoxia in suppressing large conductance Ca(2+)-activated K(+) channel activity in ovine uterine arteries via increasing oxidative stress. J. Physiol. 2016, 594, 343–356. [Google Scholar] [CrossRef]
- Poston, L.; Briley, A.L.; Seed, P.T.; Kelly, F.J.; Shennan, A.H.; Vitamins in Pre-eclampsia Trial, C. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): Randomised placebo-controlled trial. Lancet 2006, 367, 1145–1154. [Google Scholar] [CrossRef]
- Rumbold, A.R.; Crowther, C.A.; Haslam, R.R.; Dekker, G.A.; Robinson, J.S.; Group, A.S. Vitamins C and E and the risks of preeclampsia and perinatal complications. N. Engl. J. Med. 2006, 354, 1796–1806. [Google Scholar] [CrossRef]
- Roberts, J.M.; Myatt, L.; Spong, C.Y.; Thom, E.A.; Hauth, J.C.; Leveno, K.J.; Pearson, G.D.; Wapner, R.J.; Varner, M.W.; Thorp, J.M., Jr.; et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N. Engl. J. Med. 2010, 362, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.I.; Nogales, C.; Mucke, H.A.M.; Petraina, A.; Cuadrado, A.; Rojo, A.I.; Ghezzi, P.; Jaquet, V.; Augsburger, F.; Dufrasne, F.; et al. On the Clinical Pharmacology of Reactive Oxygen Species. Pharmacol. Rev. 2020, 72, 801–828. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Ndifon, C.; Lui, S.; Widdows, K.; Kotamraju, V.R.; Agemy, L.; Teesalu, T.; Glazier, J.D.; Cellesi, F.; Tirelli, N.; et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci. Adv. 2016, 2, e1600349. [Google Scholar] [CrossRef] [PubMed]
- Cureton, N.; Korotkova, I.; Baker, B.; Greenwood, S.; Wareing, M.; Kotamraju, V.R.; Teesalu, T.; Cellesi, F.; Tirelli, N.; Ruoslahti, E.; et al. Selective Targeting of a Novel Vasodilator to the Uterine Vasculature to Treat Impaired Uteroplacental Perfusion in Pregnancy. Theranostics 2017, 7, 3715–3731. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, L.; Yu, Y.; Wang, B.; Chen, Z.; Han, J.; Li, M.; Chen, J.; Xiao, T.; Ambati, B.K.; et al. Placenta-specific drug delivery by trophoblast-targeted nanoparticles in mice. Theranostics 2018, 8, 2765–2781. [Google Scholar] [CrossRef]
- Li, L.; Yang, H.; Chen, P.; Xin, T.; Zhou, Q.; Wei, D.; Zhang, Y.; Wang, S. Trophoblast-Targeted Nanomedicine Modulates Placental sFLT1 for Preeclampsia Treatment. Front. Bioeng. Biotechnol. 2020, 8, 64. [Google Scholar] [CrossRef]
- Li, M.; Cheng, W.; Luo, J.; Hu, X.; Nie, T.; Lai, H.; Zheng, X.; Li, F.; Li, H. Loss of selenocysteine insertion sequence binding protein 2 suppresses the proliferation, migration/invasion and hormone secretion of human trophoblast cells via the PI3K/Akt and ERK signaling pathway. Placenta 2017, 55, 81–89. [Google Scholar] [CrossRef]
- Duntas, L.H. Selenium and at-risk pregnancy: Challenges and controversies. Thyroid Res. 2020, 13, 16. [Google Scholar] [CrossRef]
- Vanderlelie, J.; Venardos, K.; Perkins, A.V. Selenium deficiency as a model of experimental pre-eclampsia in rats. Reproduction 2004, 128, 635–641. [Google Scholar] [CrossRef]
- Hofstee, P.; Bartho, L.A.; McKeating, D.R.; Radenkovic, F.; McEnroe, G.; Fisher, J.J.; Holland, O.J.; Vanderlelie, J.J.; Perkins, A.V.; Cuffe, J.S.M. Maternal selenium deficiency during pregnancy in mice increases thyroid hormone concentrations, alters placental function and reduces fetal growth. J. Physiol. 2019, 597, 5597–5617. [Google Scholar] [CrossRef]
- Khera, A.; Vanderlelie, J.J.; Perkins, A.V. Selenium supplementation protects trophoblast cells from mitochondrial oxidative stress. Placenta 2013, 34, 594–598. [Google Scholar] [CrossRef]
- Han, L.; Zhou, S.M. Selenium supplement in the prevention of pregnancy induced hypertension. Chin. Med. J. (Engl.) 1994, 107, 870–871. [Google Scholar]
- Tara, F.; Maamouri, G.; Rayman, M.P.; Ghayour-Mobarhan, M.; Sahebkar, A.; Yazarlu, O.; Ouladan, S.; Tavallaie, S.; Azimi-Nezhad, M.; Shakeri, M.T.; et al. Selenium supplementation and the incidence of preeclampsia in pregnant Iranian women: A randomized, double-blind, placebo-controlled pilot trial. Taiwan J. Obstet. Gynecol. 2010, 49, 181–187. [Google Scholar] [CrossRef]
- Rayman, M.P.; Searle, E.; Kelly, L.; Johnsen, S.; Bodman-Smith, K.; Bath, S.C.; Mao, J.; Redman, C.W. Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: A randomised, controlled pilot trial. Br. J. Nutr. 2014, 112, 99–111. [Google Scholar] [CrossRef]
- Sole-Navais, P.; Brantsaeter, A.L.; Caspersen, I.H.; Lundh, T.; Muglia, L.J.; Meltzer, H.M.; Zhang, G.; Jacobsson, B.; Sengpiel, V.; Barman, M. Maternal Dietary Selenium Intake during Pregnancy Is Associated with Higher Birth Weight and Lower Risk of Small for Gestational Age Births in the Norwegian Mother, Father and Child Cohort Study. Nutrients 2020, 13, 23. [Google Scholar] [CrossRef]
- Leon, J.; Acuna-Castroviejo, D.; Sainz, R.M.; Mayo, J.C.; Tan, D.X.; Reiter, R.J. Melatonin and mitochondrial function. Life Sci. 2004, 75, 765–790. [Google Scholar] [CrossRef]
- Tomas-Zapico, C.; Coto-Montes, A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J. Pineal Res. 2005, 39, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Nakazawa, K.; Sakai, J.; Kometani, K.; Iwashita, M.; Yoshimura, Y.; Maruyama, T. Melatonin as a local regulator of human placental function. J. Pineal Res. 2005, 39, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Lanoix, D.; Beghdadi, H.; Lafond, J.; Vaillancourt, C. Human placental trophoblasts synthesize melatonin and express its receptors. J. Pineal Res. 2008, 45, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; Lacasse, A.A.; Lanoix, D.; Sagrillo-Fagundes, L.; Boulard, V.; Vaillancourt, C. Placental melatonin system is present throughout pregnancy and regulates villous trophoblast differentiation. J. Pineal Res. 2015, 59, 38–46. [Google Scholar] [CrossRef]
- Lanoix, D.; Guerin, P.; Vaillancourt, C. Placental melatonin production and melatonin receptor expression are altered in preeclampsia: New insights into the role of this hormone in pregnancy. J. Pineal Res. 2012, 53, 417–425. [Google Scholar] [CrossRef]
- Berbets, A.M.; Davydenko, I.S.; Barbe, A.M.; Konkov, D.H.; Albota, O.M.; Yuzko, O.M. Melatonin 1A and 1B Receptors’ Expression Decreases in the Placenta of Women with Fetal Growth Restriction. Reprod. Sci. 2021, 28, 197–206. [Google Scholar] [CrossRef]
- Huo, X.; Wang, C.; Yu, Z.; Peng, Y.; Wang, S.; Feng, S.; Zhang, S.; Tian, X.; Sun, C.; Liu, K.; et al. Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef]
- Lanoix, D.; Lacasse, A.A.; Reiter, R.J.; Vaillancourt, C. Melatonin: The watchdog of villous trophoblast homeostasis against hypoxia/reoxygenation-induced oxidative stress and apoptosis. Mol. Cell. Endocrinol. 2013, 381, 35–45. [Google Scholar] [CrossRef]
- Sagrillo-Fagundes, L.; Clabault, H.; Laurent, L.; Hudon-Thibeault, A.A.; Salustiano, E.M.; Fortier, M.; Bienvenue-Pariseault, J.; Wong Yen, P.; Sanderson, J.T.; Vaillancourt, C. Human Primary Trophoblast Cell Culture Model to Study the Protective Effects of Melatonin Against Hypoxia/reoxygenation-induced Disruption. J. Vis. Exp. 2016. [Google Scholar] [CrossRef]
- Sagrillo-Fagundes, L.; Assuncao Salustiano, E.M.; Ruano, R.; Markus, R.P.; Vaillancourt, C. Melatonin modulates autophagy and inflammation protecting human placental trophoblast from hypoxia/reoxygenation. J. Pineal Res. 2018, 65, e12520. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Binder, N.K.; Beard, S.; Nguyen, T.V.; Kaitu’u-Lino, T.J.; Tong, S. Melatonin enhances antioxidant molecules in the placenta, reduces secretion of soluble fms-like tyrosine kinase 1 (sFLT) from primary trophoblast but does not rescue endothelial dysfunction: An evaluation of its potential to treat preeclampsia. PLoS ONE 2018, 13, e0187082. [Google Scholar] [CrossRef]
- Uzun, M.; Gencer, M.; Turkon, H.; Oztopuz, R.O.; Demir, U.; Ovali, M.A. Effects of Melatonin on Blood Pressure, Oxidative Stress and Placental Expressions of TNFalpha, IL-6, VEGF and sFlt-1 in RUPP Rat Model of Preeclampsia. Arch. Med. Res. 2017, 48, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Wakatsuki, A.; Shinohara, K.; Taniguchi, K.; Fukaya, T. Melatonin protects against oxidative mitochondrial damage induced in rat placenta by ischemia and reperfusion. J. Pineal Res. 2001, 31, 173–178. [Google Scholar] [CrossRef]
- Hobson, S.R.; Gurusinghe, S.; Lim, R.; Alers, N.O.; Miller, S.L.; Kingdom, J.C.; Wallace, E.M. Melatonin improves endothelial function in vitro and prolongs pregnancy in women with early-onset preeclampsia. J. Pineal Res. 2018, 65, e12508. [Google Scholar] [CrossRef]
- Gonzalez-Candia, A.; Veliz, M.; Araya, C.; Quezada, S.; Ebensperger, G.; Seron-Ferre, M.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Potential adverse effects of antenatal melatonin as a treatment for intrauterine growth restriction: Findings in pregnant sheep. Am. J. Obstet. Gynecol. 2016, 215, 245.e1–245.e7. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. Targeting lipophilic cations to mitochondria. Biochim. Biophys. Acta 2008, 1777, 1028–1031. [Google Scholar] [CrossRef]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef]
- Gioscia-Ryan, R.A.; LaRocca, T.J.; Sindler, A.L.; Zigler, M.C.; Murphy, M.P.; Seals, D.R. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J. Physiol. 2014, 592, 2549–2561. [Google Scholar] [CrossRef]
- Gioscia-Ryan, R.A.; Battson, M.L.; Cuevas, L.M.; Eng, J.S.; Murphy, M.P.; Seals, D.R. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J. Appl. Physiol. 2018, 124, 1194–1202. [Google Scholar] [CrossRef]
- Rossman, M.J.; Santos-Parker, J.R.; Steward, C.A.C.; Bispham, N.Z.; Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A.; Chonchol, M.; Gioscia-Ryan, R.A.; Murphy, M.P.; et al. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.J.; Scott, H.; Menassa, D.A.; Bignell, A.L.; Sood, A.; Morton, J.S.; Akagi, T.; Azuma, K.; Rogers, M.F.; Gilmore, C.E.; et al. Treating the placenta to prevent adverse effects of gestational hypoxia on fetal brain development. Sci. Rep. 2017, 7, 9079. [Google Scholar] [CrossRef] [PubMed]
- Botting, K.J.; Skeffington, K.L.; Niu, Y.; Allison, B.J.; Brain, K.L.; Itani, N.; Beck, C.; Logan, A.; Murray, A.J.; Murphy, M.P.; et al. Translatable mitochondria-targeted protection against programmed cardiovascular dysfunction. Sci. Adv. 2020, 6, eabb1929. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, A.M.; Camm, E.J.; Sferruzzi-Perri, A.N.; Ashmore, T.J.; Yung, H.W.; Cindrova-Davies, T.; Spiroski, A.M.; Sutherland, M.R.; Logan, A.; Austin-Williams, S.; et al. Placental Adaptation to Early-Onset Hypoxic Pregnancy and Mitochondria-Targeted Antioxidant Therapy in a Rodent Model. Am. J. Pathol. 2018, 188, 2704–2716. [Google Scholar] [CrossRef]
- Ganguly, E.; Aljunaidy, M.M.; Kirschenman, R.; Spaans, F.; Morton, J.S.; Phillips, T.E.J.; Case, C.P.; Cooke, C.M.; Davidge, S.T. Sex-Specific Effects of Nanoparticle-Encapsulated MitoQ (nMitoQ) Delivery to the Placenta in a Rat Model of Fetal Hypoxia. Front. Physiol. 2019, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Llurba Olive, E.; Xiao, E.; Natale, D.R.; Fisher, S.A. Oxygen and lack of oxygen in fetal and placental development, feto-placental coupling, and congenital heart defects. Birth Defects Res. 2018, 110, 1517–1530. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).