Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted from Hazelnut Skin on Advanced Glycation End-Products (AGEs) Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Polyphenols from Hazelnut Skins
2.2. HPLC-DAD/MS Analysis of Phenolic Compounds
2.3. Determination of Total Phenolic Content
2.4. Determination of Antioxidant Activity
2.5. In Vitro Glycation Assay with BSA-MGO
2.6. Measurement of AGE Fluorescence
2.7. Statistical Analysis
3. Results
3.1. HPLC-PDA/MS Quali-Quantitative Analysis of Phenolic Compounds in Methanolic and Aqueous Extracts of Hazelnut Skin
3.2. Analysis of Phenol Content and Antioxidant Activity in Hazelnut Skin Extract
3.3. AGE Quantification
3.4. Inhibitory Effect of Hazelnut Skin Extract on AGEs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morand, C.; Tomás-Barberán, F.A. Contribution of plant food bioactives in promoting health effects of plant foods: Why look at interindividual variability? Eur. J. Nutr. 2019, 58, 13–19. [Google Scholar] [CrossRef]
- Soare, A.; Khazrai, Y.M.; Fontana, L.; Del Toro, R.; Lazzaro, M.C.; Di Rosa, C.; Buldo, A.; Fioriti, E.; Maddaloni, E.; Angeletti, S.; et al. Treatment of reactive hypoglycemia with the macrobiotic Ma-PI 2 diet as assessed by continuous glucose monitoring: The MAHYP randomized crossover trial. Metabolism 2017, 69, 148–156. [Google Scholar] [CrossRef]
- Ran, X.L.; Zhang, M.; Wang, Y.; Adhikari, B. Novel technologies applied for recovery and value addition of high value compounds from plant byproducts: A review. Crit. Rev. Food. 2019, 59, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Alasalvar, C.; Liyana-Pathirana, C.M. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproducts. J. Agric. Food Chem. 2007, 4, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Calani, L.; Dall’Asta, M.; Brighenti, F. Polyphenolic composition of hazelnut skin. J. Agric. Food Chem. 2011, 59, 9935–9941. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Isom, L.D.; Hanna, M.A. Extraction, identification, and quantification of antioxidant phenolics from hazelnut (Corylus avellana L.) shells. Food Chem. 2017, 244. [Google Scholar] [CrossRef] [PubMed]
- Pelvan, E.; Olgun, E.Ö.; Karadağ, A.; Alasalvar, C. Phenolic profiles and antioxidant activity of Turkish Tombul hazelnut samples (natural, roasted, and roasted hazelnut skin). Food Chem. 2017, 244, 102–108. [Google Scholar] [CrossRef]
- Masullo, M.; Cerulli, A.; Mari, A.; De Souza Santos, C.C.; Pizza, C.; Piacente, S. LC-MS profiling highlights hazelnut (Nocciola di Giffoni PGI) shells as a byproduct rich in antioxidant phenolics. Food Res. Int. 2017, 101, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Virk, M.S.; Chen, F. Phenolic acids inhibit the formation of advanced glycation end products in food simulation systems depending on their reducing powers and structures. Int. J. Food Sci. Nutr. 2016, 67, 400–411. [Google Scholar] [CrossRef]
- Khan, M.; Liu, H.; Wang, J.; Sun, B. Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: A comprehensive review. Food Res. Int. 2019, 130, 108933. [Google Scholar] [CrossRef]
- Luevano-Contreras, C.; Chapman-Novakofski, K. Dietary advanced glycation end products and aging. Nutrient 2010, 2, 1247. [Google Scholar] [CrossRef]
- Hemmler, D.; Roullier-Gall, C.; Marshall, J.V.; Rychlik, M.; Taylor, A.J.; Schmitt-Kopplin, P. Evolution of Complex Maillard Chemical Reactions, Resolved in Time. Sci. Rep. 2017, 7, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Strauch, C.M.; Shen, V.; Monnier, V.M. 2-Aminoadipic acid is a marker of protein carbonyl oxidation in the aging human skin: Effects of diabetes, renal failure and sepsis. Biochem. J. 2007, 404, 269–277. [Google Scholar] [CrossRef]
- Schmitt, A.; Schmitt, J.; Münch, G.; Gasic-Milencovic, J. Characterization of advanced glycation end products for biochemical studies: Side chain modifications and fluorescence characteristics. Anal. Biochem. 2005, 338, 201–215. [Google Scholar] [CrossRef]
- Bidasee, K.R.; Zhang, Y.; Shao, C.H.; Wang, M.; Patel, K.P.; Dincer, U.D.; Besch, H.R. Diabetes Increases Formation of Advanced Glycation End Products on Sarco(endo)plasmic Reticulum Ca2+-ATPase. Diabetes 2004, 5, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Hegab, Z.; Gibbons, S.; Neyses, L.; A Mamas, M. Role of advanced glycation end products in cardiovascular disease. World J. Cardiol. 2012, 4, 90. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef]
- Liu, L.; Hedegaard, R.V.; Skibsted, L.H. Effect of plant polyphenols on the formation of advanced glycation end products from β-lactoglobulin. Food Sci. Biotechnol. 2017, 26, 389–391. [Google Scholar] [CrossRef]
- Wu, J.V.; Hsieh, C.L.; Wang, H.Y.; Chen, H.Y. Inhibitory effects of guava (Psidium guajava L.) leaf extracts and its active compounds on the glycation process of protein. Food Chem. 2009, 113, 78–84. [Google Scholar] [CrossRef]
- Silván, J.M.; Assar, S.H.; Srey, C.; Del Castillo, M.D.; Ames, J.M. Control of the Maillard reaction by ferulic acid. Food Chem. 2011, 128, 208–213. [Google Scholar] [CrossRef]
- Umadevi, S.; Gopi, V.; Vellaichamy, E. Inhibitory effect of gallic acid on advanced glycation end products induced up-regulation of inflammatory cytokines and matrix proteins in H9C2 (2-1) cells. Cardiovasc. Toxicol. 2013, 13, 396–405. [Google Scholar] [CrossRef]
- Mesías, M.; Navarro, M.; Gökmen, V.; Morales, F.J. Antiglycative effect of fruit and vegetable seed extracts: Inhibition of AGE formation and carbonyl-trapping abilities. J. Sci. Food Agric. 2013, 93, 2037–2044. [Google Scholar] [CrossRef]
- Contini, M.; Baccelloni, S.; Massantini, R.; Anelli, G. Extraction of natural antioxidants from hazelnut (Corylus avellana L.) shell and skin wastes by long maceration at room temperature. Food Chem. 2008, 110, 659–669. [Google Scholar] [CrossRef]
- Jakopic, J.; Petkovsek, M.M.; Likozar, A.; Solar, A.; Stampar, F.; Veberic, R. HPLC-MS identification of phenols in hazelnut (Corylus avellana L.) kernels. Food Chem. 2011, 124, 1100–1106. [Google Scholar] [CrossRef]
- Fanali, C.; Tripodo, G.; Russo, M.; Della Posta, S.; Pasqualetti, V.; De Gara, L. Effect of solvent on the extraction of phenolic compounds and antioxidant capacity of hazelnut kernel. Electrophoresis 2018, 39, 1683–1691. [Google Scholar] [CrossRef]
- Statista. Production of Tree Nuts Worldwide in 2019/2020, by Type (in 1000 Metric Tons). Available online: https://www.sttista.com/statistics/1030790/tree-nut-global-production-by-type/ (accessed on 3 March 2021).
- Martínez, M.; Moiraghi, L.; Agnese, M.; Guzman, C. Making and some properties of activated carbon produced from agricultural industrial residues from Argentina. J. Argent. Chem. Soc. 2003, 91, 104–108. [Google Scholar]
- Barbu, M.C.; Sepperer, T.; Tudor, E.M.; Petutschnigg, A. Walnut and Hazelnut Shells: Untapped Industrial Resources and Their Suitability in Lignocellulosic Composites. Appl. Sci. 2020, 10, 6340. [Google Scholar] [CrossRef]
- Alasalvar, A.; Hoffman, A.M.; Shahidi, F. Antioxidant Activities and Phytochemicals in Hazelnut (Corylus avellana L.) and Hazelnut By-Products. In Tree Nuts Composition, Phytochemicals, and Health Effects; Alasalvar, C., Shahidi, F., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 215–235. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 875–877. [Google Scholar] [CrossRef]
- Ellouze, I.; Abderrabba, M.; Sabaou, N.; Mathieu, F.; Lebrihi, A.; Bouajila, J. Season’s Variation Impact on Citrus aurantium Leaves Essential Oil: Chemical Composition and Biological Activities. J. Food Sci. 2012, 77, 9. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated β-cyclodextrin as the solubility enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M.; Zieliński, H. Inhibition of advanced glycation end-product formation by high antioxidant-leveled spices commonly used in European cuisine. Antioxidants 2019, 8, 100. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Yilmaz, C.; Durmaz, G.; Gokmen, V. Hazelnut skin powder: A new brown colored functional ingredient. Food Res. Int. 2014, 65, 291–297. [Google Scholar] [CrossRef]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. HPLC-MSn identification and quantification of phenolic compounds in hazelnut kernels, oil and bagasse pellets. Food Res. Int. 2014, 64, 36. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebron-Aguilar, R.; Gomez-Cordoves, M.C.; Rybarczyk, A.; Amarowicz, R.; Bartolome, B. Comparative flavan-3-ol profile and antioxidant capacity of roasted peanut, hazelnut, and almond skins. J. Agric. Food Chem. 2009, 57, 10590–10599. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Pagano, I.; Esposito, T.; Mencherini, T.; Porta, A.; Petrone, A.M.; Gazzerro, P.; Picerno, P.; Sansone, P.; Rastrelli, L.; et al. HRMS Profile of a Hazelnut Skin Proanthocyanidin-rich Fraction with Antioxidant and Anti-Candida albicans Activities. J. Agric. Food Chem. 2016, 64, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Grosso, G.; Yang, J.; Marventano, S.; Micek, A.; Galvano, F.; Kales, S.N. Nut consumption on all-cause, cardiovascular, and cancer mortality risk: A systematic review and meta-analysis of epidemiologic studies. Am. J. Clin. Nutr. 2015, 101, 783–793. [Google Scholar] [CrossRef]
- Mayhew, A.J.; De Souza, R.J.; Meyre, D.; Anand, S.S.; Mente, A. A systematic review and meta-analysis of nut consumption and incident risk of CVD and all-cause mortality. Br. J. Nutr. 2016, 115, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Contini, M.; Baccelloni, S.; Frangipane, M.T.; Merendino, M.; Massantini, R. Increasing espresso coffee brew antioxidant capacity using phenolic extract recovered from hazelnut skin waste. J. Funct. Foods. 2012, 4, 137–146. [Google Scholar] [CrossRef]
- Anil, M. Using of hazelnut testa as a source of dietary fiber in breadmaking. J. Food Eng. 2007, 80, 61–67. [Google Scholar] [CrossRef]
- Bertolino, M.; Belviso, S.; Dal Bello, B.; Ghirardello, D.; Giordano, M.; Rolle, L.; Gerbi, V.; Zeppa, G. Influence of the addition of different hazelnut skins on the physicochemical, antioxidant, polyphenol and sensory properties of yogurt. LWT Food Sci. Technol. 2015, 63, 1145–1154. [Google Scholar] [CrossRef]
- Montella, R.; Coisson, J.D.; Travaglia, F.; Locatelli, M.; Mafalda, P.; Martelli, A.; Arlorio, M. Bioactive compounds from hazelnut skin (Corylus avellana L.): Effects on Lactobacillus plantarum P17630 and Lactobacillus crispatus P17631. J. Funct. Foods 2013, 5, 306–315. [Google Scholar] [CrossRef]
- Caimari, A.; Puiggròs, F.; Suárez, M.; Crescenti, A.; Laos, S.; Ruiz, J.A.; Alonso, V.; Moragas, J.; Del Bas, J.M.; Arola, L. The intake of a hazelnut skin extract improves the plasma lipid profile and reduces the lithocholic/deoxycholic bile acid faecal ratio, a risk factor for colon cancer, in hamsters fed a high-fat diet. Food Chem. 2015, 167, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, S.; Avramovic, N.; Dojcinovic, B.; Trifunovic, S.; Novakovic, M.; Teševic, V.; Mandic, B. Chemical composition, total phenols and flavonoids contents and antioxidant activity as nutritive potential of roasted hazelnut skins (Corylus avellana L.). Foods 2020, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, A.; Yazdanparast, R. Cyperus rotundus suppresses AGE formation and protein oxidation in a model of fructose-mediated protein glycoxidation. Int. J. Biol. Macromol. 2007, 41, 572–578. [Google Scholar] [CrossRef]
- Ou, J.; Huang, J.; Wang, M.; Ou, S. Effect of rosmarinic acid and carnosic acid on AGEs formation in vitro. Food Chem. 2017, 221, 1057–1061. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Chen, F.; Wang, M. Naturally occurring inhibitors against the formation of advanced glycation end-product. Food Funct. 2001, 2, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, R.H.; Sarkar, P.; Mally, A.; Biemel, K.M.; Lederer, O.; Padayatti, P.S. Effect of pyridoxamine on chemical modification of proteins by carbonyls in diabetic rats: Characterization of a major product from the reaction of pyridoxamine and methylglyoxal. Arch. Biochem. Biophys. 2002, 402, 110–119. [Google Scholar] [CrossRef]
- Yoon, S.P.; Maeng, Y.H.; Hong, R.; Byung, R.L.; Lee, B.R.; Kime, G.K.; Kim, C.G.; Chung, J.H.; Shin, B.C. Protective effects of epigallocatechin gallate (EGCG) on streptozotocin-induced diabetic nephropathy in mice. Acta Histochem. 2014, 116, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Laurean, D.; Schramm, D.D.; Jacobson, E.L.; Halaweish, I.; Bruckner, G.G.; Boissonneault, G.A. Inhibition of advanced glycation end product formation on collagen by rutin and its metabolites. J. Nutr. Biochem. 2006, 17, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J. Agric. Food Chem. 2014, 62, 152–12158. [Google Scholar] [CrossRef]
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2019, 148, 54–63. [Google Scholar] [CrossRef] [PubMed]
- D’Introno, A.; Paradiso, A.; Scoditti, E.; D’Amico, L.; De Paolis, A.; Carluccio, M.A.; Nicoletti, I.; De Gara, L.; Santino, A.; Giovinazzo, G. Antioxidant and anti-inflammatory properties of tomato fruits synthesizing different amounts of stilbenes. Plant Biotechnol. J. 2009, 7, 422–429. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
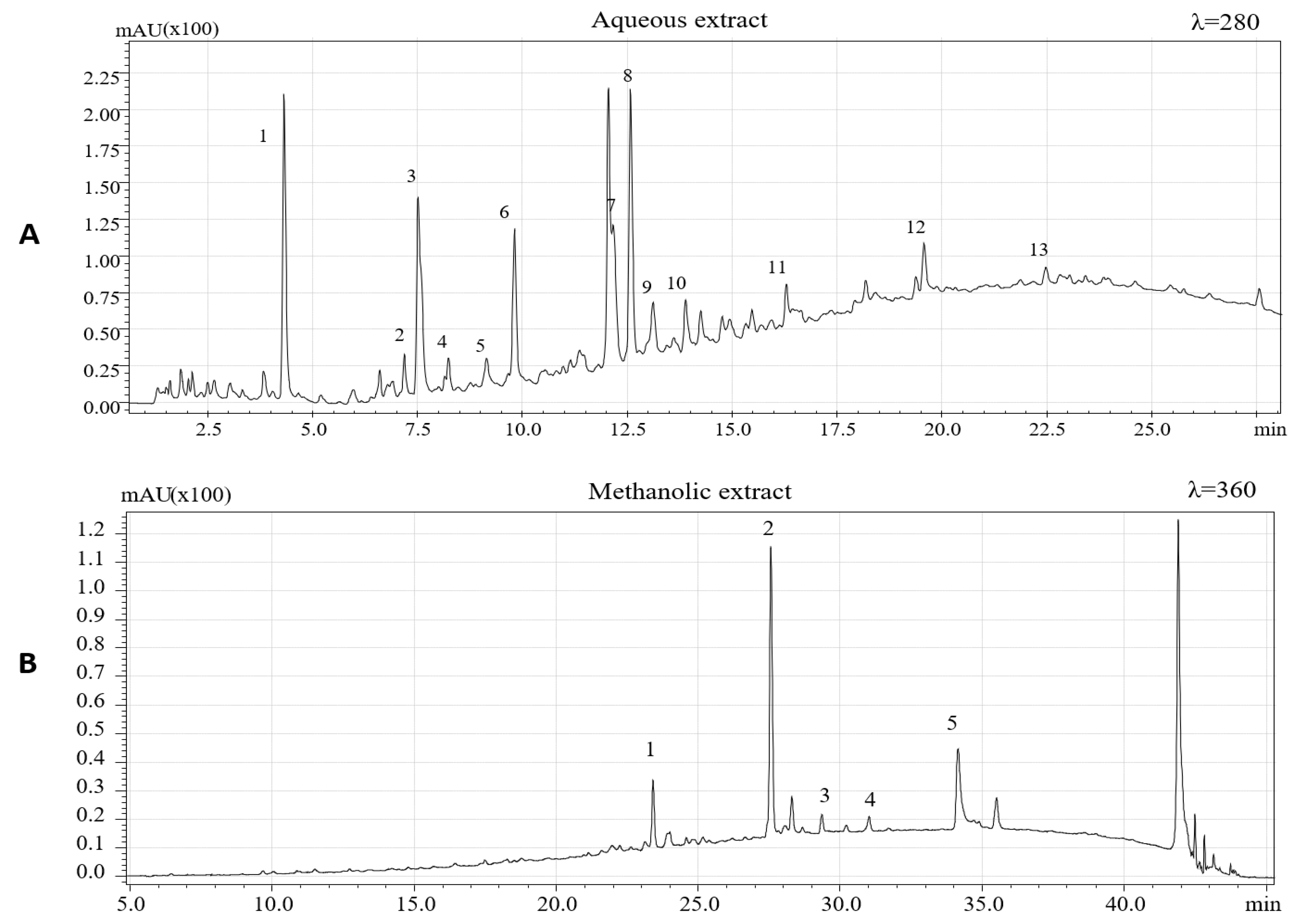




| N° | Compound Identified | Retention Time (min) | m/z (M−H)− |
|---|---|---|---|
| 1 | Gallic acid | 4.43 | 169 |
| 2 | Protocatechuic acid | 7.32 | 153 |
| 3 | Procyanidin C2 trimer | 7.55 | 865 |
| 4 | Prodelphinidin beta-type dimer | 8.19 | 593 |
| 5 | Prodelphinidin beta-type dimer | 9.05 | 593 |
| 6 | Prodelphinidin beta-type dimer | 9.59 | 593 |
| 7 | Procyanidin beta 1 dimer | 11.96 | 577 |
| 8 | (+) Catechin | 12.41 | 289 |
| 9 | Procyanidin beta-type trimer | 13.03 | 865 |
| 10 | Procyanidin beta-type trimer | 13.83 | 865 |
| 11 | (-) epicatechin | 16.20 | 289 |
| 12 | Procyanidin Beta-type dimer gallate | 19.55 | 729 |
| N° | Compound Identified | Retention Time (min) | m/z (M−H)− |
|---|---|---|---|
| 1 | Myricetin rhamnoside | 23.40 | 463 |
| 2 | Quercitin 3-0-rhamnoside | 27.52 | 447 |
| 3 | Phloretin 2-o-glucoside | 29.30 | 435 |
| 4 | Kaempferol rhamnoside | 30.96 | 431 |
| 5 | Quercetin | 34.16 | 301 |
| Compound | TPC (mg GAE/g) | TEAC (mmol TE/g) | ORAC (mmol TE/g) |
|---|---|---|---|
| Hazelnut skin 100 mg/mL | 70.07 ± 1.38 | 0.28 ± 0.03 | 0.35 ± 0.02 |
| Gallic acid 1 mg/mL | 10.98 ± 1.89 | 42 ± 3.34 |
| Compounds | IC50 (μg/mL) |
|---|---|
| Hz extract | 109.7 |
| gallic acid | 147.6 |
| AG | 117.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagnuolo, L.; Della Posta, S.; Fanali, C.; Dugo, L.; De Gara, L. Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted from Hazelnut Skin on Advanced Glycation End-Products (AGEs) Formation. Antioxidants 2021, 10, 424. https://doi.org/10.3390/antiox10030424
Spagnuolo L, Della Posta S, Fanali C, Dugo L, De Gara L. Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted from Hazelnut Skin on Advanced Glycation End-Products (AGEs) Formation. Antioxidants. 2021; 10(3):424. https://doi.org/10.3390/antiox10030424
Chicago/Turabian StyleSpagnuolo, Ludovica, Susanna Della Posta, Chiara Fanali, Laura Dugo, and Laura De Gara. 2021. "Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted from Hazelnut Skin on Advanced Glycation End-Products (AGEs) Formation" Antioxidants 10, no. 3: 424. https://doi.org/10.3390/antiox10030424
APA StyleSpagnuolo, L., Della Posta, S., Fanali, C., Dugo, L., & De Gara, L. (2021). Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted from Hazelnut Skin on Advanced Glycation End-Products (AGEs) Formation. Antioxidants, 10(3), 424. https://doi.org/10.3390/antiox10030424








