New Insights into the Metabolism of the Flavanones Eriocitrin and Hesperidin: A Comparative Human Pharmacokinetic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Analysis of Orange and Lemon Extracts by HPLC-DAD-MS/MS
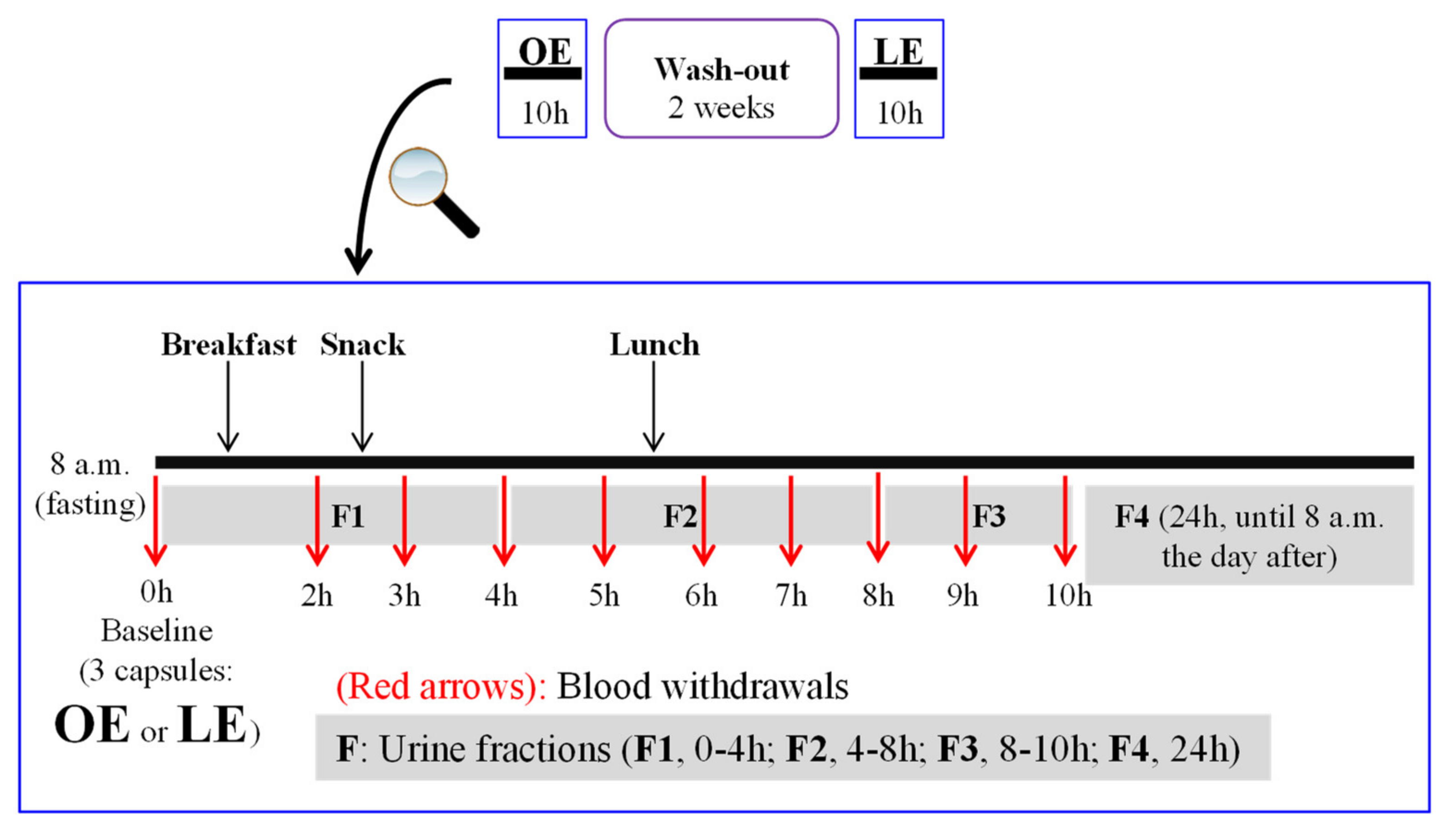
2.3. Volunteers and Study Design
2.4. Diet
2.5. Analysis of Flavanone-Derived Metabolites in Urine and Plasma by UPLC-ESI-QTOF-MS
2.6. Inflammation and Oxidative Markers in Urine and Plasma Samples
2.7. Statistical Analysis
3. Results
3.1. Analysis of Phenolic Compounds in the Citrus Extracts
3.2. Post-Prandial Response after Consumption High-Fat-High-Sugar Meal
3.2.1. Metabolic and Biochemical Markers
3.2.2. Inflammation and Oxidative Markers
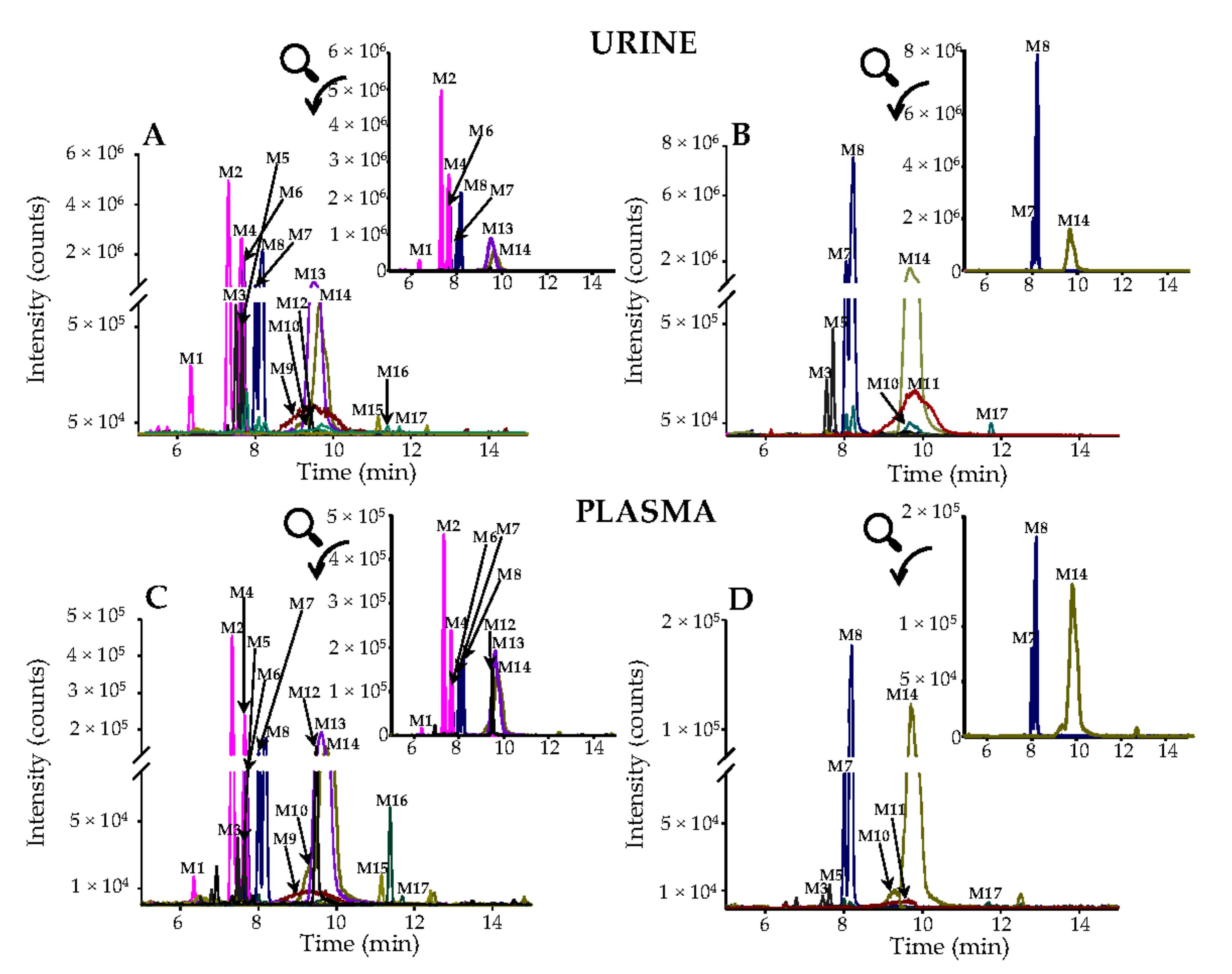
3.3. Urinary Excretion of Phenolic Metabolites
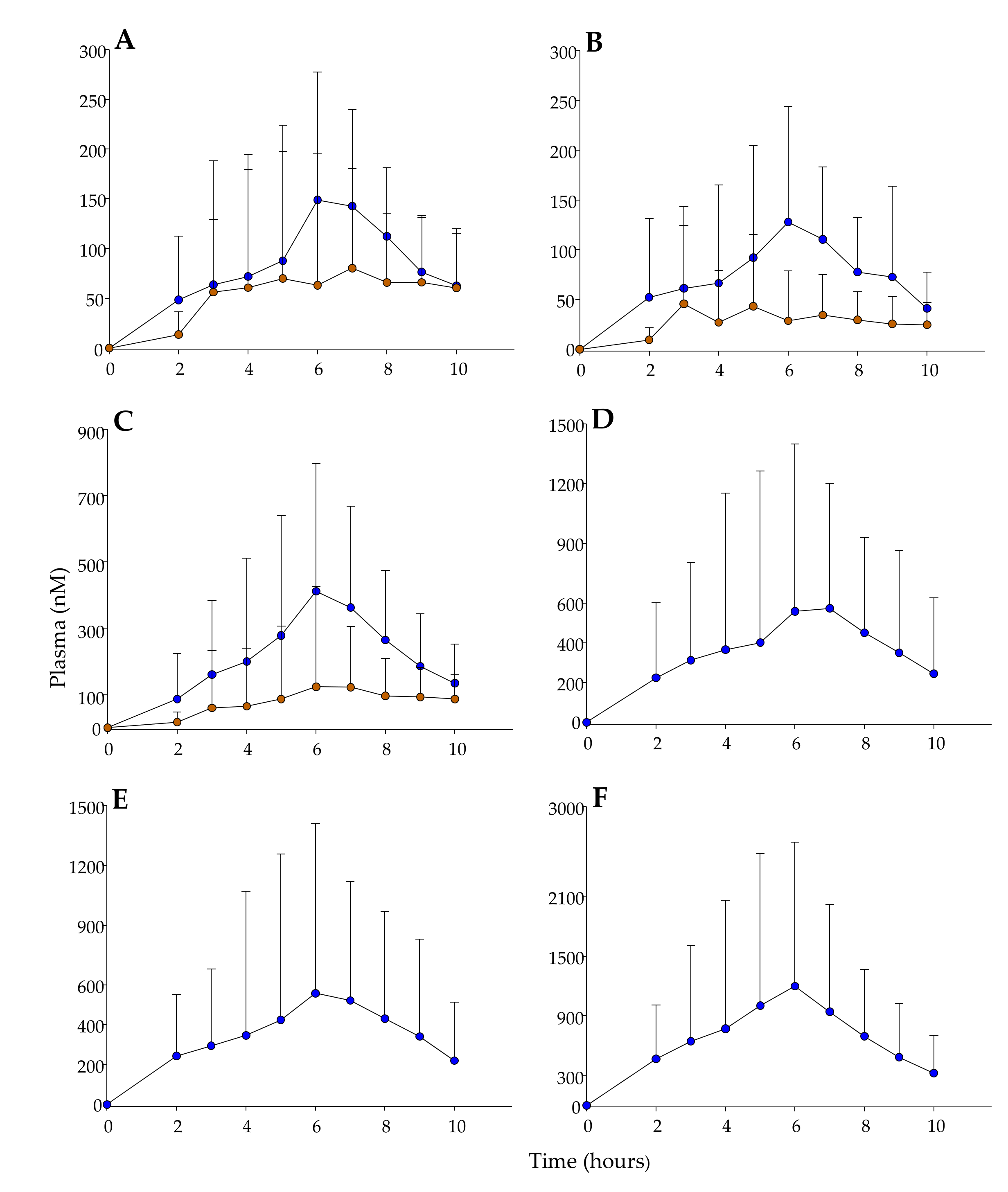
3.4. Phenolic Metabolites in Plasma and Pharmacokinetic Evaluation.
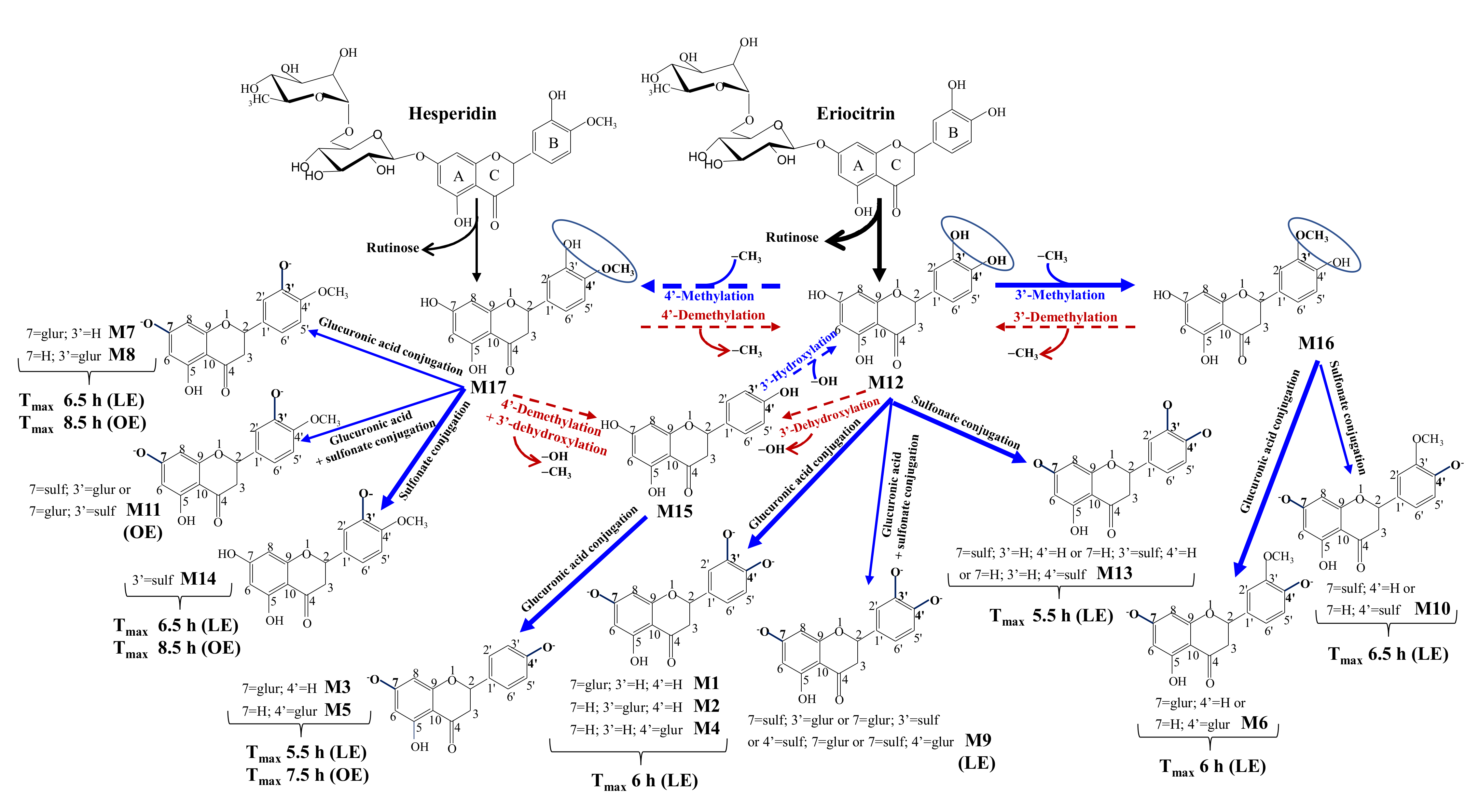
3.5. Human Metabolism of Eriocitrin and Hesperidin to Yield Phase-II Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassidy, A.; Rimm, E.B.; O’Reilly, E.J.; Logroscino, G.; Kay, C.; Chiuve, S.E.; Rexrode, K.M. Dietary Flavonoids and Risk of Stroke in Women. Stroke 2012, 43, 946–951. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Robertson, A.S.; Jacobs, E.J.; Chao, A.; Calle, E.E.; Thun, M.J. A Prospective Study of Diet and Stomach Cancer Mortality in United States Men and Women. Cancer Epidemiol. Biomark. Prev. 2001, 10, 1201. [Google Scholar]
- Gates, M.A.; Tworoger, S.S.; Hecht, J.L.; De Vivo, I.; Rosner, B.; Hankinson, S.E. A Prospective Study of Dietary Flavonoid Intake and Incidence of Epithelial Ovarian Cancer. Int. J. Cancer 2007, 121, 2225–2232. [Google Scholar] [CrossRef]
- Chanet, A.; Milenkovic, D.; Manach, C.; Mazur, A.; Morand, C. Citrus Flavanones: What Is Their Role in Cardiovascular Protection? J. Agric. Food Chem. 2012, 60, 8809–8822. [Google Scholar] [CrossRef]
- Morand, C.; Dubray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin Contributes to the Vascular Protective Effects of Orange Juice: A Randomized Crossover Study in Healthy Volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Ludwig, I.A.; Polyviou, T.; Malkova, D.; García, A.; Moreno-Rojas, J.M.; Crozier, A. Identification of Plasma and Urinary Metabolites and Catabolites Derived from Orange Juice (Poly)Phenols: Analysis by High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 5724–5735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Schluesener, H. Health-Promoting Effects of the Citrus Flavanone Hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef]
- Najmanová, I.; Vopršalová, M.; Saso, L.; Mladěnka, P. The Pharmacokinetics of Flavanones. Crit. Rev. Food Sci. Nutr. 2020, 60, 3155–3171. [Google Scholar] [CrossRef]
- Valls, R.M.; Pedret, A.; Calderón-Pérez, L.; Llauradó, E.; Pla-Pagà, L.; Companys, J.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of Hesperidin in Orange Juice on Blood and Pulse Pressures in Mildly Hypertensive Individuals: A Randomized Controlled Trial (Citrus Study). Eur. J. Nutr. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Assini, J.M.; Mulvihill, E.E.; Huff, M.W. Citrus Flavonoids and Lipid Metabolism. Curr. Opin. Lipidol. 2013, 24, 34–40. [Google Scholar] [CrossRef]
- Testai, L.; Calderone, V. Nutraceutical Value of Citrus Flavanones and Their Implications in Cardiovascular Disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakkos, S.K.; Nicolaides, A.N. Efficacy of Micronized Purified Flavonoid Fraction (Daflon®) on Improving Individual Symptoms, Signs and Quality of Life in Patients with Chronic Venous Disease: A Systematic Review and Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Int. Angiol. 2018, 37, 143–154. [Google Scholar] [CrossRef]
- Ulloa, J.H. Micronized Purified Flavonoid Fraction (MPFF) for Patients Suffering from Chronic Venous Disease: A Review of New Evidence. Adv. Ther. 2019, 36, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of a Health Claim Related to a Combination of Diosmin, Troxerutin and Hesperidin and Maintenance of Normal Venous-capillary Permeability Pursuant to Article 13 (5) of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 3511. [Google Scholar]
- González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A. Non-Extractable Polyphenols Produce Gut Microbiota Metabolites That Persist in Circulation and Show Anti-Inflammatory and Free Radical-Scavenging Effects. Trends Food Sci. Technol. 2017, 69, 281–288. [Google Scholar] [CrossRef]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F.; et al. Addressing the Inter-Individual Variation in Response to Consumption of Plant Food Bioactives: Towards a Better Understanding of Their Role in Healthy Aging and Cardiometabolic Risk Reduction. Mol. Nutr. Food Res. 2017, 61, 1600557. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, 1900952. [Google Scholar] [CrossRef]
- Vallejo, F.; Larrosa, M.; Escudero, E.; Zafrilla, M.P.; Cerdá, B.; Boza, J.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Concentration and Solubility of Flavanones in Orange Beverages Affect Their Bioavailability in Humans. J. Agric. Food Chem. 2010, 58, 6516–6524. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; Martínez-Florensa, M.; Espín, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.T. A citrus extract containing flavanones represses plasminogen activator inhibitor-1 (PAI-1) expression and regulates multiple inflammatory, tissue repair, and fibrosis genes in human colon fibroblasts. J. Agric. Food Chem. 2009, 57, 9305–9315. [Google Scholar] [CrossRef]
- Van Rymenant, E.; Salden, B.; Voorspoels, S.; Jacobs, G.; Noten, B.; Pitart, J.; Possemiers, S.; Smagghe, G.; Grootaert, C.; Van Camp, J.A. Critical Evaluation of In Vitro Hesperidin 2S Bioavailability in a Model Combining Luminal (Microbial) Digestion and Caco-2 Cell Absorption in Comparison to a Randomized Controlled Human Trial. Mol. Nutr. Food Res. 2018, 62, e1700881. [Google Scholar] [CrossRef]
- Denaro, M.; Smeriglio, A.; Trombetta, D. Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix and Its Stability after In Vitro Simulated Digestion. Antioxidants 2021, 10, 140. [Google Scholar] [CrossRef]
- Yamamoto, M.; Jokura, H.; Hashizume, K.; Ominami, H.; Shibuya, Y.; Suzuki, A.; Hase, T.; Shimotoyodome, A. Hesperidin Metabolite Hesperetin-7-O-Glucuronide, but Not Hesperetin-3′-O-Glucuronide, Exerts Hypotensive, Vasodilatory, and Anti-Inflammatory Activities. Food Funct. 2013, 4, 1346–1351. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Vallejo, F.; Espín, J.C.; Tomás-Barberán, F.A. Hesperetin and its Sulfate and Glucuronide Metabolites Inhibit TNF-α Induced Human Aortic Endothelial Cell Migration and Decrease Plasminogen Activator Inhibitor-1 (PAI-1) Levels. Food Funct. 2016, 7, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Cruz-Martins, N.; Butnariu, M.; Sarac, I.; Bagiu, I.-C.; Ezzat, S.M.; Wang, J.; Koay, A.; Sheridan, H.; Adetunji, C.O.; et al. Hesperetin’s Health Potential: Moving from Preclinical to Clinical Evidence and Bioavailability Issues, to Upcoming Strategies to Overcome Current Limitations. Crit. Rev. Food Sci. Nutr. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Schär, M.Y.; Curtis, P.J.; Hazim, S.; Ostertag, L.M.; Kay, C.D.; Potter, J.F.; Cassidy, A. Orange Juice-Derived Flavanone and Phenolic Metabolites Do Not Acutely Affect Cardiovascular Risk Biomarkers: A Randomized, Placebo-Controlled, Crossover Trial in Men at Moderate Risk of Cardiovascular Disease. Am. J. Clin. Nutr. 2015, 101, 931–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Crescenti, A. Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Navarro, M.; Vallejo, F.; Borrego, F.; Tomás-Barberán, F.A. Encapsulation and Micronization Effectively Improve Orange Beverage Flavanone Bioavailability in Humans. J. Agric. Food Chem. 2014, 62, 9458–9462. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D.; Zhang, D.; Jiang, R.; Ayer, A.; Shengule, S.; Payne, R.J.; Ward, N.C.; Stocker, R. Structural Requirements of Flavonoids to Induce Heme Oxygenase-1 Expression. Free Radic. Biol. Med. 2017, 113, 165–175. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; García-Villalba, R.; Martínez-Díaz, F.; Ocaña-Castillo, B.; Monedero-Saiz, T.; Torrecillas-Sánchez, A.; Abellán, B.; González-Sarrías, A.; Espín, J.C. Metabolic Profiling of Dietary Polyphenols and Methylxanthines in Normal and Malignant Mammary Tissues from Breast Cancer Patients. Mol. Nutr. Food Res. 2019, 63, 1801239. [Google Scholar] [CrossRef]
- González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Zafrilla, P.; Mulero, J.; Tomás-Barberán, F.A.; Espín, J.C. Identifying the Limits for Ellagic Acid Bioavailability: A Crossover Pharmacokinetic Study in Healthy Volunteers after Consumption of Pomegranate Extracts. J. Funct. Foods 2015, 19, 225–235. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Iglesias-Aguirre, C.E.; Meoro, A.; Selma, M.V.; Espín, J.C. Pharmacological Therapy Determines the Gut Microbiota Modulation by a Pomegranate Extract Nutraceutical in Metabolic Syndrome: A Randomized Clinical Trial. Mol. Nutr. Food Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Chromatographic and Spectroscopic Characterization of Urolithins for Their Determination in Biological Samples after the Intake of Foods Containing Ellagitannins and Ellagic Acid. J. Chromatogr. A 2016, 1428, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Gouveia-Figueira, S.; Späth, J.; Zivkovic, A.M.; Nording, M.L. Profiling the Oxylipin and Endocannabinoid Metabolome by UPLC-ESI-MS/MS in Human Plasma to Monitor Postprandial Inflammation. PLoS ONE 2015, 10, e0132042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansone, F.; Mencherini, T.; Picerno, P.; d’Amore, M.; Aquino, R.P.; Lauro, M.R. Maltodextrin/Pectin Microparticles by Spray Drying as Carrier for Nutraceutical Extracts. J. Food Eng. 2011, 105, 468–476. [Google Scholar] [CrossRef]
- Ribeiro, C.B.; Ramos, F.M.; Manthey, J.A.; Cesar, T.B. Effectiveness of Eriomin® in Managing Hyperglycemia and Reversal of Prediabetes Condition: A Double-Blind, Randomized, Controlled Study. Phytother. Res. 2019, 33, 1921–1933. [Google Scholar] [CrossRef] [Green Version]
- Homayouni, F.; Haidari, F.; Hedayati, M.; Zakerkish, M.; Ahmadi, K. Hesperidin Supplementation Alleviates Oxidative DNA Damage and Lipid Peroxidation in Type 2 Diabetes: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Phytother. Res. 2017, 31, 1539–1545. [Google Scholar] [CrossRef]
- Cheraghpour, M.; Imani, H.; Ommi, S.; Alavian, S.M.; Karimi-Shahrbabak, E.; Hedayati, M.; Yari, Z.; Hekmatdoost, A. Hesperidin Improves Hepatic Steatosis, Hepatic Enzymes, and Metabolic and Inflammatory Parameters in Patients with Nonalcoholic Fatty Liver Disease: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Phytother. Res. 2019, 33, 2118–2125. [Google Scholar] [CrossRef]
- Takumi, H.; Nakamura, H.; Simizu, T.; Harada, R.; Kometani, T.; Nadamoto, T.; Mukai, R.; Murota, K.; Kawai, Y.; Terao, J. Bioavailability of Orally Administered Water-Dispersible Hesperetin and Its Effect on Peripheral Vasodilatation in Human Subjects: Implication of Endothelial Functions of Plasma Conjugated Metabolites. Food Funct. 2012, 3, 389–398. [Google Scholar] [CrossRef]
- Martínez-Noguera, F.J.; Marín-Pagán, C.; Carlos-Vivas, J.; Rubio-Arias, J.A.; Alcaraz, P.E. Acute Effects of Hesperidin in Oxidant/Antioxidant State Markers and Performance in Amateur Cyclists. Nutrients 2019, 11, 1898. [Google Scholar] [CrossRef] [Green Version]
- Demonty, I.; Lin, Y.; Zebregs, Y.E.M.P.; Vermeer, M.A.; van der Knaap, H.C.M.; Jäkel, M.; Trautwein, E.A. The Citrus Flavonoids Hesperidin and Naringin Do Not Affect Serum Cholesterol in Moderately Hypercholesterolemic Men and Women. J. Nutr. 2010, 140, 1615–1620. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.C.D.; Cecatti, C.; Fidélix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef]
- Strassburg, K.; Esser, D.; Vreeken, R.J.; Hankemeier, T.; Müller, M.; van Duynhoven, J.; van Golde, J.; van Dijk, S.J.; Afman, L.A.; Jacobs, D.M. Postprandial Fatty Acid Specific Changes in Circulating Oxylipins in Lean and Obese Men after High-Fat Challenge Tests. Mol. Nutr. Food Res. 2014, 58, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Weickert, M.O.; Qureshi, S.; Kandala, N.-B.; Anwar, A.; Waldron, M.; Shafie, A.; Messenger, D.; Fowler, M.; Jenkins, G.; et al. Improved Glycemic Control and Vascular Function in Overweight and Obese Subjects by Glyoxalase 1 Inducer Formulation. Diabetes 2016, 65, 2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-Y.O.; Rasmussen, H.; Kamil, A.; Du, P.; Blumberg, J.B. Orange Pomace Improves Postprandial Glycemic Responses: An Acute, Randomized, Placebo-Controlled, Double-Blind, Crossover Trial in Overweight Men. Nutrients 2017, 9, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Crocco, P.; Montesanto, A.; Dato, S.; Geracitano, S.; Iannone, F.; Passarino, G.; Rose, G. Inter-Individual Variability in Xenobiotic-Metabolizing Enzymes: Implications for Human Aging and Longevity. Genes 2019, 10, 403. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Feng, X.; Chen, Y.; Li, S.; Sun, Y.; Zhang, L.A. Comprehensive Study of Eriocitrin Metabolism in Vivo and in Vitro Based on an Efficient UHPLC-Q-TOF-MS/MS Strategy. RSC Adv. 2019, 9, 24963–24980. [Google Scholar] [CrossRef] [Green Version]
- Spanakis, M.; Kasmas, S.; Niopas, I. Simultaneous Determination of the Flavonoid Aglycones Diosmetin and Hesperetin in Human Plasma and Urine by a Validated GC/MS Method: In Vivo Metabolic Reduction of Diosmetin to Hesperetin. Biomed. Chromatogr. 2009, 23, 124–131. [Google Scholar] [CrossRef]
- Nectoux, A.M.; Abe, C.; Huang, S.-W.; Ohno, N.; Tabata, J.; Miyata, Y.; Tanaka, K.; Tanaka, T.; Yamamura, H.; Matsui, T. Absorption and Metabolic Behavior of Hesperidin (Rutinosylated Hesperetin) after Single Oral Administration to Sprague-Dawley Rats. J. Agric. Food Chem. 2019, 67, 9812–9819. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Polyviou, T.; Ludwig, I.A.; Nastase, A.-M.; Moreno-Rojas, J.M.; Garcia, A.L.; Malkova, D.; Crozier, A. Bioavailability of Orange Juice (Poly)Phenols: The Impact of Short-Term Cessation of Training by Male Endurance Athletes. Am. J. Clin. Nutr. 2017, 106, 791–800. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, H.; Ikoma, Y.; Sugiura, M.; Yano, M.; Hasegawa, Y. Identification and Quantification of the Conjugated Metabolites Derived from Orally Administered Hesperidin in Rat Plasma. J. Agric. Food Chem. 2004, 52, 6653–6659. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.M.; Wein, S.; Blank, R.; Metges, C.C.; Wolffram, S. Bioavailability of the Flavonol Quercetin in Cows after Intraruminal Application of Quercetin Aglycone and Rutin. J. Dairy Sci. 2012, 95, 5047–5055. [Google Scholar] [CrossRef] [PubMed]
- Anacleto, S.L.; Milenkovic, D.; Kroon, P.A.; Needs, P.W.; Lajolo, F.M.; Hassimotto, N.M.A. Citrus Flavanone Metabolites Protect Pancreatic-β Cells under Oxidative Stress Induced by Cholesterol. Food Funct. 2020, 11, 8612–8624. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Jia, Z.; Zheng, X.; Cui, S.; Chen, W. A Recent Review of Citrus Flavanone Naringenin on Metabolic Diseases and Its Potential Sources for High Yield-Production. Trends Food Sci. Technol. 2018, 79, 35–54. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Selma, M.V.; Espín, J.C.; García-Villalba, R. The Human Metabolism of Nuts Proanthocyanidins Does Not Reveal Urinary Metabolites Consistent with Distinctive Gut Microbiota Metabotypes. Mol. Nutr. Food Res. 2019, 63, 1800819. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Oliver, C.M.; Weerakkody, R.; Singh, T.; Conlon, M.; Borges, G.; Sanguansri, L.; Lockett, T.; Roberts, S.A.; Crozier, A.; et al. Chronic Administration of a Microencapsulated Probiotic Enhances the Bioavailability of Orange Juice Flavanones in Humans. Free Radic. Biol. Med. 2015, 84, 206–214. [Google Scholar] [CrossRef]
| Baseline LE 2 | Baseline OE 2 | ||
|---|---|---|---|
| Subjects characteristics | |||
| Age (years) | 33.2 ± 7.2 (26–49) | ||
| Weight (kg) | 68.1 ± 13.2 (48–90) | ||
| BMI (kg/m2) | 23.0 ± 2.1 (19–25) | ||
| Sex (Female/Male) | 8/8 | ||
| Baseline values 1 | |||
| Glucose (mg/dL) | 81.8 ± 8.3 (67–98) | 79.4 ± 7.5 (69–96) | |
| Total cholesterol (mg/dL) | 169.7 ± 30.2 (129–238) | 164.7 ± 24.9 (121–217) | |
| HDL-cholesterol (mg/dL) | 56.7 ± 12.6 (33–89) | 53.4 ± 11.8 (29–81) | |
| LDL-cholesterol (mg/dL) | 97.1 ± 25.3 (64–157) | 93.6 ± 21.2 (62–140) | |
| Triglycerides (mg/dL) | 72.6 ± 43.2 (34–173) | 74.7 ± 39.0 (35–150) | |
| Insulin (µU/mL) | 4.0 ± 1.9 (2–7) | 3.5 ± 2.2 (1–9) | |
| HOMA-IR | 0.8 ± 0.4 (0.3–1.4) | 0.7 ± 0.5 (0.2–2.0) | |
| GGT (U/mL) | 15.3 ± 9.1 (6–36) | 15.3 ± 7.9 (8–34) | |
| ALT (U/mL) | 19.5 ± 8.7 (10–39) | 19.9 ± 9.4 (11–41) | |
| Diastolic blood pressure (mmHg) | 71.5 ± 8.7 (58–91) | 71.3 ± 10.8 (57–90) | |
| Systolic blood pressure (mmHg) | 110.4 ± 10.8 (96–129) | 109.9 ± 11.0 (90–127) | |
| Heart rate (bpm) | 59.4 ± 8.9 (42–76) | 55.5 ± 7.4 (43–68) |
| Peak Nº | Compound | RT | m/z- | MS/MS | λmax | mg/g Extract 1 | Extract |
|---|---|---|---|---|---|---|---|
| 1 | Ferulic acid-O-Glu | 16.94 | 355 | 193/160/134 | 294/328 | 10.40 ± 0.51 | Lemon |
| 2 | Apigenin 6,8-di-C-Glu (Vicenin-2) | 18.13 | 593 | 503/473/383/353 | 270/332 | 14.6 ± 0.4 | Lemon |
| 3 | Eriodictyol-Glu-Rha-Glu | 18.70 | 757 | 595/449/287 | 282/330 | 33.86 ± 0.91 | Lemon |
| 4 | Diosmetin 6,8-di-C-Glu | 19.00 | 623 | 605/533/503/383 | 270/346 | 9.62 ± 0.27 | Lemon |
| 5 | Crisoeriol 6,8-di-C-Glu | 19.65 | 623 | 533/503/383 | 270/346 | 11.3 ± 0.2 | Lemon |
| 6 | Eriocitrin | 22.90 | 595 | 459/329/287 | 284/334 | 83.3 ± 5.6 | Lemon |
| 7 | Apigenin 8-C-xylanopyranosil-Glu | 23.39 | 563 | 413/341/293 | 268/332 | 10.0 ± 0.15 | Lemon |
| 8 | Diosmetin 8-C-Glu | 24.88 | 461 | 371/341 | 268/334 | 14.31 ± 0.35 | Lemon |
| 9 | Naringenin 7-O-rutinoside (naringin) | 25.02 | 579 | 271 | 278/330 | 35.45 ± 6.04 | Lemon |
| 10 | Hesperidin | 28.10 | 609 | 301 | 284/336 | 6.3 ± 0.1 133.7 ± 15.9 | Lemon Orange |
| 11 | Diosmetin 7-O-rutinoside (Diosmin) | 28.40 | 607 | 299/284 | 270/334 | 4.12 ± 0.13 | Lemon |
| 12 | Limocitrin-HMG-Glu | 29.43 | 651 | 549/507/345 | 274/350 | 6.9 ± 0.2 | Lemon |
| Total: | 240.16 ± 14.86 | Lemon | |||||
| 133.7 ± 15.9 | Orange | ||||||
| Nº | Metabolites | RT | m/z- | Occurrence 2 | |||
|---|---|---|---|---|---|---|---|
| Urine LE | Plasma LE | Urine OE | Plasma OE | ||||
| M1 | Eriodictyol glucuronide-1 | 6.37 | 463.0882 | 16 | 16 | 5 | 3 |
| M2 | Eriodictyol glucuronide-2 | 7.31 | 463.0882 | 16 | 16 | 10 | 7 |
| M3 | Naringenin 7-O-glucuronide 1 | 7.52 | 447.0933 | 16 | 16 | 16 | 14 |
| M4 | Eriodictyol glucuronide-3 | 7.68 | 463.0882 | 16 | 16 | 11 | 7 |
| M5 | Naringenin 4′-O-glucuronide 1 | 7.70 | 447.0933 | 16 | 16 | 16 | 14 |
| M6 | Homoeriodictyol glucuronide | 7.72 | 477.1038 | 16 | 16 | 3 | 3 |
| M7 | Hesperetin 7-O-glucuronide 1 | 8.02 | 477.1038 | 16 | 16 | 16 | 16 |
| M8 | Hesperetin 3′-O-glucuronide 1 | 8.20 | 477.1038 | 16 | 16 | 16 | 16 |
| M9 | Eriodictyol sulfoglucuronide | 8.99 | 543.0450 | 5 | 15 | 0 | 0 |
| M10 | Homoeriodictyol sulfate | 9.02 | 381.0286 | 16 | 16 | 0 | 3 |
| M11 | Hesperetin sulfoglucuronide | 9.30 | 557.0607 | 0 | 0 | 7 | 8 |
| M12 | Eriodictyol 1 | 9.50 | 287.0561 | 14 | 16 | 4 | 0 |
| M13 | Eriodictyol sulfate | 9.75 | 367.0129 | 16 | 16 | 12 | 10 |
| M14 | Hesperetin 3′-O-sulfate 1 | 9.89 | 381.0286 | 16 | 16 | 16 | 16 |
| M15 | Naringenin 1 | 11.22 | 271.0612 | 9 | 6 | 5 | 2 |
| M16 | Homoeriodictyol 1 | 11.42 | 301.0718 | 12 | 15 | 1 | 1 |
| M17 | Hesperetin 1 | 11.73 | 301.0718 | 5 | 8 | 8 | 3 |
| Lemon Extract | Orange Extract | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nº | Metabolites | F1 | F2 | F3 | F4 | Total F | F1 | F2 | F3 | F4 | Total F |
| M1 | Eriodictyol glucuronide-1 | 3.2 ± 5.9 | 9.6 ± 15.5 | 4.5 ± 6.2 | 0.7 ± 0.7 | 18.0 ± 28.4 | – | 0.4 ± 0.3 | 0.1 ± 0.1 | 0.05 ± 0.05 | 0.6 ± 0.4 |
| M2 | Eriodictyol glucuronide-2 | 6.4 ± 11.0 | 18.0 ± 17.2 | 18.1 ± 19.7 | 4.2 ± 5.7 | 46.6 ± 53.6 ** | 0.1 ± 0.1 | 0.5 ± 0.8 | 0.5 ± 0.6 | 1.4 ± 3.4 | 2.5 ± 4.9 |
| M3 | Naringenin 7-O-glucuronide | 1.0 ± 0.7 | 2.6 ± 4.0 | 1.7 ± 1.9 | 0.3 ± 0.3 | 5.7 ± 6.9 *** | 0.1 ± 0.1 | 0.2 ± 0.6 | 0.2 ± 0.2 | 0.2 ± 0.3 | 0.7 ± 1.5 |
| M4 | Eriodictyol glucuronide-3 | 9.4 ± 16.9 | 33.8 ± 64.1 | 27.7 ± 40.1 | 4.3 ± 6.4 | 75.1 ± 128 * | 0.1 ± 0.2 | 0.5 ± 0.9 | 0.7 ± 0.7 | 2.1 ± 4.5 | 3.4 ± 6.2 |
| M5 | Naringenin 4′-O-glucuronide | 0.8 ± 0.5 | 1.7 ± 2.7 | 1.3 ± 1.5 | 0.4 ± 0.3 | 4.2 ± 5.0 *** | 0.1 ± 0.1 | 0.3 ± 0.5 | 0.3 ± 0.4 | 0.3 ± 0.5 | 1.0 ± 1.5 |
| M6 | Homoeriodictyol glucuronide | 13.9 ± 16.2 | 48.1 ± 90.0 | 37.2 ± 56.9 | 6.8 ± 8.0 | 106 ± 171 | – | – | – | – | – |
| M7 | Hesperetin 7-O-glucuronide | 1.2 ± 1.4 | 4.0 ± 4.7 | 2.7 ± 2.5 | 0.6 ± 0.6 | 8.5 ± 9.2 *** | 0.2 ± 0.6 | 2.1 ± 5.7 | 2.2 ± 3.6 | 1.1 ± 3.3 | 5.6 ± 13.2 |
| M8 | Hesperetin 3′-O-glucuronide | 2.1 ± 2.3 | 10.7 ± 11.3 | 9.2 ± 7.7 | 2.2 ± 2.4 | 24.2 ± 23.7 *** | 0.5 ± 1.5 | 4.3 ± 11.5 | 3.3 ± 4.2 | 1.8 ± 1.6 | 9.9 ± 18.9 |
| M10 | Homoeriodictyol sulfate | 3.8 ± 3.2 | 4.0 ± 5.5 | 3.6 ± 4.1 | 0.5 ± 0.4 | 9.6 ± 11.3 | – | – | – | – | – |
| M13 | Eriodictyol sulfate | 4.6 ± 5.0 | 14.6 ± 17.3 | 11.9 ± 12.6 | 1.5 ± 1.5 | 32.6 ± 36.5 *** | 0.02 ± 0.01 | 0.1 ± 0.1 | 0.2 ± 0.4 | 0.3 ± 0.4 | 0.6 ± 0.9 |
| M14 | Hesperetin 3′-O-sulfate | 1.9 ± 2.6 | 4.3 ± 4.5 | 3.3 ± 3.1 | 0.7 ± 0.6 | 9.0 ± 9.1 * | 0.1 ± 0.3 | 1.2 ± 3.3 | 1.9 ± 3.1 | 0.6 ± 0.6 | 3.8 ± 7.3 |
| Total excretion (24 h): | 339 ± 482 *** | 28 ± 55 | |||||||||
| Tmax (h) | Cmax (nM) | AUC0-t (nM·h) | |||||
|---|---|---|---|---|---|---|---|
| Nº | Metabolites | Lemon | Hesperidin | Lemon | Hesperidin | Lemon | Hesperidin |
| M1 | Eriodictyol glucuronide-1 | 5.9 ± 2.4 | — | 103.9 ± 85.8 | — | 282.6 ± 271.3 | — |
| M2 | Eriodictyol glucuronide-2 | 5.9 ± 2.4 | — | 871.3 ± 868.0 | — | 3222 ± 4118 | — |
| M3 | Naringenin 7-O-glucuronide | 5.4 ± 2.5 * | 7.3 ± 2.2 | 56.7 ± 44.2 *** | 4.7 ± 3.6 | 202.7 ± 187.1 *** | 18.8 ± 19.0 |
| M4 | Eriodictyol glucuronide-3 | 5.9 ± 2.6 | — | 638.6 ± 801.8 | — | 2291 ± 3491 | — |
| M5 | Naringenin 4′-O-glucuronide | 5.7 ± 2.3 * | 7.7 ± 2.0 | 35.8 ± 25.9 *** | 7.3 ± 5.9 | 129.4 ± 124.0 *** | 26.7 ± 27.7 |
| M6 | Homoeriodictyol glucuronide | 6.1 ± 2.4 | — | 780.5 ± 863.2 | — | 3133 ± 4170 | — |
| M7 | Hesperetin 7-O-glucuronide | 6.3 ± 2.0 * | 8.3 ± 1.6 | 169.1 ± 109.4 *** | 49.6 ± 51.7 | 703.3 ± 590.5 *** | 176.7 ± 249.5 |
| M8 | Hesperetin 3′-O-glucuronide | 6.4 ± 2.0 * | 8.4 ± 1.6 | 198.5 ± 103.7 * | 106.8 ± 128.5 | 802.8 ± 568.8 * | 408.9 ± 643.2 |
| M10 | Homoeriodictyol sulfate | 6.5 ± 2.2 | — | 479.4 ± 524.0 | — | 1778 ± 2257 | — |
| M13 | Eriodictyol sulfate | 5.7 ± 2.5 | — | 1706 ± 1510 | — | 6120 ± 6631 | — |
| M14 | Hesperetin 3′-O-sulfate | 6.5 ± 1.9 *** | 8.4 ± 1.5 | 527.0 ± 357.8 *** | 177.9 ± 285.8 | 2037 ± 1809 *** | 635.3 ± 1167 |
| Total: | 5724 ± 5432 * | 347 ± 475 | 21060 ± 24531 * | 1266 ± 2106 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; González-Sarrías, A.; Espín, J.C. New Insights into the Metabolism of the Flavanones Eriocitrin and Hesperidin: A Comparative Human Pharmacokinetic Study. Antioxidants 2021, 10, 435. https://doi.org/10.3390/antiox10030435
Ávila-Gálvez MÁ, Giménez-Bastida JA, González-Sarrías A, Espín JC. New Insights into the Metabolism of the Flavanones Eriocitrin and Hesperidin: A Comparative Human Pharmacokinetic Study. Antioxidants. 2021; 10(3):435. https://doi.org/10.3390/antiox10030435
Chicago/Turabian StyleÁvila-Gálvez, María Ángeles, Juan Antonio Giménez-Bastida, Antonio González-Sarrías, and Juan Carlos Espín. 2021. "New Insights into the Metabolism of the Flavanones Eriocitrin and Hesperidin: A Comparative Human Pharmacokinetic Study" Antioxidants 10, no. 3: 435. https://doi.org/10.3390/antiox10030435
APA StyleÁvila-Gálvez, M. Á., Giménez-Bastida, J. A., González-Sarrías, A., & Espín, J. C. (2021). New Insights into the Metabolism of the Flavanones Eriocitrin and Hesperidin: A Comparative Human Pharmacokinetic Study. Antioxidants, 10(3), 435. https://doi.org/10.3390/antiox10030435









