Metabolic Profiling Analysis Reveals the Potential Contribution of Barley Sprouts against Oxidative Stress and Related Liver Cell Damage in Habitual Alcohol Drinkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Materials
2.2. Subjects
2.3. Study Design
2.4. Liver Fat Content by Magnetic Resonance Imaging (MRI)
2.5. Biochemical Measurements
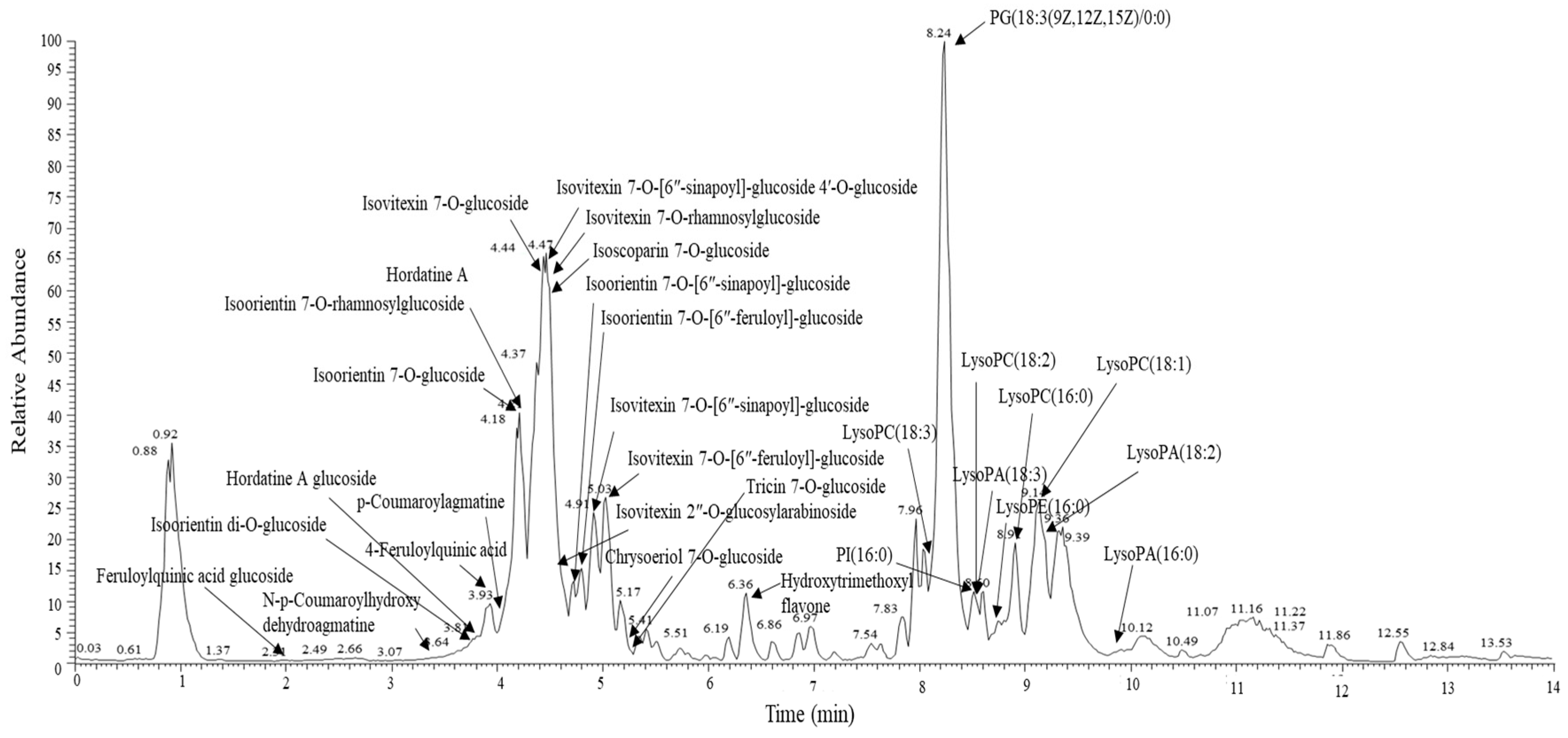
2.6. Metabolomics Analysis
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Subjects
3.2. Alterations in Alcohol-Induced Oxidative Stress by BSE Supplementation
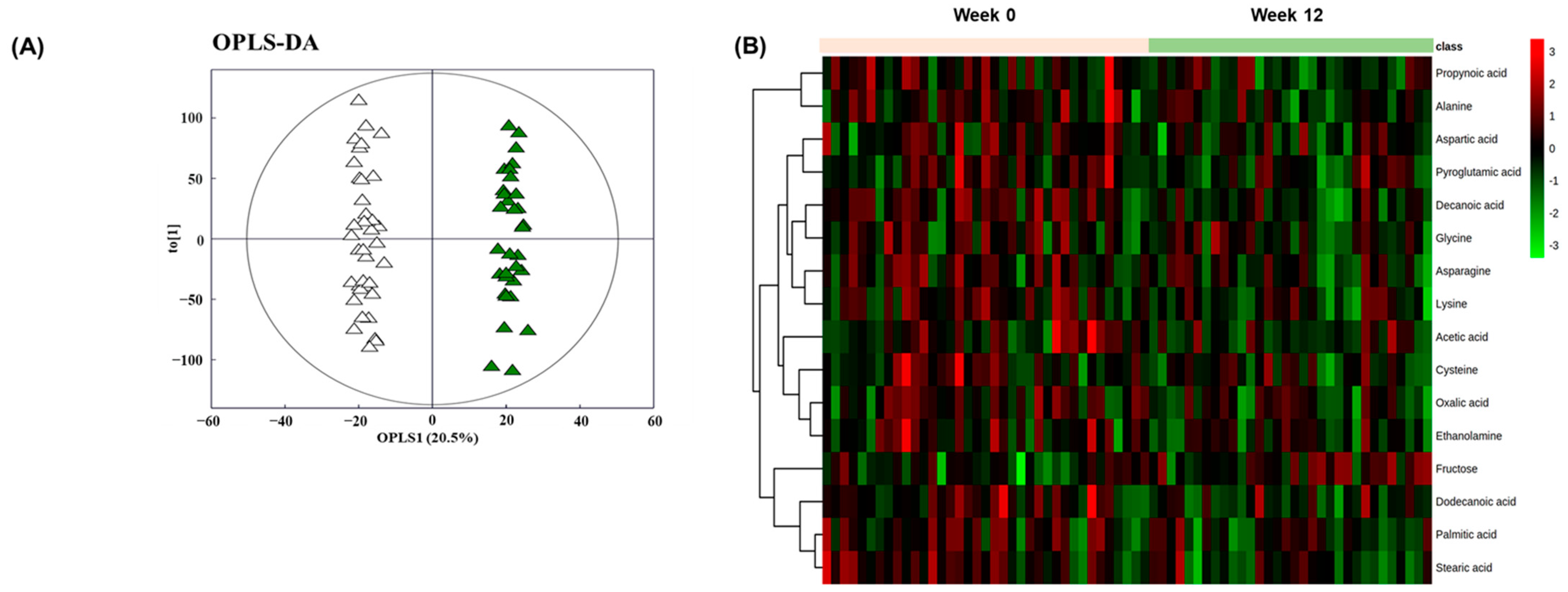
3.3. Alterations in Plasma Metabolomic Profile by BSE Supplementation
3.4. Impacts of BSE Supplementation on Alcohol-Induced Liver Cell Damages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Shaw, J.J.; Shah, S.A. Rising incidence and demographics of hepatocellular carcinoma in the USA: What does it mean? Expert Rev. Gastroenterol. Hepatol. 2011, 5, 365–370. [Google Scholar] [CrossRef]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef]
- Nagata, K.; Suzuki, H.; Sakaguchi, S. Common pathogenic mechanism in development progression of liver injury caused by non-alcoholic or alcoholic steatohepatitis. J. Toxicol. Sci 2007, 32, 453–468. [Google Scholar] [CrossRef]
- Varatharajalu, R.; Garige, M.; Leckey, L.C.; Reyes-Gordillo, K.; Shah, R.; Lakshman, M.R. Protective Role of Dietary Curcumin in the Prevention of the Oxidative Stress Induced by Chronic Alcohol with respect to Hepatic Injury and Antiatherogenic Markers. Oxidative Med. Cell. Longev. 2016. [Google Scholar] [CrossRef]
- Beier, J.I.; McClain, C.J. Mechanisms and cell signaling in alcoholic liver disease. Biol. Chem. 2010, 391, 1249–1264. [Google Scholar] [CrossRef]
- Diehl, A.M. Recent events in alcoholic liver disease V. effects of ethanol on liver regeneration. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 288. [Google Scholar] [CrossRef]
- Masalkar, P.D.; Abhang, S.A. Oxidative stress and antioxidant status in patients with alcoholic liver disease. Clin. Chim. Acta 2005, 355, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Vidali, M.; Stewart, S.F.; Albano, E. Interplay between oxidative stress and immunity in the progression of alcohol-mediated liver injury. Trends Mol. Med. 2008, 14, 63–71. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, F.; Li, C.; Li, W.; Liao, W.; Li, Q.; Qin, G.; Liu, W.; Zhao, X.J.A. White peony (fermented Camellia sinensis) polyphenols help prevent alcoholic liver injury via antioxidation. Antioxidants 2019, 8, 524. [Google Scholar] [CrossRef]
- Tavakoli, H.R.; Hull, M.; Okasinski, L.M. Review of current clinical biomarkers for the detection of alcohol dependence. Innov. Clin. Neurosci. 2011, 8, 26. [Google Scholar]
- Zhang, H.; Forman, H.J. Redox regulation of γ-glutamyl transpeptidase. Am. J. Respir. Cell Mol. Biol. 2009, 41, 509–515. [Google Scholar] [CrossRef]
- Shukla, I.; Azmi, L.; Gupta, S.S.; Upreti, D.K.; Rao, C.V. Amelioration of anti-hepatotoxic effect by Lichen rangiferinus against alcohol induced liver damage in rats. J. Ayurveda Integr. Med. 2019, 10, 171–177. [Google Scholar] [CrossRef]
- Singh, M.; Gupta, S.; Singhal, U.; Pandey, R.; Aggarwal, S. Evaluation of the oxidative stress in chronic alcoholics. J. Clin. Diagn. Res. JCDR 2013, 7, 1568. [Google Scholar]
- Benedet, J.A.; Umeda, H.; Shibamoto, T. Antioxidant activity of flavonoids isolated from young green barley leaves toward biological lipid samples. J. Agric. Food Chem. 2007, 55, 5499–5504. [Google Scholar] [CrossRef]
- Yu, Y.-M.; Wu, C.-H.; Tseng, Y.-H.; Tsai, C.E.; Chang, W.-C. Antioxidative and hypolipidemic effects of barley leaf essence in a rabbit model of atherosclerosis. Jpn. J. Pharmacol. 2002, 89, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, K.; Nakayama, N.; Shimada, M.; Bi, Y.; Fukata, H.; Ueno, K. Antidepressant-like effects of young green barley leaf (Hordeum vulgare L.) in the mouse forced swimming test. Pharmacogn. Res. 2012, 4, 22. [Google Scholar]
- Kamiyama, M.; Shibamoto, T. Flavonoids with potent antioxidant activity found in young green barley leaves. J. Agric. Food Chem. 2012, 60, 6260–6267. [Google Scholar] [CrossRef]
- Vitcheva, V.; Simeonova, R.; Krasteva, I.; Yotova, M.; Nikolov, S.; Mitcheva, M. Hepatoprotective effects of saponarin, isolated from Gypsophila trichotoma Wend. on cocaine-induced oxidative stress in rats. Redox Rep. 2011, 16, 56–61. [Google Scholar] [CrossRef]
- Simeonova, R.; Vitcheva, V.; Kondeva-Burdina, M.; Krasteva, I.; Manov, V.; Mitcheva, M. Hepatoprotective and antioxidant effects of saponarin, isolated from Gypsophila trichotoma Wend. on paracetamol-induced liver damage in rats. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Kim, J.-H.; Kim, S.H.; Oh, J.Y.; Seo, W.D.; Kim, K.-M.; Jung, J.-C.; Jung, Y.-S. Barley sprouts extract attenuates alcoholic fatty liver injury in mice by reducing inflammatory response. Nutrients 2016, 8, 440. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 2005. Available online: http://www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf (accessed on 6 March 2021).
- Ninomiya, M.; Kajiguchi, T.; Yamamoto, K.; Kinoshita, T.; Emi, N.; Naoe, T. Increased oxidative DNA products in patients with acute promyelocytic leukemia during arsenic therapy. Haematologica 2006, 91, 1571–1572. [Google Scholar] [PubMed]
- Fukunaga, K.; Takama, K.; Suzuki, T. High-performance liquid chromatographic determination of plasma malondialdehyde level without a solvent extraction procedure. Anal. Biochem. 1995, 230, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Jung, E.S.; Lee, G.M.; Lee, C.H. Distinguishing six edible berries based on metabolic pathway and bioactivity correlations by non-targeted metabolite profiling. Front. Plant. Sci. 2018, 9, 1462. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.-M.; Ahn, E.-M.; Yun, J.-M.; Cho, B.-L.; Paek, Y.-J. Angelica keiskei Koidzumi extracts improve some markers of liver function in habitual alcohol drinkers: A randomized double-blind clinical trial. J. Med. Food 2015, 18, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Dusseldorp, E.; Van Mechelen, I. Qualitative interaction trees: A tool to identify qualitative treatment–subgroup interactions. Stat. Med. 2014, 33, 219–237. [Google Scholar] [CrossRef]
- Dusseldorp, E.; Doove, L.; Van Mechelen, I. Quint: An R package for the identification of subgroups of clients who differ in which treatment alternative is best for them. Behav. Res. Methods 2016, 48, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yang, Y.J.; Yang, Y.K.; Oh, S.Y.; Hong, Y.C.; Lee, E.K.; Kwon, O. Diet quality scores and oxidative stress in Korean adults. Eur. J. Clin. Nutr. 2011, 65, 1271–1278. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Dey, A.; Cederbaum, A.I. Alcohol and oxidative liver injury. Hepatology 2006, 43, S63–S74. [Google Scholar] [CrossRef]
- Pari, L.; Suresh, A. Effect of grape (Vitis vinifera L.) leaf extract on alcohol induced oxidative stress in rats. Food Chem Toxicol. 2008, 46, 1627–1634. [Google Scholar] [CrossRef]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the flavonoid C-glycosides and health benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, S29–S45. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health 2003, 27, 277–284. [Google Scholar]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Mulder, T.P.; Manni, J.J.; Roelofs, H.M.; Peters, W.H.; Wiersma, A. Glutathione S-transferases and glutathione in human head and neck cancer. Carcinogenesis 1995, 16, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Shalini, S.; Bansal, M.P. Influence of vitamin E on alcohol-induced changes in antioxidant defenses in mice liver. Toxicol Mech Methods 2010, 20, 82–89. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.; Kim, H.-S.; Jung, B.H. Metabolomic approaches to the normal aging process. Metabolomics 2014, 10, 1268–1292. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J.; Choi, J. Gamma-glutamyl transpeptidase in glutathione biosynthesis. Methods Enzym. 2005, 401, 468–483. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.; Pappa, A.; Panayiotidis, M. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007, 113, 234–258. [Google Scholar] [CrossRef]
- Nolin, T.D.; McMenamin, M.E.; Himmelfarb, J. Simultaneous determination of total homocysteine, cysteine, cysteinylglycine, and glutathione in human plasma by high-performance liquid chromatography: Application to studies of oxidative stress. J. Chromatogr. B 2007, 852, 554–561. [Google Scholar] [CrossRef]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef]
- Willke, R.J.; Zheng, Z.; Subedi, P.; Althin, R.; Mullins, C.D. From concepts, theory, and evidence of heterogeneity of treatment effects to methodological approaches: A primer. BMC Med. Res. Methodol. 2012, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Shireen, K.F.; Pace, R.D.; Mahboob, M.; Khan, A.T. Effects of dietary vitamin E, C and soybean oil supplementation on antioxidant enzyme activities in liver and muscles of rats. Food Chem. Toxicol. 2008, 46, 3290–3294. [Google Scholar] [CrossRef] [PubMed]


 placebo;
placebo;  BSE). The interquartile range and median are shown by the vertical bar and black dash, respectively, and the mean is shown by the red dot. GR, glutathione reductase; FSS, fatigue severity scale; ROS, reactive oxygen species; AUC, area under the curve; ox-LDL, oxidized low-density lipoprotein; GGT, γ-glutamyl transferase; BSE, barley sprout extract powder.
BSE). The interquartile range and median are shown by the vertical bar and black dash, respectively, and the mean is shown by the red dot. GR, glutathione reductase; FSS, fatigue severity scale; ROS, reactive oxygen species; AUC, area under the curve; ox-LDL, oxidized low-density lipoprotein; GGT, γ-glutamyl transferase; BSE, barley sprout extract powder.
 placebo;
placebo;  BSE). The interquartile range and median are shown by the vertical bar and black dash, respectively, and the mean is shown by the red dot. GR, glutathione reductase; FSS, fatigue severity scale; ROS, reactive oxygen species; AUC, area under the curve; ox-LDL, oxidized low-density lipoprotein; GGT, γ-glutamyl transferase; BSE, barley sprout extract powder.
BSE). The interquartile range and median are shown by the vertical bar and black dash, respectively, and the mean is shown by the red dot. GR, glutathione reductase; FSS, fatigue severity scale; ROS, reactive oxygen species; AUC, area under the curve; ox-LDL, oxidized low-density lipoprotein; GGT, γ-glutamyl transferase; BSE, barley sprout extract powder.
| Variable | Placebo (n = 38) | BSE (n = 38) | p-Value |
|---|---|---|---|
| Gender (male/female) | 37/1 | 37/1 | 1.000 |
| Age (years) | 47.6 ± 1.5 | 50.3 ± 1.5 | 0.185 |
| Alcohol amount (g/week) | 294.3 ± 13.9 | 264.0 ± 12.9 | 0.115 |
| Smoker (Y/N) | 17/21 | 14/24 | 0.484 |
| Smoking amount (cigarettes/day) | 16.2 ± 2.5 | 14.1 ± 1.6 | 0.473 |
| FSS (total score) | 29.0 ± 1.6 | 30.2 ± 1.8 | 0.631 |
| Physical activity (MET-min/wk) | 2714 ± 612 | 1845 ± 340 | 0.220 |
| RFS | 19.6 ± 1.2 | 23.9 ± 1.5 | 0.028 |
| BMI (kg/m2) | 27.4 ± 0.4 | 27.6 ± 0.4 | 0.671 |
| Liver fat content (%) | 15.1 ± 1.5 | 15.1 ± 1.3 | 0.984 |
| ALT (IU/L) | 42.5 ± 3.2 | 38.6 ± 2.7 | 0.357 |
| AST (IU/L) | 31.6 ± 2.0 | 31.2 ± 1.7 | 0.857 |
| GGT (U/L) | 97.7 ± 5.3 | 95.5 ± 5.7 | 0.780 |
| Variable | Placebo (n = 38) | BSE (n = 38) | Estimate | p-Value | ||
|---|---|---|---|---|---|---|
| Week 0 | Week 12 | Week 0 | Week 12 | |||
| ROS AUC | 1861 ± 222 | 1869 ± 241 | 2254 ± 245 | 1457 ± 245 * | −805 | 0.072 |
| Urinary MDA (μmol/g creatine) | 1.94 ± 0.18 | 2.39 ± 0.19 | 2.43 ± 0.18 | 2.05 ± 0.19 | −0.82 | 0.026 |
| ox-LDL (μg/mL) | 77.3± 2.7 | 76.5 ± 2.9 | 72.5 ±2.7 | 68.4 ± 2.9 | −3.3 | 0.350 |
| SOD/CAT | 7.3 ± 1.7 | 10.1 ± 1.8 | 9.7 ± 1.8 | 9.8 ± 1.8 | −2.6 | 0.271 |
| SOD/GPx | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.21 ± 0.02 | 0.23 ± 0.02 | 0.01 | 0.498 |
| SOD/(CAT+GPx) | 0.18 ± 0.02 | 0.17 ± 0.02 | 0.20 ± 0.02 | 0.23 ± 0.02 | 0.03 | 0.180 |
| GR (nmol/min/mL) | 134.0 ± 7.9 | 134.9 ± 8.2 | 139.2 ± 7.8 | 133.0 ± 8.2 | −7.1 | 0.481 |
| GST (nmol/min/mL) | 108.5 ± 6.0 | 98.4 ± 6.5 | 100.5 ± 6.0 | 111.4 ± 6.3 | 20.9 | 0.039 |
| GSH/GSSG | 4.79 ± 0.39 | 5.81 ± 0.41 | 4.25 ± 0.39 | 4.75 ± 0.41 | −0.52 | 0.351 |
| GSSG/TGSH | 0.20 ± 0.01 | 0.17 ± 0.01 | 0.21 ± 0.01 | 0.20 ± 0.01 | 0.01 | 0.488 |
| Variable | Placebo (n = 38) | BSE (n = 38) | Estimate | p-Value | ||
|---|---|---|---|---|---|---|
| Week 0 | Week 12 | Week 0 | Week 12 | |||
| Liver fat content (%) | 13.0 ± 1.1 | 11.3 ± 1.1 ** | 14.3 ± 1.1 | 12.4 ± 1.1 *** | −0.2 | 0.725 |
| ALT (IU/L) | 37.9 ± 2.2 | 34.4 ± 2.4 | 37.8 ± 2.2 | 35.9 ± 2.3 | 1.6 | 0.581 |
| AST (IU/L) | 29.2 ± 1.4 | 29.5 ± 1.4 | 29.8 ± 1.4 | 28.9 ± 1.4 | −1.2 | 0.505 |
| AST/ALT ratio | 0.78 ± 0.03 | 0.81 ± 0.04 | 0.81 ± 0.03 | 0.81 ± 0.03 | −0.04 | 0.424 |
| GGT (U/L) | 92.1 ± 4.2 | 89.9 ± 4.5 | 90.5 ± 4.2 | 81.1 ± 4.5 * | −7.2 | 0.213 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Lee, E.; Kim, Y.; Jung, H.Y.; Kim, K.-M.; Kwon, O. Metabolic Profiling Analysis Reveals the Potential Contribution of Barley Sprouts against Oxidative Stress and Related Liver Cell Damage in Habitual Alcohol Drinkers. Antioxidants 2021, 10, 459. https://doi.org/10.3390/antiox10030459
Park H, Lee E, Kim Y, Jung HY, Kim K-M, Kwon O. Metabolic Profiling Analysis Reveals the Potential Contribution of Barley Sprouts against Oxidative Stress and Related Liver Cell Damage in Habitual Alcohol Drinkers. Antioxidants. 2021; 10(3):459. https://doi.org/10.3390/antiox10030459
Chicago/Turabian StylePark, Hyerin, Eunok Lee, Yunsoo Kim, Hye Yoon Jung, Kwang-Min Kim, and Oran Kwon. 2021. "Metabolic Profiling Analysis Reveals the Potential Contribution of Barley Sprouts against Oxidative Stress and Related Liver Cell Damage in Habitual Alcohol Drinkers" Antioxidants 10, no. 3: 459. https://doi.org/10.3390/antiox10030459
APA StylePark, H., Lee, E., Kim, Y., Jung, H. Y., Kim, K.-M., & Kwon, O. (2021). Metabolic Profiling Analysis Reveals the Potential Contribution of Barley Sprouts against Oxidative Stress and Related Liver Cell Damage in Habitual Alcohol Drinkers. Antioxidants, 10(3), 459. https://doi.org/10.3390/antiox10030459








