Abstract
Over the last several decades, hydrogen sulfide (H2S) has gained attention as a new signaling molecule, with extensive physiological and pathophysiological roles in human disorders affecting vascular biology, immune functions, cellular survival, metabolism, longevity, development, and stress resistance. Apart from its known functions in oxidative stress and inflammation, new evidence has emerged revealing that H2S carries out physiological functions by targeting proteins, enzymes, and transcription factors through a post-translational modification known as persulfidation. This review article provides a critical overview of the current state of the literature addressing the role of H2S in obesity-associated metabolic disturbances, with particular emphasis on its mechanisms of action in obesity, diabetes, non-alcoholic fatty liver disease (NAFLD), and cardiovascular diseases.
1. Introduction
Gasotransmitters are small molecules of endogenous gas that have the noticeable ability to diffuse into cells to interact with their targets, inducing an array of intracellular signaling and pathophysiological responses [1,2]. Hydrogen sulfide is the most recent addition to the gasotransmitters family, the first two being nitric oxide (NO) and carbon monoxide (CO) [3].
Instead of binding to plasma membrane receptors, the high solubility of gasotransmitters in lipids allows them to penetrate cell membranes without requiring a specific transporter or receptor. Gasotransmitters are generated endogenously by specific enzymes and can generate various functions at physiologically relevant concentrations by targeting specific cellular and molecular targets [4]. A gaseous substance is not readily stored in vesicular structures and so must be resynthesized as needed. This implies that the biosynthetic enzymes must be subject to tightly regulated mechanisms [5]. Abnormal generation and metabolism of these gasotransmitters have been extensively demonstrated to affect diverse biological processes, such as vascular biology, immune functions, cellular survival, metabolism, longevity, development, and stress resistance [4].
Perhaps the most remarkably unique feature of gasotransmitters relates to the molecular mechanisms whereby they signal to their targets. Gasotransmitters chemically modify intracellular proteins, thus affecting cellular metabolism in a more immediate fashion than other signal transduction mechanisms [5].
H2S has been traditionally considered only as a toxic agent for living organisms [6]. Nowadays, it is considered a gaseous mediator that plays important regulatory roles in innate immunity and inflammatory responses, impacting the development of cardiovascular and metabolic disorders [7,8,9,10,11]. Dysfunctional tissue H2S metabolism is increasingly implicated in different pathologies, from cardiovascular [12,13] and neurodegenerative diseases [14,15,16] to cancer [17,18]. There is emerging evidence supporting the importance of H2S in the pathophysiology of obesity, type 2 diabetes, NAFLD, and cardiovascular diseases. Understanding the precise mechanisms that control H2S homeostasis and their dysregulation is a major research focus. Despite decades of molecular and cellular studies on the enzymatic systems involved in H2S synthesis and breakdown, it appears at times that this field of biology is still in its infancy, with new targets of H2S and related species constantly being identified and new mechanistic details being revealed. In this review, we provide an overview of the current state of the literature about H2S in the context of obesity, diabetes, NAFLD, and cardiovascular diseases.
2. Biosynthesis of H2S
Hydrogen sulfide biosynthesis has been identified in a variety of mammalian tissues via enzymatic and non-enzymatic pathways [17]. In enzymatic biosynthesis, the endogenous generation of H2S from L-cysteine in the cytosol of cells is mainly mediated by two pyridoxal-5′-phosphate (PLP)-dependent enzymes known as cystathionine β-synthase (CBS) [19] and cystathionine γ-lyase (CTH or CSE) [20]. H2S is also produced by L-cysteine aminotransferase (CAT) and 3-mercapto-pyruvate sulfurtransferase (MPST) in the cytosol and mitochondria [21]. The expression of these enzymes is tissue-specific; in some tissues, CBS, CTH and MPST are all needed for the generation of H2S, whereas in others one enzyme serves this function. A small portion of endogenous H2S is derived via non-enzymatic reduction sulfur species, which are present in certain metabolites [17].
2.1. Enzymatic Synthesis of H2S
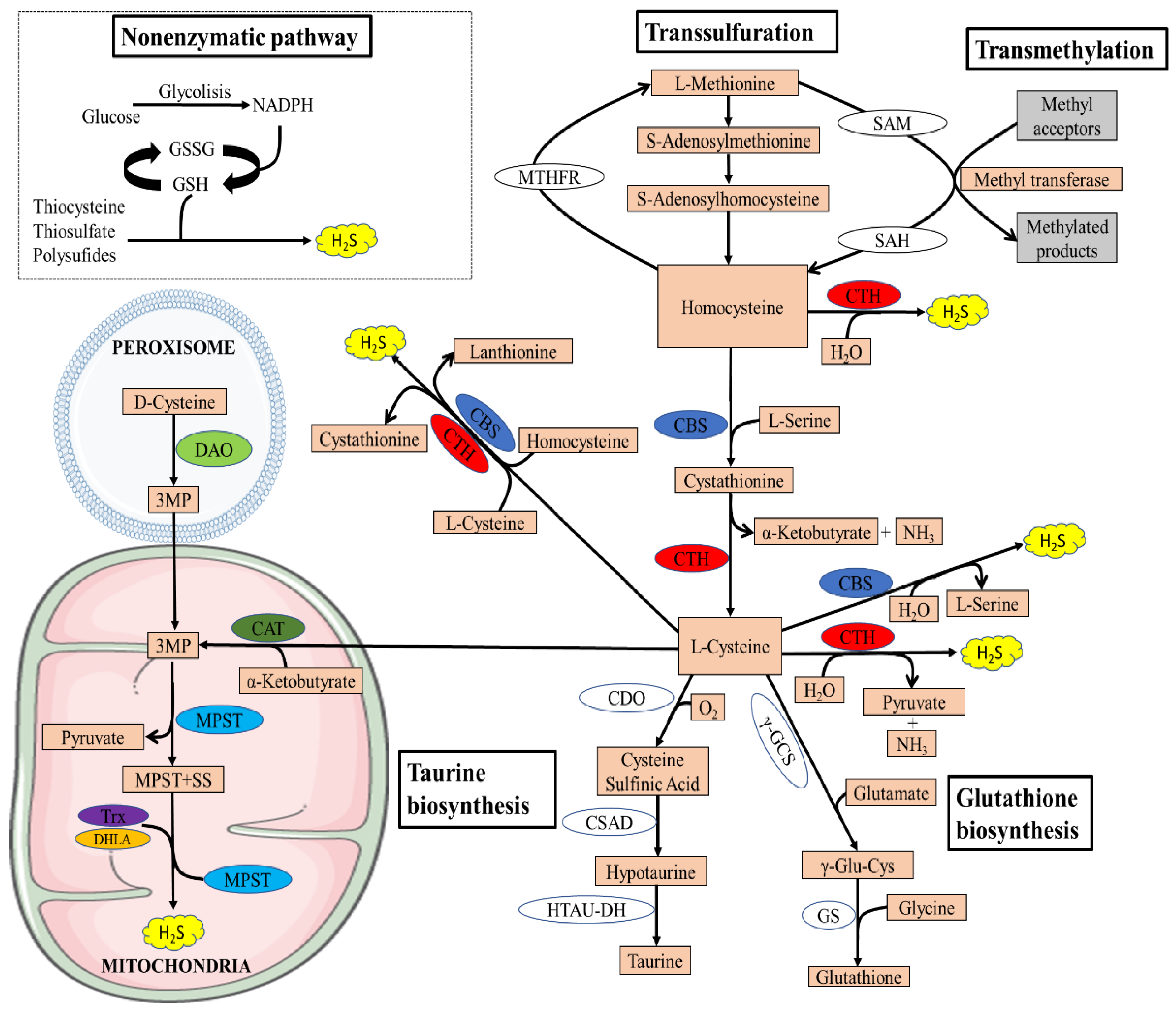
The transsulfuration pathway plays a central role in sulfur metabolism and redox regulation in cells. The pathway leads to the generation of several sulfur metabolites, which include L-cysteine, glutathione (GSH), taurine, and the gaseous signaling molecule hydrogen sulfide [22]. In mammals, the pathway involves the transfer of sulfur from homocysteine to cysteine via cystathionine and is the only route for the biosynthesis of cysteine. Homocysteine, which is derived from dietary methionine, is converted to cystathionine by cystathionine β-synthase (CBS), which is acted on by cystathionine γ-lyase (CTH) to generate L-cysteine [22] (Figure 1).
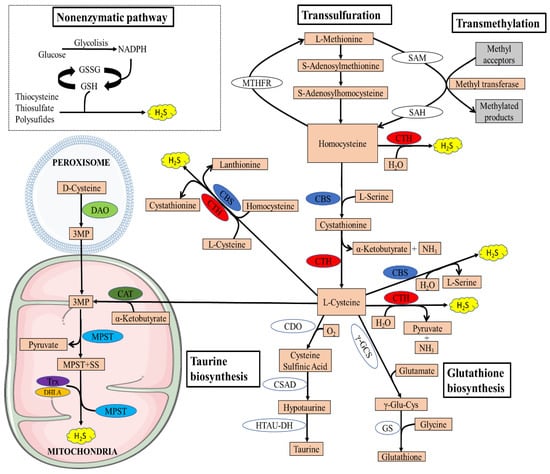
Figure 1.
Overview of the transsulfuration pathway. There are four enzymatic pathways for the biosynthesis of H2S, including CBS, CSE, MPST coupled with CAT, and MPST coupled with DAO. Non-enzymatic H2S generation occurs in the presence of reducing equivalents such as NADPH and NADH, reactive sulfur species in persulfides, thiosulfate, and polysulfides that are reduced into H2S and other metabolites. The transsulfuration pathway intersects with the transmethylation pathway at homocysteine. Methionine is an indispensable amino acid and is transmethylated intracellularly via S-adenosylmethionine (SAM), an important methyl donor for most biological methylation reactions, producing S-adenosylhomocysteine (SAH) in this process, and is then hydrolyzed to homocysteine. Homocysteine can be remethylated back to methionine by N5,N10-methylenetetrahydrofolate reductase (MTHFR). The cysteine generated by the pathway can be conducted into GSH synthesis by the action of the enzymes γ-glutamyl cysteine synthetase (γ-GCS) and glutathione synthetase (GS) or converted to other sulfur-containing molecules such as taurine. Taurine is generated by the action of three enzymes, CDO, cysteine sulfinic acid decarboxylase (CSAD), and hypotaurine dehydrogenase (HTAU-DH).
In addition to its essential role in protein synthesis, cysteine is also a component of the major antioxidant GSH and is a potent antioxidant itself [23]. Disruption of cysteine and GSH metabolism has been frequently linked to aberrant redox homeostasis and neurodegeneration [23,24]. Both CTH and CBS play important roles in the regulation of redox balance. It has been reported that approximately 50% of the cysteine generated by the transsulfuration pathway is utilized for GSH biosynthesis in hepatic cells [25,26]. Cysteine is also the precursor of the gaseous signaling molecule hydrogen sulfide and other sulfur metabolites [27,28]. In addition to GSH and H2S, cysteine is converted to the sulfur-containing molecule taurine by the action of the enzyme cysteine dioxygenase (CDO) to form cysteinensulfinic acid, which can then be decarboxylated to hypotaurine by cysteine sulfinic acid decarboxylase, and the hypotaurine generated can be oxidized to taurine [29]. Since CDO acts directly on cysteine, it can modulate H2S production by influencing substrate availability. Mice lacking CDO show an elevated cysteine and H2S production capacity [30,31]. Taurine plays a role in osmoregulation, immunomodulation, neuromodulation, Ca2+ homeostasis, and ocular function and possesses antioxidant and anti-inflammatory effects [32]. The transsulfuration pathway is intimately linked to the transmethylation pathway via homocysteine, which can be remethylated to generate methionine or can be irreversibly converted to cysteine (Figure 1).
Recently, an alternative enzymatic pathway to the transsulfuration pathway has been identified for the enzymatic generation of H2S within mitochondria, known as the 3-mercaptopyruvate pathway. The pathway requires two enzymes, 3-mercaptopyruvate sulfurtransferase and the PLP-dependent enzyme cysteine aminotransferase (CAT). 3-mercaptopyruvate (3MP) is produced by CAT from L-cysteine and α-ketoglutarate [21]. Thereafter, MPST transfers a sulfur atom from 3MP onto itself, which leads to the formation of a persulfide, MPST-SS. H2S and MPST are then released from the persulfideof MPST-SS in the presence of reductants such as thioredoxin (TRX) and dihydrolipoic acid (DHLA) [33]. Recently, another source of 3MP was found in mammals by Shibuya et al.: D-cysteine [34]. Specifically, D-cysteine is transformed into 3MP by peroxisome-located d-amino acid oxidase (DAO). Metabolite exchanges between peroxisome and mitochondria can import 3MP into mitochondria, where it is further catalyzed into H2S by MPST. Because of the exclusive location of DAO in the brain and kidney, this H2S-generating pathway is currently believed to exist only in these two organs [17].
2.2. Non-Enzymatic Synthesis of H2S
Several cases of the non-enzymatic production of H2S have been reported, and these are suspected to represent a small proportion of all generated endogenous H2S. It is postulated that coordinated activities of PLP and iron (Fe3+) catalyze the generation of H2S using cysteine as a substrate, in a non-enzymatic manner in specific circumstances. Regulation of H2S production via this pathway may contribute to the pathophysiology of conditions with iron dysregulation such as hemolysis, iron overload, and hemorrhagic disorders [35].
H2S can also be generated from sulfane sulfur via non-enzymatical reduction in the presence of an endogenous reductant, such as nicotinamide adenine dinucleotide phosphate (NADPH) and nicotinamide adenine dinucleotide (NADH), which are supplied by oxidation of glucose via glycolysis or from phosphogluconate via NADPH oxidase [36]. In the presence of such reductants, reactive sulfur species in persulfides, thiosulfate, and polysulfides can be reduced into H2S and other metabolites [37]. Essentially, all the components of this non-enzymatic route are available in mammals, including reducible sulfur, suggesting the necessity of this pathway in mammalian systems. In accordance with this, hyperglycemia is demonstrated to promote H2S generation by enhancing this pathway [38].
3. The Relationship between H2S and Obesity
Obesity is characterized by the excessive accumulation and storage of fat in the body (regionally, globally, or both) that may be harmful to health and is defined by a body mass index (BMI) of 30 kg/m2 or greater, being considered morbidly obesity when BMI is over 35 kg/m2.
The epidemic of obesity presents a serious threat to human health around the world. The worldwide prevalence of obesity has increased dramatically over the past 30 years, fueled by economic growth, industrialization, mechanized transport, urbanization, an increasingly sedentary lifestyle, and a nutritional transition to processed foods and high-calorie diets [39]. According to the World Health Organization (WHO), 30% of Americans and 10%–20% of Europeans are obese, and on estimate more than 1.9 billion adults worldwide are overweight. High body mass carries with it an increased risk of the development of a number of serious cardiovascular and metabolic diseases, such as type 2 diabetes, hypertension, dyslipidemia, stroke, osteoarthritis, as well as several different forms of cancer [40]. There is emerging evidence supporting the importance of H2S in the pathophysiology of obesity.
3.1. The Importance of H2S in Obesity
Few studies have evaluated circulating sulfide in humans, with discrepant results. Whiteman et al. were the first ones to demonstrate the involvement of H2S in obesity [41]. These authors found that plasma H2S levels were significantly decreased in non-obese individuals with type-2 diabetes and in overweight participants with altered glucose [41]. However, the mechanisms that mediate the loss of H2S were not elucidated in that early study.
Additionally, other animal studies have demonstrated that exogenous H2S administration led to increased insulin sensitivity and improved glucose tolerance after a high-fat diet was fed to mice in parallel to weight gain [42,43]. Supplementation with H2S donors or increasing endogenous H2S biosynthesis was sufficient to stimulate fat mass accumulation in mice and fruit flies, whereas the depletion of endogenous H2S biosynthesis prevented high fat diet-induced fat mass (HFD-induced fat mass) [43,44]. Later, Alkhouri et al. reported that the H2S concentration in exhaled air was higher by 1/3 in obese children compared to lean children [45].
Recently, Comas and colleagues demonstrated that serum sulfide concentrations were increased in subjects with morbid obesity in proportion to fat mass [46]. Longitudinally, weight gain resulted in increased serum sulfide concentration, whereas weight loss had opposite effects, being the percent change in serum sulfide positively correlated with the percent change in BMI and waist circumference [46]. Ren and colleagues demonstrated that a milk protein concentrate diet prevents obesity induced by HFD in Sprague Dawley rats. This protection against obesity was associated with increased transsulfuration pathway and plasma H2S levels, which in turn maintained redox homeostasis and reduced lipid disorders induced by HFD [47].
Due to the lack of standardized methodology and variation in models and study cohorts, we see contradicting reports about H2S in obesity (Table 1). Depending on the technique used to measure sulfide, reported circulating levels of sulfide vary widely in obese and lean individuals. Furthermore, more human and animal studies are needed to fully comprehend the physiological and pathophysiological roles of H2S in obesity.

Table 1.
Findings present in the literature related to the reported circulating levels of sulfide in obesity.
3.2. Persulfidation Might Prevent the Negative Effects of Obesity-Associated Oxidative Stress
Signaling by H2S is proposed to occur via persulfidation, a posttranslational modification of cysteine residues (RSH) to persulfides (RSSH) [48], thought to be one of its main beneficial mechanisms of action [28]. Like other posttranslational modifications, persulfidation potentially alters a protein’s structure, function, stability, and/or macromolecular interactions [48,49,50]. Nonetheless, due to their enhanced nucleophilicity, persulfides react readily with reactive oxygen species (ROS), whereas H2S itself is a poor ROS scavenger. When exposed to ROS, proteins undergo oxidation to form sulfenic acids (P-SOH), sulfinic acids (P-SO2H), and sulphonic acids (P-SO3H), which cause the irreversible inactivation of the protein [51].
The detrimental actions of ROS are well-known. ROS are physiologically important, acting as second messengers in cell signaling, and they also play a pivotal role in cellular homeostasis [52,53] and are associated with cellular damage by oxidizing cellular constituents such as proteins, lipids, and DNA [54]. These physiological effects are mainly mediated by changes in the redox state of crucial intracellular and/or surface thiols. In the last few years, several studies have shown a strong association between obesity and altered redox state, consistent with the idea that an increased caloric intake and/or obesity are associated with a pro-oxidant environment and increased oxidative damage [55,56,57,58,59]. A lot of evidence has shown that obesity is a state of chronic oxidative stress, although it is not completely understood if the alteration in redox balance is a trigger rather than a result of obesity [57,60,61,62,63].
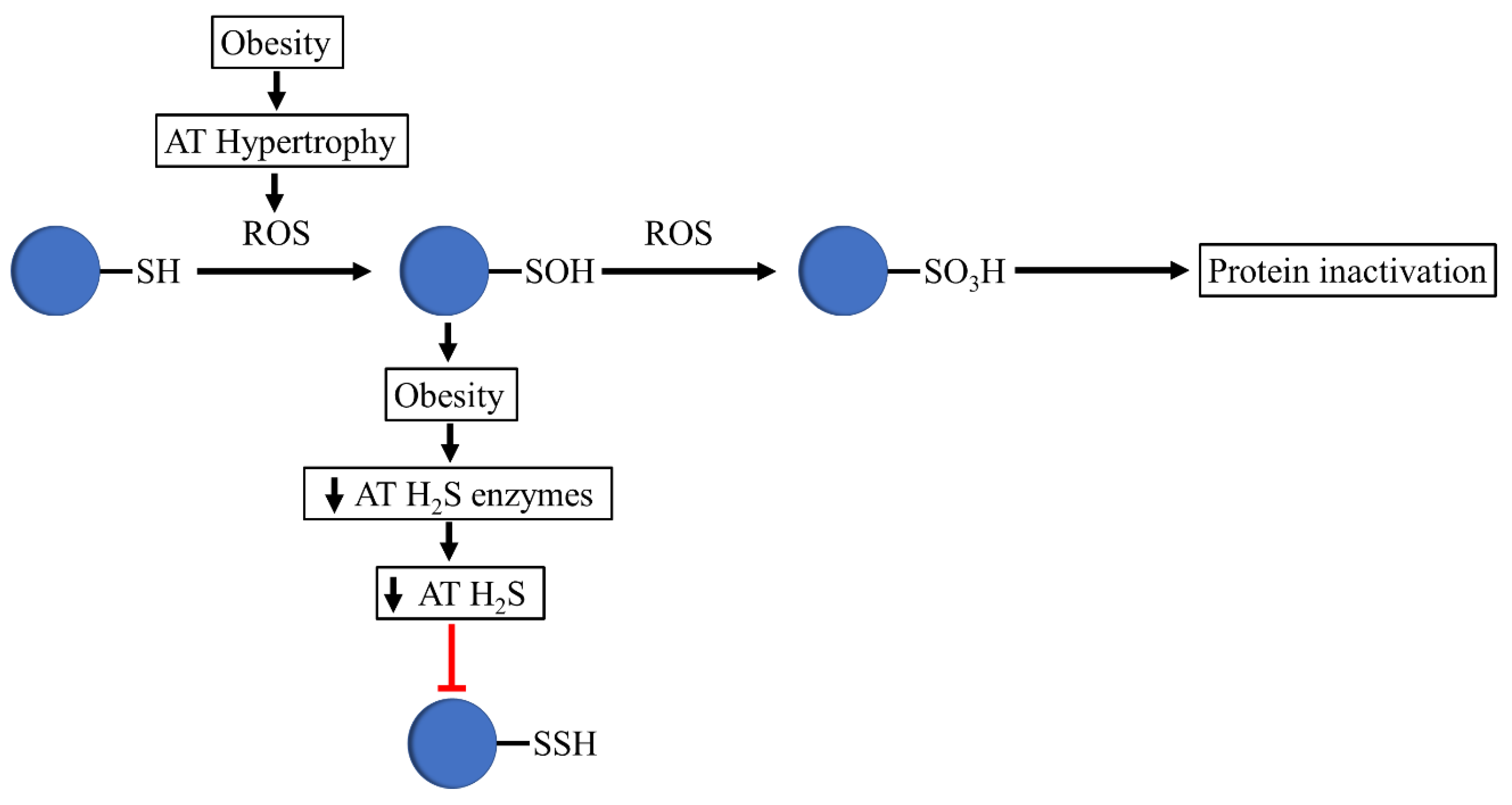
Given the fact that adipose tissue expansion during the progression of obesity can result in the excess production of toxic radical species that can cause the generation of oxidative stress through ROS, it is tempting to speculate that increased ROS in obesity leads to reduced persulfidation, resulting in cysteine thiol overoxidation, altering the integrity and activity of relevant adipose-tissue-related proteins (Figure 2).
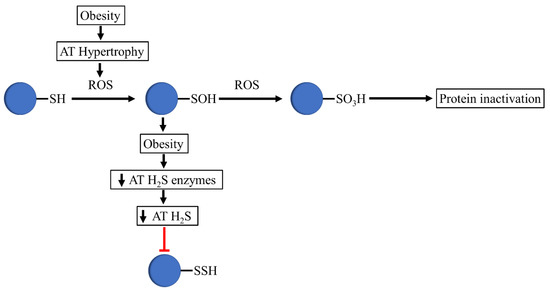
Figure 2.
Proposed mechanism that occurs in thiols in obesogenic conditions. In obesity, adipose tissue hypertrophy is associated with increased ROS levels, which oxidizes protein thiols to sulfenic acids. The decreased expression of H2S-synthesizing enzymes and H2S production in adipose tissue avoids the formation of persulfides by sulfenic acid. The oxidizing environment of obesity mediates the conversion of sulfenic acids to sulfonate, altering the integrity and activity of relevant adipose-tissue-related proteins.
Aside from decreased persulfidation in obesity due to ROS, alternative mechanisms have also been proposed. Dietary restriction (DR) regimens are known to reduce adiposity. Hine et al. reported that dietary restriction of sulfur amino acids (SAAs) methionine (Met) and cysteine (Cys) and decreased mTORC1 activation led to the increased expression of CTH, resulting in increased H2S production in the liver, and an increased lifespan [9]. Methionine and cysteine are considered to be the principal SAAs in the diet because they are incorporated into proteins. Elevation of plasma SAAs is generally associated with an unfavorable lipid profile, and plasma total cysteine is independently associated with body mass index [64]. Another study reported that higher plasma total cysteine was associated with an increased risk of obesity and IR in Hispanic children and adolescents [65]. These studies suggest that a pro-oxidant environment and increased oxidative damage, paired with increased SAAs in obesity, lead to decreased transsulfuration pathway (TSP) activity and decreased H2S, which could result in decreased persulfidation in obesity.
Aging is known to be associated with decreased persulfidated protective pools, associated with the loss of protein expression of the three H2S-producing enzymes [66]. Hine et al. showed that hepatic-produced H2S was elevated in long-lived hypopituitary mouse models, and that the thyroid hormone (TH) and growth hormone (GH) negatively regulate hepatic H2S via the repression of CTH [67]. In addition to the onset of age-related diseases in senior adults, there are some conserved aging phenotypes in human and animals—redox imbalance, mitochondrial dysfunction, increased apoptosis, cellular senescence, insufficient autophagy, and increased inflammation [68]. Similar to aging, obesity and excess calorie intake appear to perpetuate the onset of age-related diseases through similar mechanisms [69]. These findings indicate that obesity accelerates aging, allowing for cysteine overoxidation and reduced persulfidation.
Supporting the importance of H2S and persulfidation in the physiology of adipose tissue (Figure 2), Comas et al. recently demonstrated that proteins required for an appropriate adipogenesis and adipose tissue functionality present higher persulfidation levels in adipocytes in comparison to preadipocytes, sustaining the idea that persulfidation preserves the function of adipogenesis-related proteins [70].
Altogether, the evidence collectively suggests that obesity might be associated with reduced protein persulfidation, paving the way to cysteine overoxidation and impairing the activity and function of adipogenesis-related proteins.
4. H2S in Adipose Tissue
Adipose tissue is one of the most abundant organs in the human body, but for a long time it has been given little importance and it was considered only as a passive energy storage site [71]. The worldwide epidemic of obesity and type 2 diabetes has greatly increased interest in the biology and physiology of adipose tissues. It is now known that apart from the energy reservoir function, adipose tissue functions as a thermal insulator, mechanical shock absorber, and more importantly as an endocrine organ [72].
Feng et al. first identified the endogenous CTH and CBS gene expression pathways in adipose tissue, and suggested CTH as the primary pathway of H2S generation in adipose tissue [73]. The same year, Fang et al. also demonstrated that CTH protein expression and endogenous H2S production in rat perivascular adipose tissue were detectable and that the endogenous H2S generated was predominantly CTH-catalyzed [74]. Subsequent studies confirmed that CBS, CTH, and MPST genes were expressed in adipose tissue depots [75,76], and suggested that H2S affects diverse metabolisms that take place in the adipose tissue, such as lipid, glucose, and mitochondrial metabolism. H2S is also involved in the regulation of inflammatory and oxidative stress-associated responses in adipose tissue through adipokine and antioxidant control [77]. Studies of an in vitro 3T3-L1 mouse cell line model pointed to a possible role of H2S in adipocyte differentiation through the modulation of peroxisome proliferator-activated receptor gamma (PPARγ) activity [43,44,78]. The overexpression of the H2S generation enzyme CTH and the administration of the H2S donor sodium hydrosulfide (NaHS) to 3T3-L1 cells in an environment of high glucose restored adiponectin secretion and decreased the secretion of proinflammatory cytokines [79]. Recently, Comas et al. first reported the relevance of H2S and human adipose tissue physiology in the context of obesity [70]. Experiments in human adipose tissue explants and in isolated preadipocytes demonstrated that exogenous H2S or the activation of endogenous H2S biosynthesis resulted in increased adipogenesis, insulin action, sirtuin deacetylase, and PPARγ transcriptional activity, whereas inhibition through chemical compound or gene knockdown of one of the H2S-generating enzymes (CTH, CBS, MPST) led to altered adipocyte differentiation, cellular senescence, and increased inflammation. In morbidly obese subjects, reduced gene expression of H2S-synthesising enzymes in visceral and subcutaneous adipose tissue depots was reported, whereas weight loss interventions improved the expression of these enzymes. In human preadipocytes, the expression of CTH, CBS, and MPST genes and hydrogen sulfide production were dramatically increased during adipocyte differentiation [70]. In addition, a recent study demonstrated the relevance of selenium-binding-protein 1 (SELENBP1) in 3T3-L1 adipocyte differentiation through the modulation of cellular H2S levels and the expression of H2S-producing enzymes [80].
4.1. H2S and Lipid Metabolism
The effect of H2S on the regulation of lipid metabolism in adipose tissue is controversial. In primary adipocytes, inhibition of the CTH/H2S system was achieved with stimulated adipocyte lipolysis by means of increased activation of the PKA-perilipin1/hormone sensitive lipase (HSL) pathway, whereas GYY4137 or L-cysteine reversed this effect. In adipose tissue of normal chow and HFD mice, the CTH inhibitor DL-propargylglycine (PAG or PPG) increased lipolysis, evidenced by elevated serum glycerol, whereas H2S donors lowered lipolysis only in HFD mice [42]. Different studies have reported the use of slow hydrogen sulfide-releasing agents derived from garlic with effects on lipid metabolism. In isolated human adipocytes and Wistar rats, diallyl sulfide (DAS) downregulated the mRNA and protein expression of lipolytic genes like hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL), whereas it upregulated the expression of lipogenic gens like PPARγ [81]. Aged black garlic (AGB) extract suppressed lipogenesis by reducing the expression of PPARγ, whereas it enhanced lipolysis by upregulating HSL phosphorylation at Ser563 and downregulating perilipin in mature 3T3-L1 adipocytes [82].
On the other hand, infusing Na2S in adipose tissue of rats resulted in increased glycerol and cAMP release in control and obese rats, whereas PAG partially reduced glycerol levels in obese rats, suggesting an H2S-cAMP-PKA lipolysis mechanism [77].
A mechanistic way by which hydrogen sulfide could regulate lipid metabolism is persulfidation. Ding et al. showed that H2S post-translationally modified perilipin 1 (Plin-1) via cysteine persulfidation, resulting in increased Plin1 activity, which blocks HSL translocation to lipid droplets, resulting in decreased lipolysis and promoting adipocyte lipid accumulation [83].
4.2. H2S and Adipose Tissue Inflammation
Obesity predisposes patients to a pro-inflammatory state via increased inflammatory mediators interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), as well as reduced levels of adiponectin, which has a totally anti-inflammatory function [84]. In recent years, substantial basic scientific research has led to a reasonably clear understanding of the role of H2S as an inflammatory mediator implicated in different inflammatory conditions, such as acute pancreatitis, sepsis, joint inflammation, and chronic obstructive pulmonary disease (COPD) [85]. Nevertheless, our understanding of the molecular mechanisms by which adipose tissue H2S contributes to inflammation in the obesity context is still scarce.
Adipose tissue macrophages (ATMs) are able to adopt either a proinflammatory (M1) or an anti-inflammatory (M2) phenotype. During obesity, the proinflammatory M1 phenotype is predominant. Store-operated calcium entry (SOCE), causing an influx of Ca2+, occurs in macrophage polarization to the M1 phenotype [86]. Increased amounts of CSE mRNA and protein and reduced H2S concentrations were found in ATMs isolated from obese mice and RAW264.7 macrophages under inflammatory conditions. Nonetheless, the H2S production capacity was found to be markedly increased in the previous conditions, leading to the increased consumption of H2S and reducing its bioavailability in proinflammatory conditions. This decrease in the concentration of H2S was associated with increased Ca2+ entry through the amplification of SOCE activity, facilitating the proinflammatory M1 phenotype in obese adipose tissue [87].
Recently, we examined the role of H2S in the regulation of inflammation during adipogenesis. Treatment with slow-releasing H2S donor GYY4137 attenuated the negative effect of inflammation on adipogenesis in 3T3-L1 during differentiation [88].
Active research on the role of H2S in inflammation will unravel the pathophysiology of its actions in adipose tissue inflammatory conditions and may help to develop novel therapeutic approaches.
5. The Possible Role of H2S on Glucose Metabolism
Most human cells utilize glucose as the primary substrate, with cellular uptake requiring insulin. Insulin signaling is therefore critical for these tissues. However, a decrease in insulin sensitivity due to the disruption of various molecular pathways causes insulin resistance (IR) [89]. Insulin resistance is one of the main characteristics of pathological manifestations associated with type 2 diabetes mellitus. In general, several intrinsic and extrinsic cellular mechanisms have been identified, which display a cause-effect relationship between weight gain and peripheral insulin resistance [90]. Intrinsic cell-signaling pathways include mitochondrial dysfunction, oxidative stress, and endoplasmic reticulum (ER) stress, whereas alterations in adipokines and fatty acids levels and the presence of inflammation in metabolic tissue are the dominant extrinsic mechanisms that modulate insulin’s peripheral actions [90].
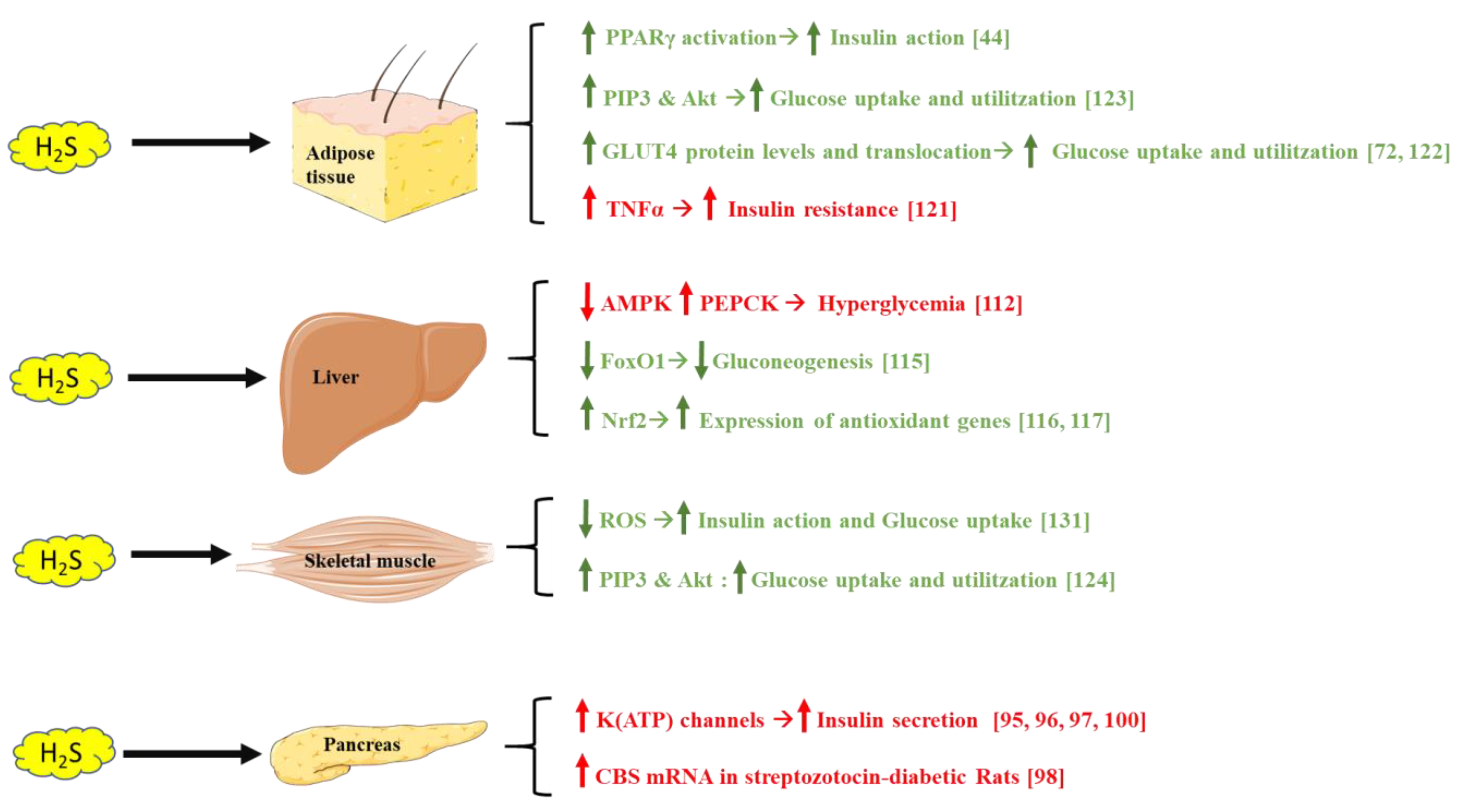
H2S has emerged as a regulator of glucose metabolism and energy homeostasis, through different mechanisms targeting the pancreas, liver, adipose tissue, and skeletal muscle (Figure 3).
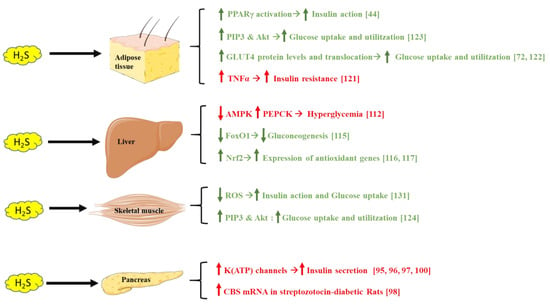
Figure 3.
Different mechanisms showing how H2S affects adipose tissue, liver, skeletal muscle, and the pancreas, modulating insulin sensitivity and blood glucose levels. Green text indicates beneficial H2S effects on glucose metabolism, whereas red text indicates a negative impact on this process. Parts of the figure were drawn or modified by using pictures from Servier Medical Art (http://smart.servier.com/, accession: 13 April 2021), licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/, accession: 13 April 2021).
5.1. H2S and the Pancreas
The pancreas is a complex gland, active in digestion and metabolism through the secretion of digestive enzymes, including the secretion of the blood sugar-lowering hormone insulin by β-cells and its opponent glucagon via the secretion of α-cells [91].
Insulin release from pancreatic β-cells is tightly regulated and allows the sensitive response of insulin levels to calorigenic nutrients in the body. Glucose, free fatty acids, and amino acids serve as fuel stimuli for insulin release, promoting insulin granule exocytosis. Additional hormonal factors influence the regulation pathway [92]. Insulin therapy is the most effective method of lowering blood glucose, thus pharmacological agents that augment insulin release are a key part of the treatment of diabetes.
All three enzymes, CBS, CTH, and MPST, are reported to be present at detectable mRNA and protein levels in rat pancreatic tissues or in cloned rat pancreatic β-cell lines (HIT-T15, INS-1E, and MIN6) [93,94,95].
At the start of the century, the pathophysiological implications of the CTH/H2S system in diabetes were reported. Yusuf et al. first demonstrated that CBS mRNA and H2S synthesis were increased in the pancreas from streptozotocin-induced diabetic rats [96]. Later, CSE and CBS gene expression were reported in mouse pancreatic acini [97]. In HIT-T15, a hamster insulin-secreting cell line, low levels of CBS were reported and no CTH mRNA transcripts were found [94]. In Zucker diabetic fatty (ZDF) rats, both CSE and CBS transcripts were detected in pancreatic islet tissues, with the expression level of CSE being significantly higher than that of CBS [98]. The expression of CBS protein in mouse pancreatic islets and a mouse β-cell line MIN6 was reported by Kaneko et al. [99,100]. INS-1E rat insulinoma cells expressed CTH, and DL-proparglycine mostly depleted H2S synthesis, indicating that CTH was the principal enzyme for H2S in INS-1E cells [93]. No MPST protein expression was detected by Western blot pancreases from C57BL/6J; however, later, strong levels of MPST protein were detected in the pancreas of C57/BL mice via immunohistochemistry using anti-rabbit IgG conjugated with HRP polymer and diaminobenzidine–hydrogen peroxide. Specifically, islets were strongly stained, whereas acinar wells were not stained [101]. Interestingly, Bronowicka-Adamska et al. reported for the first time the expression and activity of all enzymes involved in H2S production in the pancreas and suggested the most important role of MPST in Wistar Kyoto rats [102]. More human and animal studies are needed to elucidate the role of the interplay between H2S synthesizing enzymes, which seems to be a species-specific system.
5.2. H2S and the Liver
The human liver possesses the remarkable ability to produce glucose that is released to the systemic circulation and used by other tissues, particularly during periods of fasting. Hepatic glucose production derives from glycogen breakdown (glycogenolysis) and de novo synthesis of glucose (gluconeogenesis). In the fed state, plasma glucose is derived from the ingestion of nutrients, and the liver maintains normal plasma glucose levels by promoting glycogen synthesis and inhibiting gluconeogenesis [103]. All H2S-producing enzymes are expressed in the liver, which plays an important role in glucose and lipid homeostasis, xenobiotic metabolism, and antioxidant defense [104,105,106,107]. The published literature regarding the role of H2S in regulating glucose metabolism is controversial. Compared with non-diabetic rats, H2S production and CSE and CBS mRNA levels in the liver were increased in STZ diabetic rats, whereas insulin treatment reversed these effects [96]. Alternatively, H2S formation and CSE activity and protein expression in the liver were suppressed in STZ-induced type 1 diabetic rats [108]. Livers taken from insulin-sensitizer metformin-treated SJL mice (100 mg/kg b.w. per day) exhibited increased H2S concentrations [109].
In HepG2 hepatocytes, H2S has been shown to downregulate glucose uptake and glycogen storage, mediated through decreased AMP-activated protein kinase (AMPK) activation, resulting in increased activity of the gluconeogenic enzyme known as phosphoenolpyruvate carboxykinase (PEPCK), leading to hyperglycemia [110]. The same group also reported a stimulatory effect of H2S on liver glucose production under physiologic conditions [111]. On the other hand, Kundu et al. showed that H2S mitigates hyperglycemia in the liver by remodeling kinase B1-adenosine monophosphate-activated protein kinase signaling [112]. Another mechanism suggested by Guo et al. implies that CSE deficiency promoted liver gluconeogenesis in HepG2 via forkhead Box O1 (FoxO1) accumulation [113]. Other hepatoprotective effects of H2S donors were correlated with Nrf2 translocation and increased expression of antioxidant genes, which is regulated by kelch-like ECH-associated protein 1 (Keap1) persulfidation [114,115].
Further study will be needed to explore the regulatory mechanism of diabetes mellitus and its related conditions on the production of H2S in the liver.
5.3. H2S and Adipose Tissue
Adipose tissue plays a central role in regulating whole-body energy and glucose homeostasis through its subtle functions at both organ and systemic levels. On one hand, adipose tissue stores energy in the form of lipids and controls the lipid mobilization and distribution in the body. On the other hand, adipose tissue acts as an endocrine organ and produces numerous bioactive factors such as adipokines that communicate with other organs and modulate a range of metabolic pathways [116]. As explained, previous studies indicate that H2S plays an important role in adipose tissue, and all three enzymes are expressed in human adipose tissue [70].
Insulin exerts a critical control on anabolic responses in adipose tissue (AT) by stimulating glucose and free fatty acid uptake, inhibiting lipolysis and stimulating de novo fatty acid synthesis in adipocytes. In addition, insulin regulates AT growth and differentiation by enhancing the gene expression of various fat-specific transcription factors, including sterol regulatory element-binding protein 1 (SREBP-1c) and PPARγ [117]. Insulin increases glucose uptake in adipocytes by regulating the intracellular localization of glucose transporter 4 (GLUT4), the main glucose transporter involved in the insulin-regulated glucose transport from the cytosol compartment to the plasma membrane [118].
Various studies have shown that H2S regulates insulin sensitivity in adipocytes. In the adipose tissue of fructose induced-diabetic rats, the CTH/H2S system was upregulated and negatively associated with glucose uptake in AT, suggesting a pathological role of H2S in insulin resistance [73]. Huang et al. reported that H2S mediated TNF-α-stimulated insulin resistance, as the treatment of 3T3-L1 adipocytes with TNF-α lead to a deficiency in insulin-stimulated glucose consumption and uptake and an increase in endogenous H2S generation [119]. Chemical inhibition of CTH with PPG and β-cyano-L-alanine attenuated TNF-α-induced insulin resistance in 3T3-L1 adipocytes [119].
Other findings have suggested a beneficial role of H2S in glucose metabolism. Manna et al. showed a molecular mechanism in 3T3-L1 adipocytes by which H2S acts on 1,25-dihydroxyvitamin D3, upregulating GLUT4 protein levels and the translocation of GLUT4, essential for normal glucose metabolism [120]. Through persulfidation of PPARγ (Cys139) in 3T3-L1 cells and in the adipose tissue of HFD-mice, Cai et al. demonstrated that the CSE/H2S system attenuates insulin resistance and promotes the conversion of glucose into triglyceride storage through persulfidation [43]. Manna and Jain found that the administration of exogenous H2S using Na2S donors or stimulating the synthesis of H2S with L-cysteine increased phosphatidylinositol 3,4,5-trisphosphate (PIP3), which phosphorylated Akt and promoted glucose uptake and utilization in 3T3-L1 cells [121]. They also showed that exogenous H2S or L-cysteine supplementation increased insulin receptor substrate 1 (IRS1) phosphorylation and GLUT4 activation, resulting in upregulation of the metabolic actions of insulin and an improvement in glucose metabolism [121]. In another in vivo study, administration of exogenous H2S through NaHS or H2S gas solution promoted adipocytes to uptake glucose, thus reducing fasting blood glucose levels and increasing glucose tolerance [122]. H2S can protect adipocytes against high concentrations of glucose-induced adipocyte dysfunction, evidenced by the restored monocyte chemotactic protein (MCP)-1 and adiponectin secretion [79].
Recently Comas et al., showed that in ex vivo adipose tissue explants the stimulation of H2S synthesis via L-cysteine and pyridoxal 5′-phosphate (PLP) enhanced the expression of adipogenic genes in association with the insulin sensitivity, indicating a beneficial role of adipose tissue H2S in insulin pathway. In different human cohorts, insulin sensitivity was positively associated mainly with subcutaneous CTH and CBS, and weight loss resulted in increased CTH, CBS, and MPST mRNA, in parallel with improved insulin sensitivity [70]. In line with this, it is known that subcutaneous adipose tissue, VAT, is associated with insulin resistance, whereas SAT is associated with a decreased risk of insulin resistance [123]. In human adipocytes, exogenous administration of H2S using GYY4137 increased insulin action, whereas chemical inhibition with PPG attenuated insulin-induced Ser473Akt phosphorylation. Altogether, these findings suggest a mechanism by which H2S increases insulin action through the activation of PPARγ transcriptional activity in differentiated adipocytes [70]. Further study will be needed to explore the regulatory mechanism of adipose tissue H2S and glucose metabolism.
5.4. H2S and Skeletal Muscle
Skeletal muscle differentiation follows an organized sequence of events, including commitment, cell cycle withdrawal, and cell fusion to form multinucleated myotubes [124]. The skeletal muscle is the largest organ in the body by mass. It is also the regulator of glucose homeostasis, responsible for 80% of postprandial glucose uptake from the circulation. Skeletal muscle is essential for metabolism, both for its role in glucose uptake and its importance in exercise and metabolic disease [125]. Human skeletal muscles express significant amounts of CBS and CSE, whereas mouse skeletal muscles completely lack these enzymes [126]. A recent report suggested that all the three enzymes (CBS, CSE, MPST) were present in detectable levels in rat skeletal muscles [127]. Nonetheless, their expression is very low when compared to that of the liver and kidney. Only human skeletal muscles express CBS and CSE enzymes that are comparable to the expression levels in the liver in relative abundance [128].
Xue et al. provided evidence of the insulin-sensitizing effect of exogenous H2S administration (NaHS) in an in vitro model of myotubes (L6) and in vivo in Wistar Rats [122]. Using C2C12 mouse myotubes, exogenous H2S administration (NaHS) upregulated CSE expression and genes involved in GSH biosynthesis in parallel to increased H2S and glucose uptake and decreased ROS, whereas CSE knockdown had the opposite effects [129]. Chronic NaHS treatment (30 μmol·kg−1·day−1) in Goto-Kakizaki diabetic rats decreased fasting blood glucose, increased insulin sensitivity, and increased glucose tolerance with increased phosphorylation of PI3K and Akt in muscles [122]. Expression of CSE was declined at 20 weeks in skeletal muscle of db/db mice, compared to the control group, whereas administration of NaHS restores the expression of CSE at 20 weeks. In C2C12 myoblasts high glucose and palmitate and oleate reduced H2S and expression of CSE significantly [130]. It seems that H2S plays an important role in skeletal muscle, improving glucose homeostasis and increasing glucose uptake.
6. Implication of H2S in NAFLD
Non-alcoholic fatty liver disease (NAFLD) has become the principal cause of chronic liver disease worldwide, involving a spectrum of disturbances mainly characterized by fatty acid infiltration and fat deposition in the liver parenchyma, which can further progress to fibrosis, inflammation, and eventually develop into cirrhosis and hepatocellular carcinoma [131]. Identifying altered molecular pathways that trigger the onset and progression of the disease is a key point in ensuring early diagnoses and developing treatments.
In the liver, although the three H2S-generating enzymes are detectable, their roles in endogenous H2S generation are differently described [108,132,133]. It was found that CTH expression is about 60-fold greater than that of CBS in the murine liver [133]. Apart from endogenous hepatic synthesis, the liver is likely exposed to high levels of H2S exogenous sources as a consequence of its location, making the liver a key regulator of H2S levels by maintaining a high capacity for H2S clearance from the circulation [134].
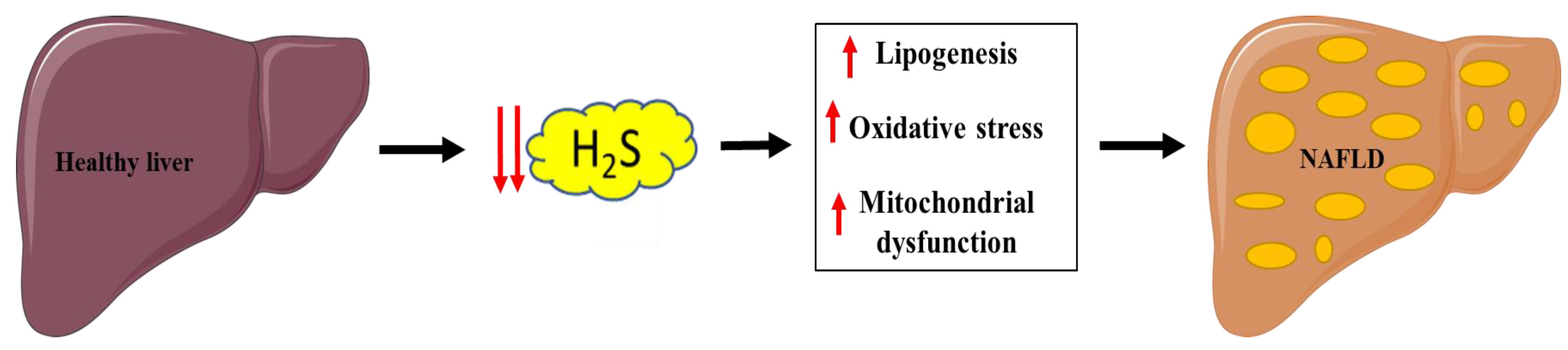
Pathological processes involved with NAFLD are linked with hepatic H2S pathways and include lipid metabolism dysfunction, oxidative stress, insulin resistance, inflammation, and mitochondrial dysfunction. Malfunction of hepatic H2S metabolism is involved in the pathogenesis of many liver diseases, such as hepatic fibrosis and cirrhosis [135] (Figure 4).
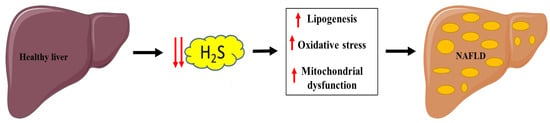
Figure 4.
Link between decreased H2S production and NAFLD. Parts of the figure were drawn or modified by using pictures from Servier Medical Art (http://smart.servier.com/ accessed on 13 April 2021), licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/ accessed on 13 April 2021).
6.1. Mitochondrial and Lipid Metabolism Dysfunction
Since the first studies on NAFLD, many researchers have pointed out that it is primarily characterized by the presence of mitochondrial dysfunction [136]. Mitochondria play a pivotal role in hepatocyte metabolism, being the primary site for the oxidation of fatty acids and oxidative phosphorylation [137].
The CBS/CSE system, which may be regulated by several fatty acids, has been actively investigated in the pathogenesis of NAFLD and has been proposed as a potential therapeutic target for NAFLD [138].
Impaired endogenous H2S synthesis was reported to be associated with fatty liver induced by HFD feeding [106,139] or methionine and choline-deficient (MCD) diet feeding [104]. Treatment with H2S donor sodium hydrosulfide (NaHS), prevented nonalcoholic steatohepatitis (NASH) by reducing hepatic triglyceride and cholesterol levels in rodents fed with the MCD diet through increasing peroxisome proliferator-activated receptor alpha (PPARα) and reducing SREBP-1c gene expression in the liver, suggesting an antisteatogenic effect of H2S through the prevention of oxidative stress and inflammation [104]. In fatty livers of mice induced by HFD, administration of NaHS significantly reduced hypertriglyceridemia and improved NAFLD by activating liver autophagy flux and the AMPK-mTOR signaling pathway [139]. However, a recent study suggested that hepatic MPST promoted hepatic steatosis in HFD fed mice and the knockdown of MPST-stimulated H2S production, whereas overexpression of MPST markedly reduced the formation of H2S via inhibition of CSE/H2S and subsequent upregulation of SREBP-1c, c-Jun N-terminal kinase phosphorylation, and oxidative stress [138]. In addition, that study also demonstrated that the inhibition of MPST in L02 cells reduced free fatty acids (FFAs) and increased the expression of CTH and H2S [138].
Garlic oil derivates diallyl trisulfide/disulfide (DATS/DADS), used as H2S donors in mice, reduced fatty acid synthase (FAS) protein levels, which suppressed ethanol-induced hepatic mitochondrial dysfunction, suggesting the regulation of SREBP1, PPARα, and cytochrome p450 2E1 (CYP2E1) [140].
6.2. Oxidative Stress
Oxidative stress is an important pathophysiological mechanism in NAFLD pathogenesis. Growing evidence supports a key role for oxidative stress caused by the generation of ROS in the progression of NAFLD [141]. Disturbances in lipid metabolism lead to hepatic lipid accumulation, which affects various reactive oxygen species (ROS) generators, including mitochondria, the endoplasmic reticulum, and NADPH oxidase [142].
Oxidative stress involves molecular or cellular damage, resulting from deficiency of antioxidants and/or antioxidant enzyme systems, and disrupting the cellular reduction-oxidation balance [143]. The human body is equipped with a variety of antioxidants that serve to counterbalance the effect of oxidants, with H2S being one of the most important. In fact, H2S protects cells in various diseases by acting as an antioxidant that reduces excessive amounts of reactive oxygen species (ROS) and reactive nitrogen species (RNS) [144]. ROS are highly reactive molecules and can damage cell structures such as carbohydrates, nucleic acids, lipids, and proteins, and alter their functions [145].
Several studies have highlighted the role of H2S in cellular redox homeostasis, which can be summarized in two main mechanisms: i) modulating levels and activity of classic cellular antioxidants, such as glutathione (GSH) and thioredoxin (TRX) [146], and ii) increasing the activity or expression of the transcription nuclear factor (erythroid-derived 2)-like 2 (NRF2) and the histone deacetylase protein family of sirtuins (SIRTs), which in turn increase the expression of antioxidant enzymes (AOE) [114,115].
7. H2S on Cardiovascular Disease
The epidemiological tendency in the 20th century was accompanied by an increase in noncommunicable diseases, of which cardiovascular diseases (CVDs) are now the leading cause of mortality and morbidity worldwide [147]. CVDs are a cluster of diseases and injuries that affect the cardiovascular system, including the heart and blood vessels. Multiple factors are contributing to the epidemic of CVDs, including the rapid aging of the population, improved survival rate from other illnesses, progressive urbanization, increased calorie consumption, decreased physical activity, mental stress, and air pollution [148,149,150].
The main condition underlying CVD is atherosclerosis, a chronic inflammatory condition that involves different cell types, and several cytokines and adhesion molecules.
Atherosclerosis is a chronic and slowly progressive cardiovascular disease that affects arterial blood vessels by thickening and hardening as consequences of high plasma cholesterol concentrations, especially cholesterol in the form of low-density lipoprotein (LDL) [151]. In these processes, reactive oxygen species play a pivotal role, as they can cause the oxidation of lipids such as low-density lipoprotein (LDL) and polyunsaturated fatty acids that are deposited in the vascular wall, allowing plaque calcification, directly damaging cellular components, and further promoting inflammation by activating several pro-atherogenic transcriptional factors [152]. The accumulation of plaques consequentially narrows the arterial lumen and restricts the blood supply, causing atherosclerotic lesions, which through the action of several cytokines can rupture and lead to occlusion of the vascular lumen. Depending on the area of rupture, these can manifest as acute myocardial infractions or stroke or acute ischemia of any nearby organ [153].
Over the last few years, our understanding of atherosclerotic processes has vastly improved; however, there are still many mechanisms that have not been fully elucidated. One of the first reported physiological roles of H2S was its capacity to induce vasorelaxation, acting as a K(ATP) channel opener, and displaying important antihypertensive effects and cardioprotection properties [11,154,155,156]. In line with this, there is a growing body of evidence linking H2S with cardioprotection, including decreasing heart rate, exerting inotropic and proangiogenic effects, decreasing blood pressure, and causing vasodilation [157]. Reduced levels of H2S have been found in patients with acute or stable coronary artery disease [158], hypertension [159], and heart failure [160]. The impact of H2S on cardiovascular disease has been extensively updated and reviewed in recent studies [161,162,163,164,165].
7.1. Hypertension
In 2003, Zhang et al. demonstrated that H2S could exert beneficial effects on the pathogenesis of hypoxic pulmonary hypertension in rats [166]. Later, other groups using hypertensive experimental animal models showed that in rats which had spontaneous hypertension (SHR), the level of hydrogen sulfide and the gene expression and activity of CSE were reduced [167]. In SHR rats, reduced arterial pressure after systemic treatment with NaHS or after intracerebroventricular NaHS treatment were also reported [168].
CSE levels are reduced in the vessel wall of spontaneously hypertensive rats and both CSE and CBS are reduced in the resistance vessels of rats rendered hypertensive after dexamethasone treatment [167,169,170]. In addition, animals with salt-sensitive hypertension have lower levels of CBS [171]. A causal link between low CSE levels and high blood pressure was established following the observation that CSE KO mice exhibit hypertension [172].
Moreover, administration of sulfide salts or the slowly releasing H2S donor GYY4137 reduced blood pressure in a genetic model of hypertension, as well as in rats rendered hypertensive by angiotensin-II or L-NAME administration [173,174,175].
Other naturally occurring H2S donors such as garlic-derived compounds or isothiocyanates present in vegetables also demonstrated cardioprotective effects [176]. Allicin, a garlic-derived H2S donor, is known for lowering arterial blood pressure, among other cardiovascular protection systems [177]. Isothiocyanates, which are abundant in cruciferae like mustard and broccoli, are also considered important in the cardiovascular system. In the rat aortic ring, H2S derived from isothiocyanates was responsible for observed vasorelaxant effects [178]. Other synthetic H2S donors like thioamino acids, including thioglycine and thiovaline, were found to induce significant relaxation of the pre-contracted mouse aortic ring [179].
A great number of studies have been carried on the investigation of the modulation of blood pressure by means of exogenous and endogenous H2S. However, in humans, few studies have measured plasma H2S levels in association with hypertension. In a small cohort of patients with type 2 diabetes, lower plasma levels of H2S were associated with higher systolic blood pressure (SBP) and diastolic blood pressure (DBP) [41]. Reduced H2S plasma levels have been confirmed in a human cohort of hypertensive patients [180,181].
7.2. Atherosclerosis
There are strong indications that the loss of H2S contributes to the establishment and progression of the disease of atherosclerosis. Hydrogen sulfide shows an anti-atherosclerotic action by attenuating oxidative stress, reducing blood platelet activation, reducing the inflammation process, and preventing the proliferation of vascular smooth muscle cells [182,183,184].
Atherosclerosis is a lipoprotein-driven disease that leads to plaque formation at specific sites of the arterial tree through intimal inflammation, necrosis, fibrosis, and calcification [185]. H2S is known to reduce endothelial dysfunction by preventing plaque formation by counteracting the main aspects of atherosclerosis, such as oxidation, adhesion, proliferation, and calcification [165].
Increased oxidative stress can accelerate the development of atherosclerosis by means of endothelial cell dysfunction, adhesion of molecules, vascular smooth muscle proliferation and migration, platelet activation, lipid oxidation, matrix metalloproteinase activation, and alterations in vasomotor activity [186]. Several studies have highlighted the role of H2S in cellular redox homeostasis, which can be summarized in two main mechanisms: (i) modulating the levels and activity of classic cellular antioxidants, such as glutathione (GSH) and thioredoxin (TRX), and (ii) increasing the activity or expression of transcription nuclear factor (erythroid-derived 2)-like 2 (NRF2) and the histone deacetylase protein family of sirtuins (SIRTs), which in turn increase the expression of antioxidant enzymes.
H2S can ameliorate vascular calcification by decreasing the activation of alkaline phosphatase and reducing the gene expression of osteopontin [187].
Plasma H2S and aortic H2S levels were decreased in apolipoprotein E knockout (ApoE(−/−)) mice. CSE expression was reduced in oxidized LDL (Ox-LDL)-stimulated human aortic endothelial cells (HAEC) and in the aorta of high-fat-diet-induced ApoE(−/−) mice [188]. The expression of CSE is mainly upregulated in macrophages, foam cells, and myofibroblasts from the atherosclerotic lesions of human patients with carotid specimens. In mouse and human atherosclerosis, CSE expression is upregulated, but circulating and plasma levels of H2S are reduced, a phenomenon that can be attributed to the inhibition of CSE enzyme activity [189].
The main therapeutic strategy to lower or reverse atherosclerosis is statins, but the clinical benefits of statins are somewhat limited [190,191]. Surprisingly, recent reports have mentioned that treatment using statins can improve H2S production, but it is the lipophilic atorvastatin, rather than the hydrophilic pravastatin, that increases the net H2S production [192,193,194,195,196].
8. Conclusions
This review summarizes and discusses the literature concerning the roles and mechanisms of H2S on obesity-associated metabolic disturbances, including insulin resistance, NAFLD, and cardiovascular diseases. Impaired H2S metabolism is involved in obesity and adipose tissue disturbances; however, scientific understandings of the role of H2S and the mechanisms by which it is altered in obesity remain somewhat contradictory. Significant progress has been made in this field during recent years, but more research is warranted to address this discrepancy in the future. The majority of studies suggest that the deficiency of endogenous H2S synthesis is associated with NAFLD, due to altered pathways that include lipid metabolism dysfunction, oxidative stress, insulin resistance, inflammation, and mitochondrial dysfunction. However, the mechanisms involved are complex and are still incompletely understood. Based on the evidence, it is clear that H2S plays a protective role in cardiovascular diseases, including atherosclerosis. However, there are still many controversies surrounding the signaling pathways, beneficial roles, and harmful effects of H2S in cardiovascular diseases. H2S therapy has only entered a preliminary stage in terms of basic medical research or preclinical research. Nevertheless, based on the existing knowledge about the beneficial effects of H2S, the future of H2S as a potential therapy against obesity-associated metabolic disturbances is expected to be promising and exciting.
Author Contributions
Conceptualization, F.C. and J.M.M.-N.; formal analysis, F.C. and J.M.M.-N.; investigation, F.C. and J.M.M.-N.; writing—review and editing, F.C. and J.M.M.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by research grants PI19/01712 from the Instituto de Salud Carlos III and Fondo Europeo de Desarrollo Regional (FEDER) from Spain and VII Spanish Diabetes Association grants to Basic Diabetes Research Projects led by young researchers. CIBEROBN Fisiopatología de la Obesidad y Nutrición is an initiative from the Instituto de Salud Carlos III.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kolluru, G.K.; Shen, X.; Yuan, S.; Kevil, C.G. Gasotransmitter Heterocellular Signaling. Antioxid. Redox Signal. 2017, 26, 936–960. [Google Scholar] [CrossRef]
- Wang, R. The Gasotransmitter Role of Hydrogen Sulfide. Antioxid. Redox Signal. 2003, 5, 493–501. [Google Scholar] [CrossRef]
- Hendriks, K.D.; Maassen, H.; van Dijk, P.R.; Henning, R.H.; van Goor, H.; Hillebrands, J.L. Gasotransmitters in Health and Disease: A Mitochondria-Centered View. Curr. Opin. Pharmacol. 2019, 45, 87–93. [Google Scholar] [CrossRef]
- Yang, G.; Sener, A.; Ji, Y.; Pei, Y.; Pluth, M.D. Gasotransmitters in Biology and Medicine: Molecular Mechanisms and Drug Targets. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Snyder, S.H. Signaling by Gasotransmitters. Sci. Signal. 2009, 2, re2. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Physiological Role of Hydrogen Sulfide and Polysulfide in the Central Nervous System. Neurochem. Int. 2013, 63, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen Sulfide Mediates the Vasoactivity of Garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Huang, Y.; Yan, H.; Zhang, Q.; Zhao, M.; Zhu, M.; Liu, J.; Chen, S.X.; Bu, D.; Tang, C.; et al. Hydrogen Sulfide Suppresses Oxidized Low-Density Lipoprotein (Ox-LDL)-Stimulated Monocyte Chemoattractant Protein 1 Generation from Macrophages via the Nuclear Factor Κb (NF-κB) Pathway. J. Biol. Chem. 2014, 289, 9741–9753. [Google Scholar] [CrossRef]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.; Longchamp, A.; Treviño-Villarreal, J.H.; Mejia, P.; Ozaki, C.K.; et al. Endogenous Hydrogen Sulfide Production Is Essential for Dietary Restriction Benefits. Cell 2015, 160, 132–144. [Google Scholar] [CrossRef]
- Szabõ, C. Hydrogen Sulphide and Its Therapeutic Potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine γ-Lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lu, M.; Hu, L.F.; Wong, P.T.H.; Webb, G.D.; Bian, J.S. Hydrogen Sulfide in the Mammalian Cardiovascular System. Antioxid. Redox Signal. 2012, 17, 141–185. [Google Scholar] [CrossRef]
- Nagpure, B.V.; Bian, J.S. Interaction of Hydrogen Sulfide with Nitric Oxide in the Cardiovascular System. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Hu, L.F.; Lu, M.; Hon Wong, P.T.; Bian, J.S. Hydrogen Sulfide: Neurophysiology and Neuropathology. Antioxid. Redox Signal. 2011, 15, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Nagpure, B.V.; Bian, J.S. Brain, Learning, and Memory: Role of H2S in Neurodegenerative Diseases. Handb. Exp. Pharmacol. 2015, 230, 193–215. [Google Scholar] [CrossRef]
- Gong, Q.H.; Shi, X.R.; Hong, Z.Y.; Pan, L.L.; Liu, X.H.; Zhu, Y.Z. A New Hope for Neurodegeneration: Possible Role of Hydrogen Sulfide. J. Alzheimer’s Dis. 2011, 24, 173–182. [Google Scholar] [CrossRef]
- Cao, X.; Ding, L.; Xie, Z.Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid. Redox Signal. 2019, 31, 1. [Google Scholar] [CrossRef]
- Hellmich, M.R.; Szabo, C. Hydrogen Sulfide and Cancer. Handb. Exp. Pharmacol. 2015, 230, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Jhee, K.H.; Kruger, W.D. The Role of Cystathionine β-Synthase in Homocysteine Metabolism. Antioxid. Redox Signal. 2005, 7, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S Biogenesis by Human Cystathionine γ-Lyase Leads to the Novel Sulfur Metabolites Lanthionine and Homolanthionine and Is Responsive to the Grade of Hyperhomocysteinemia. J. Biol. Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the Transsulfuration Pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Sbodio, J.I.; Snyder, S.H. Cysteine Metabolism in Neuronal Redox Homeostasis. Trends Pharmacol. Sci. 2018, 39, 513–524. [Google Scholar] [CrossRef] [PubMed]
- McBean, G.J.; Aslan, M.; Griffiths, H.R.; Torrão, R.C. Thiol Redox Homeostasis in Neurodegenerative Disease. Redox Biol. 2015, 5, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Mosharov, E.; Cranford, M.R.; Banerjee, R. The Quantitatively Important Relationship between Homocysteine Metabolism and Glutathione Synthesis by the Transsulfuration Pathway and Its Regulation by Redox Changes. Biochemistry 2000, 39, 13005–13011. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Zou, C.G. Redox Regulation and Reaction Mechanism of Human Cystathionine-β-Synthase: A PLP-Dependent Hemesensor Protein. Arch. Biochem. Biophys. 2005, 433, 144–156. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. H2S Signalling through Protein Sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. H2S: A Novel Gasotransmitter That Signals by Sulfhydration. Trends Biochem. Sci. 2015, 40, 687–700. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Ueki, I. Dealing with Methionine/homocysteine Sulfur: Cysteine Metabolism to Taurine and Inorganic Sulfur. J. Inherit. Metab. Dis. 2011, 34, 17–32. [Google Scholar] [CrossRef]
- Rose, P.; Moore, P.K.; Zhu, Y.Z. H2S Biosynthesis and Catabolism: New Insights from Molecular Studies. Cell. Mol. Life Sci. 2017, 74, 1391–1412. [Google Scholar] [CrossRef]
- Jurkowska, H.; Roman, H.B.; Hirschberger, L.L.; Sasakura, K.; Nagano, T.; Hanaoka, K.; Krijt, J.; Stipanuk, M.H. Primary Hepatocytes from Mice Lacking Cysteine Dioxygenase Show Increased Cysteine Concentrations and Higher Rates of Metabolism of Cysteine to Hydrogen Sulfide and Thiosulfate. Amino Acids 2014, 46, 1353–1365. [Google Scholar] [CrossRef]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef]
- Shibuya, N.; Kimura, H. Production of Hydrogen Sulfide from D-Cysteine and Its Therapeutic Potential. Front. Endocrinol. (Lausanne) 2013, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A Novel Pathway for the Production of Hydrogen Sulfide from D-Cysteine in Mammalian Cells. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Minkler, P.; Grove, D.; Wang, R.; Willard, B.; Dweik, R.; Hine, C. Non-Enzymatic Hydrogen Sulfide Production from Cysteine in Blood Is Catalyzed by Iron and Vitamin B6. Commun. Biol. 2019, 2, 1–14. [Google Scholar] [CrossRef]
- Searcy, D.G.; Lee, S.H. Sulfur Reduction by Human Erythrocytes. J. Exp. Zool. 1998, 282, 310–322. [Google Scholar] [CrossRef]
- Olson, K.R.; DeLeon, E.R.; Gao, Y.; Hurley, K.; Sadauskas, V.; Batz, C.; Stoy, G.F. Thiosulfate: A Readily Accessible Source of Hydrogen Sulfide in Oxygen Sensing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, H.R.; Mu, Q.; Rose, P.; Zhu, Y.Z. S-Propargyl-Cysteine Protects Both Adult Rat Hearts and Neonatal Cardiomyocytes from Ischemia/hypoxia Injury: The Contribution of the Hydrogen Sulfide-Mediated Pathway. J. Cardiovasc. Pharmacol. 2009, 54, 139–146. [Google Scholar] [CrossRef]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Whiteman, M.; Gooding, K.M.; Whatmore, J.L.; Ball, C.I.; Mawson, D.; Skinner, K.; Tooke, J.E.; Shore, A.C. Adiposity Is a Major Determinant of Plasma Levels of the Novel Vasodilator Hydrogen Sulphide. Diabetologia 2010, 53, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Cai, B.; Liao, F.; Zheng, Y.; Zeng, Q.; Fan, X.; Gong, Y.; Yang, J.; Cui, Q.H.; Tang, C.; et al. Increase or Decrease Hydrogen Sulfide Exert Opposite Lipolysis, but Reduce Global Insulin Resistance in High Fatty Diet Induced Obese Mice. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Cai, J.; Shi, X.; Wang, H.; Fan, J.; Feng, Y.; Lin, X.; Yang, J.; Cui, Q.; Tang, C.; Xu, G.; et al. Cystathionine γ Lyase-Hydrogen Sulfide Increases Peroxisome Proliferator-Activated Receptor γ Activity by Sulfhydration at C139 Site Thereby Promoting Glucose Uptake and Lipid Storage in Adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ju, Y.; Fu, M.; Zhang, Y.; Pei, Y.; Racine, M.; Baath, S.; Merritt, T.J.S.; Wang, R.; Wu, L. Cystathionine Gamma-Lyase/hydrogen Sulfide System Is Essential for Adipogenesis and Fat Mass Accumulation in Mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 165–176. [Google Scholar] [CrossRef]
- Alkhouri, N.; Eng, K.; Cikach, F.; Patel, N.; Yan, C.; Brindle, A.; Rome, E.; Hanouneh, I.; Grove, D.; Lopez, R.; et al. Breathprints of Childhood Obesity: Changes in Volatile Organic Compounds in Obese Children Compared with Lean Controls. Pediatr. Obes. 2015, 10, 23–29. [Google Scholar] [CrossRef]
- Comas, F.; Latorre, J.; Ortega, F.; Arnoriaga Rodríguez, M.; Lluch, A.; Sabater, M.; Rius, F.; Ribas, X.; Costas, M.; Ricart, W.; et al. Morbidly Obese Subjects Show Increased Serum Sulfide in Proportion to Fat Mass. Int. J. Obes. 2021, 45, 415–426. [Google Scholar] [CrossRef]
- Ren, H.; Liu, T.C.; Lu, Y.; Zhang, K.; Xu, Y.; Zhou, P.; Tang, X. A Comparison Study of the Influence of Milk Protein versus Whey Protein in High-Protein Diets on Adiposity in Rats. Food Funct. 2021, 12, 1008–1019. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. HS Signals through Protein S-Sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef]
- Kimura, H. Signaling by Hydrogen Sulfide (H2S) and Polysulfides (H2SN) in the Central Nervous System. Neurochem. Int. 2019, 126, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R. Persulfidation (S-Sulfhydration) and H2S. Handb. Exp. Pharmacol. 2015, 230, 29–59. [Google Scholar] [CrossRef]
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in Innate Immune Responses. Nat. Rev. Immunol. 2011, 11, 389–402. [Google Scholar] [CrossRef]
- Kamiński, M.M.; Röth, D.; Krammer, P.H.; Gülow, K. Mitochondria as Oxidative Signaling Organelles in T-Cell Activation: Physiological Role and Pathological Implications. Arch. Immunol. Ther. Exp. (Warsz.) 2013, 61, 367–384. [Google Scholar] [CrossRef]
- Hekimi, S.; Lapointe, J.; Wen, Y. Taking A “good” look at Free Radicals in the Aging Process. Trends Cell Biol. 2011, 21, 569–576. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive Oxygen Species Have a Causal Role in Multiple Forms of Insulin Resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Sindhu, K.K. Oxidative Stress and Metabolic Syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Sankhla, M.; Sharma, T.K.; Mathur, K.; Rathor, J.S.; Butolia, V.; Gadhok, A.K.; Vardey, S.K.; Sinha, M.; Kaushik, G.G. Relationship of Oxidative Stress with Obesity and Its Role in Obesity Induced Metabolic Syndrome. Clin. Lab. 2012, 58, 385–392. [Google Scholar] [PubMed]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Skoumas, J.; Krinos, X.; Chloptsios, Y.; Nikolaou, V.; Stefanadis, C. Long-Term Fish Consumption Is Associated with Protection against Arrhythmia in Healthy Persons in a Mediterranean Region—The ATTICA Study. Am. J. Clin. Nutr. 2007, 85, 1385–1391. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative Stress and Inflammation Interactions in Human Obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Sfar, S.; Boussoffara, R.; Sfar, M.T.; Kerkeni, A. Antioxidant Enzymes Activities in Obese Tunisian Children. Nutr. J. 2013, 12, 18. [Google Scholar] [CrossRef]
- Gunanti, I.R.; Marks, G.C.; Al-Mamun, A.; Long, K.Z. Low Serum Concentrations of Carotenoids and Vitamin E Are Associated with High Adiposity in Mexican-American Children. J. Nutr. 2014, 144, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Amirkhizi, F.; Siassi, F.; Djalali, M.; Shahraki, S.H. Impaired Enzymatic Antioxidant Defense in Erythrocytes of Women with General and Abdominal Obesity. Obes. Res. Clin. Pract. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Valdivia-Garcia, M.; Graham, I.M.; Palma Reis, R.; Sales Luis, A.; Smith, A.D.; Refsum, H. The Association of Fasting Plasma Sulfur-Containing Compounds with BMI, Serum Lipids and Apolipoproteins. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Valdivia-Garcia, M.; Refsum, H.; Butte, N. The Association of Cysteine with Obesity, Inflammatory Cytokines and Insulin Resistance in Hispanic Children and Adolescents. PLoS ONE 2012, 7, 44166. [Google Scholar] [CrossRef] [PubMed]
- Zivanovic, J.; Kouroussis, E.; Kohl, J.B.; Adhikari, B.; Bursac, B.; Schott-Roux, S.; Petrovic, D.; Miljkovic, J.L.; Thomas-Lopez, D.; Jung, Y.; et al. Selective Persulfide Detection Reveals Evolutionarily Conserved Antiaging Effects of S-Sulfhydration. Cell Metab. 2019, 30, 1152–1170.e13. [Google Scholar] [CrossRef]
- Hine, C.; Kim, H.J.; Zhu, Y.; Harputlugil, E.; Longchamp, A.; Matos, M.S.; Ramadoss, P.; Bauerle, K.; Brace, L.; Asara, J.M.; et al. Hypothalamic-Pituitary Axis Regulates Hydrogen Sulfide Production. Cell Metab. 2017, 25, 1320–1333.e5. [Google Scholar] [CrossRef] [PubMed]
- Vijg, J.; Campisi, J. Puzzles, Promises and a Cure for Ageing. Nature 2008, 454, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Tam, B.T.; Morais, J.A.; Santosa, S. Obesity and Ageing: Two Sides of the Same Coin. Obes. Rev. 2020, 21. [Google Scholar] [CrossRef]
- Comas, F.; Latorre, J.; Ortega, F.; Arnoriaga Rodríguez, M.; Kern, M.; Lluch, A.; Ricart, W.; Blüher, M.; Gotor, C.; Romero, L.C.; et al. Activation of Endogenous H2S Biosynthesis or Supplementation with Exogenous H2S Enhances Adipose Tissue Adipogenesis and Preserves Adipocyte Physiology in Humans. Antioxid. Redox Signal. 2021. [Google Scholar] [CrossRef]
- Cypess, A.M.; Kahn, C.R. The Role and Importance of Brown Adipose Tissue in Energy Homeostasis. Curr. Opin. Pediatr. 2010, 22, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Beattie, J.H. Physiological Role of Adipose Tissue: White Adipose Tissue as an Endocrine and Secretory Organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, Y.; Zhao, J.; Tang, C.; Jiang, Z.; Geng, B. Hydrogen Sulfide from Adipose Tissue Is a Novel Insulin Resistance Regulator. Biochem. Biophys. Res. Commun. 2009, 380, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhao, J.; Chen, Y.; Ma, T.; Xu, G.; Tang, C.; Liu, X.; Geng, B. Hydrogen Sulfide Derived from Periadventitial Adipose Tissue Is a Vasodilator. J. Hypertens. 2009, 27, 2174–2185. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J. Endogenous Hydrogen Sulfide in Perivascular Adipose Tissue: Role in the Regulation of Vascular Tone in Physiology and pathology1. Can. J. Physiol. Pharmacol. 2013, 91, 889–898. [Google Scholar] [CrossRef]
- Katsouda, A.; Szabo, C.; Papapetropoulos, A. Reduced Adipose Tissue H2S in Obesity. Pharmacol. Res. 2018, 128, 190–199. [Google Scholar] [CrossRef]
- Bełtowski, J.; Jamroz-Wísniewska, A. Hydrogen Sulfide in the Adipose Tissue-Physiology, Pathology and a Target for Pharmacotherapy. Molecules 2017, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Peh, M.T.; Feng, W.; Dymock, B.W.; Moore, P.K. Hydrogen Sulfide Promotes Adipogenesis in 3T3L1 Cells. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, H.; Liu, Y.; Yu, C.; Zhang, Y.; Chen, J.; Wang, X.; Guan, Q. Involvement of CSE/ H2S in High Glucose Induced Aberrant Secretion of Adipokines in 3T3-L1 Adipocytes. Lipids Health Dis. 2014, 13, 155. [Google Scholar] [CrossRef]
- Randi, E.B.; Casili, G.; Jacquemai, S.; Szabo, C. Selenium-Binding Protein 1 (Selenbp1) Supports Hydrogen Sulfide Biosynthesis and Adipogenesis. Antioxidants 2021, 10, 361. [Google Scholar] [CrossRef]
- Kema, V.H.; Khan, I.; Jamal, R.; Vishwakarma, S.K.; Lakki Reddy, C.; Parwani, K.; Patel, F.; Patel, D.; Khan, A.A.; Mandal, P. Protective Effects of Diallyl Sulfide Against Ethanol-Induced Injury in Rat Adipose Tissue and Primary Human Adipocytes. Alcohol. Clin. Exp. Res. 2017, 41, 1078–1092. [Google Scholar] [CrossRef]
- Nam, H.; Jung, H.; Kim, Y.; Kim, B.; Kim, K.H.; Park, S.J.; Suh, J.G. Aged Black Garlic Extract Regulates Lipid Metabolism by Inhibiting Lipogenesis and Promoting Lipolysis in Mature 3T3-L1 Adipocytes. Food Sci. Biotechnol. 2018, 27, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, H.; Geng, B.; Xu, G. Sulfhydration of Perilipin 1 Is Involved in the Inhibitory Effects of Cystathionine Gamma Lyase/hydrogen Sulfide on Adipocyte Lipolysis. Biochem. Biophys. Res. Commun. 2020, 521, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity & Inflammation: The Linking Mechanism & the Complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M. Role of Hydrogen Sulfide in the Pathology of Inflammation. Scientifica 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Sun, Y.; Sukumaran, P.; Quenum Zangbede, F.O.; Jondle, C.N.; Sharma, A.; Evans, D.L.; Chauhan, P.; Szlabick, R.E.; Aaland, M.O.; et al. M1 Macrophage Polarization Is Dependent on TRPC1-Mediated Calcium Entry. iScience 2018, 8, 85–102. [Google Scholar] [CrossRef]
- Velmurugan, G.V.; Huang, H.; Sun, H.; Candela, J.; Jaiswal, M.K.; Beaman, K.D.; Yamashita, M.; Prakriya, M.; White, C. Depletion of H2S during Obesity Enhances Store-Operated Ca2+ Entry in Adipose Tissue Macrophages to Increase Cytokine Production. Sci. Signal. 2015, 8, ra128. [Google Scholar] [CrossRef]
- Comas, F.; Latorre, J.; Cussó, O.; Ortega, F.; Lluch, A.; Sabater, M.; Castells-Nobau, A.; Ricart, W.; Ribas, X.; Costas, M.; et al. Hydrogen Sulfide Impacts on Inflammation-Induced Adipocyte Dysfunction. Food Chem. Toxicol. 2019, 131. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin Resistance: Review of the Underlying Molecular Mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Chawla, A. Pleiotropic Actions of Insulin Resistance and Inflammation in Metabolic Homeostasis. Science 2013, 339, 172–177. [Google Scholar] [CrossRef]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic Regulation of Glucose Homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef]
- Mann, E.; Sunni, M.; Bellin, M.D. Secretion of Insulin in Response to Diet and Hormones. Pancreapedia Exocrine Pancreas Knowl. Base 2020. [Google Scholar] [CrossRef]
- Yang, W.; Yang, G.; Jia, X.; Wu, L.; Wang, R. Activation of KATP Channels by H2S in Rat Insulin-Secreting Cells and the Underlying Mechanisms. J. Physiol. 2005, 569, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Whiteman, M.; Low, C.M.; Moore, P.K. Hydrogen Sulphide Reduces Insulin Secretion from HIT-T15 Cells by a KATP Channel-Dependent Pathway. J. Endocrinol. 2007, 195, 105–112. [Google Scholar] [CrossRef]
- Taniguchi, S.; Niki, I. Significance of Hydrogen Sulfide Production in the Pancreatic β-Cell. J. Pharmacol. Sci. 2011, 116, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Huat, B.T.K.; Hsu, A.; Whiteman, M.; Bhatia, M.; Moore, P.K. Streptozotocin-Induced Diabetes in the Rat Is Associated with Enhanced Tissue Hydrogen Sulfide Biosynthesis. Biochem. Biophys. Res. Commun. 2005, 333, 1146–1152. [Google Scholar] [CrossRef]
- Tamizhselvi, R.; Moore, P.K.; Bhatia, M. Hydrogen Sulfide Acts as a Mediator of Inflammation Inacute Pancreatitis: In Vitro Studies Using Isolated Mouse Pancreatic Acinar Cells. J. Cell. Mol. Med. 2007, 11, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, W.; Jia, X.; Yang, G.; Duridanova, D.; Cao, K.; Wang, R. Pancreatic Islet Overproduction of H2S and Suppressed Insulin Release in Zucker Diabetic Rats. Lab. Investig. 2009, 89, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Kimura, T.; Taniguchi, S.; Souma, M.; Kojima, Y.; Kimura, Y.; Kimura, H.; Niki, I. Glucose-Induced Production of Hydrogen Sulfide May Protect the Pancreatic Beta-Cells from Apoptotic Cell Death by High Glucose. FEBS Lett. 2008, 583, 377–382. [Google Scholar] [CrossRef]
- Kaneko, Y.; Kimura, Y.; Kimura, H.; Niki, I. L-Cysteine Inhibits Insulin Release from the Pancreatic β-Cell: Possible Involvement of Metabolic Production of Hydrogen Sulfide, a Novel Gasotransmitter. Diabetes 2006, 55, 1391–1397. [Google Scholar] [CrossRef]
- Tomita, M.; Nagahara, N.; Ito, T. Expression of 3-Mercaptopyruvate Sulfurtransferase in the Mouse. Molecules 2016, 21, 1707. [Google Scholar] [CrossRef]
- Bronowicka-Adamska, P.; Hutsch, T.; Gawrys-Kopczynska, M.; Maksymiuk, K.; Wróbel, M. Hydrogen Sulfide Formation in Experimental Model of Acute Pancreatitis. Acta Biochim. Pol. 2019, 66. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Pérez-Felpete, N.; Fernández-Fernández, C.; Donapetry-García, C.; Pazos-García, C. Liver Glucose Metabolism in Humans. Biosci. Rep. 2016, 36, 416. [Google Scholar] [CrossRef]
- Luo, Z.L.; Tang, L.J.; Wang, T.; Dai, R.W.; Ren, J.D.; Cheng, L.; Xiang, K.; Tian, F.Z. Effects of Treatment with Hydrogen Sulfide on Methionine-Choline Deficient Diet-Induced Non-Alcoholic Steatohepatitis in Rats. J. Gastroenterol. Hepatol. 2014, 29, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Shirozu, K.; Tokuda, K.; Marutani, E.; Lefer, D.; Wang, R.; Ichinose, F. Cystathionine γ-Lyase Deficiency Protects Mice from Galactosamine/lipopolysaccharide-Induced Acute Liver Failure. Antioxid. Redox Signal. 2014, 20, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Peh, M.T.; Anwar, A.B.; Ng, D.S.W.; Shirhan Bin Mohamed Atan, M.; Kumar, S.D.; Moore, P.K. Effect of Feeding a High Fat Diet on Hydrogen Sulfide (H2S) Metabolism in the Mouse. Nitric Oxide Biol. Chem. 2014, 41, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Sarna, L.K.; Siow, Y.L.; Karmin, O. High-Fat Diet Stimulates Hepatic Cystathionine β-Synthase and Cystathionine γ-Lyase Expression. Can. J. Physiol. Pharmacol. 2013, 91, 913–919. [Google Scholar] [CrossRef]
- Manna, P.; Gungor, N.; McVie, R.; Jain, S.K. Decreased Cystathionine-γ-Lyase (CSE) Activity in Livers of Type 1 Diabetic Rats and Peripheral Blood Mononuclear Cells (PBMC) of Type 1 Diabetic Patients. J. Biol. Chem. 2014, 289, 11767–11778. [Google Scholar] [CrossRef]
- Wiliński, B.; Wiliński, J.; Somogyi, E.; Piotrowska, J.; Opoka, W. Metformin Raises Hydrogen Sulfide Tissue Concentrations in Various Mouse Organs. Pharmacol. Reports 2013, 65, 737–742. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Untereiner, A.; Ju, Y.; Wu, L.; Wang, R. Hydrogen Sulfide Impairs Glucose Utilization and Increases Gluconeogenesis in Hepatocytes. Endocrinology 2013, 154, 114–126. [Google Scholar] [CrossRef]
- Untereiner, A.A.; Wang, R.; Ju, Y.; Wu, L. Decreased Gluconeogenesis in the Absence of Cystathionine Gamma-Lyase and the Underlying Mechanisms. Antioxid. Redox Signal. 2016, 24, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Pushpakumar, S.; Khundmiri, S.J.; Sen, U. Hydrogen Sulfide Mitigates Hyperglycemic Remodeling via Liver Kinase B1-Adenosine Monophosphate-Activated Protein Kinase Signaling. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, D.; You, V.; Li, W.; Hu, B.; Zhang, S.; Miao, L.; Xian, M.; Zhu, Y.; Shen, X. Cystathionine Γ-lyase Deficiency Aggravates Obesity-related Insulin Resistance via FoxO1-dependent Hepatic Gluconeogenesis. FASEB J. 2019, 33, 4212–4224. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen Sulfide Protects against Cellular Senescence via S-Sulfhydration of keap1 and Activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef]
- Hourihan, J.M.; Kenna, J.G.; Hayes, J.D. The Gasotransmitter Hydrogen Sulfide Induces Nrf2-Target Genes by Inactivating the keap1 Ubiquitin Ligase Substrate Adaptor through Formation of a Disulfide Bond between Cys-226 and Cys-613. Antioxid. Redox Signal. 2013, 19, 465–481. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adipose Tissue in Control of Metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Laviola, L.; Perrini, S.; Cignarelli, A.; Giorgino, F. Insulin Signalling in Human Adipose Tissue. Archives of Physiology and Biochemistry. J. Metab. Dis. 2006, 112, 82–88. [Google Scholar] [CrossRef]
- Rea, S.; James, D.E. Moving GLUT4: The Biogenesis and Trafficking of GLUT4 Storage Vesicles. Diabetes 1997, 46, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Yao, W.F.; Wu, W.-g.; Lu, Y.L.; Wan, H.; Wang, W. Endogenous CSE/H2S System Mediates TNF-α-Induced Insulin Resistance in 3T3-L1 Adipocytes. Cell Biochem. Funct. 2013, 31, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Vitamin D up-Regulates Glucose Transporter 4 (GLUT4) Translocation and Glucose Utilization Mediated by Cystathionine-γ-Lyase (CSE) Activation and H2S Formation in 3T3L1 Adipocytes. J. Biol. Chem. 2012, 287, 42324–42332. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Hydrogen Sulfide and L-Cysteine Increase Phosphatidylinositol 3,4,5-Trisphosphate (PIP3) and Glucose Utilization by Inhibiting Phosphatase and Tensin Homolog (PTEN) Protein and Activating Phosphoinositide 3-Kinase (PI3K)/serine/threonine Protein Kinase (AKT)/protein Kinase Cς/λ (PKCς/λ) in 3T3l1 Adipocytes. J. Biol. Chem. 2011, 286, 39848–39859. [Google Scholar] [CrossRef]
- Xue, R.; Hao, D.D.; Sun, J.P.; Li, W.W.; Zhao, M.M.; Li, X.H.; Chen, Y.; Zhu, J.H.; Ding, Y.J.; Liu, J.; et al. Hydrogen Sulfide Treatment Promotes Glucose Uptake by Increasing Insulin Receptor Sensitivity and Ameliorates Kidney Lesions in Type 2 Diabetes. Antioxid. Redox Signal. 2013, 19, 5–23. [Google Scholar] [CrossRef]
- McLaughlin, T.; Lamendola, C.; Liu, A.; Abbasi, F. Preferential Fat Deposition in Subcutaneous versus Visceral Depots Is Associated with Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2011, 96, E1756–E1760. [Google Scholar] [CrossRef] [PubMed]
- Araya, R.; Riquelme, M.A.; Brandan, E.; Sáez, J.C. The Formation of Skeletal Muscle Myotubes Requires Functional Membrane Receptors Activated by Extracellular ATP. Brain Res. Brain Res. Rev. 2004, 47, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.C.; Yang, F.; Capecci, L.M.; Gu, Z.; Schafer, A.I.; Durante, W.; Yang, X.; Wang, H. Regulation of Homocysteine Metabolism and Methylation in Human and Mouse Tissues. FASEB J. 2010, 24, 2804–2817. [Google Scholar] [CrossRef] [PubMed]
- Du, J.T.; Li, W.; Yang, J.Y.; Tang, C.S.; Li, Q.; Jin, H.F. Hydrogen Sulfide Is Endogenously Generated in Rat Skeletal Muscle and Exerts a Protective Effect against Oxidative Stress. Chin. Med. J. 2013, 126, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Veeranki, S.; Tyagi, S.C. Role of Hydrogen Sulfide in Skeletal Muscle Biology and Metabolism. Nitric Oxide Biol. Chem. 2015, 46, 66–71. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Hydrogen Sulfide Increases Glutathione Biosynthesis, and Glucose Uptake and Utilisation in C2C12 Mouse Myotubes. Free Radic. Res. 2018, 52, 288–303. [Google Scholar] [CrossRef]
- Lu, F.; Lu, B.; Zhang, L.; Wen, J.C.; Wang, M.; Zhang, S.; Li, Q.; Shu, F.; Sun, Y.; Liu, N.; et al. Hydrogen Sulphide Ameliorating Skeletal Muscle Atrophy in Db/db Mice via Muscle RING Finger 1 S-Sulfhydration. J. Cell. Mol. Med. 2020, 24, 9362–9377. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.U.; Cusi, K. Non-Alcoholic Fatty Liver Disease: Causes, Diagnosis, Cardiometabolic Consequences, and Treatment Strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Ahmad, A.; Gerö, D.; Olah, G.; Szabo, C. Effect of Endotoxemia in Mice Genetically Deficient in Cystathionine-γ-Lyase, Cystathionine-β-Synthase or 3-Mercaptopyruvate Sulfurtransferase. Int. J. Mol. Med. 2016, 38, 1683–1692. [Google Scholar] [CrossRef]
- Kabil, O.; Vitvitsky, V.; Xie, P.; Banerjee, R. The Quantitative Significance of the Transsulfuration Enzymes for H 2S Production in Murine Tissues. Antioxid. Redox Signal. 2011, 15, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Norris, E.J.; Culberson, C.R.; Narasimhan, S.; Clemens, M.G. The Liver as a Central Regulator of Hydrogen Sulfide. Shock 2011, 36, 242–250. [Google Scholar] [CrossRef]
- Mani, S.; Cao, W.; Wu, L.; Wang, R. Hydrogen Sulfide and the Liver. Nitric Oxide Biol. Chem. 2014, 41, 62–71. [Google Scholar] [CrossRef]
- Caldwell, S.H.; Swerdlow, R.H.; Khan, E.M.; Iezzoni, J.C.; Hespenheide, E.E.; Parks, J.K.; Parker, W.D. Mitochondrial Abnormalities in Non-Alcoholic Steatohepatitis. J. Hepatol. 1999, 31, 430–434. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, C.; Shi, J.; Ding, J.; Wan, X.; Chen, D.; Gao, J.; Li, C.; Zhang, J.; Lin, Y.; et al. Fatty Acids Promote Fatty Liver Disease via the Dysregulation of 3-Mercaptopyruvate Sulfurtransferase/hydrogen Sulfide Pathway. Gut 2017, 67, 2169. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, S.; Yu, C.; Pan, Z.; Liu, Y.; Zhao, J.; Wang, X.; Yun, F.; Zhao, H.; Yan, S.; et al. Hydrogen Sulfide Reduces Serum Triglyceride by Activating Liver Autophagy via the AMPK-mTOR Pathway. Am. J. Physiol. Metab. 2015, 309, E925–E935. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, C.L.; Song, F.Y.; Zhao, X.L.; Xie, K.Q. Garlic Oil Alleviated Ethanol-Induced Fat Accumulation via Modulation of SREBP-1, PPAR-A, and CYP2E1. Food Chem. Toxicol. 2012, 50, 485–491. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial Oxidative Stress and Antioxidants Balance in Fatty Liver Disease. Hepatol. Commun. 2018, 2, 1425–1439. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.-D.; Huang, P. Redox Regulation of Cell Survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Ying, J.; Xiang, L.; Zhang, C. The Biologic Effect of Hydrogen Sulfide and Its Function in Various Diseases. Medicine (USA) 2018, 97, e13065. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Kimura, Y.; Goto, Y.I.; Kimura, H. Hydrogen Sulfide Increases Glutathione Production and Suppresses Oxidative Stress in Mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef]
- Shen, C.; Ge, J. Epidemic of Cardiovascular Disease in China Current Perspective and Prospects for the Future. Circulation 2018, 138, 342–344. [Google Scholar] [CrossRef]
- Evans, M.A.; Sano, S.; Walsh, K. Cardiovascular Disease, Aging, and Clonal Hematopoiesis. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 419–438. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, Indicators, Risk Factors and New Hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Zhong, G.Z.; Chen, F.R.; Cheng, Y.Q.; Tang, C.S.; Du, J.B. The Role of Hydrogen Sulfide Generation in the Pathogenesis of Hypertension in Rats Induced by Inhibition of Nitric Oxide Synthase. J. Hypertens. 2003, 21, 1879–1885. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The Vasorelaxant Effect of H2S as a Novel Endogenous Gaseous KATP Channel Opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Cheng, Y.; Ndisang, J.F.; Tang, G.; Cao, K.; Wang, R. Hydrogen Sulfide-Induced Relaxation of Resistance Mesenteric Artery Beds of Rats. Am. J. Physiol. Hear. Circ. Physiol. 2004, 287. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.J.; Lefer, D.J. Emergence of Hydrogen Sulfide as an Endogenous Gaseous Signaling Molecule in Cardiovascular Disease. Circ. Res. 2014, 114, 730–737. [Google Scholar] [CrossRef]
- Gao, L.; Xu, Z.; Yin, Z.; Chen, K.; Wang, C.; Zhang, H. Association of Hydrogen Sulfide with Alterations of Monocyte Chemokine Receptors, CCR2 and CX3CR1 in Patients with Coronary Artery Disease. Inflamm. Res. 2015, 64, 627–635. [Google Scholar] [CrossRef]
- Van Goor, H.; Van Den Born, J.C.; Hillebrands, J.L.; Joles, J.A. Hydrogen Sulfide in Hypertension. Curr. Opin. Nephrol. Hypertens. 2016, 25, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Kovačić, D.; Glavnik, N.; Marinšek, M.; Zagožen, P.; Rovan, K.; Goslar, T.; Marš, T.; Podbregar, M. Total Plasma Sulfide in Congestive Heart Failure. J. Card. Fail. 2012, 18, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Casin, K.M.; Calvert, J.W. Harnessing the Benefits of Endogenous Hydrogen Sulfide to Reduce Cardiovascular Disease. Antioxidants 2021, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Scammahorn, J.J.; Nguyen, I.T.N.; Bos, E.M.; van Goor, H.; Joles, J.A. Fighting Oxidative Stress with Sulfur: Hydrogen Sulfide in the Renal and Cardiovascular Systems. Antioxidants 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, X.; Li, X.; Wei, X.; Wang, H. Hydrogen Sulfide Plays an Important Role in Diabetic Cardiomyopathy. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Citi, V.; Martelli, A.; Brogi, S.; Calderone, V. Role of Hydrogen Sulfide in Cardiovascular Ageing. Pharmacol. Res. 2020, 160. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Martelli, A.; Gorica, E.; Brogi, S.; Testai, L.; Calderone, V. Role of Hydrogen Sulfide in Endothelial Dysfunction: Pathophysiology and Therapeutic Approaches. J. Adv. Res. 2021, 27, 99–113. [Google Scholar] [CrossRef]
- Zhang, C.; Du, J.; Bu, D.; Yan, H.; Tang, X.; Tang, C. The Regulatory Effect of Hydrogen Sulfide on Hypoxic Pulmonary Hypertension in Rats. Biochem. Biophys. Res. Commun. 2003, 302, 810–816. [Google Scholar] [CrossRef]
- Yan, H.; Du, J.; Tang, C. The Possible Role of Hydrogen Sulfide on the Pathogenesis of Spontaneous Hypertension in Rats. Biochem. Biophys. Res. Commun. 2004, 313, 22–27. [Google Scholar] [CrossRef]
- Sikora, M.; Drapala, A.; Ufnal, M. Exogenous Hydrogen Sulfide Causes Different Hemodynamic Effects in Normotensive and Hypertensive Rats via Neurogenic Mechanisms. Pharmacol. Reports 2014, 66, 751–758. [Google Scholar] [CrossRef]
- D’Emmanuele Di Villa Bianca, R.; Mitidieri, E.; Donnarumma, E.; Tramontano, T.; Brancaleone, V.; Cirino, G.; Bucci, M.; Sorrentino, R. Hydrogen Sulfide Is Involved in Dexamethasone-Induced Hypertension in Rat. Nitric Oxide Biol. Chem. 2015, 46, 80–86. [Google Scholar] [CrossRef]
- Bucci, M.; Vellecco, V.; Cantalupo, A.; Brancaleone, V.; Zhou, Z.; Evangelista, S.; Calderone, V.; Papapetropoulos, A.; Cirino, G. Hydrogen Sulfide Accounts for the Peripheral Vascular Effects of Zofenopril Independently of ACE Inhibition. Cardiovasc. Res. 2014, 102, 138–147. [Google Scholar] [CrossRef]
- Huang, P.; Chen, S.; Wang, Y.; Liu, J.; Yao, Q.; Huang, Y.; Li, H.; Zhu, M.; Wang, S.; Li, L.; et al. Down-Regulated CBS/H2S Pathway Is Involved in High-Salt-Induced Hypertension in Dahl Rats. Nitric Oxide Biol. Chem. 2015, 46, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Kanagy, N.L.; Szabo, C.; Papapetropoulos, A. Vascular Biology of Hydrogen Sulfide. Am. J. Physiol. Cell Physiol. 2017, 312, C537–C549. [Google Scholar] [CrossRef]
- Al-Magableh, M.R.; Kemp-Harper, B.K.; Hart, J.L. Hydrogen Sulfide Treatment Reduces Blood Pressure and Oxidative Stress in Angiotensin II-Induced Hypertensive Mice. Hypertens. Res. 2015, 38, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a Novel, Water-Soluble Hydrogen Sulfide-Releasing Molecule (GYY4137): New Insights into the Biology of Hydrogen Sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.K.; Zhang, C.Y.; Zeng, X.J.; Yan, H.; Jin, H.F.; Tang, C.S.; Du, J.B. Regulatory Effect of Hydrogen Sulfide on Vascular Collagen Content in Spontaneously Hypertensive Rats. Hypertens. Res. 2008, 31, 1619–1630. [Google Scholar] [CrossRef]
- Corvino, A.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Scognamiglio, A.; Caliendo, G.; Fiorino, F.; Severino, B. Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants 2021, 10, 429. [Google Scholar] [CrossRef]
- Chan, J.Y.Y.; Yuen, A.C.Y.; Chan, R.Y.K.; Chan, S.W. A Review of the Cardiovascular Benefits and Antioxidant Properties of Allicin. Phyther. Res. 2013, 27, 637–646. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Bellagambi, F.G.; Ghimenti, S.; Breschi, M.C.; Calderone, V. Pharmacological Characterization of the Vascular Effects of Aryl Isothiocyanates: Is Hydrogen Sulfide the Real Player? Vascul. Pharmacol. 2014, 60, 32–41. [Google Scholar] [CrossRef]
- Zhou, Z.; Von Wantoch Rekowski, M.; Coletta, C.; Szabo, C.; Bucci, M.; Cirino, G.; Topouzis, S.; Papapetropoulos, A.; Giannis, A. Thioglycine and L-Thiovaline: Biologically Active H 2S-Donors. Bioorganic Med. Chem. 2012, 20, 2675–2678. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.L.; Xi, Y.; Yang, S.N.; Ma, Z.; Tang, C. shu Plasma Hydrogen Sulfide and Homocysteine Levels in Hypertensive Patients with Different Blood Pressure Levels and Complications. Zhonghua Xin Xue Guan Bing Za Zhi [Chin. J. Cardiovasc. Dis.] 2007, 35, 1145–1148. [Google Scholar] [PubMed]
- Kutz, J.L.; Greaney, J.L.; Santhanam, L.; Alexander, L.M. Evidence for a Functional Vasodilatatory Role for Hydrogen Sulphide in the Human Cutaneous Microvasculature. J. Physiol. 2015, 593, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Hydrogen Sulfide in Hemostasis: Friend or Foe? Chem. Biol. Interact. 2014, 217, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Chaoshu, T.; Hongfang, J.; Junbao, D. Endogenous Hydrogen Sulfide Is Involved in the Pathogenesis of Atherosclerosis. Biochem. Biophys. Res. Commun. 2010, 396, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Li, H.; Untereiner, A.; Wu, L.; Yang, G.; Austin, R.C.; Dickhout, J.G.; Lhoták, Š.; Meng, Q.H.; Wang, R. Decreased Endogenous Production of Hydrogen Sulfide Accelerates Atherosclerosis. Circulation 2013, 127, 2523–2534. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Pan, C.-S.; Geng, B.; Zhao, J.; Yu, F.; Pang, Y.-Z.; Tang, C.-S.; Qi, Y.-F.; Wang, X. Hydrogen Sulfide Ameliorates Vascular Calcification Induced by Vitamin D3 plus Nicotine in Rats 1. Acta Pharmacol. Sin. 2006, 27, 299–306. [Google Scholar] [CrossRef]
- Leucker, T.M.; Nomura, Y.; Kim, J.H.; Bhatta, A.; Wang, V.; Wecker, A.; Jandu, S.; Santhanam, L.; Berkowitz, D.; Romer, L.; et al. Cystathionine γ-Lyase Protects Vascular Endothelium: A Role for Inhibition of Histone Deacetylase 6. Am. J. Physiol. Hear. Circ. Physiol. 2017, 312, H711–H720. [Google Scholar] [CrossRef]
- Bibli, S.I.; Hu, J.; Sigala, F.; Wittig, I.; Heidler, J.; Zukunft, S.; Tsilimigras, D.I.; Randriamboavonjy, V.; Wittig, J.; Kojonazarov, B.; et al. Cystathionine γ Lyase Sulfhydrates the RNA Binding Protein Human Antigen R to Preserve Endothelial Cell Function and Delay Atherogenesis. Circulation 2019, 139, 101–114. [Google Scholar] [CrossRef]
- Libby, P. Mechanisms of Acute Coronary Syndromes and Their Implications for Therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef]
- Thompson, J.B.; Blaha, M.; Resar, J.R.; Blumenthal, R.S.; Desai, M.Y. Strategies to Reverse Atherosclerosis: An Imaging Perspective. Curr. Treat. Options Cardiovasc. Med. 2008, 10, 283–293. [Google Scholar] [CrossRef]
- Wiliński, B.; Wiliński, J.; Somogyi, E.; Piotrowska, J.; Góralska, M. Atorvastatin Affects the Tissue Concentration of Hydrogen Sulfide in Mouse Kidneys and Other Organs. Pharmacol. Reports 2011, 63, 184–188. [Google Scholar] [CrossRef]
- Wójcicka, G.; Jamroz-Wiśniewska, A.; Atanasova, P.; Chaldakov, G.N.; Chylińska-Kula, B.; Bełtowski, J. Differential Effects of Statins on Endogenous H2S Formation in Perivascular Adipose Tissue. Pharmacol. Res. 2011, 63, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, H.P.; Li, J.; Xu, R.; Wang, Y.L.; You, S.J.; Liu, H.; Wang, F.; Cao, Y.J.; Liu, C.F.; et al. Statins Upregulate Cystathionine γ-Lyase Transcription and H 2S Generation via Activating Akt Signaling in Macrophage. Pharmacol. Res. 2014, 87, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, H.; Li, M.; Gu, X. A Novel Fluorescent Probe for Imaging Endogenous Hydrogen Sulphide via the CSE Enzymatic Pathway. Chem. Commun. 2015, 51, 13135–13137. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, X.; Li, K.; Zhu, S.; Guo, Z.; Zhang, L.; Wang, F.; Fei, Q.; Luo, S.; Shi, P.; et al. Förster Resonance Energy Transfer Switchable Self-Assembled Micellar Nanoprobe: Ratiometric Fluorescent Trapping of Endogenous H2S Generation via Fluvastatin-Stimulated Upregulation. J. Am. Chem. Soc. 2015, 137, 8490–8498. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).