Ozone Pollution Alters Olfaction and Behavior of Pollinators
Abstract
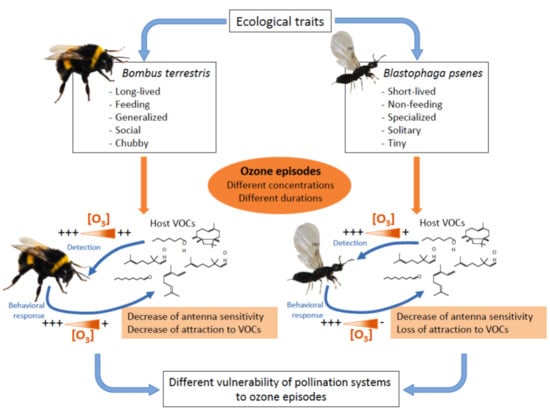
:1. Introduction
2. Materials and Methods
2.1. Model Systems
2.1.1. Fig Wasp System
2.1.2. Bumblebee System
2.2. Ozone Exposure
2.3. Does O3 Concentration Affect Pollinator Antenna Sensitivity?
2.4. Does O3 Concentration Affect the Attraction of Pollinators to VOCs?
2.5. Statistical Analyses
2.5.1. Pollinator Antenna Sensitivity
2.5.2. Attraction of Pollinators to VOCs
3. Results
3.1. Does O3 Concentration Affect Pollinator Antenna Sensitivity?
3.1.1. Fig Wasp System
3.1.2. Bumblebee System
3.2. Does O3 Concentration Affect the Attraction of Pollinators to VOCs?
3.2.1. Fig Wasp System
3.2.2. Bumblebee System
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.S.; Himanen, S.J.; Holopainen, J.K.; Chen, F.; Stewart, C.N., Jr. Smelling global climate change: Mitigation of function for plant volatile organic compounds. Trends Ecol. Evol. 2009, 24, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B. Economic valuation of the vulnerability of world agriculture confronted to pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- IPBES. The Methodological Assessment Report on Scenarios and Models of Biodiversity and Ecosystem Services: Summary for Policymakers; Ferrier, S., Ninan, K.N., Leadley, P., Alkemade, R., Acosta, L.A., Akçakaya, H.R., Brotons, L., Cheung, W., Christensen, V., Harhash, K.A., Eds.; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2016. [Google Scholar]
- Girling, R.D.; Lusebrink, I.; Farthing, E.; Newman, T.A.; Poppy, G.M. Diesel exhaust rapidly degrades floral odours used by honeybees. Sci. Rep. 2013, 3, 2779. [Google Scholar] [CrossRef] [PubMed]
- Lusebrink, I.; Girling, R.D.; Farthing, E.; Newman, T.A.; Jackson, C.W.; Poppy, G.M. The effects of diesel exhaust pollution on floral volatiles and the consequences for honey bee olfaction. J. Chem. Ecol. 2015, 41, 904–912. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F.D., Qin, G.-K., Plattner, M., Tignor, S.K., Allen, J., Boschung, A., Nauels, Y., Xia, V.B., Midgley, P.M., Eds.; Cambridge University Press: New York, NY, USA, 2013; 1535p. [Google Scholar]
- Mills, G.; Pleijel, H.; Malley, C.S.; Sinha, B.; Cooper, O.R.; Schultz, M.G.; Neufeld, H.S.; Simpson, D.; Sharps, K.; Feng, Z.; et al. Tropospheric Ozone Assessment Report: Present-day tropospheric ozone distribution and trends relevant to vegetation. Elem. Sci. Anthrop. 2018, 6, 47. [Google Scholar] [CrossRef]
- Paoletti, E. Impact of ozone on Mediterranean forests: A review. Environ. Pollut. 2006, 144, 463–474. [Google Scholar] [CrossRef] [PubMed]
- The Royal Society. Ground-Level Ozone in the 21st Century: Future Trends, Impacts and Policy Implications; The Royal Society: London, UK, 2008; 132p. [Google Scholar]
- Cooper, O.R.; Parrish, D.D.; Ziemke, J.; Balashov, N.V.; Cupeiro, M.; Galbally, I.E.; Gilge, S.; Horowitz, L.; Jensen, N.R.; Lamarque, J.-F.; et al. Global distribution and trends of tropospheric ozone: An observation-based review. Elem. Sci. Anthrop. 2014, 2, 000029. [Google Scholar] [CrossRef]
- Lei, H.; Wuebbles, D.J.; Liang, X.Z. Projected risk of high ozone episodes in 2050. Atmos. Environ. 2012, 59, 567–577. [Google Scholar] [CrossRef]
- Gryparis, A.; Forsberg, B.; Katsouyanni, K.; Analitis, A.; Touloumi, G.; Schwartz, J.; Samoli, E.; Medina, S.; Andersib, H.R.; Niciu, E.M.; et al. Acute effects of ozone on mortality from the «air pollution and health: A European approach» project. Am. J. Respir. Crit. Care Med. 2004, 170, 1080–1087. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Oxidative stress, the paradigm of ozone toxicity in plants and animals. Water Air Soil Pollut. 2008, 187, 285–301. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. Review of Evidence on Health Aspects of Air Pollution-REVIHAAP Project; Technical Report; WHO Regional Office for Europe: Copenhagen, Denmark, 2013; pp. 1–309. [Google Scholar]
- Babadjouni, R.M.; Hodis, D.M.; Radwanski, R.; Durazo, R.; Patel, A.; Liu, Q.; Mack, W.J. Clinical effects of air pollution on the central nervous system: A review. J. Clin. Neurosci. 2017, 43, 16–24. [Google Scholar] [CrossRef]
- Mills, G.; Sharps, K.; Simpson, D.; Pleijel, H.; Broberg, M.; Uddling, J.; Jaramillo, F.; Davies, W.J.; Dentener, F.; van den Berg, M.; et al. Ozone pollution will compromise efforts to increase global wheat production. Glob. Chang. Biol. 2018, 24, 3560–3574. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Oksanen, E.; Sicard, P.; Wang, Q.; Saitanis, C.J.; Araminiene, V.; Blande, J.D.; Hayes, F.; Calatayud, V.; et al. Ozone affects plant, insect, and soil microbial communities: A threat to terrestrial ecosystems and biodiversity. Sci. Adv. 2020, 6, eabc1176. [Google Scholar] [CrossRef]
- Blande, J.D. Effects of air pollution on plant–insect interactions mediated by olfactory and visual cues. Curr. Opin. Environ. Sci. Health 2021, 19, 100228. [Google Scholar]
- Williams, N.M.; Crone, E.E.; Roulston, T.H.; Minckley, R.L.; Packer, L.; Potts, S.G. Ecological and life-history traits predict bee species responses to environmental disturbances. Biol. Conserv. 2010, 143, 2280–2291. [Google Scholar] [CrossRef]
- Bartomeus, I.; Ascher, J.S.; Gibbs, J.; Danforth, B.N.; Wagner, D.L.; Hedtke, S.M.; Winfree, R. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc. Natl. Acad. Sci. USA 2013, 110, 4656–4660. [Google Scholar] [CrossRef] [Green Version]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee foraging ranges and their relationship to body size. Oecologia 2007, 153, 589–596. [Google Scholar] [CrossRef]
- Bommarco, R.; Biesmeijer, J.C.; Meyer, B.; Potts, S.G.; Pöyry, J.; Roberts, S.P.M.; Steffan-dewenter, I.; Öckinger, E. Dispersal capacity and diet breadth modify the response of wild bees to habitat loss. Proc. R. Soc. B Biol. Sci. 2010, 277, 2075–2082. [Google Scholar] [CrossRef] [Green Version]
- Jauker, B.; Krauss, J.; Jauker, F.; Steffan-Dewenter, I. Linking life history traits to pollinator loss in fragmented calcareous grasslands. Landsc. Ecol. 2013, 28, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Hopfenmüller, S.; Steffan-Dewenter, I.; Holzschuh, A. Trait-specific responses of wild bee communities to landscape composition, configuration and local factors. PLoS ONE 2014, 9, e104439. [Google Scholar] [CrossRef] [Green Version]
- Li-Byarlay, H.; Huang, M.H.; Simone-Finstrom, M.; Strand, M.K.; Tarpy, D.R.; Rueppell, O. Honey bee (Apis mellifera) drones survive oxidative stress due to increased tolerance instead of avoidance or repair of oxidative damage. Exp. Gerontol. 2016, 83, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Tasaki, E.; Kobayashi, K.; Matsuura, K.; Iuchi, Y. An efficient antioxidant system in a long-lived termite queen. PLoS ONE 2017, 12, e0167412. [Google Scholar] [CrossRef]
- Aličić, D.; Šubarić, D.; Jašić, M.; Pašalić, H.; Ačkar, Đ. Antioxidant properties of pollen. Hrana u Zdravlju i Bolesti 2014, 3, 6–12. [Google Scholar]
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Hossaert-McKey, M.; Soler, C.; Schatz, B.; Proffit, M. Floral scents: Their roles in nursery pollination mutualisms. Chemoecology 2010, 20, 75–88. [Google Scholar] [CrossRef]
- Conchou, L.; Lucas, P.; Meslin, C.; Proffit, M.; Staudt, M.; Renou, M. Insect odorscapes: From plant volatiles to natural olfactory scenes. Front. Physiol. 2019, 10, 425. [Google Scholar] [CrossRef]
- Laothawornkitkul, J.; Taylor, J.E.; Paul, N.D.; Hewitt, C.N. Biogenic volatile organic compounds in the Earth system. New Phytol. 2009, 183, 27–51. [Google Scholar] [CrossRef]
- McFrederick, Q.S.; Fuentes, J.D.; Roulston, T.; Kathilankal, J.C.; Lerdau, M. Effects of air pollution on biogenic volatiles and ecological interactions. Oecologia 2009, 160, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.M.; Blande, J.D.; Souza, S.R.; Nerg, A.-M.; Holopainen, J.K. Plant volatile organic compounds (VOCs) in ozone (O3) polluted atmospheres: The ecological effects. J. Chem. Ecol. 2010, 36, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Blande, J.D.; Holopainen, J.K.; Niinemets, Ü. Plant volatiles in polluted atmospheres: Stress responses and signal degradation. Plant Cell Environ. 2014, 37, 1892–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.K.; Kessler, A.; Woods, H.A. Noisy communication via airborne infochemicals. BioScience 2015, 65, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Loreto, F.; Schnitzler, J.-P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Jamieson, M.A.; Burkle, L.A.; Manson, J.S.; Runyon, J.B.; Trowbridge, A.M.; Zientek, J. Global change effects on plant–insect interactions: The role of phytochemistry. Curr. Opin. Insect Sci. 2017, 23, 70–80. [Google Scholar] [CrossRef]
- Saunier, A.; Blande, J.D. The effect of elevated ozone on floral chemistry of Brassicaceae species. Environ. Pollut. 2019, 255, 113257. [Google Scholar] [CrossRef]
- Blande, J.D.; Holopainen, J.K.; Li, T. Air pollution impedes plant-to-plant communication by volatiles. Ecol. Lett. 2010, 13, 1172–1181. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Peñuelas, J.; Li, T.; Yli-Pirilä, P.; Filella, I.; Llusia, J.; Blande, J.D. Ozone degrades floral scent and reduces pollinator attraction to flowers. New Phytol. 2016, 209, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, J.; Chamecki, M.; Roulston, T.; Chen, B.; Pratt, K.R. Air pollutants degrade floral scents and increase insect foraging times. Atmos. Environ. 2016, 141, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Bruce, T.J.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Proffit, M.; Lapeyre, B.; Buatois, B.; Deng, X.; Arnal, P.; Gouzerth, F.; Carrasco, D.; Hossaert-McKey, M. Chemical signal is in the blend: Bases of plant-pollinator encounter in a highly specialized interaction. Sci. Rep. 2020, 10, 10071. [Google Scholar] [CrossRef]
- Hansson, B.S.; Stensmyr, M.C. Evolution of insect olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef] [Green Version]
- Owald, D.; Felsenberg, J.; Talbot, C.B.; Das, G.; Perisse, E.; Huetteroth, W.; Waddell, S. Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron 2015, 86, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Schultzhaus, J.; Saleem, S.; Iftokhar, H.; Carney, G.E. The role of the Drosophila lateral horn in olfactory information processing and behavioral response. J. Insect Physiol. 2017, 98, 29–37. [Google Scholar] [CrossRef]
- Dötterl, S.; Vater, M.; Rupp, T.; Held, A. Ozone differentially affects perception of plant volatiles in western honey bees. J. Chem. Ecol. 2016, 42, 486–489. [Google Scholar] [CrossRef] [Green Version]
- Kjellberg, F.; Doumesche, B.; Bronstein, J.L. Longevity of a fig wasps (Blastophaga psenes). Proc. K. Ned. Akd. Wet. Ser. C Biol. Med. Sci. 1988, 91, 117–122. [Google Scholar]
- Kjellberg, F.; Lesne, A. Ficus carica and Its Pollination. Master, France. 2020. hal-02516888. Available online: https://hal.archives-ouvertes.fr/hal-02516888/file/Ficus_carica_and_its_pollination_2020_03_21.pdf (accessed on 7 January 2021).
- Rodd, F.H.; Plowright, R.C.; Owen, R.E. Mortality rates of adult bumble bee workers (Hymenoptera: Apidae). Can. J. Zool. 1980, 58, 1718–1721. [Google Scholar] [CrossRef]
- Schmid-Hempel, P.; Heeb, D. Worker mortality and colony development in bumblebees, B. lucorum L. Mitt. Schweiz Entomol. Ges. 1991, 64, 93–108. [Google Scholar]
- Kleijn, D.; Raemakers, I. A retrospective analysis of pollen host plant use by stable and declining bumble bee species. Ecology 2008, 89, 1811–1823. [Google Scholar] [CrossRef]
- Rasmont, P.; Coppée, A.; Michez, D.; de Meleumeester, T. An overview of the Bombus terrestris (L. 1758) subspecies (Hymenoptera: Apidae). Ann. Soc. Entomol. Fr. 2008, 44, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Leonhardt, S.D.; Blüthgen, N. The same, but different: Pollen foraging in honeybee and bumblebee colonies. Apidologie 2012, 43, 449–464. [Google Scholar] [CrossRef]
- Mazzeo, G.; Bella, S.; Seminara, A.R.; Longo, S. Bumblebees in natural and agro-ecosystems at different altitudes from Mount Etna, Sicily (Hymenoptera apidae bombinae): Long-term faunistic and ecological observations. Redia 2016, 98, 123–131. [Google Scholar]
- Vanderplanck, M.; Declèves, S.; Roger, N.; Decro, C.; Caulier, G.; Glauser, G.; Gerbaux, P.; Lognay, G.; Richel, A.; Escaravage, N.; et al. Is non-host pollen suitable for generalist bumblebees? Insect Sci. 2018, 25, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Hudon, T.M.; Plowright, C.M.S. Trapped: Assessing attractiveness of potential food sources to bumblebees. J. Insect Behav. 2011, 24, 144–158. [Google Scholar] [CrossRef]
- Burger, H.; Dötterl, S.; Ayasse, M. Host-plant finding and recognition by visual and olfactory floral cues in an oligolectic bee. Funct. Ecol. 2010, 24, 1234–1240. [Google Scholar] [CrossRef]
- Leonard, A.S.; Dornhaus, A.; Papaj, D.R. Flowers help bees cope with uncertainty: Signal detection and the function of floral complexity. J. Exp. Biol. 2011, 214, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chittka, L.; Raine, N.E. Recognition of flowers by pollinators. Curr. Opin. Plant Biol. 2006, 9, 428–435. [Google Scholar] [CrossRef]
- Vautard, R.; Honoré, C.; Beelmann, M.; Rouïl, L. Simulation of ozone during the August 2003 heat wave and emission control scenarios. Atmos. Environ. 2005, 39, 2957–2967. [Google Scholar] [CrossRef]
- Solberg, S.; Hov, O.; Sovde, A.; Isaksen, I.S.A.; Coddeville, P.; de Backer, H.; Forster, C.; Orsolini, Y.; Uhse, K. European surface ozone in the extreme summer 2003. J. Geophys. Res. 2008, 113, D07307. [Google Scholar] [CrossRef] [Green Version]
- Roelofs, W.L. Electroantennogram assay: Rapid and convenient screening procedures for pheromones. In Techniques in Pheromone Research; Hummel, H.E., Miller, T.A., Eds.; Springer: New York, NY, USA, 1984; pp. 131–160. [Google Scholar]
- Wright, G.A.; Lutmerding, A.; Dudareva, N.; Smith, B.H. Intensity and the ratios of compounds in the scent of snapdragon flowers affect scent discrimination by honey bees (Apis mellifera). J. Comp. Physiol. 2005, 191, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Suchet, C.; Dormont, L.; Schatz, B.; Giurfa, M.; Simon, V.; Raynaud, C.; Chave, J. Floral scent variation in two Antirrhinum majus subspecies influences the choice of naïve bumblebees. Behav. Ecol. Sociobiol. 2011, 65, 1015–1027. [Google Scholar] [CrossRef]
- Fonta, C.; Masson, C. Comparative study by electrophysiology of olfactory responses in bumblebees (Bombus hypnorum and Bombus terrestris). J. Chem. Ecol. 1984, 10, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Laloi, D.; Sandoz, J.C.; Picard-Nizou, A.L.; Marchesi, A.; Povreau, A.; Taséi, J.N.; Poppy, G.; Pham-Delègue, M.H. Olfactory conditioning of the proboscis extension in bumble bees. Entomol. Exp. Appl. 1999, 90, 123–129. [Google Scholar] [CrossRef]
- Anfora, G.; Rigosi, E.; Frasnelli, E.; Ruga, V.; Trona, F.; Vallortigara, G. Lateralization in the invertebrate brain: Left-right asymmetry of olfaction in bumble bee, Bombus terrestris. PLoS ONE 2011, 6, e18903. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2017. Available online: https://www.R-project.org/ (accessed on 21 April 2017).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, M.; Weston, S.; Wing, J.; Forester, J.; Thaler, T. Contrast: A Collection of Contrast Methods. R Package Version 0.21. 2016. Available online: https://CRAN.R-project.org/package=contrast (accessed on 31 March 2017).
- Schad, D.J.; Vasishth, S.; Hohenstein, S.; Kliegl, R. How to capitalize on a priori contrasts in linear (mixed) models: A tutorial. J. Mem. Lang. 2020, 110, 104038. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Kelly, F.J.; Mudway, I.S. Protein oxidation at the air lung interface. Amino Acids 2003, 25, 375–396. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Kadala, A.; Charreton, M.; Jakob, I.; Le Conte, Y.; Collet, C. A use-dependent sodium current modification induced by type I pyrethroid insecticides in honeybee antennal olfactory receptor neurons. Neurotoxicology 2011, 32, 320–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felton, G.W.; Summers, C.B. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 1995, 29, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Perić-Mataruga, V.; Nenadović, V.; Ivanović, J. Neurohormones in insect stress: A review. Arch. Biol. Sci. 2006, 58, 1–12. [Google Scholar] [CrossRef]
- Krishnan, N.; Kodrík, D. Endocrine control of oxidative stress in insects. In Oxidative Stress in Vertebrates and Invertebrates: Molecular Aspects of Cell Signaling; Farooqui, T., Farooqui, A.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 261–270. [Google Scholar]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Kodrik, D.; Bednarova, A.; Zemanova, M.; Krishnan, N. Hormonal regulation of response to oxidative stress in insects—An Update. Int. J. Mol. Sci. 2015, 16, 25788–25816. [Google Scholar] [CrossRef] [Green Version]
- Bonvehí, J.S.; Torrentó, M.S.; Lorente, E.C. Evaluation of polyphenolic and flavonoid compounds in honeybee-collected pollen produced in Spain. J. Agric. Food Chem. 2001, 49, 1848–1853. [Google Scholar] [CrossRef]
- Johnson, K.S.; Felton, G.W. Plant phenolics as dietary antioxidants for herbivorous insects: A test with genetically modified tobacco. J. Chem. Ecol. 2001, 27, 2579–2597. [Google Scholar] [CrossRef]
- Monaghan, P.; Metcalfe, N.B.; Torres, R. Oxidative stress as a mediator of life history trade-offs: Mechanisms, measurements and interpretation. Ecol. Lett. 2009, 12, 75–92. [Google Scholar] [CrossRef]
- Beaulieu, M.; Schaefer, H.M. Rethinking the role of dietary antioxidants through the lens of self-medication. Anim. Behav. 2013, 86, 17–24. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanderplanck, M.; Lapeyre, B.; Brondani, M.; Opsommer, M.; Dufay, M.; Hossaert-McKey, M.; Proffit, M. Ozone Pollution Alters Olfaction and Behavior of Pollinators. Antioxidants 2021, 10, 636. https://doi.org/10.3390/antiox10050636
Vanderplanck M, Lapeyre B, Brondani M, Opsommer M, Dufay M, Hossaert-McKey M, Proffit M. Ozone Pollution Alters Olfaction and Behavior of Pollinators. Antioxidants. 2021; 10(5):636. https://doi.org/10.3390/antiox10050636
Chicago/Turabian StyleVanderplanck, Maryse, Benoît Lapeyre, Margot Brondani, Manon Opsommer, Mathilde Dufay, Martine Hossaert-McKey, and Magali Proffit. 2021. "Ozone Pollution Alters Olfaction and Behavior of Pollinators" Antioxidants 10, no. 5: 636. https://doi.org/10.3390/antiox10050636
APA StyleVanderplanck, M., Lapeyre, B., Brondani, M., Opsommer, M., Dufay, M., Hossaert-McKey, M., & Proffit, M. (2021). Ozone Pollution Alters Olfaction and Behavior of Pollinators. Antioxidants, 10(5), 636. https://doi.org/10.3390/antiox10050636