Supplementary Light Differently Influences Physico-Chemical Parameters and Antioxidant Compounds of Tomato Fruits Hybrids
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Plant Materials and Growing Conditions
2.3. Supplemental Light Treatment and Daily Light Integral (DLI)
2.4. Yield and Average Fruits Weight
2.5. Dry Weight, Total Soluble Solids, pH and Tritratable Acidity Measurements
2.6. Vitamin C and Total Antioxidant Activity
2.7. Carotenoids and Tocopherols Analysis
2.8. Elemental Analysis
2.9. Statistical Analysis
3. Results
3.1. Supplemental Light Treatment
3.2. Yield and Average Fruits Weight
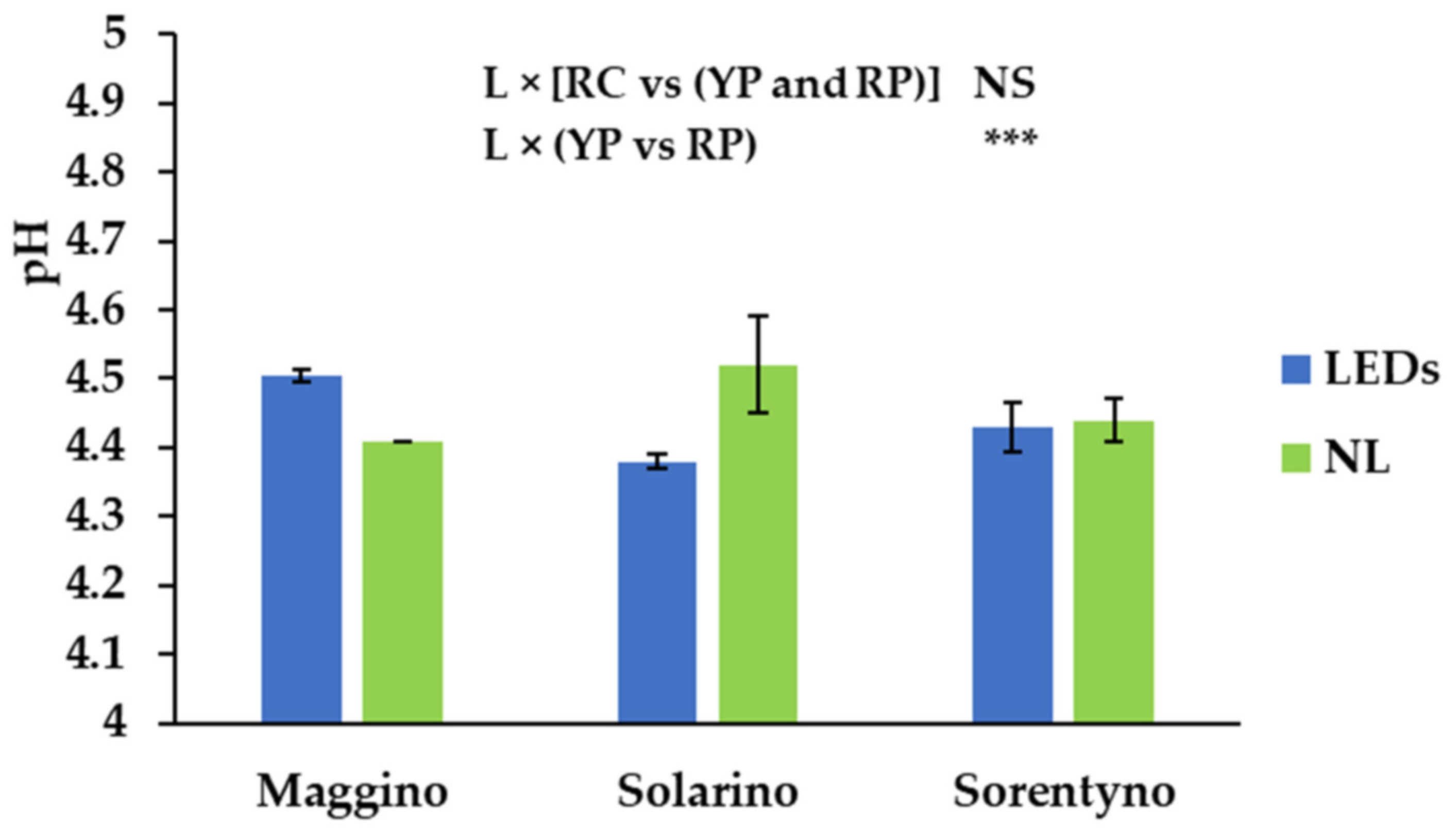
3.3. Dry Weight, Total Soluble Solids, pH, and Titratable Acidity Measurements
3.4. Vitamin C and Total Antioxidant Activity
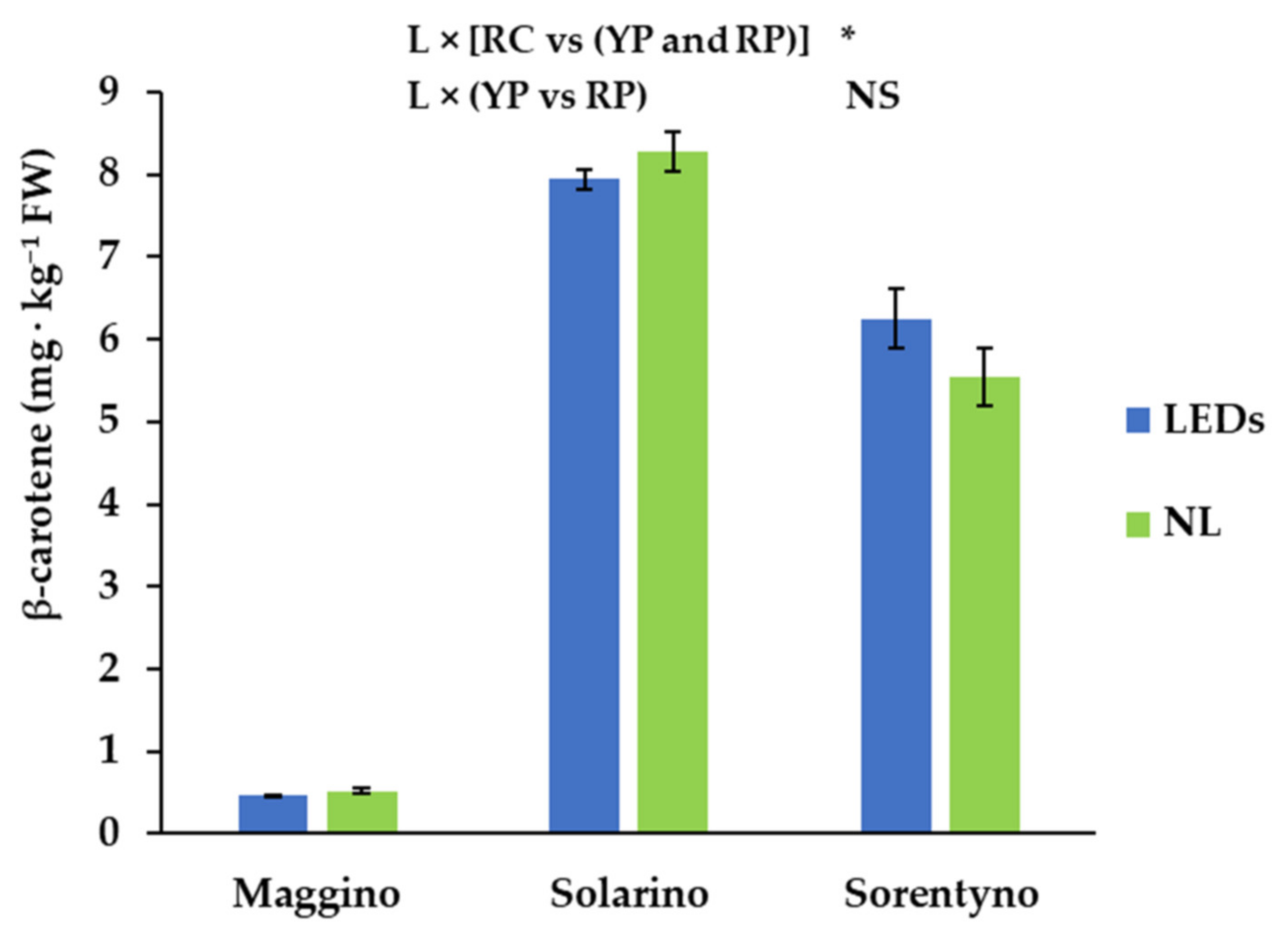
3.5. Analysis of Carotenoids and Tocopherols
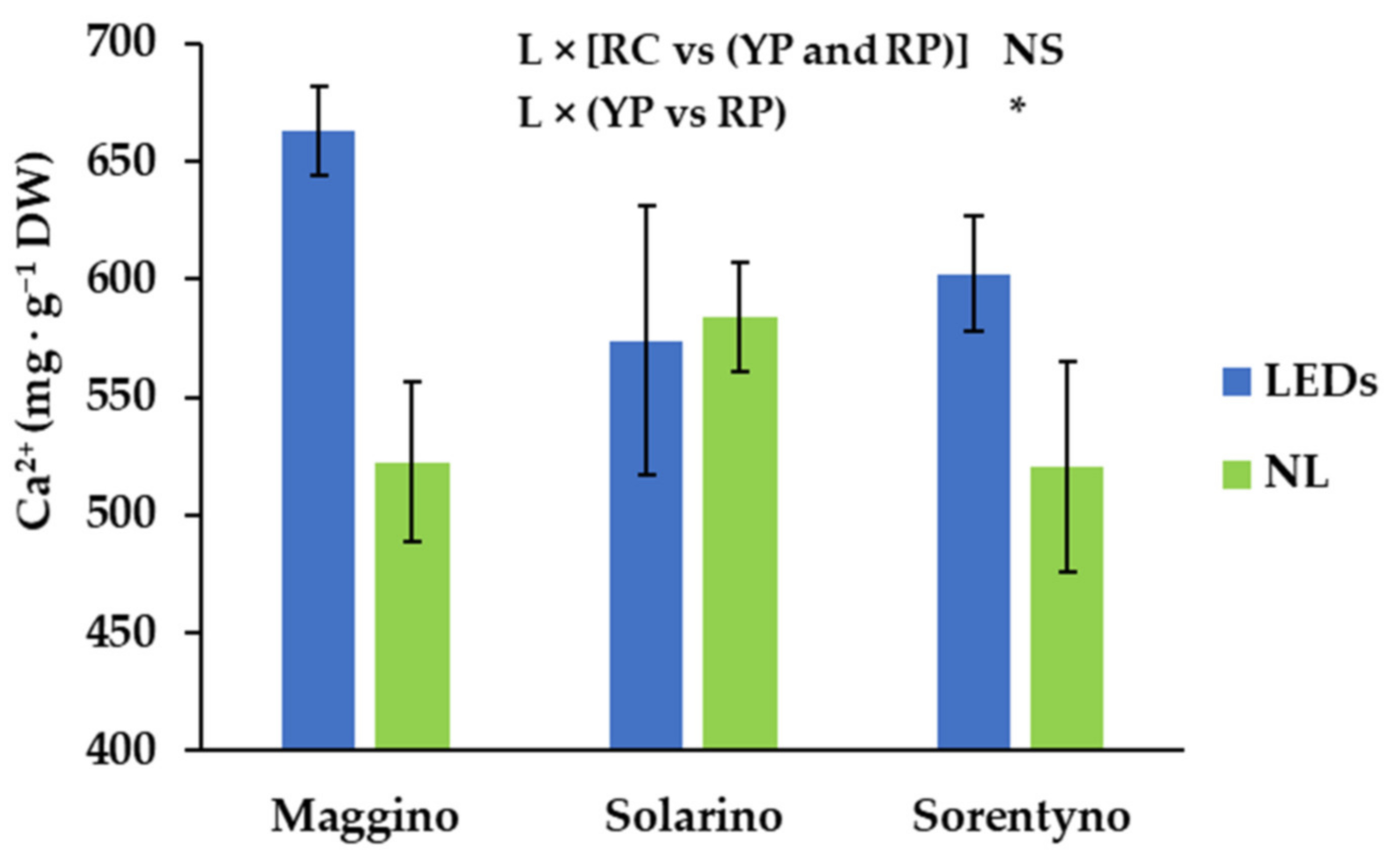
3.6. Mineral Analysis
4. Discussion
5. Conclusions
- Increased tomato fruit production;
- Maintained the antioxidant property of the hydrophilic fraction and increased that of the lipophilic fraction as well as the α-tocopherol content (particularly for yellow plum and cherry tomato types);
- Maintained or increased (depending on the tomato hybrids) the mineral profile of the tomato fruits;
- Increased DW, TSS, and TA of tomato fruits;
- Could be used as an innovative method for producing tomato fruits biofortified with Ca2+ and Mg2+.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Help Eliminate Hunger and Malnutrition. Available online: http://www.fao.org/3/ca3923en/ca3923en.pdf (accessed on 4 February 2021).
- Costa, J.M.; Heuvelink, E. The Global Tomato Industry. In Tomatoes; CABI Publishing: Wallingford, UK, 2018; pp. 1–27. ISBN 9781780641942. [Google Scholar]
- ISTAT. Coltivazioni: Ortive. Available online: http://dati.istat.it/Index.aspx?QueryId=33654 (accessed on 6 April 2021).
- Dueck, T.A.; Janse, J.; Eveleens, B.A.; Kempkes, F.L.K.; Marcelis, L.F.M. Growth of tomatoes under hybrid LED and HPS lighting. Acta Hortic. 2012, 952, 335–342. [Google Scholar] [CrossRef]
- Hovi-Pekkanen, T.; Tahvonen, R. Effects of interlighting on yield and external fruit quality in year-round cultivated cucumber. Sci. Hortic. 2008, 116, 152–161. [Google Scholar] [CrossRef]
- Dieleman, J.A.; Kruidhof, H.M.; Weerheim, K.; Leiss, K. Physiology and Resilience to Pathogens and Pests in Eggplant (Solanum melongena L.). Front. Plant Sci. 2021, 11, 610046. [Google Scholar] [CrossRef]
- Paucek, I.; Pennisi, G.; Pistillo, A.; Appolloni, E.; Crepaldi, A.; Calegari, B.; Spinelli, F.; Cellini, A.; Gabarrell, X.; Orsini, F.; et al. Supplementary LED Interlighting Improves Yield and Precocity of Greenhouse Tomatoes in the Mediterranean. Agronomy 2020, 10, 1002. [Google Scholar] [CrossRef]
- Buttaro, D.; Santamaria, P.; Signore, A.; Cantore, V.; Boari, F.; Montesano, F.F.; Parente, A. Irrigation Management of Greenhouse Tomato and Cucumber Using Tensiometer: Effects on Yield, Quality and Water Use. Ital. Oral Surg. 2015, 4, 440–444. [Google Scholar] [CrossRef]
- Palmitessa, O.D.; Paciello, P.; Santamaria, P. Supplemental LED Increases Tomato Yield in mediterranean Semi-Closed Greenhouse. Agronomy 2020, 10, 1353. [Google Scholar] [CrossRef]
- Peralta, I.E.; Peppi, D.; Sance, M.; Asis, R.; Asprelli, P.D.; Galmarini, C.R. Nutritional quality of orange tomatoes for fresh consumption and processing products. Acta Hortic. 2017, 1159, 205–214. [Google Scholar] [CrossRef]
- Taylor, P.; Borguini, R.G.; Ferraz, E.A.; Silva, D.; Ferraz, E.A.; Silva, D.A. Tomatoes and Tomato Products as Dietary Sources of Antioxidants Tomatoes and Tomato Products as Dietary Sources of Antioxidants. Food Rev. Int. 2009, 25, 313–325. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens. Food Chem. Toxicol. 2012, 50, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Milano, F.; de Caroli, M.; Giotta, L.; Piro, G.; Mita, G.; Frigione, M.; Lenucci, M.S. Tomato oil encapsulation by α-, β-, and γ-Cyclodextrins: A comparative study on the formation of supramolecular structures, antioxidant activity, and carotenoid stability. Foods 2020, 9, 1553. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Salvatore, M.; Paolo, P.; Rizzi, V.; Caroli, M.; De Piro, G.; Fini, P.; Luigi, G.; Mita, G. A-Cyclodextrin encapsulation of supercritical CO2 extracted oleoresins from different plant matrices: A stability study. Food Chem. 2016, 199, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.L.; Schwartz, J. Lycopene Stability During Food Processing. Exp. Biol. Med. 1998, 218, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Manela-Azulay, M.; Filgueira, A.L.; Mandarim-de-lacerda, C.A.; Cuzzi, T.; Perez, M. Vitamina C. An. Bras. Dermatol. 2003, 78, 265–274. [Google Scholar] [CrossRef]
- Levine, M.; Downing, D. New Concepts in the Biology and Biochemistry of Ascorbic Acid. J. Nutr. Med. 1992, 3, 361–362. [Google Scholar] [CrossRef]
- Greco, M.; Chiappetta, A.; Bruno, L.; Bitonti, M.B. The role of l-ascorbic acid recycling in responding to methylation and chromatin patterning environmental stress and in promoting plant growth. J. Exp. Bot. 2012, 63, 695–709. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Serio, F.; De Gara, L.; Caretto, S.; Leo, L.; Santamaria, P. Influence of an increased NaCl concentration on yield and quality of cherry tomato grown in posidonia (Posidonia oceanica (L.) Delile). J. Sci. Food Agric. 2004, 84, 1885–1890. [Google Scholar] [CrossRef]
- Caretto, S.; Parente, A.; Serio, F.; Santamaria, P. Influence of potassium and genotype on vitamin E content and reducing sugar of tomato fruits. HortScience 2008, 43, 2048–2051. [Google Scholar] [CrossRef]
- Serio, F.; Leo, L.; Parente, A.; Santamaria, P. Potassium nutrition increases the lycopene content of tomato fruit. J. Hortic. Sci. Biotechnol. 2007, 82, 941–945. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef] [PubMed]
- Bao-xing, X.I.E.; Jing-jing, W.E.I.; Yi-ting, Z.; Shi-wei, S.; Wei, S.U.; Guang-wen, S.U.N.; Yan-wei, H.A.O. Supplemental blue and red light promote lycopene synthesis in tomato fruits. J. Integr. Agric. 2019, 18, 590–598. [Google Scholar] [CrossRef]
- Lu, N.; Maruo, T.; Johkan, M.; Hohjo, M. Effects of supplemental lighting with light-emitting diodes (Leds) on tomato yield and quality of single-truss tomato plants grown at high planting density. Environ. Control Biol. 2012, 50, 63–74. [Google Scholar] [CrossRef]
- Paponov, M.; Kechasov, D.; Lacek, J.; Verheul, M.J.; Paponov, I.A. Supplemental Light-Emitting Diode Inter-Lighting Increases Tomato Fruit Growth Through Enhanced Photosynthetic Light Use Efficiency and Modulated Root Activity. Front. Plant Sci. 2020, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Ouzounis, T.; Heuvelink, E.; Ji, Y.; Schouten, H.J.; Visser, R.G.F.; Marcelis, L.F.M. Blue and red LED lighting effects on plant biomass, stomatal conductance, and metabolite content in nine tomato genotypes. Acta Hortic. 2016, 1134, 251–258. [Google Scholar] [CrossRef]
- Straver, N.; De Kreij, C.; Verberkt, H. Bemestingsadviesbasis Substraten. In Glasgroente; Tuinbow, P., Ed.; Research Report Wageningen University & Research; Proefstation voor Bloemisterij en Glasgroente: Naaldwijk, The Netherlands, 1999; pp. 1–55. ISBN 1387-2427. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Paradiso, A.; Cecchini, C.; Gara, L.; De Grazia, M.; Egidio, D. Functional, antioxidant and rheological properties of meal from immature durum wheat. J. Cereal Sci. 2006, 43, 216–222. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Palmitessa, O.D.; Signore, A.; Caretto, S.; Durante, M.; Mita, G.; Paradiso, A.; Serio, F.; Santamaria, P. Nutritional value of five new tomato (Solanum lycopersicum L.) cultivars in a Mediterranean commercial glasshouse. In Proceedings of the III International Symposium on Soilless Culture and Hydroponics: Innovation and Advanced Technology for Circular Horticulture—Hydro 2020, Lemesos, Cyprus, 19–22 March 2021. [Google Scholar]
- D’Imperio, M.; Montesano, F.F.; Renna, M.; Leoni, B.; Buttaro, D.; Parente, A.; Serio, F. NaCl stress enhances silicon tissue enrichment of hydroponic “baby leaf” chicory under biofortification process. Sci. Hortic. 2018, 235, 258–263. [Google Scholar] [CrossRef]
- Dzakovich, M.P.; Gómez, C.; Mitchell, C.A. Tomatoes grown with light-emitting diodes or high-pressure sodium supplemental lights have similar fruit-quality attributes. HortScience 2015, 50, 1498–1502. [Google Scholar] [CrossRef]
- Davies, J.N.; Hobson, G.E. The constituents of tomato fruit—The influence of environment, nutrition, and genotype. CRC Crit. Rev. Food Sci. Nutr. 1981, 15, 205–280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ebihara, M.; Nakaminami, A.; Maruo, T. Photosynthesis, plant growth, and fruit production of single-truss tomato improves with supplemental lighting provided from underneath or within the inner canopy. Sci. Hortic. 2017, 222, 221–229. [Google Scholar] [CrossRef]
- Nájera, C.; Guil-Guerrero, J.L.; Enríquez, L.J.; Álvaro, J.E.; Urrestarazu, M. LED-enhanced dietary and organoleptic qualities in postharvest tomato fruit. Postharvest Biol. Technol. 2018, 145, 151–156. [Google Scholar] [CrossRef]
- Loi, M.; Villani, A.; Paciolla, F.; Mul, G.; Paciolla, C. Challenges and Opportunities of Light-Emitting Diode (LED) as Key to Modulate Antioxidant Compounds in Plants: A Review. Antioxidant 2021, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Gautier, H.; Massot, C.; Stevens, R.; Sérino, S.; Génard, M. Regulation of tomato fruit ascorbate content is more highly dependent on fruit irradiance than leaf irradiance. Ann. Bot. 2009, 103, 495–504. [Google Scholar] [CrossRef]
- Alba, R.; Cordonnier-Pratt, M.M.; Pratt, L.H. Fruit-localized phytochromes regulate lycopene accumulation independently of ethylene production in tomato. Plant Physiol. 2000, 123, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.; Pék, Z.; Barna, É.; Lugasi, A.; Helyes, L. Lycopene content and colour of ripening tomatoes as affected by environmental conditions. J. Sci. Food Agric. 2006, 86, 568–572. [Google Scholar] [CrossRef]
- Raiola, A.; Tenore, G.C.; Barone, A.; Frusciante, L.; Rigano, M.M. Vitamin E content and composition in tomato fruits: Beneficial roles and bio-fortification. Int. J. Mol. Sci. 2015, 16, 29250–29264. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.; Imperio, M.D.; Gonnella, M.; Durante, M.; Parente, A.; Mita, G.; Santamaria, P.; Serio, F. Morphological and Chemical Profile of Three Tomato (Solanum lycopersicum L.) Landraces of A Semi-Arid Mediterranean Environment. Plants 2019, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Pinho, P.; Jokinen, K.; Halonen, L. The influence of the LED light spectrum on the growth and nutrient uptake of hydroponically grown lettuce. Light. Res. Technol. 2016, 49, 866–881. [Google Scholar] [CrossRef]
- D’Imperio, M.; Renna, M.; Cardinali, A.; Serio, F.; Santamaria, P.; Imperio, M.D.; Renna, M. Calcium biofortification and bioaccessibility in soilless “baby leaf” vegetable production. Food Chem. 2016, 213, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Elless, M.P.; Park, J.; Jenkins, A.; Lim, W.; Iv, E.C.; Hirschi, K.D. Sensory analysis of calcium-biofortified lettuce. Plant Biotechnol. J. 2009, 106–117. [Google Scholar] [CrossRef]


| Day after Transplant (DAT) | Natural Light (DLINL) | LEDs (DLISL) | Total (DLINL + SL) | % SL |
|---|---|---|---|---|
| (mol m−2 d−1) | ||||
| 121–150 | 10.21 | 9.52 | 19.73 | 48 |
| 151–180 | 14.69 | 8.91 | 23.60 | 38 |
| 181–210 | 20.63 | 6.57 | 27.20 | 24 |
| 211–243 | 27.70 | 2.46 | 30.16 | 8 |
| Average Fruit Weight | Yield | Dry Weight (DW) | TSS | pH | Titratable Acidity | |
|---|---|---|---|---|---|---|
| g | g·Plant−1 | (g·100 g−1 FW) | (°Brix) | (g of Citric Acid·100 mL−1) | ||
| Light (L) | ||||||
| LEDs | 8.5 ± 0.8 | 4684 ± 150 | 10.4 ± 0.8 | 10.8 ± 0.8 | 4.44 ± 0.06 | 0.64 ± 0.06 |
| Natural Light | 8.4 ± 0.8 | 3865 ± 96 | 10.1 ± 0.7 | 9.4 ± 0.6 | 4.46 ± 0.08 | 0.55 ± 0.05 |
| Cultivar (CV) | ||||||
| Maggino (Y) | 8.5 ± 0.4 | 4801 ± 130 | 9.5 ± 0.3 | 9.2 ± 0.6 | 4.46 ± 0.05 | 0.58 ± 0.08 |
| Solarino (P) | 8.1 ± 0.3 | 4220 ± 102 | 10.2 ± 0.4 | 10.7 ± 0.9 | 4.45 ± 0.11 | 0.56 ± 0.07 |
| Sorentyno (C) | 8.6 ± 0.4 | 3517 ± 89 | 11.1 ± 0.1 | 10.4 ± 0.6 | 4.44 ± 0.05 | 0.64 ± 0.03 |
| Significance 1 | ||||||
| L | NS | *** | ** | *** | NS | *** |
| CV | * | *** | *** | *** | NS | * |
| C × (Y and P) | * | *** | *** | ** | NS | * |
| Y vs P | ** | *** | ** | *** | ** | NS |
| L × CV | NS | NS | NS | NS | * | NS |
| L × [(Y and P) vs C] | NS | NS | NS | NS | NS | NS |
| L × (Y vs P) | NS | NS | NS | NS | NS | NS |
| Total Antioxidant Activity | |||
|---|---|---|---|
| Vitamin C | Lipophilic Fraction | Hydrophilic Fraction | |
| (mg·kg−1 FW) | (TEAC meq·kg−1 FW) | ||
| Light (L) | |||
| LEDs | 263 | 115 | 1432 |
| Natural Light | 260 | 106 | 1376 |
| Cultivar (CV) | |||
| Maggino (YP) | 210 | 107 | 1354 |
| Solarino (RP) | 286 | 122 | 1628 |
| Sorentyno (RC) | 288 | 101 | 1249 |
| Significance 1 | |||
| L | NS | ** | NS |
| CV | *** | * | * |
| RC vs (YP and RP) | *** | * | * |
| YP vs RP | *** | NS | * |
| L × CV | ** | NS | NS |
| α-Tocopherol | β-Tocopherol | Lutein | Zeaxanthin | β-Cryptoxanthin | β-Carotene | Trans-Lycopene | |
|---|---|---|---|---|---|---|---|
| (mg·kg−1 FW) | |||||||
| Light (L) | |||||||
| LEDs | 6.47 | 3.85 | 0.86 | 0.04 | 1.22 | 4.89 | 13.5 |
| Natural Light | 5.63 | 4.04 | 0.83 | 0.02 | 1.15 | 4.78 | 13.1 |
| Cultivar (CV) | |||||||
| Maggino (YP) | 3.69 | 0.00 | 0.55 | 0.06 | 0.11 | 0.49 | 0.5 |
| Solarino (RP) | 7.25 | 3.95 | 1.10 | 0.03 | 0.11 | 8.11 | 19.0 |
| Sorentyno (RC) | 7.21 | 0.00 | 0.88 | 0.00 | 3.33 | 5.89 | 20.8 |
| Significance 1 | |||||||
| L | ** | NS | NS | ** | NS | NS | NS |
| CV | *** | *** | *** | *** | *** | *** | *** |
| RC vs (YP and RP) | *** | *** | NS | *** | *** | *** | *** |
| YP vs RP | *** | *** | *** | *** | NS | *** | *** |
| L × CV | NS | NS | NS | *** | NS | NS | NS |
| Ca | Fe | K | Mg | Al | B | Cu | Mn | Mo | Na | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg·kg−1 DW) | |||||||||||
| Light (L) | |||||||||||
| LEDs | 613 | 139 | 44,192 | 1059 | 14.40 | 9.80 | 12.80 | 12.90 | 3.60 | 132 | 26.40 |
| Natural Light | 545 | 106 | 44,530 | 944 | 13.80 | 9.10 | 11.50 | 12.90 | 2.40 | 152 | 24.20 |
| Cultivar (CV) | |||||||||||
| Maggino (YP) | 592 | 58 | 46,425 | 952 | 13.30 | 8.50 | 13.10 | 11.50 | 4.60 | 129 | 27.90 |
| Solarino (RP) | 579 | 193 | 40,442 | 942 | 11.20 | 12.10 | 9.80 | 14.30 | 2.10 | 161 | 24.60 |
| Sorentyno (RC) | 569 | 118 | 46,552 | 1143 | 18.70 | 7.60 | 14.00 | 12.90 | 2.30 | 134 | 23.20 |
| Significance 1 | |||||||||||
| L | NS | NS | NS | ** | NS | NS | NS | NS | * | NS | NS |
| CV | NS | * | * | *** | NS | NS | NS | * | ** | NS | NS |
| RC vs. (YP and RP) | NS | NS | NS | *** | NS | NS | NS | NS | * | NS | NS |
| YP vs RP | NS | * | * | NS | NS | NS | NS | * | ** | NS | NS |
| L × CV | * | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmitessa, O.D.; Durante, M.; Caretto, S.; Milano, F.; D'Imperio, M.; Serio, F.; Santamaria, P. Supplementary Light Differently Influences Physico-Chemical Parameters and Antioxidant Compounds of Tomato Fruits Hybrids. Antioxidants 2021, 10, 687. https://doi.org/10.3390/antiox10050687
Palmitessa OD, Durante M, Caretto S, Milano F, D'Imperio M, Serio F, Santamaria P. Supplementary Light Differently Influences Physico-Chemical Parameters and Antioxidant Compounds of Tomato Fruits Hybrids. Antioxidants. 2021; 10(5):687. https://doi.org/10.3390/antiox10050687
Chicago/Turabian StylePalmitessa, Onofrio Davide, Miriana Durante, Sofia Caretto, Francesco Milano, Massimiliano D'Imperio, Francesco Serio, and Pietro Santamaria. 2021. "Supplementary Light Differently Influences Physico-Chemical Parameters and Antioxidant Compounds of Tomato Fruits Hybrids" Antioxidants 10, no. 5: 687. https://doi.org/10.3390/antiox10050687
APA StylePalmitessa, O. D., Durante, M., Caretto, S., Milano, F., D'Imperio, M., Serio, F., & Santamaria, P. (2021). Supplementary Light Differently Influences Physico-Chemical Parameters and Antioxidant Compounds of Tomato Fruits Hybrids. Antioxidants, 10(5), 687. https://doi.org/10.3390/antiox10050687












