Spinach Methanolic Extract Attenuates the Retinal Degeneration in Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Methanolic Extract of Spinach (SME)
2.2. In Vitro Assays of SME Anti-Glycation Activity
2.2.1. Formation of Advanced Glycation End Products Derived from Bovine Serum Albumin (BSA-AGEs)
2.2.2. Fructosamine Assay
2.2.3. Determination of Carbonyl Groups Formation and Thiol Groups Depletion in BSA-AGEs
2.3. Effect of SME on the Retina of Diabetic Rats
2.3.1. Diabetes Induction in the Rats
2.3.2. Experimental Design
2.3.3. Histological and Immunohistochemistry Evaluations
2.3.4. Apoptosis Evaluation
2.3.5. Lipid Peroxidation Assay
2.4. Statistical Analysis
3. Results
3.1. Methanolic Extract of Spinach (SME) Inhibits Bovine Serum Albumin (BSA) Glycation In Vitro
3.1.1. Effect of SME on the Formation of Advanced Glycation End Products (AGEs) and Fructosamine
3.1.2. Effect of SME on Levels of Carbonyl and Reduced-Thiol Groups
3.2. Studies of SME in the Retina of STZ-Induced Diabetic Rats (STZ)
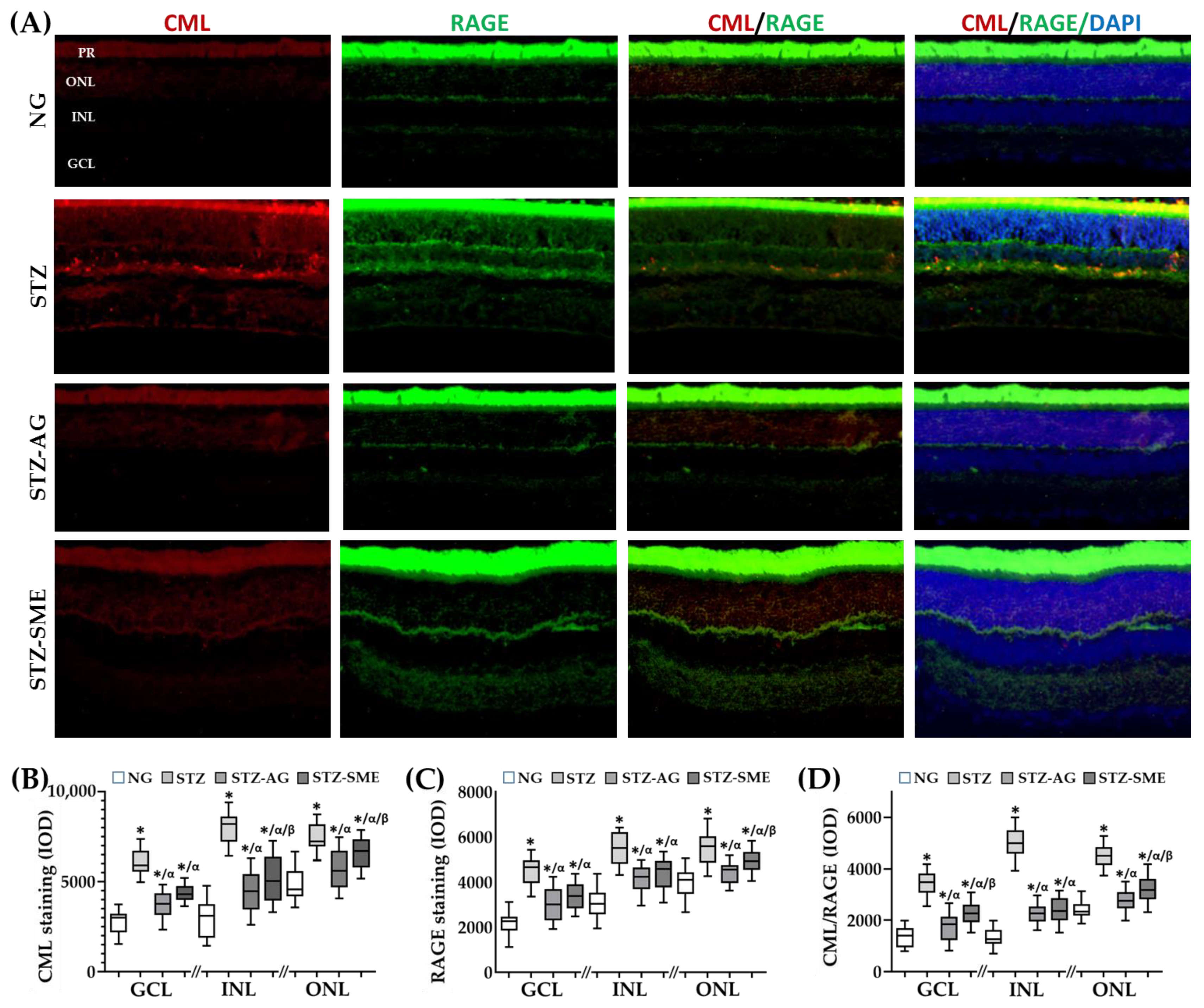
3.2.1. Effect of SME on Carboxymethyl-Lysine (CML) Formation and Its Interaction with the AGEs Receptor (RAGE) in STZ
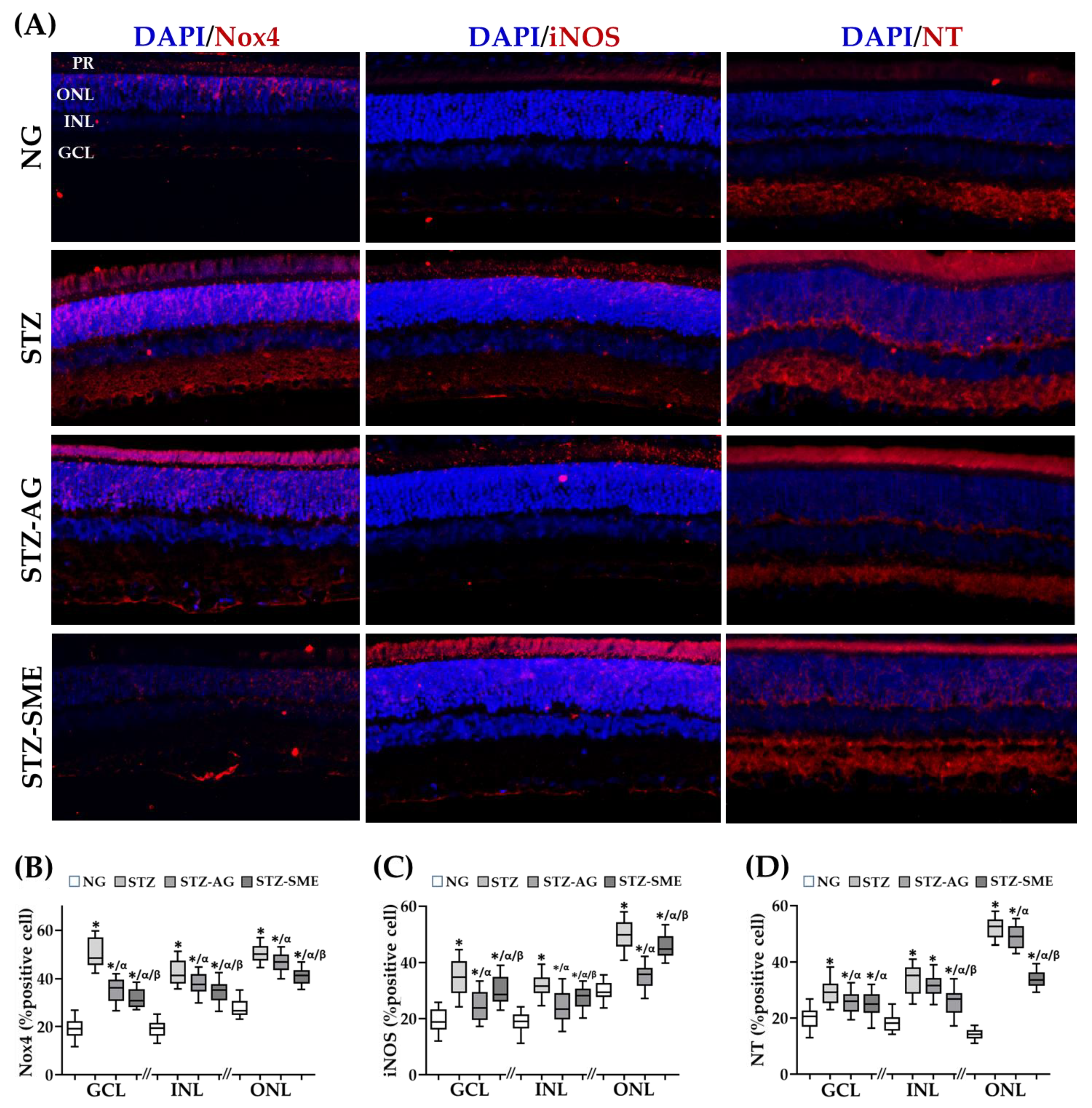
3.2.2. Antioxidant Effect of SME in the Retina of STZ
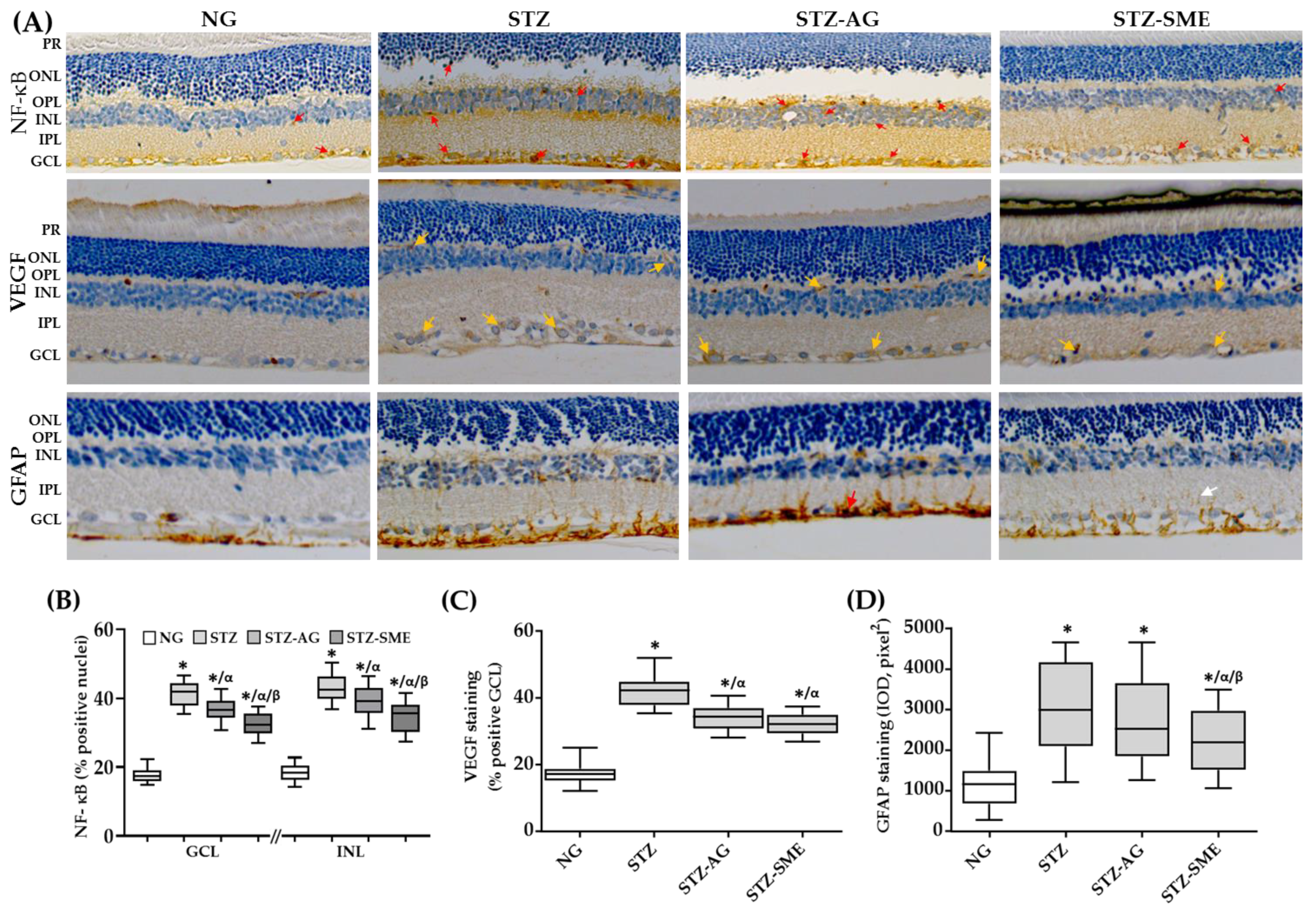
3.2.3. Effect of SME on the Retinal Inflammation of STZ
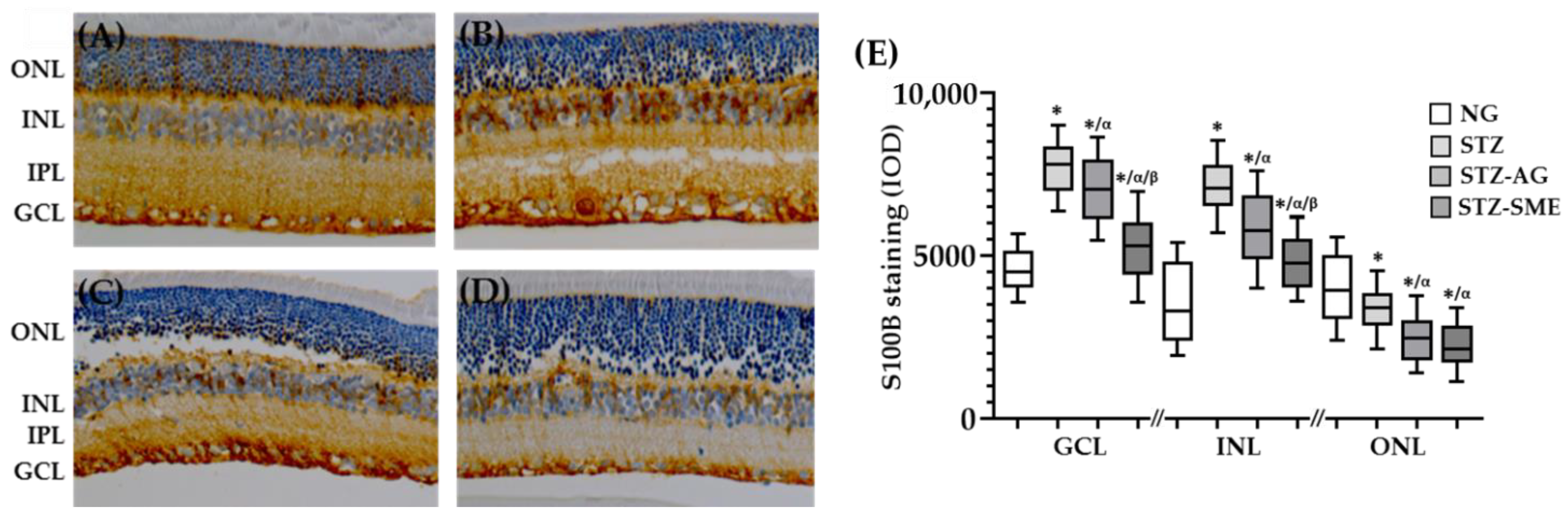
3.2.4. SME Effect on the S100 Calcium-Binding Protein B (S100B) Levels in the Retina of STZ
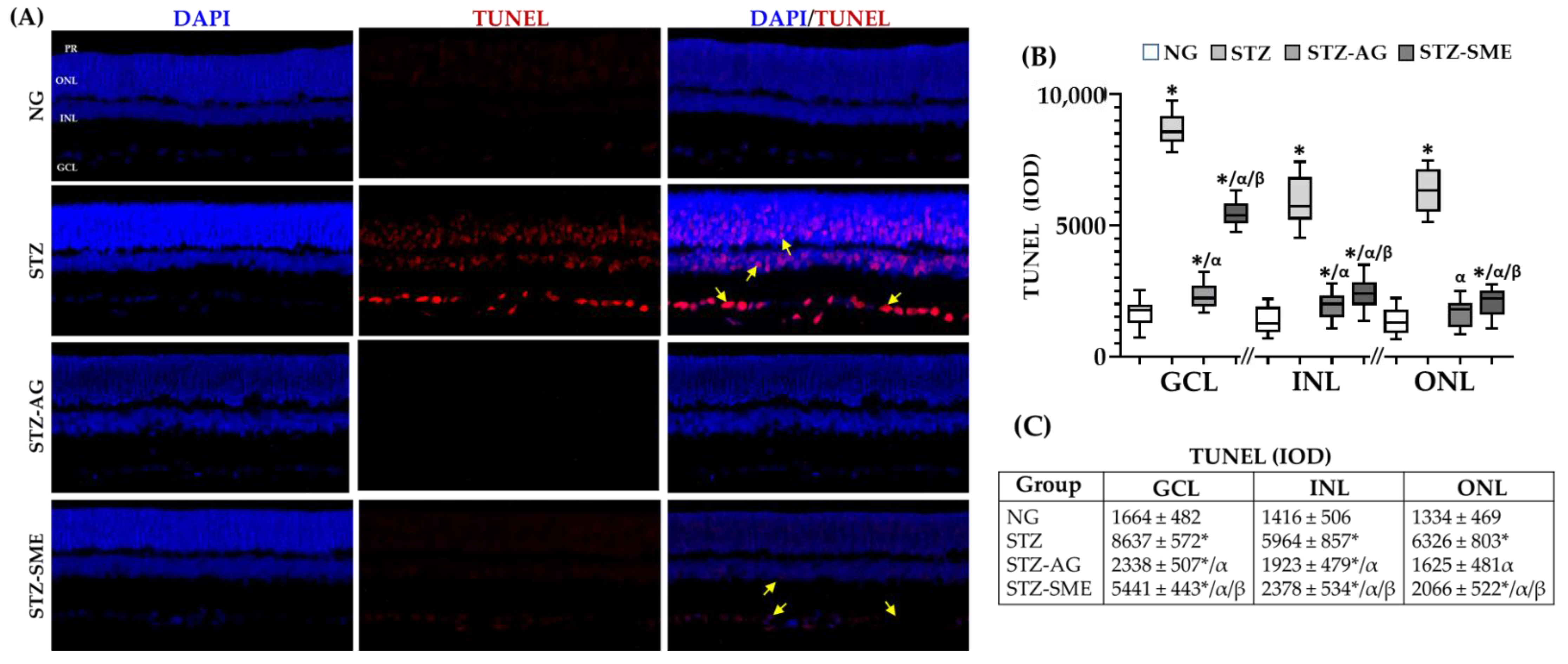
3.2.5. SME Effect on the Retinal Degeneration in STZ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Integral Optical Density (IOD) | |||
|---|---|---|---|
| CML | |||
| Group | GCL | INL | ONL |
| NG | 2766 ± 657 | 3002 ± 961 | 4812 ± 949 |
| STZ | 6066 ± 651 * | 7978 ± 871 * | 7442 ± 732 * |
| STZ-AG | 3772 ± 719 */α | 4482 ± 1153 */α | 5674 ± 1076 */α |
| STZ-SME | 4318 ± 443 */α | 5227 ± 982 */α/β | 6534 ± 826 */α/β |
| RAGE | |||
| Group | GCL | INL | ONL |
| NG | 2137 ± 527 | 3093 ± 653 | 3950 ± 604 |
| STZ | 4473 ± 597 * | 5502 ± 706 * | 5549 ± 721 * |
| STZ-AG | 2998 ± 734 */α | 4147 ± 522 */α | 4447 ± 461 */α |
| STZ-SME | 3376 ± 584 */α | 4391 ± 655 */α | 4951 ± 449 */α/β |
| CML/RAGE | |||
| Group | GCL | INL | ONL |
| NG | 1369 ± 395 | 1338 ± 353 | 2408 ± 324 |
| STZ | 3439 ± 483 * | 5002 ± 604 * | 4489 ± 427 * |
| STZ-AG | 1721 ± 512 */α | 2262 ± 351 */α | 2776 ± 387 */α |
| STZ-SME | 2253 ± 446 */α/β | 2430 ± 485 */α | 3234 ± 483 */α/β |
| % of Cell Positive Immunostaining | |||
|---|---|---|---|
| NADPH-Nox4 | |||
| Group | GCL | INL | ONL |
| NG | 19.3 ± 3.9 | 19.0 ± 3.2 | 27.5 ± 3.4 |
| STZ | 50.7 ± 5.8 * | 42.6 ± 5.0 * | 50.5 ± 3.6 * |
| STZ-AG | 35.1 ± 4.8* /α | 37.8 ± 3.8 */α | 47.0 ± 3.6 */α |
| STZ-SME | 31.8 ± 3.7 */α/β | 34.5 ± 982 */α/β | 41.1 ± 3.4 */α/β |
| iNOS | |||
| Group | GCL | INL | ONL |
| NG | 19.2 ± 4.2 | 18.6 ± 3.3 | 29.7 ± 3.2 |
| STZ | 34.8 ± 6.3 * | 31.9 ± 3.7 * | 49.9 ± 5.3 * |
| STZ-AG | 24.9 ± 5.0 */α | 24.1 ± 5.3 */α | 34.9 ± 4.3 */α |
| STZ-SME | 29.8 ± 5.1 */α/β | 27.3 ± 3.7 */α/β | 45.9 ± 4.0 */α/β |
| Nitrotyrosine | |||
| Group | GCL | INL | ONL |
| NG | 19.9 ± 3.9 | 18.5 ± 3.2 | 14.2 ± 1.8 |
| STZ | 29.3 ± 4.1 * | 33.9 ± 4.8 * | 52.0 ± 3.5 * |
| STZ-AG | 25.5 ± 3.6 */α | 31.5 ± 3.6 * | 48.9 ± 3.9 */α |
| STZ-SME | 25.1 ± 4.2 */α | 25.6 ± 4.6 */α/β | 33.8 ± 2.8 */α/β |
| Nuclear NF-kB (%) | |||
|---|---|---|---|
| Group | GCL | INL | ONL |
| NG | 17.3 ± 2.9 | 17.1 ± 1.9 | 15.3 ± 2.1 |
| STZ | 42.1 ± 4.4 * | 42.4 ± 5.1 * | 36.1 ± 3.2 * |
| STZ-AG | 35.3 ± 3.8 */α | 39.4 ± 6.6 */α | 17.8 ± 2.2 */α |
| STZ-SME | 32.3 ± 3.1 */α/β | 27.5 ± 6.9 */α/β | 15.0 ± 2.7 α/β |
| S100B (IOD) | |||
| Group | GCL | INL | ONL |
| NG | 4356 ± 849 | 3464 ± 1170 | 4207 ± 1160 |
| STZ | 7721 ± 916 * | 7916 ± 1103 * | 3317 ± 827 * |
| STZ-AG | 7081 ± 1037 */α | 5836 ± 1041 */α | 2438 ± 729 */α |
| STZ-SME | 5248 ± 903 */α/β | 3862 ± 736 */α/β | 2233 ± 735 */α |
References
- Punna, R.; Paruchuri, U.R. Effect of maturity and processing on total, insoluble and soluble dietary fiber contents of Indian green leafy vegetables. Int. J. Food Sci. Nutr. 2004, 55, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Imaeda, N.; Tokudome, Y.; Ikeda, M.; Kitagawa, I.; Fujiwara, N.; Tokudome, S. Foods Contributing to Absolute Intake and Variance in Intake of Selected Vitamins, Minerals and Dietary Fiber in Middle-Aged Japanese. J. Nutr. Sci. Vitaminol. 1999, 45, 519–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshminarayana, R.; Raju, M.; Krishnakantha, T.P.; Baskaran, V. Determination of Major Carotenoids in a Few Indian Leafy Vegetables by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2005, 53, 2838–2842. [Google Scholar] [CrossRef]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhé, R.; Van Camp, J. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef]
- Singh, A.; Singh, P.; Kumar, B.; Kumar, S.; Dev, K.; Maurya, R. Detection of flavonoids from Spinacia oleracea leaves using HPLC-ESI-QTOF-MS/MS and UPLC-QqQLIT-MS/MS techniques. Nat. Prod. Res. 2018, 33, 2253–2256. [Google Scholar] [CrossRef]
- Bergquist, S.Å.M.; Gertsson, U.E.; Knuthsen, P.; Olsson, M.E. Flavonoids in Baby Spinach (Spinacia oleracea L.): Changes during Plant Growth and Storage. J. Agric. Food Chem. 2005, 53, 9459–9464. [Google Scholar] [CrossRef]
- Bergman, M.; Varshavsky, L.; Gottlieb, H.E.; Grossman, S. The antioxidant activity of aqueous spinach extract: Chemical identification of active fractions. Phytochemistry 2001, 58, 143–152. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.L.; Moreau, R. Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef]
- Ko, S.-H.; Park, J.-H.; Kim, S.-Y.; Lee, S.W.; Chun, S.-S.; Park, E. Antioxidant Effects of Spinach (Spinacia oleracea L.) Supplementation in Hyperlipidemic Rats. Prev. Nutr. Food Sci. 2014, 19, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, R.M.P.; Velazquez, E.G. Glucopyranoside flavonoids isolated from leaves of Spinacia oleracea (spinach) inhibit the formation of advanced glycation end products (AGEs) and aldose reductase activity (RLAR). Biomed. Pharmacother. 2020, 128, 110299. [Google Scholar] [CrossRef] [PubMed]
- Vutharadhi, S.; Jolapuram, U.; Kodidhela, L.D. Nutraceutical inherent of Spinacia oleracea Linn. methanolic leaf extract ameliorates isoproterenol induced myocardial necrosis in male albino Wistar rats via mitigating inflammation. Biomed. Pharmacother. 2017, 85, 239–247. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Gardner, T.W. An Integrated Approach to Diabetic Retinopathy Research. Arch. Ophthalmol. 2011, 129, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, J.M. Determination ofNɛ-(Carboxymethyl)lysine in Foods and Related Systems. Ann. N. Y. Acad. Sci. 2008, 1126, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Saxena, S.; Shukla, R.K.; Singh, V.; Meyer, C.H.; Kruzliak, P.; Khanna, V.K. Association of serum Nε-Carboxy methyl lysine with severity of diabetic retinopathy. J. Diabetes Complicat. 2016, 30, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, S.; Dutta, D.; Sen, A.; Chowdhury, I.H.; Mitra, B.; Mondal, L.K.; Saha, A.; Bhadhuri, G.; Bhattacharya, B. Role of N–epsilon-carboxy methyl lysine, advanced glycation end products and reactive oxygen species for the development of nonproliferative and proliferative retinopathy in type 2 diabetes mellitus. Mol. Vis. 2013, 19, 100–113. [Google Scholar] [PubMed]
- Zhao, Y.; Luo, C.; Chen, J.; Sun, Y.; Pu, D.; Lv, A.; Zhu, S.; Wu, J.; Wang, M.; Zhou, J.; et al. High glucose-induced complement component 3 up-regulation via RAGE-p38MAPK-NF-κB signalling in astrocytes: In vivo and in vitro studies. J. Cell. Mol. Med. 2018, 22, 6087–6098. [Google Scholar] [CrossRef]
- Tobon-Velasco, J.C.; Cuevas, E.; Torres-Ramos, M.A. Receptor for AGEs (RAGE) as Mediator of NF-kB Pathway Activation in Neuroinflammation and Oxidative Stress. CNS Neurol. Disord. Drug Targets 2014, 13, 1615–1626. [Google Scholar] [CrossRef]
- Villarreal, A.; Reyes, R.X.A.; Angelo, M.F.; Reines, A.G.; Ramos, A.J. S100B alters neuronal survival and dendrite extension via RAGE-mediated NF-κB signaling. J. Neurochem. 2011, 117, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, R.; Kastrisianaki, E.; Giambanco, I.; Donato, R. S100B Protein Stimulates Microglia Migration via RAGE-dependent Up-regulation of Chemokine Expression and Release. J. Biol. Chem. 2011, 286, 7214–7226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Kim, J.; Kim, K.M.; Jung, D.H.; Choi, S.; Kim, C.-S.; Kim, J.S. Myricetin inhibits advanced glycation end product (AGE)-induced migration of retinal pericytes through phosphorylation of ERK1/2, FAK-1, and paxillin in vitro and in vivo. Biochem. Pharmacol. 2015, 93, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, Y.; Song, Z.; Shang, S.; Chen, X. Influence of dietary flavonoids on the glycation of plasma proteins. Mol. BioSyst. 2012, 8, 2183–2187. [Google Scholar] [CrossRef]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Ojha, S.; Arya, D.S. Molecular Pathways Involved in the Amelioration of Myocardial Injury in Diabetic Rats by Kaempferol. Int. J. Mol. Sci. 2017, 18, 1001. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Liu, J.; Zeng, X.; Huangfu, J.; Jiang, Y.; Wang, M.; Chen, F. Protective actions of microalgae against endogenous and exogenous advanced glycation endproducts (AGEs) in human retinal pigment epithelial cells. Food Funct. 2011, 2, 251–258. [Google Scholar] [CrossRef]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxidative Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Kowluru, R.; Zhong, Q.; Santos, J.M.; Thandampallayam, M.; Putt, D.; Gierhart, D.L. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr. Metab. 2014, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Newman, E.A. Aminoguanidine Reverses the Loss of Functional Hyperemia in a Rat Model of Diabetic Retinopathy. Front. Neuroenerget. 2012, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Bolton, W.K.; Cattran, D.C.; Williams, M.E.; Adler, S.G.; Appel, G.B.; Cartwright, K.; Foiles, P.G.; Freedman, B.I.; Raskin, P.; Ratner, R.E.; et al. Randomized Trial of an Inhibitor of Formation of Advanced Glycation End Products in Diabetic Nephropathy. Am. J. Nephrol. 2004, 24, 32–40. [Google Scholar] [CrossRef]
- Seri, A.; Khorsand, M.; Rezaei, Z.; Hamedi, A.; Takhshid, M.A. Inhibitory Effect of Bunium Persicum Hydroalcoholic Extract on Glucose-Induced Albumin Glycation, Oxidation, and Aggregation In Vitro. Iran. J. Med. Sci. 2017, 42, 369–376. [Google Scholar]
- Yanagisawa, K.; Makita, Z.; Shiroshita, K.; Ueda, T.; Fusegawa, T.; Kuwajima, S.; Takeuchi, M.; Koike, T. Specific fluorescence assay for advanced glycation end products in blood and urine of diabetic patients. Metabolism 1998, 47, 1348–1353. [Google Scholar] [CrossRef]
- Baker, J.R.; Zyzak, D.V.; Thorpe, S.R.; Baynes, J.W. Chemistry of the fructosamine assay: D-glucosone is the product of oxidation of Amadori compounds. Clin. Chem. 1994, 40, 1950–1955. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. [49] Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.R.; Jamshidi, S.; Farhangi, A.; Verdi, A.A.; Mofidian, S.M.A.; Rad, B.L. Induction of diabetes by Streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, O.K.; Kim, D.-O.; Smith, N.; Schroeder, D.; Han, J.T.; Lee, C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sci. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Agoston, D.V. How to Translate Time? The Temporal Aspect of Human and Rodent Biology. Front. Neurol. 2017, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Pérez, J.; Martínez-Rosas, M.; Conde-Castañón, C.A.; Toscano-Garibay, J.D.; Ruiz-Pérez, N.J.; Flores, P.L.; Jiménez, E.M.; Flores-Estrada, J. Epigallocatechin 3-Gallate Has a Neuroprotective Effect in Retinas of Rabbits with Ischemia/Reperfusion through the Activation of Nrf2/HO-1. Int. J. Mol. Sci. 2020, 21, 3716. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Pharmacology of methylglyoxal: Formation, modification of proteins and nucleic acids, and enzymatic detoxification-A role in pathogenesis and antiproliferative chemotherapy. Gen. Pharmacol. Vasc. Syst. 1996, 27, 565–573. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344 Pt 1, 109–116. [Google Scholar] [CrossRef]
- Lo, T.W.; Westwood, M.; McLellan, A.C.; Selwood, T.; Thornalley, P.J. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994, 269, 32299–32305. [Google Scholar] [CrossRef]
- Vivekanadan-Giri, A.; Wang, J.H.; Byun, J.; Pennathur, S. Mass spectrometric quantification of amino acid oxidation products identifies oxidative mechanisms of diabetic end-organ damage. Rev. Endocr. Metab. Disord. 2008, 9, 275–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossin, N.; Wautier, M.-P.; Meas, T.; Guillausseau, P.-J.; Massin, P.; Wautier, J.-L. Severity of diabetic microvascular complications is associated with a low soluble RAGE level. Diabetes Metab. 2008, 34, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Akileshwari, C.; Reddy, V.S.; Reddy, G.B. Attenuation of diabetic retinopathy in rats by ellagic acid through inhibition of AGE formation. J. Food Sci. Technol. 2017, 54, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Renier, G. Activation of nicotinamide adenine dinucleotide phosphate (reduced form) oxidase by advanced glycation end products links oxidative stress to altered retinal vascular endothelial growth factor expression. Metabolism 2006, 55, 1516–1523. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.M.; Kim, C.-S.; Sohn, E.; Lee, Y.M.; Jo, K.; Kim, J.S. Puerarin inhibits the retinal pericyte apoptosis induced by advanced glycation end products in vitro and in vivo by inhibiting NADPH oxidase-related oxidative stress. Free. Radic. Biol. Med. 2012, 53, 357–365. [Google Scholar] [CrossRef]
- Martín, A.S.; Du, P.; Dikalova, A.; Lassègue, B.; Aleman, M.; Góngora, M.C.; Brown, K.; Joseph, G.; Harrison, D.G.; Taylor, W.R.; et al. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am. J. Physiol. Circ. Physiol. 2007, 292, H2073–H2082. [Google Scholar] [CrossRef]
- Yokota, T.; Kamimura, N.; Igarashi, T.; Takahashi, H.; Ohta, S.; Oharazawa, H. Protective effect of molecular hydrogen against oxidative stress caused by peroxynitrite derived from nitric oxide in rat retina. Clin. Exp. Ophthalmol. 2015, 43, 568–577. [Google Scholar] [CrossRef]
- Cantó, A.; Olivar, T.; Romero, F.J.; Miranda, M. Nitrosative Stress in Retinal Pathologies: Review. Antioxidants 2019, 8, 543. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Du, Y.; Miller, C.; Gubitosi-Klug, R.A.; Kern, T.S.; Ball, S.; Berkowitz, B.A. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia 2007, 50, 1987–1996. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.-X.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. The Advanced Glycation End Product, Nepsilon-(Carboxymethyl)lysine, Is a Product of both Lipid Peroxidation and Glycoxidation Reactions. J. Biol. Chem. 1996, 271, 9982–9986. [Google Scholar] [CrossRef] [Green Version]
- Stitt, A.W.; Curtis, T.M. Diabetes-related adduct formation and retinopathy. J. Ocul. Biol. Dis. Inform. 2011, 4, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammes, H.P.; Hoerauf, H.; Alt, A.; Schleicher, E.; Clausen, J.T.; Bretzel, R.G.; Laqua, H. N(epsilon)(carboxymethyl)lysin and the AGE receptor RAGE colocalize in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1855–1859. [Google Scholar]
- Haque, R.; Iuvone, P.M.; He, L.; Hur, E.H.; Choi, K.S.C.; Park, D.; Farrell, A.N.; Ngo, A.; Gokhale, S.; Aseem, M.; et al. Prorenin receptor (PRR)-mediated NADPH oxidase (Nox) signaling regulates VEGF synthesis under hyperglycemic condition in ARPE-19 cells. J. Recept. Signal Transduct. 2017, 37, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Antonetti, D.; Gardner, T.W. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research Group. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3561–3568. [Google Scholar]
- Berner, A.K.; Brouwers, O.; Pringle, R.; Klaassen, I.; Colhoun, L.; McVicar, C.; Brockbank, S.; Curry, J.W.; Miyata, T.; Brownlee, M.; et al. Protection against methylglyoxal-derived AGEs by regulation of glyoxalase 1 prevents retinal neuroglial and vasodegenerative pathology. Diabetologia 2012, 55, 845–854. [Google Scholar] [CrossRef]
- Zong, H.; Ward, M.; Madden, A.; Yong, P.H.; Limb, G.A.; Curtis, T.M.; Stitt, A.W. Hyperglycaemia-induced pro-inflammatory responses by retinal Müller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia 2010, 53, 2656–2666. [Google Scholar] [CrossRef] [Green Version]
- Reinehr, S.; Reinhard, J.; Gandej, M.; Gottschalk, I.; Stute, G.; Faissner, A.; Dick, H.B.; Joachim, S.C. S100B immunization triggers NFκB and complement activation in an autoimmune glaucoma model. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Rambotti, M.; Giambanco, I.; Spreca, A.; Donato, R. S100B and S100A1 proteins in bovine retina: Their calcium-dependent stimulation of a membrane-bound guanylate cyclase activity as investigated by ultracytochemistry. Neuroscience 1999, 92, 1089–1101. [Google Scholar] [CrossRef]
- Sokal, I.; Alekseev, A.; Palczewski, K. Photoreceptor guanylate cyclase variants: cGMP production under control. Acta Biochim. Polonica 2003, 50, 1075–1095. [Google Scholar] [CrossRef] [Green Version]
- Morozzi, G.; Beccafico, S.; Bianchi, R.; Riuzzi, F.; Bellezza, I.; Giambanco, I.; Arcuri, C.; Minelli, A.; Donato, R. Oxidative stress-induced S100B accumulation converts myoblasts into brown adipocytes via an NF-κB/YY1/miR-133 axis and NF-κB/YY1/BMP-7 axis. Cell Death Differ. 2017, 24, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Feenstra, D.J.; Yego, E.C.; Mohr, S. Modes of Retinal Cell Death in Diabetic Retinopathy. J. Clin. Exp. Ophthalmol. 2013, 4, 298. [Google Scholar] [CrossRef] [Green Version]
- Howes, K.A.; Liu, Y.; Dunaief, J.L.; Milam, A.; Frederick, J.M.; Marks, A.; Baehr, W. Receptor for Advanced Glycation End Products and Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2004, 45, 3713–3720. [Google Scholar] [CrossRef]

| AGEs formation (FIU) | ||||
|---|---|---|---|---|
| Mixture | Glucose | Fructose | Methylglyoxal | |
| BSA | - | 184.7 ± 29.47 | 317.3 ±30.73 | 286.2 ± 31,78 |
| SME-50 | 167.9 ± 12.49 | 295.0 ± 13.71 | 267.2 ± 11.23 | |
| SME-100 | 158.4 ± 12.11 | 281.4 ± 17.45 | 256.2 ± 12.95 | |
| SME-200 | 149.1 ± 11.83 * | 267.5 ± 18.11 * | 243.2 ± 15.01 * | |
| AG | 128.4 ± 13.22 * | 250.6 ± 14.65 * | 229.1 ± 12.78 * | |
| Fructosamine formation (nmol/L) | ||||
| Mixture | Glucose | Fructose | Methylglyoxal | |
| BSA | - | 8.81 ± 1.46 | 4.55 ± 1.15 | 0.69 ± 0.21 |
| SME-50 | 7.37 ± 1.39 | 3.76 ± 0.93 | 0.49 ± 0.19 | |
| SME-100 | 6.94 ± 0.97 | 3.11 ± 0.62 | 0.33 ± 0.17 | |
| SME-200 | 6.16 ± 0.81 * | 2.53 ± 0.53 * | 0.28 ± 0.07 */α | |
| AG | 5.58 ± 0.71 * | 2.11 ± 0.53 * | 0.33 ± 0.05 * | |
| Carbonyl Groups formation (nmol/mg protein) | ||||
| Mixture | Glucose | Fructose | Methylglyoxal | |
| BSA | - | 2.57 ±1.46 | 3.77 ± 0.6 | 2.12 ± 0.54 |
| SME-50 | 2.33 ± 1.39 | 3.11 ± 0.45 | 2.76 ± 1.15 | |
| SME-100 | 2.02 ± 0.97 | 2.92 ± 0.45 | 1.82 ± 0.79 | |
| SME-200 | 1.95 ± 0.8 * | 2.84 ± 0.45 * | 1.88 ± 0.52 * | |
| AG | 1.40 ± 0.71 * | 2.12 ± 0.54 * | 1.64 ± 0.33 * | |
| Reduced thiol groups (nmol/mg) | ||||
| Mixture | Glucose | Fructose | Methylglyoxal | |
| BSA | - | 0.72 ± 0.14 | 0.62 ± 0.1 | 0.66 ± 0.09 |
| SME-50 | 0.75 ± 0.09 | 0.68 ± 0.07 | 0.74 ± 0.01 | |
| SME-100 | 0.84 ± 0.1 | 0.75 ± 0.09 | 0.83 ± 0.12 | |
| SME-200 | 0.95 ± 0.14 * | 0.84 ± 0.11 * | 0.98 ± 0.01 */α | |
| AG | 1.08 ± 0.15 * | 0.93 ± 0.08 * | 0.87 ± 0.12 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bautista-Pérez, R.; Cano-Martínez, A.; Gutiérrez-Velázquez, E.; Martínez-Rosas, M.; Pérez-Gutiérrez, R.M.; Jiménez-Gómez, F.; Flores-Estrada, J. Spinach Methanolic Extract Attenuates the Retinal Degeneration in Diabetic Rats. Antioxidants 2021, 10, 717. https://doi.org/10.3390/antiox10050717
Bautista-Pérez R, Cano-Martínez A, Gutiérrez-Velázquez E, Martínez-Rosas M, Pérez-Gutiérrez RM, Jiménez-Gómez F, Flores-Estrada J. Spinach Methanolic Extract Attenuates the Retinal Degeneration in Diabetic Rats. Antioxidants. 2021; 10(5):717. https://doi.org/10.3390/antiox10050717
Chicago/Turabian StyleBautista-Pérez, Rocio, Agustina Cano-Martínez, Elisa Gutiérrez-Velázquez, Martín Martínez-Rosas, Rosa M. Pérez-Gutiérrez, Francisco Jiménez-Gómez, and Javier Flores-Estrada. 2021. "Spinach Methanolic Extract Attenuates the Retinal Degeneration in Diabetic Rats" Antioxidants 10, no. 5: 717. https://doi.org/10.3390/antiox10050717
APA StyleBautista-Pérez, R., Cano-Martínez, A., Gutiérrez-Velázquez, E., Martínez-Rosas, M., Pérez-Gutiérrez, R. M., Jiménez-Gómez, F., & Flores-Estrada, J. (2021). Spinach Methanolic Extract Attenuates the Retinal Degeneration in Diabetic Rats. Antioxidants, 10(5), 717. https://doi.org/10.3390/antiox10050717






