High Resolution Mass Spectroscopy-Based Secondary Metabolite Profiling of Nymphaea nouchali (Burm. f) Stem Attenuates Oxidative Stress via Regulation of MAPK/Nrf2/HO-1/ROS Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Extraction
2.2. Electro Spray Ionization (ESI)-Mass Spectroscopy Analysis
2.3. Data Processing
2.4. Radical Scavenging Activity Assays
2.5. Cell Culture and Intracellular ROS Generation Assay
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Secondary Metabolite Profiling of NNSE
3.1.1. Phenolic Acids
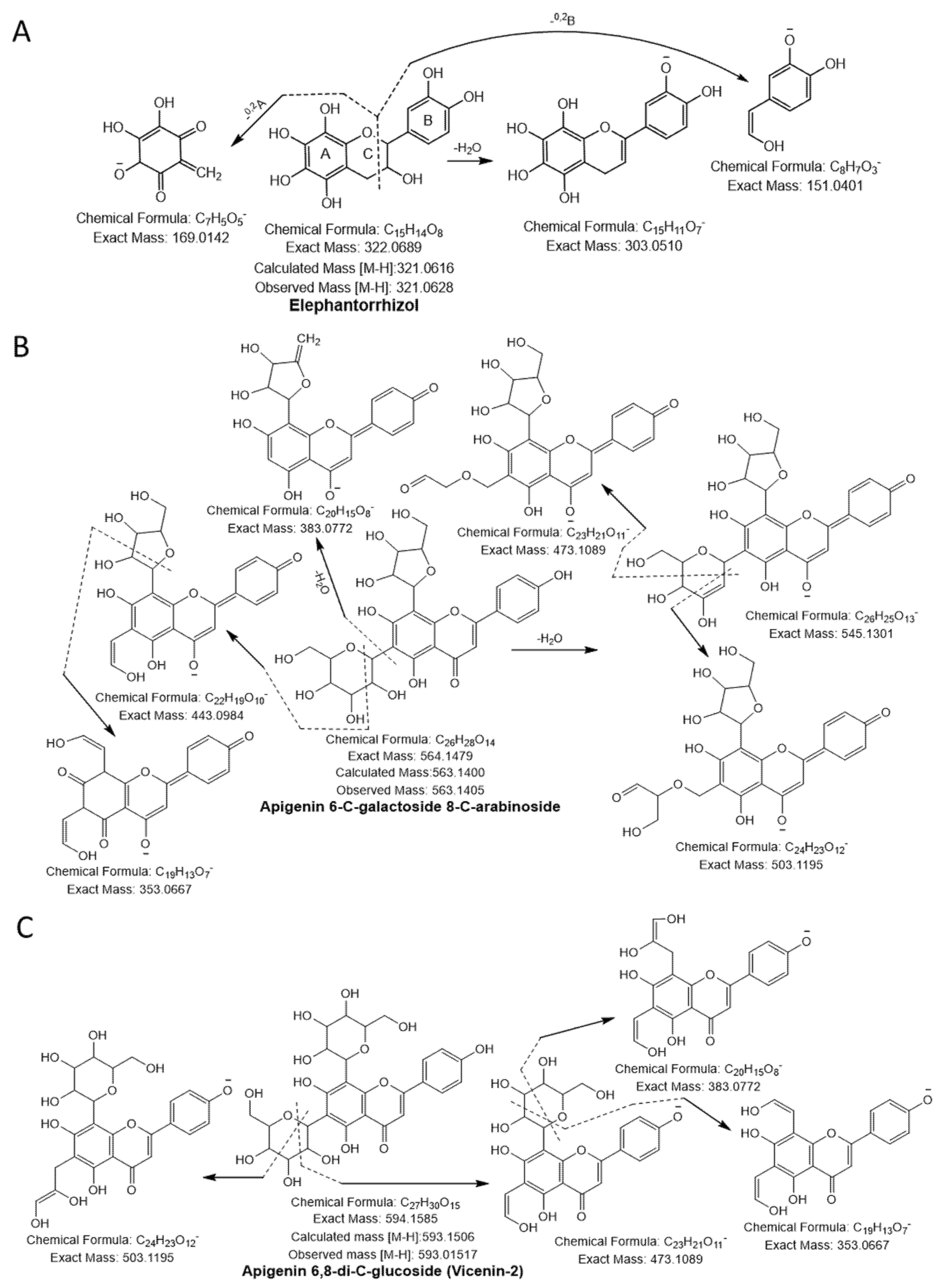
3.1.2. Flavonoids
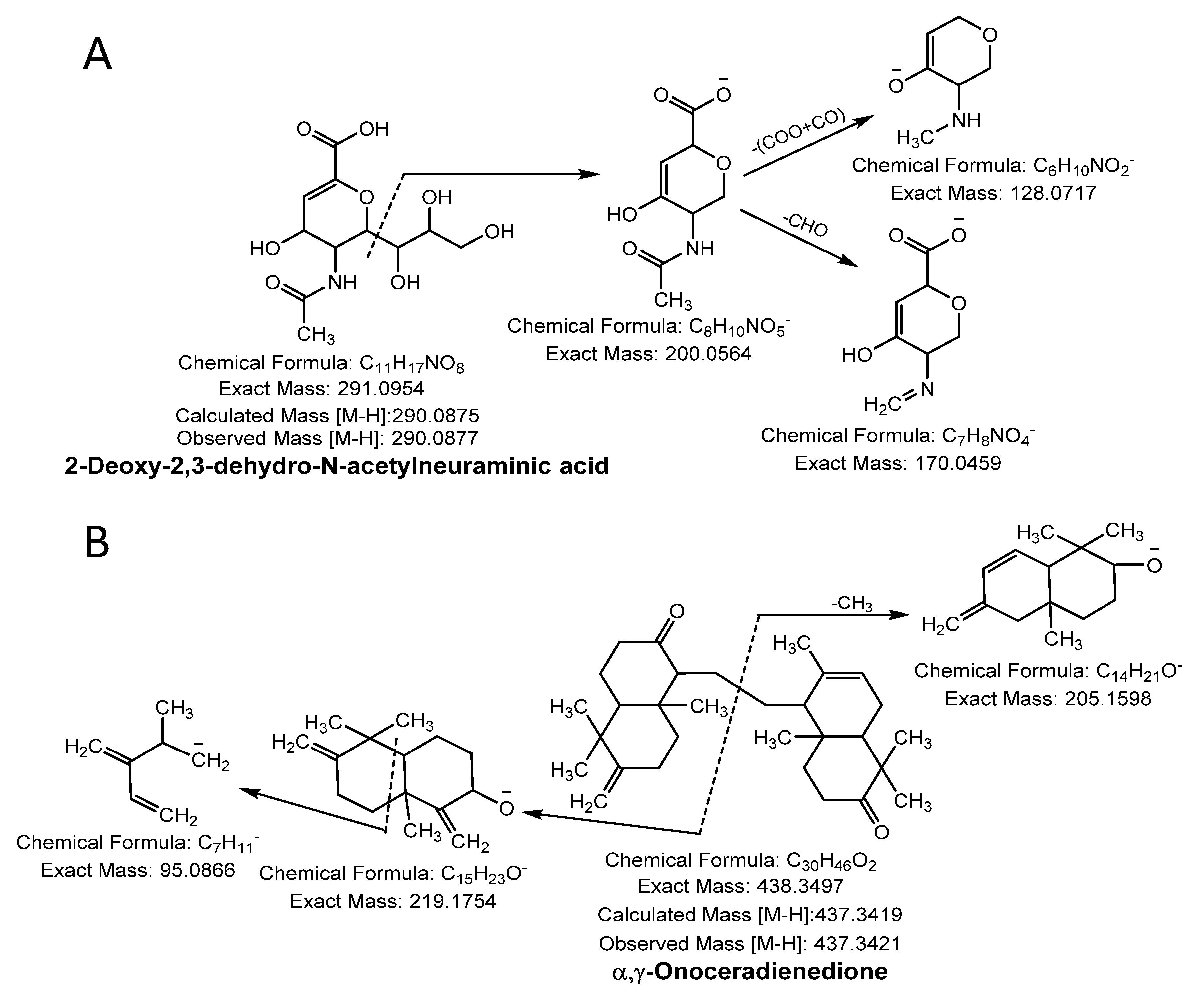
3.1.3. Sialic Acid
3.1.4. Terpenoid
3.1.5. Dicarboxylic Acids
3.1.6. Others
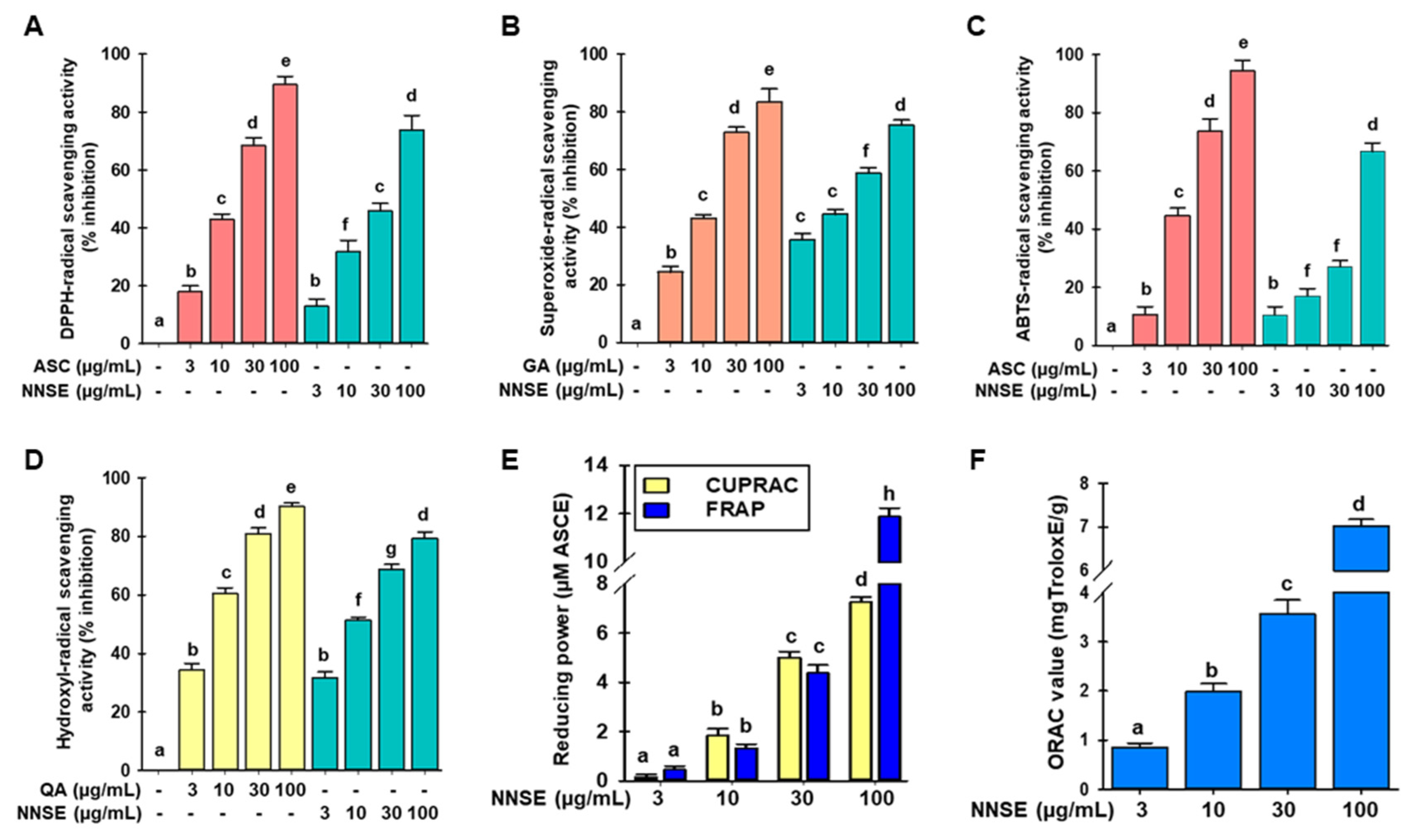
3.2. Radical Scavenging Activities of NNSE Extracts
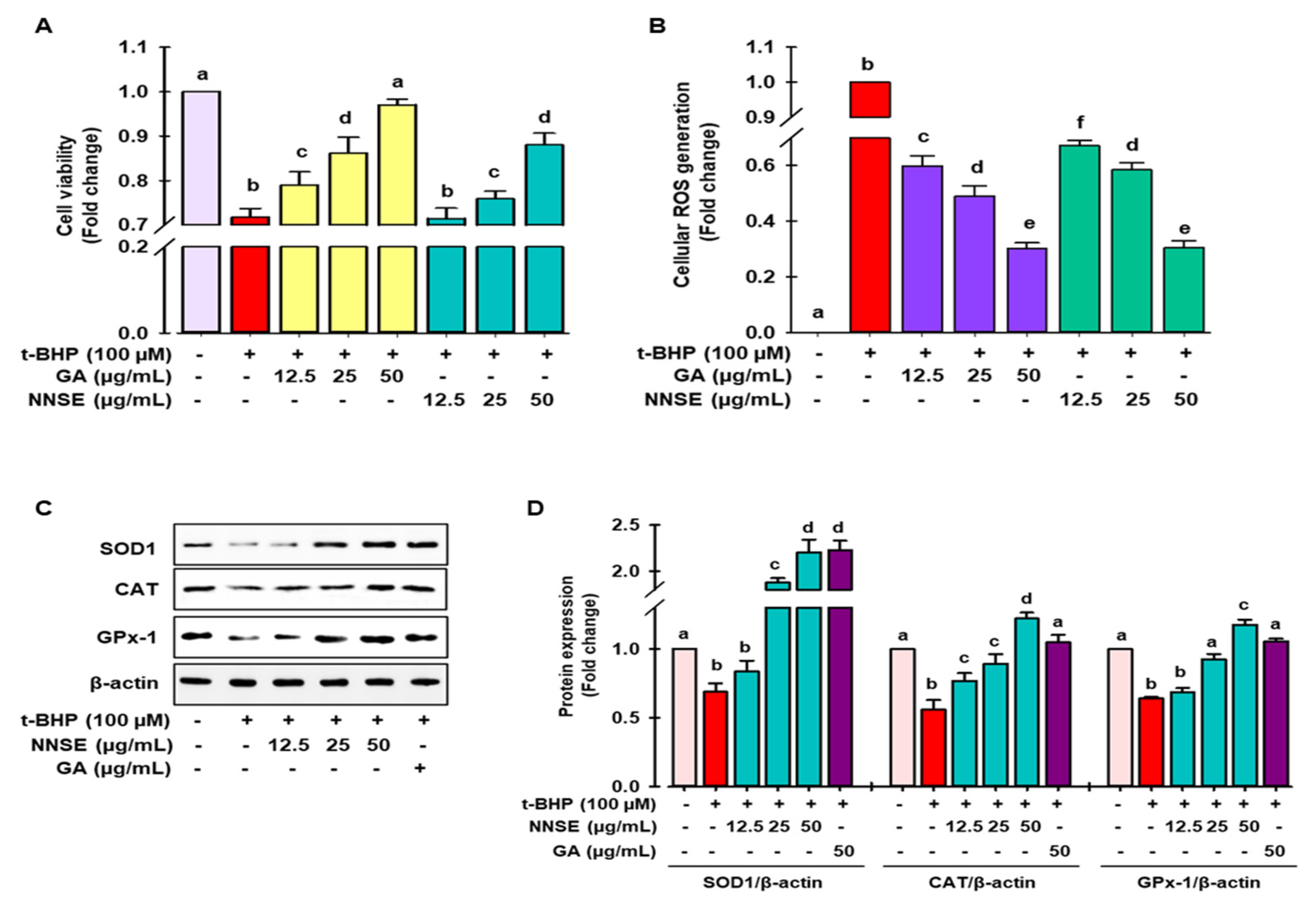
3.3. Attenuation of t-BHP Induced Cellular Oxidative Stress by NNSE
3.4. NNSE Induces Phase II Enzymes through Nrf2 Regulation
3.5. NNSE Activates MAPKs and Regulates Nuclear Translocation of Nrf2, Leading to Reduced Oxidative Stress
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, J.; Das, A.; Sinha, M.; Saha, S. Biological efficacy of medicinal plant extracts in preventing oxidative damage. Oxid. Med. Cell. Longev. 2018, 2018, 7904349. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.B.; Ahmed, A.; Islam, S.; Choi, H.-J.; Motin, M.A.; Kim, S.; Lee, S.-H. Phytochemical Characterization of Dillenia indica L. bark by paper spray ionization-mass spectrometry and evaluation of its antioxidant potential against t-BHP-induced oxidative stress in RAW 264.7 cells. Antioxidants 2020, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, O.A.; Lee, S.C.; Ho, C.T.; Huang, T.C. Macrophages in oxidative stress and models to evaluate the antioxidant function of dietary natural compounds. J. Food Drug Anal. 2017, 25, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.B.; Ju, M.K.; Lee, S.H. DNA Protecting activities of Nymphaea nouchali (Burm. f) flower extract attenuate t-BHP-induced oxidative stress cell death through Nrf2-mediated induction of heme oxygenase-1 expression by activating MAP-kinases. Int. J. Mol. Sci. 2017, 18, 2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar]
- Raja, M.M.M.; Sethiya, N.K.; Mishra, S. A comprehensive review on Nymphaea stellata: A traditionally used bitter. J. Adv. Pharm. Technol. Res. 2010, 1, 311. [Google Scholar] [CrossRef] [Green Version]
- Bhandarkar, M.R.; Khan, A. Antihepatotoxic effect of Nymphaea stellata willd., against carbon tetrachloride-induced hepatic damage in albino rats. J. Ethnopharmacol. 2004, 91, 61–64. [Google Scholar] [CrossRef]
- Kabir, S.R.; Zubair, M.A.; Nurujjaman, M.; Haque, M.A.; Hasan, I.; Islam, M.F.; Hossain, M.T.; Hossain, M.A.; Rakib, M.A.; Alam, M.T. Purification and characterization of a Ca2+-dependent novel lectin from Nymphaea nouchali tuber with antiproliferative activities. Biosci. Rep. 2011, 31, 465–475. [Google Scholar] [CrossRef]
- Alam, M.B.; Ahmed, A.; Motin, M.A.; Kim, S.; Lee, S.-H. Attenuation of melanogenesis by Nymphaea nouchali (Burm. f) flower extract through the regulation of cAMP/CREB/MAPKs/MITF and proteasomal degradation of tyrosinase. Sci. Rep. 2018, 8, 13928. [Google Scholar] [CrossRef]
- Islam, S.; Alam, M.B.; Ahmed, A.; Lee, S.; Lee, S.-H.; Kim, S. Identification of secondary metabolites in Averrhoa carambola L. bark by high-resolution mass spectrometry and evaluation for α-glucosidase, tyrosinase, elastase, and antioxidant potential. Food Chem. 2020, 332, 127377. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Alam, M.B.; Ann, H.-J.; Park, J.-H.; Lee, S.-H.; Kim, S. Metabolite profiling of Manilkara zapota L. leaves by high-resolution mass spectrometry coupled with ESI and APCI and in vitro antioxidant activity, α-glucosidase, and elastase inhibition assays. Int. J. Mol. Sci. 2021, 22, 132. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Dars, A.G.; Liu, Q.; Xie, B.; Sun, Z. Phytochemical profiling of the ripening of Chinese mango (Mangifera indica L.) cultivars by real-time monitoring using UPLC-ESI-QTOF-MS and its potential benefits as prebiotic ingredients. Food Chem. 2018, 256, 171–180. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Ma, X.; Chu, Y.; Li, S.; Guo, J.; Jia, Y.; Zhou, S.; Zhu, Y.; Liu, C. Simultaneous determination of caffeic acid and its major pharmacologically active metabolites in rat plasma by LC-MS/MS and its application in pharmacokinetic study. Biomed. Chromatogr. BMC 2015, 29, 552–559. [Google Scholar] [CrossRef]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS profiling of Australian mango peel by-product polyphenols and their potential antioxidant activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterization of Phenolic Compounds from Medicinal Plants (Hops and Juniper Berries) and Their Antioxidant Activity. Foods 2019, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Buiarelli, F.; Coccioli, F.; Merolle, M.; Jasionowska, R.; Terracciano, A. Identification of hydroxycinnamic acid–tartaric acid esters in wine by HPLC–tandem mass spectrometry. Food Chem. 2010, 123, 827–833. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K.; Gilani, A. Isolation and structure elucidation of novel hypotensive agents, niazinin A, niazinin B, niazimicin and niaziminin A+ B from Moringa oleifera: The first naturally occurring thiocarbamates. J. Chem. Soc. Perkin Trans. 1 1992, 3237–3241. [Google Scholar] [CrossRef]
- Othman, M.R.; Othman, R.; Ismail, A.A.; Hazni, H.; Ahmad, K.; Abd Razzak, M.; Yusoff, Z.M.; Awang, K. High-performance liquid chromatography quadrupole time-of-flight mass spectrometry (HPLC-QTOFMS) analysis on the ethanol: Water (80: 20) extract of Lawsonia inermis leaves. Sains Malays. 2020, 49, 1597–1613. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, H.; Xu, X.; Xue, X.; Liu, M.; Xu, S.; Liu, H.; Gao, Y.; Zhang, H.; Li, X. Compounds identification in semen cuscutae by ultra-high-performance liquid chromatography (UPLCs) coupled to electrospray ionization mass spectrometry. Molecules 2018, 23, 1199. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Salces, R.; Serra, F.; Reniero, F.; Heberger, K. Botanical and geographical characterization of green coffee (Coffea arabica and Coffea canephora): Chemometric evaluation of phenolic and methylxanthine contents. J. Agric. Food Chem. 2009, 57, 4424–4435. [Google Scholar] [CrossRef]
- Galaverna, R.S.; Sampaio, P.T.B.; Barata, L.E.S.; Eberlin, M.N.; Fidelis, C.H.V. Differentiation of two morphologically similar Amazonian Aniba species by mass spectrometry leaf fingerprinting. Anal. Methods 2015, 7, 1984–1990. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Xu, F.; Li, H.-F.; Wang, Y.; Li, F.-C.; Shang, M.-Y.; Liu, G.-X.; Wang, X.; Cai, S.-Q. Detection of 191 taxifolin metabolites and their distribution in rats using HPLC-ESI-IT-TOF-MS(n). Molecules 2016, 21, 1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, M.U.; Mumtaz, M.W.; Mukhtar, H.; Rashid, U.; Akhtar, M.T.; Raza, S.A.; Nadeem, M. UHPLC-QTOF-MS/MS based phytochemical characterization and anti-hyperglycemic prospective of hydro-ethanolic leaf extract of Butea monosperma. Sci. Rep. 2020, 10, 3530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and quantification by LC-MS/MS of the chemical components of the heating products of the flavonoids extract in Pollen Typhae for Transformation Rule Exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef] [Green Version]
- Baskaran, R.; Pullencheri, D.; Somasundaram, R. Characterization of free, esterified and bound phenolics in custard apple (Annona squamosa L) fruit pulp by UPLC-ESI-MS/MS. Food Res. Int. 2016, 82, 121–127. [Google Scholar] [CrossRef]
- Sørensen, L.K.; Rittig, N.F.; Holmquist, E.F.; Jørgensen, K.A.; Jørgensen, J.O.; Møller, N.; Johannsen, M. Simultaneous determination of β-hydroxybutyrate and β-hydroxy-β-methylbutyrate in human whole blood using hydrophilic interaction liquid chromatography electrospray tandem mass spectrometry. Clin. Biochem. 2013, 46, 1877–1883. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Alam, M.B.; Ju, M.K.; Kwon, K.R.; Huh, Y.S.; Han, Y.K.; Lee, S.H. Antioxidant mechanism of polyphenol-rich Nymphaea nouchali leaf extract protecting DNA damage and attenuating oxidative stress-induced cell death via Nrf2-mediated heme-oxygenase-1 induction coupled with ERK/p38 signaling pathway. Biomed. Pharmacother. 2018, 103, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Cudalbeanu, M.; Furdui, B.; Cârâc, G.; Barbu, V.; Iancu, A.V.; Marques, F.; Leitão, J.H.; Sousa, S.A.; Dinica, R.M. Antifungal, Antitumoral and Antioxidant Potential of the Danube Delta Nymphaea alba Extracts. Antibiotics 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnihotri, V.K.; Elsohly, H.N.; Khan, S.I.; Smillie, T.J.; Khan, I.A.; Walker, L.A. Antioxidant constituents of Nymphaea caerulea flowers. Phytochemistry 2008, 69, 2061–2066. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Kabra, A.; Hano, C. Enrichment in antioxidant flavonoids of stamen extracts from Nymphaea lotus L. using ultrasonic-assisted extraction and macroporous resin adsorption. Antioxidants 2020, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Antonisamy, P.; Subash-Babu, P.; Alshatwi, A.A.; Aravinthan, A.; Ignacimuthu, S.; Choi, K.C.; Kim, J.H. Gastroprotective effect of nymphayol isolated from Nymphaea stellata (Willd.) flowers: Contribution of antioxidant, anti-inflammatory and anti-apoptotic activities. Chem. Biol. Interact. 2014, 224, 157–163. [Google Scholar] [CrossRef]
- Yuan, J.; Li, B.; Qin, F.G.; Tu, J. Analysis of ethyl acetate extract of enzymatic hydrolysate from high purity oleuropein and DPPH radical scavenging capacity. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 301, pp. 012–021. [Google Scholar]
- Hsu, F.-L.; Huang, W.-J.; Wu, T.-H.; Lee, M.-H.; Chen, L.-C.; Lu, H.-J.; Hou, W.-C.; Lin, M.-H. Evaluation of antioxidant and free radical scavenging capacities of polyphenolics from pods of Caesalpinia pulcherrima. Int. J. Mol. Sci. 2012, 13, 6073–6088. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-N.; Su, B.-J.; Wang, Y.-Q.; Liao, H.-B.; Chen, Z.-F.; Liang, D. Isolation, absolute configuration, and biological activities of chebulic acid and brevifolincarboxylic acid derivatives from Euphorbia hirta. J. Nat. Prod. 2020, 83, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Hsouna, A.B.; Trigui, M.; Culioli, G.; Blache, Y.; Jaoua, S. Antioxidant constituents from Lawsonia inermis leaves: Isolation, structure elucidation and antioxidative capacity. Food Chem. 2011, 125, 193–200. [Google Scholar] [CrossRef]
- Li, X.; Xie, H.; Jiang, Q.; Wei, G.; Lin, L.; Li, C.; Ou, X.; Yang, L.; Xie, Y.; Fu, Z.; et al. The mechanism of (+) taxifolin’s protective antioxidant effect for •OH-treated bone marrow-derived mesenchymal stem cells. Cell. Mol. Biol. Lett. 2017, 22, 31. [Google Scholar] [CrossRef] [Green Version]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G.; Bórquez, J.; Kennelly, E.J. The Passiflora tripartita (Banana Passion) fruit: A source of bioactive flavonoid C-glycosides isolated by HSCCC and characterized by HPLC–DAD–ESI/MS/MS. Molecules 2013, 18, 1672–1692. [Google Scholar] [CrossRef]
- Zou, X.; Gao, J.; Zheng, Y.; Wang, X.; Chen, C.; Cao, K.; Xu, J.; Li, Y.; Lu, W.; Liu, J. Zeaxanthin induces Nrf2-mediated phase II enzymes in protection of cell death. Cell Death Dis. 2014, 5, e1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.S.; Berná, G.; Otaolaurruchi, E.; Troncoso, A.M.; Martín, F.; García-Parrilla, M.C. Changes in antioxidant endogenous enzymes (activity and gene expression levels) after repeated red wine intake. J. Agric. Food Chem. 2009, 57, 6578–6583. [Google Scholar] [CrossRef]
- Crespo, I.; García-Mediavilla, M.V.; Almar, M.; González, P.; Tuñón, M.J.; Sánchez-Campos, S.; González-Gallego, J. Differential effects of dietary flavonoids on reactive oxygen and nitrogen species generation and changes in antioxidant enzyme expression induced by proinflammatory cytokines in Chang Liver cells. Food Chem. Toxicol. 2008, 46, 1555–1569. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Ghosh, S.; Hazra, B. Inhibitory effect of Nymphaea pubescens Willd. flower extract on carrageenan-induced inflammation and CCl4-induced hepatotoxicity in rats. Food Chem. Toxicol. Int. J. Publ. Br. Industr. Biol. Res. Assoc. 2013, 59, 485–491. [Google Scholar] [CrossRef]
- Bakr, R.O.; El-Naa, M.M.; Zaghloul, S.S.; Omar, M.M. Profile of bioactive compounds in Nymphaea alba L. leaves growing in Egypt: Hepatoprotective, antioxidant and anti-inflammatory activity. BMC Complement. Altern. Med. 2017, 17, 52. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.-F.; An, L.-J.; Jiang, B.; Guan, S.; Bao, Y.-M. Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo. Neurosci. Lett. 2006, 403, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.U.; Shah, S.A.; Kim, M.O. Vanillic acid attenuates Aβ1-42-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 2017, 7, 40753. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.B.; Wang, Y.; He, C.; Yang, Y.; Wan, J.B. Gallic acid, a natural polyphenol, protects against tert-butyl hydroperoxide- induced hepatotoxicity by activating ERK-Nrf2-Keap1-mediated antioxidative response. Food Chem. Toxicol. Int. J. Publ. Br. Industr. Biol. Res. Assoc. 2018, 119, 479–488. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.H.; Cho, S.S.; Kim, J.H.; Xu, J.; Seo, K.; Ki, S.H. 5-Caffeoylquinic acid ameliorates oxidative stress-mediated cell death via Nrf2 activation in hepatocytes. Pharm. Biol. 2020, 58, 999–1005. [Google Scholar] [CrossRef]
- Suraweera, T.L.; Rupasinghe, H.P.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by dietary flavonoids: A friend or foe for cancer management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef]
- Kuang, H.; Tang, Z.; Zhang, C.; Wang, Z.; Li, W.; Yang, C.; Wang, Q.; Yang, B.; Kong, A.-N. Taxifolin activates the Nrf2 anti-oxidative stress pathway in mouse skin epidermal JB6 P+ cells through epigenetic modifications. Int. J. Mol. Sci. 2017, 18, 1546. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Wu, T.; Liu, T.; Yang, H.; Ding, X.; Chen, Y.; Mu, Y. Vicenin-2 ameliorates oxidative damage and photoaging via modulation of MAPKs and MMPs signaling in UVB radiation exposed human skin cells. J. Photochem. Photobiol. B Biol. 2019, 190, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Natural Nrf2 modulators for skin protection. Antioxidants 2020, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Kaspar, J.W.; Shen, J.; Jaiswal, A.K. Nrf2 signaling and cell survival. Toxicol. Appl. Pharmacol. 2010, 244, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.J.; Kim, E.H.; Hannan, M.A.; Ha, H. Pharmacotherapy against Oxidative stress in chronic kidney disease: Promising small molecule natural products targeting Nrf2-HO-1 signaling. Antioxidants 2021, 10, 258. [Google Scholar] [CrossRef] [PubMed]



| Groups | No. | Compound Name | EF | Observed m/z | Calculated m/z | Adducts | MS/MS Fragments | CE (eV) |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids | 1 | Salicylic acid | C7H6O3 | 137.0227 | 137.0238 | [M − H]− | 93.03 | 10 |
| 2 | Methyl benzoic acid | C8H8O2 | 135.0444 | 135.0446 | 91.05 | 10 | ||
| 3 | Protocatechuic acid | C7H6O4 | 153.0186 | 153.0187 | 135.00,109.02 | 10 | ||
| 4 | Vanillic acid | C8H8O4 | 167.0344 | 167.0344 | 151.00, 123.04,107.01 | 10 | ||
| 5 | Gallic acid | C7H6O5 | 169.0134 | 169.0137 | 125.02 | 10 | ||
| 6 | Methoxygallate | C8H8O5 | 183.0290 | 183.0293 | 166.99, 139.04, 123.01, 111.01 | 30 | ||
| 7 | Brevifolincarboxylic acid | C13H8O8 | 291.0141 | 291.0140 | 247.02,219.02,203.03,191.03,175.04 | 20 | ||
| 8 | Pyrogallol gallate | C13H10O8 | 293.0300 | 293.0297 | 169.01, 125.02 | 10 | ||
| 9 | p-coumaroyltartaric acid | C13H12O8 | 295.0456 | 295.0453 | 163.04,149.00,145.02 | 20 | ||
| 10 | Methyl brevifolincarboxylic acid | C14H10O8 | 305.0300 | 305.0297 | 245.00,217.01, 201.01, 189.01,161.02,145.02 | 20 | ||
| 11 | Galloylglucose | C13H16O10 | 331.0667 | 331.0665 | 241.03, 211.02, 169.01, 125.02 | 20 | ||
| 12 | Niazinin B | C15H21NO6S | 342.1115 | 342.1011 | 310.07,196.04,166.03,164.01 | 20 | ||
| 13 | Lalioside | C14H18O10 | 345.0822 | 345.0822 | 301.05,211.02,183.02,165.01 | 20 | ||
| 14 | 5-O-caffeoylquinic acid | C16H18O9 | 353.0865 | 353.0872 | 309.09,191.05,179.03,173.04,161.02 | 20 | ||
| 15 | 3-Feruloylquinic acid | C17H20O9 | 367.1050 | 367.1029 | 193.05, 173.04 | 20 | ||
| 16 | Isoferulic acid 3-O-glucuronide | C16H18O10 | 369.0822 | 369.0821 | 193.05,177.04 | 10 | ||
| 17 | Ethyl 5-O-caffeoylquinic acid | C18H22O9 | 381.1204 | 381.1186 | 201.07,191.05,179.03,161.02 | 20 | ||
| 18 | Gallic acid-O-rutinoside | C19H26O14 | 477.1249 | 477.1244 | 315.07, 297.06, 283.04, 211.02, 169.01, 105.05, 125.02, 93.03 | 30 | ||
| 19 | Digalloylglucose | C20H20O14 | 483.0779 | 483.0774 | 331.06, 313.05, 303.07, 271.04, 241.03, 211.02, 169.01, 125.02 | 30 | ||
| Flavonoids | 20 | Catechin | C15H14O6 | 289.0692 | 289.0712 | [M − H]− | 245.04, 205.05,151.04,137.02 | 30 |
| 21 | Taxifolin | C15H12O7 | 303.0470 | 303.0504 | 285.04,241.05,177.01,151.04 | 20 | ||
| 22 | Elephantorrhizol | C15H14O8 | 321.0628 | 321.0616 | 303.05,169.01,151.04 | 30 | ||
| 23 | Naringenin-7-sulfate | C15H12O8S | 351.0175 | 351.0174 | 271 | 30 | ||
| 24 | Apigenin-6-C-galactoside-8-C-arabinoside | C26H28O14 | 563.1405 | 563.1400 | 545.13, 503.11, 473.10, 443.09, 383.07, 353.06 | 40 | ||
| 25 | Apigenin 6,8-di-C-glucoside (Vicenin-2) | C27H30O15 | 593.1517 | 593.1506 | 503.11,473.10, 383.07,353.06 | 40 | ||
| 26 | Quercetin-3-neohesperidoside | C27H30O16 | 609.1460 | 609.1455 | 245.04, 205.05,151.04,137.02 | 30 | ||
| Sugars | 27 | L-arabinofuranose | C5H10O5 | 149.0445 | 149.0450 | [M − H]− | 131.03, 89.02, 75.01, 71.01 | 10 |
| 28 | Glucose | C6H12O6 | 179.0553 | 179.0555 | [M − H]− | 113.02, 101.02, 89.02, 71.01 | 10 | |
| 215.0322 | 215.0324 | [M + Cl]− | 179.05, 113.02, 101.02, 89.02, 71.01 | 10 | ||||
| 217.0293 | 217.0295 | [M + K-2H]− | ||||||
| 29 | 6-O-β-D-galactopyranosyl-D-galactose | C12H22O11 | 341.1087 | 341.1083 | [M − H]− | 179.05, 113.02, 101.02, 89.02, 71.01 | 10 | |
| 377.0854 | 377.0857 | [M + Cl]− | 341.10, 179.05, 113.02, 101.02, 89.02, 71.01 | 20 | ||||
| 379.2159 | 379.2161 | [M + K-2H]− | ||||||
| Dicarboxylic acids | 30 | Succinic acid | C4H6O4 | 117.0175 | 117.0187 | [M − H]− | 99.00, 73.02 | 10 |
| 31 | Malic Acid | C4H6O5 | 133.0124 | 133.0137 | 115.00, 89.02, 71.01 | 10 | ||
| 32 | Citramalic acid | C5H8O5 | 147.0292 | 147.0293 | 133.01, 115.00, 87.00 | 20 | ||
| 33 | Hydroxyadipic acid | C6H10O5 | 161.0450 | 161.0450 | 143.03, 101.02, 99.04 | 20 | ||
| Amino acids | 34 | α-amino-β-hydroxybutyric acid | C4H9NO3 | 118.0141 | 118.0140 | [M − H]− | 100.04, 96.00, 74.02 | 10 |
| 35 | Pyroglutamic acid | C5H7NO3 | 128.0336 | 128.0347 | 82.03, 71.01, 69.00 | 20 | ||
| Flavoring agents | 36 | Maltol | C6H6O3 | 125.0227 | 125.0238 | [M − H]− | 97.03, 95.01, 83.04, 79.01, | 20 |
| 37 | Kahweofuran | C7H8OS | 139.0213 | 139.0217 | 111.02, 109.01, 68.98, 67.01 | 20 | ||
| 38 | Cetone V | C16H24O | 231.1748 | 231.1748 | 173.09, 155.08, 137.13,93.03 | 20 | ||
| Fatty acids | 39 | Methylcaproic acid | C7H14O2 | 129.0916 | 129.0915 | [M − H]− | 99.04, 85.10, 71.01, 69.07 | 20 |
| 40 | Ethyl-β-hydroxybutyric acid | C6H12O3 | 131.0696 | 131.0708 | 113.06, 87.08, 85.06 | 10 | ||
| 41 | Caprylic acid | C8H16O2 | 143.1061 | 143.1072 | 125.10, 113.06, 99.05, 85.03, | 10 | ||
| 42 | Dodecadienoic acid | C12H20O2 | 195.1384 | 195.1385 | 179.11, 161.10, 97.10, 71.09 | 30 | ||
| 43 | Dodecanoic acid | C12H24O2 | 199.1697 | 199.1698 | 181.16, 165.13, 163.11, 139.11, 135.11 | 20 | ||
| Sialic acid | 44 | 2-Deoxy-2,3-dehydro-N-acetylneuraminic acid | C11H17NO8 | 290.0877 | 290.0875 | [M − H]− | 200.05, 170.04, 128.07 | 10 |
| Terpenoid | 45 | α-γ-Onoceradienedione | C30H46O2 | 437.3421 | 437.3419 | [M − H]− | 219.17, 205.15, 95.08 | 40 |
| others | 46 | Glyceric acid | C3H6O4 | 105.0184 | 105.0187 | [M − H]− | 87.00, 75.00, 61.03 | 10 |
| 47 | Sorbic acid | C6H8O2 | 111.0443 | 111.0446 | 67.05 | 10 | ||
| 48 | Salicylaldehyde | C7H6O2 | 121.0278 | 121.0289 | 93.03, 65.03 | 30 | ||
| 49 | Methyl benzoic acid | C8H8O2 | 135.0444 | 135.0446 | 91.05 | 10 | ||
| 50 | Hydroxynicotinic acid | C6H5NO3 | 138.0189 | 138.0191 | 94.02 | 20 | ||
| 51 | Ribonic acid | C5H10O6 | 165.0395 | 165.0399 | 149.04, 105.01, 87.00, 75.00 | 10 | ||
| 52 | Shikimic acid | C7H10O5 | 173.0445 | 173.0450 | 155.03, 137.02, 111.04, 93.03 | 10 | ||
| 53 | Quinic acid | C7H12O6 | 191.0553 | 191.0555 | 173.05, 127.04, 93.03, 85.03 | 10 | ||
| 54 | N-undecanoylglycine | C13H25NO3 | 242.1756 | 242.1756 | 224.1656, 182.1550 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.B.; Naznin, M.; Islam, S.; Alshammari, F.H.; Choi, H.-J.; Song, B.-R.; Kim, S.; Lee, S.-H. High Resolution Mass Spectroscopy-Based Secondary Metabolite Profiling of Nymphaea nouchali (Burm. f) Stem Attenuates Oxidative Stress via Regulation of MAPK/Nrf2/HO-1/ROS Pathway. Antioxidants 2021, 10, 719. https://doi.org/10.3390/antiox10050719
Alam MB, Naznin M, Islam S, Alshammari FH, Choi H-J, Song B-R, Kim S, Lee S-H. High Resolution Mass Spectroscopy-Based Secondary Metabolite Profiling of Nymphaea nouchali (Burm. f) Stem Attenuates Oxidative Stress via Regulation of MAPK/Nrf2/HO-1/ROS Pathway. Antioxidants. 2021; 10(5):719. https://doi.org/10.3390/antiox10050719
Chicago/Turabian StyleAlam, Md Badrul, Marufa Naznin, Syful Islam, Fanar Hamad Alshammari, Hee-Jeong Choi, Bo-Rim Song, Sunghwan Kim, and Sang-Han Lee. 2021. "High Resolution Mass Spectroscopy-Based Secondary Metabolite Profiling of Nymphaea nouchali (Burm. f) Stem Attenuates Oxidative Stress via Regulation of MAPK/Nrf2/HO-1/ROS Pathway" Antioxidants 10, no. 5: 719. https://doi.org/10.3390/antiox10050719
APA StyleAlam, M. B., Naznin, M., Islam, S., Alshammari, F. H., Choi, H.-J., Song, B.-R., Kim, S., & Lee, S.-H. (2021). High Resolution Mass Spectroscopy-Based Secondary Metabolite Profiling of Nymphaea nouchali (Burm. f) Stem Attenuates Oxidative Stress via Regulation of MAPK/Nrf2/HO-1/ROS Pathway. Antioxidants, 10(5), 719. https://doi.org/10.3390/antiox10050719







