Coenzyme Q10 and Immune Function: An Overview
Abstract
:1. Introduction
2. CoQ10 and Animal Models of Immune Function
3. CoQ10 and Susceptibility to Infection
4. CoQ10 and Immune Function in Athletes
5. CoQ10 and Immune Cell Activation
6. CoQ10 and Inflammation
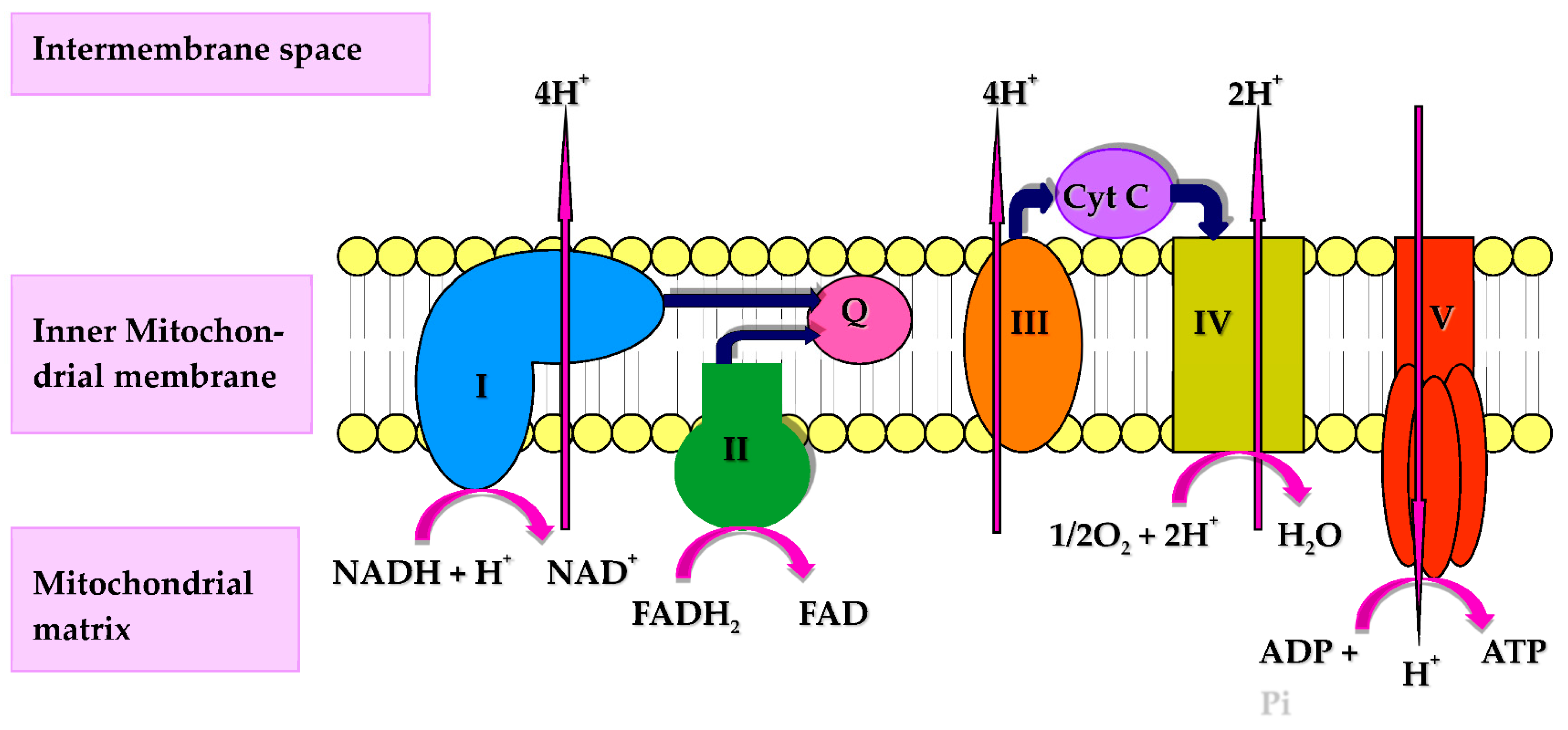
7. CoQ10, Mitochondria, and Immune Function
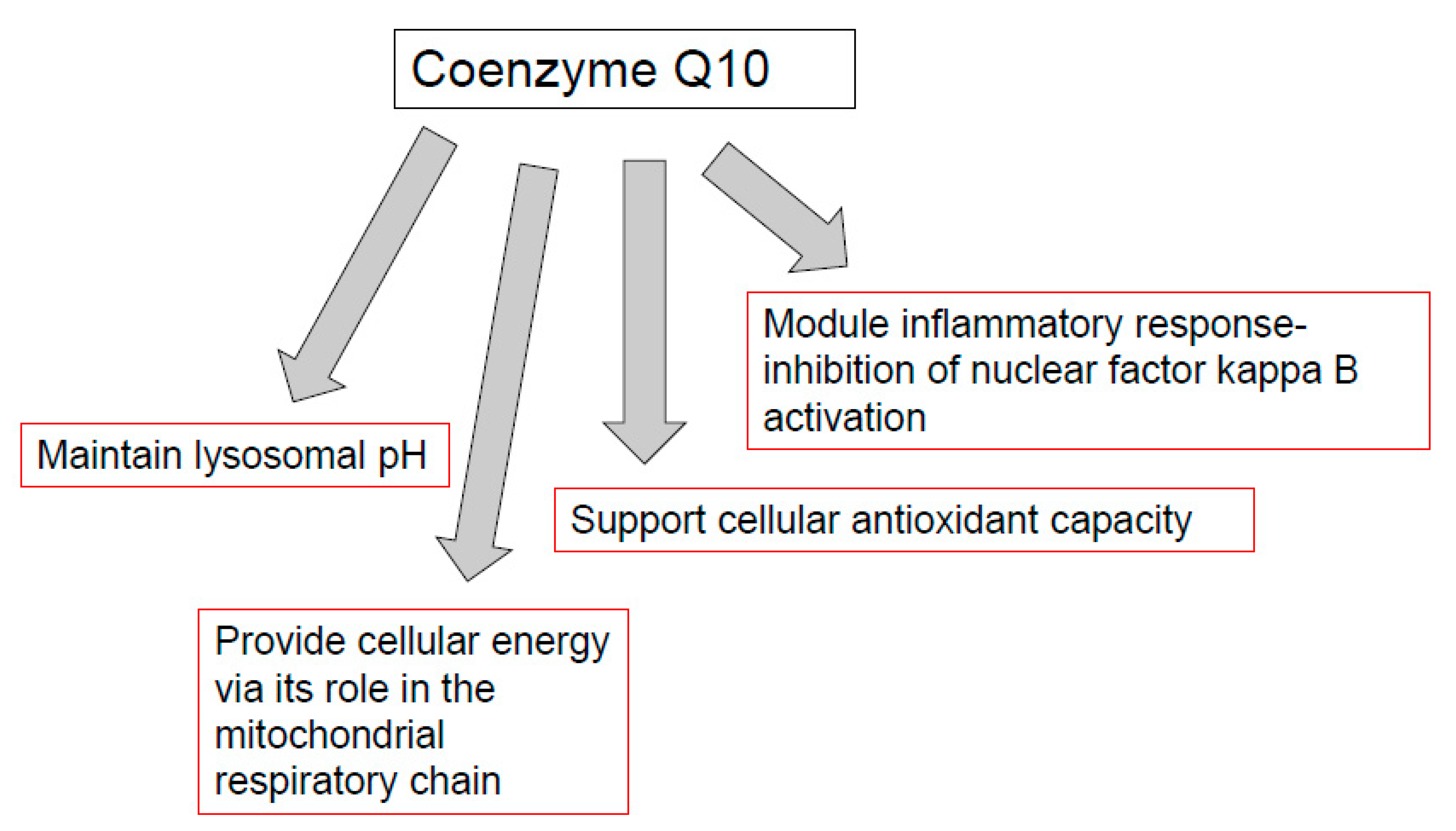
8. CoQ10, Lysosomes and Immune Function
9. CoQ10, Peroxisomes and Immune Function
10. CoQ10 and Immune Function in Cancer
11. Coenzyme Q10 Monitoring and Dosage
12. Safety and Bioavailability of CoQ10
13. Discussion
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. et Biophys. Acta (bba)-Mol. Cell Biol. Lipids 2015, 1851, 397–413. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, I.P. Ubiquinone: Cholesterol’s reclusive cousin. Ann. Clin. Biochem. 2003, 40, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.M.; Pou, S.; Ramos, C.L.; Cohen, M.S.; Britigan, B.E. Free radicals and phagocytic cells. FASEB J. 1995, 9, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Schmelzer, C.; Lindner, I.; Rimbach, G.; Niklowitz, P.; Menke, T.; Döring, F. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors 2008, 32, 179–183. [Google Scholar] [CrossRef]
- Fülöp, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The role of immunosenescence in the development of age-related diseases. Rev. de Investig. Clin. 2016, 68, 84–91. [Google Scholar]
- Blasco, M.A. Immunosenescence phenotypes in the telomerase knockout mouse. Springer Semin. Immunopathol. 2002, 24, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Maue, A.C.; Yager, E.J.; Swain, S.L.; Woodland, D.L.; Blackman, M.A.; Haynes, L. T-cell immunosenescence: Lessons learned from mouse models of aging. Trends Immunol. 2009, 30, 301–305. [Google Scholar] [CrossRef]
- Heidrick, M.L.; Makinodan, T. Nature of cellular deficiencies in age-related decline of the immune system. Gerontology 1972, 18, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Segre, D.; Segre, M. Age-related changes in B and T lymphocytes and decline of humoral immune responsiveness in aged mice. Mech. Ageing Dev. 1977, 6, 115–129. [Google Scholar] [CrossRef]
- Bliznakov, E.G. Immunological senescence in mice and its reversal by coenzyme Q10. Mech. Ageing Dev. 1978, 7, 189–197. [Google Scholar] [CrossRef]
- Bliznakov, E.; Watanabe, T.; Saji, S.; Folkers, K. Coenzyme Q deficiency in aged mice. J. Med. 1978, 9, 337–346. [Google Scholar]
- Tian, G.; Sawashita, J.; Kubo, H.; Nishio, S.-Y.; Hashimoto, S.; Suzuki, N.; Yoshimura, H.; Tsuruoka, M.; Wang, Y.; Liu, Y. Ubiquinol-10 supplementation activates mitochondria functions to decelerate senescence in senescence-accelerated mice. Antioxid. Redox Signal. 2014, 20, 2606–2620. [Google Scholar] [CrossRef]
- Warren, J.L.; MacIver, N.J. Regulation of adaptive immune cells by sirtuins. Front. Endocrinol. 2019, 10, 466. [Google Scholar] [CrossRef]
- Jhun, J.; Lee, S.H.; Byun, J.-K.; Jeong, J.-H.; Kim, E.-K.; Lee, J.; Jung, Y.-O.; Shin, D.; Park, S.H.; Cho, M.-L. Coenzyme Q10 suppresses Th17 cells and osteoclast differentiation and ameliorates experimental autoimmune arthritis mice. Immunol. Lett. 2015, 166, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Licitra, F.; Puccio, H. An overview of current mouse models recapitulating coenzyme q10 deficiency syndrome. Mol. Syndromol. 2014, 5, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Caobi, A.; Dutta, R.K.; Garbinski, L.D.; Esteban-Lopez, M.; Ceyhan, Y.; Andre, M.; Manevski, M.; Ojha, C.R.; Lapierre, J.; Tiwari, S.; et al. The Impact of CRISPR-Cas9 on Age-related Disorders: From Pathology to Therapy. Aging Dis. 2020, 11, 895–915. [Google Scholar] [CrossRef]
- Cheng, W.; Song, C.; Anjum, K.; Chen, M.; Li, D.; Zhou, H.; Wang, W.; Chen, J. Coenzyme Q plays opposing roles on bacteria/fungi and viruses in Drosophila innate immunity. Int. J. Immunogenet. 2011, 38, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Yu, S.; Sun, H.; Chen, L.; Wang, R.; Wu, X.; Goltzman, D.; Miao, D. 1, 25-Dihydroxyvitamin D insufficiency accelerates age-related bone loss by increasing oxidative stress and cell senescence. Am. J. Transl. Res. 2020, 12, 507. [Google Scholar]
- Kishimoto, C.; Tomioka, N.; Nakayama, Y.; Miyamoto, M. Anti-oxidant effects of coenzyme Q10 on experimental viral myocarditis in mice. J. Cardiovasc. Pharmacol. 2003, 42, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, G.; Varin, F. Determination of ubiquinone-9 and 10 levels in rat tissues and blood by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. Sci. 1998, 36, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Novoselova, E.; Safonova, M.; Gordon, R.Y.; Semiletova, N. Immune functions of spleen lymphocytes of rats subjected to chronic irradiation and antioxidant (ubiquinone Q-9) diet. Int. J. Radiat. Biol. 1995, 67, 469–476. [Google Scholar] [CrossRef]
- Kamzalov, S.; Sumien, N.; Forster, M.J.; Sohal, R.S. Coenzyme Q intake elevates the mitochondrial and tissue levels of coenzyme Q and α-tocopherol in young mice. J. Nutr. 2003, 133, 3175–3180. [Google Scholar] [CrossRef] [Green Version]
- Chase, M.; Cocchi, M.N.; Liu, X.; Andersen, L.W.; Holmberg, M.J.; Donnino, M.W. Coenzyme Q10 in acute influenza. Influenza Other Respir. Viruses 2019, 13, 64–70. [Google Scholar] [CrossRef]
- Kelekçi, S.; Evliyaoğlu, O.; Sen, V.; Yolbaş, I.; Uluca, U.; Tan, I.; Gürkan, M. The relationships between clinical outcome and the levels of total antioxidant capacity (TAC) and coenzyme Q (CoQ 10) in children with pandemic influenza (H 1 N1) and seasonal flu. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1033–1038. [Google Scholar]
- Farazi, A.; Sofian, M.; Jabbariasl, M.; Nayebzadeh, B. Coenzyme Q10 administration in community-acquired pneumonia in the elderly. Iran. Red Crescent Med. J. 2014, 16, e18852. [Google Scholar] [CrossRef] [Green Version]
- Israel, A.; Schäffer, A.A.; Cicurel, A.; Feldhamer, I.; Tal, A.; Cheng, K.; Sinha, S.; Schiff, E.; Lavie, G.; Ruppin, E. Large population study identifies drugs associated with reduced COVID-19 severity. MedRxiv 2020. [Google Scholar] [CrossRef]
- Ayala, D.J.M.-F.; Navas, P.; López-Lluch, G. Age-related mitochondrial dysfunction as a key factor in COVID-19 disease. Exp. Gerontol. 2020, 111147. [Google Scholar] [CrossRef]
- Gvozdjakova, A.; Klauco, F.; Kucharska, J.; Sumbalova, Z. Is mitochondrial bioenergetics and coenzyme Q10 the target of a virus causing COVID-19? Bratisl. Lek. Listy 2020, 121, 775–778. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M.; Pedersen, J.Z.; Incerpi, S. Computational studies reveal mechanism by which quinone derivatives can inhibit SARS-CoV-2. Study of embelin and two therapeutic compounds of interest, methyl prednisolone and dexamethasone. J. Infect. Public Health 2020, 13, 1868–1877. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Folkers, K.; Morita, M.; McRee, J. The activities of coenzyme Q10 and vitamin B6 for immune responses. Biochem. Biophys. Res. Commun. 1993, 193, 88–92. [Google Scholar] [CrossRef]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the regulation of immune functions. Prog. Mol. Biol. Transl. Sci. 2015, 135, 355–380. [Google Scholar]
- Emami, A. The Impact of Pre-Cooling and CoQ10 Supplementation on Mediators of Inflammatory Cytokines in Elite Swimmers. Nutr. Cancer 2020, 72, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kon, M.; Tanimura, Y.; Hanaoka, Y.; Kimura, F.; Akama, T.; Kono, I. Coenzyme Q10 supplementation downregulates the increase of monocytes expressing toll-like receptor 4 in response to 6-day intensive training in kendo athletes. Appl. Physiol. Nutr. Metab. 2015, 40, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Trushina, E.N.; Vybornov, V.D.; Riger, N.A.; Mustafina, O.K.; Solntseva, T.N.; Timonin, A.N.; Zilova, I.S.; Rajabkadiev, R.M. Immunomodulating effects of using L-carnitine and coenzyme Q(10) in the nutrition of junior athletes. Vopr. Pitan. 2019, 88, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Brauner, H.; Lüthje, P.; Grünler, J.; Ekberg, N.; Dallner, G.; Brismar, K.; Brauner, A. Markers of innate immune activity in patients with type 1 and type 2 diabetes mellitus and the effect of the anti-oxidant coenzyme Q 10 on inflammatory activity. Clin. Exp. Immunol. 2014, 177, 478–482. [Google Scholar] [CrossRef]
- Barbieri, B.; Lund, B.; Lundström, B.; Scaglione, F. Coenzyme Q10 administration increases antibody titer in hepatitis B vaccinated volunteers—A single blind placebo-controlled and randomized clinical study. Biofactors 1999, 9, 351–357. [Google Scholar] [CrossRef]
- Farough, S.; Karaa, A.; Walker, M.; Slate, N.; Dasu, T.; Verbsky, J.; Fusunyan, R.; Canapari, C.; Kinane, T.; Van Cleave, J. Coenzyme Q10 and immunity: A case report and new implications for treatment of recurrent infections in metabolic diseases. Clin. Immunol. 2014, 155, 209–212. [Google Scholar] [CrossRef]
- Zhai, J.; Bo, Y.; Lu, Y.; Liu, C.; Zhang, L. Effects of coenzyme Q10 on markers of inflammation: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0170172. [Google Scholar]
- Barden, A.E.; Shinde, S.; Burke, V.; Puddey, I.B.; Beilin, L.J.; Irish, A.B.; Watts, G.F.; Mori, T.A. The effect of n-3 fatty acids and coenzyme Q10 supplementation on neutrophil leukotrienes, mediators of inflammation resolution and myeloperoxidase in chronic kidney disease. Prostaglandins Other Lipid Mediat. 2018, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farsi, F.; Mohammadshahi, M.; Alavinejad, P.; Rezazadeh, A.; Zarei, M.; Engali, K.A. Functions of coenzyme Q10 supplementation on liver enzymes, markers of systemic inflammation, and adipokines in patients affected by nonalcoholic fatty liver disease: A double-blind, placebo-controlled, randomized clinical trial. J. Am. Coll. Nutr. 2016, 35, 346–353. [Google Scholar] [CrossRef]
- Lee, B.-J.; Tseng, Y.-F.; Yen, C.-H.; Lin, P.-T. Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: A randomized, placebo-controlled trial. Nutr. J. 2013, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, E.; Jamilian, M.; Samimi, M.; Zarezade Mehrizi, M.; Aghadavod, E.; Akbari, E.; Tamtaji, O.R.; Asemi, Z. The effects of coenzyme Q10 supplementation on gene expression related to insulin, lipid and inflammation in patients with polycystic ovary syndrome. Gynecol. Endocrinol. 2018, 34, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Alexander, J.; Aaseth, J.; Larsson, A. Decrease in inflammatory biomarker concentration by intervention with selenium and coenzyme Q10: A subanalysis of osteopontin, osteoprotergerin, TNFr1, TNFr2 and TWEAK. J. Inflamm. 2019, 16, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Feng, Y.; Chen, G.-C.; Qin, L.-Q.; Fu, C.-l.; Chen, L.-H. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017, 119, 128–136. [Google Scholar] [CrossRef]
- Mohanty, A.; Tiwari-Pandey, R.; Pandey, N.R. Mitochondria: The indispensable players in innate immunity and guardians of the inflammatory response. J. Cell Commun. Signal. 2019, 13, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, C.; Horner, S.M. MAVS coordination of antiviral innate immunity. J. Virol. 2015, 89, 6974–6977. [Google Scholar] [CrossRef] [Green Version]
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011, 11, 389–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsing, L.C.; Rudensky, A.Y. The lysosomal cysteine proteases in MHC class II antigen presentation. Immunol. Rev. 2005, 207, 229–241. [Google Scholar] [CrossRef]
- Schmid, D.; Münz, C. Immune surveillance of intracellular pathogens via autophagy. Cell Death Differ. 2005, 12, 1519–1527. [Google Scholar] [CrossRef]
- Radoja, S.; Frey, A.B.; Vukmanovic, S. T-cell receptor signaling events triggering granule exocytosis. Crit. Rev. Immunol. 2006, 26, 265–290. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, J.A.; Lim, M.J.; Cooper, J.D.; Pearce, D.A. Immune system irregularities in lysosomal storage disorders. Acta Neuropathol. 2008, 115, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Mindell, J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gille, L.; Nohl, H. The existence of a lysosomal redox chain and the role of ubiquinone. Arch. Biochem. Biophys. 2000, 375, 347–354. [Google Scholar] [CrossRef]
- Heaton, R.A.; Heales, S.; Rahman, K.; Sexton, D.W.; Hargreaves, I. The Effect of Cellular Coenzyme Q(10) Deficiency on Lysosomal Acidification. J. Clin. Med. 2020, 9, 1923. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dellibovi-Ragheb, T.A.; Kerviel, A.; Pak, E.; Qiu, Q.; Fisher, M.; Takvorian, P.M.; Bleck, C.; Hsu, V.; Fehr, A.R. β-Coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell 2020, 183, 1520–1535. [Google Scholar] [CrossRef]
- Di Cara, F.; Sheshachalam, A.; Braverman, N.E.; Rachubinski, R.A.; Simmonds, A.J. Peroxisome-mediated metabolism is required for immune response to microbial infection. Immunity 2017, 47, 93–106.e107. [Google Scholar] [CrossRef] [Green Version]
- Odendall, C.; Dixit, E.; Stavru, F.; Bierne, H.; Franz, K.M.; Durbin, A.F.; Boulant, S.; Gehrke, L.; Cossart, P.; Kagan, J.C. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nat. Immunol. 2014, 15, 717. [Google Scholar] [CrossRef]
- Crane, F.L.; Sun, I.L.; Sun, E.; Morré, D.J. Alternative functions for coenzyme Q in endomembranes. In Biomedical and Clinical Aspects of Coenzyme Q; Folkers, K., Littarru, G.P., Yamagami, T., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991; pp. 59–70. [Google Scholar]
- Tekle, M.; Bentinger, M.; Nordman, T.; Appelkvist, E.-L.; Chojnacki, T.; Olsson, J.M. Ubiquinone biosynthesis in rat liver peroxisomes. Biochem. Biophys. Res. Commun. 2002, 291, 1128–1133. [Google Scholar] [CrossRef]
- Folkers, K.; Osterborg, A.; Nylander, M.; Morita, M.; Mellstedt, H. Activities of vitamin Q10 in animal models and a serious deficiency in patients with cancer. Biochem. Biophys. Res. Commun. 1997, 234, 296–299. [Google Scholar] [CrossRef] [Green Version]
- Folkers, K. Relevance of the Biosynthesis of Coenzyme Q10 and of the Four Bases of DNA as a Rationale for the Molecular Causes of Cancer and a Therapy. Biochem. Biophys. Res. Commun. 1996, 224, 358–361. [Google Scholar] [CrossRef]
- Jolliet, P.; Simon, N.; Barre, J.; Pons, J.; Boukef, M.; Paniel, B.; Tillement, J. Plasma coenzyme Q10 concentrations in breast cancer: Prognosis and therapeutic consequences. Int. J. Clin. Pharmacol. Ther. 1998, 36, 506–509. [Google Scholar] [PubMed]
- Chai, W.; Cooney, R.V.; Franke, A.A.; Caberto, C.P.; Wilkens, L.R.; Le Marchand, L.; Goodman, M.T.; Henderson, B.E.; Kolonel, L.N. Plasma coenzyme Q10 levels and prostate cancer risk: The multiethnic cohort study. Cancer Epidemiol. Prev. Biomark. 2011, 20, 708–710. [Google Scholar] [CrossRef] [Green Version]
- Reichenbach, J.; Schubert, R.; Schwan, C.; Zielen, S. Antioxidative Capacity in Patients with CommonVariable Immunodeficiency. J. Clin. Immunol. 2000, 20, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Littarru, G.; Lippa, S.; Oradei, A.; Fiorni, R.; Mazzanti, L. Metabolic and diagnostic implications of blood CoQ10 levels. Biomed. Clin. Asp. Coenzyme Q 1991, 6, 167–178. [Google Scholar]
- Kumari, N.; Dwarakanath, B.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Hodges, S.; Hertz, N.; Lockwood, K.; Lister, R. CoQ10: Could it have a role in cancer management? Biofactors 1999, 9, 365–370. [Google Scholar] [CrossRef]
- Folkers, K.; Brown, R.; Judy, W.V.; Morita, M. Survival of cancer patients on therapy with coenzyme Q10. Biochem. Biophys. Res. Commun. 1993, 192, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, K.; Moesgaard, S.; Folkers, K. Partial and complete regression of breast cancer in patients in relation to dosage of coenzyme Q10. Biochem. Biophys. Res. Commun. 1994, 199, 1504–1508. [Google Scholar] [CrossRef]
- Yang, H.-L.; Lin, M.-W.; Korivi, M.; Wu, J.-J.; Liao, C.-H.; Chang, C.-T.; Liao, J.-W.; Hseu, Y.-C. Coenzyme Q0 regulates NFκB/AP-1 activation and enhances Nrf2 stabilization in attenuation of LPS-induced inflammation and redox imbalance: Evidence from in vitro and in vivo studies. Biochim. et Biophys. Acta (bba)-Gene Regul. Mech. 2016, 1859, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. Combination therapy with glucan and coenzyme Q10 in murine experimental autoimmune disease and cancer. Anticancer Res. 2018, 38, 3291–3297. [Google Scholar] [CrossRef] [Green Version]
- Iarussi, D.; Auricchio, U.; Agretto, A.; Murano, A.; Giuliano, M.; Casale, F.; Indolfi, P.; Iacono, A. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: Control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Mol. Asp. Med. 1994, 15, s207–s212. [Google Scholar] [CrossRef]
- Judy, W.; Hall, J.; Dugan, W.; Toth, P.; Folkers, K. Coenzyme Q10 reduction of adriamycin cardiotoxicity. Biomed. Clin. Asp. Coenzyme Q 1984, 4, 231–241. [Google Scholar]
- Beyer, R.; Ernster, L. The Antioxidant Role of Coenzyme Q; Taylor and Francis: London, UK, 1990; pp. 191–213. [Google Scholar]
- Yakin, M.; Eksioglu, U.; Sadic, M.; Koca, G.; Ozkan-Uney, G.; Yumusak, N.; Husniye Telek, H.; Demir, A.; Yazihan, N.; Ornek, F. Coenzyme Q10 for the protection of lacrimal gland against high-dose radioiodine therapy-associated oxidative damage: Histopathologic and tissue cytokine level assessments in an animal model. Curr. Eye Res. 2017, 42, 1590–1596. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Said, R.S. Coenzyme Q10 attenuates inflammation and fibrosis implicated in radiation enteropathy through suppression of NF-kB/TGF-β/MMP-9 pathways. Int. Immunopharmacol. 2021, 92, 107347. [Google Scholar] [CrossRef]
- Li, T.-Y.; Chiang, B.-H. 4-Acetylantroquinonol B from Antrodia cinnamomea enhances immune function of dendritic cells against liver cancer stem cells. Biomed. Pharmacother. 2019, 109, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, S.L.; Young, J.M.; Florkowski, C.M.; Lever, M.; George, P.M. Coenzyme Q10: Is there a clinical role and a case for measurement? Clin. Biochem. Rev. 2008, 29, 71. [Google Scholar]
- Yubero, D.; Montero, R.; Artuch, R.; Land, J.M.; Heales, S.J.; Hargreaves, I.P. Biochemical diagnosis of coenzyme Q10 deficiency. Mol. Syndromol. 2014, 5, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Shults, C.W.; Beal, M.F.; Song, D.; Fontaine, D. Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson’s disease. Exp. Neurol. 2004, 188, 491–494. [Google Scholar] [CrossRef]
- Hidaka, T.; Fujii, K.; Funahashi, I.; Fukutomi, N.; Hosoe, K. Safety assessment of coenzyme Q10 (CoQ10). Biofactors 2008, 32, 199–208. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; del Pozo-Cruz, J.; Sánchez-Cuesta, A.; Cortés-Rodríguez, A.B.; Navas, P. Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition 2019, 57, 133–140. [Google Scholar] [CrossRef]
- Bhagavan, H.N.; Chopra, R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 2007, 7, S78–S88. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.V.; Patterson, B.J.; Schapiro, M.B.; Hickey, F.J.; Chalfonte-Evans, M.; Horn, P.S.; Hotze, S.L. Coenzyme Q10 absorption and tolerance in children with Down syndrome: A dose-ranging trial. Pediatric Neurol. 2006, 35, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, J.; Nyyssönen, K.; Tomasi, A.; Iannone, A.; Tuomainen, T.-P.; Porkkala-Sarataho, E.; Salonen, J.T. Antioxidative efficacy of parallel and combined supplementation with coenzyme Q10 and d-α-tocopherol in mildly hypercholesterolemic subjects: A randomized placebo-controlled clinical study. Free Radic. Res. 2000, 33, 329–340. [Google Scholar] [CrossRef]
- Sanoobar, M.; Eghtesadi, S.; Azimi, A.; Khalili, M.; Khodadadi, B.; Jazayeri, S.; Gohari, M.R.; Aryaeian, N. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: A double blind, placebo, controlled randomized clinical trial. Nutr. Neurosci. 2015, 18, 169–176. [Google Scholar] [CrossRef]
- Wainwright, L.; Hargreaves, I.P.; Georgian, A.R.; Turner, C.; Dalton, R.N.; Abbott, N.J.; Heales, S.J.; Preston, J.E. CoQ10 Deficient Endothelial Cell Culture Model for the Investigation of CoQ10 Blood–Brain Barrier Transport. J. Clin. Med. 2020, 9, 3236. [Google Scholar] [CrossRef]
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Dahlström, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013, 167, 1860–1866. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P.; Investigators, Q.-S.S. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef]
- Mantle, D. Coenzyme Q10 supplementation for diabetes and its complications: An overview. Br. J. Diabetes 2017, 17, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Mantle, D.; Hargreaves, I.P. Coenzyme Q10 supplementation in non-alcoholic fatty liver disease: An overview. J. Prescr. Pract. 2020, 2, 200–204. [Google Scholar] [CrossRef]
- Hargreaves, I.; Mantle, D.; Milford, D. Chronic kidney disease and coenzyme Q10 supplementation. J. Kidney Care 2019, 4, 82–90. [Google Scholar] [CrossRef]
- Mantle, D.; Hargreaves, I.P. Ataxia and coenzyme Q10: An overview. Br. J. Neurosci. Nurs. 2018, 14, 108–114. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10 and Immune Function: An Overview. Antioxidants 2021, 10, 759. https://doi.org/10.3390/antiox10050759
Mantle D, Heaton RA, Hargreaves IP. Coenzyme Q10 and Immune Function: An Overview. Antioxidants. 2021; 10(5):759. https://doi.org/10.3390/antiox10050759
Chicago/Turabian StyleMantle, David, Robert A. Heaton, and Iain P. Hargreaves. 2021. "Coenzyme Q10 and Immune Function: An Overview" Antioxidants 10, no. 5: 759. https://doi.org/10.3390/antiox10050759
APA StyleMantle, D., Heaton, R. A., & Hargreaves, I. P. (2021). Coenzyme Q10 and Immune Function: An Overview. Antioxidants, 10(5), 759. https://doi.org/10.3390/antiox10050759





