Oxidative Stability, Microbial Safety, and Sensory Properties of Flaxseed (Linum usitatissimum L.) Oil Infused with Spices and Herbs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reagents and Standards
2.3. Preparation of Flaxseed Oil Samples Infused with Herbs and Spices
2.4. Determination of Oil Quality Parameters
2.5. HPLC Analysis of Polyphenolic Compounds in Oil Samples
2.6. Determination of Antioxidant Activity in Oil Samples with the 2,2′-Diphenyl-1-picrylhydrazyl (DPPH) Method
2.7. Microbiological Analysis of Oil Samples
2.8. Sensory Analysis of Oil Samples
2.9. Statistical Analysis
3. Results and Discussion
3.1. Oil Quality
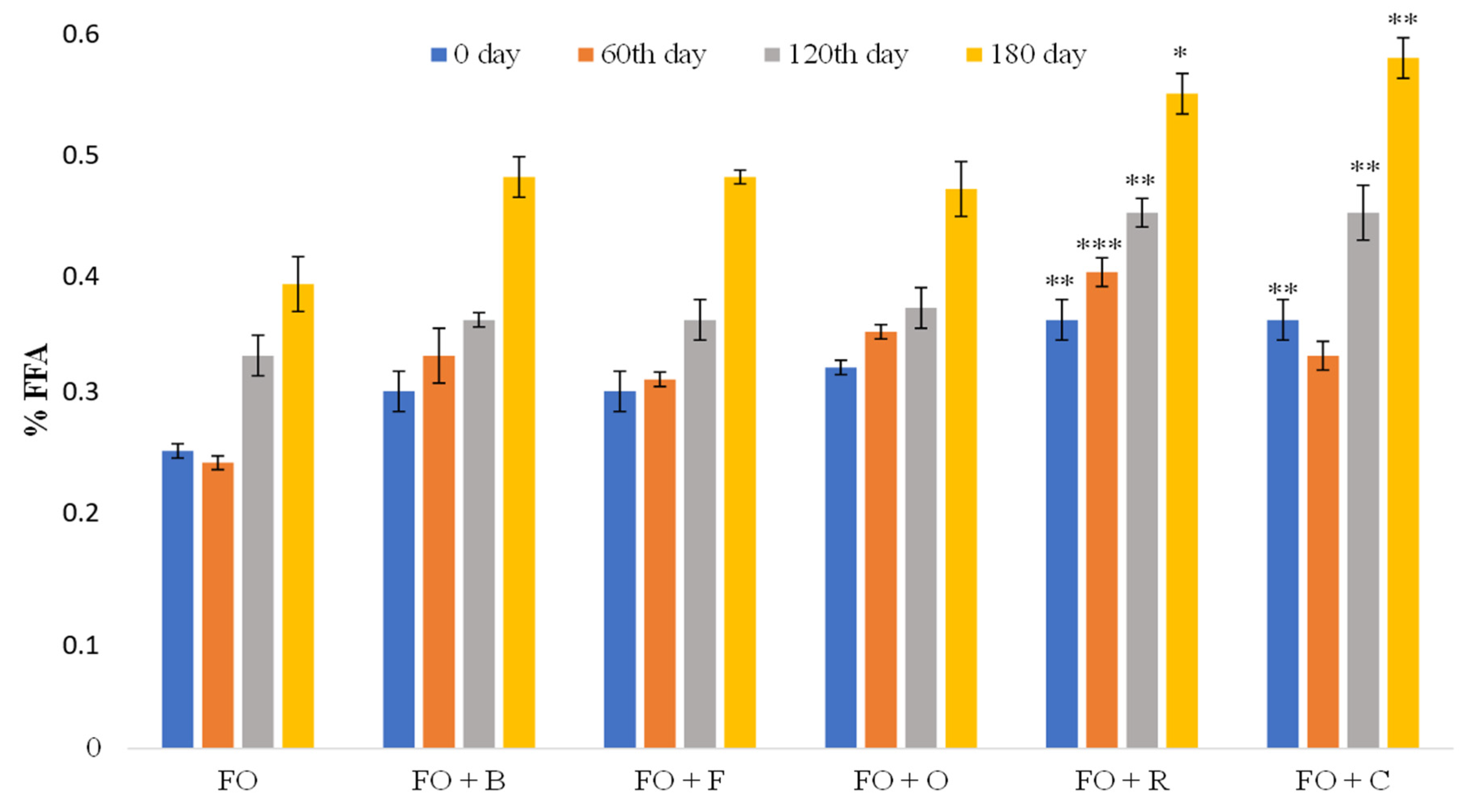
3.1.1. Content of Free Fatty Acids (FFAs)
3.1.2. Changes in Peroxide Value (PV)
3.2. Content and Composition of Polyphenols and DPPH Radical-Scavenging Activity of Oil Samples
3.3. Microbial Safety of the Oil Samples
3.4. Sensory Properties of Oil Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Przybylski, R. Flax oil and high linolenic oils. In Bailey’s Industrial Oil and Fat Products, Edible Oil and Fat Products: Edible Oils, 6th ed.; Shahidi, F., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2005; Volume 1, pp. 289–292. [Google Scholar]
- Koshta, N.; Yadav, P.; Tetwar, S. Omega-3 in linseed and its role in human diet. Asian J. Biol. Sci. 2014, 9, 104–108. [Google Scholar]
- Žanetić, M.; Gugić, M. Čuvanje djevičanskog maslinovog ulja. Pomol. Croat. 2005, 11, 31–41. [Google Scholar]
- Šarkanj, B.; Kipčić, D.; Vašić-Rački, Ð.; Delaš, F.; Galić, K.; Katalenić, M.; Dimitrov, N.; Klapec, T. Kemijske i Fizikalne Opasnosti u Hrani (Chemical and Physical Hazards in Food), 1st ed.; Hrvatska Agencija za Hranu: Osijek, Croatia, 2010; pp. 119–121.
- Rade, D.; Mokrovčak, Ž.; Štrucelj, D. Priručnik za Vježbe Kemije i Tehnologije Lipida (Handbook of Lipid Chemistry and Technology), 1st ed.; Durieux: Zagreb, Croatia, 2001; pp. 1–71. [Google Scholar]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Oxidative stability of flaxseed oil: Effect of hydrophilic, hydrophobic and intermediate polarity antioxidants. Food Chem. 2018, 266, 524–533. [Google Scholar] [CrossRef]
- Horbańczuk, O.K.; Kurek, M.A.; Atanasov, A.G.; Brnčić, M.; Rimac Brnčić, S. The Effect of Natural Antioxidants on Quality and Shelf Life of Beef and Beef Products. Food Technol. Biotechnol. 2019, 57, 439–447. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Pezeshk, S.; Ojagh, S.M.; Alishahi, A. Effect of Plant Antioxidant and Antimicrobial Compounds on the Shelf-life of Seafood—A Review. Czech. J. Food. Sci. 2015, 33, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Chathuri, S.M.; Harshani Algama, C.; Wimalasekara, R.L.; Weerakoon, W.N.M.T.D.N.; Jayathilaka, N.; Seneviratne, K.N. Improvement of Oxidative Stability and Microbial Shelf Life of Vanilla Cake by Coconut Oil Meal and Sesame Oil Meal Phenolic Extracts. J. Food Qual. 2019, 2019, 1–8. [Google Scholar]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [Green Version]
- Blasi, F.; Cossignani, L. An Overview of Natural Extracts with Antioxidant Activity for the Improvement of the Oxidative Stability and Shelf Life of Edible Oils. Processes 2020, 8, 956. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis L.): A review. Medicines 2018, 3, 98. [Google Scholar] [CrossRef] [Green Version]
- Taghvaei, M.; Jafari, S.M. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J. Food Sci. Technol. 2015, 52, 1272–1282. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, R.N.; Moura, L.S.; Rosa, P.T.V.; Meireles, M.A.A. Supercritical fluid extraction from rosemary (Rosmarinus officinalis): Kinetic data, extract’s global yield, composition, and antioxidant activity. J. Supercrit. Fluids 2005, 35, 197–204. [Google Scholar] [CrossRef]
- Lu, T.; Shen, Y.; Wang, J.H.; Xie, H.K.; Wang, Y.F.; Zhao, Q.; Zhou, D.Y.; Shahidi, F. Improving oxidative stability of flaxseed oil with a mixture of antioxidants. J. Food Process. Preserv. 2019, e14355. [Google Scholar] [CrossRef]
- Kalogianni, A.I.; Lazou, T.; Bossis, I.; Gelasakis, A.I. Natural Phenolic Compounds for the Control of Oxidation, Bacterial Spoilage, and Foodborne Pathogens in Meat. Foods 2020, 9, 794. [Google Scholar] [CrossRef]
- Pop, A.; Muste, S.; Păucean, A.; Chis, S.; Man, S.; Salanță, L.; Marc, R.; Mureșan, A.; Marțiș, G. herbs and spices in terms of food preservation and shelf life. Hop. Med. Plants 2019, 1, 57–65. [Google Scholar]
- Gottardi, D.; Bukvicki, D.; Prasad, S.; Tyagi, A.K. Beneficial Effects of Spices in Food Preservation and Safety. Front. Microbiol. 2016, 7, 1394. [Google Scholar] [CrossRef] [Green Version]
- Dadalioglu, I.; Evrendilek, G.A. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8255–8260. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. Biomed. Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [Green Version]
- Paduano, A.; Caporaso, N.; Santini, A.; Sacchi, R. Microwave and Ultrasound-Assisted Extraction of Capsaicinoids From Chili Peppers (Capsicum annuum L.) in Flavored Olive Oil. J. Food Res. 2014, 3, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Gad, A.S.; Sayd, A.F. Antioxidant properties of rosemary and its potential uses as natural antioxidant in dairy products—A review. Food Sci. Nutr. 2015, 6, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Duraković, S. Prehrambena Mikrobiologija (Nutritional Microbiology), 1st ed.; Medicinska naklada: Zagreb, Croatia, 1991; pp. 1–297. [Google Scholar]
- Lorenzo, J.M.; Munekata, P.E.S.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main groups of microorganisms of relevance for food safety and stability: General aspects and overall description. In Innovative Technologies for Food Preservation Inactivation of Spoilage and Pathogenic Microorganisms, 1st ed.; Barba, F.J., Sant’Ana, A.S., Orlie, V., Koubaa, M., Eds.; Academic Press: London, UK, 2017; pp. 53–107. [Google Scholar]
- Duraković, S.; Delaš, F.; Duraković, L. Moderna mikrobiologija namirnica–knjiga prva (Modern Food Microbiology–Book one), 1st ed.; Kugler: Zagreb, Croatia, 2002; pp. 1–442. [Google Scholar]
- Škevin, D.; Neđeral, S.; Kraljić, K.; Petričević, S.; Rade, D. The influence of rosemary leaves addition procedure on the properties of virgin olive oil during storage. In Proceedings of the 6th Croatian Congress of Food Technologists, Biotechnologists and Nutritionists, Cavtat, Croatia, 15–17 May 2008; Ćurić, D., Ed.; Croatian Chamber of Economy: Zagreb, Croatia, 2008; pp. 155–163. [Google Scholar]
- International Organization for Standardization (ISO). Animal and Vegetable Fats and Oils–Determination of Acid Value and Acidity; ISO 660:2020; ISO: Geneva, Switzerland, 2020. [Google Scholar]
- International Organization for Standardization (ISO). Animal and Vegetable Fats and Oils-Determination of Peroxide Value–Iodometric (Visual) Endpoint Determination; ISO 3960:2017; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Kraljić, K.; Škevin, D.; Pospišil, M.; Obranović, M.; Neđeral, S.; Bosolt, T. Quality of rapeseed oil produced by conditioning seeds at modest temperatures. J. Am. Oil Chem. Soc. 2013, 90, 589–599. [Google Scholar] [CrossRef]
- Panić, M.; Radić Stojković, M.; Kraljić, K.; Škevin, D.; Radojčić Redovniković, I.; Gaurina Srček, V.; Radošević, K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Kalantzakis, G.; Blekas, G.; Pegklidou, K.; Boskou, D. Stability and radical scavenging activity of heated olive oil and other vegetable oils. Eur. J. Lipid Sci. Technol. 2006, 108, 329–335. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Microbiology of the Food Chain-Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria Spp.-Part. 1: Detection Method; ISO 11290-1:2017; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- International Organization for Standardization (ISO). Microbiology of the Food Chain–Horizontal Method for the Enumeration of Microorganisms—Part. 1: Colony Count at 30 Degrees C by the Pour Plate Technique; ISO 4833-1:2013; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- International Organization for Standardization (ISO). Microbiology of Food and Animal Feeding Stuffs–Horizontal Method for the Enumeration of Yeasts and Moulds—Part. 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0,95; ISO 21527-2:2008; ISO: Geneva, Switzerland, 2008. [Google Scholar]
- International Organization for Standardization (ISO). Microbiology of the Food Chain–Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part. 2: Colony-Count Technique; ISO 21528-2:2017; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Meilgaard, M.C.; Vance Civille, G.; Carr, B.T. Sensory Evaluation Techniques, 3rd ed.; CRE Press Inc.: London, UK, 1991; pp. 59–122. [Google Scholar]
- International Organization for Standardization (ISO). Sensory Analysis-Methodology-Method of Investigating Sensitivity of Taste; ISO 3972:2011; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- International Organization for Standardization (ISO). Sensory Analysis-Methodology-Triangle Test; ISO 4120:2004; ISO: Geneva, Switzerland, 2004. [Google Scholar]
- International Organization for Standardization (ISO). Sensory Analysis-Methodology-Ranking; ISO 8587:2006; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- International Organization for Standardization (ISO). Sensory Analysis-Methodology-Sequential Analysis; ISO 16820:2019; ISO: Geneva, Switzerland, 2019. [Google Scholar]
- Abuzaytoun, R.; Shahidi, F. Oxidative stability of flax and hemp oil. J. Am. Oil Chem. Soc. 2006, 83, 855–861. [Google Scholar] [CrossRef]
- Bozan, B.; Temelli, F. Chemical composition and oxidative stability of flax, safflower and poppy seed and seed oils. Bioresour. Technol. 2008, 99, 6354–6359. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Narodne, N. Pravilnik o Jestivim Uljima i Mastima (Regulation on Edible Oils and Fats); Narodne Novine: Zagreb, Croatia, 2019; 11/19. [Google Scholar]
- Codex Alimentarius. Codex Standard for Named Vegetable Oils; Codex-Stan 210; FAO/WHO: Rome, Italy, 2013. [Google Scholar]
- de Almeida, D.T.; Viana, T.V.; Costa, M.M.; Silva, C.S.; Feitosa, S. Effects of different storage conditions on the oxidative stability of crude and refined palm oil, olein and stearin (Elaeis guineensis). Food Sci. Technol. 2019, 39, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Sharav, O.; Shim, Y.Y.; Okinyo-Owiti, D.P.; Sammynaiken, R.; Reaney, M.J.T. Effect of cyclolinopeptides on the oxidative stability of flaxseed oil. J. Agric. Food Chem 2014, 62, 88–96. [Google Scholar] [CrossRef]
- Juita, J.; Dlugogorski, B.Z.; Kennedy, E.M.; Mackie, J.C. Low temperature oxidation of linseed oil: A review. Fire Sci. Rev. 2012, 1, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Gambacorta, G.; Faccia, M.; Pati, S.; Lamacchia, C.; Baiano, A.; La Notte, E. Changes in the chemicaland sensorial profile of extravirgin olive oils flavored with herbs and spices during storage. J. Food Lipids 2007, 14, 202–215. [Google Scholar] [CrossRef]
- Hasiewicz-Derkacz, K.; Kulma, A.; Czuj, T.; Prescha, A.; Zuk, M.; Grajzer, M.; Lukaszewicz, M.; Szopa, J. Natural phenolics greatly increase flax (Linum usitatissimum) oil stability. BMC Biotechnol 2015, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.J. Optimizing lipid stability with natural antioxidants. In Natural Antioxidants, Chemistry, Health Effects, and Applications, 1st ed.; Shahidi, F., Ed.; AOCS Press: Champaign, IL, USA, 1997; Volume 13, pp. 224–244. [Google Scholar]
- Parkash Kochhar, S. Stabilisation of frying oils with natural antioxidative components. Eur. J. Lipid Sci. Technol. 2000, 102, 552–559. [Google Scholar] [CrossRef]
- Dameckhi, M.; Sotriopolou, S.; Tsimidou, M. Antioxidant and pro-oxidant factors in oregano, rosemary gourmet olive oils. Grasas. y Aceites. 2001, 52, 207–213. [Google Scholar]
- De Leonardis, A.; Macciola, V.; Di Rocco, A. Oxidative stabilization of cold pressed sunflower oil using phenolic compounds of the same seeds. J. Sci. Food Agric. 2003, 83, 523–528. [Google Scholar] [CrossRef]
- Persson, E.; Graziani, R.; Ferracane, R.; Fogliano, V.; Skog, K. Influence of antioxidants in virgin olive oil on the formation of heterocyclic amines in fried beefburgers. Food Chem. Toxicol 2003, 41, 1587–1597. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.; Pokorný, J. Natural antioxidants from herbs and spices. Eur. J. Lipid Sci. Technol. 2006, 108, 776–793. [Google Scholar] [CrossRef]
- Ocakoglu, D.; Tokatli, F.; Ozen, B.; Korel, F. Distribution of simple phenols, phenolic acids and flavonoids in Turkish monovarietal extra virgin olive oils for two harvest years. Food Chem. 2009, 113, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Al-Maskari, M.Y.; Hanif, M.A.; Al-Maskri, A.Y.; Al-Adawi, S. Basil: A natural source of antioxidants and neutraceuticals. In Natural Products and Their Active Compounds on Disease Prevention, 1st ed.; Essa, M.M., Manickavasagan, A., Sukumar, E., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2012; pp. 463–471. [Google Scholar]
- Deng, M.; Deng, Y.; Dong, L.; Ma, Y.; Liu, L.; Huang, F.; Wei, Z.; Zhang, Y.; Zhang, M.; Zhang, R. Effect of Storage Conditions on Phenolic Profiles and Antioxidant Activity of Litchi Pericarp. Molecules 2018, 23, 2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del-Toro-Sanchez, C.L.; Gutiérrez-Lomelí, M.; Lugo-Cervantes, E.; Zurita, F.; Robles-García, M.A.; Ruiz-Cruz, S.; Aguilar, J.A.; Morales-Del Rio, J.A.; Guerrero-Medina, P.J. Storage effect on phenols and on the antioxidant activity of extracts from Anemopsis californica and inhibition of elastase enzyme. J. Chem. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic Acid and Carnosol, Two Major Antioxidants of Rosemary, Act through Different Mechanisms. Plant. Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siger, A.; Nogala-Kalucka, M.; Lampart-Szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Schwarz, K.; Bertelsen, G.; Nissen, L.R.; Gardner, P.T.; Heinonen, M.I.; Hopia, A.; Huynh- Ba, T.; Lambelet, P.; McPhail, D.; Skibsted, L.H.; et al. Investigation of plant extracts for the protection of processed foods against lipid oxidation. Comparison of antioxidant assay based on radical scavenging, lipid oxidation and analysis of the principal antioxidant compounds. Eur. Food Res. Technol. 2001, 21, 319–328. [Google Scholar] [CrossRef]
- Vodič za Mikrobiološke Kriterije za Hranu, 3. Izmijenjeno Izdanje. 2011. Ministarstvo Poljoprivrede, Ribarstva i Ruralnog Razvoja (Guideline on Microbiological Food Criteria of the Ministry of Agriculture of the Republic of Croatia, 2011). Available online: https://cdn.agroklub.com/upload/documents/vodic-za-mikrobioloske-kriterije-za-hranu.pdf (accessed on 14 March 2020).
- Official Journal of the European Union. European Commission Regulation 2073/2005 on Microbiological Criteria for Foodstuffs; Official Journal of the European Union: Brussels, Belgium, 2005. [Google Scholar]
- Ferracane, R.; Tafuri, A.; Logieco, A.; Galvano, F.; Balzano, D.; Ritieni, A. Simultaneous determination of aflatoxin B1 and ochratoxin A and natural occurrence in Mediterranean virgin olive oil. Food Addit. Contam. 2007, 24, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oršolić, N.; Jembrek, M.J.; Terzi’c, S. Honey and Quercetin Reduce Ochratoxin A-Induced Dna Damage in the Liver and the Kidney through the Modulation of Intestinal Microflora. Food Agric. Immunol. 2017, 28, 812–833. [Google Scholar] [CrossRef] [Green Version]
- Bažok, R.; Ðugum, J.; Grbeša, D.; Hadžiosmanovic, M.; Havranek, J.; Ivankovic, A.; Jakopovic, I.; Oreškovic, S.; Rupic, V.; Samaržija, D.; et al. Sigurnost Hrane: Od Polja do Stola, 1st ed.; M.E.P. d.o.o.: Zgreb, Croatia, 2014; pp. 1–454. [Google Scholar]
- Ciafardini, G.; Zullo, B.A.; Peca, G. Presence of microorganisms in flavoured extra virgin olive oil. Ann. Microbiol. 2004, 54, 161–168. [Google Scholar]
- Giese, J. Spices and seasoning blends: A taste for all seasons. Food Technol. 1994, 48, 87–98. [Google Scholar]
- Kneifel, W.; Berger, E. Microbial criteria of random samples of spices and herbs retailed on the Austrian market. J. Food Prot. 1994, 57, 893–901. [Google Scholar] [CrossRef]
- Gobena, W.; Girma, S.; Legesse, T.; Abera, F.; Gonfa, A.; Muzeyin, R.; Fekade, R.; Yohannes, T. Microbial safety and quality of edible oil examined at Ethiopian public health institute, Addis Ababa, Ethiopia: A retrospective study. J. Microbiol. Exp. 2018, 6, 136–139. [Google Scholar] [CrossRef] [Green Version]
- Tesfaye, L.; Sahile, S.; Madhusudhan, A. Microbial quality and chemical characteristics evaluation of edible oil sold at Gondar Town Markets, North West Ethiopia. Int. J. Mod. Chem. Appl. Sci. 2015, 2, 238–247. [Google Scholar]
- Bruhl, L.; Matthaus, B.; Fehling, E.; Wiege, B.; Lehmann, B.; Luftmann, H.; Bergander, K.; Quiroga, K.; Scheipers, A.; Frank, O.; et al. Identification of Bitter Off-Taste Compounds in the Stored Cold Pressed Linseed Oil. J. Agric. Food Chem. 2007, 55, 7864–7868. [Google Scholar] [CrossRef] [PubMed]
- Antoun, N.; Tsimidou, M. Gourmet olive oils: Stability and consumer acceptability studies. Food Res. Int. 1997, 30, 131–136. [Google Scholar] [CrossRef]


| Polyphenol (mg/kg) | FO | FO + B | FO + F | FO + O | FO + R | FO + C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Day | 120th Day | 180th Day | 0 Day | 120th Day | 180th Day | 0 Day | 120th Day | 180th Day | 0 Day | 120th Day | 180th Day | 0 Day | 120th Day | 180th Day | 0 Day | 120th Day | 180th Day | |
| Vanillic acid | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 3.47 ± 0.04 | nd | nd | nd | nd | nd |
| Vanillin | nd | nd | nd | 5.54 ± 0.01 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Rosmarinic acid | nd | nd | nd | 6.89 ± 1.99 | nd | nd | nd | nd | nd | 6.8 ± 1.05 | 5.87 ± 0.03 | nd | 5.57 ± 0.02 | 5.86 ± 0.03 | nd | nd | nd | nd |
| SDG | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 5. 55 ± 0.05 | nd | nd | nd | nd | nd |
| trans-cinnamic acid | nd | nd | nd | 2.89 ± 0.13 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| un.c. | 5.4 ± 0.31 | 5.62 ± 0.31 | 10.58 ± 4.9 | 7.56 ± 3.86 | 7.54 ± 4.63 | 11.4 ± 9.26 | 9.17 ± 5.10 | 8.74 ± 6.19 | 7.05 ± 1.54 | 12.88 ± 13.3 | 13.49 ± 15.7 | 9.53 ± 4.77 | 8.33 ± 2.92 | 8.86 ± 4.05 | 7.46 ± 4.43 | 6.81 ± 2.05 | 6.07 ± 1.19 | 6.29 ± 1.11 |
| Total polyphenols (mg/kg) | 5.44 | 5.62 | 10.58 | 22.88 ** | 7.54 | 11.47 | 9.17 | 8.74 * | 7.05 * | 19.68 * | 19.36 *** | 9.53 | 22.92 *** | 14.72 ** | 7.46 | 6.81 | 6.07 | 6.29 * |
| Experimental Oils a | Enterobacteriacae (CFU/mL) | Aerobic Mesophilic Bacteria (CFU/mL) | Molds (CFU/mL) | Listeria monocytogenes (CFU/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Day | 60th Day | 120th Day | 180th DAY | 0 Day | 60th Day | 120th Day | 180th Day | 0 Day | 60th Day | 120th Day | 180th Day | 0 Day | 60th Day | 120th Day | 180th Day | |
| FO | 1 | <1 | <1 | <1 | 10 | <1 | 5 | <1 | 3 | <1 | <1 | <1 | n.d. | n.d. | n.d. | n.d. |
| FO + B | 9 | <1 | 10 | <1 | 10 | 80 | 80 | 30 | 56 | 56 | 60 | 16 | n.d. | n.d. | n.d. | n.d. |
| FO + F | <1 | <1 | <1 | 2 | 5 | 2 | 10 | 4 | 2 | <1 | 7 | 6 | n.d. | n.d. | n.d. | n.d. |
| FO + O | 10 | <1 | 10 | 10 | 10 | 10 | 10 | 10 | 2 | 5 | 4 | 4 | n.d. | n.d. | n.d. | n.d. |
| FO + R | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | 1 | 3 | n.d. | n.d. | n.d. | n.d. |
| FO + C | 10 | <1 | <1 | <1 | 6 | 8 | 10 | 10 | 1 | 3 | <1 | 5 | n.d. | n.d. | n.d. | n.d. |
| Experimental Oils a | 0 Day (Score) | 60th Day (Score) | 120th Day (Score) | 180th Day (Score) | Total Score |
|---|---|---|---|---|---|
| FO | 32 | 28 | 19 | 19 | 98 |
| FO + F | 22 | 27 | 15 | 24 | 88 |
| FO + O | 26 | 19 | 16 | 24 | 85 |
| FO + R | 22 | 24 | 15 | 21 | 82 |
| FO + C | 23 | 33 | 22 | 37 | 115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odeh, D.; Kraljić, K.; Benussi Skukan, A.; Škevin, D. Oxidative Stability, Microbial Safety, and Sensory Properties of Flaxseed (Linum usitatissimum L.) Oil Infused with Spices and Herbs. Antioxidants 2021, 10, 785. https://doi.org/10.3390/antiox10050785
Odeh D, Kraljić K, Benussi Skukan A, Škevin D. Oxidative Stability, Microbial Safety, and Sensory Properties of Flaxseed (Linum usitatissimum L.) Oil Infused with Spices and Herbs. Antioxidants. 2021; 10(5):785. https://doi.org/10.3390/antiox10050785
Chicago/Turabian StyleOdeh, Dyana, Klara Kraljić, Andrea Benussi Skukan, and Dubravka Škevin. 2021. "Oxidative Stability, Microbial Safety, and Sensory Properties of Flaxseed (Linum usitatissimum L.) Oil Infused with Spices and Herbs" Antioxidants 10, no. 5: 785. https://doi.org/10.3390/antiox10050785
APA StyleOdeh, D., Kraljić, K., Benussi Skukan, A., & Škevin, D. (2021). Oxidative Stability, Microbial Safety, and Sensory Properties of Flaxseed (Linum usitatissimum L.) Oil Infused with Spices and Herbs. Antioxidants, 10(5), 785. https://doi.org/10.3390/antiox10050785