Dietary ω-3 Fatty Acid Supplementation Improves Murine Sickle Cell Bone Disease and Reprograms Adipogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Design of the Study
2.2. Measurements of Bone Homeostasis and Turnover
2.3. Bone Total RNA and microRNA Extraction and Reverse Transcription
2.4. Bone Immunohistochemistry and Bone Immune-Microscopy
2.5. Colony Forming Units Osteogenic and Adipogenic Assays
2.6. Total RNA Extraction and Reverse Transcription of CFU-Osteoblasts or CFU-Adipocytes
2.7. Statistical Analysis
3. Results
3.1. FD Supplementation Improves Bone Structure and Reduces Bone Turnover in Humanized Sickle Cell Mice
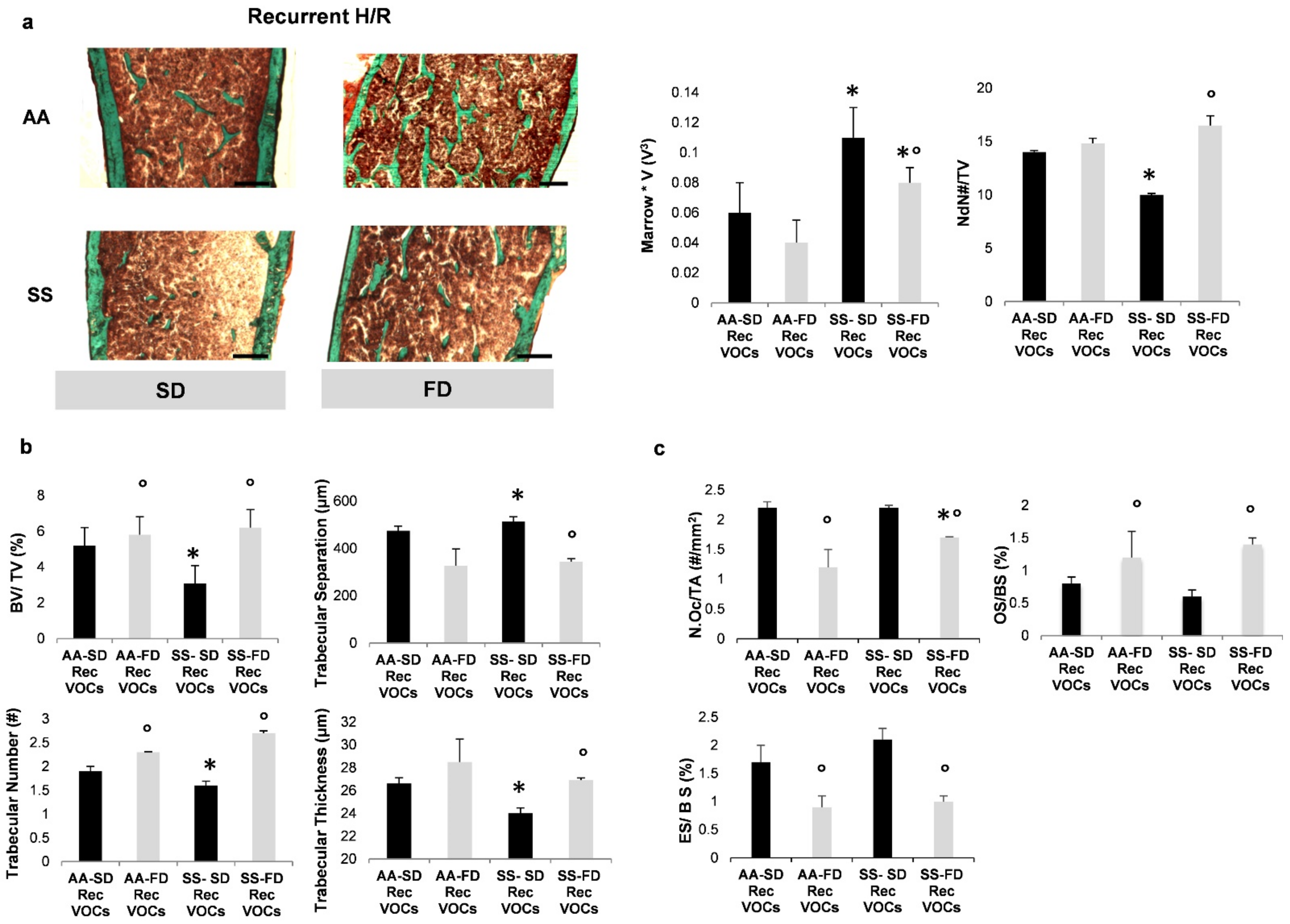
3.2. In SCD Mice Exposed to Recurrent Hypoxia/Reoxygenation, FD Protects Bone Microarchitecture and Reduces Bone Turnover
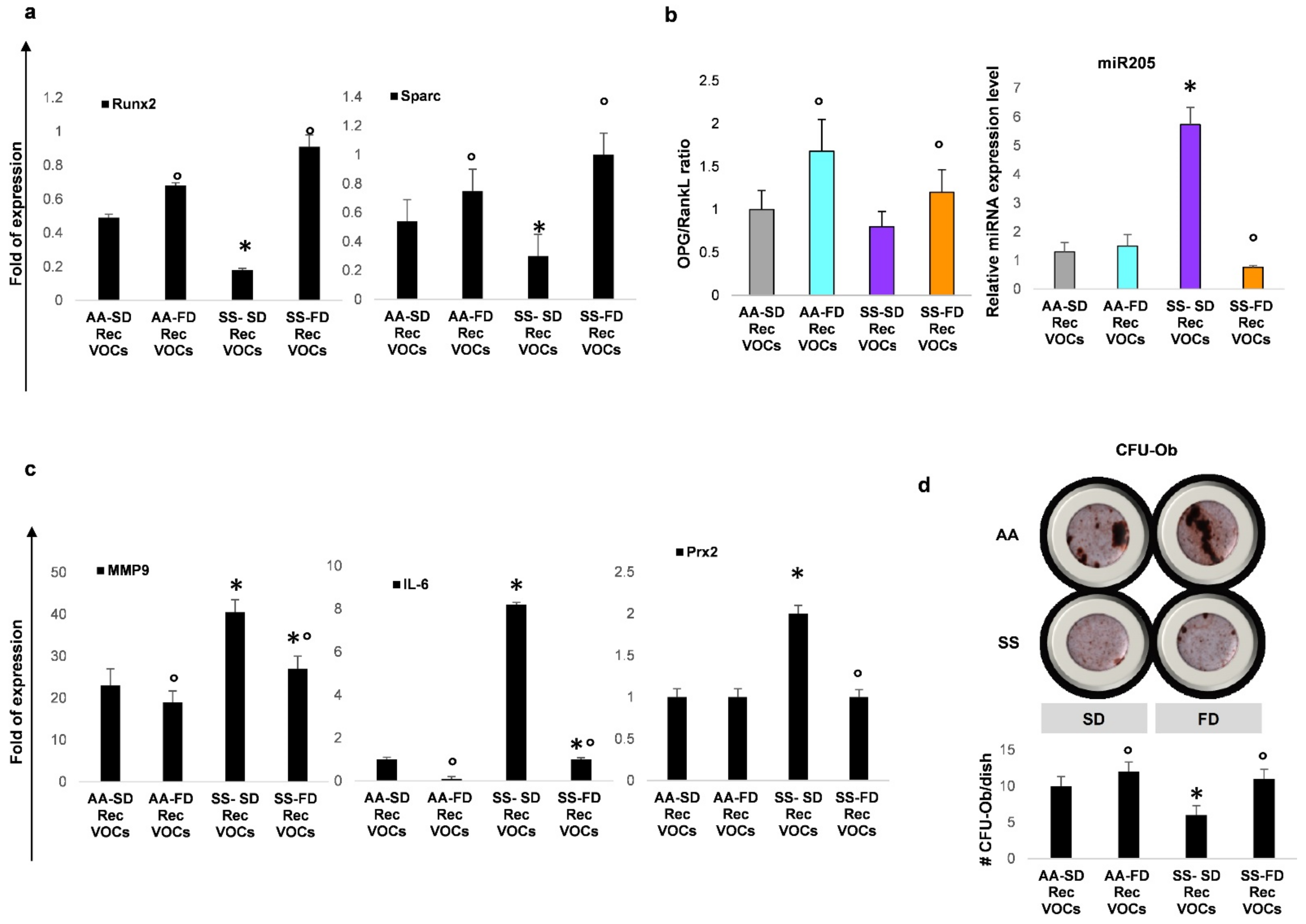
3.3. In SCD Mice Exposed to Recurrent H/R, FD Improves Osteogenesis and Reduces Bone Resorption
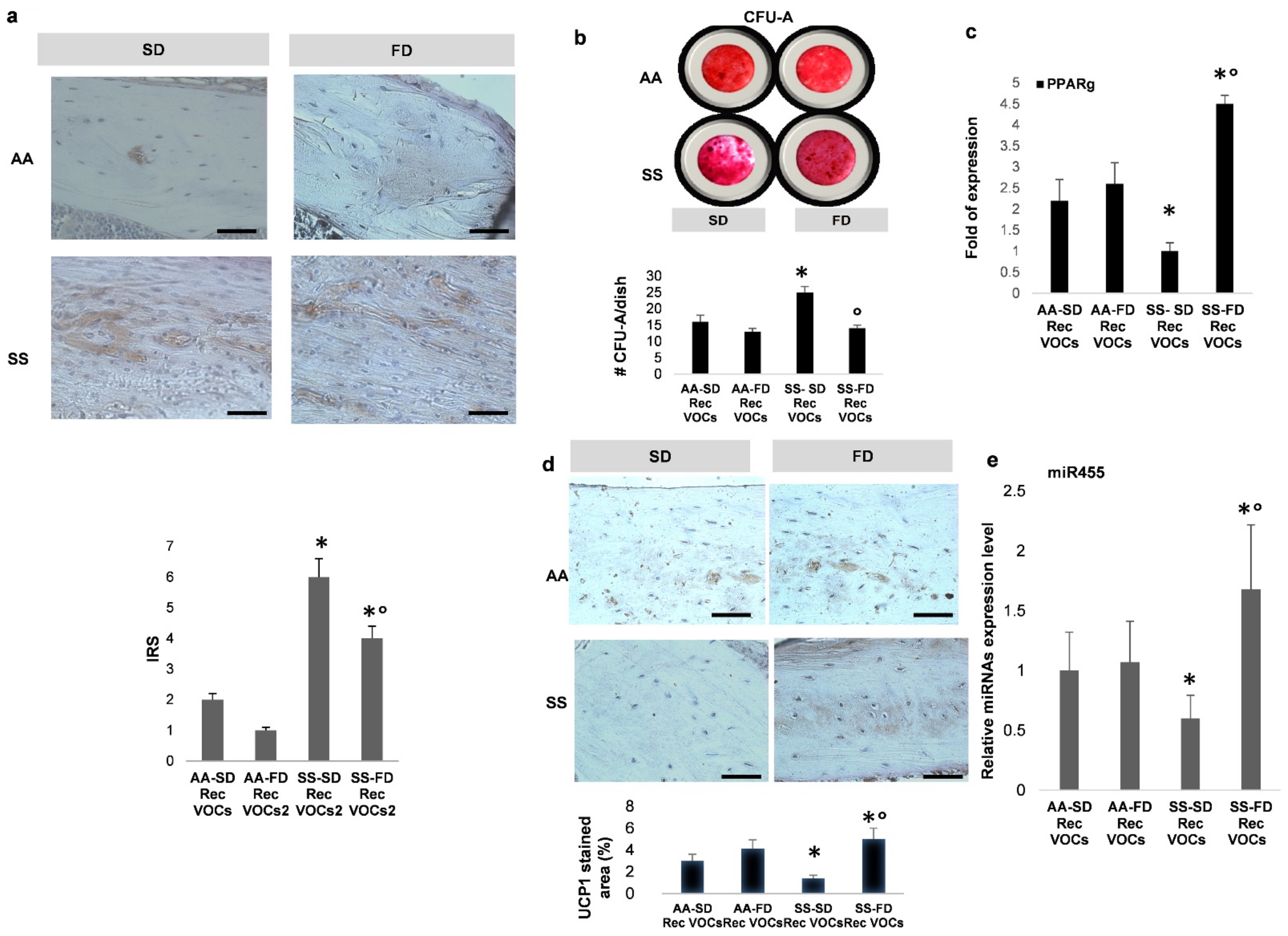
3.4. In SCD Mice Exposed to Recurrent H/R, FD Supplementation Induces Brown Adipogenesis
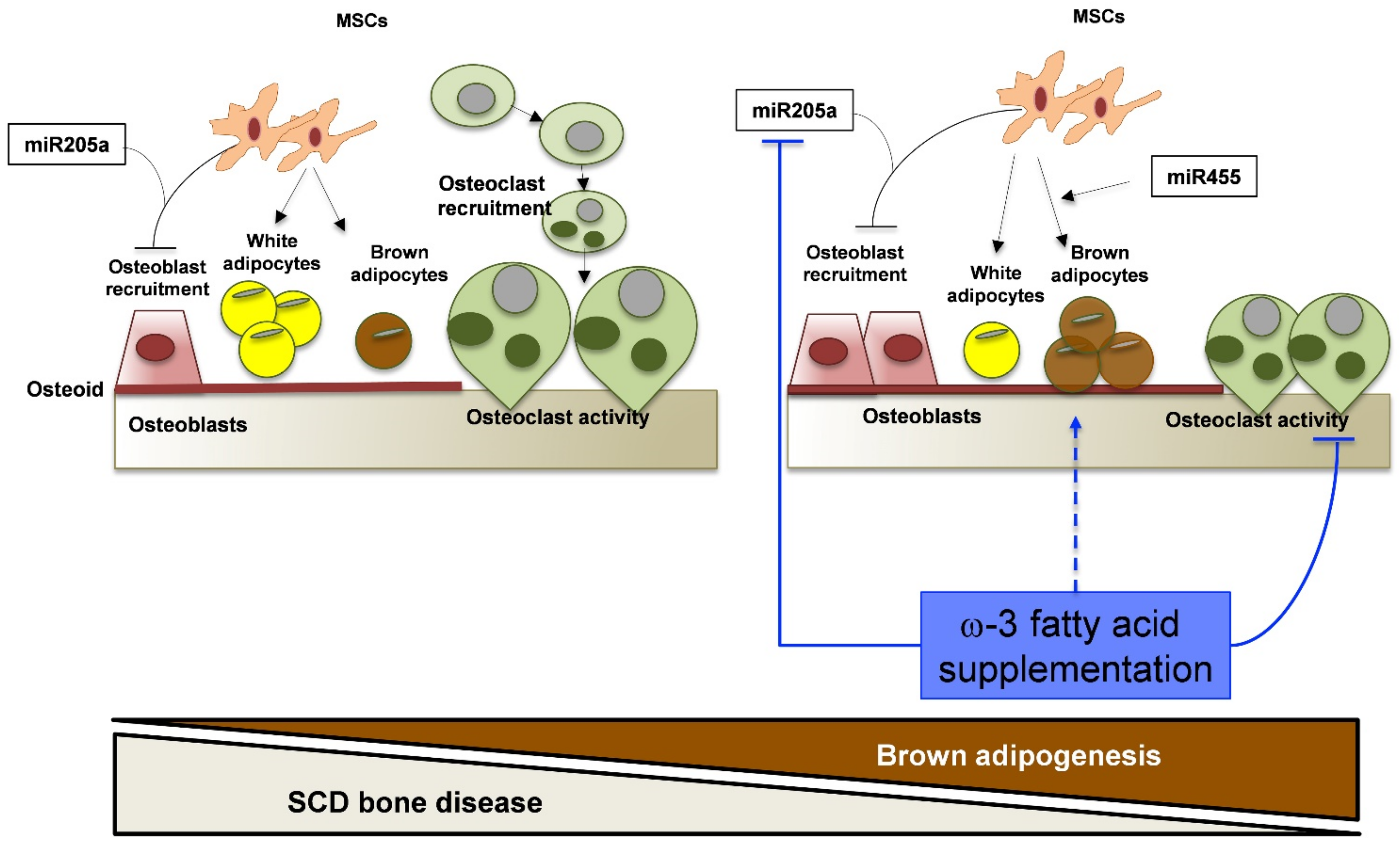
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maitra, P.; Caughey, M.; Robinson, L.; Desai, P.C.; Jones, S.; Nouraie, M.; Gladwin, M.T.; Hinderliter, A.; Cai, J.; Ataga, K.I. Risk factors for mortality in adult patients with sickle cell disease: A meta-analysis of studies in North America and Europe. Haematology 2017, 102, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Elmariah, H.; Garrett, M.E.; De Castro, L.M.; Jonassaint, J.C.; Ataga, K.I.; Eckman, J.R.; Ashley-Koch, A.E.; Telen, M.J. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am. J. Hematol. 2014, 89, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Prim. 2018, 4, 18010. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Matte’, A.; Valenti, M.T.; Siciliano, A.; Mori, A.; Schweiger, V.; Zampieri, G.; Perbellini, L.; De Franceschi, L. Hypoxia-reperfusion affects osteogenic lineage and promotes sickle cell bone disease. Blood 2015, 126, 2320–2328. [Google Scholar] [CrossRef]
- Garadah, T.S.; Hassan, A.B.; Jaradat, A.A.; Diab, D.E.; Kalafalla, H.O.; Kalifa, A.K.; Sequeira, R.P.; Alawadi, A.H.A.; Hassan, A.B. Predictors of Abnormal Bone Mass Density in Adult Patients with Homozygous Sickle-Cell Disease. Clin. Med. Insights Endocrinol. Diabetes 2015, 8, 35–40. [Google Scholar] [CrossRef]
- Miller, R.G.; Segal, J.B.; Ashar, B.H.; Leung, S.; Ahmed, S.; Siddique, S.; Rice, T.; Lanzkron, S. High prevalence and correlates of low bone mineral density in young adults with sickle cell disease. Am. J. Hematol. 2006, 81, 236–241. [Google Scholar] [CrossRef]
- Matte, A.; Recchiuti, A.; Federti, E.; Koehl, B.; Mintz, T.; El Nemer, W.; Tharaux, P.-L.; Brousse, V.; Andolfo, I.; Lamolinara, A.; et al. Resolution of sickle cell disease–associated inflammation and tissue damage with 17R-resolvin D1. Blood 2019, 133, 252–265. [Google Scholar] [CrossRef]
- Kalish, B.T.; Matte, A.; Andolfo, I.; Iolascon, A.; Weinberg, O.; Ghigo, A.; Cimino, J.; Siciliano, A.; Hirsch, E.; Federti, E.; et al. Dietary -3 fatty acids protect against vasculopathy in a transgenic mouse model of sickle cell disease. Haematology 2015, 100, 870–880. [Google Scholar] [CrossRef]
- De Franceschi, L.; Gabbiani, D.; Giusti, A.; Forni, G.; Stefanoni, F.; Pinto, V.M.; Sartori, G.; Balocco, M.; Zotto, C.D.; Valenti, M.T.; et al. Development of Algorithm for Clinical Management of Sickle Cell Bone Disease: Evidence for a Role of Vertebral Fractures in Patient Follow-up. J. Clin. Med. 2020, 9, 1601. [Google Scholar] [CrossRef] [PubMed]
- Fallon, E.M.; Nazarian, A.; Nehra, D.; Pan, A.H.; O’Loughlin, A.A.; Nosé, V.; Puder, M. The effect of docosahexaenoic acid on bone microstructure in young mice and bone fracture in neonates. J. Surg. Res. 2014, 191, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.S.; Pan, X.; Cheek, F.; Ing, S.W.; Jackson, R.D. A systematic review of omega-3 fatty acids and osteoporosis. Br. J. Nutr. 2012, 107, S253–S260. [Google Scholar] [CrossRef] [PubMed]
- Selenscig, D.; Ferreira, M.D.R.; Chicco, A.G.; Lombardo, Y.B. Dietary fish oil ameliorates adipose tissue dysfunction in insulin-resistant rats fed a sucrose-rich diet improving oxidative stress, peroxisome proliferator-activated receptor γ and uncoupling protein 2. Food Funct. 2018, 9, 2496–2507. [Google Scholar] [CrossRef]
- Vinchi, F.; De Franceschi, L.; Ghigo, A.; Townes, T.; Cimino, J.; Silengo, L.; Hirsch, E.; Altruda, F.; Tolosano, E. Hemopexin Therapy Improves Cardiovascular Function by Preventing Heme-Induced Endothelial Toxicity in Mouse Models of Hemolytic Diseases. Circulation 2013, 127, 1317–1329. [Google Scholar] [CrossRef]
- Wu, L.-C.; Sun, C.-W.; Ryan, T.M.; Pawlik, K.M.; Ren, J.; Townes, T.M. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood 2006, 108, 1183–1188. [Google Scholar] [CrossRef]
- Watkins, B.A.; Li, Y.; Lippman, H.E.; Feng, S. Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 387–398. [Google Scholar] [CrossRef]
- Casado-Diaz, A.; Ferreiro-Vera, C.; Priego-Capote, F.; Dorado, G.; Luque-De-Castro, M.D.; Quesada-Gómez, J.M. Effects of arachidonic acid on the concentration of hydroxyeicosatetraenoic acids in culture media of mesenchymal stromal cells differentiating into adipocytes or osteoblasts. Genes Nutr. 2013, 9, 375. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Zhang, Z.-M.; Zheng, X.-C.; Wang, L.; Huang, M.-J.; Qin, S.; Chen, J.; Lai, P.-L.; Yang, C.-L.; Liu, J.; et al. Endogenous n 3 polyunsaturated fatty acids PUFAs mitigate ovariectomy-induced bone loss by attenuating bone marrow adipogenesis in FAT1 transgenic mice. Drug Des. Dev. Ther. 2013, 7, 545–552. [Google Scholar] [CrossRef]
- Högström, M.; Nordström, P.; Nordström, A. n − 3 Fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: The NO2 Study. Am. J. Clin. Nutr. 2007, 85, 803–807. [Google Scholar] [CrossRef]
- Moerman, E.J.; Teng, K.; Lipschitz, D.A.; Lecka-Czernik, B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 2004, 3, 379–389. [Google Scholar] [CrossRef]
- Weiss, L.A.; Barrett-Connor, E.; Von Mühlen, D. Ratio of n–6 to n–3 fatty acids and bone mineral density in older adults: The Rancho Bernardo Study. Am. J. Clin. Nutr. 2005, 81, 934–938. [Google Scholar] [CrossRef]
- Rosen, C.J.; Ackert-Bicknell, C.; Rodriguez, J.P.; Pino, A.M. Marrow fat and the bone microenvironment: Developmental, functional, and pathological implications. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 109–124. [Google Scholar] [CrossRef]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 2009, 66, 236–253. [Google Scholar] [CrossRef]
- Varela-López, A.; Ochoa, J.J.; Llamas-Elvira, J.M.; López-Frías, M.; Planells, E.; Ramirez-Tortosa, M.; Ramirez-Tortosa, C.L.; Giampieri, F.; Battino, M.; Quiles, J.L. Age-Related Loss in Bone Mineral Density of Rats Fed Lifelong on a Fish Oil-Based Diet Is Avoided by Coenzyme Q10 Addition. Nutrients 2017, 9, 176. [Google Scholar] [CrossRef]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef]
- Kishikawa, A.; Kitaura, H.; Kimura, K.; Ogawa, S.; Qi, J.; Shen, W.-R.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; et al. Docosahexaenoic Acid Inhibits Inflammation-Induced Osteoclast Formation and Bone Resorption in vivo Through GPR120 by Inhibiting TNF-α Production in Macrophages and Directly Inhibiting Osteoclast Formation. Front. Endocrinol. 2019, 10, 175. [Google Scholar] [CrossRef]
- Philippe, C.; Wauquier, F.; Landrier, J.-F.; Bonnet, L.; Miot-Noirault, E.; Rochefort, G.Y.; Sadoine, J.; Asrih, M.; Jornayvaz, F.R.; Bernalier, A.; et al. GPR40 mediates potential positive effects of a saturated fatty acid enriched diet on bone. Mol. Nutr. Food Res. 2016, 61, 1600219. [Google Scholar] [CrossRef]
- Wauquier, F.; Philippe, C.; Léotoing, L.; Mercier, S.; Davicco, M.-J.; Lebecque, P.; Guicheux, J.; Pilet, P.; Miot-Noirault, E.; Poitout, V.; et al. The Free Fatty Acid Receptor G Protein-coupled Receptor 40 (GPR40) Protects from Bone Loss through Inhibition of Osteoclast Differentiation. J. Biol. Chem. 2013, 288, 6542–6551. [Google Scholar] [CrossRef]
- De Franceschi, L.; Turrini, F.; Honczarenko, M.; Ayi, K.; Rivera, A.; Fleming, M.D.; Law, T.; Mannu, F.; Kuypers, F.A.; Bast, A.; et al. In vivo reduction of erythrocyte oxidant stress in a murine model of beta-thalassemia. Haematologica 2004, 89, 1287–1298. [Google Scholar]
- Zheng, X.; Zhang, Y.; Guo, S.; Zhang, W.; Wang, J.; Lin, Y. Dynamic expression of matrix metalloproteinases 2, 9 and 13 in ovariectomy-induced osteoporosis rats. Exp. Ther. Med. 2018, 16, 1807–1813. [Google Scholar] [CrossRef]
- Hu, N.; Feng, C.; Jiang, Y.; Miao, Q.; Liu, H. Regulative Effect of Mir-205 on Osteogenic Differentiation of Bone Mesenchymal Stem Cells (BMSCs): Possible Role of SATB2/Runx2 and ERK/MAPK Pathway. Int. J. Mol. Sci. 2015, 16, 10491–10506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, H.; Zhao, W.; Li, M.; Tian, L.; Ju, W.; Li, X. miR-205 regulates bone turnover in elderly female patients with type 2 diabetes mellitus through targeted inhibition of Runx2. Exp. Ther. Med. 2020, 20, 1557–1565. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, R.-L.; Croce, C.M.; Stein, J.L.; Lian, J.B.; van Wijnen, A.J.; Stein, G.S. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc. Natl. Acad. Sci. USA 2011, 108, 9863–9868. [Google Scholar] [CrossRef]
- Andersen, T.L.; Ovejero, M.D.C.; Kirkegaard, T.; Lenhard, T.; Foged, N.T.; Delaissé, J.-M. A scrutiny of matrix metalloproteinases in osteoclasts: Evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone 2004, 35, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, K.; Tezuka, Y.; Maejima, A.; Sato, T.; Nemoto, K.; Kamioka, H.; Hakeda, Y.; Kumegawa, M. Molecular cloning of a possible cysteine proteinase predominantly expressed in osteoclasts. J. Biol. Chem. 1994, 269, 1106–1109. [Google Scholar] [CrossRef]
- Varghese, S.; Canalis, E. Alendronate Stimulates Collagenase 3 Expression in Osteoblasts by Posttranscriptional Mechanisms. J. Bone Miner. Res. 2000, 15, 2345–2351. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef]
- Justesen, J.; Stenderup, K.; Ebbesen, E.; Mosekilde, L.; Steiniche, T.; Kassem, M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001, 2, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Meunier, P.; Aaron, J.; Edouard, C.; Vlgnon, G. Osteoporosis and the Replacement of Cell Populations of the Marrow by Adipose Tissue. Clin. Orthop. Relat. Res. 1971, 80, 147–154. [Google Scholar] [CrossRef]
- Pino, A.M.; Miranda, M.; Figueroa, C.; Rodríguez, J.P.; Rosen, C.J. Qualitative Aspects of Bone Marrow Adiposity in Osteoporosis. Front. Endocrinol. 2016, 7, 139. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPARγ Is Required for Placental, Cardiac, and Adipose Tissue Development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; A Graves, R.; Budavari, A.I.; Spiegelman, B.M. mPPAR gamma 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994, 8, 1224–1234. [Google Scholar] [CrossRef]
- Pahlavani, M.; Razafimanjato, F.; Ramalingam, L.; Kalupahana, N.S.; Moussa, H.; Scoggin, S.; Moustaid-Moussa, N. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes. J. Nutr. Biochem. 2017, 39, 101–109. [Google Scholar] [CrossRef]
- Hardouin, P.; Rharass, T.; Lucas, S. Bone Marrow Adipose Tissue: To Be or Not To Be a Typical Adipose Tissue? Front. Endocrinol. 2016, 7, 85. [Google Scholar] [CrossRef]
- Paccou, J.; Hardouin, P.; Cotten, A.; Penel, G.; Cortet, B. The Role of Bone Marrow Fat in Skeletal Health: Usefulness and Perspectives for Clinicians. J. Clin. Endocrinol. Metab. 2015, 100, 3613–3621. [Google Scholar] [CrossRef]
- Järvinen, R.; Tuppurainen, M.; Erkkilä, A.T.; Penttinen, P.; Kärkkäinen, M.; Salovaara, K.; Jurvelin, J.S.; Kröger, H. Associations of dietary polyunsaturated fatty acids with bone mineral density in elderly women. Eur. J. Clin. Nutr. 2011, 66, 496–503. [Google Scholar] [CrossRef]
- Wongdee, K. Osteoporosis in diabetes mellitus: Possible cellular and molecular mechanisms. World J. Diabetes 2011, 2, 41–48. [Google Scholar] [CrossRef]
- Infante, A.; Rodríguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Valenti, M.T.; Garbin, U.; Pasini, A.; Zanatta, M.; Stranieri, C.; Manfro, S.; Zucal, C.; Carbonare, L.D. Role of Ox-PAPCs in the Differentiation of Mesenchymal Stem Cells (MSCs) and Runx2 and PPARγ2 Expression in MSCs-Like of Osteoporotic Patients. PLoS ONE 2011, 6, e20363. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.A.; Sun, L. Turning WAT into BAT: A review on regulators controlling the browning of white adipocytes. Biosci. Rep. 2013, 33, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Lasar, D.; Rosenwald, M.; Kiehlmann, E.; Balaz, M.; Tall, B.; Opitz, L.; Lidell, M.E.; Zamboni, N.; Krznar, P.; Sun, W.; et al. Peroxisome Proliferator Activated Receptor Gamma Controls Mature Brown Adipocyte Inducibility through Glycerol Kinase. Cell Rep. 2018, 22, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Feng, J.; Zhang, H.; Li, P.; Zhang, Y.; Zeng, Y.; Cai, Y.; Lin, X.; Xue, Y.; Guan, M. MiR-455 targeting SOCS3 improve liver lipid disorders in diabetic mice. Adipocyte 2020, 9, 179–188. [Google Scholar] [CrossRef]
- Martínez-Fernández, L.; Laiglesia, L.M.; Huerta, A.E.; Martínez, J.A.; Moreno-Aliaga, M.J. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 2015, 121, 24–41. [Google Scholar] [CrossRef]
- Schulz, T.J.; Tseng, Y.-H. Brown adipose tissue: Development, metabolism and beyond. Biochem. J. 2013, 453, 167–178. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Puzianowska-Kuźnicka, M. Induction of Adipose Tissue Browning as a Strategy to Combat Obesity. Int. J. Mol. Sci. 2020, 21, 6241. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenti, M.T.; Mattè, A.; Federti, E.; Puder, M.; Anez-Bustillos, L.; Deiana, M.; Cheri, S.; Minoia, A.; Brugnara, C.; Di Paolo, M.L.; et al. Dietary ω-3 Fatty Acid Supplementation Improves Murine Sickle Cell Bone Disease and Reprograms Adipogenesis. Antioxidants 2021, 10, 799. https://doi.org/10.3390/antiox10050799
Valenti MT, Mattè A, Federti E, Puder M, Anez-Bustillos L, Deiana M, Cheri S, Minoia A, Brugnara C, Di Paolo ML, et al. Dietary ω-3 Fatty Acid Supplementation Improves Murine Sickle Cell Bone Disease and Reprograms Adipogenesis. Antioxidants. 2021; 10(5):799. https://doi.org/10.3390/antiox10050799
Chicago/Turabian StyleValenti, Maria Teresa, Alessandro Mattè, Enrica Federti, Mark Puder, Lorenzo Anez-Bustillos, Michela Deiana, Samuele Cheri, Arianna Minoia, Carlo Brugnara, Maria Luisa Di Paolo, and et al. 2021. "Dietary ω-3 Fatty Acid Supplementation Improves Murine Sickle Cell Bone Disease and Reprograms Adipogenesis" Antioxidants 10, no. 5: 799. https://doi.org/10.3390/antiox10050799
APA StyleValenti, M. T., Mattè, A., Federti, E., Puder, M., Anez-Bustillos, L., Deiana, M., Cheri, S., Minoia, A., Brugnara, C., Di Paolo, M. L., Dalle Carbonare, L., & De Franceschi, L. (2021). Dietary ω-3 Fatty Acid Supplementation Improves Murine Sickle Cell Bone Disease and Reprograms Adipogenesis. Antioxidants, 10(5), 799. https://doi.org/10.3390/antiox10050799








