Oxidative Stress and Its Consequences in the Blood of Rats Irradiated with UV: Protective Effect of Cannabidiol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiment
2.2. Methods
2.2.1. Determination of Blood Plasma Basic Biochemical Parameters
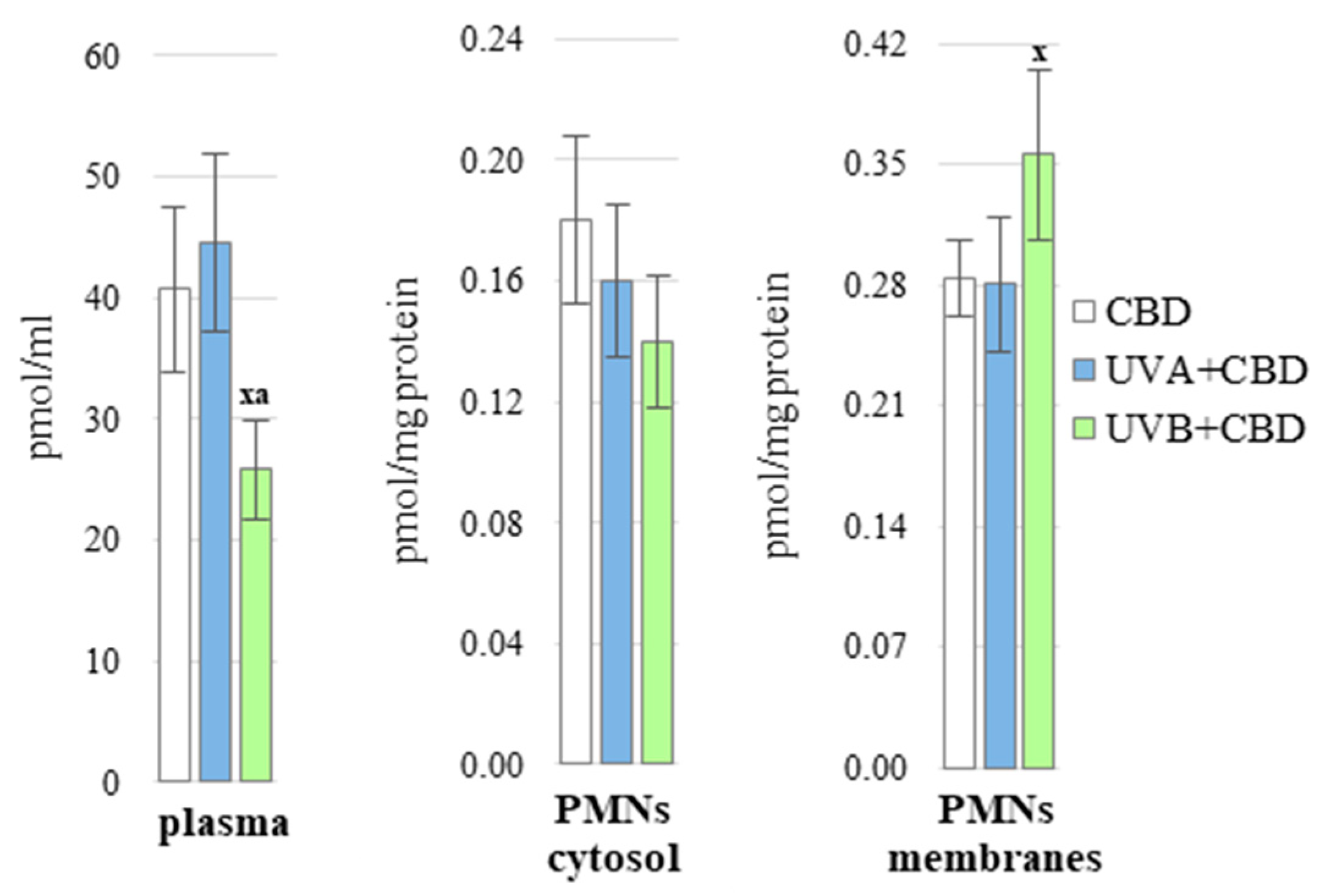
2.2.2. Determination of CBD Level in Plasma and in the Cytosolic/Membrane Fraction of PMNs
2.3. Redox Balance
2.3.1. Determination of ROS Levels
2.3.2. Determination of the Activity of Antioxidant Enzymes
2.3.3. Determination of the Level of Non-Enzymatic Antioxidants
2.4. Macromolecule Modifications
2.4.1. Determination of Protein Modifications
2.4.2. Determination of the Level of Lipid Peroxidation Products
2.4.3. Determination of the Level of Endocannabinoids
2.4.4. Determination of Protein Expression
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. UV Effect on Redox Status in Nude Rats Plasma
4.2. CBD Protection against UV-Induced Disturbances
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, S.; Rogers, L.J.; Halliday, G.M. Immunosuppressive Ultraviolet-A Radiation Inhibits the Development of Skin Memory CD8 T Cells. Photochem. Photobiol. Sci. 2010, 9, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Fernandez, B.O.; Hamilton, A.; Lang, N.N.; Gallagher, J.M.C.; Newby, D.E.; Feelisch, M.; Weller, R.B. UVA Irradiation of Human Skin Vasodilates Arterial Vasculature and Lowers Blood Pressure Independently of Nitric Oxide Synthase. J. Investig. Dermatol. 2014, 134, 1839–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gęgotek, A.; Biernacki, M.; Ambrożewicz, E.; Surażyński, A.; Wroński, A.; Skrzydlewska, E. The Cross-Talk between Electrophiles, Antioxidant Defence and the Endocannabinoid System in Fibroblasts and Keratinocytes after UVA and UVB Irradiation. J. Dermatol. Sci. 2016, 81, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Effects on Phospholipid Metabolism in Keratinocytes from Patients with Psoriasis Vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef] [Green Version]
- Wölfle, U.; Esser, P.R.; Simon-Haarhaus, B.; Martin, S.F.; Lademann, J.; Schempp, C.M. UVB-Induced DNA Damage, Generation of Reactive Oxygen Species, and Inflammation Are Effectively Attenuated by the Flavonoid Luteolin in Vitro and in Vivo. Free Radic. Biol. Med. 2011, 50, 1081–1093. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z. Crosstalk of Reactive Oxygen Species and NF-ΚB Signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting Molecular Cross-Talk between Nrf2 and NF-ΚB Response Pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Brand, R.M.; Wipf, P.; Durham, A.; Epperly, M.W.; Greenberger, J.S.; Falo, L.D. Targeting Mitochondrial Oxidative Stress to Mitigate UV-Induced Skin Damage. Front. Pharmacol. 2018, 9, 920. [Google Scholar] [CrossRef]
- Pajares, M.; Jiménez-Moreno, N.; Dias, I.H.K.; Debelec, B.; Vucetic, M.; Fladmark, K.E.; Basaga, H.; Ribaric, S.; Milisav, I.; Cuadrado, A. Redox Control of Protein Degradation. Redox Biol. 2015, 6, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Sander, C.S.; Chang, H.; Salzmann, S.; Müller, C.S.L.; Ekanayake-Mudiyanselage, S.; Elsner, P.; Thiele, J.J. Photoaging Is Associated with Protein Oxidation in Human Skin in Vivo. J. Investig. Dermatol. 2002, 118, 618–625. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.-W.; Hung, Y.-C.; Lin, T.-Y.; Fang, J.-Y.; Yang, P.-M.; Chen, M.-H.; Pan, T.-L. Comparison of the Biological Impact of UVA and UVB upon the Skin with Functional Proteomics and Immunohistochemistry. Antioxidants 2019, 8, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sample, A.; He, Y.-Y. Mechanisms and Prevention of UV-Induced Melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef]
- Paolino, G.; Panetta, C.; Cota, C.; Didona, D.; Moliterni, E.; Di Mattia, C.; De Vita, G.; Bottoni, U.; Donati, P.; Calvieri, S. Vitamin D Receptor Immunohistochemistry Variability in Sun-Exposed and Non-Sun-Exposed Melanomas. Melanoma Res. 2017, 27, 17–23. [Google Scholar] [CrossRef]
- Koh, H.K.; Geller, A.C.; Miller, D.R.; Grossbart, T.A.; Lew, R.A. Prevention and Early Detection Strategies for Melanoma and Skin Cancer: Current Status. Arch. Dermatol. 1996, 132, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, M.X. A Clinical Review of Phototherapy for Psoriasis. Lasers Med. Sci. 2018, 33, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Carrara, I.M.; Melo, G.P.; Bernardes, S.S.; Neto, F.S.; Ramalho, L.N.Z.; Marinello, P.C.; Luiz, R.C.; Cecchini, R.; Cecchini, A.L. Looking beyond the Skin: Cutaneous and Systemic Oxidative Stress in UVB-Induced Squamous Cell Carcinoma in Hairless Mice. J. Photochem. Photobiol. B Biol. 2019, 195, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Rankin, L.; Fowler, C.J. The Basal Pharmacology of Palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millar, S.A.; Stone, N.L.; Bellman, Z.D.; Yates, A.S.; England, T.J.; O’Sullivan, S.E. A Systematic Review of Cannabidiol Dosing in Clinical Populations. Br. J. Clin. Pharmacol. 2019, 85, 1888–1900. [Google Scholar] [CrossRef]
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Protects Keratinocyte Cell Membranes Following Exposure to UVB and Hydrogen Peroxide. Redox Biol. 2020, 36, 101613. [Google Scholar] [CrossRef]
- Łuczaj, W.; Domingues, M.D.R.; Domingues, P.; Skrzydlewska, E. Changes in Lipid Profile of Keratinocytes from Rat Skin Exposed to Chronic UVA or UVB Radiation and Topical Application of Cannabidiol. Antioxidants 2020, 9, 1178. [Google Scholar] [CrossRef] [PubMed]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol Induces Antioxidant Pathways in Keratinocytes by Targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, K.A.K.; Peiris, H.; Wallace, G.; Holland, O.J.; Mitchell, M.D. The Interplay between the Endocannabinoid System, Epilepsy and Cannabinoids. Int. J. Mol. Sci. 2019, 20, 6079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef]
- Palmieri, B.; Laurino, C.; Vadalà, M. A Therapeutic Effect of Cbd-Enriched Ointment in Inflammatory Skin Diseases and Cutaneous Scars. Clin. Ter. 2019, 170, e93–e99. [Google Scholar] [CrossRef]
- Xu, D.H.; Cullen, B.D.; Tang, M.; Fang, Y. The Effectiveness of Topical Cannabidiol Oil in Symptomatic Relief of Peripheral Neuropathy of the Lower Extremities. Curr. Pharm. Biotechnol. 2020, 21, 390–402. [Google Scholar] [CrossRef]
- Katica, M.; Gradascevic, N. Hematologic profile of laboratory rats fed with bakery products. Int. J. Res. Granthaalayah 2017, 5, 221–231. [Google Scholar] [CrossRef]
- Hammell, D.C.; Zhang, L.P.; Ma, F.; Abshire, S.M.; McIlwrath, S.L.; Stinchcomb, A.L.; Westlund, K.N. Transdermal Cannabidiol Reduces Inflammation and Pain-Related Behaviours in a Rat Model of Arthritis. Eur. J. Pain 2016, 20, 936–948. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Luque-Córdoba, D.; Calderón-Santiago, M.; Luque de Castro, M.D.; Priego-Capote, F. Study of Sample Preparation for Determination of Endocannabinoids and Analogous Compounds in Human Serum by LC-MS/MS in MRM Mode. Talanta 2018, 185, 602–610. [Google Scholar] [CrossRef]
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of Peroxynitrite, Tetrahydrobiopterin, Ascorbic Acid, and Thiols: Implications for Uncoupling Endothelial Nitric-Oxide Synthase. J. Biol. Chem. 2003, 278, 22546–22554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Sykes, J.A.; McCormack, F.X.; O’Brien, T.J. A Preliminary Study of the Superoxide Dismutase Content of Some Human Tumors. Cancer Res. 1978, 38, 2759–2762. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the Quantitative and Qualitative Characterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Mize, C.E.; Langdon, R.G. Hepatic Glutathione Reductase. I. Purification and General Kinetic Properties. J. Biol. Chem. 1962, 237, 1589–1595. [Google Scholar] [CrossRef]
- Holmgren, A.; Björnstedt, M. Thioredoxin and Thioredoxin Reductase. Methods Enzymol. 1995, 252, 199–208. [Google Scholar] [CrossRef]
- Maeso, N.; García-Martínez, D.; Rupérez, F.J.; Cifuentes, A.; Barbas, C. Capillary Electrophoresis of Glutathione to Monitor Oxidative Stress and Response to Antioxidant Treatments in an Animal Model. J. Chromatogr. B 2005, 822, 61–69. [Google Scholar] [CrossRef]
- Lovell, M.A.; Xie, C.; Gabbita, S.P.; Markesbery, W.R. Decreased Thioredoxin and Increased Thioredoxin Reductase Levels in Alzheimer’s Disease Brain. Free Radic. Biol. Med. 2000, 28, 418–427. [Google Scholar] [CrossRef]
- Ivanović, D.; Popović, A.; Radulović, D.; Medenica, M. Reversed-Phase Ion-Pair HPLC Determination of Some Water-Soluble Vitamins in Pharmaceuticals. J. Pharm. Biomed. Anal. 1999, 18, 999–1004. [Google Scholar] [CrossRef]
- De Leenheer, A.P.; De Bevere, V.O.; De Ruyter, M.G.; Claeys, A.E. Simultaneous Determination of Retinol and Alpha-Tocopherol in Human Serum by High-Performance Liquid Chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1979, 162, 408–413. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of Carbonyl Content in Oxidatively Modified Proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Milkovic, L.; Bennett, S.J.; Griffiths, H.R.; Zarkovic, N.; Grune, T. Measurement of HNE-Protein Adducts in Human Plasma and Serum by ELISA-Comparison of Two Primary Antibodies. Redox Biol. 2013, 1, 226–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coolen, S.A.J.; van Buuren, B.; Duchateau, G.; Upritchard, J.; Verhagen, H. Kinetics of Biomarkers: Biological and Technical Validity of Isoprostanes in Plasma. Amino Acids 2005, 29, 429–436. [Google Scholar] [CrossRef]
- Luo, X.P.; Yazdanpanah, M.; Bhooi, N.; Lehotay, D.C. Determination of Aldehydes and Other Lipid Peroxidation Products in Biological Samples by Gas Chromatography-Mass Spectrometry. Anal. Biochem. 1995, 228, 294–298. [Google Scholar] [CrossRef]
- Eissa, S.; Seada, L.S. Quantitation of Bcl-2 Protein in Bladder Cancer Tissue by Enzyme Immunoassay: Comparison with Western Blot and Immunohistochemistry. Clin. Chem. 1998, 44, 1423–1429. [Google Scholar] [CrossRef] [Green Version]
- Vangipuram, R.; Feldman, S.R. Ultraviolet Phototherapy for Cutaneous Diseases: A Concise Review. Oral Dis. 2016, 22, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Kremslehner, C.; Miller, A.; Nica, R.; Nagelreiter, I.-M.; Narzt, M.-S.; Golabi, B.; Vorstandlechner, V.; Mildner, M.; Lachner, J.; Tschachler, E.; et al. Imaging of Metabolic Activity Adaptations to UV Stress, Drugs and Differentiation at Cellular Resolution in Skin and Skin Equivalents—Implications for Oxidative UV Damage. Redox Biol. 2020, 37, 101583. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and Ascorbic Acid Cooperation in Antioxidant and Antiapoptotic Effect on Human Skin Keratinocytes and Fibroblasts Exposed to UVA and UVB Radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Atalay, S.; Gęgotek, A.; Wroński, A.; Domigues, P.; Skrzydlewska, E. Therapeutic Application of Cannabidiol on UVA and UVB Irradiated Rat Skin. A Proteomic Study. J. Pharm. Biomed. Anal. 2021, 192, 113656. [Google Scholar] [CrossRef] [PubMed]
- Pudlarz, A.M.; Czechowska, E.S.; Karbownik, M.; Ranoszek-Soliwoda, K.; Tomaszewska, E.; Celichowski, G.; Grobelny, J.; Chabielska, E.; Gromotowicz-Popławska, A.; Szemraj, J. The Effect of Immobilized Antioxidant Enzymes on the Oxidative Stress in UV-Irradiated Rat Skin. Nanomedicine 2020, 15, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Markowska, A.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Anti-Oxidant and Anti-Inflammatory Effect of Cannabidiol Contributes to the Decreased Lipid Peroxidation of Keratinocytes of Rat’s Skin Exposed to UV Radiation. Oxidative Med. Cell. Longev. 2021, 2021, 6647222. [Google Scholar] [CrossRef]
- Svobodová, A.R.; Galandáková, A.; Sianská, J.; Doležal, D.; Ulrichová, J.; Vostálová, J. Acute Exposure to Solar Simulated Ultraviolet Radiation Affects Oxidative Stress-Related Biomarkers in Skin, Liver and Blood of Hairless Mice. Biol. Pharm. Bull. 2011, 34, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juzeniene, A.; Moan, J. Beneficial Effects of UV Radiation Other than via Vitamin D Production. Dermatoendocrinology 2012, 4, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omer, S.A.E.; Badi, R.M.; Garelnabi, M.E.M.; Altayeb, O.A.; Hussein, M.O.; Fadul, E.A.; Saeed, A.M. Effects of Acute and Chronic Exposure to Natural Sunlight and UVB on CD4/CD8 Ratio and Circulating pro-Inflammatory and Anti-Inflammatory Cytokine Levels in Mice. Sci. Afr. 2019, 4, e00102. [Google Scholar] [CrossRef]
- Erden Inal, M.; Kahraman, A. The Protective Effect of Flavonol Quercetin against Ultraviolet a Induced Oxidative Stress in Rats. Toxicology 2000, 154, 21–29. [Google Scholar] [CrossRef]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg 2016, 138, S18–S28. [Google Scholar] [CrossRef]
- Ryšavá, A.; Čížková, K.; Franková, J.; Roubalová, L.; Ulrichová, J.; Vostálová, J.; Vrba, J.; Zálešák, B.; Rajnochová Svobodová, A. Effect of UVA Radiation on the Nrf2 Signalling Pathway in Human Skin Cells. J. Photochem. Photobiol. B 2020, 209, 111948. [Google Scholar] [CrossRef]
- Verma, A.; Kushwaha, H.N.; Srivastava, A.K.; Srivastava, S.; Jamal, N.; Srivastava, K.; Ray, R.S. Piperine Attenuates UV-R Induced Cell Damage in Human Keratinocytes via NF-KB, Bax/Bcl-2 Pathway: An Application for Photoprotection. J. Photochem. Photobiol. B 2017, 172, 139–148. [Google Scholar] [CrossRef]
- Sakurai, H.; Suzuki, S.; Kawasaki, N.; Nakano, H.; Okazaki, T.; Chino, A.; Doi, T.; Saiki, I. Tumor Necrosis Factor-Alpha-Induced IKK Phosphorylation of NF-KappaB P65 on Serine 536 Is Mediated through the TRAF2, TRAF5 and TAK1 Signaling Pathway. J. Biol. Chem. 2003, 278, 36916–36923. [Google Scholar] [CrossRef] [Green Version]
- Anrather, J.; Racchumi, G.; Iadecola, C. NF-KappaB Regulates Phagocytic NADPH Oxidase by Inducing the Expression of Gp91phox. J. Biol. Chem. 2006, 281, 5657–5667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gęgotek, A.; Skrzydlewska, E. Biological Effect of Protein Modifications by Lipid Peroxidation Products. Chem. Phys. Lipids 2019, 221, 46–52. [Google Scholar] [CrossRef]
- Zaidi, S.M.K.R.; Al-Qirim, T.M.; Banu, N. Effects of Antioxidant Vitamins on Glutathione Depletion and Lipid Peroxidation Induced by Restraint Stress in the Rat Liver. Drugs R D 2005, 6, 157–165. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kaneko, F.; Niwa, Y. Changes in Lipid Peroxide Levels and Activity of Reactive Oxygen Scavenging Enzymes in Skin, Serum and Liver Following UVB Irradiation in Mice. Life Sci. 1992, 50, 1893–1903. [Google Scholar] [CrossRef]
- Gęgotek, A.; Atalay, S.; Rogowska-Wrzesińska, A.; Skrzydlewska, E. The Effect of Cannabidiol on UV-Induced Changes in Intracellular Signaling of 3D-Cultured Skin Keratinocytes. Int. J. Mol. Sci. 2021, 22, 1501. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carsberg, C.J.; Ohanian, J.; Friedmann, P.S. Ultraviolet Radiation Stimulates a Biphasic Pattern of 1,2-Diacylglycerol Formation in Cultured Human Melanocytes and Keratinocytes by Activation of Phospholipases C and D. Biochem. J. 1995, 305, 471–477. [Google Scholar] [CrossRef] [Green Version]
- Nicolaou, A.; Pilkington, S.M.; Rhodes, L.E. Ultraviolet-Radiation Induced Skin Inflammation: Dissecting the Role of Bioactive Lipids. Chem. Phys. Lipids 2011, 164, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.-W.; Kim, Y.; Kang, J.-H.; Kang, S.-S.; Ahn, Y.K.; Park, C.-S.; Kim, J.J. CB1 and CB2 Cannabinoid Receptors Differentially Regulate the Production of Reactive Oxygen Species by Macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tronino, D.; Russo, R.; Ostacolo, C.; Mazzolari, A.; De Caro, C.; Avagliano, C.; Laneri, S.; La Rana, G.; Sacchi, A.; Della Valle, F.; et al. Improvement of Topical Palmitoylethanolamide Anti-Inflammatory Activity by Pegylated Prodrugs. Mol. Pharm. 2015, 12, 3369–3379. [Google Scholar] [CrossRef]
- Rundle, C.W.; Rietcheck, H.R.; Maghfour, J.; Dercon, S.; Fernandez, J.; Lio, P.; Dellavalle, R.P.; Fujita, M.; Yardley, H. Anti-Inflammatory Effect of Cannabidiol and Palmitoylethanolamide Containing Topical Formulation on Skin in a 12-O-Tetradecanoylphorbol-13-Acetate-Induced Dermatitis Model in Mice. Dermat. Contact Atopic Occup. Drug 2021. [Google Scholar] [CrossRef]
- Itin, C.; Barasch, D.; Domb, A.J.; Hoffman, A. Prolonged Oral Transmucosal Delivery of Highly Lipophilic Drug Cannabidiol. Int. J. Pharm. 2020, 581, 119276. [Google Scholar] [CrossRef] [PubMed]
- Vacek, J.; Vostalova, J.; Papouskova, B.; Skarupova, D.; Kos, M.; Kabelac, M.; Storch, J. Antioxidant Function of Phytocannabinoids: Molecular Basis of Their Stability and Cytoprotective Properties under UV-Irradiation. Free Radic. Biol. Med. 2021, 164, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Chularojanamontri, L.; Charoenpipatsin, N.; Silpa-Archa, N.; Wongpraparut, C.; Thongboonkerd, V. Proteomics in Psoriasis. Int. J. Mol. Sci. 2019, 20, 1141. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Mukhopadhyay, P.; Rajesh, M.; Patel, V.; Mukhopadhyay, B.; Gao, B.; Haskó, G.; Pacher, P. Cannabidiol Attenuates Cisplatin-Induced Nephrotoxicity by Decreasing Oxidative/Nitrosative Stress, Inflammation, and Cell Death. J. Pharmacol. Exp. Ther. 2009, 328, 708–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Mukhopadhyay, P.; Cao, Z.; Wang, H.; Feng, D.; Haskó, G.; Mechoulam, R.; Gao, B.; Pacher, P. Cannabidiol Attenuates Alcohol-Induced Liver Steatosis, Metabolic Dysregulation, Inflammation and Neutrophil-Mediated Injury. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-Y.; Huang, C.-H.; Lin, Y.-H.; Wang, C.-C.; Jan, T.-R. Cannabidiol Induced Apoptosis in Human Monocytes through Mitochondrial Permeability Transition Pore-Mediated ROS Production. Free Radic. Biol. Med. 2018, 124, 311–318. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Nabissi, M.; Santoni, G.; Ligresti, A. Actions and Regulation of Ionotropic Cannabinoid Receptors. Adv. Pharmacol. 2017, 80, 249–289. [Google Scholar] [CrossRef]
- Ghovanloo, M.-R.; Shuart, N.G.; Mezeyova, J.; Dean, R.A.; Ruben, P.C.; Goodchild, S.J. Inhibitory Effects of Cannabidiol on Voltage-Dependent Sodium Currents. J. Biol. Chem. 2018, 293, 16546–16558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Di Marzo, V. Anti-Inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef] [PubMed]
- Bielawiec, P.; Harasim-Symbor, E.; Chabowski, A. Phytocannabinoids: Useful Drugs for the Treatment of Obesity? Special Focus on Cannabidiol. Front. Endocrinol. 2020, 11, 114. [Google Scholar] [CrossRef]
- Li, H.; Wood, J.T.; Whitten, K.M.; Vadivel, S.K.; Seng, S.; Makriyannis, A.; Avraham, H.K. Inhibition of Fatty Acid Amide Hydrolase Activates Nrf2 Signalling and Induces Heme Oxygenase 1 Transcription in Breast Cancer Cells. Br. J. Pharmacol. 2013, 170, 489–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, S.E. An Update on PPAR Activation by Cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [Green Version]
- Nahler, G. Cannabidiol and Contributions of Major Hemp Phytocompounds to the “Entourage Effect”; Possible Mechanisms. Altern. Complementary Integr. Med. 2019, 5, 1–16. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Kozela, E.; Juknat, A.; Vogel, Z. Modulation of Astrocyte Activity by Cannabidiol, a Nonpsychoactive Cannabinoid. Int. J. Mol. Sci. 2017, 18, 1669. [Google Scholar] [CrossRef] [Green Version]
- Pucci, M.; Rapino, C.; Di Francesco, A.; Dainese, E.; D’Addario, C.; Maccarrone, M. Epigenetic Control of Skin Differentiation Genes by Phytocannabinoids. Br. J. Pharmacol. 2013, 170, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Moreau, F.; Chadee, K. PPARγ Is an E3 Ligase That Induces the Degradation of NFκB/P65. Nat. Commun. 2012, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Δ9-Tetrahydrocannabinol and Cannabidiol Differentially Inhibit the Lipopolysaccharide-Activated NF-ΚB and Interferon-β/STAT Proinflammatory Pathways in BV-2 Microglial Cells. J. Biol. Chem. 2010, 285, 1616–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1-M2 Polarization Balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, R.L.; Silveira, G.T.; Wanderlei, C.W.; Cecilio, N.T.; Maganin, A.G.M.; Franchin, M.; Marques, L.M.M.; Lopes, N.P.; Crippa, J.A.; Guimarães, F.S.; et al. DMH-CBD, a Cannabidiol Analog with Reduced Cytotoxicity, Inhibits TNF Production by Targeting NF-KB Activity Dependent on A2A Receptor. Toxicol. Appl. Pharmacol. 2019, 368, 63–71. [Google Scholar] [CrossRef] [PubMed]
- McAllister, S.D.; Murase, R.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Allison, J.; Almanza, C.; Pakdel, A.; Lee, J.; Limbad, C.; et al. Pathways Mediating the Effects of Cannabidiol on the Reduction of Breast Cancer Cell Proliferation, Invasion and Metastasis. Breast Cancer Res. Treat. 2011, 129, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Oghumu, S.; Satoskar, A.R.; Shilo, K.; Carson, W.E.; et al. Modulation of the Tumor Microenvironment and Inhibition of EGF/EGFR Pathway: Novel Anti-Tumor Mechanisms of Cannabidiol in Breast Cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef] [Green Version]
- Lee, C. Collaborative Power of Nrf2 and PPARγ Activators against Metabolic and Drug-Induced Oxidative Injury. Oxidative Med. Cell Longev. 2017, 2017, 1378175. [Google Scholar] [CrossRef] [Green Version]
- Gęgotek, A.; Atalay, S.; Domingues, P.; Skrzydlewska, E. The Differences in the Proteome Profile of Cannabidiol-Treated Skin Fibroblasts Following UVA or UVB Irradiation in 2D and 3D Cell Cultures. Cells 2019, 8, 995. [Google Scholar] [CrossRef] [Green Version]
- Ruhaak, L.R.; Felth, J.; Karlsson, P.C.; Rafter, J.J.; Verpoorte, R.; Bohlin, L. Evaluation of the Cyclooxygenase Inhibiting Effects of Six Major Cannabinoids Isolated from Cannabis Sativa. Biol. Pharm. Bull. 2011, 34, 774–778. [Google Scholar] [CrossRef] [Green Version]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Schaur, R.J.; Siems, W.; Bresgen, N.; Eckl, P.M. 4-Hydroxy-Nonenal-A Bioactive Lipid Peroxidation Product. Biomolecules 2015, 5, 2247–2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis Phenolics and Their Bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef] [PubMed]
- Simmerman, E.; Qin, X.; Yu, J.C.; Baban, B. Cannabinoids as a Potential New and Novel Treatment for Melanoma: A Pilot Study in a Murine Model. J. Surg. Res. 2019, 235, 210–215. [Google Scholar] [CrossRef] [PubMed]









| Parameters | Control | CBD | UVA | UVA + CBD | UVB | UVB + CBD |
|---|---|---|---|---|---|---|
| total protein [g/dL] | 5.41 ± 0.44 | 5.40 ± 0.48 | 5.25 ± 0.55 | 5.35 ± 0.56 | 5.40 ± 0.58 | 5.35 ± 0.53 |
| Na+ [mmol/L] | 85.1 ± 3.1 | 85.3 ± 3.2 | 86.9 ± 3.4 | 85.2 ± 3.2 | 86.9 ± 3.5 | 86.6 ± 3.2 |
| K+ [mmol/L] | 4.52 ± 0.22 | 4.51 ± 0.21 | 4.54 ± 0.24 | 4.45 ± 0.23 | 4.62 ± 0.26 | 4.54 ± 0.23 |
| glucose [mg/dL] | 220 ± 12 | 224 ± 13 | 214 ± 12 | 224 ± 13 | 227 ± 13 | 226 ± 13 |
| AST [U/L] | 81 ± 4 | 78 ± 5 | 86 ± 5 | 82 ± 4 | 79 ± 5 | 80 ± 5 |
| ALT [U/L] | 43 ± 2 | 41 ± 3 | 46 ± 3 | 45 ± 3 | 46 ± 4 | 43 ± 3 |
| triglycerides [mg/dL] | 85 ± 6 | 85 ± 7 | 88 ± 7 | 87 ± 8 | 93 ± 8 | 87 ± 7 |
| cholesterol [mg/dL] | 74 ± 5 | 74 ± 6 | 73 ± 6 | 73 ± 5 | 75 ± 7 | 73 ± 5 |
| urea [mg/dL] | 43 ± 3 | 41 ± 4 | 46 ± 4 | 46 ± 4 | 45 ± 4 | 43 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biernacki, M.; Brzóska, M.M.; Markowska, A.; Gałażyn-Sidorczuk, M.; Cylwik, B.; Gęgotek, A.; Skrzydlewska, E. Oxidative Stress and Its Consequences in the Blood of Rats Irradiated with UV: Protective Effect of Cannabidiol. Antioxidants 2021, 10, 821. https://doi.org/10.3390/antiox10060821
Biernacki M, Brzóska MM, Markowska A, Gałażyn-Sidorczuk M, Cylwik B, Gęgotek A, Skrzydlewska E. Oxidative Stress and Its Consequences in the Blood of Rats Irradiated with UV: Protective Effect of Cannabidiol. Antioxidants. 2021; 10(6):821. https://doi.org/10.3390/antiox10060821
Chicago/Turabian StyleBiernacki, Michał, Małgorzata Michalina Brzóska, Agnieszka Markowska, Małgorzata Gałażyn-Sidorczuk, Bogdan Cylwik, Agnieszka Gęgotek, and Elżbieta Skrzydlewska. 2021. "Oxidative Stress and Its Consequences in the Blood of Rats Irradiated with UV: Protective Effect of Cannabidiol" Antioxidants 10, no. 6: 821. https://doi.org/10.3390/antiox10060821
APA StyleBiernacki, M., Brzóska, M. M., Markowska, A., Gałażyn-Sidorczuk, M., Cylwik, B., Gęgotek, A., & Skrzydlewska, E. (2021). Oxidative Stress and Its Consequences in the Blood of Rats Irradiated with UV: Protective Effect of Cannabidiol. Antioxidants, 10(6), 821. https://doi.org/10.3390/antiox10060821







