Purification and Structural Characterization of Sulfated Polysaccharides Derived from Brown Algae, Sargassum binderi: Inhibitory Mechanism of iNOS and COX-2 Pathway Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polysaccharide
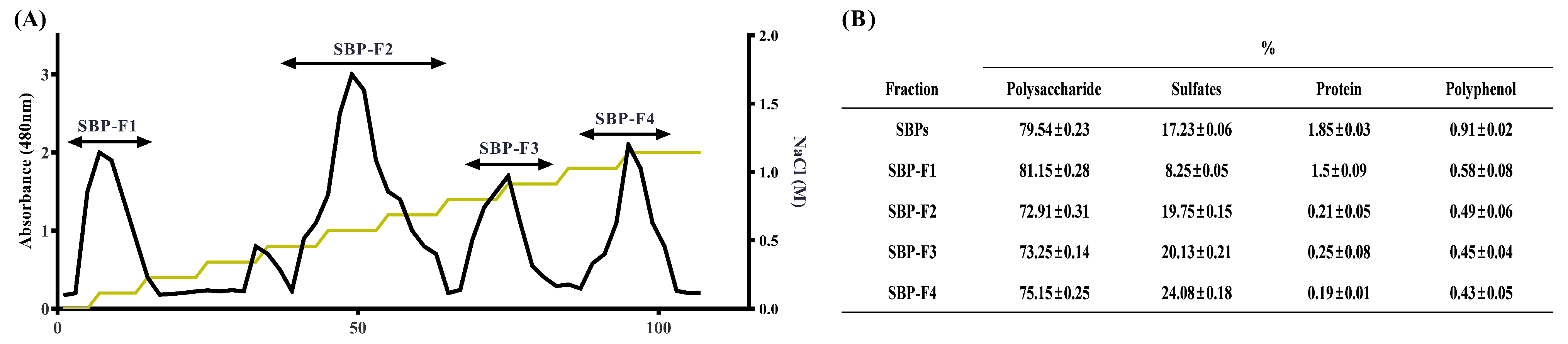
2.3. Polysaccharide Separation by Anion-Exchange Chromatography
2.4. Characterization of Polysaccharides
2.4.1. FTIR Analysis
2.4.2. NMR Analysis
2.4.3. Monosaccharide Composition Analysis
2.4.4. Characterization of Average Molecular Weight
2.4.5. Morphological Analysis
2.5. Cell Culture
2.6. Measurement of NO Production in RAW 264.7 Cells
2.7. Western Blot Analysis
2.8. Measurement of Survival Rate, Cell Death, and NO Production in Zebrafish Larvae
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of the SBPs
3.2. FTIR Spectrum of Sulfated Polysaccharides
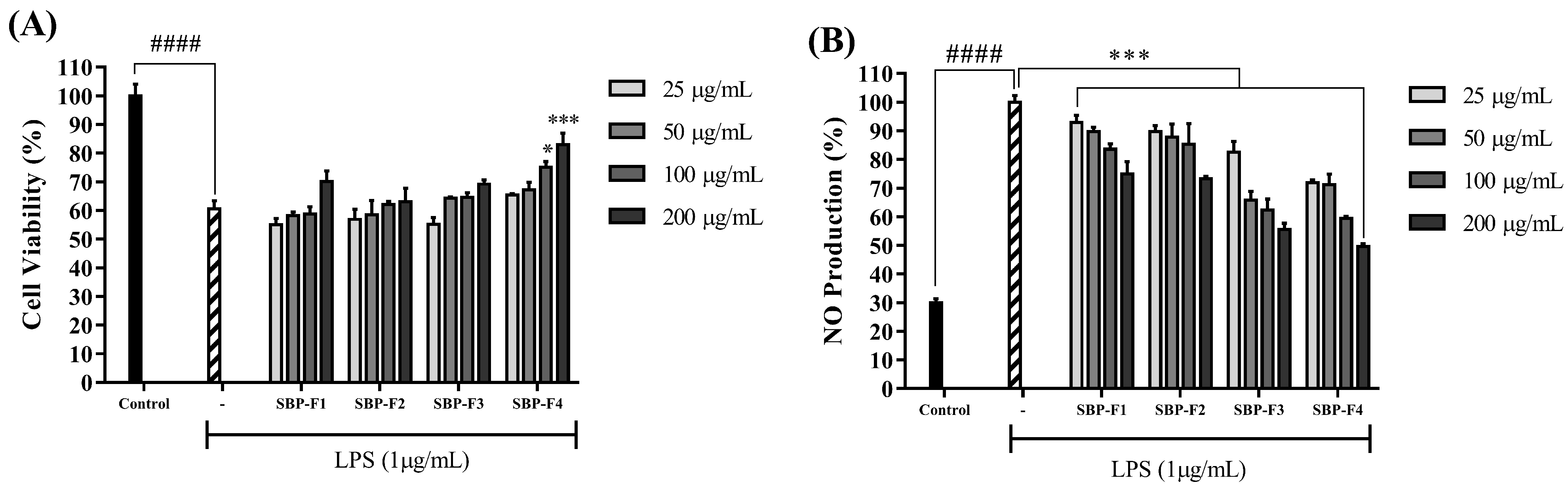
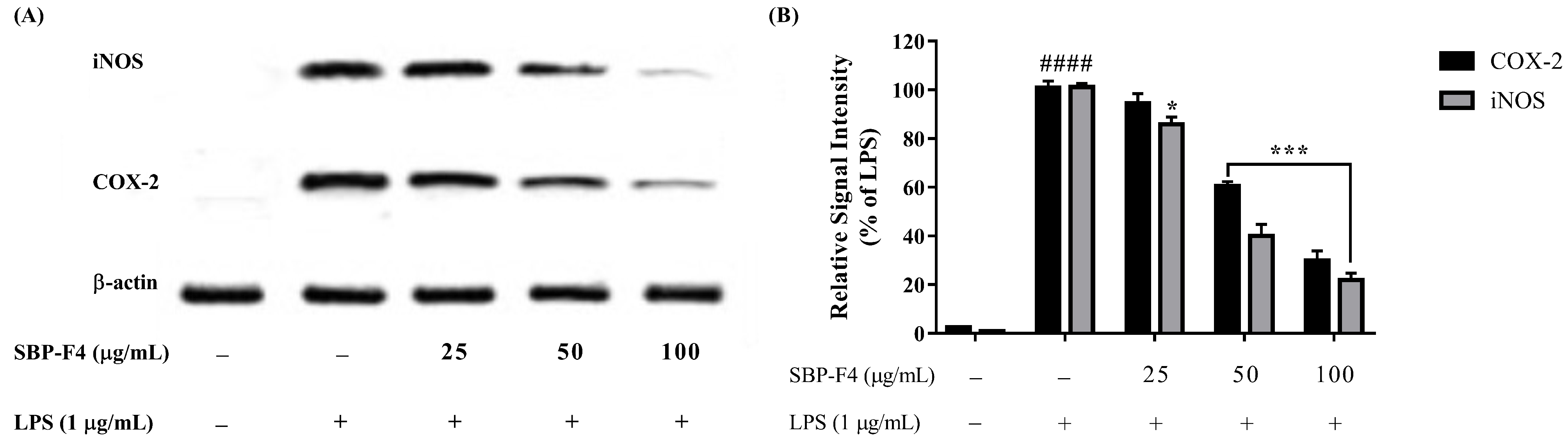
3.3. Anti-Inflammatory Effect of Sulfated Polysaccharides on LPS-Activated RAW 264.7
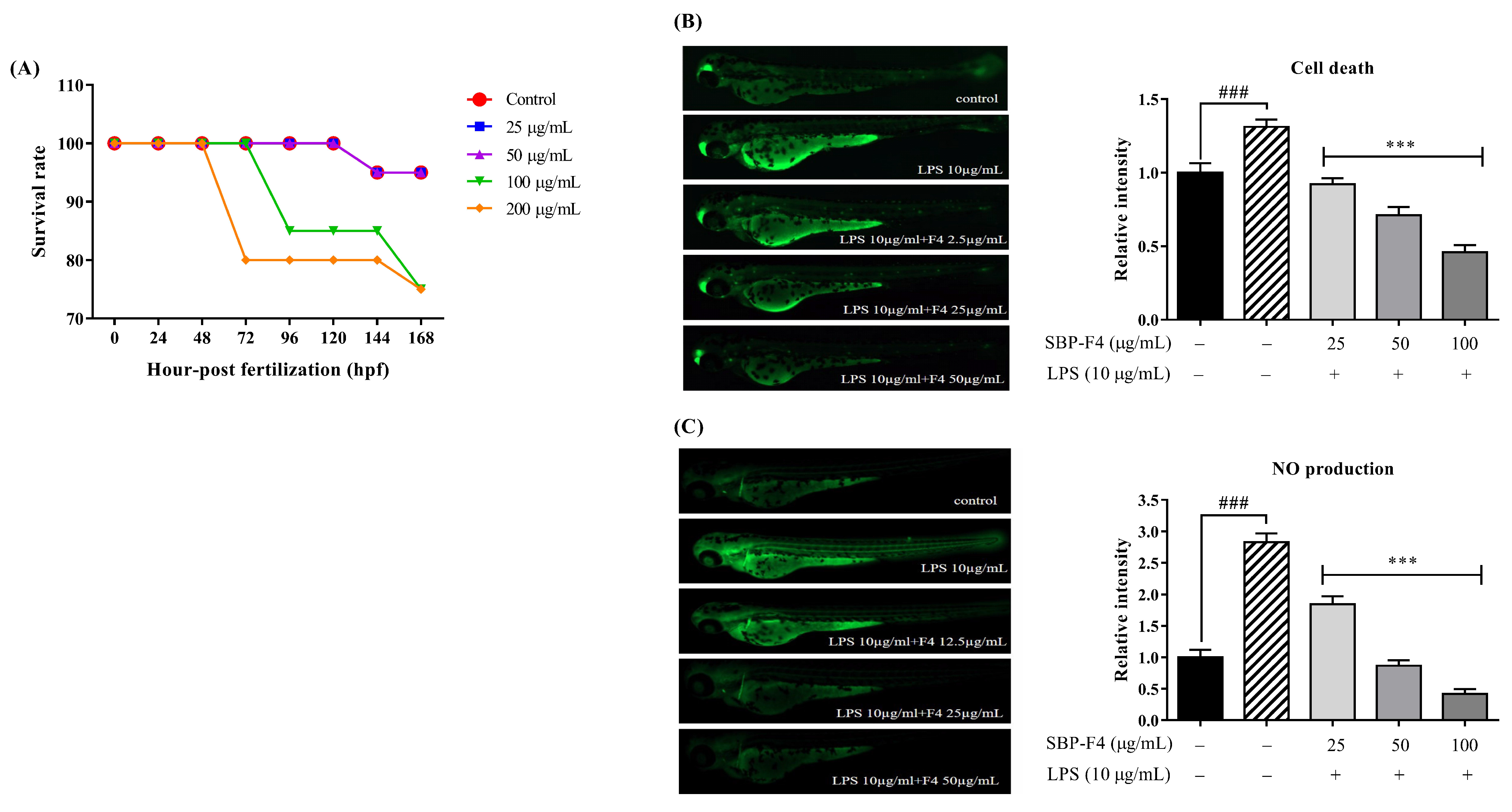
3.4. Anti-Inflammatory Effect of Sulfated Polysaccharide on LPS-Treated Zebrafish Larvae
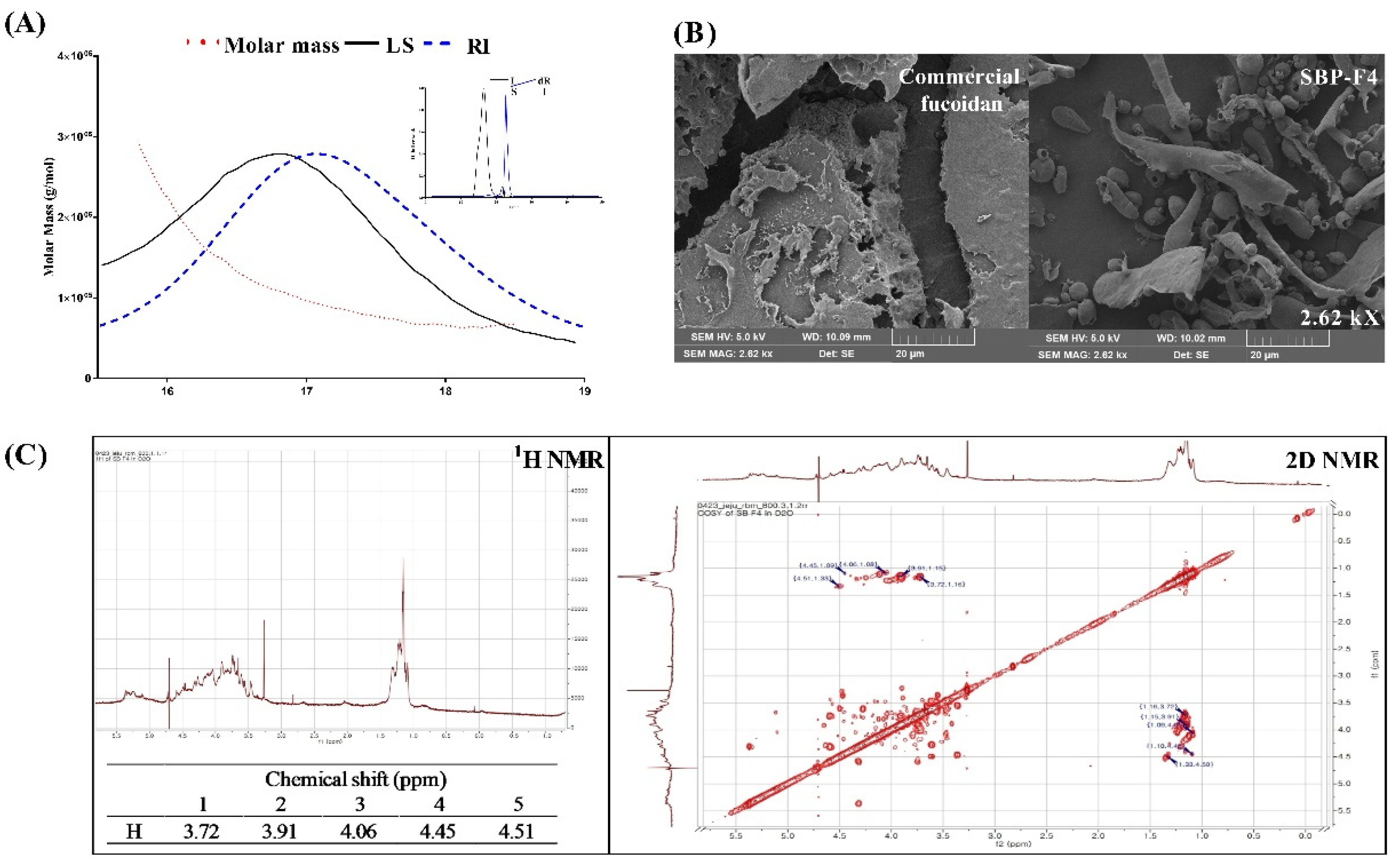
3.5. Characterization of SBP-F4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Husni, A.; Lailatussifa, R.; Isnansetyo, A. Sargassum hystrix as a source of functional food to improve blood biochemistry profiles of rats under stress. Prev. Nutr. Food Sci. 2019, 24, 150. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Shevchenko, N.M.; Malyarenko, O.S.; Anastyuk, S.D.; Kasprik, A.E.; Zvyagintsev, N.V.; Ermakova, S.P. Fucoidans from brown algae Laminaria longipes and Saccharina cichorioides: Structural characteristics, anticancer and radiosensitizing activity in vitro. Carbohydr. Polym. 2019, 221, 157–165. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Bursać Kovačević, D.; Elez Garofulić, I.; Dragović-Uzelac, V. Advanced technologies for the extraction of marine brown algal polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Yugay, Y.A.; Usoltseva, R.V.; Silant’ev, V.E.; Egorova, A.E.; Karabtsov, A.A.; Kumeiko, V.V.; Ermakova, S.P.; Bulgakov, V.P.; Shkryl, Y.N. Synthesis of bioactive silver nanoparticles using alginate, fucoidan and laminaran from brown algae as a reducing and stabilizing agent. Carbohydr. Polym. 2020, 245, 116547. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, B.; Liu, Q.; Kong, B.; Wang, H. Fabrication and characterization of a novel polysaccharide based composite nanofiber films with tunable physical properties. Carbohydr. Polym. 2020, 236, 116054. [Google Scholar] [CrossRef]
- Venkatesan, M.; Arumugam, V.; Pugalendi, R.; Ramachandran, K.; Sengodan, K.; Vijayan, S.R.; Sundaresan, U.; Ramachandran, S.; Pugazhendhi, A. Antioxidant, anticoagulant and mosquitocidal properties of water soluble polysaccharides (WSPs) from Indian seaweeds. Process Biochem. 2019, 84, 196–204. [Google Scholar] [CrossRef]
- de Araújo, I.W.F.; Vanderlei, E.D.S.O.; Rodrigues, J.A.G.; Coura, C.O.; Quinderé, A.L.G.; Fontes, B.P.; de Queiroz, I.N.L.; Jorge, R.J.B.; Bezerra, M.M.; e Silva, A.A.R.; et al. Effects of a sulfated polysaccharide isolated from the red seaweed Solieria filiformis on models of nociception and inflammation. Carbohydr. Polym. 2011, 86, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K. The functional effects of anti-microbial activity and anti-inflammatory of seaweed polysaccharide extracts. J. Converg. Cult. Technol. 2018, 4, 161–169. [Google Scholar]
- Sanjeewa, K.K.A.; Lee, J.-S.; Kim, W.-S.; Jeon, Y.-J. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.; Gao, X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef]
- Jo, B.W.; Choi, S.-K. Degradation of fucoidans from Sargassum fulvellum and their biological activities. Carbohydr. Polym. 2014, 111, 822–829. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Macquarrie, D.J.C.P. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan extracted from Undaria pinnatifida: Source for nutraceuticals/functional foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, H.-L.; Tai, C.-J.; Huang, C.-W.; Chang, F.-R.; Wang, J.-Y. Efficacy of low-molecular-weight fucoidan as a supplemental therapy in metastatic colorectal cancer patients: A double-blind randomized controlled trial. Mar. Drugs 2017, 15, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.; Trang, V.T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S. Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Mar. Drugs 2020, 18, 296. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Kelly, M.; Sanderson, C.J.; Nifantiev, N.E.; Usov, A.I. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr. Res. 2010, 345, 2038–2047. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Shevchenko, N.M.; Zvyagintseva, T.N.; Imbs, T.I. Monthly changes in the content and monosaccharide composition of fucoidan from Undaria pinnatifida (Laminariales, Phaeophyta). J. Appl. Phycol. 2010, 22, 79–86. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, S.-Y.; Kim, H.-S.; Ahn, G.; Jee, Y.; Jeon, Y.-J. In vitro and in vivo anti-inflammatory activities of high molecular weight sulfated polysaccharide; containing fucose separated from Sargassum horneri: Short communication. Int. J. Biol. Macromol. 2018, 107, 803–807. [Google Scholar] [CrossRef]
- Yip, W.H.; Joe, L.S.; Mustapha, W.A.W.; Maskat, M.Y.; Said, M. Characterisation and stability of pigments extracted from Sargassum binderi obtained from Semporna, Sabah. Sains Malays. 2014, 43, 1345–1354. [Google Scholar]
- Stegenga, H. Sri Lankan seaweeds: Methodologies and field guide to the dominant species. Bot. Mar. 2011. [Google Scholar] [CrossRef]
- Peranginangin, R.; Saepudin, E.; Postharvest, F. Purification and characterization of fucoidan from the brown seaweed Sargassum binderi Sonder. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2016, 10, 79–87. [Google Scholar]
- Fernando, I.S.; Jayawardena, T.U.; Kim, H.-S.; Lee, W.W.; Vaas, A.; De Silva, H.; Abayaweera, G.; Nanayakkara, C.; Abeytunga, D.; Lee, D.-S. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 172, 150–158. [Google Scholar] [CrossRef]
- Moni, S.S.; Alam, M.F.; Safhi, M.M.; Jabeen, A.; Sanobar, S.; Siddiqui, R.; Moochikkal, R. Potency of nano-antibacterial formulation from Sargassum binderi against selected human pathogenic bacteria. Braz. J. Pharm. Sci. 2018, 54. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.J.; Mustapha, W.A.W.; Maskat, M.Y.; Latip, J.; Badri, K.H.; Hassan, O. Chemical properties and toxicology studies of fucoidan extracted from Malaysian Sargassum binderi. Food Sci. Biotechnol. 2016, 25, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Aida, W.M.W.; Maskat, M.Y.; Latip, J.; Badri, K.H.; Hassan, O.; Yamin, B.M. Characterisation of fucoidan extracted from Malaysian Sargassum binderi. Food Chem. 2016, 209, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.S.; Sanjeewa, K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kang, N.; Ranasinghe, P.; Lee, H.-S.; Jeon, Y.-J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef]
- Wang, Q.; Song, Y.; He, Y.; Ren, D.; Kow, F.; Qiao, Z.; Liu, S.; Yu, X. Structural characterisation of algae Costaria costata fucoidan and its effects on CCl4-induced liver injury. Carbohydr. Polym. 2014, 107, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Lee, H.G.; Kim, H.-S.; Vaas, A.P.; De Silva, H.I.C.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.-S.; Lee, J.-S. Fucoidan purified from Sargassum polycystum induces apoptosis through mitochondria-mediated pathway in HL-60 and MCF-7 cells. Mar. Drugs 2020, 18, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.-C.; Lee, H.; Choi, H.-D.; Jeon, Y.-J. Antioxidant properties of a sulfated polysaccharide isolated from an enzymatic digest of Sargassum thunbergii. Int. J. Biol. Macromol. 2019, 132, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wang, C.; Yu, Y.-B.; Wu, L.-X.; Chen, T.-T.; Wang, Z.-W. Physicochemical characteristics and in vitro biological activities of polysaccharides derived from raw garlic (Allium sativum L.) bulbs via three-phase partitioning combined with gradient ethanol precipitation method. Food Chem. 2021, 339, 128081. [Google Scholar] [CrossRef]
- Yoon, W.-J.; Moon, J.; Song, G.; Lee, Y.; Han, M.; Lee, J.; Ihm, B.; Lee, W.; Lee, N.; Hyun, C.J.F.; et al. Artemisia fukudo essential oil attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 1222–1229. [Google Scholar] [PubMed]
- Ko, E.-Y.; Cho, S.-H.; Kwon, S.-H.; Eom, C.-Y.; Jeong, M.S.; Lee, W.; Kim, S.-Y.; Heo, S.-J.; Ahn, G.; Lee, K.P.; et al. The roles of NF-κB and ROS in regulation of pro-inflammatory mediators of inflammation induction in LPS-stimulated zebrafish embryos. Fish Shellfish Immunol. 2017, 68, 525–529. [Google Scholar] [CrossRef]
- Kim, E.-A.; Kim, S.-Y.; Ye, B.-R.; Kim, J.; Ko, S.-C.; Lee, W.W.; Kim, K.-N.; Choi, I.-W.; Jung, W.-K.; Heo, S.-J. Anti-inflammatory effect of Apo-9′-fucoxanthinone via inhibition of MAPKs and NF-kB signaling pathway in LPS-stimulated RAW 264.7 macrophages and zebrafish model. Int. Immunopharmacol. 2018, 59, 339–346. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Kim, H.S.; Lee, W.; Cui, Y.; Lee, H.G.; Kim, Y.-T.; Ko, J.Y.; Jeon, Y.-J. Protective effect of polysaccharides from Celluclast-assisted extract of Hizikia fusiforme against hydrogen peroxide-induced oxidative stress in vitro in Vero cells and in vivo in zebrafish. Int. J. Biol. Macromol. 2018, 112, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.P.; Mourão, P.J. Occurrence of a unique fucose-branched chondroitin sulfate in the body wall of a sea cucumber. J. Biol. Chem. 1988, 263, 18176–18183. [Google Scholar] [CrossRef]
- Takamatsu, S.; Hodges, T.W.; Rajbhandari, I.; Gerwick, W.H.; Hamann, M.T.; Nagle, D.G. Marine natural products as novel antioxidant prototypes. J. Nat. Prod. 2003, 66, 605–608. [Google Scholar] [CrossRef] [Green Version]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.-J.; Xu, J.; Gao, X. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef]
- Dore, C.M.P.G.; Faustino Alves, M.G.d.C.; Pofírio Will, L.S.E.; Costa, T.G.; Sabry, D.A.; de Souza Rêgo, L.A.R.; Accardo, C.M.; Rocha, H.A.O.; Filgueira, L.G.A.; Leite, E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, E.-A.; Ahn, G.; Jee, Y.; Jeon, Y.-J. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr. Res. Pract. 2017, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Sanjeewa, K.A.; Jayawardena, T.U.; Kim, S.-Y.; Kim, H.-S.; Ahn, G.; Kim, J.; Jeon, Y.-J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Wang, L.; Sanjeewa, K.K.A.; Kang, S.I.; Lee, J.-S.; Jeon, Y.-J. Antioxidant Potential of Sulfated Polysaccharides from Padina boryana; Protective Effect against Oxidative Stress in In Vitro and In Vivo Zebrafish Model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.-H.; Heo, S.-J.; Yang, H.-W.; Ko, E.-Y.; Jung, M.S.; Cha, S.-H.; Ahn, G.; Jeon, Y.-J.; Kim, K.-N. Protective effect of 3-bromo-4, 5-dihydroxybenzaldehyde from Polysiphonia morrowii harvey against hydrogen peroxide-induced oxidative stress in vitro and in vivo. J. Microbiol. Biotechnol. 2019, 29, 1193–1203. [Google Scholar] [CrossRef]
- He, K.; Mergens, B.; Yatcilla, M.; Zheng, Q.; Bao, Z.; Zhang, Y.; Li, X.; Xie, Z. Molecular weight determination of aloe polysaccharides using size exclusion chromatography coupled with multi-angle laser light scattering and refractive index detectors. J. Aoac Int. 2018, 101, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jayawardena, T.U.; Yang, H.-W.; Lee, H.G.; Kang, M.-C.; Sanjeewa, K.K.A.; Oh, J.Y.; Jeon, Y.-J. Isolation, Characterization, and Antioxidant Activity Evaluation of a Fucoidan from an Enzymatic Digest of the Edible Seaweed, Hizikia fusiforme. Antioxidants 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.K.; Balakrishnan, M.; Ulbricht, M. Sugarcane juice ultrafiltration: FTIR and SEM analysis of polysaccharide fouling. J. Membr. Sci. 2007, 306, 287–297. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Fernando, I.P.S.; Lee, W.W.; Sanjeewa, K.K.A.; Kim, H.-S.; Lee, D.-S.; Jeon, Y.-J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef] [PubMed]

| Monosaccharide composition (molar ratio) | SBP-F1 | SBP-F2 | SBP-F3 | SBP-F4 | |
| Fucose | 1.67 | 1.24 | 4.35 | 8.00 | |
| Galactose | 1.25 | 1.34 | 5.84 | 7.61 | |
| Glucose | 1 | 1 | 1 | 1 | |
| Mannose | 0.98 | 1.56 | 2.51 | N.D. | |
| Arabinose | N.D. | 0.17 | 0.40 | 0.19 | |
| Rhamnose | 0.37 | 0.41 | 0.52 | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Je, J.-G.; Lee, H.-G.; Fernando, K.H.N.; Jeon, Y.-J.; Ryu, B. Purification and Structural Characterization of Sulfated Polysaccharides Derived from Brown Algae, Sargassum binderi: Inhibitory Mechanism of iNOS and COX-2 Pathway Interaction. Antioxidants 2021, 10, 822. https://doi.org/10.3390/antiox10060822
Je J-G, Lee H-G, Fernando KHN, Jeon Y-J, Ryu B. Purification and Structural Characterization of Sulfated Polysaccharides Derived from Brown Algae, Sargassum binderi: Inhibitory Mechanism of iNOS and COX-2 Pathway Interaction. Antioxidants. 2021; 10(6):822. https://doi.org/10.3390/antiox10060822
Chicago/Turabian StyleJe, Jun-Geon, Hyo-Geun Lee, Kurukulasuriya H. N. Fernando, You-Jin Jeon, and Bomi Ryu. 2021. "Purification and Structural Characterization of Sulfated Polysaccharides Derived from Brown Algae, Sargassum binderi: Inhibitory Mechanism of iNOS and COX-2 Pathway Interaction" Antioxidants 10, no. 6: 822. https://doi.org/10.3390/antiox10060822
APA StyleJe, J. -G., Lee, H. -G., Fernando, K. H. N., Jeon, Y. -J., & Ryu, B. (2021). Purification and Structural Characterization of Sulfated Polysaccharides Derived from Brown Algae, Sargassum binderi: Inhibitory Mechanism of iNOS and COX-2 Pathway Interaction. Antioxidants, 10(6), 822. https://doi.org/10.3390/antiox10060822







