Environmental Factors-Induced Oxidative Stress: Hormonal and Molecular Pathway Disruptions in Hypogonadism and Erectile Dysfunction
Abstract
:1. Introduction
2. Methods
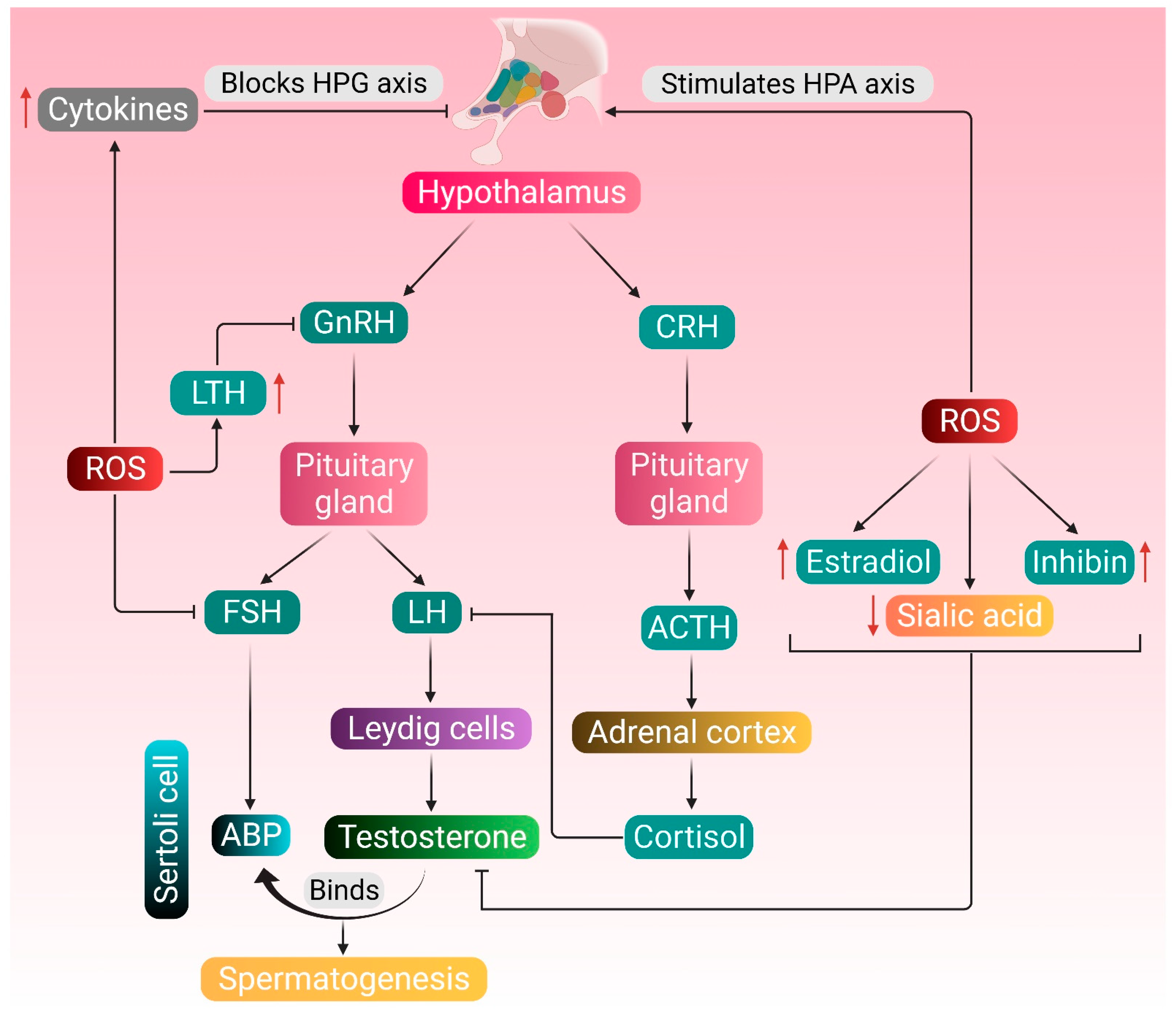
3. Testosterone Metabolism, Spermatogenesis, and Reactive Oxygen Species (ROS)
4. Etiology and Pathogenesis
4.1. Hypogonadism
4.2. Erectile Dysfunction (ED)
5. Environmental Factors
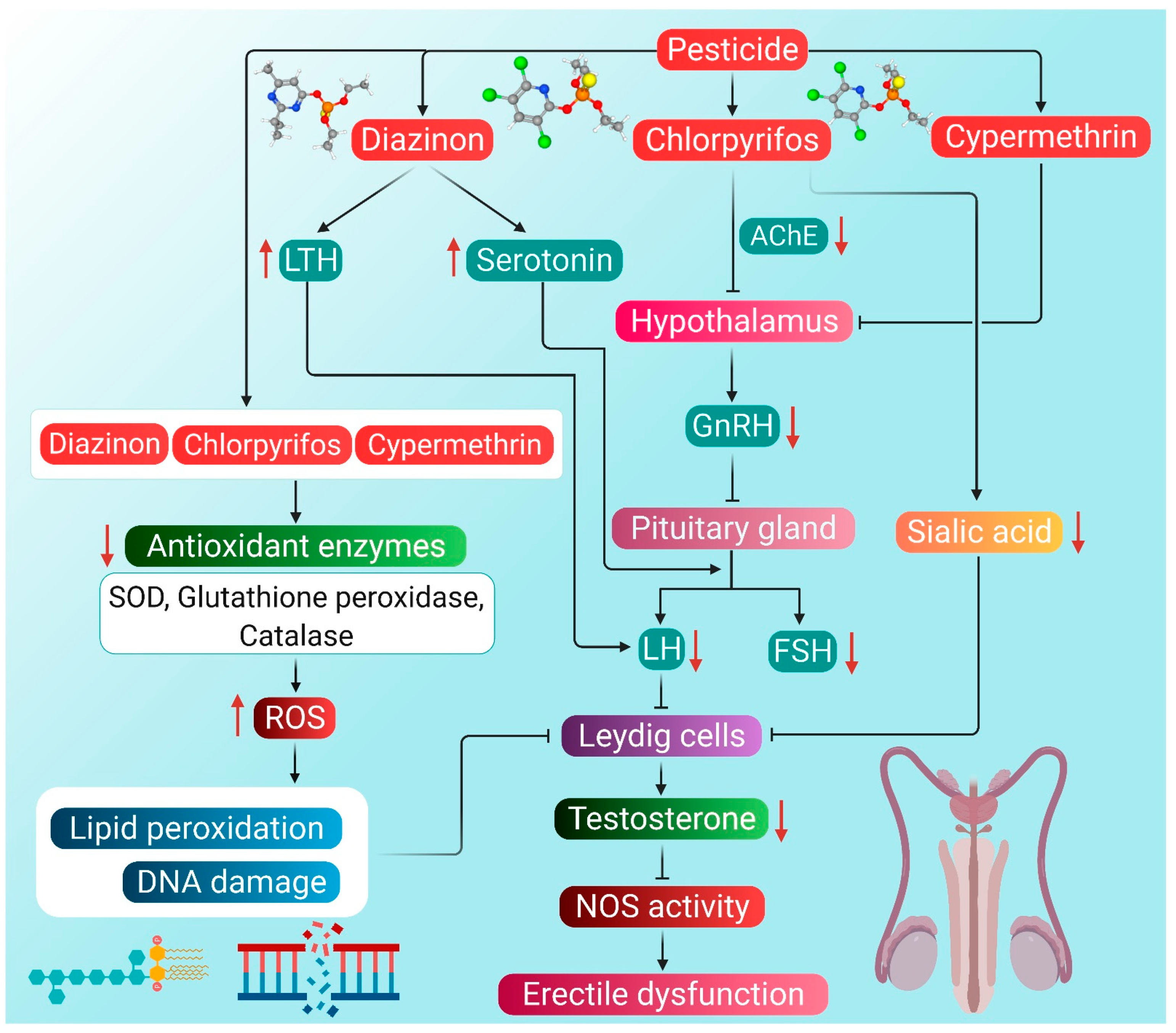
5.1. Pesticides
5.2. Radiation
5.2.1. Nonionizing Radiation
5.2.2. Ionizing Radiation
5.3. Air Pollution
5.3.1. Heavy Metal Pollution
5.3.2. Particulate Matter (PM2.5) Pollution
5.4. Other Endocrine-Disrupting Chemicals
5.4.1. Agents Originating in Plastics
5.4.2. Polychlorinated Biphenyl (PCB)
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jungwirth, A.; Giwercman, A.; Tournaye, H.; Diemer, T.; Kopa, Z.; Dohle, G.; Krausz, C. European Association of Urology guidelines on male infertility: The 2012 update. Eur. Urol. 2012, 62, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Tüttelmann, F.; Nieschlag, E. Classification of andrological disorders. In Andrology, Male Reproductive Health and Dysfucntion, 3rd ed.; Nieschlag, E., Behre, H.M., Nieschlag, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 87–92. [Google Scholar]
- Salonia, A.; Rastrelli, G.; Hackett, G.; Seminara, S.B.; Huhtaniemi, I.T.; Rey, R.A.; Hellstorm, W.J.G.; Palmert, M.R.; Corona, G.; Dohle, G.R.; et al. Paediatric and adult-onset hypogonadism. Nat. Rev. Dis. Primers 2019, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Sizar, O.; Schwartz, J. Hypogonadism; Stat Pearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Kim, K.M. Late-onset hypogonadism. Korean J. Fam. Pract. 2013, 3, 245–254. [Google Scholar]
- European Association of Urology. Guidelines on Male Hypogonadism. Available online: http://uroweb.org/wp-content/uploads/EAU-Guidelines-Male-Hypogonadism-2015.pdf (accessed on 15 February 2021).
- Huhtaniemi, I. Late-onset hypogonadism: Current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J. Androl. 2014, 16, 192–202. [Google Scholar] [CrossRef]
- Huhtaniemi, I.; Makinen, J.I.; Perheentupa, A.; Raitakari, O.T. Late-onset hypogonadism in men. Experience form Turku Male Aging Study (TuMAS). Hormones 2008, 7, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.Y. Awareness and knowledge of andropause among Chinese males in Hong Kong. Am. J. Men’s Health 2009, 4, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Guay, A.; Seftel, A.D.; Traish, A. Hypogonadism in men with erectile dysfunction may be related to a host of chronic illness. Int. J. Impot. Res. 2010, 22, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Shamloul, R.; Ghanem, H. Erectile dysfunction. Lancet 2013, 381, 153–165. [Google Scholar] [CrossRef]
- Agarwal, A.; Nandipati, K.C.; Sharma, R.K.; Zippe, C.D.; Raina, R. Role of oxidative stress in the pathophysiological mechanisms of erectile dysfunction. J. Androl. 2006, 27, 335–347. [Google Scholar] [CrossRef] [Green Version]
- Yafi, F.A.; Jenkins, L.; Albersen, M.; Corona, G.; Isidori, A.M.; Goldfarb, S.; Maggi, M.; Nelson, C.J.; Parish, S.; Salonia, A.; et al. Erectile dysfunction. Nat. Rev. Dis. Primers 2016, 2, 16003. [Google Scholar] [CrossRef]
- Rew, K.T.; Heidelbaugh, J.J. Erectile dysfunction. Am. Fam. Phys. 2016, 94, 820–827. [Google Scholar]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Darbandi, S.; Darbandi, M. Lifestyle modifications on further reproductive problems. Cresco J. Reprod. Sci. 2016, 1, 1–2. [Google Scholar]
- Zirkin, B.R.; Chen, H. Regulation of Leydig cell steroidogenic function during aging. Biol. Reprod. 2000, 63, 977–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, T.T.; Bang, H.J.; Lysiak, J.J. Experimental testicular torsion: Reperfusion blood flow and subsequent testicular venous plasma testosterone concentrations. Urology 2005, 65, 390–394. [Google Scholar] [CrossRef]
- Cartledge, J.; Minhas, S.; Eardley, I. The role of nitric oxide in penile erection. Expert Opin. Pharmacother. 2001, 2, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.W.A.; Rees, R.W.; Minhas, S.; Ralph, D.; Persad, R.A.; Jeremy, J.Y. Oxygen free radicals and the penis. Expert Opin. Pharmacother. 2002, 3, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, J.Y.; Jones, R.A.; Koupparis, A.J.; Hotston, M.; Persad, R.; Angelini, G.D.; Shukla, N. Reactive oxygen species and erectile dysfunction: Possible role of NADPH oxidase. Int. J. Impot. Res. 2007, 19, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, E.; Parivar, K.; Jorsaraei, S.G.A.; Moghadamnia, A.A. The effects of diazinon on testosterone, FSH and LH levels and testicular tissue in mice. Int. J. Reprod. Biomed. 2009, 7, 59–64. [Google Scholar]
- Kesari, K.K.; Kumar, S.; Behari, J. Effects of radiofrequency electromagnetic wave exposure from cellular phones on the reproductive pattern in male wistar rats. Appl. Biochem. Biotechnol. 2011, 164, 546–559. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Li, J.; Bai, Y.; Tang, Y.; Han, Y. Effects of fine particulate matter (PM2.5) on erectile function and its potential mechanism in rats. Urology 2016, 102, e9–e265. [Google Scholar] [CrossRef]
- Alaee, S.; Talaiekhozani, A.; Rezaei, S.; Alaee, K.; Yousefian, E. Cadmium and male fertility. J. Int. Reprod. Biol. 2014, 2, 62–69. [Google Scholar]
- Rahman, M.S.; Pang, M.G. Understanding the molecular mechanisms of bisphenol A action in spermatozoa. Clin. Exp. Reprod. Med. 2019, 46, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Clavijo, R.I.; Hsiao, W. Update on male reproductive endocrinology. Trans. Androl. Urol. 2018, 7, 367–372. [Google Scholar] [CrossRef]
- Tsai, M.-Y.; Yeh, S.-D.; Wang, R.-S.; Yeh, S.; Zhang, C.; Lin, H.-Y.; Tzeng, C.-R.; Chang, C. Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells. Proc. Natl. Acad. Sci. USA 2006, 103, 18975–18980. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.C.; Mathur, R.; Gulati, N. Testicular toxicity of chlorpyrifos (an organophosphate pesticide) in albino rat. Toxicol. Ind. Health 2007, 23, 439. [Google Scholar] [CrossRef]
- Chinoy, N.J.; Bhattacharya, S. Effects of chronic administration of aluminium chloride on reproductive function of testis and some accessory sex organs of male mice. Ind. J. Environ. Toxicol. 1997, 7, 12–22. [Google Scholar]
- Johnson, L.; Thompson, D.L., Jr.; Varner, D.D. Role of Sertoli cell number and function on regulation of spermatogenesis. Anim. Reprod. Sci. 2008, 105, 23–51. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Wong, E.W.P.; Lie, P.P.Y.; Li, M.W.M.; Su, L.; Siu, E.R.; Yan, H.H.N.; Mannu, J.; Mathur, P.P.; Bonanomi, M.; et al. Environmental toxicants and male reproductive functions. Spermatogenesis 2010, 1, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Li, M.W.M.; Mruk, D.D.; Cheng, C.Y. Gap junctions and blood-testis barriers. Adv. Exp. Med. Biol. 2012, 763, 260–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, E.R.; Mruk, D.D.; Porto, C.S.; Cheng, C.Y. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009, 238, 240–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mruk, D.D.; Cheng, C.Y. Environmental contaminants: Is male reproductive health at risk? Spermatogenesis 2011, 1, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pointis, G.; Gilleron, J.; Carette, D.; Segretain, D. Testicular connexin 43, a precocious molecular target for the effect of environmental toxicants on male fertility. Spermatogenesis 2011, 1, 303–317. [Google Scholar] [CrossRef] [Green Version]
- Pierantoni, R.; Cobellis, G.; Meccariello, R.; Fasano, S. Evolutionary aspects of cellular communication in the vertebrate hypothalamo-hypophysio-gonadal axis. Int. Rev. Cytol. 2002, 218, 69–141. [Google Scholar] [CrossRef]
- Madhukar, D.; Rajender, S. hormonal treatment of male infertility: Promises and pitfalls. J. Androl. 2009, 30, 95–112. [Google Scholar] [CrossRef]
- Magon, N.; Singh, S.; Saxena, A.; Sahay, R. Growth hormone in male infertility. Indian J. Endocrinol. Metab. 2011, 15, 248–249. [Google Scholar] [CrossRef]
- Gangwar, P.K.; Sankhwar, S.N.; Pant, S.; Krishna, A.; Singh, B.P.; Mahdi, A.A.; Singh, R. Increased gonadotropins and prolactin are linked to infertility in males. Bioinformation 2020, 16, 176–182. [Google Scholar] [CrossRef]
- Lu, C.; Yang, W.; Chen, M.; Liu, T.; Yang, J.; Tan, P.; Li, L.; Hu, X.; Fan, C.; Hu, Z.; et al. Inhibin A inhibits follicle-stimulating hormone (FSH) action by supressing its receptor expression in cultured rat granulosa cells. Mol. Cell Endocrinol. 2009, 298, 48–56. [Google Scholar] [CrossRef]
- Vermeulen, A. Androgens in the aging male. J. Clin. Endocrinol. Metab. 1991, 73, 221–224. [Google Scholar] [CrossRef]
- Morley, J.E.; Kaiser, F.E.; Perry, H.M., III; Patrick, P.; Morley, P.M.; Stauber, P.M.; Vellas, B.; Baumgartenr, R.N.; Garry, P.J. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism 1997, 46, 410–413. [Google Scholar] [CrossRef]
- Seidman, S.N. Testosterone deficiency and mood in aging men: Pathogenic and therapeutic interactions. World J. Biol. Psychiatry 2003, 4, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.; Pangas, S.A.; Bernard, D.J.; Wang, E.; Gitch, J.; Chen, W.; Draper, L.B.; Cox, E.T.; Woodruff, T.K. Structure and expression of a membrane component of the inhibin receptor system. Endocrinology 2000, 141, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, M.; Juul, A.; Feldt-Rasmussen, U.; Jorgensen, N. Semen quality in patients with pituitary disease and adult-onset hypogonadotropic hypogonadism. Endocr. Connect 2018, 7, 523–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, I.D.; Fronczak, C.; Roth, L.; Meacham, R.B. Fertility and the aging male. Rev. Urol. 2011, 13, e184–e190. [Google Scholar] [PubMed]
- Schwartz, D.; Mayaux, M.J.; Spira, A.; Moscato, M.L.; Jouannet, P.; Czyglik, F.; David, G. Semen characteristics as a function of age in 833 fertile men. Fertil. Steril. 1983, 39, 530–535. [Google Scholar] [CrossRef]
- Hellstorm, W.J.G.; Sikka, S.C. Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. J. Urol. 2006, 176, 1529–1533. [Google Scholar] [CrossRef]
- Elzanaty, S.; Erenpreiss, J.; Becker, C. Seminal plasma albumin: Origin and relation to the male reproductive parameters. Andrologia 2007, 39, 60–65. [Google Scholar] [CrossRef]
- Tang, W.H.; Zhuang, X.J.; Shu, R.M.; Guan, D.; Ji, Y.D.; Zhang, B.L.; Wang, C.G.; Zhuang, L.H.; Yang, Z.; Hong, K.; et al. The prevalence of erectile dysfunction among subjects with late-onset hypogonadism: A population-based study in China. Int. J. Clin. Exp. Med. 2015, 8, 13901–13910. [Google Scholar]
- Wu, F.C.W.; Tajar, A.; Beynon, J.M.; Pye, S.R.; Silman, A.J.; Finn, J.D.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Forti, G.; et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Eugster, E.A. Etiology and treatment of hypogonadism in adolescents. Pediatr. Clin. N. Am. 2011, 58, 1181–1200. [Google Scholar] [CrossRef] [Green Version]
- Brauner, R.; Neve, M.; Allali, S.; Trivin, C.; Lottmann, H.; Bashaamboo, A.; McElreavey, K. Clinical, biological and genetic analysis of anorchia in 26 boys. PLoS ONE 2011, 6, e23292. [Google Scholar] [CrossRef] [PubMed]
- Rodprasert, W.; Virtanen, H.E.; Makela, J.-A.; Toppari, J. Hypogonadism and cryptorchidism. Front. Endocrinol. 2020, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, N.; Thakur, D.S.; Patidar, A. Male hypogonadism: Symptoms and treatment. J. Adv. Pharm. Technol. Res. 2010, 1, 297–301. [Google Scholar] [CrossRef]
- Achermann, J.C.; Gu, W.X.; Kotlar, T.J.; Meeks, J.J.; Sabacan, L.P.; Seminara, S.B.; Habiby, R.L.; Hindmarsh, P.C.; Bick, D.P.; Sherins, R.J.; et al. Mutational analysis of DAX1 in patients with hypogonadotropic hypogonadism or pubertal delay. J. Clin. Endocrinol. Metab. 1999, 84, 4497–4500. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, K.L.; Stewart, F.; Larson, B.M. Approaches to male hypogonadism in primary care. Nurse Pract. 2017, 42, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Pelusi, C.; Gasparini, D.I.; Bianchi, N.; Pasquali, R. Endocrine dysfunction in hereditary hemochromatosis. J. Endocrinol. Investig. 2016, 39, 837–847. [Google Scholar] [CrossRef]
- Osta, R.E.; Grandpre, N.; Monnin, N.; Hubert, J.; Koscinski, I. Hypogonadotropic hypogonadism in men with hereditary hemochromatosis. Basic Clin. Androl. 2017, 27, 13. [Google Scholar] [CrossRef] [Green Version]
- Leichtmann-Bardoogo, Y.; Cohen, L.A.; Weiss, A.; Marohn, B.; Schubert, S.; Meinhardt, A.; Meyron-Holtz, E.G. Compartmentalization and regulation of iron metabolism proteins protect male germ cells from iron overload. Am. J. Physiol. Endocrinol. Metab. 2012, 302, 1519–1530. [Google Scholar] [CrossRef] [Green Version]
- Nieschlag, E. Late-onset hypogonadism: A concept comes of age. Andrologia 2019, 8, 1506–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaglione, F.; Donde, S.; Hassan, T.A.; Jannini, E.A. Phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction: Pharmacology and clinical impact of the sildenafil citrate orodispersible tablet formulation. Clin. Ther. 2017, 39, 370–377. [Google Scholar] [CrossRef]
- Shridharani, A.N.; Brant, W.O. The treatment of erectile dysfunction in patients with neurogenic disease. Transl. Androl. Urol. 2016, 5, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Hussain, N.H.N.; Noor, N.M.; Mohamed, M.; Sidi, H.; Ismail, S.B. Epidemiology of male sexual dysfunction in Asian and European regions: A systematic review. Am. J. Mens Health 2020, 14, 1557988320937200. [Google Scholar] [CrossRef]
- Dowsett, G.W.; Lyons, A.; Duncan, D.; Wassersug, R.J. Flexibility in men’s sexual practices in response to iatrogenic erectile dysfunction after prostate cancer treatment. Sex. Med. 2014, 2, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, K.; Yajima, M.; Carrier, S.; Morgan, D.M.; Nunes, L.; Lue, T.F.; Iwamoto, T. Delayed testosterone replacement restores nitric oxide synthase-containing nerve fibres and the erectile response in rat penis. BJU Int. 2000, 85, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Soran, H.; Wu, F.C.W. Endocrine causes of erectile dysfunction. Int. J. Androl. 2005, 28, 28–34. [Google Scholar] [CrossRef]
- Lunenfeld, B.; Mskhalaya, G.; Kalinchenko, S.; Tishova, Y. Recommendations on the diagnosis, treatment and monitoring of late-onset hypogonadism in men—A suggested update. Aging Male 2013, 16, 143–150. [Google Scholar] [CrossRef]
- Attia, A.A.; Hassan, F.A.; Kamel, M.I.; Ayoub, M.R. Quality of life in erectile dysfunction patients and their partners responding to tadalafil versus sildenafil citrate. Egypt. J. Dermatol. Venerol. 2013, 33, 32–36. [Google Scholar] [CrossRef]
- Bai, W.J.; Li, H.J.; Jin, J.J.; Xu, W.P.; Sebastian, S.; Wang, X.F. A randomized clinical trial investigating treatment choice in Chinese men receiving sildenafil citrate and tadalafil for treating erectile dysfunction. Asian J. Androl. 2017, 19, 500–504. [Google Scholar] [CrossRef]
- Selvin, E.; Burnett, A.L.; Platz, E.A. Prevalence and risk factors for erectile dysfunction in the US. Am. J. Med. 2007, 120, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Sand, M.S.; Fisher, W.; Rosen, R.; Heiman, J.; Eardly, I. Erectile dysfunction and constructs of masculinity and quality of life in the multinational Men’s Attitudes to Life Events and Sexuality (MALES) study. J. Sex. Med. 2008, 5, 583–594. [Google Scholar] [CrossRef]
- Corona, G.; Boddi, V.; Balercia, G.; Rastrelli, G.; De Vita, G.; Sforza, A.; Forti, G.; Mannucci, E.; Maggi, M. The effect of statin therapy on testosterone levels in subjects consulting for erectile dysfunction. J. Sex. Med. 2010, 4, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Hafez, E.S.E.; Hafez, S.D. Erectile dysfunction: Anatomical parameters, etiology, diagnosis, and therapy. Arch. Androl. 2005, 51, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, A.A.; Mohamed, M.A.; Seftel, A.D.; Neiderberger, C. Hypogonadism is associated with overt depression in men with erectile dysfuncion. Int. J. Impot. Res. 2008, 20, 157–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeulen, A. Environment, human reproduction, menopause, and andropause. Environ. Health Perspect. 1993, 101, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Roychoudhury, S.; Bhattacharjee, R. Environmental issues resulting in andropause and hypogonadism. In Bioenvironmental Issues Affecting Men’s Reproductive and Sexual Health, 1st ed.; Sikka, S.C., Hellstrorm, W.J.G., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 262–273. [Google Scholar]
- Park, S. Genetic factors and environmental factors affecting male infertility. Int. Res. J. Adv. Eng. Sci. 2016, 1, 115–118. [Google Scholar]
- Da Ros, C.T.; Graziottin, T.M. Environmental issues resulting in hypogonadism in Brazilian men. In Bioenvironmental Issues Affecting Men’s Reproductive and Sexual Health, 1st ed.; Sikka, S.C., Hellstrorm, W.J.G., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 33–40. [Google Scholar]
- Tallon, L.A.; Manjourides, J.; Pun, V.C.; Mittleman, M.A.; Kioumourtzoglou, M.A.; Coull, B.; Suh, H. Erectile dysfunction and exposure to ambient Air pollution in a nationally representative cohort of older. Men Environ. Health 2017, 16, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafez, E.M.; Issa, S.Y.; AI-Mazroua, M.K.; Ibrahim, K.T.; Rahman, S.M.A. The neonicotinoid insecticide imidacloprid: A male reproductive system toxicity inducer-human and experimental study. Toxicol. Open Access 2016, 1, 109. [Google Scholar] [CrossRef] [Green Version]
- Wilson, V.S.; Blystone, C.R.; Hotchkiss, A.K.; Rider, C.V.; Gray, L.E., Jr. Diverse mechanisms of anti-androgen action: Impact on male rat reproductive tract development. Int. J. Androl. 2008, 31, 178–187. [Google Scholar] [CrossRef]
- Kaur, R.P.; Gupta, V.; Christopher, A.F.; Bansal, P. Potential pathways of pesticide action on erectile function—A contributory factor in male infertility. Asian Pac. J. Reprod. 2015, 4, 322–330. [Google Scholar] [CrossRef]
- Toman, R.; Tunegová, M. Selenium, cadmium and diazinon insecticide in tissues of rats after peroral exposure. Potr. Slovak J. Food Sci. 2017, 11, 718–724. [Google Scholar] [CrossRef] [Green Version]
- Krockova, J.; Massanyi, P.; Toman, R.; Danko, J.; Roychoudhury, S. In vivo and in vitro effect of bendiocarb on rabbit testicular structure and spermatozoa motility. J. Environ. Sci. Health 2012, 47, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Slimani, S.; Boulakoud, M.S.; Abdennour, C. Pesticide exposure and reproductive biomarkers among male farmers from north-east Algeria. Ann. Biol. Res. 2011, 2, 290–297. [Google Scholar]
- Brook, J.S.; Brook, D.W.; Rosa, M.D.L.; Whiteman, M.; Johnson, E.; Montoya, I. Adolescent illegal drug use: The impact of personality, family, and environmental factors. J. Behav. Med. 2001, 24, 183–203. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyan, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar] [CrossRef]
- Costa, C.; Virag, R. The endothelial-erectile dysfunction connection: An essential update. J. Sex. Med. 2009, 6, 2390–2404. [Google Scholar] [CrossRef]
- Traish, A.M.; Park, K.; Dhir, V.; Kim, N.N.; Moreland, R.B.; Goldstein, I. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology 1999, 140, 1861–1868. [Google Scholar] [CrossRef]
- ElMazoudy, R.H.; Attia, A.A. Endocrine-disrupting and cytotoxic potential of anticholinesterase insecticide, diazinon in reproductive toxicity of male mice. J. Hazard. Mater. 2012, 209–210, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, A.; Cabaleiro, T.; Caride, A.; Esquifino, A.I. Toxic effects of methoxychlor administered subcutaneously on the hypothalamic-pituitary-testicular axis in adult rats. Food Chem. Toxicol. 2008, 46, 1570–1575. [Google Scholar] [CrossRef]
- Lafuente, A.; Gonzalez-Carracedo, A.; Romero, A.; Cano, P.; Esquifino, A.I. Effect of nitric oxide on prolactin secretion and hypothalamic biogenic amine contents. Life Sci. 2004, 74, 1681–1690. [Google Scholar] [CrossRef]
- Svechnikov, K.; Izzo, G.; Landreh, L.; Weisser, J.; Soder, O. Endocrine disruptors and Leydig cell function. J. Biomed. Biotechnol. 2010, 2010, 684504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, C.T.; D’Souza, U.J.A.; Iqbal, M.; Mustapha, Z.A. Lipid peroxidation and decline in antioxidant status as one of the toxicity measures of diazinon in the testis. Redox Rep. 2013, 18, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Bedwal, R.S.; Nair, N.; Mathur, R.S. Effects of zinc deficiency and toxicity on reproductive organs, pregnancy and lactation—A review. Trace Elem. Med. 1991, 8, 89–100. [Google Scholar]
- Farag, A.T.; Radwan, A.H.; Sorour, F.; Okazy, A.E.; El-Agamy, E.; El-Sebae, A.E. Chlorpyrifos induced reproductive toxicity in male mice. Reprod. Toxicol. 2010, 29, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, J.Y.; Tyndale, R.F. Rat brain CYP2B-enzymatic activation of chlorpyrifos to the oxon mediates cholinergic neurotoxicity. Toxicol. Sci. 2012, 126, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Adedara, I.A.; Owoeye, O.; Ajayi, B.O.; Awogbindin, I.O.; Rocha, J.B.T.; Farombi, E.O. Diphenyl diselenide abrogates chlorpyrifos-induced hypothalamic-pituitary-testicular axis impairment in rats. Biochem. Biophys. Res. Commun. 2018, 503, 171–176. [Google Scholar] [CrossRef]
- Sai, L.; Li, X.; Liu, Y.; Guo, Q.; Xie, L.; Yu, G.; Bo, C.; Zhang, Z.; Li, L. Effects of chlorpyrifos on reproductive toxicology of male rats. Environ. Toxicol. 2014, 29, 1083–1088. [Google Scholar] [CrossRef]
- Slimen, S.; Saloua, E.F.; Najoua, G. Oxidative stress and cytotoxic potential of anticholinesterase insecticide, malathion in reproductive toxicology of male adolescent mice after acute exposure. Iran. J. Basic Med. Sci. 2014, 17, 522–530. [Google Scholar]
- Janssens, L.; Stoks, R. Chlorpyrifos-induced oxidative damage is reduced under warming and predation risk: Explaining antagonistic interactions with a pesticide. Environ. Pollut. 2017, 226, 79–88. [Google Scholar] [CrossRef]
- Mandal, T.K.; Das, N.S. Correlation of testicular toxicity and oxidative stress induced by chlorpyrifos in rats. Hum. Exp. Toxicol. 2011, 30, 1529–1539. [Google Scholar] [CrossRef]
- Mandal, T.K.; Das, N.S. Testicular gametogenic and steroidogenic activities in chlorpyrifos insecticide-treated rats: A correlational study with testicular oxidative stress and role of antioxidant enzyme defence systems in Sprague-Dawley rats. Andrologia 2012, 44, 102–115. [Google Scholar] [CrossRef]
- Peiris, D.C.; Dhanushka, T. Low doses of chlorpyrifos interfere with spermatogenesis of rats through reduction of sex hormones. Environ. Sci. Pollut. Res. Int. 2017, 24, 20859–20867. [Google Scholar] [CrossRef]
- Watkins, S.S.; Koob, G.F.; Markou, A. Neural mechanisms underlying nicotine addiction: Acute positive reinforcement and withdrawal. Nicotine Tob. Res. 2000, 2, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Ala-Eldin, E.A.; El-Safei, D.A.; Abouhashem, N.S. Individual and combined effect of chlorpyrifos and cypermethrin on reproductive system of adult male albino rats. Environ. Sci. Pollut. Res. 2017, 24, 1532–1543. [Google Scholar] [CrossRef]
- Sharma, P.; Huq, A.U.; Singh, R. Cypermethrin-induced reproductive toxicity in the rat is preserved by resveratrol. J. Hum. Reprod. Sci. 2014, 7, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Civen, M.; Brown, C.B. The effect of organophosphate insecticides on adrenal corticosterone formation. Pestic. Biochem. Phys. 1947, 4, 254–259. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Sarkar, M.; Biswas, N.M. Dose-dependent effect of copper chloride on male reproductive function in immature rats. Kathmandu Univ. Med. J. 2005, 3, 392–400. [Google Scholar]
- Joshi, S.C.; Bansal, B.; Jasuja, N.D. Evaluation of reproductive and developmental toxicity of cypermethrin in male albino rats. Toxicol. Environ. Chem. 2011, 93, 593–602. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Liu, S.-S.; Sun, Y.; Wu, J.-Y.; Zhou, Y.-L.; Zhang, J.-H. Beta-cypermethrin impairs reproductive function in male mice by inducing oxidative stress. Theriogenology 2009, 72, 599–611. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.-X.; Shen, J.-Y.; Zhang, R.; Hong, J.-W.; Li, Z.; Chen, G.; Li, M.-X.; Ding, Z.; Li, J.; et al. The anti-androgenic effects of cypermethrin mediated by non-classical testosterone pathway activation of mitogen-activated protein kinase cascade in mouse Sertoli cells. Ecotoxicol. Environ. Saf. 2019, 177, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Solati, J.; Hajikhani, R.; Zaeim, R.T. Effects of cypermethrin on sexual behaviour and plasma concentrations of pituitary-gonadal hormones. Int. J. Fertil. Steril. 2010, 4, 23–28. [Google Scholar]
- Avendano, C.; Mata, A.; Sarmiento, C.A.S.; Doncel, G.F. Use of laptop computers connected to internet through Wi-Fi decreases human sperm motility and increases sperm DNA fragmentation. Fertil. Steril. 2012, 97, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.; Kumar, S.; Behari, J. Mobile phone usage and male infertility in Wistar rats. Indian J. Exp. Biol. 2010, 48, 987–992. [Google Scholar] [PubMed]
- Kesari, K.K.; Kumar, S.; Nirala, J.; Siddiqui, M.H.; Behari, J. Biophysical evaluation of radiofrequency electromagnetic field effects on male reproductive pattern. Cell Biochem. Biophys. 2013, 65, 85–96. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.J.; Agarwal, A. The impact of cell phone, laptop computer, and microwave oven usage on male fertility. In Male Infertility: A Complete Guide to Lifestyle and Environmental Factors; du Plessis, S.S., Agarwal, A., Sabanegh, E.S., Jr., Eds.; Springer: New York, NY, USA, 2014; pp. 161–177. [Google Scholar] [CrossRef]
- Kesari, K.K.; Agarwal, A.; Henkel, R. Radiations and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 118. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Tang, Z.; Chen, H.; Wang, L.; Cao, H.; Wang, G.; Xing, J.; Shen, H.; Chen, Q.; Li, D.; et al. Long-term exposure to 4G smartphone radiofrequency electromagneticradiation diminished male reproductive potential by directly disruptingSpock3–MMP2-BTB axis in the testes of adult rats. Sci. Total Environ. 2020, 698, 133860. [Google Scholar] [CrossRef]
- Daniell, H.W.; Clark, J.C.; Pereira, S.E.; Niazi, Z.A.; Ferguson, D.W.; Dunn, S.R.; Figueroa, M.L.; Stratte, P.T. Hypogonadism following prostate-bed radiation therapy for prostate carcinoma. Cancer 2001, 91, 1889–1895. [Google Scholar] [CrossRef]
- Incrocci, L. Radiotherapy for prostate cancer and sexual health. Transl. Androl. Urol. 2015, 4, 124–130. [Google Scholar]
- Huyghe, E.; Matsuda, T.; Daudin, M.; Chevreau, C.; Bachaud, J.M.; Plante, P.; Bujan, L.; Thonneau, P. Fertility after testicular cancer treatments: Results of a large multicenter study. Cancer 2004, 100, 732–737. [Google Scholar] [CrossRef]
- Brydøy, M.; Fosså, S.D.; Klepp, O.; Bremnes, R.M.; Wist, E.A.; Wentzel-Larsen, T.; Dahl, O. Paternity following treatment for testicular cancer. J. Natl. Cancer Inst. 2005, 97, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Huddart, R.A.; Norman, A.; Moynihan, C.; Horwich, A.; Parker, C.; Nicholls, E.; Dearnaley, D.P. Fertility, gonadal and sexual function in survivors of testicular cancer. Br. J. Cancer. 2005, 93, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasqualotto, F.F.; Agarwal, A. Impact of cancers and treatment on male fertility: Radiation effects on spermatogenesis. In Fertility Preservation in Male Cancer Patients; Mulhall, J.P., Applegarth, L.D., Oates, R.D., Schlegel, P.N., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 104–109. [Google Scholar]
- Arnon, J.; Meirow, D.; Lewis-Roness, H.; Ornoy, A. Genetic and teratogenic effects of cancer treatments on gametes and embryos. Hum. Reprod. Update 2001, 7, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Meistrich, M.L. The effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil. Steril. 2013, 100, 1180–1186. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.H.; Hildebrand, K.; Yamamoto, J. An increased frequency of human sperm chromosomal abnormalities after radiotherapy. Mutat. Res. 1986, 174, 219–225. [Google Scholar] [CrossRef]
- Behari, J. Biological correlates of low-level electromagnetic-field exposure. In General, Applied and Systems Toxicology; Wiley: Hoboken, NJ, USA, 2009; p. 109. [Google Scholar] [CrossRef]
- Kesari, K.K.; Behari, J. Evidence for mobile phone radiation exposure effects on reproductive pattern of male rats: Role of ROS. Electromagn. Biol. Med. 2012, 31, 13–222. [Google Scholar] [CrossRef]
- Kumar, S.; Nirala, J.P.; Behari, J.; Paulraj, R. Effect of electromagnetic irradiation produced by 3G mobile phone on male rat reproductive system in a simulated scenario. Indian J. Exp. Biol. 2014, 52, 890–897. [Google Scholar] [PubMed]
- Zilberlicht, A.; Weiner-Megnazi, Z.; Sheinfeld, Y.; Grach, B.; Lahav-Baratz, S.; Dirnfeld, M. Habits of cell phone usage and sperm quality–Does it warrant attention? Reprod. Biomed. 2015, 31, 421–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Deepinder, F.; Sharma, R.K. Effect of cell phone usage on semen analysis in men attending infertility clinic: An observational study. Fertil. Steril. 2008, 89, 124–128. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Jedlicka, J.; Ondruska, L.; Bulla, J.; Massanyi, P.; Kolesarova, A. Does 50 Hz extra low frequency electromagnetic field affect rabbit spermatozoa motility in vitro? Res. J. Biotechnol. 2008, 3, 244–249. [Google Scholar]
- Roychoudhury, S.; Jedlicka, J.; Parkanya, V.; Ondruska, L.; Massanyi, P.; Bulla, J. Influence of a 50 Hz extra low frequency electromagnetic field on spermatozoa motility and fertilization rates in rabbits. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2009, 44, 1041–1047. [Google Scholar] [CrossRef]
- Lukac, N.; Massanyi, P.; Roychoudhury, S.; Capcarova, M.; Tvrda, E.; Knazicka, Z.; Kolesarova, A.; Danko, J. In vitro effects of radiofrequency electromagnetic waves on bovine spermatozoa motility. J. Environ. Sci. Health 2011, 46, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, F.H.F.; Osman, K.; Ismail, H.N.; Chin, K.Y.; Ibrahim, S.F. Adverse effects of wifi–Radiation on male reproductive system: A systematic review. Tohoku J. Exp. Med. 2019, 248, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saygin, M.; Asci, H.; Ozmen, O.; Cankara, F.N.; Dincoglu, D.; Ilhan, I. Impact of 2.45 GHz microwave radiation on testicular inflammatory pathway biomarkers in young rats: Role of gallic acid. Environ. Toxicol. 2016, 31, 1771–1784. [Google Scholar] [CrossRef]
- Jonwal, C.; Sisodia, R.; Saxena, V.K.; Kesari, K.K. Effect of 2.45GHz microwave radiation on fertility pattern in male mice. Gen. Physiol. Biophys. 2018, 37, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.; Kumari, K.; Kumar, J.; Rajamani, P.; Verma, H.N.; Kesari, K.K. Therapeutic approaches of melatonin in microwave radiations-induced oxidative stress-mediated toxicity on male fertility pattern of Wistar rats. Electromagn. Biol. Med. 2013, 1–11. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Wu, T.; Liu, J.Y.; Gao, P.; Li, K.C.; Guo, Q.Y.; Yuan, M.; Lang, H.Y.; Zeng, L.H.; Guo, G.Z. 1950MHz radio frequency electromagnetic radiation inhibits testosterone secretion of mouse Leydig cells. Int. J. Environ. Res. Public Health 2017, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Qin, F.; Cao, H.; Yuan, H.; Guo, W.; Pei, H.; Cao, Y.; Tong, J. 1800MHz radiofrequency fields inhibitstestosteroneproduction viaCaMKI /RORα pathway. Reprod. Toxicol. 2018. [Google Scholar] [CrossRef]
- Song, B.; Wang, F.; Wang, W. Effect of aqueous extract from Morindaofficinalis F. C. Howon microwave-induced hypothalamic-pituitary-testis axis impairment in male Sprague-Dawley rats. Evid. Based Complement. Alternat. Med. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Walker, W.H. Non-classical actions of testosterone and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol.Sci. 2010, 365, 1557–1569. [Google Scholar] [CrossRef] [Green Version]
- Ramaswamy, S.; Weinbauer, G.F. Endocrine control of spermatogenesis: Role of FSH and LH/testosterone. Spermatogenesis 2014, 4, e996025. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Li, T.; Zhang, M.; Feng, Y.; Li, W.; Zhu, X.; Gu, R.; Zhou, L. Effect and safety evaluation of XETHRU X4 radar radiation on sexual hormone levels in mice. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Shahin, S.; Singh, S.P.; Chaturvedi, C.M. 2.45 GHz microwave radiation induced oxidative and nitrosativestress mediated testicular apoptosis: Involvement of a p53 dependent bax-caspase-3 mediated pathway. Environ. Toxicol. 2018, 33, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Behari, J. Microwave exposure affecting reproductive system in male rats. Appl. Biochem. Biotechnol 2010, 162, 416–428. [Google Scholar] [CrossRef]
- Condell, R.A.; Tappel, A.L. Evidence for suitability of glutathione peroxidase as a protective enzyme: Studies of oxidative damage, restoration and proteolysis. Arch. Biochem. Biophy. 1993, 223, 407. [Google Scholar] [CrossRef]
- Russo, A.; Troncoso, N.; Sanchez, F.; Vanella, A. Propolis protects human spermatozoa from DNA damage caused by benzopyrene and exogenous reactive oxygen species. Life Sci. 2006, 78, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Baker, M.A. Oxidative stress, sperm survival and fertility control. Mol. Cell. Endocrinol. 2006, 250, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.; Bansal, M.P. P53 is involved in inducing testicular apoptosis in mice by the altered redox status following tertiary butyl hydroperoxide treatment. Chem. Biol. Interact. 2008, 174, 193–200. [Google Scholar] [CrossRef]
- Li, D.; Ueta, E.; Kimura, T.; Yamamoto, T.; Osaki, T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004, 95, 644–650. [Google Scholar] [CrossRef]
- Mishra, D.P.; Pal, R.; Shaha, C. Changes in cytosolic Ca2+ levels regulate Bcl-xSandBcl-xL expression in spermatogenic cells during apoptotic death. J. Biol. Chem. 2006, 281, 2133–2143. [Google Scholar] [CrossRef] [Green Version]
- Chandra, J.; Samali, A.; Orrenius, S. Triggering and modulation of apo-ptosis by oxidative stress. Free Radic. Biol. Med. 2000, 29, 323–333. [Google Scholar] [CrossRef]
- Lazebnik, Y.A.; Kaufmann, S.H.; Desnoyers, S.; Poirier, G.G.; Earnshaw, W.C. Cleavageofpoly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 1994, 371, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.J.; Leach, D.R.; Warner, G.A.; Heller, C.G. Effect of graded doses of ionizing radiation on the human testis. Radiat. Res. 1974, 59, 665–678. [Google Scholar] [CrossRef]
- Nichols, R.C.; Hu, C.; Bahary, J.P.; Zeitzer, K.L.; Souhami, L.; Leibenhaut, M.H.; Rotman, M.; Gore, E.M.; Balogh, A.G.; McGowan, D.; et al. Serum testosterone changes in patients treated with radiation therapy alone for prostate cancer on NRG oncology RTOG 9408. Adv. Radiat. Oncol. 2017, 2, 608–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiyama, H.; Teh, B.S.; Paulino, A.C.; Yogeswarern, S.; Mai, W.; Xu, B.; Butler, E.B. Serum testosterone level after intensity-modulated radio therapy in low-risk prostate cancer patients: Does testicular dose correlate with testosterone level? J. Radiat. Oncol. 2012, 1, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Pickles, T.; Graham, P.; Members of the British Columbia Cancer Agency Prostate Cohort Outcomes Initiative. What happens to testosterone after prostate radiation mono therapy, and does it matter? J. Urol. 2002, 167, 2448–2452. [Google Scholar] [CrossRef]
- Pompe, R.S.; Karakiewicz, P.I.; Zaffuto, E.; Smith, A.; Bandini, M.; Marchioni, M.; Tian, Z.; Ley-Bannurah, S.; Schiffmann, J.; Delouya, G.; et al. External beam radiotherapy affects serum testosterone in patients with localized prostate cancer. J. Sex. Med. 2017, 14, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Filchenkov, G.N.; Popoff, E.H.; Naumov, A.D. The low dose gamma ionizing radiation impact upon cooperativity of androgen specific proteins. J. Environ. Radioact. 2013. [Google Scholar] [CrossRef]
- Zagars, G.K.; Pollack, A. Serum testosterone levels after external beam radiation for clinically localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 85–89. [Google Scholar] [CrossRef]
- Zagars, G.K. Management of stage I seminoma: Radiotherapy. In Testicular Cancer, Investigation and Management; Horwich, A., Ed.; Chapman & Hall Medical: London, UK, 1991; pp. 83–107. [Google Scholar]
- Shapiro, E.; Kinsella, T.J.; Makuch, R.W.; Fraass, B.A.; Glatstein, E.; Rosenberg, S.A.; Sherins, R.J. Effects of fractionated irradiation on endocrine aspects of testicular function. J. Clin. Oncol. 1985, 3, 1232–1239. [Google Scholar] [CrossRef]
- Oberley-Deegan, R.E.; Steffan, J.J.; Rove, K.O.; Pate, K.M.; Weaver, M.W.; Spasojevic, I.; Frederick, B.; Raben, D.; Meacham, R.B.; Crapo, J.D.; et al. The antioxidant, MnTE-2-PyP, prevents side-effects incurred by prostate cancer irradiation. PLoS ONE 2012, 7, e44178. [Google Scholar] [CrossRef]
- Kimura, M.; Rabbani, Z.N.; Zodda, A.R.; Yan, H.; Jackson, I.L.; Polascik, T.J.; Donatucci, C.F.; Moul, J.W.; Vujaskovic, Z.; Koontz, B.F. Role of oxidative stress in a rat model of radiation-induced erectile dysfunction. J. Sex. Med. 2012, 9, 1535–1549. [Google Scholar] [CrossRef]
- Ji, H.J.; Wang, D.M.; Wu, Y.P.; Niu, Y.Y.; Jia, L.L.; Liu, B.W.; Feng, Q.J.; Feng, M.L. Wuzi Yanzong pill, a Chinese poly herbal formula, alleviates testicular damage in mice induced by ionizing radiation. BMC Complement. Altern. Med. 2016, 16, 509. [Google Scholar] [CrossRef] [Green Version]
- Ezz, M.K.; Ibrahim, N.K.; Said, M.M.; Farrag, M.A. The beneficial radioprotective effect of tomato seed oil against gamma radiation–induced damage in male rats. J. Diet Suppl. 2018, 15, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Chugh, N.A.; Bansal, S.C.; Garg, M.L.; Koul, A. Protective role of Aloe vera against X-ray induced testicular dysfunction. Andrologia 2016, 1–12. [Google Scholar] [CrossRef]
- Chatterjee, A.; Kosmacek, E.A.; Oberly-Deegan, R.E. MnTE-2-PyP treatment, or NOX4 inhibition, protects against radiation-, induced damage in mouse primary prostate fibroblasts by inhibiting the TGF-Beta 1 signaling pathway. Radiat. Res. 2017, 187, 367–381. [Google Scholar] [CrossRef] [Green Version]
- Gorbunov, N.V.; Sharma, P. Protracted oxidative alterations in the mechanism of hematopoietic acute radiation syndrome. Antioxidants 2015, 4, 134–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batinic-Haberle, I.; Benov, L.; Spasojevic, I.; Fridovich, I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl) porphyrin a powerful and potentially useful superoxide dismutase mimic. J. Biol. Chem. 1998, 273, 24521–24528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. 1996, 271, C1424–C1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, M.; Martin, C.; Ullrich, V. Tyrosine nitration as a mechanism of selective inactivation of prostacyclin synthase by peroxynitrite. Biol. Chem. 1997, 378, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Thompsom, C.S.; Mumtaz, F.H.; Mikhailidis, D.P.; Morgan, R.J.; Bruckdorfer, R.K.; Naseem, K.M. The effect of nitric oxide and peroxynitrate on rabbit cavernosal smooth muscle relaxation. World J. Urol. 2001, 19, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Steers, W.D. Pharmacologic treatment of erectile dysfunction. Rev. Urol. 2002, 4, S17–S25. [Google Scholar]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A review. Front. Public Health 2002, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Luo, D.; Liu, X.; Zhu, J.; Wang, F.; Li, B.; Li, L. Effects of PM2.5 exposure on reproductive system and its mechanisms. Chemosphere 2021, 264, 128436. [Google Scholar] [CrossRef] [PubMed]
- El-Maraghy, S.A.; Nasssar, N.N. Modulatory effects of lipoic acid and selenium against cadmium-induced biochemical alterations in testicular steroidogenesis. Biochem. Mol. Toxicol. 2011, 25, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Alkhedaide, A.; Alsheri, Z.S.; Sabry, A.; Abdel-Gaffer, T.; Soliman, M.M.; Attia, S. Protective effect of grape seed extract against cadmium-induced testicular dysfunction. Mol. Med. Rep. 2016, 13, 3101–3109. [Google Scholar] [CrossRef]
- Zhang, Q.; Zou, P.; Zhan, H.; Zhang, M.; Zhang, M.; Ge, R.S.; Huang, Y. Dihydrolipoamide dehydrogenase and cAMP are associated with cadmium mediated Leydig cell damage. Toxicol. Lett. 2011, 205, 183–189. [Google Scholar] [CrossRef]
- Kresovich, J.K.; Argos, M.; Turyk, M.E. Associations of lead and cadmium with sex hormones in adult males. Environ. Res. 2015, 142, 25–33. [Google Scholar] [CrossRef]
- El-Magd, M.A.; Kahilo, K.A.; Nasr, N.E.; Kamal, T.; Shukry, M.; Saleh, A.A. A potential mechanism associated with lead-induced testicular toxicity in rats. Andrologia 2017, 49. [Google Scholar] [CrossRef]
- Cacciola, G.; Chioccarelli, T.; Fasano, S.; Pierantoni, R.; Cobellis, G. Estrogens and spermiogenesis: New insights from type 1 cannabinoid receptor knockout mice. Int. J. Endocrinol. 2013, 2013, 501350. [Google Scholar] [CrossRef]
- Carreau, S.; Hess, R.A. Oestrogens and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1517–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreau, S.; de Vienne, C.; Galeraud-Denis, I. Aromatase and estrogens in man reproduction: A review and latest advances. Adv. Med. Sci. 2008, 53, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Elmallah, M.I.Y.; Elkhadragy, M.F.; Al-Olayan, E.M.; Moneim, A.E.A. Protective effect of Fragaria ananassa crude extract on cadmium-induced lipid peroxidation, antioxidant enzymes suppression, and apoptosis in rat testes. Int. J. Mol. Sci. 2017, 18, 957. [Google Scholar] [CrossRef] [Green Version]
- Mouro, V.G.S.; de Melo, F.C.S.A.; Martins, A.L.P.; Gomes, M.L.M.; de Oliveria, J.M.; de Freitas, M.B.D.; Demuner, A.J.; Leite, J.P.V.; de Matta, S.L.P. Euterpe oleracea (Martius) oil reverses testicular alterations caused after cadmium administration. Biol. Trace Elem. Res. 2020, 197, 555–570. [Google Scholar] [CrossRef]
- Chatterjee, P.K.; Anantharaya, V.N.M.; Shiva, R.K.; Kumar, N.A.; Shetty, S.B.; Budihal, S.V.; Bhat, M.R.; Kunal. Pre- and post-treatment effects: Estimation of serum testosterone and lipid peroxidation levels on Moringaolifera extract induced cadmium exposed rats. Pharmacogn. J. 2017, 9, 846–849. [Google Scholar] [CrossRef] [Green Version]
- Ekhoye, E.I.; Olerimi, S.M.; Ehebha, S.E. Comparison of the deleterious effects of yajiandcadmium chloride on testicular physio morphological and oxidative stress status: The gonado protective effects of an omega-3 fatty acid. Clin. Exp. Reprod. Med. 2020, 47, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Koriem, K.M.M.; Fathi, G.E.; Salem, H.A.; Akram, N.H.; Gamil, S.A. Protective role of pectin against cadmium induced testiculartoxicity and oxidative stress in rats. Toxicol. Mech. Method. 2013. [Google Scholar] [CrossRef] [PubMed]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem. Biol. Interact. 2014, 211, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Marzec-Wróblewska, U.; Kaminski, P.; Lakota, P. Influence of chemical elements on mammalian spermatozoa. Folia Biol. 2012, 58, 7–15. [Google Scholar]
- Khanna, S.; Mitra, S.; Lakhera, P.C.; Khandelwal, S. N-acetylcysteine effectively mitigates cadmium induced oxidative damage and cell death in Leydig cells in vitro. Drug Chem. Toxicol. 2016, 39, 74–80. [Google Scholar] [CrossRef]
- Kelainy, E.G.; Laila, I.M.I.; Ibrahim, S.R. The effect of ferulic acid against lead-induced oxidative stress and DNA damage in kidney and testes of rats. Environ. Sci. Pollut. Res. 2019, 26, 31675–31684. [Google Scholar] [CrossRef] [PubMed]
- Dorostghoal, M.; Seyyednejad, S.M.; Nejad, M.N.T. Cichorium intybus L. extract ameliorates testicular oxidative stress induced by lead acetate in male rats. Clin. Exp. Reprod. Med. 2020, 47, 161–167. [Google Scholar] [CrossRef]
- Olayaki, L.A.; Alagbonsi, I.A.; Abdulrahim, A.H.; Adeyami, W.J.; Bakare, M.; Omeiza, M. Melatonin prevents and ameliorates lead-induced gonado toxicity through antioxidative and hormonal mechanisms. Toxicol. Ind. Health 2018, 34, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Jiang, X.; Sun, J.; Li, X.; Li, X.; Tian, L.; Li, Y. Cyanidin-3-O-glucoside promotes the biosynthesis of progesterone throughthe protection of mitochondrial function in Pb-exposed rat leydig cells. Food Chem. Toxicol. 2018, 112, 427–434. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, Y.S.; El-Neweshy, M.S. Impact of lead toxicity on male ratreproduction at hormonal and histopathological levels. Toxicol. Environ. Chem. 2010, 4, 765–774. [Google Scholar] [CrossRef]
- Rubio, J.; Riqueros, M.I.; Gasco, M.; Yucra, S.; Miranda, S.; Gonzales, G.F. Lepidiummeyenii (Maca) reversed the lead acetate induced-damage on reproductive function in male rats. Food Chem. Toxicol. 2006, 44, 1114–1122. [Google Scholar] [CrossRef]
- Kasperczyk, A.; Kasperczyk, S.; Horak, S.; Ostalowska, A.; Grucka- Mamczar, E.; Romuk, E.; Olejek, A.; Birkner, E. Assessment of semen function and lipid peroxidation among lead exposed men. Toxicol. Appl. Pharmacol. 2008, 228, 378–384. [Google Scholar] [CrossRef]
- Ghaffari, M.A.; Motlagh, B. In vitro effect of lead, silver, tin, mercury, indium and bismuth on human sperm creatine kinase activity: A presumable mechanism for men infertility. Iran. Biomed. J. 2011, 15, 38–43. [Google Scholar]
- Abdel-Wahhab, M.A.; Aly, S.E. Antioxidant property of Nigella sativa (Black cumin) and Syzygium aromaticum (Cloves) in rats during aflatoxicosis. J. Appl. Toxicol. 2005, 25, 218–223. [Google Scholar] [CrossRef]
- Nita, M.; Grybowski, A. The role of reactive oxygen species and oxidative stress in the patho mechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Szweda, P.A.; Friguet, B.; Szweda, L.I. Proteolysis, free radicals, and aging. Free Radic. Biol. Med. 2002, 33, 29–36. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Waalkes, M.; Rice, J.M. Lead as a carcinogen: Experimental evidence and mechanisms of action. Am. J. Ind. Med. 2000, 38, 316–323. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (EPA). Particulate Matter (PM) Pollution. Available online: https://www.epa.gov/pm-pollution (accessed on 20 January 2021).
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C., Jr.; et al. Expert panel on population and prevention science of the American Heart Association: Air pollution and cardiovascular disease: A statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A.; Speakman, M. Erectile dysfunction and atherosclerosis. Curr. Atheroscler. Rep. 2002, 4, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Xu, X.; Bai, Y.; Zhong, J.; Chen, M.; Liang, Y.; Zhao, J.; Liu, D.; Morishita, M.; Sun, Q.; et al. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: A role for hypothalamic inflammation. Environ. Health Perspect. 2014, 122, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, L.; Chen, M.; Wang, X.; Qin, X.; Chen, S.; Qian, Y.; Liu, Z.; Cao, Q.; Ying, Z. Exposure to concentrated ambient PM2.5 compromises spermatogenesis in a mouse model: Role of suppression of hypothalamus-pituitary-gonads axis. Toxicol. Sci. 2018, 162, 318–326. [Google Scholar] [CrossRef]
- Jeng, H.A.; Yu, L. Alteration of sperm quality and hormone levels by polycyclic aromatic hydrocarbons on airborne particulate particles. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2008, 43, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Corradi, P.F.; Corradi, R.B.; Greene, L.W. Physiology of the hypothalamic pituitary gonadal axis in the male. Urol. Clin. N. Am. 2016, 43, 151–162. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, T.; Liu, S.; Cao, Z.; Zhao, Y.; Su, X.; Liao, Z.; Teng, X.; Hua, J. Concentrated ambient PM2.5 exposure affects mice sperm quality and testosterone biosynthesis. PeerJ 2019, 7, e8109. [Google Scholar] [CrossRef] [Green Version]
- Albersen, M.; Orabi, H.; Lue, T.F. Evaluation and treatment of erectile dysfunction in the aging male: A mini review. Gerontology 2012, 58, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhoutte, P.M. Ageing and endothelial dysfunction. Eur. Heart J. Suppl. 2002, 4, A8–A17. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J.; Baker, M.A. Reactive oxygen species generation by human spermatozoa: A continuing enigma. Int. J. Androl. 2002, 25, 191–194. [Google Scholar] [CrossRef]
- Mishra, D.P.; Shaha, C. Estrogen-induced spermatogenic cell apoptosis occurs via the mitochondrial pathway: Role of superoxide and nitric oxide. J. Biol. Chem. 2005, 280, 6181–6196. [Google Scholar] [CrossRef] [Green Version]
- Iremashvili, V.; Brackett, N.L.; Lynne, C.M. Impact of spinal cord injury. In Male Infertility, 1st ed.; Parekattil, S., Agarwal, A., Eds.; Springer: New York, NY, USA, 2012; pp. 337–345. [Google Scholar]
- Brackett, N.L.; Ferrell, S.M.; Aballa, T.C.; Amador, M.J.; Lynne, C.M. Semen quality in spinal cord injured men: Does it progressively decline postinjury? Arch. Phys. Med. Rehabil. 1998, 79, 625–628. [Google Scholar] [CrossRef]
- Gillon, G.; Barnea, O. Erection mechanism of the penis: A model-based analysis. J. Urol. 2002, 168, 2711–2715. [Google Scholar] [CrossRef]
- Wang, W.; Deng, Z.; Feng, Y.; Liao, F.; Feng, S.; Wang, X. PM2.5 induced apoptosis in endothelial cell through the activation of p53-bax-caspase pathway. Chemosphere 2017, 177, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Künzli, N.; Jerrett, M.; Garcia-Esteban, R.; Basagaña, X.; Beckermann, B.; Gilliland, F.; Medina, M.; Peters, J.; Hodis, H.N.; Mack, W.J. Ambient air pollution and the progression of atherosclerosis in adults. PLoS ONE 2010, 5, e9096. [Google Scholar] [CrossRef]
- Xie, L.N.; Wang, X.C.; Dong, X.J.; Su, L.Q.; Zhu, H.J.; Wang, C.; Zhang, D.P.; Liu, F.Y.; Hou, S.S.; Dong, B.; et al. Concentration, spatial distribution, and health risk assessment of PFASs in serum of teenagers, tap water and soil near a Chinese fluorochemical industrial plant. Environ. Int. 2021, 146, 106166. [Google Scholar] [CrossRef]
- Castellini, C.; Totaro, M.; Parisi, A.; D’Andrea, S.; Lucente, L.; Cordeschi, G.; Francavilla, S.; Francevilla, F.; Barbonetti, A. Bisphenol A and male fertility: Myths and realities. Front. Endocrinol. 2020, 11, 353. [Google Scholar] [CrossRef]
- Pan, G.; Hanaoka, T.; Yoshimura, M.; Zhang, S.; Wang, P.; Tsukino, H.; Inoue, K.; Nakazawa, H.; Tsugane, S.; Takahasi, K. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): A cross-sectional study in China. Environ. Health Perspect. 2006, 114, 1643–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, J.Y.; Blachman-Braun, R.; Patel, A.S.; Patel, P.; Ramasamy, R. Association between polychlorinated biphenyl 153 exposure and serum testosterone levels: Analysis of the National Health and Nutrition Examination Survey. Transl. Androl. Urol. 2019, 8, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Espinosa, M.J.; Fletcher, T.; Armstrong, B.; Genser, B.; Dhatariya, K.; Mondal, D.; Ducatman, A.; Leonardi, G. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ. Sci. Technol. 2011, 45, 8160–8166. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, Z.; Qing, D.; He, Y.; Wu, T.; Miao, M.; Wang, J.; Weng, X.; Ferber, J.R.; Herrinton, L.J.; et al. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum. Reprod. 2010, 255, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Groves-Kirkby, N. BPA worsens male sexual function. Nat. Rev. Urol. 2010, 7, 60. [Google Scholar] [CrossRef]
- Hanaoka., T.; Kawamura, N.; Hara, K.; Tsugane, S. Urinary bisphenol A and plasma hormone concentrations in male workers exposed to bisphenol A diglycidyl ether and mixed organic solvents. Occup. Environ. Med. 2002, 59, 625–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, W.; Lee, C.K.; Yeung, W.S.; Giesy, J.P.; Wong, M.H.; Zhang, X.; Hecker, M.; Wong, C.K. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reprod. Toxicol. 2011, 31, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, D.; Yanagiba, Y.; Duan, Z.; Ito, Y.; Okamura, A.; Asaeda, N.; Tagawa, Y.; Li, C.; Taya, K.; Zhang, S.Y.; et al. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol. Lett. 2010, 194, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Pirzada, M.; Jahan, S.; Ullah, H.; Turi, N.; Ullah, W.; Siddiqui, M.F.; Zakria, M.; Lodhi, K.Z.; Khan, M.M. Impact of low-dose chronic exposure to bisphenol A and its analogue bisphenol B, bisphenol F and bisphenol S on hypothalamic-pituitary-testicular activities in adult rats: A focus on the possible hormonal mode of action. Food Chem. Toxicol. 2018, 121, 24–36. [Google Scholar] [CrossRef]
- Ha, M.; Guan, X.; Wei, L.; Li, P.; Yang, M.; Liu, C. Di-(2-ethylhexyl) phthalate inhibits testosterone level through disturbed hypothalamic-pituitary-testis axis and ERK-mediated 5α-Reductase 2. Sci. Total Environ. 2016, 563–564, 566–575. [Google Scholar] [CrossRef]
- Akingbemi, B.T.; Youker, R.T.; Sottas, C.M.; Ge, R.; Katz, E.; Klinefelter, G.R.; Zirkin, B.R.; Hardy, M.P. Modulation of rat Leydig cell steroidogenic function by di(2-ethylhexyl)phthalate. Biol. Reprod. 2001, 65, 1252–1259. [Google Scholar] [CrossRef] [Green Version]
- Barlow, N.J.; Philips, S.L.; Wallace, D.G.; Sar, M.; Gaido, K.W.; Foster, P.M.D. Quantitatve changes in gene expression in fetal rat testes following exposure to di(n-butyl) phthalate. Toxicol. Sci. 2003, 73, 431–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedha, S.; Kumar, S.; Shukla, S. Role of oxidative stress in male reproductive dysfunctions with reference to phthalate compounds. Urol. J. 2015, 12, 2304–2316. [Google Scholar] [PubMed]
- Meroni, S.B.; Galardo, M.N.; Rindone, G.; Gorga, A.; Riera, M.F.; Cigorraga, S.B. Molecular mechanisms and signaling pathways involved in Sertoli cell proliferation. Front. Endocrinol. 2019, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Barr, D.B.; Hauser, R. Pyrethroid insecticide metabolites are associated with serum hormone levels in adult men. Reprod. Toxicol. 2009, 27, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansone, A.; Romanelli, F.; Gianfrilli, D.; Lenzi, A. Endocrine evaluation of erectile dysfunction. Endocrine 2014, 46, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Seftel, A.D.; Kathrins, M.; Niederberger, C. Critical update of the 2010 endocrine society clinical practice guidelines for male hypogonadism: A systematic analysis. Mayo Clin. Proc. 2015, 90, 1104–1115. [Google Scholar] [CrossRef] [Green Version]
- Traish, A.M.; Miner, M.M.; Morgentaler, A.; Zitzmann, M. Testosterone deficiency. Am. J. Med. 2011, 124, 578–587. [Google Scholar] [CrossRef]
- Talsness, C.E.; Andrade, A.J.; Kuriyama, S.N.; Taylor, J.A.; vom Saal, F.S. Components of plastic: Experimental studies in animals and relevance for human health. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2079–2096. [Google Scholar] [CrossRef] [Green Version]
- Fromme, H.; Tittlemier, S.A.; Volkel, W.; Wilhelm, M.; Twardella, D. Perfluorinated compounds--exposure assessment for the general population in Western countries. Int. J. Hyg. Environ. Health 2009, 212, 239–270. [Google Scholar] [CrossRef]
- Joensen, U.N.; Veyrand, B.; Antignac, J.P.; Blomberg Jensen, M.; Petersen, J.H.; Marchand, P.; Skakkebaek, N.E.; Andersson, A.M.; Le Bizec, B.; Jorgensen, N. PFOS (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Hum. Reprod. 2013, 28, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Kraugerud, M.; Zimmer, K.E.; Ropstad, E.; Verhaegen, S. Perfluorinated compounds differentially affect steroidogenesis and viability in the human adrenocortical carcinoma (H295R) in vitro cell assay. Toxicol. Lett. 2011, 205, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chu, Y.; Hardy, D.O.; Li, X.K.; Ge, R.S. Inhibition of 3beta- and 17beta-hydroxysteroid dehydrogenase activities in rat Leydig cells by perfluoro octane acid. J. Steroid Biochem. Mol. Biol. 2010, 118, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, H.; Gao, J.; Li, Z.; Bao, S.; Chen, X.; Wang, Y.; Ge, R.; Ye, L. Effects of perfluorooctanoic acid on stem Leydig cell functions in the rat. Environ. Pollut. 2019, 250, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Eggert, A.; Cisneros-Montalvo, S.; Anandan, S.; Musilli, S.; Stukenborg, J.B.; Adamsson, A.; Nurmio, M.; Toppari, J. The effects of perfluorooctanoic acid (PFOA) on fetal and adult rat testis. Reprod. Toxicol. 2019, 90, 68–76. [Google Scholar] [CrossRef]
- Wielsoe, M.; Long, M.; Ghisari, M.; Bonefeld-Jorgensen, E.C. Perfluoro alkylated substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere 2015, 129, 239–245. [Google Scholar] [CrossRef]
- Zeng, Z.; Song, B.; Xiao, R.; Zeng, G.; Gong, J.; Chen, M.; Xu, P.; Zhang, P.; Shen, M.; Yi, H. Assessing the human health risk of perfluorooctane sulfonate by in vivo and in vitro studies. Environ. Int. 2019, 126, 598–610. [Google Scholar] [CrossRef]
- Ivanciuc., T.; Ivanciuc, O.; Klein, D.J. Modeling the bioconcentration factors and bioaccumulation factors of polychlorinated biphenyls with posetic quantitative super-structure/activity relationships (QSSAR). Mol. Divers. 2006, 10, 133–145. [Google Scholar] [CrossRef]
- Murugesan, P.; Muthusamy, T.; Balasubramanian, K.; Arunakaran, J. Polychlorinated biphenyl (Aroclor 1254) inhibits testosterone biosynthesis and antioxidant enzymes in cultured rat Leydig cells. Reprod. Toxicol. 2008, 25, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Oskam, I.C.; Skaare, J.U.; Reksen, O.; Sweeney, T.; Dahl, E.; Farstad, W.; Ropstad, E. Effects of gestational and lactational exposure to low doses of PCBs 126 and 153 on anterior pituitary and gonadal hormones and on puberty in mice. Reprod. Toxicol. 2004, 19, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Okino, S.T.; Whitlock, J.P. The aromatic hydrocarbon receptor, transcription, and endocrine aspects of dioxin action. Vitam. Horm. 2000, 59, 241–264. [Google Scholar] [CrossRef]
- Chen, H.; Cangello, D.; Benson, S.; Folmer, J.; Zhu, H.; Trush, M.A.; Zirkin, B.R. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: Relationship to reduced steroidogenic function? Exp. Gerontol. 2001, 36, 1361–1373. [Google Scholar] [CrossRef]
- Cao, L.; Leers-Sucheta, S.; Azhar, S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J. Steroid Biochem. Mol. Biol. 2004, 88, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Toppari., J.; Larsen, J.C.; Christiansen, P. Male reproductive health and environmental xenoestrogens. Environ. Health Perspect. 1996, 104, 741–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, M.S.; Camann, D.; Gammon, M.; Stellman, S.D. Proposed PCB congener groupings for epidemiological studies. Environ. Health Perspect. 1997, 105, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.R.; Argiolas, A. Nitric oxide donors induce penile erection and yawning when injected in the central nervous system of male rats. Eur. J. Pharmacol. 1995, 294, 1–9. [Google Scholar] [CrossRef]
- Lugg, J.A.; Rajfer, J.; González-Cadavid, N.F. Dihydrotestosterone is the active androgen in the maintenance of nitric oxide-mediated penile erection in the rat. Endocrinology 1995, 136, 1495–1501. [Google Scholar] [CrossRef]
- Mills, T.M. Vasoconstriction and vasodilation in erectile physiology. Curr. Urol. Rep. 2002, 3, 477–483. [Google Scholar] [CrossRef]
- Polsky, J.Y.; Aronson, K.J.; Heaton, J.P.; Adams, M.A. Pesticides and polychlorinated biphenyls as potential risk factors for erectile dysfunction. J. Androl. 2007, 28, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Farokhi, F.; Shalijar-Jalali, A.; Najafi, G. Protective effect of vitamin E on sperm quality and in vitro fertilizing potential and testosterone concentration in polyvinyl chloride treated male rats. Vet. Res. Forum 2020, 11, 257–263. [Google Scholar] [CrossRef]
- Lamb, D.J. An approach that someday may boost testosterone biosynthesis in males with late-onset hypogonadism (low testosterone). Proc. Natl. Acad. Sci. USA 2019, 116, 22904–22906. [Google Scholar] [CrossRef] [Green Version]
- Ankerst, D.P.; Hoefler, J.; Bock, S.; Goodman, P.J.; Vickers, A.; Hernandez, J.; Sokoll, L.J.; Sanda, M.G.; Wei, J.T.; Leach, R.J.; et al. Prostate cancer prevention trial risk calculator 2.0 for the prediction of low-vs-high grade prostate cancer. Urology 2014, 83, 1362–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francomano, D.; Bruzziches, R.; Barbaro, G.; Lenzi, A.; Aversa, A. Effects of testosterone undecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: A pilot study. J. Endocrinol. Investig. 2014, 37, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Ide, V.; Vanderschueren, D.; Antonio, L. Treatment of men with central hypogonadism: Alternatives for testosterone replacement therapy. Int. J. Mol. Sci. 2021, 22, 21. [Google Scholar] [CrossRef]
- Sharma, R.; Oni, O.A.; Gupta, K.; Sharma, M.; Sharma, R.; Singh, V.; Parashara, D.; Kamalakar, S.; Dawn, B.; Chen, G. Normalization of testosterone levels after testosterone replacement therapy is associated with decreased incidence of atrial fibrillation. J. Am. Heart. Assoc. 2017, e004880. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Yang, Y.; Wang, X.; Nie, M.; Wu, X.; Mao, J. Metabolic effects of testosterone replacement therapy in patients with type 2 diabetes mellitus or metabolic syndrome: A meta-analysis. Int. J. Endocrinol. 2020, 2020, 4732021. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.D.; McMahon, C.G.; Guay, A.T.; Morgentaler, A.; Althof, S.E.; Becher, E.F.; Bivalacqua, T.J.; Burnett, A.L.; Buvat, J.; Meliegy, A.E.; et al. The international society for sexual medicine’s process of care for the assessment and management of testosterone deficiency in adult men. J. Sex. Med. 2015, 12, 1660–1686. [Google Scholar] [CrossRef]
- Khera, M.; Adaikan, G.; Buvat, J.; Carrier, S.; El-Meliegy, A.; Hatzimouratidis, K.; McCoullough, A.; Morgentaler, A.; Torres, L.O.; Salonia, A. Diagnosis and treatment of testosterone deficiency: Recommendations from the fourth international consultation for sexual medicine (ICSM 2015). J. Sex. Med. 2016, 13, 1787–1804. [Google Scholar] [CrossRef] [PubMed]
- Üçer, O.; Gümüş, B. The treatment of late-onset hypogonadism. Turk. J. Urol. 2014, 40, 170–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, K.M.; Pastuzak, A.W. A history of penile implants. Transl. Androl. Urol. 2017, 6, S851–S857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, L.; de Souza, R.R.; Maifrino, L.B.; Caperuto, É.C.; Carbone, P.O.; Rodrigues, B.; Gama, E.F. Resistance exercise and testosterone treatment alters the proportion of numerical density of capillaries of the left ventricle of aging Wistar rats. Aging Male 2014, 17, 243–247. [Google Scholar] [CrossRef]
- Hayes, L.D.; Sculthorpe, N.; Herbert, P.; Baker, J.S.; Hullin, D.A.; Kilduff, L.P.; Grace, F.M. Resting steroid hormone concentrations in lifetime exercisers and lifetime sedentary males. Aging Male 2015, 18, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Drugs FDA. FDA-Approved Drugs. U.S Food and Drug Administration. Available online: https://www.fda.gov/scripts/cder/daf/ (accessed on 15 March 2021).
- Guiliano, F.; Peña, B.M.; Mishra, A.; Smith, M.D. Efficacy results and quality-of-life measures in men receiving sildenafil citrate for the treatment of erectile dysfunction. Qual. Life Res. 2001, 10, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Janini, E.A.; Isidori, A.M.; Gravina, G.L.; Aversa, A.; Balercia, G.; Bocchio, M.; Boscaro, M.; Carini, C.; Corona, G.; Fabbari, A.; et al. The ENDOTRIAL study: A spontaneous, open-label, randomized, multicenter, crossover study on the efficacy of sildenafil, tadalafil, and vardenafil in the treatment of erectile dysfunction. J. Sex. Med. 2009, 6, 2547–2560. [Google Scholar] [CrossRef]
- Kim, E.D.; Owen, R.C.; White, G.S.; Elkelany, O.O.; Rahnema, C.D. Endovascular treatment of vasculogenic erectile dysfunction. Asian J. Androl. 2015, 17, 40–43. [Google Scholar] [CrossRef]
- Sanchez-Borrego, R.; Molero, F.; Castaño, R.; Castelo-Branco, C.; Honrado, M.; Jurado, A.R.; Laforet, E.; Prieto, R.; Cabello, F.; Larrazabal, M.; et al. Spanish consensus on sexual health in men and women over 50. Maturitas 2014, 78, 138–145. [Google Scholar] [CrossRef]
- Urology Care Foundation, American Urological Association. Erectile Dysfunction: Diagnosis. Available online: www.urologyhealth.org (accessed on 20 March 2021).
- Coulson, C.; Jenkins, J. Complementary and alternative medicine utilisation in NHS and private clinic settings: A United Kingdom survey of 400 infertility patients. J. Exp. Clin. Assist. Reprod. 2005, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.; Yadav, A.; Kumar, A.; Singh, J.P. Understanding of andropause and its ayurvedic management: Conceptual study. IAMJ 2019, 7, 446–450. [Google Scholar]
- Singh, S.K.; Rajoria, K. Review of andropause in ayurveda, rasayan, vajikarana and panchakarma perspective. RRJoAsYN 2014, 1, 19–26. [Google Scholar]
- George, A.; Liske, E. Acceptance of herbal medicine in andrology. In Herbal Medicine in Andrology an Evidence-Based Update, 1st ed.; Henkel, R., Agarwal, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 215–255. [Google Scholar] [CrossRef]
- Sengupta, P.; Agarwal, A.; Pogrebetskaya, M.; Roychoudhury, S.; Durairajanayagam, D.; Henkel, R. Role of Withania somnifera (Ashwagandha) in the management of male infertility. Reprod. Biomed. 2017, 36, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Lohiya, N.K.; Balasubramanian, K.; Ansari, A.S. Indian folklore medicine in managing men’s health and wellness. Andrologia 2016, 48, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Amano, T.; Imao, T.; Takemae, K. Clinical efficacy of Japanese herbal medicine (Kampo) in patients with late-onset hypogonadism. Aging Male 2010, 13, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Jeon, S.H.; Kwon, E.B.; Zhu, G.Q.; Lee, K.W.; Choi, J.B.; Jeong, H.C.; Kim, K.S.; Bae, S.R.; Bae, W.J.; et al. Effect of Korean herbal formula (modified ojayeonjonghwan) on androgen receptor expression in an aging rat model of late onset hypogonadism. World J. Mens Health 2019, 37, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Ji, S.Y.; Dong, W.; Zhang, Y.N.; Zhang, E.H.; Bin, Z. A herbal medicine, saikokaryokotsuboreito, improves serum testosterone levels and affects sexual behavior of in old male mice. Aging Male 2015, 18, 106–111. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Chakraborty, S.; Das, A.; Guha, P.; Agarwal, A.; Henkel, R. Herbal medicine used to treat andrological problems: Asian and Indian subcontinent: Ginkgo biloba, Curcuma longa, and Camellia sinensis. In Herbal Medicine in Andrology an Evidence-Based Update, 1st ed.; Henkel, R., Agarwal, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 129–146. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Agarwal, A.; Cho, C.L. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod. Biomed. 2017, 34, 487–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.K.C.; Tan, R.B.W.; Chung, E. Erectile dysfunction treatment and traditional medicine—Can East and West medicine coexist? Transl. Androl. Urol. 2017, 6, 91–100. [Google Scholar] [CrossRef] [Green Version]



| Environmental Factor | Experimental Model | Experimental Type | Exposure Parameters | Findings | Comments | References |
| Pesticides | Human | In vivo | Exposure to various pesticides for 2–11 years | Decreased serum testosterone level and LH | Decrease in sperm count and viability | [89] |
| Rat | In vivo | Chlorpyrifos at 17.5 mg/kg bodyweight for 30 days | Decrease in serum testosterone level, sperm count and motility, but increase in cholesterol level | High cholesterol level in the testes decrease the androgen level and hampers spermatogenesis | [31] | |
| Rat | In vivo | Diazinon at 30 mg/kg body weight at 5 consecutive days for 30 days | Decrease in serum testosterone level | Reduction in sperm count, diameter of seminiferous tubules | [23] | |
| Rat | In vivo | 12.5 mg/kg cypermethrin for 12 weeks | Decrease in serum testosterone level | Reduction in testicular weight, sperm count, viability, motility | [110] | |
| Radiation | Human | In vivo | Ionizing radiation from external beam radiation therapy (EBRT) for 3 months, at the median 68Gy, as a part of prostate cancer treatment | Decrease in serum testosterone level | Suppression of spermatogenesis | [167] |
| Rat | In vivo | Exposure to 900 MHz nonionizing radiation of cell phones | Increase in SOD activity | Production of ROS, lipid peroxidation, damaged spermatozoa | [24] | |
| Rat | In vivo | Exposure to 10 GHz non-ionizing radiation of XeThru X4 radar for 90 days | Decrease in serum testosterone and sex-hormone-binding protein | Effect on the male reproductive system | [150] | |
| Rat | In vivo | Exposure to 2.45 GHz of non-ionizing radiation | Decrease in serum testosterone level and increase in ROS, NO and MDA levels, expression of p53, Bax and active caspase in testes upregulated, while the expression of Bcl-xL, Bcl-2, procaspase and PARP-1 were downregulated | Decrease in seminiferous tubule diameter, sperm count, sperm motility and viability | [151] | |
| Rat | In vivo | Exposure to 7.5 Gy ionizing radiation for 5 days | Decrease in intracavernosal pressure | Reduced potential of attaining and maintaining prolonged penile erection | [170] | |
| Rat | In vivo | Exposure to 20 Gy ionizing radiation for 9 weeks | Decrease in intracavernosal pressure, increase in DNA oxidative stress in corpora cavernosa and prostate and increase in lipid peroxidation in corpora cavernosa | Erectile dysfunction may occur | [171] | |
| Rat | In vivo | Single dose of 4Gy ionizing radiation from X-rays | Decrease in serum testosterone level | Decrease in sperm count and motility, weight of testes, distortion in the architecture of seminiferous tubules | [172] | |
| Rat | In vivo | γ ionizing radiation | Decrease in serum testosterone level, SOD activity and a sharp rise in testicular MDA levels | Induction of oxidative stress | [173] | |
| Mice | In vivo | 0.25 Gy ionizing radiation from X-ray twice a day for 4 days | Decrease in testosterone level, glutathione concentration and increase in ROS level, lipid peroxidation, serum LDH activity, antioxidant enzyme activities | Decrease in sperm count and motility. Testicular damage | [174] | |
| Air Pollution | Rats | In vivo | 6.5 mg/kg CdCl2 intraperitoneal injection for 5 days | Decrease in Testosterone level, antioxidant enzyme activities, PCNA antigen and increase in Cd concentration, lipid peroxidation, NO and MDA levels. Upregulation of BAX, TNFα factor, downregulation of BCL 2 gene | Decrease in weight of testes, depletion of DNA contents due to oxidative stress | [192] |
| Rat | In vivo | 4.28 mg/kg CdCl2 for 7 days | Decrease in serum testosterone concentration, SOD activity | Damage in the epithelium of seminiferous tubules | [193] | |
| Rat | In vivo | Single oral supplementation of 10 mg/kg/bodyweight of CdCl2 | Decrease in serum testosterone level, increase in MDA level | Testicular damage due to Cd-induced oxidative stress | [194] | |
| Rat | In vivo | 2.5 mg/kg/bodyweight oral supplementation of CdCl2 | Decrease in serum testosterone level, FSH, LH level | Decrease in semen quality parameters and gonadosomatic index | [195] | |
| Rat | In vivo | Oral supplementation of 20 mg/kg PbAc for 10 days | Decrease in the levels of serum testosterone, FSH, LH levels, catalase activity and total antioxidant capacity. Increase in lipid peroxidation and levels of three lysosomal enzymes, including ACP, ß-NAG, and β-GAL in testes | Oxidative stress due to increase of ROS in testes. Accumulation of Pb in the testis tissues | [200] | |
| Rat | In vivo | 0.1% PbAc in drinking water for 70 days | Decrease in serum testosterone level, SOD and glutathione peroxidase level | Reduction in weight of testes, the diameter of seminiferous tubules, epididymal sperm count | [201] | |
| Rat | In vivo | Oral supplementation of 50 mg/kg/bodyweight of Pb for 4 weeks | Decreased testosterone and GnRH level, glutathione, SOD, catalase activity | Imbalance in testosterone, GnRH levels and antioxidant enzymes can lead to male infertility | [202] | |
| Rat Leydig cell line R2C | In vitro | Cell lines incubated for 24 h in different concentration of Pb (50, 100, 200, 400 μM) | Decreased production of progesterone (precursor of testosterone), protein expression level of StAR, CYP11A1, 3β-HSD | Pb-induced oxidative stress can change the expression of antioxidant enzymes | [203] | |
| Rat | In vivo | Exposure to different concentration of PM2.5 once each week for 6 weeks | Ratio of intracavernosal pressure to mean atrial pressure decreased, ratio of smooth muscles to collagen decreased and ROS production increased | Testicular necrosis, hemorrhage, reduction in testicular size, degeneration of seminiferous tubules | [25] | |
| Mice | In vivo | Exposure to concentrated ambient PM2.5 (CAP) | Decrease in sperm count, circulating FSH and testosterone level, hypothalamic GnRH level | Adverse effects on testicular spermatogenesis resulting in sperm alterations | [217] | |
| Other endocrine-disrupting chemicals | Human | In vivo | Exposure to BPA from working in factories | Decrease in testosterone level | Reduced sexual function, coitus frequency, inability to achieve an erection, ED | [235] |
| Human | In vivo | Workers exposed to BPA in BPA and resin manufacturing companies | Lower sexual functions | Orgasmic function lowered leading to ED | [235] | |
| Human | In vivo | Exposure to BPA and BADGE) | Changes in endogenous sex hormone levels elevated urinary BPA concentration | Altered level of estrogen, androgen, gonadotropin, SHBG | [237] | |
| Human | In vivo | Exposure to DBP and DEHP in polyvinyl chloride flooring producing factory | High levels of MBP and MEHP in body and decrease in testosterone, FSH, LH level. | Reduction in steroidogenic activity and spontaneous erection that might lead to ED. | [232] | |
| Human | In vivo | Exposure to PFAS | Negative association between PFOS with testosterone | Decrease in the testosterone level with no effect on semen quality | [254] | |
| Rat | In vivo | Administration of DEHP for 30 days | Affects testicular physiology and testosterone production | Deformation of seminiferous tubules along with an increase in Leydig cell number | [242] | |
| Rat | In vivo | Exposure of Leydig cells to different concentrations of PCB | Inhibition of basal and LH-stimulated testosterone production | Testosterone level decreases with decreased the activity of steroidogenic enzymes, enzymatic and nonenzymatic antioxidants | [262] | |
| Mice Leydig cell line TM3 | In vitro | DEHP treatment to Leydig cell TM3 for 24 h | Disturbance in the HPG axis | Reduction in LH and FSH level as well as testosterone level | [242] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roychoudhury, S.; Chakraborty, S.; Choudhury, A.P.; Das, A.; Jha, N.K.; Slama, P.; Nath, M.; Massanyi, P.; Ruokolainen, J.; Kesari, K.K. Environmental Factors-Induced Oxidative Stress: Hormonal and Molecular Pathway Disruptions in Hypogonadism and Erectile Dysfunction. Antioxidants 2021, 10, 837. https://doi.org/10.3390/antiox10060837
Roychoudhury S, Chakraborty S, Choudhury AP, Das A, Jha NK, Slama P, Nath M, Massanyi P, Ruokolainen J, Kesari KK. Environmental Factors-Induced Oxidative Stress: Hormonal and Molecular Pathway Disruptions in Hypogonadism and Erectile Dysfunction. Antioxidants. 2021; 10(6):837. https://doi.org/10.3390/antiox10060837
Chicago/Turabian StyleRoychoudhury, Shubhadeep, Saptaparna Chakraborty, Arun Paul Choudhury, Anandan Das, Niraj Kumar Jha, Petr Slama, Monika Nath, Peter Massanyi, Janne Ruokolainen, and Kavindra Kumar Kesari. 2021. "Environmental Factors-Induced Oxidative Stress: Hormonal and Molecular Pathway Disruptions in Hypogonadism and Erectile Dysfunction" Antioxidants 10, no. 6: 837. https://doi.org/10.3390/antiox10060837
APA StyleRoychoudhury, S., Chakraborty, S., Choudhury, A. P., Das, A., Jha, N. K., Slama, P., Nath, M., Massanyi, P., Ruokolainen, J., & Kesari, K. K. (2021). Environmental Factors-Induced Oxidative Stress: Hormonal and Molecular Pathway Disruptions in Hypogonadism and Erectile Dysfunction. Antioxidants, 10(6), 837. https://doi.org/10.3390/antiox10060837













