Mitochondrial Succinate Metabolism and Reactive Oxygen Species Are Important but Not Essential for Eliciting Carotid Body and Ventilatory Responses to Hypoxia in the Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Recordings of CB Chemoafferent Nerve Activity
2.3. Ventilatory Responses to Hypoxia and Hypercapnia
2.4. Data Analysis
3. Results
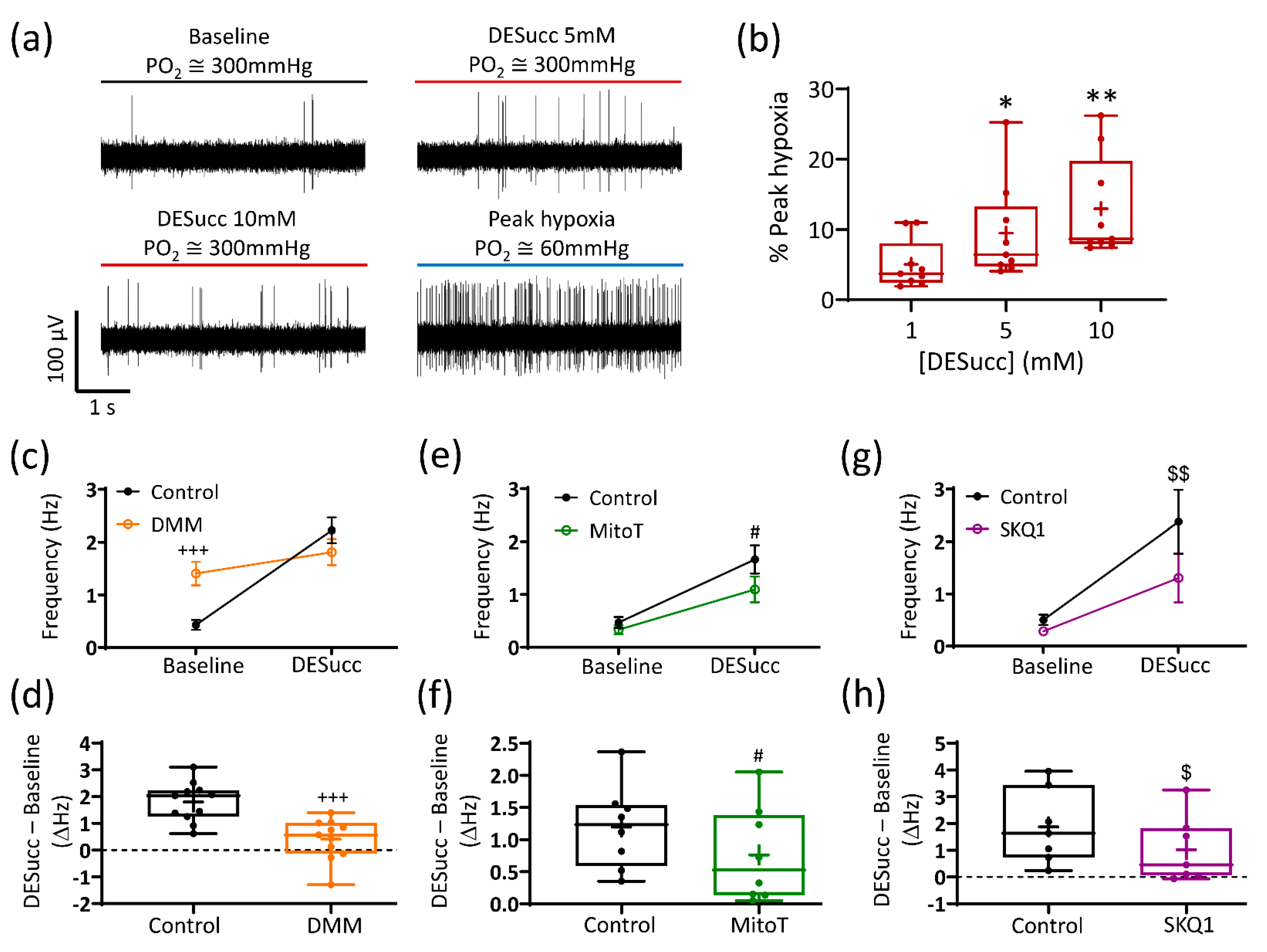
3.1. Succinate Causes Significant CB Chemoafferent Excitation
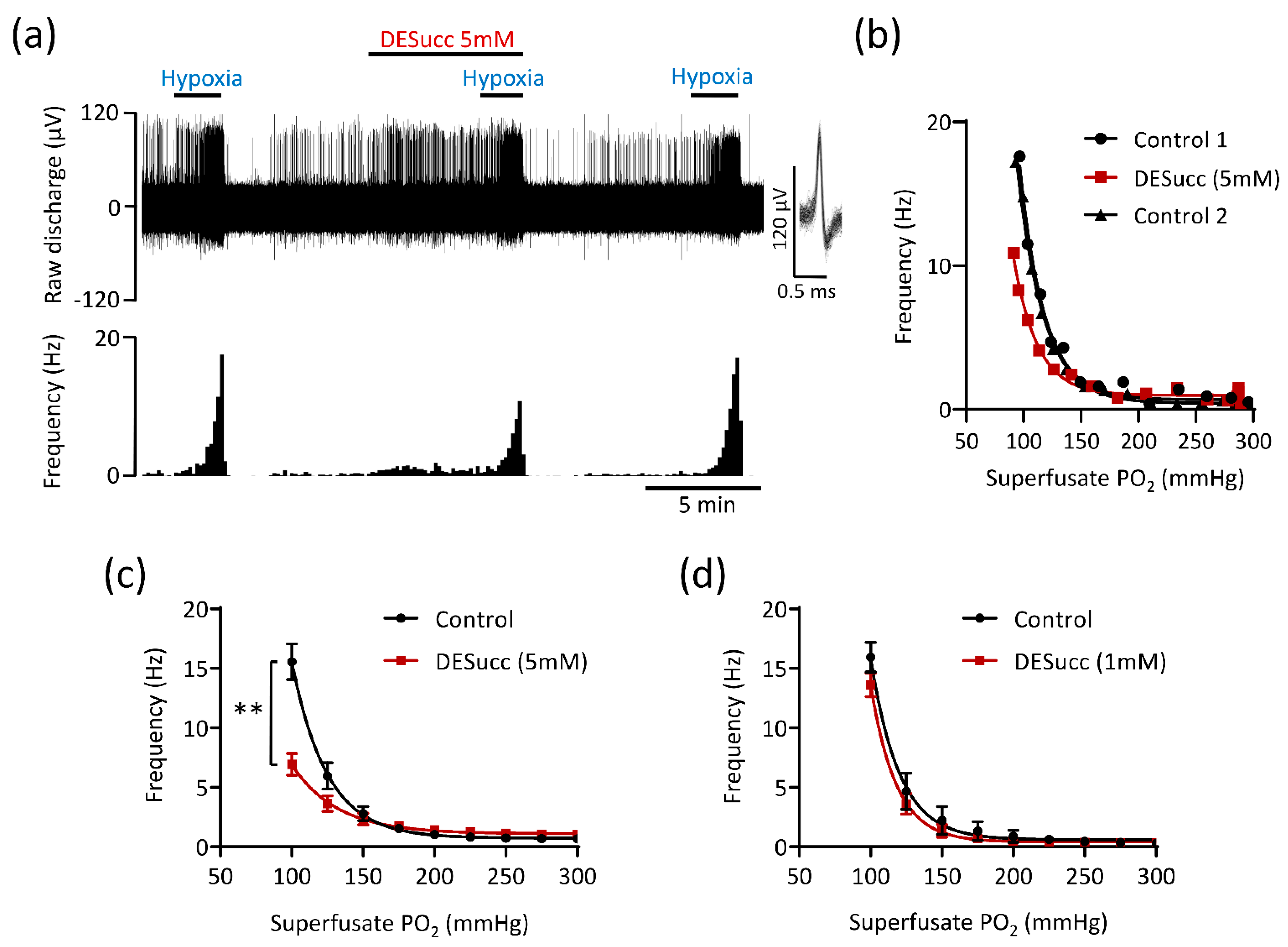
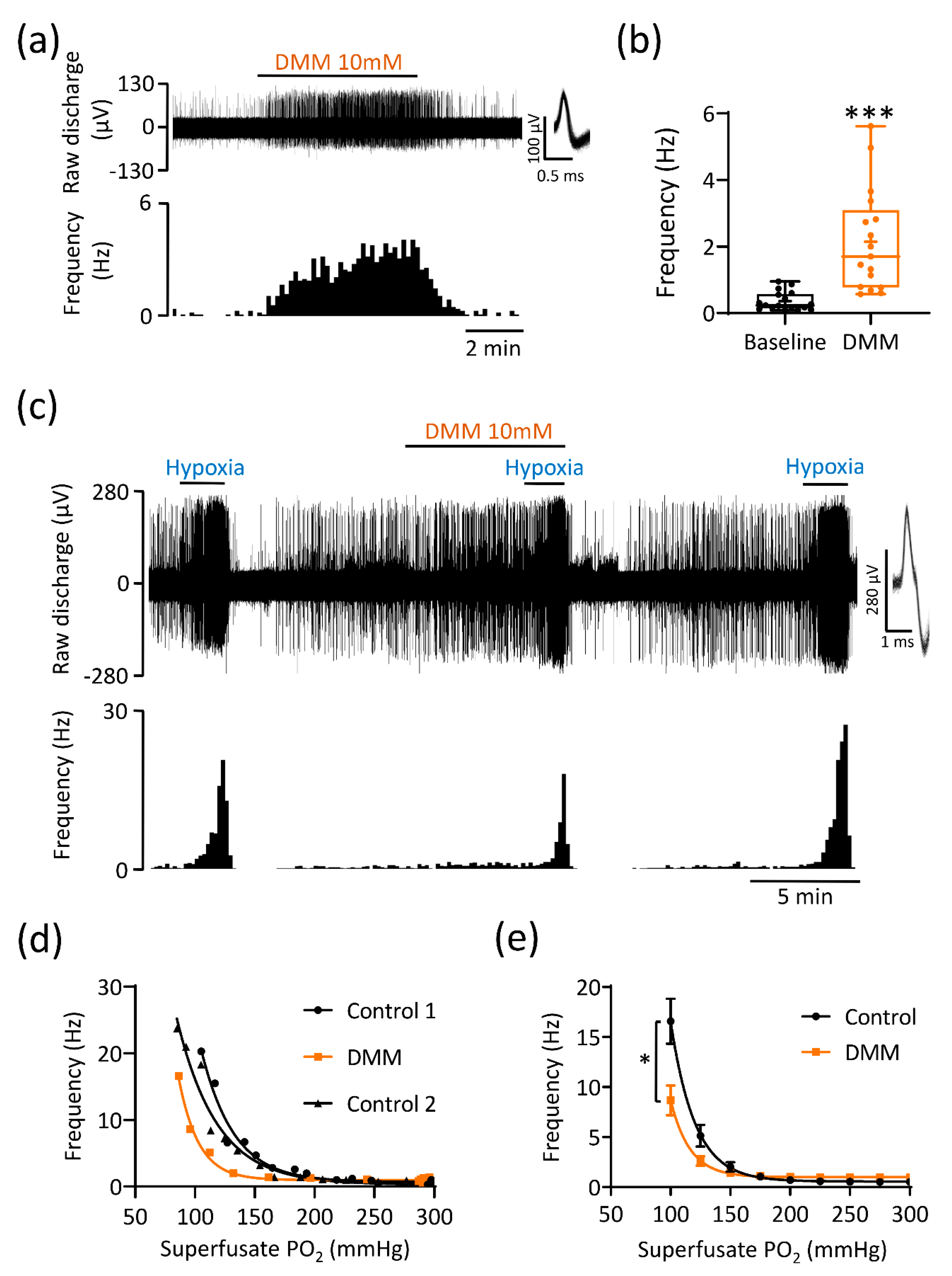
3.2. Excessive Succinate Metabolism Attenuates CB Hypoxic Sensitivity
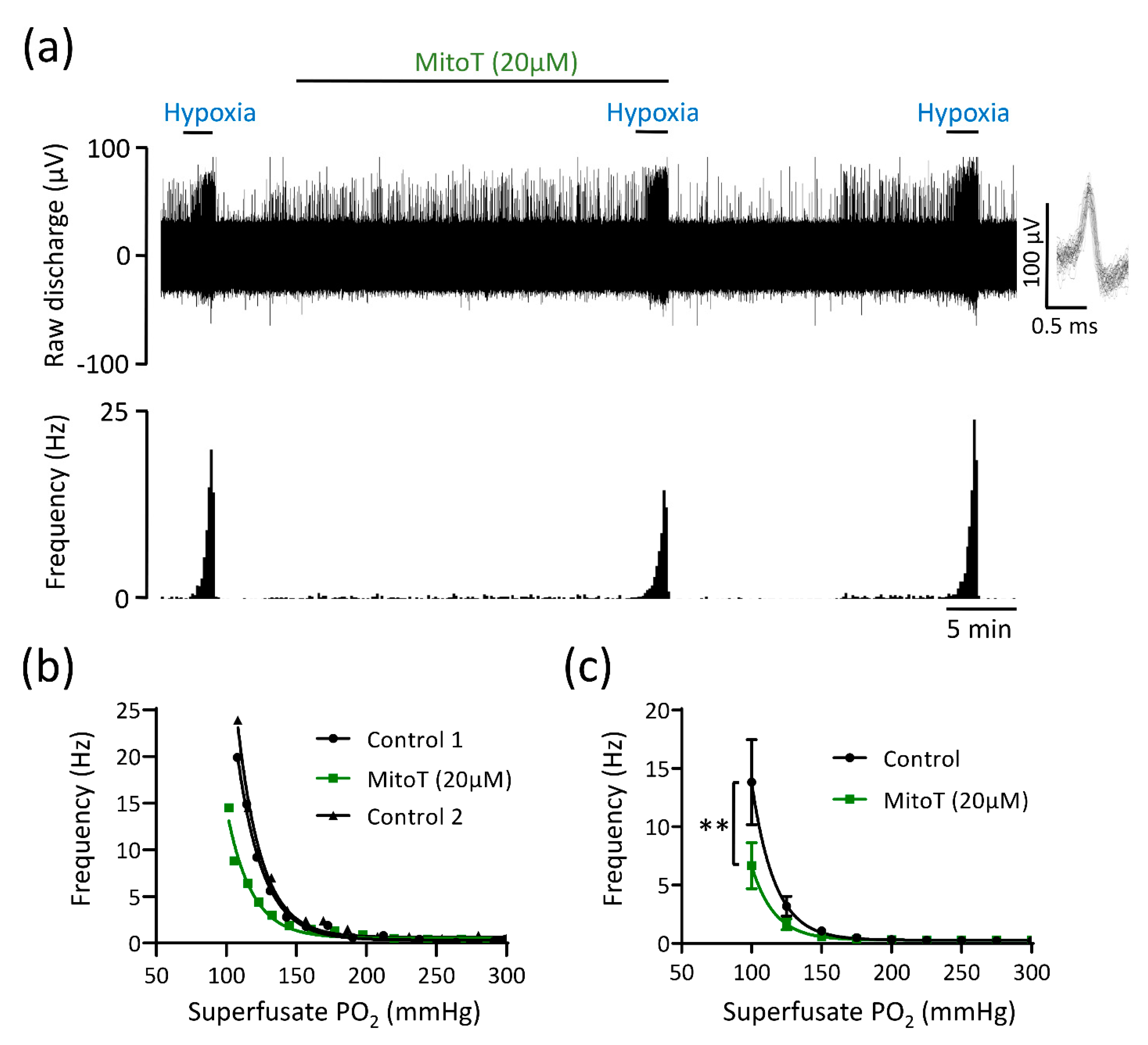
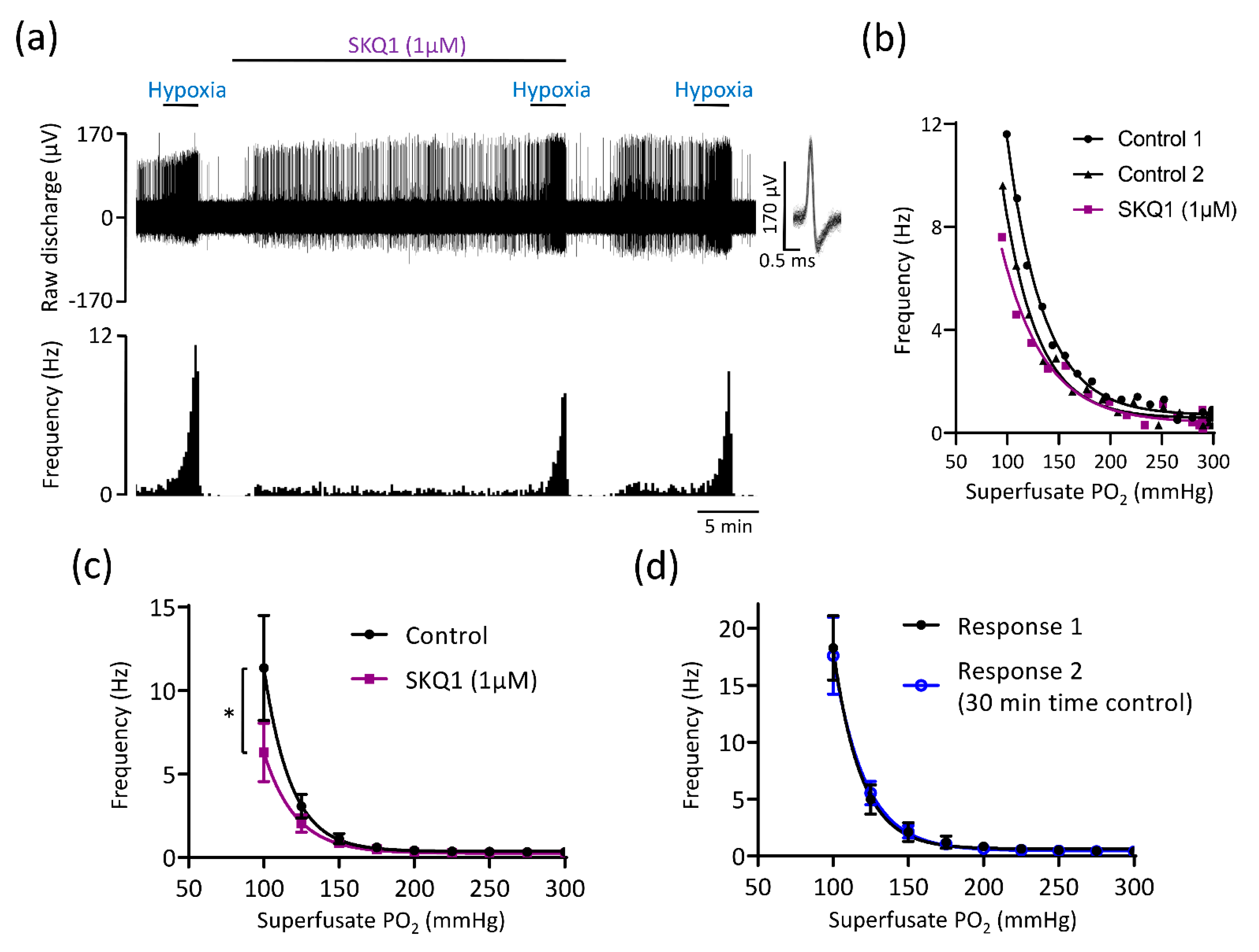
3.3. Mitochondrial Antioxidants and Inhibition of Succinate Metabolism Decrease but Do Not Abolish CB Chemoafferent Responses to Hypoxia
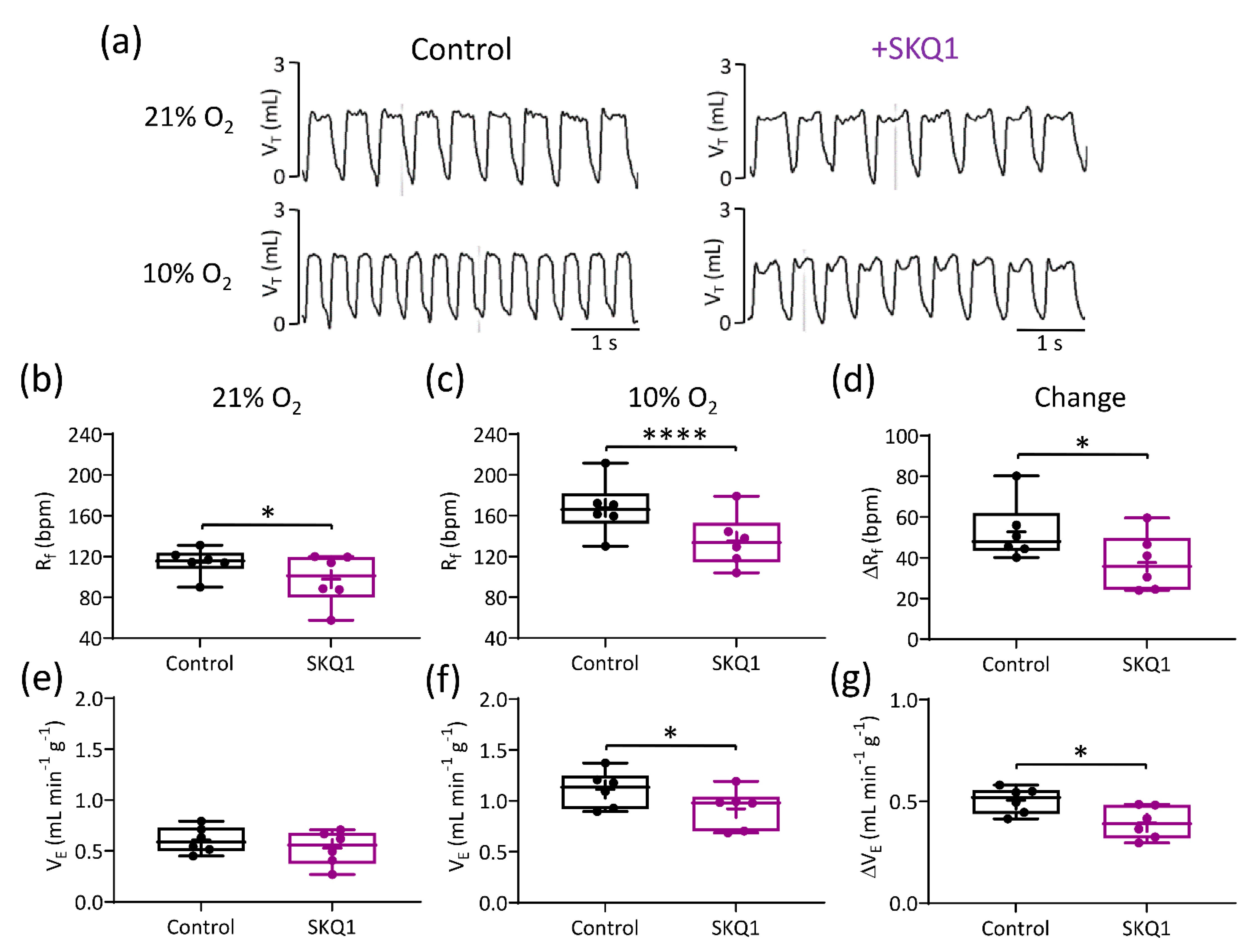
3.4. Mitochondrial Antioxidants Decrease Ventilatory Responses to Hypoxia but Not Hypercapnia
4. Discussion
4.1. Main Findings
4.2. Succinate and mitoROS Contribute to CB and Whole-Body Responses to Hypoxia in the Rat
4.3. Implications of the Current Study
4.4. Translational Relevance
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19) A Review. JAMA J. Am. Med. Assoc. 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Ortega-Saenz, P.; Lopez-Barneo, J. Physiology of the Carotid Body: From Molecules to Disease. In Annual Review of Physiology; Nelson, M.T., Walsh, K., Eds.; Annual Review of Physiology; Annual Reviews: Palo Alto, CA, USA, 2020; Volume 82, pp. 127–149. [Google Scholar]
- Kumar, P.; Prabhakar, N.R. Peripheral Chemoreceptors: Function and Plasticity of the Carotid Body. Compr. Physiol. 2012, 2, 141–219. [Google Scholar] [PubMed] [Green Version]
- Prabhakar, N.R.; Semenza, G.L. Regulation of carotid body oxygen sensing by hypoxia-inducible factors. Pflug. Arch. 2016, 468, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, A.P.; Ray, C.J.; Thompson, E.L.; Alshehri, Z.; Coney, A.M.; Kumar, P. Adrenaline activation of the carotid body: Key to CO2 and pH homeostasis in hypoglycaemia and potential pathological implications in cardiovascular disease. Respir. Physiol. Neurobiol. 2019, 265, 92–99. [Google Scholar] [CrossRef]
- Kumar, P. Systemic effects resulting from carotid body stimulation-invited article. Adv. Exp. Med. Biol. 2009, 648, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy, R.J.; Wyatt, C.N. Acute oxygen sensing by the carotid body: A rattlebag of molecular mechanisms. J. Physiol. 2018, 596, 2969–2976. [Google Scholar] [CrossRef] [Green Version]
- Duchen, M.R.; Biscoe, T.J. Mitochondrial function in type I cells isolated from rabbit arterial chemoreceptors. J. Physiol. 1992, 450, 13–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckler, K.J.; Turner, P.J. Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J. Physiol. 2013, 591, 3549–3563. [Google Scholar] [CrossRef] [Green Version]
- Mills, E.; Jobsis, F.F. Simultaneous measurement of cytochrome a3 reduction and chemoreceptor afferent activity in the carotid body. Nature 1970, 225, 1147–1149. [Google Scholar] [CrossRef]
- Holmes, A.P.; Ray, C.J.; Coney, A.M.; Kumar, P. Is Carotid Body Physiological O2 Sensitivity Determined by a Unique Mitochondrial Phenotype? Front. Physiol. 2018, 9, 562. [Google Scholar] [CrossRef]
- Chang, A.J. Acute oxygen sensing by the carotid body: From mitochondria to plasma membrane. J. Appl. Physiol. 2017, 123, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.J.; Ortega, F.E.; Riegler, J.; Adison, D.V.M.; Krasnow, M.A. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature 2015, 527, 240–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varas, R.; Buckler, K.J. Regulation of a TASK-like potassium channel in rat carotid body type I cells by ATP. Adv. Exp. Med. Biol. 2006, 580, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Varas, R.; Wyatt, C.N.; Buckler, K.J. Modulation of TASK-like background potassium channels in rat arterial chemoreceptor cells by intracellular ATP and other nucleotides. J. Physiol. 2007, 583, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, C.N.; Evans, A.M. AMP-activated protein kinase and chemotransduction in the carotid body. Respir. Physiol. Neurobiol. 2007, 157, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Aguera, M.C.; Gao, L.; Gonzalez-Rodriguez, P.; Pintado, C.O.; Arias-Mayenco, I.; Garcia-Flores, P.; Garcia-Perganeda, A.; Pascual, A.; Ortega-Saenz, P.; Lopez-Barneo, J. Oxygen Sensing by Arterial Chemoreceptors Depends on Mitochondrial Complex I Signaling. Cell Metab. 2015, 22, 825–837. [Google Scholar] [CrossRef] [Green Version]
- Arias-Mayenco, I.; Gonzalez-Rodriguez, P.; Torres-Torrelo, H.; Gao, L.; Fernandez-Aguera, M.C.; Bonilla-Henao, V.; Ortega-Saenz, P.; Lopez-Barneo, J. Acute O2 Sensing: Role of Coenzyme QH2/Q Ratio and Mitochondrial ROS Compartmentalization. Cell Metab. 2018, 28, 145–158.e144. [Google Scholar] [CrossRef] [Green Version]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijevic, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [Green Version]
- McBryde, F.D.; Abdala, A.P.; Hendy, E.B.; Pijacka, W.; Marvar, P.; Moraes, D.J.; Sobotka, P.A.; Paton, J.F. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat. Commun. 2013, 4, 2395. [Google Scholar] [CrossRef] [Green Version]
- Brognara, F.; Felippe, I.S.A.; Salgado, H.C.; Paton, J.F.R. Autonomic innervation of the carotid body as a determinant of its sensitivity: Implications for cardiovascular physiology and pathology. Cardiovasc. Res. 2021, 117, 1015–1032. [Google Scholar] [CrossRef]
- Wyatt, C.N.; Buckler, K.J. The effect of mitochondrial inhibitors on membrane currents in isolated neonatal rat carotid body type I cells. J. Physiol. 2004, 556, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Piruat, J.I.; Pintado, C.O.; Ortega-Saenz, P.; Roche, M.; Lopez-Barneo, J. The mitochondrial SDHD gene is required for early embryogenesis, and its partial deficiency results in persistent carotid body glomus cell activation with full responsiveness to hypoxia. Mol. Cell Biol. 2004, 24, 10933–10940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, A.R.; Holmes, A.P.; Sample, V.; Kumar, P.; Cann, M.J.; Monteiro, E.C.; Zhang, J.; Gauda, E.B. Bicarbonate-sensitive soluble and transmembrane adenylyl cyclases in peripheral chemoreceptors. Respir. Physiol. Neurobiol. 2013, 188, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, A.P.; Turner, P.J.; Carter, P.; Leadbeater, W.; Ray, C.J.; Hauton, D.; Buckler, K.J.; Kumar, P. Glycogen metabolism protects against metabolic insult to preserve carotid body function during glucose deprivation. J. Physiol. 2014, 592, 4493–4506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintero, M.; Olea, E.; Conde, S.V.; Obeso, A.; Gallego-Martin, T.; Gonzalez, C.; Monserrat, J.M.; Gomez-Nino, A.; Yubero, S.; Agapito, T. Age protects from harmful effects produced by chronic intermittent hypoxia. J. Physiol. 2016, 594, 1773–1790. [Google Scholar] [CrossRef] [Green Version]
- Sacramento, J.F.; Olea, E.; Ribeiro, M.J.; Prieto-Lloret, J.; Melo, B.F.; Gonzalez, C.; Martins, F.O.; Monteiro, E.C.; Conde, S.V. Contribution of adenosine and ATP to the carotid body chemosensory activity in ageing. J. Physiol. 2019, 597, 4991–5008. [Google Scholar] [CrossRef]
- Holmes, A.P.; Nunes, A.R.; Cann, M.J.; Kumar, P. Ecto-5′-Nucleotidase, Adenosine and Transmembrane Adenylyl Cyclase Signalling Regulate Basal Carotid Body Chemoafferent Outflow and Establish the Sensitivity to Hypercapnia. In Arterial Chemoreceptors in Physiology and Pathophysiology; Peers, C., Kumar, P., Wyatt, C.N., Gauda, E., Nurse, C.A., Prabhakar, N., Eds.; Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2015; Volume 860, pp. 279–289. [Google Scholar]
- Holmes, A.P.; Ray, C.J.; Pearson, S.A.; Coney, A.M.; Kumar, P. Ecto-5′-nucleotidase (CD73) regulates peripheral chemoreceptor activity and cardiorespiratory responses to hypoxia. J. Physiol. 2018, 596, 3137–3148. [Google Scholar] [CrossRef] [Green Version]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e413. [Google Scholar] [CrossRef] [Green Version]
- Dikalova, A.E.; Bikineyeva, A.T.; Budzyn, K.; Nazarewicz, R.R.; McCann, L.; Lewis, W.; Harrison, D.G.; Dikalov, S.I. Therapeutic Targeting of Mitochondrial Superoxide in Hypertension. Circ. Res. 2010, 107, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Antonenko, Y.N.; Avetisyan, A.V.; Bakeeva, L.E.; Chernyak, B.V.; Chertkov, V.A.; Domnina, L.V.; Ivanova, O.Y.; Izyumov, D.S.; Khailova, L.S.; Klishin, S.S.; et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: Synthesis and in vitro studies. Biochemistry 2008, 73, 1273–1287. [Google Scholar] [CrossRef]
- Severin, F.F.; Severina, I.I.; Antonenko, Y.N.; Rokitskaya, T.I.; Cherepanov, D.A.; Mokhova, E.N.; Vyssokikh, M.Y.; Pustovidko, A.V.; Markova, O.V.; Yaguzhinsky, L.S.; et al. Penetrating cation/fatty acid anion pair as a mitochondria-targeted protonophore. Proc. Natl. Acad. Sci. USA 2010, 107, 663–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzahrani, A.A.; Cao, L.L.; Aldossary, H.S.; Nathanael, D.; Fu, J.R.; Ray, C.J.; Brain, K.L.; Kumar, P.; Coney, A.M.; Holmes, A.P. beta-Adrenoceptor blockade prevents carotid body hyperactivity and elevated vascular sympathetic nerve density induced by chronic intermittent hypoxia. Pflug. Arch. 2021, 473, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.P.; Turner, P.J.; Buckler, K.J.; Kumar, P. Moderate inhibition of mitochondrial function augments carotid body hypoxic sensitivity. Pflug. Arch. 2016, 468, 143–155. [Google Scholar] [CrossRef]
- Gao, L.; Bonilla-Henao, V.; Garcia-Flores, P.; Arias-Mayenco, I.; Ortega-Saenz, P.; Lopez-Barneo, J. Gene expression analyses reveal metabolic specifications in acute O-2-sensing chemoreceptor cells. J. Physiol. 2017, 595, 6091–6120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Dominguez, A.; Ortega-Saenz, P.; Gao, L.; Colinas, O.; Garcia-Flores, P.; Bonilla-Henao, V.; Aragones, J.; Huttemann, M.; Grossman, L.I.; Weissmann, N.; et al. Acute O-2 sensing through HIF2 alpha-dependent expression of atypical cytochrome oxidase subunits in arterial chemoreceptors. Sci. Signal. 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosqueira, M.; Iturriaga, R. Carotid body chemosensory excitation induced by nitric oxide: Involvement of oxidative metabolism. Respir. Physiol. Neurobiol. 2002, 131, 175–187. [Google Scholar] [CrossRef]
- Zhang, M.; Buttigieg, J.; Nurse, C.A. Neurotransmitter mechanisms mediating low-glucose signalling in cocultures and fresh tissue slices of rat carotid body. J. Physiol. 2007, 578, 735–750. [Google Scholar] [CrossRef]
- Pardal, R.; Lopez-Barneo, J. Low glucose-sensing cells in the carotid body. Nat. Neurosci. 2002, 5, 197–198. [Google Scholar] [CrossRef]
- Conde, S.V.; Obeso, A.; Gonzalez, C. Low glucose effects on rat carotid body chemoreceptor cells’ secretory responses and action potential frequency in the carotid sinus nerve. J. Physiol. 2007, 585, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Martin, T.; Fernandez-Martinez, S.; Rigual, R.; Obeso, A.; Gonzalez, C. Effects of low glucose on carotid body chemoreceptor cell activity studied in cultures of intact organs and in dissociated cells. Am. J. Physiol. Cell Physiol. 2012, 302, C1128–C1140. [Google Scholar] [CrossRef] [Green Version]
- Conde, S.V.; Monteiro, E.C.; Rigual, R.; Obeso, A.; Gonzalez, C. Hypoxic intensity: A determinant for the contribution of ATP and adenosine to the genesis of carotid body chemosensory activity. J. Appl. Physiol. 2012, 112, 2002–2010. [Google Scholar] [CrossRef] [Green Version]
- Spiller, P.F.; da Silva, M.P.; Moraes, D.J.A. Lactate does not activate the carotid body of Wistar rat. Respir. Physiol. Neuro. 2021, 285, 7. [Google Scholar] [CrossRef]
- Peng, Y.J.; Nanduri, J.; Raghuraman, G.; Souvannakitti, D.; Gadalla, M.M.; Kumar, G.K.; Snyder, S.H.; Prabhakar, N.R. H2S mediates O2 sensing in the carotid body. Proc. Natl. Acad. Sci. USA 2010, 107, 10719–10724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallego-Martin, T.; Prieto-Lloret, J.; Aaronson, P.I.; Rocher, A.; Obeso, A. Hydroxycobalamin Reveals the Involvement of Hydrogen Sulfide in the Hypoxic Responses of Rat Carotid Body Chemoreceptor Cells. Antioxidants 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardini, A.; Brockmeier, U.; Metzen, E.; Berchner-Pfannschmidt, U.; Harde, E.; Acker-Palmer, A.; Papkovsky, D.; Acker, H.; Fandrey, J. Type I cell ROS kinetics under hypoxia in the intact mouse carotid body ex vivo: A FRET-based study. Am J. Physiol. Cell Physiol. 2015, 308, C61–C67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardini, A.; Wolf, A.; Brockmeier, U.; Riffkin, H.; Metzen, E.; Acker-Palmer, A.; Fandrey, J.; Acker, H. Carotid body type I cells engage flavoprotein and Pin1 for oxygen sensing. Am. J. Physiol. Cell Physiol. 2020, 318, C719–C731. [Google Scholar] [CrossRef]
- Wang, J.J.; Hogan, J.O.; Wang, R.; White, C.; Kim, D. Role of cystathionine-gamma-lyase in hypoxia-induced changes in TASK activity, intracellular Ca2+ and ventilation in mice. Respir. Physiol. Neuro. 2017, 246, 98–106. [Google Scholar] [CrossRef]
- Peng, Y.J.; Makarenko, V.V.; Gridina, A.; Chupikova, I.; Zhang, X.; Kumar, G.K.; Fox, A.P.; Prabhakar, N.R. H2S mediates carotid body response to hypoxia but not anoxia. Respir. Physiol. Neurobiol. 2019, 259, 75–85. [Google Scholar] [CrossRef]
- Li, Y.L.; Gao, L.; Zucker, I.H.; Schultz, H.D. NADPH oxidase-derived superoxide anion mediates angiotensin II-enhanced carotid body chemoreceptor sensitivity in heart failure rabbits. Cardiovasc. Res. 2007, 75, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Marcus, N.J.; Li, Y.L.; Bird, C.E.; Schultz, H.D.; Morgan, B.J. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: Role of the angiotensin II type 1 receptor. Respir. Physiol. Neurobiol. 2010, 171, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Farnham, M.M.J.; Derakhshan, F.; Pilowsky, P.M.; Wilson, R.J.A. Acute intermittent hypoxia with concurrent hypercapnia evokes P2X and TRPV1 receptor-dependent sensory long-term facilitation in naive carotid bodies. J. Physiol. 2018, 596, 3149–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.J.; Nanduri, J.; Yuan, G.; Wang, N.; Deneris, E.; Pendyala, S.; Natarajan, V.; Kumar, G.K.; Prabhakar, N.R. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J. Neurosci. 2009, 29, 4903–4910. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Peng, Y.-J.; Khan, S.A.; Nanduri, J.; Singh, A.; Vasavda, C.; Semenza, G.L.; Kumar, G.K.; Snyder, S.H.; Prabhakar, N.R. H2S production by reactive oxygen species in the carotid body triggers hypertension in a rodent model of sleep apnea. Sci. Signal. 2016, 9, ra80. [Google Scholar] [CrossRef] [Green Version]
- Aldossary, H.S.; Alzahrani, A.A.; Nathanael, D.; Alhuthail, E.A.; Ray, C.J.; Batis, N.; Kumar, P.; Coney, A.M.; Holmes, A.P. G-Protein-Coupled Receptor (GPCR) Signaling in the Carotid Body: Roles in Hypoxia and Cardiovascular and Respiratory Disease. Int. J. Mol. Sci. 2020, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Moya, E.A.; Arias, P.; Iturriaga, R. Nitration of MnSOD in the Carotid Body and Adrenal Gland Induced by Chronic Intermittent Hypoxia. J. Histochem. Cytochem. 2018, 66, 753–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluckova, K.; Tennant, D.A. Metabolic implications of hypoxia and pseudohypoxia in pheochromocytoma and paraganglioma. Cell Tissue Res. 2018, 372, 367–378. [Google Scholar] [CrossRef] [Green Version]


| Exposure | Intervention | N | ΔRf (bpm) | ΔVT (mL g−1) | ΔVE (mL min−1 g−1) | |
|---|---|---|---|---|---|---|
| Hypoxia (10% O2) | Vehicle control (saline) | − | 5 | 42.2 ± 7 | 0.0012 ± 0.0004 | 0.39 ± 0.06 |
| + | 5 | 43.7 ± 6 | 0.002 ± 0.0003 | 0.5 ± 0.02 | ||
| MitoTEMPO (1.96 μM kg−1) | − | 6 | 57.8 ± 10.7 | 0.0012 ± 0.0002 | 0.47 ± 0.055 | |
| + | 6 | 50.6 ± 7.9 | 0.0014 ± 0.00025 | 0.4 ± 0.03 | ||
| MitoTEMPO (19.6 μM kg−1) | − | 8 | 53 ± 5.3 | 0.001 ± 0.0002 | 0.53 ± 0.07 | |
| + | 8 | 41.6 ± 7.2 * | 0.0015 ± 0.0004 | 0.44 ± 0.027 | ||
| SKQ1 (500 nM kg−1) | − | 6 | 52.8 ± 6 | 0.0014 ± 0.00025 | 0.5 ± 0.03 | |
| + | 6 | 37.7 ± 5.7 * | 0.0016 ± 0.0002 | 0.4 ± 0.03 * | ||
| Hypercapnia (6% CO2) | Vehicle control (saline) | − | 5 | 81 ± 7 | 0.003 ± 0.0006 | 0.87 ± 0.14 |
| + | 5 | 84.7 ± 11 | 0.003 ± 0.0005 | 0.86 ± 0.12 | ||
| MitoTEMPO (1.96 μM kg−1) | − | 6 | 87 ± 8.5 | 0.003 ± 0.0003 | 0.94 ± 0.11 | |
| + | 6 | 80.9 ± 7.7 | 0.0018 ± 0.0002 * | 0.75 ± 0.09 | ||
| MitoTEMPO (19.6 μM kg−1) | − | 8 | 71.6 ± 5.5 | 0.003 ± 0.00025 | 0.98 ± 0.07 | |
| + | 8 | 66.7 ± 3.8 | 0.003 ± 0.0002 | 0.89 ± 0.05 | ||
| SKQ1 (500 nM kg−1) | − | 6 | 68.6 ± 3.8 | 0.0025 ± 0.0005 | 0.78 ± 0.06 | |
| + | 6 | 72.6 ± 8 | 0.003 ± 0.0005 | 0.82 ± 0.07 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swiderska, A.; Coney, A.M.; Alzahrani, A.A.; Aldossary, H.S.; Batis, N.; Ray, C.J.; Kumar, P.; Holmes, A.P. Mitochondrial Succinate Metabolism and Reactive Oxygen Species Are Important but Not Essential for Eliciting Carotid Body and Ventilatory Responses to Hypoxia in the Rat. Antioxidants 2021, 10, 840. https://doi.org/10.3390/antiox10060840
Swiderska A, Coney AM, Alzahrani AA, Aldossary HS, Batis N, Ray CJ, Kumar P, Holmes AP. Mitochondrial Succinate Metabolism and Reactive Oxygen Species Are Important but Not Essential for Eliciting Carotid Body and Ventilatory Responses to Hypoxia in the Rat. Antioxidants. 2021; 10(6):840. https://doi.org/10.3390/antiox10060840
Chicago/Turabian StyleSwiderska, Agnieszka, Andrew M. Coney, Abdulaziz A. Alzahrani, Hayyaf S. Aldossary, Nikolaos Batis, Clare J. Ray, Prem Kumar, and Andrew P. Holmes. 2021. "Mitochondrial Succinate Metabolism and Reactive Oxygen Species Are Important but Not Essential for Eliciting Carotid Body and Ventilatory Responses to Hypoxia in the Rat" Antioxidants 10, no. 6: 840. https://doi.org/10.3390/antiox10060840
APA StyleSwiderska, A., Coney, A. M., Alzahrani, A. A., Aldossary, H. S., Batis, N., Ray, C. J., Kumar, P., & Holmes, A. P. (2021). Mitochondrial Succinate Metabolism and Reactive Oxygen Species Are Important but Not Essential for Eliciting Carotid Body and Ventilatory Responses to Hypoxia in the Rat. Antioxidants, 10(6), 840. https://doi.org/10.3390/antiox10060840







