DNA Protection by an Aronia Juice-Based Food Supplement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Food Supplement Supply
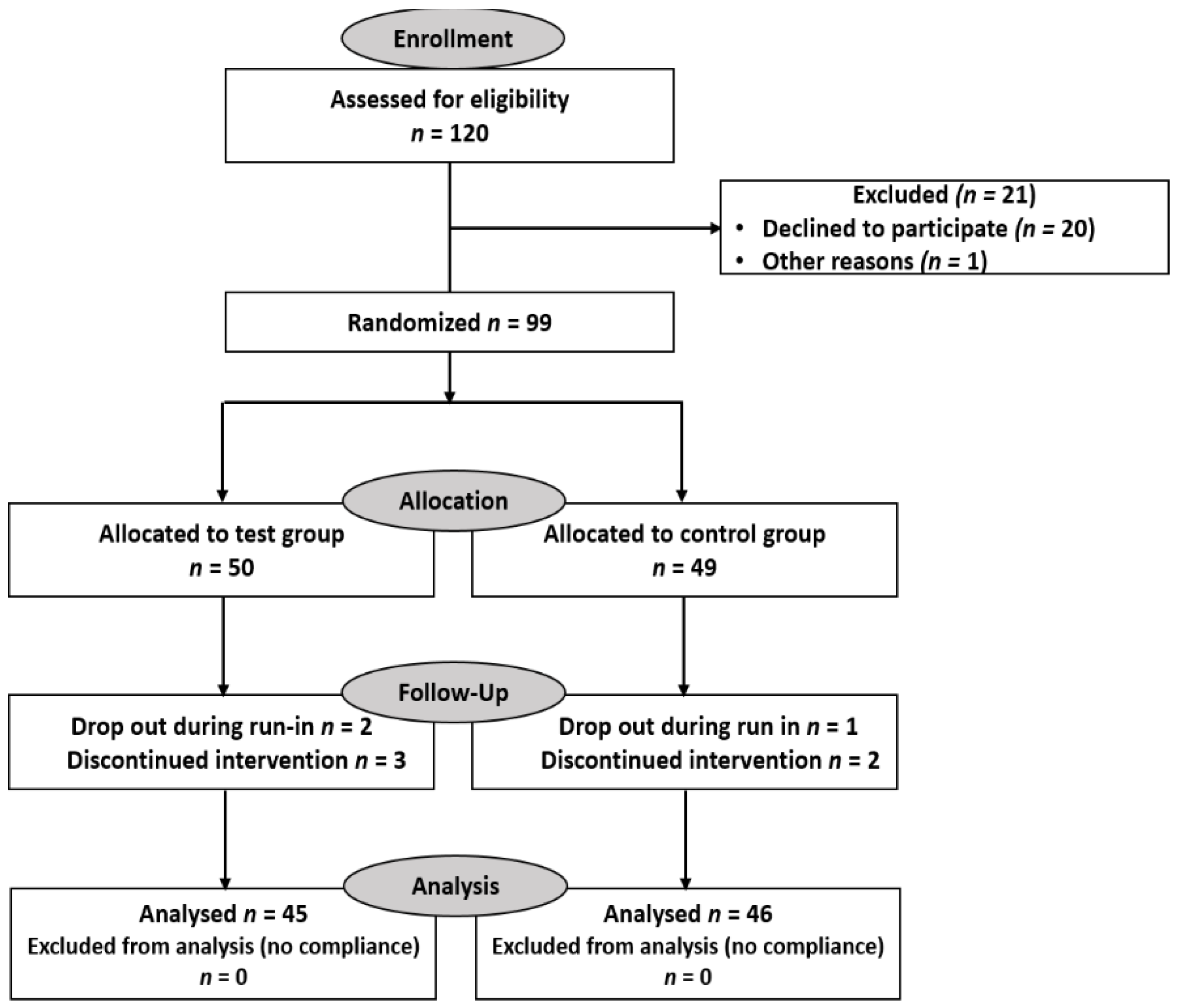
2.3. Subjects and Study Design
2.4. Blood Sample Collection and Isolation of Human Peripheral Blood Lymphocytes (PBL)
2.5. Body Weight Measurement
2.6. Single Cell Gel Electrophoresis Experiments (Comet Assay)
2.6.1. Comet Assay with Whole Blood Samples
2.6.2. Comet Assay in Isolated PBLs after H2O2 Challenge
2.6.3. Statistical Analysis
3. Results
3.1. Demographic Data, Baseline Characteristics, and Health Status of Volunteer Participants
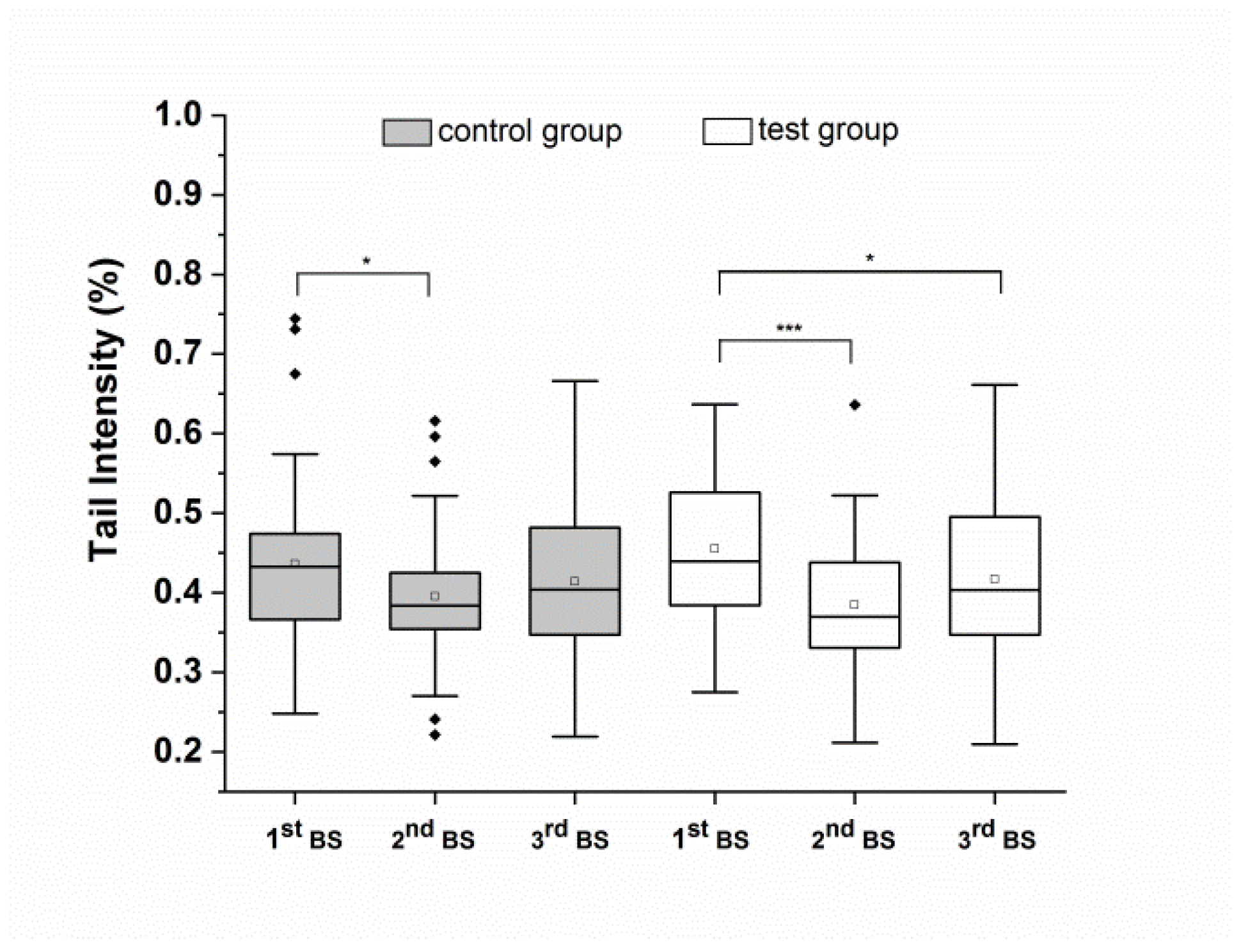
3.2. Aronia Juice-Based Food Supplement Lowers Whole Blood Background DNA Strand Breaks in Healthy Adults
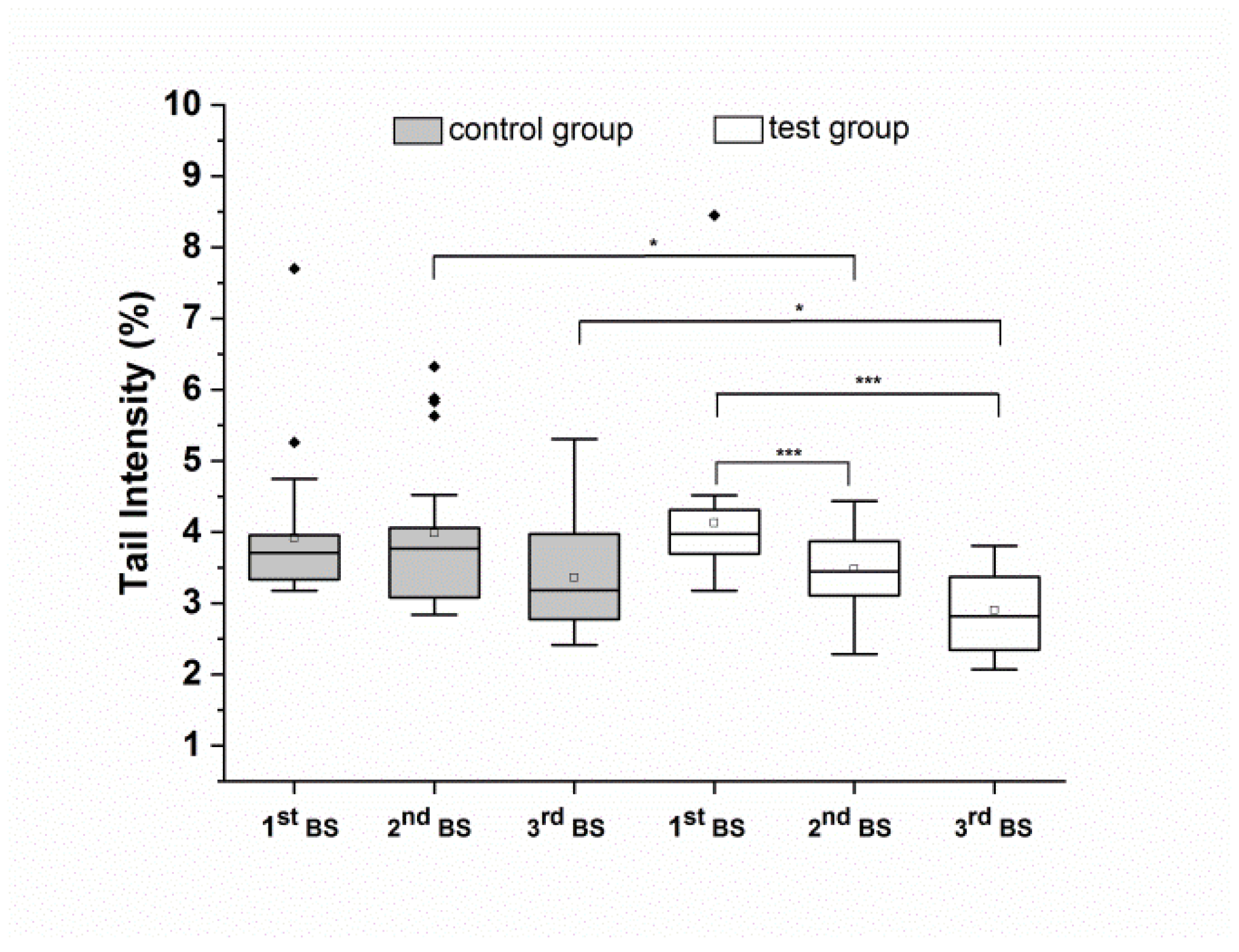
3.3. Intake of the Food Supplement Prevents Total DNA Damaging of Isolated Peripheral Blood Lymphocytes after H2O2 Challenge
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, C.; Li, G.; Chrubasik, S. The clinical effectiveness of chokeberry: A systematic review. Phytother. Res. 2010, 24, 1107–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [Green Version]
- Broncel, M.; Kozirog, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010, 16, CR28-34. [Google Scholar]
- Kuntz, S.; Kunz, C.; Herrmann, J.; Borsch, C.H.; Abel, G.; Fröhling, B.; Dietrich, H.; Rudloff, S. Anthocyanins from fruit juices improve the antioxidant status of healthy young female volunteers without affecting anti-inflammatory parameters: Results from the randomised, double-blind, placebo-controlled, cross-over ANTHONIA (ANTHOcyanins in Nutrition Investigation Alliance) study. Br. J. Nutr. 2014, 112, 925–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakuradze, T.; Tausend, A.; Galan, J.; Groh, I.A.M.; Berry, D.; Tur, J.A.; Marko, D.; Richling, E. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radic. Res. 2019, 53, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Weisel, T.; Baum, M.; Eisenbrand, G.; Dietrich, H.; Will, F.; Stockis, J.-P.; Kulling, S.; Rüfer, C.; Johannes, C.; Janzowski, C. An anthocyanin/polyphenolic-rich fruit juice reduces oxidative DNA damage and increases glutathione level in healthy probands. Biotechnol. J. 2006, 1, 388–397. [Google Scholar] [CrossRef]
- Spormann, T.M.; Albert, F.W.; Rath, T.; Dietrich, H.; Will, F.; Stockis, J.-P.; Eisenbrand, G.; Janzowski, C. Anthocyanin/polyphenolic-rich fruit juice reduces oxidative cell damage in an intervention study with patients on hemodialysis. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3372–3380. [Google Scholar] [CrossRef] [Green Version]
- Kropat, C.; Mueller, D.; Boettler, U.; Zimmermann, K.; Heiss, E.H.; Dirsch, V.M.; Rogoll, D.; Melcher, R.; Richling, E.; Marko, D. Modulation of Nrf2-dependent gene transcription by bilberry anthocyanins in vivo. Mol. Nutr. Food Res. 2013, 57, 545–550. [Google Scholar] [CrossRef]
- Collins, A.R. Measuring oxidative damage to DNA and its repair with the comet assay. Biochim. Biophys. Acta 2014, 1840, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.; Klimis-Zacas, D.; Del Bo’, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; de Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Eisenbrand, G.; Schipp, D.; Galan, J.; Richling, E. Consumption of a dark roast coffee decreases the level of spontaneous DNA strand breaks: A randomized controlled trial. Eur. J. Nutr. 2015, 54, 149–156. [Google Scholar] [CrossRef]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Schipp, D.; Galan, J.; Eisenbrand, G.; Richling, E. Coffee consumption rapidly reduces background DNA strand breaks in healthy humans: Results of a short-term repeated uptake intervention study. Mol. Nutr. Food Res. 2016, 60, 682–686. [Google Scholar] [CrossRef]
- Adamczak, M.; Wiecek, A. Food Products That May Cause an Increase in Blood Pressure. Curr. Hypertens. Rep. 2020, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Gołąbek, K.D.; Regulska-Ilow, B. Dietary support in insulin resistance: An overview of current scientific reports. Adv. Clin. Exp. Med. 2019, 28, 1577–1585. [Google Scholar] [CrossRef]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef] [Green Version]
- Bellinger, F.P.; Raman, A.V.; Reeves, M.A.; Berry, M.J. Regulation and function of selenoproteins in human disease. Biochem. J. 2009, 422, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Skalny, A.A.; Tinkov, A.A.; Medvedeva, Y.S.; Alchinova, I.B.; Karganov, M.Y.; Skalny, A.V.; Nikonorov, A.A. Effect of short-term zinc supplementation on zinc and selenium tissue distribution and serum antioxidant enzymes. Acta Sci. Pol. Technol. Aliments 2015, 14, 269–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakuradze, T.; Becker, D.; Reischmann, J.; Meiser, P.; Galan, J.; Richling, E. Protection from DNA Damage by Use of an Aronia Food Supplement—Results from a Pilot Human Intervention Study. Curr. Pharmacol. Rep. 2019, 5, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Appel, K.; Meiser, P.; Millán, E.; Collado, J.A.; Rose, T.; Gras, C.C.; Carle, R.; Muñoz, E. Chokeberry (Aronia melanocarpa (Michx.) Elliot) concentrate inhibits NF-κB and synergizes with selenium to inhibit the release of pro-inflammatory mediators in macrophages. Fitoterapia 2015, 105, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nio, C.; Payne, M.J.; Reed, J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. MB 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Bøhn, S.K.; Vebraite, V.; Shaposhnikov, S.; Collins, A.R. Isolation of leukocytes from frozen buffy coat for comet assay analysis of DNA damage. Mutat. Res. 2019, 843, 18–23. [Google Scholar] [CrossRef]
- Razavi-Azarkhiavi, K.; Behravan, J.; Mosaffa, F.; Sehatbakhsh, S.; Shirani, K.; Karimi, G. Protective effects of aqueous and ethanol extracts of rosemary on H2O2-induced oxidative DNA damage in human lymphocytes by comet assay. J. Complement. Integr. Med. 2014, 11, 27–33. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.-C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Meyskens, F.L.; Omenn, G.S.; Valanis, B.; Williams, J.H. The Beta-Carotene and Retinol Efficacy Trial: Incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J. Natl. Cancer Inst. 2004, 96, 1743–1750. [Google Scholar] [CrossRef] [Green Version]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groh, I.A.M.; Bakuradze, T.; Pahlke, G.; Richling, E.; Marko, D. Consumption of anthocyanin-rich beverages affects Nrf2 and Nrf2-dependent gene transcription in peripheral lymphocytes and DNA integrity of healthy volunteers. BMC Chem. 2020, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Schipp, D.; Tulinska, J.; Sustrova, M.; Liskova, A.; Spustova, V.; Lehotska Mikusova, M.; Krivosikova, Z.; Rausova, K.; Collins, A.; Vebraite, V.; et al. Consumption of a dark roast coffee blend reduces DNA damage in humans: Results from a 4-week randomised controlled study. Eur. J. Nutr. 2019, 58, 3199–3206. [Google Scholar] [CrossRef]
- Wilms, L.C.; Boots, A.W.; de Boer, V.C.J.; Maas, L.M.; Pachen, D.M.F.A.; Gottschalk, R.W.H.; Ketelslegers, H.B.; Godschalk, R.W.L.; Haenen, G.R.M.M.; van Schooten, F.J.; et al. Impact of multiple genetic polymorphisms on effects of a 4-week blueberry juice intervention on ex vivo induced lymphocytic DNA damage in human volunteers. Carcinogenesis 2007, 28, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Del Bó, C.; Riso, P.; Campolo, J.; Møller, P.; Loft, S.; Klimis-Zacas, D.; Brambilla, A.; Rizzolo, A.; Porrini, M. A single portion of blueberry (Vaccinium corymbosum L) improves protection against DNA damage but not vascular function in healthy male volunteers. Nutr. Res. 2013, 33, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, A.; Kaplan Dor, Y.; Nambara, K.; Pollina, E.A.; Lin, C.; Greenberg, M.E.; Rogulja, D. Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell 2020, 181, 1307–1328.e15. [Google Scholar] [CrossRef] [PubMed]
- Dusinská, M.; Barancoková, M.; Kazimírová, A.; Harrington, V.; Volkovová, K.; Staruchová, M.; Horská, A.; Wsólová, L.; Collins, A. Does occupational exposure to mineral fibres cause DNA or chromosome damage? Mutat. Res. 2004, 553, 103–110. [Google Scholar] [CrossRef]
- Dusinska, M.; Collins, A.R. The comet assay in human biomonitoring: Gene-environment interactions. Mutagenesis 2008, 23, 191–205. [Google Scholar] [CrossRef]


| Amount per 25 mL Drinking Ampule | Amount per 2 × 25 mL Drinking Ampule (Daily Study Dosage) | |
|---|---|---|
| Vitamin D3 | 10 µg | 20 µg |
| Vitamin B1 | 1.1 mg | 2.2 mg |
| Vitamin B2 | 1.4 mg | 2.8 mg |
| Niacin | 16 mg | 32 mg |
| Pantothenic acid | 6 mg | 12 mg |
| Vitamin B6 | 1.4 mg | 2.8 mg |
| Zinc | 4.5 mg | 9.0 mg |
| Selenium | 50 µg | 100 µg |
| Aronia juice concentrate | 6.75 g | 13.50 g |
| Test Group | Control Group | Test vs. Control p-Value | |
|---|---|---|---|
| Number (n) | 50 | 49 | - |
| Sex (women/men) | 25/25 | 25/24 | - |
| Age (years) | 26.3 ± 6.6 | 25.1 ± 5.9 | 0.35 |
| Weight (kg) | 72.9 ± 13.7 | 71.5 ± 11.2 | 0.41 |
| Height (m) | 1.7 ± 0.1 | 1.7 ± 0.1 | 0.75 |
| BMI (kg/m2) | 23.8 ± 3.4 | 23.6 ± 2.8 | 0.51 |
| Group | 1st BS | 2nd BS | 3rd BS | 2nd vs. 1st p-Value | 3rd vs. 1st p-Value | 2nd BS p-Value | 3rd BS p-Value |
|---|---|---|---|---|---|---|---|
| Control | 0.44 ± 0.11 | 0.40 ± 0.1 | 0.41 ± 0.1 | 0.03 | 0.32 | ||
| Test | 0.46 ± 0.09 | 0.39 ± 0.08 | 0.42 ± 0.1 | 0.001 | 0.02 | ||
| Test vs. Control | 0.46 | 0.49 |
| Group | 1st BS | 2nd BS | 3rd BS | 2nd vs. 1st p-Value | 3rd vs. 1st p-Value | 2nd BS p-Value | 3rd BS p-Value |
|---|---|---|---|---|---|---|---|
| Control | 1.24 ± 0.35 | 1.31 ± 0.35 | 1.32 ± 0.4 | 0.32 | 0.27 | ||
| Test | 1.27 ± 0.35 | 1.25 ± 0.34 | 1.28 ± 0.32 | 0.75 | 0.95 | ||
| test vs. control | 0.39 | 0.48 |
| Group | 1st BS | 2nd BS | 3rd BS | 2nd vs. 1st p-Value | 3rd vs. 1st p-Value | 2nd BS p-Value | 3rd BS p-Value |
|---|---|---|---|---|---|---|---|
| Control | 3.91 ± 0.96 | 3.98 ± 1.0 | 3.36 ± 0.74 | 0.8 | 0.06 | ||
| Test | 4.13 ± 0.35 | 3.48 ± 0.51 | 2.9 ± 0.57 | 0.001 | 0.001 | ||
| Test vs. Control | 0.05 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakuradze, T.; Meiser, P.; Galan, J.; Richling, E. DNA Protection by an Aronia Juice-Based Food Supplement. Antioxidants 2021, 10, 857. https://doi.org/10.3390/antiox10060857
Bakuradze T, Meiser P, Galan J, Richling E. DNA Protection by an Aronia Juice-Based Food Supplement. Antioxidants. 2021; 10(6):857. https://doi.org/10.3390/antiox10060857
Chicago/Turabian StyleBakuradze, Tamara, Peter Meiser, Jens Galan, and Elke Richling. 2021. "DNA Protection by an Aronia Juice-Based Food Supplement" Antioxidants 10, no. 6: 857. https://doi.org/10.3390/antiox10060857
APA StyleBakuradze, T., Meiser, P., Galan, J., & Richling, E. (2021). DNA Protection by an Aronia Juice-Based Food Supplement. Antioxidants, 10(6), 857. https://doi.org/10.3390/antiox10060857






